OBJECTIVES:
To summarize the most impactful articles relevant to the pharmacotherapy of critically ill adult patients published in 2021.
DATA SOURCE:
PubMed/MEDLINE.
STUDY SELECTION:
Randomized controlled trials, prospective studies, or systematic review/meta-analyses of adult critical care patients assessing a pharmacotherapeutic intervention and reporting clinical endpoints published between January 1, 2021, and December 31, 2021.
DATA EXTRACTION:
Candidate articles were organized by clinical domain based on the emerging themes from all studies. A modified Delphi process was applied to obtain consensus on the most impactful publication within each clinical domain based on overall contribution to scientific knowledge and novelty to the literature.
DATA SYNTHESIS:
The search revealed 830 articles, of which 766 were excluded leaving 64 candidate articles for the Delphi process. These 64 articles were organized by clinical domain including: emergency/neurology, cardiopulmonary, nephrology/fluids, infectious diseases, metabolic, immunomodulation, and nutrition/gastroenterology. Each domain required the a priori defined three Delphi rounds. The resultant most impactful articles from each domain included five randomized controlled trials and two systematic review/meta-analyses. Topics studied included sedation during mechanical ventilation, anticoagulation in COVID-19, extended infusion beta-lactams, interleukin-6 antagonists in COVID-19, balanced crystalloid resuscitation, vitamin C/thiamine/hydrocortisone in sepsis, and promotility agents during enteral feeding.
CONCLUSIONS:
This synoptic review provides a summary and perspective of the most impactful articles relevant to the pharmacotherapy of critically ill adults published in 2021.
Keywords: COVID-19, critical care, drug therapy, pharmacotherapy, review, sepsis
KEY POINTS
Question: What were the most impactful publications to the pharmacotherapy of critically ill patients in 2021?
Findings: In this comprehensive search and modified Delphi process, five randomized trials and two systematic review/meta-analysis were identified. These articles spanned various critical care topics across multiple clinical domains.
Meaning: There is an evolving and growing body of evidence of treating critically ill patients, and this review provided insight to the most impactful works and additional framework for future research.
The number of medical publications has been exponentially increasing over the years (1), and in 2021 alone, there were nearly 1.8 million new articles indexed in PubMed/MEDLINE. This has left clinicians and researchers with an unrealistic quantity of new knowledge to review and synthesize, often relying on alternative strategies to stay up to date such as social media, webinars, podcasts, and journal clubs, among others (2). The Clinical Pharmacy and Pharmacology Literature Update working group in the Society of Critical Care Medicine, Section on Clinical Pharmacy and Pharmacology, reviews major critical care journals and provides synopses to its members on a monthly basis in addition to an annual review of most impactful articles relevant to critical care pharmacotherapy from the year (3–11). Our objective was to identify the most impactful pharmacotherapy articles published in 2021 as it relates to critical care and provide synopses of these articles with their relevance to bedside care.
METHODS
We performed a systematic search of PubMed/MEDLINE for articles published from January 1, 2021, to December 31, 2021. The search criteria were designed to capture articles relevant to the care of critical care patients, and the full search details can be found in Appendix 1. Two independent reviewers (P.M.W., B.D.B.) screened the titles and abstracts to exclude additional articles that were not relevant to critical care pharmacotherapy. Full-text documents were reviewed to assess final eligibility criteria including: 1) randomized controlled trial, prospective study, or systematic review/meta-analysis design; 2) adult critical care population; 3) pharmacotherapeutic intervention; and 4) clinical endpoints reported. The title, abstract, and full-text of the final included publications were organized into major clinical domain-based categories relevant to critical care pharmacotherapy based on emerging themes when considered in aggregate.
We then applied a modified Delphi process to obtain consensus on the most impactful publications. The Delphi process consisted of a Qualtrics survey containing the title and full-text for each included article, organized by the clinical domain-based categories. Participants (the authors, n = 15) were asked to rank articles, within each domain, specifically in terms of 1) overall contribution to scientific knowledge (morbidity/expense) and 2) novelty to the literature. Individual responses from participants were submitted independently. With the first round of the Delphi process, participants were allowed to recommend additional articles that may not have been captured by the systematic search. We aimed to perform three rounds of the Delphi process and would terminate early if an 80% level of consensus was attained. With each subsequent Delphi round, the articles with less than 50% agreement were removed. At the end of the third round, the article with the highest percentage agreement within each domain was selected to be included in this synoptic review.
RESULTS
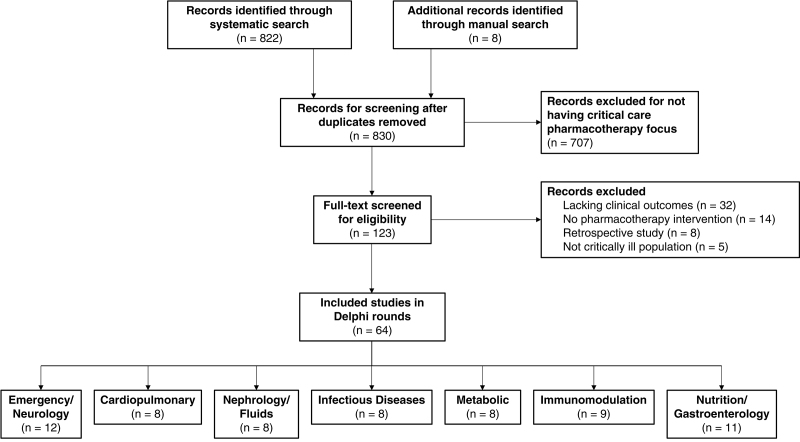
A total of 830 citations were screened for potential inclusion. A large majority of these (n = 707) were excluded for lacking a focus of critical care pharmacotherapy. An additional 59 articles were excluded following full-text review, leaving 64 articles for inclusion in the Delphi process (Fig. 1). No article achieved the prespecified 80% level of consensus agreement during any round. Therefore, each domain underwent the full three rounds of the Delphi process to determine the final articles included in this review (12–18).
Figure 1.
Flow diagram of article screening for inclusion in Delphi rounds.
DISCUSSION
Neurology
Dexmedetomidine or Propofol for Sedation in Mechanically Ventilated Adults with Sepsis (MENDS2)
This was a multicenter, double-blind trial that randomized mechanically ventilated adults with sepsis to receive dexmedetomidine (n = 214) or propofol (n = 208) titrated to target Richmond Agitation–Sedation Scale (RASS) (12). There was no difference in the median number of days alive without delirium or coma at 14 days in the dexmedetomidine compared with propofol arm (10.7 vs 10.8 d; odds ratio [OR] 0.96; 95% CI 0.74–1.26). Secondary outcomes were not significantly different, including ventilator-free days at 28 days, death at 90 days, and global cognition at 6 months. The median daily dose of dexmedetomidine and propofol was 0.27 µg/kg/hr and 10.2 µg/kg/min, respectively, with a median RASS score of –2 in both groups.
The previous MENDS trial found that patients sedated with dexmedetomidine had more days alive without delirium or coma (7 vs 3 d; p = 0.001) when compared with lorazepam (19). In an a priori-designed subgroup analysis, these benefits were more pronounced in septic patients compared with nonseptic patients (20). Furthermore, a reduction in mortality was noted in the septic patients, potentially from dexmedetomidine’s purported anti-inflammatory, immunomodulatory, and neuroprotective properties. However, Kawazoe et al (21) did not find a difference in 28-day mortality or ventilator-free days in patients receiving dexmedetomidine compared with usual care in septic patients. In a larger, open-label trial with 3,904 all-comers, Shehabi et al (22) compared patients randomized early to dexmedetomidine to usual care and again found no difference in 90-day mortality, including a subgroup of septic patients.
This MENDS2 trial lauds several strengths that had limited previous studies, including power, a low rate of unblinding and drug crossover, and assessment of both short-term and long-term outcomes (23). However, the median dosing rates of both dexmedetomidine and propofol were surprisingly low despite nearly half of all patients requiring an antipsychotic during the admission, making it difficult to conclude how much impact these study drugs had on the outcomes, although use was well-balanced between the groups in MENDS2. Furthermore, the 13 study centers had high rates of “ABCDE bundle” adherence, limiting generalizability to practice sites where coordination of the entire bundle may be lacking (24). Taken altogether, at sites where the ABCDE bundle and light sedation are routine, choice of dexmedetomidine or propofol is unlikely to impact outcomes.
Cardiopulmonary
Therapeutic Anticoagulation with Heparin in Critically Ill Patients with COVID-19 (REMAP-CAP, ACTIV-4a, ATTACC)
This open-label, multiplatform trial randomized critically ill patients with severe COVID-19 to receive therapeutic-dose heparin anticoagulation for up to 14 days or recovery, or low or intermediate-dose prophylaxis, and evaluated survival to hospital discharge and organ support-free days up to day 21 (13). This trial was stopped at the interim analysis due to futility. Baseline characteristics were similar. Therapeutic anticoagulation did not increase the probability of survival to hospital discharge (62.7% vs 64.5%; adjacent OR 0.84; 95% CI 0.64–1.11; probability of inferiority 89.2%) or organ support-free days up to day 21 (1 vs 4 d; adjusted OR 0.83; 95% CI 0.67–1.03; probability of inferiority 95%). Major bleeding was numerically greater in those who received therapeutic anticoagulation (3.8% vs 2.3%; adjusted OR 1.48; 95% CI 0.75–3.04; probability of inferiority 87.2%); however, major thrombotic events were similar.
The benefit of therapeutic anticoagulation in critically ill COVID-19 patients has been evaluated in several trials due to the increased incidence of venous thromboembolism in this population (25). Kuno et al (26) found no difference in in-hospital mortality in a retrospectively reviewed subgroup of patients requiring endotracheal intubation who received therapeutic anticoagulation (23.8% vs 20.6%; p = 0.42). Similarly, the ACTION trial did not demonstrate a difference in the hierarchical analysis of time to death, or duration of hospitalization or supplemental oxygen to day 30, with rivaroxaban in stable (win ratio 0.86; 95% CI 0.59–1.22; p = 0.4), or unstable critically ill patients initially started on enoxaparin (win ratio 1.12; 95% CI 0.29–4.29). Bleeding, however, was significantly increased with therapeutic anticoagulation (risk ratio [RR] 3.64; 95% CI 1.61–8.27; p = 0.001) (27).
Similarly, in a subgroup of ICU patients in a systematic review and meta-analysis, although intermediate-to-therapeutic anticoagulation was not associated with reduced in-hospital mortality (RR 0.94; 95% CI 0.79–1.1; p = 0.42), bleeding risk was significantly increased (RR 1.66; 95% CI 1.37–2; p < 0.01) (28). A recently published retrospective analysis further demonstrated a five-fold greater risk of death with therapeutic anticoagulation after 3 or more weeks of ICU stay (adjusted hazard ratio [HR] 4.89; 95% CI 1.71–14; p = 0.003) (29). Intermediate-dose anticoagulation also did not improve outcomes in the INSPIRATION trial at either 30 (OR 1.06; 95% CI 0.76–1.48; p = 0.7) or 90 days (HR 1.21; 95% CI 0.95–1.55; p = 0.11) compared with prophylactic anticoagulation but resulted in numerically greater major bleeding events (HR 1.82; 95% CI 0.53–6.24) (30, 31). Taken altogether, these studies support the avoidance of therapeutic or intermediate-dose anticoagulation in critically ill patients with COVID-19 due to lack of benefit and increased harm, aligning with current National Institutes of Health guidance in favor of prophylactic anticoagulation doses (32).
Infectious Diseases
Loading Dose and Efficacy of Continuous or Extended Infusion of Beta-lactams Compared With Intermittent Administration in Patients With Critical Illnesses: A Subgroup Meta-Analysis and Meta-Regression Analysis
This systematic review and meta-analysis of 31 studies, of which 18 were randomized controlled trials, evaluated clinical outcomes associated with continuous/extended infusion versus intermittent administration of beta-lactam antibiotics in critically ill patients (14). All the included studies used beta-lactams (piperacillin/tazobactam, ticarcillin/clavulanate, cefepime, ceftazidime, ceftriaxone, cefoperazone, doripenem, imipenem/cilastatin, or meropenem). Overall, continuous/extended infusion beta-lactams were associated with a significant reduction in mortality (RR 0.82; 95% CI 0.72–0.94) and improved clinical cure (RR 1.31; 95% CI 1.15–1.49) when compared with intermittent administration. In the subgroup analyses, an increase in clinical cure was associated with continuous/extended infusion loading-dose subgroups (RR 1.44; 95% CI 1.22–1.69), but no significant difference in overall mortality rates was found across loading-dose subgroups. After adjusting for beta-lactam type in the multiple meta-regression analysis, the association between clinical cure and loading dose with continuous/extended infusion was found to be significant (RR 1.43; 95% CI 1.12–1.80; p = 0.006). A significant decrease in overall mortality was observed in the continuous/extended infusion carbapenem, penicillin, and beta-lactamase inhibitor groups but not in the cephalosporin group. Clinical cure was only significantly improved in the continuous/extended infusion carbapenem group. Importantly, no significant heterogeneity was found among the studies for mortality (I2 = 13.1%); however, there was substantial heterogeneity present for rates of clinical cure (I2 = 72.7%). Despite this, the authors aggregated retrospective studies with randomized trials, as such there was still a wide range of quality of evidence included and varying severity of illnesses represented across the studies. However, a strength of the study by Wu et al (14) is the use of subgroup analysis to identify signals of benefit among sicker patients and, unsurprisingly, higher quality studies.
Many factors including augmented renal clearance, acute kidney injury, and renal replacement therapy may alter antibiotic concentrations in critically ill patients, and dosing strategies remain unclear. The Surviving Sepsis Campaign suggests using prolonged infusion beta-lactam antibiotics over conventional bolus infusions for adults with sepsis or septic shock; however, ensuring the initial loading dose precedes this to ensure timely achievement of target beta-lactam concentrations (33). Accordingly, extending the infusion time of beta-lactam antibiotics has been shown to improve clinical outcomes and achievement of concentrations above the minimum inhibitory concentration in critically ill patients (34–36). Using prolonged infusions of beta-lactams in critically ill patients should be considered if the necessary equipment is available without delaying timely administration, also considering local or geographical susceptibility and antimicrobial resistance patterns. Further research involving loading doses, extended versus continuous beta-lactam infusions, cost effectiveness, and development of antimicrobial resistance is needed. Specifically, based on the findings of the study by Wu et al(14), definitive trials should focus on more consistent dosing protocols and patients with high severity of illness at greater risk of mortality, as well as strict inclusion of high quality of evidence if additional meta-analysis is pursued.
Immunomodulation
Interleukin-6 Receptor Antagonists in Critically Ill Patients with COVID-19 (REMAP-CAP)
This international, randomized, open-label, multifactorial, adaptive platform trial encompasses numerous treatment domains (e.g., glucocorticoids, interleukin [IL]–6 antagonist) and in each domain multiple interventions (15). Within the immune modulation domain, severely ill, COVID-19 confirmed adults were assigned to tocilizumab 8 mg/kg (n = 353), sarilumab 400 mg (n = 48), or standard of care (n = 402) within 24 hours of requiring organ support (e.g., vasopressors, mechanical ventilation). The primary outcome was an ordinal composite of organ (cardiovascular and respiratory) support free days up to 21. At baseline, 99.6% and 18.8% of patients were requiring respiratory and cardiovascular support, respectively. Over 90% of patients enrolled received glucocorticoids. Both agents showed probability of superiority to control with the median organ support free days of 10 for tocilizumab (interquartile range [IQR] –1 to 16, adjusted OR 1.64; 95% CI 1.25–2.14; probability of superiority > 99.9%), 11 for sarilumab (IQR 0–16; adjusted OR 1.3; 95% CI 1.17–2.91; probability of superiority > 99.5%), and 0 for control (IQR –1 to 15). There was a 9% absolute reduction of in-hospital mortality with IL-6 antagonists as compared with control (27% vs 36%).
Prior to the publication of REMAP-CAP, numerous studies evaluating tocilizumab for treatment of COVID-19 did not demonstrate benefit regarding mortality or prevention of progression of disease (37–40). This could be in part due to the low usage of dexamethasone since RECOVERY-dexamethasone had not been published. Several studies evaluated the use of tocilizumab in less severe patients. The Boston Area COVID-19 Consortium Bay Tocilizumab Trial did not find a difference between tocilizumab and control in patients requiring supplemental oxygen in regard to preventing death or intubation (HR 0.83; 95% CI 0.38–1.81; p = 0.64) (40).
RECOVERY-tocilizumab published results after REMAP-CAP with a larger sample size (n = 4,116) which demonstrated mortality benefit of tocilizumab in patients who are not only critically ill, but also in patients who are hypoxic (oxygen saturation [Spo2] < 93% without support) with systemic inflammation (e.g., C-reactive protein ≥ 75 mg/L). Mortality at 28 days was lower with tocilizumab (31%) than control (35%; RR 0.85; 95% CI 0.76–0.94; p = 0.0028) (41). REMAP-CAP and RECOVERY were pinnacle in reshaping the current National Institutes of Health guideline on COVID-19 which provides grade BII1 recommendation for tocilizumab (in combination with dexamethasone) for patients 1) with rapidly increasing oxygen requirements signs of systemic inflammation or 2) within 24 hours of ICU admission and requiring mechanical ventilation (32).
Fluids/Nephrology
Effect of Early Balanced Crystalloids Before ICU Admission on Sepsis Outcomes
This was a secondary analysis of the Isotonic Solutions and Major Adverse Renal Events Trial (SMART) dataset to determine whether controlling the selection of IV fluids from the time septic patients present to the emergency department and throughout their ICU stay was associated with any differences compared with when IV fluids were not controlled until septic patients were admitted to the ICU (16). SMART was a single-center, open-label, cluster-randomized, multiple cross-over trial, which compared the use of balanced crystalloids with 0.9% sodium chloride in critically ill adults in the ICU setting (42). The primary outcome of this secondary analysis was 30-day in-hospital mortality. Among patients whose IV fluids were only controlled in the ICU, 30-day in-hospital mortality occurred in 33.1% of patients who received balanced crystalloids compared with 32.9% of patients who received 0.9% sodium chloride (OR 1.14; 95% CI 0.70–1.88). Among patients whose IV fluids were controlled in both the emergency department and ICU, 30-day in-hospital mortality occurred in 24.9% of patients who received balanced crystalloids compared with 30.6% of patients who received 0.9% sodium chloride (OR 0.68; 95% CI 0.52–0.89; p = 0.07).
Whether the use of balanced versus unbalanced crystalloid fluids in patients with sepsis and septic shock leads to differences in clinical outcomes has been subject to debate. Predating the availability of this SMART secondary analysis, the 2021 Surviving Sepsis Campaign Guidelines favor using balanced crystalloids instead of normal saline (33). The results of Jackson et al (43) suggest that septic patients will experience a more substantial mortality benefit when controlled to receive balanced crystalloids in both the emergency department and ICU compared with later when they are admitted to the ICU. This is consistent with other aspects of sepsis care, wherein a faster time to appropriate antibiotic administration and source control are also associated with improved mortality (43, 44). A previous analysis showed no correlation between time to completion of a 30 mL/kg IV fluid bolus and mortality, but the type of fluid was not tracked (45). Additionally, since the guideline recommendation, the Plasma-Lyte 148 versus Saline and the Balanced Solutions in Intensive Care Study trials performed in Australia/New Zealand and Brazil, respectively, did not find any difference in 90-day mortality between balanced solutions and normal saline (46, 47). A recent meta-analysis including these studies, and in total comprising nearly 35,000 patients, found a risk ratio of 0.96 (95% CI 0.91–1.01) for 90-day mortality with balanced solutions as compared with normal saline and 0.93 (95% CI 0.86–1.01) in a subgroup analysis of 6,754 septic patients (48). Bayesian meta-analysis was also performed for the overall 90-day mortality with balanced solutions compared with normal saline and demonstrated nearly 90% probability of reduced mortality with balanced solutions (RR 0.96; credible interval 0.88–1.04). Collectively, the evidence to date appears to support the use of balanced crystalloids over unbalanced crystalloids when administering IV fluids to patients with sepsis and septic shock.
Metabolic
Effect of Vitamin C, Thiamine, and Hydrocortisone on Ventilator- and Vasopressor-free Days in Patients With Sepsis: The VICTAS Randomized Clinical Trial
The use of metabolic resuscitators in sepsis and septic shock with the so-called “HAT (hydrocortisone, ascorbic acid, thiamine) Therapy” has remained controversial. This multicenter, randomized, double-blind, placebo-controlled trial enrolled patients with cardiac and/or respiratory dysfunction due to sepsis to receive HAT therapy (vitamin C 1.5 g IV, thiamine 100 mg IV, and hydrocortisone 50 mg IV) or placebo every 6 hours for 96 hours (17). The study was initially planned to use an adaptive design for flexible sample size but was terminated early at n equals to 500 interim analysis due to abrupt withdrawal of the funding source. Of the 501 enrolled persons, there were no differences between intervention and control groups in vasopressor and ventilator-free days (25 vs 26 d; median difference –1; 95% CI –4 to 2 d; p = 0.85) or 30-day (22% vs 24%; p not reported) or 180-day (40.5% vs 37.8%; 95% CI –11.3% to 5.8%; p = 0.81) all-cause mortality.
The physiologic rationale for using HAT therapy includes: 1) vitamin C’s antioxidant scavenging capacity and improvement and protection of microcirculatory perfusion, 2) thiamine’s crucial role in aerobic metabolism and entry of pyruvate to the Krebs cycle, and 3) hydrocortisone’s benefit for a relative adrenal insufficiency and purported synergistic effects among the agents (49). The VICTAS trial adds to the growing body of clinical trial evidence demonstrating a lack of benefit of the HAT therapy in sepsis and septic shock (50–53). Although previous studies have suggested faster shock reversal, those results are confounded by less corticosteroid administration in control arms (50, 53). Accordingly, the Surviving Sepsis Campaign weakly recommends against the routine use of vitamin C in patients with sepsis or septic shock, albeit, prior to publication of the VICTAS results (33). However, important questions remain regarding timing and dosing of the intervention that are unanswered by current available evidence. The median time to treatment in the VICTAS trial from a qualifying organ dysfunction onset was nearly 15 hours, which is similar to other studies assessing this intervention (17). Whether there are putative benefits of HAT therapy in early sepsis, including prior to the progression to organ dysfunction where theoretically the effects on oxidative stress and the microvascular endothelium integrity may be most pronounced, remains to be determined. Similarly, most studies concluding a lack of benefit have assessed similar dosing strategies of vitamin C (i.e., 1.5 g IV every 6 hr), which was initially proposed on the basis of being sufficient to restore plasma concentrations in those deficient from critical illness (54). A higher dosage (50 mg/kg IV every 6 hr) was used in the CITRIS-ALI trial that, despite finding no differences in organ dysfunction scores, suggested a reduced 28-day mortality compared with placebo (29.8% vs 46.3%; p = 0.03) (52). This study was limited to septic patients with acute lung injury, so the potential for beneficial effect of higher dosages cannot be excluded from the available evidence.
Nutrition/Gastroenterology
The Efficacy and Safety of Prokinetics in Critically Ill Adults Receiving Gastric Feeding Tubes: A Systematic Review and Meta-Analysis
This systematic review and meta-analysis evaluated the effect of prokinetic regimens on feeding intolerance using a gastric residual volume (GRV) threshold of greater than or equal to 500 mL (52). Random-effects model pooled 15 randomized controlled trials comparing prokinetic treatment (metoclopramide, erythromycin, or other prokinetic agents at any dose, frequency, duration, or combination) and herbal or natural medication to control. Ten trials including 846 critically ill patients provided quantitative results. Ten of 13 studies (76%) showed a beneficial effect of prokinetic agents on gastric feeding tolerance and symptoms assessed by GRV, gastric emptying, diarrhea, constipation, feeding complications, and intolerance. Trials included prokinetic agents like metoclopramide (6/15; 40%), erythromycin (2/15; 13%), and herbal or natural medicines (5/15; 33%). Prokinetic agents decreased hospital length of stay by a mean difference of –3.21 days (95% CI –5.35 to –1.06; p = 0.003) in five studies (I2 = 28%) and ICU length of stay by a mean difference of –2.03 days (95% CI –3.96 to –0.10, p = 0.04) in three studies (I2 = 0%). There was no difference in adverse events (RR 1.13; 95% CI 0.92–1.38; p = 0.25) or all-cause mortality (RR 0.96; 95% CI 0.81–1.14; p = 0.64). Certainty of evidence was moderate for all-cause mortality and low for adverse events, ICU, and hospital length of stay due to risk of bias and imprecision.
GRV monitoring as a sole reason to withhold enteral nutrition is not recommended, but GRV greater than or equal to 500 mL/6 hr or greater than or equal to500 mL in combination with other feeding intolerance symptoms should prompt enteral nutrition interruption and prokinetic agents or postpyloric feeding (55, 56). Previous meta-analysis showed favorable feeding tolerance, reduction in GRV, and increased postpyloric feeding tube placement with prokinetic agents (57). However, feeding intolerance included GRV of greater than or equal to 150 mL, and routine approach to postpyloric feeding was not established. Peng et al (18) restricted trial inclusion to reflect updated guideline recommendations including GRV greater than or equal to 500 mL combined with feeding intolerance symptoms and a standardized approach to postpyloric or cessation of gastric feeding. Prokinetic agents’ beneficial effect on feeding tolerance is consistent with previous studies (58, 59). However various outcome definitions for feeding tolerance limited quantitative assessment, so the magnitude of effect is unknown. Caution should be applied to the conclusion of prokinetic agent benefit on hospital or ICU length of stay, which differs from previously published trails, due to low certainty of evidence based on GRADE methodology (55–57, 60). No conclusion on prokinetic agent of choice can be gleaned from the results, as the subgroup analysis found no significant subgroup differences.
CONCLUSIONS
In this review, we provided synopses of the most impactful articles relative to the pharmacotherapy of critically ill patients published in 2021. The studies presented herein add the growing body of evidence of treating the critically ill and provide additional framework for future research.
ACKNOWLEDGMENTS
This Society of Critical Care Medicine (SCCM) work-product was commissioned by the SCCM Section on Clinical Pharmacy and Pharmacology (CPP). We thank the members of the CPP Pharmacotherapy Literature Update working group including George Abdallah, Beth Israel Deaconess; Tori Adams, Northwestern Memorial; Diana Altshuler, NYU Langone; Mahmoud Ammar, Yale; Brooke Barlow, UK Healthcare; Alyson Basting, IU Health Arnett; Allison Boyd, Rhode Island Hospital; Judah Brown, Thomas Jefferson University Hospital; Lisa Burry, Mount Sinai; Tyler Chanas, Vidant Med Center; Laura Cole, Wake Forest; Reagan Collins, MD Anderson; Patrick Costello, U of Chicago; Aubrey Defayette, Roswell Park Comprehensive Cancer Center; Dharati Desai, Advocate Christ Med Center; Payal Desai, UCMC; Elisabeth Donahey, Lexicomp; Chris Droege, UCMC Cincinnati; Mary Eche, Beth Israel Deaconess; Michael Erdman, UF Health Jacksonville; Joel Feih, Froedtert; Daniela Fernandez, Nova Southeastern U COP; Mallory Fiorenza, Lee Memorial; David Gagnon, Maine Med Center; Gabrielle Gibson, Barnes-Jewish; Brian Gilbert, Wesley Med Center; Kasey Greathouse, Northwestern Memorial; Leslie Hamilton, U Tennessee; Jennifer Hanify, Duke U; Mai Hashhoush, King Fahad Spec Hosp Dammam; Michelle Henninger, Cleveland Clinic; Olivia Henton, Ohio Health Riverside Methodist; Benjamin Hohlfelder, Cleveland Clinic; Randy Hollins, Tufts; Michelle Horng, MD Anderson; Ah Hyun Jun, Augusta U Med Center; Janelle Juul, Froedtert; Kristi Kim, UPMC Harrisburg; Brian Kopp, Banner—UMC Tucson; Kinsey Kowalski, UC Health Mem Central; Carolyn Magee, MUSC; Courtney Makowski, Northwestern Memorial; Chris Miller, St Anthony Hospital; Megan Moore, Sanford Medl Center; Mandy Morris, UCSF; Andrea Newsome, Augusta U Med Center; Bill Olney, UK Healthcare; Mona Patel, NY-Presbyterian; Sarah Peppard, Concordia University; Haley Peters, IU Health Methodist; Caitlin Pfaff, UC Health; Carolyn Philpott, UCMC; Angela Plewa-Rusiecki, Stroger of Cook County; Nicole Reardon, Ocala Regional; Brianne Ritchie, Mayo Clinic; Ryan Rivosecchi, U Pitt Med Center; Lauren Roller, Touro U California; Melissa Santibanez, Nova Southeastern U COP; Samantha Say, U Virginia Med Center; Mike Semanco, Lakeland Regional Health; Angela Slampak-Cindric, Geisinger Med Center; Adam Smith, Riverside Methodist; Melanie Smith, MUSC; Ryan Szaniawski, Froedtert; Colleen Teevan, Hartford Healthcare; William Tidwell, Vanderbilt; Rachel LaBianca Toler, Duke Regional; Megan Van Berkel Patel, Erlanger; Amanda Wiebe, Memorial Regional Med Center; Corey Witenko, NY-Presbyterian; and Jason Yerke, Cleveland Clinic.
APPENDIX 1. Systematic Search Criteria
Search: ((critical care[MeSH Terms]) OR (care unit, intensive[MeSH Terms]) OR (critical illness)[MeSH Terms])).
Filters: Full text, Clinical Study, Clinical Trial, Clinical Trial, Phase I, Clinical Trial, Phase II, Clinical Trial, Phase III, Clinical Trial, Phase IV, Comparative Study, Controlled Clinical Trial, Meta-Analysis, Pragmatic Clinical Trial, Randomized Controlled Trial, Validation Study.
Footnotes
This study was a work product of the Society of Critical Care Medicine and endorsed by the Section on Clinical Pharmacy and Pharmacology.
The authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Chi Y: Global trends in medical journal publishing. J Korean Med Sci 2013; 28:1120–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kamtchum-Tatuene J, Zafack JG: Keeping up with the medical literature: Why, how, and when? Stroke 2021; 52:e746–e748 [DOI] [PubMed] [Google Scholar]
- 3.Turck CJ, Frazee E, Kram B, et al. : Major publications in the critical care pharmacotherapy literature: February 2012 through February 2013. Am J Heal Pharm 2014; 71:68–77 [DOI] [PubMed] [Google Scholar]
- 4.Rech MA, Day SA, Kast JM, et al. : Major publications in the critical care pharmacotherapy literature: January–December 2013. Am J Heal Pharm 2015; 72:224–236 [DOI] [PubMed] [Google Scholar]
- 5.Day SA, Cucci M, Droege ME, et al. : Major publications in the critical care pharmacotherapy literature: January–December 2014. Am J Heal Pharm 2015; 72:1974–1985 [DOI] [PubMed] [Google Scholar]
- 6.Wong A, Erdman M, Hammond DA, et al. : Major publications in the critical care pharmacotherapy literature in 2015. Am J Heal Pharm 2017; 74:295–311 [DOI] [PubMed] [Google Scholar]
- 7.Horner D, Altshuler D, Droege C, et al. : Major publications in the critical care pharmacotherapy literature: January–December 2016. J Crit Care 2018; 43:327–339 [DOI] [PubMed] [Google Scholar]
- 8.Hammond DA, Baumgartner L, Cooper C, et al. : Major publications in the critical care pharmacotherapy literature: January–December 2017. J Crit Care 2018; 45:239–246 [DOI] [PubMed] [Google Scholar]
- 9.Newsome AS, Bissell BD, Burry LD, et al. : Major publications in critical care pharmacotherapy literature in 2018. J Crit Care 2019; 52:200–207 [DOI] [PubMed] [Google Scholar]
- 10.Condeni MS, Basting AT, Costello PG, et al. : Major publications in the critical care pharmacotherapy literature: 2019. J Crit Care 2021; 62:197–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bissell BD, Campbell J, Collins R, et al. : Major publications in the critical care pharmacotherapy literature: 2020. Crit Care Explor 2021; 3:e0590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hughes CG, Mailloux PT, Devlin JW, et al. ; MENDS2 Study Investigators: Dexmedetomidine or propofol for sedation in mechanically ventilated adults with sepsis. N Engl J Med 2021; 384:1424–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.REMAP-CAP Investigators, ACTIV-4a Investigators, ATTACC Investigators et al. : Therapeutic anticoagulation with heparin in critically ill patients with COVID-19. N Engl J Med 2021; 385:777–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu C-C, Su Y-C, Wu K-S, et al. : Loading dose and efficacy of continuous or extended infusion of beta-lactams compared with intermittent administration in patients with critical illnesses: A subgroup meta-analysis and meta-regression analysis. J Clin Pharm Ther 2021; 46:424–432 [DOI] [PubMed] [Google Scholar]
- 15.REMAP-CAP Investigators: Interleukin-6 receptor antagonists in critically ill patients with covid-19. N Engl J Med 2021; 384:1491–1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jackson KE, Wang L, Casey JD, et al. ; SMART Investigators and the Pragmatic Critical Care Research Group: Effect of early balanced crystalloids before ICU admission on sepsis outcomes. Chest 2021; 159:585–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sevransky JE, Rothman RE, Hager DN, et al. ; VICTAS Investigators: Effect of vitamin C, thiamine, and hydrocortisone on ventilator- and vasopressor-free days in patients with sepsis: The VICTAS randomized clinical trial. JAMA 2021; 325:742–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peng R, Li H, Yang L, et al. : The efficacy and safety of prokinetics in critically ill adults receiving gastric feeding tubes: A systematic review and meta-analysis. PLoS One 2021; 16:e0245317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pandharipande PP, Pun BT, Herr DL, et al. : Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients. JAMA 2007; 298:2644–2653 [DOI] [PubMed] [Google Scholar]
- 20.Pandharipande PP, Sanders RD, Girard TD, et al. ; MENDS investigators: Effect of dexmedetomidine versus lorazepam on outcome in patients with sepsis: An a priori-designed analysis of the MENDS randomized controlled trial. Crit Care 2010; 14:R38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawazoe Y, Miyamoto K, Morimoto T, et al. ; Dexmedetomidine for Sepsis in Intensive Care Unit Randomized Evaluation (DESIRE) Trial Investigators: Effect of dexmedetomidine on mortality and ventilator-free days in patients requiring mechanical ventilation with sepsis. JAMA 2017; 317:1321–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shehabi Y, Howe BD, Bellomo R, et al. ; ANZICS Clinical Trials Group and the SPICE III Investigators: Early sedation with dexmedetomidine in critically ill patients. N Engl J Med 2019; 380:2506–2517 [DOI] [PubMed] [Google Scholar]
- 23.Girard TD, Exline MC, Carson SS, et al. ; MIND-USA Investigators: Haloperidol and ziprasidone for treatment of delirium in critical illness. N Engl J Med 2018; 379:2506–2516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsieh SJ, Otusanya O, Gershengorn HB, et al. : Staged implementation of awakening and breathing, coordination, delirium monitoring and management, and early mobilization bundle improves patient outcomes and reduces hospital costs*. Crit Care Med 2019; 47:885–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klok FA, Kruip MJHA, van der Meer NJM, et al. : Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res 2020; 191:145–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuno T, So M, Takahashi M, et al. : Prophylactic versus therapeutic anticoagulation for survival of patients with COVID-19 on steroid. J Thromb Thrombolysis 2022; 53:352-358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lopes RD, de Barros E Silva PGM, Furtado RHM, et al. ; ACTION Coalition COVID-19 Brazil IV Investigators: Therapeutic versus prophylactic anticoagulation for patients admitted to hospital with COVID-19 and elevated D-dimer concentration (ACTION): An open-label, multicentre, randomised, controlled trial. Lancet 2021; 397:2253–2263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang S, Li Y, Liu G, et al. : Intermediate-to-therapeutic versus prophylactic anticoagulation for coagulopathy in hospitalized COVID-19 patients: A systemic review and meta-analysis. Thromb J 2021; 19:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoogenboom WS, Lu JQ, Musheyev B, et al. : Prophylactic versus therapeutic dose anticoagulation effects on survival among critically ill patients with COVID-19. PLoS One 2022; 17:e0262811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bikdeli B, Talasaz AH, Rashidi F, et al. : Intermediate-dose versus standard-dose prophylactic anticoagulation in patients with COVID-19 admitted to the intensive care unit: 90-day results from the INSPIRATION randomized trial. Thromb Haemost 2022; 122:131–141 [DOI] [PubMed] [Google Scholar]
- 31.Sadeghipour P, Talasaz AH, Rashidi F, et al. ; INSPIRATION Investigators: Effect of intermediate-dose vs standard-dose prophylactic anticoagulation on thrombotic events, extracorporeal membrane oxygenation treatment, or mortality among patients with COVID-19 admitted to the intensive care unit: The INSPIRATION randomized clinic. JAMA 2021; 325:1620–1630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.National Institutes of Health: Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. Available at: https://www.covid19treatmentguidelines.nih.gov. Accessed February 2, 2022 [PubMed]
- 33.Evans L, Rhodes A, Alhazzani W, et al. : Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Crit Care Med 2021; 49:e1063–e1143 [DOI] [PubMed] [Google Scholar]
- 34.Kondo Y, Ota K, Imura H, et al. : Prolonged versus intermittent β-lactam antibiotics intravenous infusion strategy in sepsis or septic shock patients: A systematic review with meta-analysis and trial sequential analysis of randomized trials. J Intensive Care 2020; 8:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roberts JA, Abdul-Aziz M-H, Davis JS, et al. : Continuous versus intermittent β-lactam infusion in severe sepsis. A meta-analysis of individual patient data from randomized trials. Am J Respir Crit Care Med 2016; 194:681–691 [DOI] [PubMed] [Google Scholar]
- 36.Abdul-Aziz MH, Sulaiman H, Mat-Nor M-B, et al. : Beta-lactam infusion in severe sepsis (BLISS): A prospective, two-centre, open-labelled randomised controlled trial of continuous versus intermittent beta-lactam infusion in critically ill patients with severe sepsis. Intensive Care Med 2016; 42:1535–1545 [DOI] [PubMed] [Google Scholar]
- 37.Hermine O, Mariette X, Tharaux P-L, et al. ; CORIMUNO-19 Collaborative Group: Effect of tocilizumab vs usual care in adults hospitalized with COVID-19 and moderate or severe pneumonia. JAMA Intern Med 2021; 181:32–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salvarani C, Dolci G, Massari M, et al. ; RCT-TCZ-COVID-19 Study Group: Effect of tocilizumab vs standard care on clinical worsening in patients hospitalized with COVID-19 pneumonia. JAMA Intern Med 2021; 181:24–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosas IO, Bräu N, Waters M, et al. : Tocilizumab in hospitalized patients with severe COVID-19 pneumonia. N Engl J Med 2021; 384:1503–1516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stone JH, Frigault MJ, Serling-Boyd NJ, et al. ; BACC Bay Tocilizumab Trial Investigators: Efficacy of tocilizumab in patients hospitalized with covid-19. N Engl J Med 2020; 383:2333–2344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.RECOVERY Collaborative Group: Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial. Lancet 2021; 397:1637–1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Semler MW, Self WH, Wanderer JP, et al. ; SMART Investigators and the Pragmatic Critical Care Research Group: Balanced crystalloids versus saline in critically ill adults. N Engl J Med 2018; 378:829–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kumar A, Roberts D, Wood KE, et al. : Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 2006; 34:1589–1596 [DOI] [PubMed] [Google Scholar]
- 44.Karvellas CJ, Dong V, Abraldes JG, et al. : The impact of delayed source control and antimicrobial therapy in 196 patients with cholecystitis-associated septic shock: A cohort analysis. Can J Surg 2019; 62:189–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seymour CW, Gesten F, Prescott HC, et al. : Time to treatment and mortality during mandated emergency care for sepsis. N Engl J Med 2017; 376:2235–2244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Finfer S, Micallef S, Hammond N, et al. ; PLUS Study Investigators and the Australian New Zealand Intensive Care Society Clinical Trials Group: Balanced multielectrolyte solution versus saline in critically ill adults. N Engl J Med 2022; 386:815–826 [DOI] [PubMed] [Google Scholar]
- 47.Zampieri FG, Machado FR, Biondi RS, et al. : Effect of intravenous fluid treatment with a balanced solution vs 0.9% saline solution on mortality in critically ill patients. JAMA 2021; 326:818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hammond NE, Zampieri FG, Di Tanna GL, et al. : Balanced crystalloids versus saline in critically ill adults—A systematic review with meta-analysis. NEJM Evid 2022; 1:1-12 [DOI] [PubMed] [Google Scholar]
- 49.Marik PE: Hydrocortisone, ascorbic acid and thiamine (HAT therapy) for the treatment of sepsis. Focus on ascorbic acid. Nutrients 2018; 10:1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moskowitz A, Huang DT, Hou PC, et al. ; ACTS Clinical Trial Investigators: Effect of ascorbic acid, corticosteroids, and thiamine on organ injury in septic shock: The ACTS randomized clinical trial. JAMA 2020; 324:642–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fujii T, Luethi N, Young PJ, et al. ; VITAMINS Trial Investigators: Effect of vitamin C, hydrocortisone, and thiamine vs hydrocortisone alone on time alive and free of vasopressor support among patients with septic shock: The VITAMINS randomized clinical trial. JAMA 2020; 323:423–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fowler AA, Truwit JD, Hite RD, et al. : Effect of vitamin C infusion on organ failure and biomarkers of inflammation and vascular injury in patients with sepsis and severe acute respiratory failure: The CITRIS-ALI randomized clinical trial. JAMA 2019; 322:1261–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Iglesias J, Vassallo AV, Patel VV, et al. : Outcomes of metabolic resuscitation using ascorbic acid, thiamine, and glucocorticoids in the early treatment of sepsis: The ORANGES trial. Chest 2020; 158:164–173 [DOI] [PubMed] [Google Scholar]
- 54.Marik PE, Khangoora V, Rivera R, et al. : Hydrocortisone, vitamin C, and thiamine for the treatment of severe sepsis and septic shock: A retrospective before-after study. Chest 2017; 151:1229–1238 [DOI] [PubMed] [Google Scholar]
- 55.McClave SA, Taylor BE, Martindale RG, et al. : Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient. J Parenter Enter Nutr 2016; 40:159–211 [DOI] [PubMed] [Google Scholar]
- 56.Singer P, Blaser AR, Berger MM, et al. : ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr 2019; 38:48–79 [DOI] [PubMed] [Google Scholar]
- 57.Lewis K, Alqahtani Z, Mcintyre L, et al. : The efficacy and safety of prokinetic agents in critically ill patients receiving enteral nutrition: A systematic review and meta-analysis of randomized trials. Crit Care 2016; 20:259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.MacLaren R, Kiser TH, Fish DN, et al. : Erythromycin vs metoclopramide for facilitating gastric emptying and tolerance to intragastric nutrition in critically ill patients. J Parenter Enter Nutr 2008; 32:412–419 [DOI] [PubMed] [Google Scholar]
- 59.Reignier J, Bensaid S, Perrin-Gachadoat D, et al. : Erythromycin and early enteral nutrition in mechanically ventilated patients*. Crit Care Med 2002; 30:1237–1241 [DOI] [PubMed] [Google Scholar]
- 60.Booth CM, Heyland DK, Paterson WG: Gastrointestinal promotility drugs in the critical care setting: A systematic review of the evidence*. Crit Care Med 2002; 30:1429–1435 [DOI] [PubMed] [Google Scholar]