Abstract
Objective
This study compared small dense low-density lipoprotein cholesterol (sdLDL-C) with apolipoprotein B (apo B), and low-density lipoprotein particles (LDL-P) in predicting CHD risk in generally healthy adults with normal fasting glucose (NFG).
Methods
This study was conducted among participants with NFG in the Multi-Ethnic Study of Atherosclerosis (MESA) prospective cohort with measurements of sdLDL-C, LDL-P, and apo B available at baseline (2000–2002) and follow-up CHD data (through 2015) (N = 3,258). Biomarkers were evaluated as quartiles, and in categories using clinically and 75th percentile-defined cut-points. Discordance/concordance of sdLDL-C relative to other biomarkers was calculated using 75th percentile cut-points and linear regression residuals. Associations between individual biomarkers, sdLDL-C discordance and CHD incidence were evaluated using Cox proportional hazards regression.
Results
There were 241 incident CHD events in this population through 2015. Higher sdLDL-C, apo B, LDL-P were similarly associated with increased CHD in individuals with NFG. Discordance of sdLDL-C with apo B or LDL-P by 75th percentiles was not significantly associated with CHD. Residuals discordantly higher/lower sdLDL-C relative to apo B (discordant high HR=1.26, 95% CI: 0.89, 1.78; discordant low HR=0.94, 95% CI: 0.68, 1.29) and LDL-P (discordant high HR=1.25, 95% CI: 0.88, 1.75; discordant low HR=0.84, 95% CI:0.60, 1.16), compared to those with concordant measures, had non-statistically significant higher/lower risk of CHD.
Conclusions
Results suggest sdLDL-C, apo B and LDL-P are generally comparable for predicting CHD events in normoglycemic individuals. Larger studies are needed to confirm findings and to investigate whether measurement of sdLDL-C may be beneficial to evaluate as an additional risk-enhancing factor.
Keywords: Small dense low-density lipoprotein cholesterol, Apolipoprotein B (apo B), Low-density lipoprotein particles, Coronary heart disease
1. Introduction
Despite advances in coronary heart disease (CHD) risk prediction, limitations remain in reducing CHD. Elevated low-density lipoprotein cholesterol (LDL-C) is associated with CHD risk, and has historically been used as part of CHD risk assessment [1,2]. However, LDL particle (LDL-P) sizes are heterogeneous with varying amounts of cholesterol contained in each particle. Therefore, individuals may have similar measurements for LDL-C, but differing numbers of LDL-P. It is theorized that the number of particles (higher number more atherogenic) [3,4] and the size of particles (smaller size more atherogenic) [5], [6], [7] may impact level of risk, indicating that biomarkers that best capture this information may be better at predicting risk. This has resulted in the proposed usage of biomarkers that better reflect particle number, including LDL-P as a direct measurement of particle number [8]; and apolipoprotein B (apo B), a protein component present on all types of atherogenic lipoproteins with one molecule per particle providing an indirect particle number measurement [9].
Another more recent biomarker of interest is small dense low-density lipoprotein cholesterol (sdLDL-C). Previous studies have shown that sdLDL-C is associated with CHD risk [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], including in individuals considered lower risk for CHD based on current clinical standards, but was expensive and difficult to compare across studies due to variations in methods until the development of an automated assay in recent years [12,16,25]. Additionally, recent genetic research indicates an association of sdLDL-C with SNPs linked to CHD risk, which could also be a unique pathway by which sdLDL-C contributes to CHD incidence [16,26]. Therefore, measurement of sdLDL-C could have clinical significance for identifying populations that do not screen as higher risk, but who could benefit from treatment intervention, such as statins, but research is currently limited.
The 2018 multi-society guideline recommends measurement of Apo B as a risk-enhancing factor and recently published recommendations from the National Lipid Association (NLA) conclude that measurement of apo B or LDL-P may be warranted to guide lipid therapy in select populations [1,8]. However, neither set of guidelines currently recommends measurement of sdLDL-C, but NLA recommendations specifically state that future studies are needed to reveal how best to utilize sdLDL assays [1]. SdLDL-C has previously been shown to better predict CHD risk than LDL-C and non-HDL-C, but it is unclear if sdLDL provides the same or additional risk prediction beyond Apo B and/or LDL-P [25]. It has been previously shown that sdLDL-C and apo B are only associated with CHD risk among participants with normal fasting glucose (NFG) in this MESA population [12]. Therefore, the primary objective of this study is to compare these biomarkers for predicting CHD risk among individuals with NFG. We hypothesized that, as a more direct measure of small dense particles, sdLDL-C would better predict risk for CHD compared to LDL-P and apo B.
2. Materials and methods
The study population was derived from 6814 subjects enrolled in the Multi-Ethnic Study of Atherosclerosis (MESA), a multi-center cohort developed by the National Heart, Lung, and Blood Institute (NHLBI) to investigate atherosclerotic risk factors and subclinical disease progression [27]. Between July 2000 and August 2002, men and women aged 45 to 84 years and free of clinically apparent cardiovascular disease were recruited from four ethnic/racial groups (White, Black, Hispanic, or Chinese American) at six centers in the United States (Baltimore, MD; Chicago, IL; Forsyth County, NC; Los Angeles County, CA; New York, NY; St. Paul, MN). The present study utilizes baseline exposure and covariate data and outcome follow-up through 2015 with a median follow-up time of 14.1 years. Follow-up was stopped in 2015 for the present study due to duration of time between baseline measurements and follow-up time. Longer follow-up duration results in attenuation of associations, which would be expected as participants health and medication usage would change over time, but ends up obscuring any potential true associations.
Participants who were on statins at baseline or who were a part of the “MESA 1000″ sub-study do not have measurements of sdLDL-C, so were excluded from the current analysis (N = 2143). Individuals with missing outcome data (N = 21), a non-CHD atherosclerotic cardiovascular disease event (N = 215) or probable angina without myocardial infarction, a coronary bypass graft (CABG) or percutaneous transluminal coronary angioplasty (PTCA) (N = 9) during follow-up were also excluded. Participants missing key covariate or lipoprotein biomarker measurements were additionally excluded (N = 145). As previously noted, sdLDL-C and apo B are only associated with CHD among participants with normal fasting glucose in this MESA population; therefore, the present analysis includes only non-diabetic individuals with normal fasting glucose (fasting glucose <100 mg/dl) (analytic N = 3258). Those that were included/excluded were generally similar, but those excluded were slightly older and were more likely to be diabetic and have hypertension. The study was approved by all institutional review boards for the MESA field centers and all participants provided informed consent.
Lipids were measured on EDTA plasma at a central laboratory using 12-hour fasted blood samples collected at baseline study visits and stored at −70 °C. Total cholesterol and triglycerides were measured on a Roche COBAS FARA centrifugal analyzer (Roche Diagnostics, Indianapolis, IN) using cholesterol oxidase methods and triglyceride GB, respectively. Following precipitation of non-HDL-C (magnesium/dextran), high-density lipoprotein cholesterol (HDL-C) was quantified by cholesterol oxidase methods (Roche Diagnostics, Indianapolis, IN). Non-HDL-C concentrations were obtained by subtracting measured HDL-C from total cholesterol. The Friedewald equation was used to calculate LDL-C [28]. Apo B concentrations were quantified using the Tina-quant Apolipoprotein B ver.2 immunoassay on a Roche Modular P analyzer (Roche Diagnostics, Indianapolis, IN) at Health Diagnostics Laboratory Inc. (Richmond, VA). Lipoprotein particles were measured on plasma specimens frozen at −70 °C by NMR spectroscopy using the LipoProfile-3 algorithm at LipoScience, Inc. (Raleigh, N.C.), as described previously [3,4]. Direct measurement of cholesterol within small dense LDL particles (15.0 nm-20.0 nm) was completed using a previously validated automated homogenous assay (Denka Seiken Co., Ltd., Tokyo) and analyzed on a Roche/Hitachi Modular P Chemistry Analyzer [25]. Coefficients of variation for lipid biomarkers were 1.6% (total cholesterol), 4.0% (triglycerides), 2.9% (HDL-C), <5% (Apo B), <4% (LDL-P), and 3.2% (sdLDL-C). SdLDL-C and apo B are presented as mg/dl throughout the manuscript and LDL-P as nmol/L.
Incident CHD (NFG event N = 241) through 2015 was used for this study and was defined as the first occurrence of any of the following: myocardial infarction, resuscitated cardiac arrest, CHD death, or definite angina. Definite angina was defined as symptoms of typical chest pain and physician diagnosis of angina followed by CABG and PTCA, evidence of ischemia by stress tests or resting ECG, or ≥70% obstruction on coronary angiography. Probable angina was defined as chest pain or atypical symptoms with a physician diagnosis of angina followed by treatment, including CABG or PTCA.
At the baseline study visit, demographic, personal and medical history, and lifestyle behaviors data were obtained via questionnaire or interview and study staff measured height, weight, waist and hip circumferences, blood pressure and fasting glucose. Body mass index (BMI) (kg/m2) was calculated from measured height and weight. Hypertension was defined as diastolic blood pressure ≥90 mmHG or a systolic blood pressure ≥140 mmHG.
Population characteristics were summarized as medians and proportions according to sdLDL-C quartile. Univariate associations were evaluated using one-way ANOVA and cross-tabulation Wald X2 tests. Spearman correlations were conducted between sdLDL-C, apo B and LDL-P.
Lipoprotein biomarkers were evaluated as quartiles with the following values at cut-points for each: sdLDL-C = 26.1, 35.4, 48.7 mg/dl; apo B = 90.9, 106.7, 124.3 mg/dl; LDL-P = 1020.0, 1232.0, 1473.0 nmol/L. Due to their similarities, and limited studies comparing the three measures, sdLDL-C, apo B and LDL-P were additionally evaluated as binary variables using both clinical cut-points and 75th percentile cut-points. The clinical cut-points used were as follows: sdLDL-C = 50 mg/dl; apo B = 130 mg/dl; and LDL-P = 1300 nmol/L with sdLDL-C and LDL-P cut-points based on company recommended cut-points. In addition, we also compared 75th percentile cut-points for each of the three biomarkers, which were 48.7 mg/dl for sdLDL-C, 124.3 mg/dl for apo B and 1473.0 nmol/L for LDL-P to allow for evaluation of the biomarkers at a similar point in the population distribution.
Discordance of sdLDL-C with apo B and LDL-P was evaluated using two approaches: (1) 75th percentile cut-points (previously outlined); and (2) the residuals approach conducted in prior analyses of lipoprotein biomarkers [29], [30], [31]. Percentile cut-points were used as the clinical cut-points corresponded with different population percentiles and resulted in insufficient numbers of participants in certain clinical cut-point discordant categories. Differences in linear regression residuals (observed-expected) were computed between sdLDL-C and apo B and LDL-P. Discordance was defined as: discordant low (sdLDL-C residual <25th percentile); concordant (sdLDL-C residual between 25th – 75th percentiles); discordant high (sdLDL-C residual >75th percentile).
Cox proportional hazards regression was used to evaluate associations of individual measures (quartiles and binary categories) and concordance/discordance of sdLDL-C with incidence of CHD. Three versions of models were conducted (1) Model 0=unadjusted; (2) Model 1=age, sex and race/ethnicity; (3) Model 2=Model 1 + hypertension, hypertension medication usage, log-transformed triglycerides, waist circumference and HDL-C. Covariates in the final model (Model 2) were those chosen a priori for known associations with the exposure or outcome and were retained if they remained associated in the final Model 2 and/or changed the magnitude of the association between the exposure and outcome. Additional covariates that were considered, but not included because they did not change study results (data not shown) include: lipid lowering medication use, metabolic syndrome, smoking status, alcohol intake, body mass index, and total cholesterol. AUC concordance statistics (c-statistics) were tabulated in Cox proportional hazards regression models using Harrell's c-statistic for sdLDL-C, apo B and LDL-P as quartiles and using clinical cut-points. As a sensitivity analysis, initiation of statin usage after baseline was evaluated as a potential covariate, but did not change study findings (data not presented). Statistical analysis was conducted using SAS 9.4 (SAS Institute Inc, Cary, NC) and a p-value <0.05 was defined as statistical significance.
3. Results
Baseline population characteristics among participants with NFG by sdLDL-C quartile are presented in Table 1. Female sex appeared to be inversely associated with sdLDL-C, while age generally did not differ across quartiles. Blacks were more likely to have lower sdLDL-C, while Hispanics and Chinese were somewhat more likely to be in the top quartiles. BMI, WC, fasting glucose, LDL-C, non-HDL-C, apo B, LDL-P, total cholesterol and triglycerides all increased across increasing sdLDL-C quartiles. HDL-C decreased across quartiles and lbLDL-C was lowest in the lowest and highest sdLDL-C quartiles. Baseline concentrations of sdLDL-C were statistically significantly (P<0.001) correlated with apo B (Spearman r = 0.84) and LDL-P (Spearman r = 0.74).
Table 1.
Baseline characteristics of the Multi-Ethnic Study of Atherosclerosis study population across quartiles of small dense low-density lipoprotein cholesterol levels (sdLDL-C) among participants with normal fasting glucosed (N = 3258).
| sdLDL-C Quartile 1 | sdLDL-C Quartile 2 | sdLDL-C Quartile 3 | sdLDL-C Quartile 4 | |
|---|---|---|---|---|
| Overallb | 887 (27.2) | 841 (25.8) | 791 (24.3) | 739 (22.7) |
| Agec | 61.0 (51.0, 70.0) | 60.0 (52.0, 69.0) | 61.0 (53.0, 69.0) | 59.0 (52.0, 67.0) |
| Gender (female)b | 520 (58.6) | 513 (61.0) | 410 (51.8) | 370 (50.1) |
| Race/Ethnicityb | 341 (38.4) | 343 (40.8) | 325 (41.1) | 308 (41.7) |
| White | 318 (35.8) | 264 (31.4) | 180 (22.8) | 118 (16.0) |
| Black | 95 (10.7) | 89 (10.6) | 100 (12.6) | 110 (14.9) |
| Chinese | 133 (15.0) | 145 (17.2) | 186 (23.5) | 203 (30.4) |
| Hispanic | 438 (49.6) | 412 (49.2) | 416 (52.7) | 378 (51.3) |
| Smokingb | 320 (36.2) | 328 (39.1) | 265 (33.5) | 277 (37.6) |
| Never | 125 (14.2) | 98 (11.7) | 109 (13.8) | 82 (11.1) |
| Former | 171 (19.4) | 152 (18.3) | 157 (19.9) | 167 (22.7) |
| Current | 212 (24.1) | 203 (24.4) | 162 (20.6) | 151 (20.5) |
| Alcoholb | 497 (56.5) | 476 (57.3) | 468 (59.5) | 419 (56.8) |
| Never | 294 (33.2) | 291 (34.6) | 308 (38.9) | 275 (37.2) |
| Former | 248 (28.0) | 224 (26.6) | 236 (29.8) | 180 (24.4) |
| Current | 25.7 (23.1, 29.6) | 26.6 (23.5, 30.1) | 27.0 (24.3, 30.6) | 27.5 (24.9, 30.8) |
| Hypertensionb | 92.2 (82.0, 101.5) | 93.7 (84.5, 103.5) | 95.6 (88.0, 104.5) | 97.1 (89.5, 105.0) |
| Hypertension medication useb | 85.0 (80.0, 91.0) | 86.0 (81.0, 91.0) | 87.0 (82.0, 92.0) | 88.0 (83.0, 93.0) |
| BMI (kg/m2)c | 21.0 (17.5, 23.5) | 30.6 (28.4, 33.0) | 41.0 (38.1, 44.7) | 58.4 (53.1, 68.1) |
| Waist (cm)c | 75.3 (62.3, 88.0) | 86.8 (72.5, 99.1) | 87.8 (71.1, 104.2) | 79.7 (63.6, 100.0) |
| Fasting glucose (mg/dl)c | 82.4 (72.2, 91.7) | 100.8) (92.8, 108.6) | 115.2 (104.9, 125.3) | 134.8 (122.3, 148.2) |
| sdLDL-C (mg/dL)c | 96.0 (81.0, 110.0) | 117.0 (103.0, 130.0) | 129.0 (113.0, 146.0) | 142.0 (123.0, 164.0) |
| lbLDL-C (mg/dL)a,c | 939.0 (808.0, 1070.0) | 1146.0 (1033.0, 1283.0) | 1329.0 (1189.0, 1492.0) | 1561.0 (1387.0, 1780.0) |
| Apo B (mg/dL)c | 111.0 (97.0, 125.0) | 136.0 (122.0, 149.0) | 153.0 (139.0, 168.0) | 178.0 (159.0, 197.0) |
| LDL-C (mg/dL)c | 56.0 (46.0, 67.0) | 53.0 (44.0, 64.0) | 48.0 (39.0, 59.0) | 44.0 (38.0, 52.0) |
| LDL-P, (nmol/L)c | 169.0 (152.0, 185.0) | 191.0 (178.0, 206.0) | 204.0 (187.0, 223.0) | 224.0 (204.0, 245.0) |
| Non-HDL-C (mg/dL)c | 71.0 (55.0, 92.0) | 91.0 (69.0, 118.0) | 119.0 (92.0, 156.0) | 171.0 (132.0, 221.0) |
| HDL-C (mg/dL)c | ||||
| Total Cholesterol (mg/dL)c | ||||
| Triglycerides (mg/dL)c |
Abbreviations: Apolipoprotein-B, apo B; body mass index, BMI; high-density lipoprotein cholesterol, HDL-C; interquartile range, IQR; large buoyant low-density lipoprotein cholesterol, lbLDL-C; low-density lipoprotein cholesterol, LDL-C; low-density lipoprotein particle, LDL-P; non-high-density lipoprotein cholesterol, non-HDL-C; small dense-low-density lipoprotein cholesterol, sdLDL-C.
LDL-C minus sdLDL-C.
n (%).
Median (IQR).
Normal fasting glucose (NFG)=non-diabetic with fasting glucose <100.
Among individuals with NFG sdLDL-C (quartile 4 vs. quartile 1 HR=1.74, 95% CI: 1.11, 2.73; P = 0.01) and apo B (quartile 4 vs. quartile 1 HR=1.50, 95% CI: 1.02, 2.25; P = 0.03), but not LDL-P, were positively associated with CHD incidence in Model 2 (Table 2). Further evaluations of sdLDL-C, apo B, and LDL-P using clinical and 75th percentile cut-points indicated significant associations with CHD events (Table 3) among NFG participants. Hazard ratios for sdLDL-C (HR=1.72, 95% CI: 1.23, 2.40; P = 0.001) were somewhat higher when clinical cut-points were used compared to apo B (HR=1.63, 95% CI:1.19, 2.24; P = 0.002) and LDL-P (HR=1.46, 95% CI: 1.11, 1.92; P = 0.007) in adjusted Model 2. However, when defined using comparably-derived 75th percentile cut-points HRs were consistent between sdLDL-C (HR=1.49, 1.07, 2.08; P = 0.01), apo B (HR=1.47, 95%: 1.09, 1.98; P = 0.008) and LDL-P (HR=1.51, 95% CI: 1.12 2.03; P = 0.006). C-statistics were similar for sdLDL-C (quartile c-statistic=0.7431, SE=0.0149; clinical cut-point c-statistic=0.7412, SE=0.0147), apo B C (quartile c-statistic=0.7411, SE=0.0152; clinical cut-point c-statistic=0.7372, SE=0.0151) and LDL-P C (quartile c-statistic=0.7372, SE=0.0150; clinical cut-point c-statistic=0.7322, SE=0.0153) modeled both as quartiles and using clinical cut-points.
Table 2.
Associations between baseline sdLDL-C, apo B and LDL-P with incident CHD through 2015 among participants with normal fasting glucosea (N = 3258).
| Model 1c | Model 2d | ||
|---|---|---|---|
| Quartiles | N Events/ Total N | HR (95% CI) | HR (95% CI) |
| sdLDL-Cb | |||
| Q1 | 57/887 | Reference | Reference |
| Q2 | 57/841 | 1.07 (0.74, 1.55) | 1.08 (0.74, 1.57) |
| Q3 | 59/791 | 1.17 (0.81, 1.69) | 1.21 (0.80, 1.81) |
| Q4 | 68/739 | 1.60 (1.12, 2.29) | 1.74 (1.11, 2.74) |
| Apo Bb | |||
| Q1 | 56/862 | Reference | Reference |
| Q2 | 56/829 | 1.00 (0.69, 1.45) | 0.96 (0.66, 1.40) |
| Q3 | 58/793 | 1.10 (0.76, 1.59) | 1.07 (0.73, 1.58) |
| Q4 | 71/774 | 1.50 (1.05, 2.14) | 1.49 (1.01, 2.20) |
| LDL-Pb | |||
| Q1 | 68/861 | Reference | Reference |
| Q2 | 42/852 | 0.61 (0.41, 0.89) | 0.58 (0.39, 0.85) |
| Q3 | 56/782 | 0.88 (0.62, 1.26) | 0.82 (0.57, 1.19) |
| Q4 | 75/763 | 1.26 (0.90, 1.76) | 1.17 (0.81, 1.70) |
Abbreviations: Apolipoprotein-B, apo B; confidence interval, CI; hazard ratio, HR; high-density lipoprotein cholesterol, HDL-C; interquartile range, IQR; low-density lipoprotein particle, LDL-P; small dense-low-density lipoprotein cholesterol, sdLDL-C.
Normal fasting glucose (NFG)=non-diabetic with fasting glucose <100. bQuartiles cut-point: sdLDL-C = 26.1, 35.4, 48.7 mg/dl; LDL-C = 99.0, 119.0, 139.0 mg/dl; apoB=90.9, 106.7, 124.3 mg/dl; LDL-P = 1020.0, 1232.0, 1473.0 nmol/L.
Cox proportional hazard regression model adjusted for age (continuous), sex (female/male), race/ethnicity (White/Black/Chinese/Hispanic).
Cox proportional hazard regression model adjusted for age, sex, race/ethnicity, hypertension, blood pressure medication use, triglycerides, high-density lipoprotein cholesterol, waist circumference.
P-value for quartile 4 versus quartile 1.
Table 3.
Associations between clinical and 75th percentile cut-points for baseline sdLDL, apo B and LDL-P and CHD incidence through 2015 among participants with normal fasting glucosea (N = 3258).
| Model 1c | Model 2d | ||
|---|---|---|---|
| N Events/ Total N | HR (95% CI) | HR (95% CI) | |
| sdLDL-C | |||
| Clinical Cut-pointb | 173/2574 | Reference | Reference |
| <50 mg/dl | 68/684 | 1.61 (1.21, 2.14) | 1.75 (1.25, 2.44) |
| ≥50 mg/dl | 173/2511 | P = 0.001 | P = 0.001 |
| 75th Percentile | 68/747 | Reference | Reference |
| <48.7 mg/dl | 1.45 (1.09, 1.92) | 1.52 (1.09, 2.13) | |
| ≥48.7 mg/dl | P = 0.01 | P = 0.01 | |
| Apo B | |||
| Clinical Cut-pointb | 182/2655 | Reference | Reference |
| <130 mg/dl | 59/603 | 1.58 (1.17, 2,12) | 1.63 (1.19, 2.23) |
| ≥130 md/dl | 170/2480 | P = 0.003 | P = 0.003 |
| 75th Percentile | 71/778 | Reference | Reference |
| <124.3 mg/dl | 1.44 (1.09, 1.91) | 1.48 (1.10, 1.99) | |
| ≥124.3 mg/dl | P = 0.01 | P = 0.001 | |
| LDL-P | |||
| Clinical Cut-pointb | 122/1968 | Reference | Reference |
| <1300 nmol/L | 119/1290 | 1.51 (1.17, 1.94) | 1.45 (1.10, 1.91) |
| ≥1300 nmol/L | 166/2495 | P = 0.002 | P = 0.008 |
| 75th Percentile | 75/763 | Reference | Reference |
| <1473 nmol/L | 1.53 (1.16, 2.01) | 1.51 (1.12, 2.03) | |
| ≥1473 nmol/L | P = 0.03 | P = 0.007 |
Abbreviations: Apolipoprotein-B, apo B; confidence interval, CI; hazard ratio, HR; high-density lipoprotein cholesterol, HDL-C; interquartile range, IQR; low-density lipoprotein particle, LDL-P; small dense-low-density lipoprotein cholesterol, sdLDL-C.
Normal fasting glucose (NFG)=non-diabetic with fasting glucose <100.
Cut-points based on clinical recommendations (apo B) or recommended cut-points from test provider (for LDL-P and sdLDL-C).
Cox proportional hazard regression model adjusted for age, sex, race/ethnicity.
Cox proportional hazard regression model adjusted for age, sex, race/ethnicity, hypertension, blood pressure medication use, triglycerides, high-density lipoprotein cholesterol, waist circumference.
Discordance analyses by 75th percentile cut-points are outlined in Table 4. Concordantly high sdLDL-C and apo B or LDL-P was associated with increased risk of CHD. Discordantly low or high sdLDL-C relative to high or low, respectively, apo B or LDL-P was not associated with CHD risk, however, numbers in these categories were also relatively small.
Table 4.
Associations between lipid markers concordance/discordance using 75th percentile and clinical cut-points for sdLDL-C, apo B and LDL-P with CHD incidence through 2015 among participants with normal fasting glucosea (N = 3258).
| Model 1b | Model 2c | ||
|---|---|---|---|
| N Events/ Total N | HR (95% CI) | HR (95% CI | |
| 75th Percentile | |||
| sdLDL-C/Apob | |||
| <48.7 mg/dl/<124.3 mg/dl | 155/2264 | Reference | Reference |
| <48.7 mg/dl/≥124.3 mg/dl | 18/247 | 1.09 (0.67, 1.78) | 1.11 (0.68, 1.81) |
| ≥48.7 mg/dl/<124.3 mg/dl | 15/216 | 1.06 (0.62, 1.81) | 1.07 (0.61, 1.89) |
| ≥48.7 mg/dl/≥124.3 mg/dl | 53/531 | 1.64 (1.19, 2.25) | 1.75 (1.22, 2.50) |
| sdLDL-C/LDL-P | |||
| <48.7 mg/dl/<1473 nmol/L | 147/2223 | Reference | Reference |
| <48.7 mg/dl/≥1473 nmol/L | 26/288 | 1.33 (0.87, 2.02) | 1.28 (0.83, 1.96) |
| ≥48.7 mg/dl/<1473 nmol/L | 19/272 | 1.15 (0.71, 1.86) | 1.21 (0.72, 2.04) |
| ≥48.7 mg/dl/≥1473 nmol/L | 49/475 | 1.71 (1.23, 2.37) | 1.82 (1.25, 2.64) |
Abbreviations: Apolipoprotein-B, apo B; confidence interval, CI; hazard ratio, HR; high-density lipoprotein cholesterol, HDL-C; interquartile range, IQR; low-density lipoprotein particle, LDL-P; small dense-low-density lipoprotein cholesterol, sdLDL-C.
Normal fasting glucose (NFG)=non-diabetic with fasting glucose <100.
Cox proportional hazard regression model adjusted for age, sex, race/ethnicity.
Cox proportional hazard regression model adjusted for age, sex, race/ethnicity, hypertension, blood pressure medication use, triglycerides, high-density lipoprotein cholesterol, waist circumference.
Using residual defined categories, discordantly low sdLDL-C relative to apo B (HR=0.94, 95% CI: 0.68, 1.29) and LDL-P HR=0.84, 95% CI: 0.60, 1.16) was associated with a non-statistically significant lower incidence of CHD compared to NFG individuals with concordant levels (Table 5). Conversely, sdLDL-C discordantly higher than apo B (HR=1.26 95% CI: 0.89, 1.78) and LDL-P (HR=1.25, 95% CI: 0.88, 1.75) were associated with a non-statistically significant higher incidence of CHD than observed in individuals with concordant measurements in this population.
Table 5.
Associations between baseline sdLDL-C concordance/discordance with apo B and LDL-P defined by residuals and CHD incidence through 2015 among participants with normal fasting glucosea (N = 3258).
| Model 1c | Model 2d | ||
|---|---|---|---|
| N Events/ Total N | HR (95% CI) | HR (95% CI | |
| sdLDL-C/apo Bb | |||
| Discordant Low | 62/871 | 0.99 (0.73, 1.36) | 0.93 (0.67, 1.28) |
| Concordant | 113/1658 | Reference | Reference |
| Discordant High | 66/729 | 1.33 (0.98, 1.81) P = 0.14 |
1.25 (0.89, 1.77) P = 0.33 |
| sdLDL-C/LDL-Pb | |||
| Discordant Low | 62/831 | 0.96 (0.70, 1.30) | 0.83 (0.60, 1.15) |
| Concordant | 115/1676 | Reference | Reference |
| Discordant High | 64/751 | 1.27 (0.93, 1.73) P = 0.22 |
1.26 (0.89, 1.77) P = 0.15 |
Abbreviations: Apolipoprotein-B, apo B; confidence interval, CI; hazard ratio, HR; high-density lipoprotein cholesterol, HDL-C; interquartile range, IQR; low-density lipoprotein particle, LDL-P; small dense-low-density lipoprotein cholesterol, sdLDL-C.
Normal fasting glucose (NFG)=non-diabetic with fasting glucose <100.
Discordant low=residual difference <25th percentile; discordant high=residual difference >75th percentile; concordant=residual difference 25th-75th percentile.
Cox proportional hazard regression model adjusted for age, sex, race/ethnicity.
Cox proportional hazard regression model adjusted for age, sex, race/ethnicity, hypertension, blood pressure medication use, triglycerides, high-density lipoprotein cholesterol, waist circumference.
4. Discussion
This study evaluated the relationship between sdLDL-C and CHD and compared sdLDL-C in relation to other particle-based lipoprotein biomarkers for predicting CHD events. SdLDL-C, apo B and LDL-P were all positively associated with increased risk for CHD in individuals with NFG. Results from this study indicate that sdLDL-C, apo B and LDL-P may be similarly predictive of CHD risk among individuals with NFG. SdLDL-C has previously been shown to be associated with CHD risk, but until a recently developed standardized assay, measurement was expensive and challenging to directly compare due to variations in measurement across labs [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24]. To our knowledge, there are not previously published studies comparing apo B and LDL-P with sdLDL-C. SdLDL-C was recently shown to be better at predicting CHD risk than directly measured LDL-C, however, LDL-C does not directly measure the more atherogenic particles, which may be better measured by sdLDL-C, apo B and LDL-P [11]. A previous MESA study demonstrated that elevated sdLDL-C was associated with CHD risk, but only in normoglycemic, nondiabetic participants [12]. Thus, in the current study, we limited the comparison of these three biomarkers to the subset of individuals with NFG. The lack of association among individuals with T2D or IFG may be that the increase in risk imparted by these risk- enhancers is proportionately more significant in lower risk individuals compared to high risk individuals.
The assay for sdLDL-C was recently approved by the FDA for measurement of cholesterol content of small dense LDL particles (< 20 nm). The importance of assessing LDL particle size/number is acknowledged by the 2018 Multi-Society Guideline for the Management of Cholesterol which now includes apo B as a risk enhancing factor [1]. By quantifying the amount of cholesterol in the small dense LDL particles which are known to be more atherogenic compared to the larger more buoyant particles, this assay may improve CHD risk assessment beyond that provided by LDL-C alone by better quantifying atherogenic particles. Two other assays, apo B and LDL-P, are used in the clinical laboratories for the determination of LDL particle numbers, similarly providing better quantification of the level of atherogenicity. Given the similarities of these assays, knowledge on the comparability of these measures is important to establish whether one of these measures is universally superior to the others for assessing CHD risk and/or whether there are complementary roles for each assay in terms of identifying CHD risk in select populations, such as individuals with NFG.
While many other prior studies evaluating sdLDL-C have compared effect sizes or included other lipoprotein biomarkers as covariates in models, interpreting effect sizes can be subjective. Including other biomarkers as a covariate works well for some types of biomarker evaluations, but for very highly correlated biomarkers like sdLDL-C and LDL-C it dilutes any associations because the measurements are not different enough from each other in terms of their relation to CHD risk. An alternative approach, discordance analysis, has been frequently utilized for evaluating the benefits of measuring apo B [30], [31], [32], [33]. Discordance analysis facilitates assessment of the potential additional benefit of measuring sdLDL-C by addressing the dilution that occurs when biomarkers are correlated [31,34,35], and makes it possible to directly evaluate associations between discordant/concordant measurements and CHD at the individual level [36]. In the present study, suggestive but not statistically significant results were obtained among individuals with NFG. Discordantly higher sdLDL-C relative to other lipoprotein biomarkers were non-significantly associated with higher CHD risk, while discordantly lower sdLDL-C was non-significantly associated with lower CHD risk. However, results were not statistically significant and apparent only in those with NFG. Overall these results do not clearly show additional benefit of measuring sdLDL-C versus apo B, but this approach should be explored in future studies with a larger population to further investigate whether sdLDL-C measurement can significantly improve CHD risk prediction in certain populations.
This study has several important strengths and limitations. A major strength is the gender-balanced and racially/ethnically diverse MESA population and the cohort study's rigorous, standardized data collection and measurement procedures. The large number of biomarkers measured in this study population is another strength. This allowed for the evaluation of sdLDL-C relative to a comprehensive number of other lipoprotein biomarkers and the most thorough evaluation to date. This is also the first study to measure all three particle-based biomarkers to allow for a direct comparison of sdLDL-C, apo B and LDL-P in CHD risk prediction.
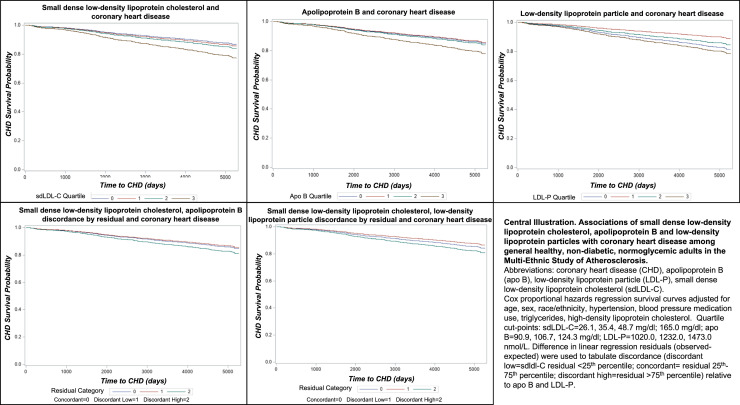
The primary limitation of this study is the number of participants excluded from this analysis due to lack of sdLDL-C measurement resulting in a smaller available sample size, which could have limited our ability to observe more modest, but potentially important differences in associations. This study included only non-statin users at baseline, which meant we could not evaluate the role of statin. While this can be considered a limitation, it is also a strength in that guidelines are built around identifying at risk populations who may benefit from lipid-lowering medication, which makes this the relevant population for studying whether adding measurement of sdLDL-C provides additional risk prediction benefit (Fig. 1).
Fig. 1.
Central Illustration. Associations of small dense low-density lipoprotein cholesterol, apolipoprotein B and low-density lipoprotein particles with coronary heart disease among general healthy, non-diabetic, normoglycemic adults in the Multi-Ethnic Study of Atherosclerosis. Abbreviations: coronary heart disease (CHD), apolipoprotein B (apo B), low-density lipoprotein particle (LDL-P), small dense low-density lipoprotein cholesterol (sdLDL-C). Cox proportional hazards regression survival curves adjusted for age, sex, race/ethnicity, hypertension, blood pressure medication use, triglycerides, high-density lipoprotein cholesterol. Quartile cut-points: sdLDL-C = 26.1, 35.4, 48.7 mg/dl; apo B = 90.9, 106.7, 124.3 mg/dl; LDL-P = 1020.0, 1232.0, 1473.0 nmol/L. Difference in linear regression residuals (observed-expected) were used to tabulate discordance (discordant low=sdLDL-C residual <25th percentile; concordant= residual 25th-75th percentile; discordant high=residual >75th percentile) relative to apo B and LDL-P.
5. Conclusions
Study results indicate that apo B, LDL-P and sdLDL-C are all significantly associated with CHD risk in normoglycemic, non-diabetic individuals. Overall, sdLDL-C is comparable to apo B and LDL-P in prediction of CHD risk among generally healthy adults with NFG. Additional, larger studies are needed to confirm findings. This could have clinical implications for current practices in that sdLDL-C assays which can be performed with existing equipment in standard clinical laboratories, could be a potentially important additions to screening panels for identifying individuals who may not be captured using current clinical practices. Future research should further evaluate whether particle-based biomarkers, such as sdLDL-C, improve identification of patients at risk for CHD events not captured using standard biomarkers whose risk for CHD may be overlooked based on traditional lipid measures and guidelines.
Author contribution statement
Sarah O. Nomura, Weihua Guan, and Michael Y. Tsai contributed to study design, data analysis and manuscript writing and editing. Edward K. Duran, Daniel Duprez, Parveen Garg, Jing Cao, Harpreet Bhatia, and Amy B. Karger contributed to manuscript writing and editing.
Declaration of Competing Interest
Authors have no conflicts of interest to disclose.
Acknowledgments
This research was supported by contracts 75N92020D00001, HHSN268201500003I, N01-HC-95159, 75N92020D00005, N01-HC-95160, 75N92020D00002, N01-HC-95161, 75N92020D00003, N01-HC-95162, 75N92020D00006, N01-HC-95163, 75N92020D00004, N01-HC-95164, 75N92020D00007, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute, and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from the National Center for Advancing Translational Sciences (NCATS). The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services.
References
- 1.Grundy S.M., Stone N.J., Bailey A.L., Beam C., Birtcher K.K., Blumenthal R.S., et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary: a report of the american college of cardiology/american heart association task force on clinical practice guidelines. J Am Coll Cardiol. 2018 [Google Scholar]
- 2.National Cholesterol Education Program Expert Panel on Detection E Treatment of high blood cholesterol in A. third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) final report. Circulation. 2002;106(25):3143–3421. [PubMed] [Google Scholar]
- 3.Sniderman A.D., Lawler P.R., Williams K., Thanassoulis G., de Graaf J., Furberg C.D. The causal exposure model of vascular disease. Clin Sci (Lond) 2012;122(8):369–373. doi: 10.1042/CS20110449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tabas I., Williams K.J., Boren J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation. 2007;116(16):1832–1844. doi: 10.1161/CIRCULATIONAHA.106.676890. [DOI] [PubMed] [Google Scholar]
- 5.Chait A., Brazg R.L., Tribble D.L., Krauss R.M. Susceptibility of small, dense, low-density lipoproteins to oxidative modification in subjects with the atherogenic lipoprotein phenotype, pattern B. Am J Med. 1993;94(4):350–356. doi: 10.1016/0002-9343(93)90144-e. [DOI] [PubMed] [Google Scholar]
- 6.Gerber P.A., Thalhammer C., Schmied C., Spring S., Amann-Vesti B., Spinas G.A., et al. Small, dense LDL particles predict changes in intima media thickness and insulin resistance in men with type 2 diabetes and prediabetes–a prospective cohort study. PLoS One. 2013;8(8):e72763. doi: 10.1371/journal.pone.0072763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Younis N., Charlton-Menys V., Sharma R., Soran H., Durrington P.N. Glycation of LDL in non-diabetic people: small dense LDL is preferentially glycated both in vivo and in vitro. Atherosclerosis. 2009;202(1):162–168. doi: 10.1016/j.atherosclerosis.2008.04.036. [DOI] [PubMed] [Google Scholar]
- 8.Wilson P.W.F., Jacobson T.A., Martin S.S., Jackson E.J., Le N.A., Davidson M.H., et al. Lipid measurements in the management of cardiovascular diseases: practical recommendations a scientific statement from the national lipid association writing group. J Clin Lipidol. 2021;15(5):629–648. doi: 10.1016/j.jacl.2021.09.046. [DOI] [PubMed] [Google Scholar]
- 9.Wilson D.P., Jacobson T.A., Jones P.H., Koschinsky M.L., McNeal C.J., Nordestgaard B.G., et al. Use of Lipoprotein(a) in clinical practice: a biomarker whose time has come. A scientific statement from the national lipid association. J Clin Lipidol. 2019;13(3):374–392. doi: 10.1016/j.jacl.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 10.Duran E.K., Aday A.W., Cook N.R., Buring J.E., Ridker P.M., Pradhan A.D. Triglyceride-rich lipoprotein cholesterol, small dense LDL cholesterol, and incident cardiovascular disease. J Am Coll Cardiol. 2020;75(17):2122–2135. doi: 10.1016/j.jacc.2020.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ikezaki H., Lim E., Cupples L.A., Liu C.T., Asztalos B.F., Schaefer E.J. Small dense low-density lipoprotein cholesterol is the most atherogenic lipoprotein parameter in the prospective framingham offspring study. J Am Heart Assoc. 2021;10(5) doi: 10.1161/JAHA.120.019140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsai M.Y., Steffen B.T., Guan W., McClelland R.L., Warnick R., McConnell J., et al. New automated assay of small dense low-density lipoprotein cholesterol identifies risk of coronary heart disease: the Multi-ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2014;34(1):196–201. doi: 10.1161/ATVBAHA.113.302401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Austin M.A., Breslow J.L., Hennekens C.H., Buring J.E., Willett W.C., Krauss R.M. Low-density lipoprotein subclass patterns and risk of myocardial infarction. JAMA. 1988;260(13):1917–1921. [PubMed] [Google Scholar]
- 14.Arai H., Kokubo Y., Watanabe M., Sawamura T., Ito Y., Minagawa A., et al. Small dense low-density lipoproteins cholesterol can predict incident cardiovascular disease in an urban Japanese cohort: the Suita study. J Atheroscler Thromb. 2013;20(2):195–203. doi: 10.5551/jat.14936. [DOI] [PubMed] [Google Scholar]
- 15.Ai M., Otokozawa S., Asztalos B.F., Ito Y., Nakajima K., White C.C., et al. Small dense LDL cholesterol and coronary heart disease: results from the Framingham Offspring Study. Clin Chem. 2010;56(6):967–976. doi: 10.1373/clinchem.2009.137489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoogeveen R.C., Gaubatz J.W., Sun W., Dodge R.C., Crosby J.R., Jiang J., et al. Small dense low-density lipoprotein-cholesterol concentrations predict risk for coronary heart disease: the Atherosclerosis Risk In Communities (ARIC) study. Arterioscler Thromb Vasc Biol. 2014;34(5):1069–1077. doi: 10.1161/ATVBAHA.114.303284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higashioka M., Sakata S., Honda T., Hata J., Shibata M., Yoshida D., et al. The association of small dense low-density lipoprotein cholesterol and coronary heart disease in subjects at high cardiovascular risk. J Atheroscler Thromb. 2021;28(1):79–89. doi: 10.5551/jat.55350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higashioka M., Sakata S., Honda T., Hata J., Yoshida D., Hirakawa Y., et al. Small dense low-density lipoprotein cholesterol and the risk of coronary heart disease in a japanese community. J Atheroscler Thromb. 2020;27(7):669–682. doi: 10.5551/jat.51961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liou L., Kaptoge S. Association of small, dense LDL-cholesterol concentration and lipoprotein particle characteristics with coronary heart disease: a systematic review and meta-analysis. PLoS One. 2020;15(11) doi: 10.1371/journal.pone.0241993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arsenault B.J., Lemieux I., Despres J.P., Wareham N.J., Luben R., Kastelein J.J., et al. Cholesterol levels in small LDL particles predict the risk of coronary heart disease in the EPIC-Norfolk prospective population study. Eur Heart J. 2007;28(22):2770–2777. doi: 10.1093/eurheartj/ehm390. [DOI] [PubMed] [Google Scholar]
- 21.Siddiqui M.B., Arshad T., Patel S., Lee E., Albhaisi S., Sanyal A.J., et al. Small dense low-density lipoprotein cholesterol predicts cardiovascular events in liver transplant recipients. Hepatology. 2019;70(1):98–107. doi: 10.1002/hep.30518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koba S., Yokota Y., Hirano T., Ito Y., Ban Y., Tsunoda F., et al. Small LDL-cholesterol is superior to LDL-cholesterol for determining severe coronary atherosclerosis. J Atheroscler Thromb. 2008;15(5):250–260. doi: 10.5551/jat.e572. [DOI] [PubMed] [Google Scholar]
- 23.Balling M., Nordestgaard B.G., Langsted A., Varbo A., Kamstrup P.R. Small dense low-density lipoprotein cholesterol predicts atherosclerotic cardiovascular disease in the copenhagen general population study. J Am Coll Cardiol. 2020;75(22):2873–2875. doi: 10.1016/j.jacc.2020.03.072. [DOI] [PubMed] [Google Scholar]
- 24.Ishii J., Kashiwabara K., Ozaki Y., Takahashi H., Kitagawa F., Nishimura H., et al. Small dense low-density lipoprotein cholesterol and cardiovascular risk in statin-treated patients with coronary artery disease. J Atheroscler Thromb. 2022;29(10):1458–1474. doi: 10.5551/jat.63229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ito Y., Fujimura M., Ohta M., Hirano T. Development of a homogeneous assay for measurement of small dense LDL cholesterol. Clin Chem. 2011;57(1):57–65. doi: 10.1373/clinchem.2010.149559. [DOI] [PubMed] [Google Scholar]
- 26.Consortium C.A.D., Deloukas P., Kanoni S., Willenborg C., Farrall M., Assimes T.L., et al. Large-scale association analysis identifies new risk loci for coronary artery disease. Nat Genet. 2013;45(1):25–33. doi: 10.1038/ng.2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bild D.E., Bluemke D.A., Burke G.L., Detrano R., Diez Roux A.V., Folsom A.R., et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 28.Friedewald W.T., Levy R.I., Fredrickson D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 29.Cao J., Nomura S.O., Steffen B.T., Guan W., Remaley A.T., Karger A.B., et al. Apolipoprotein B discordance with low-density lipoprotein cholesterol and non-high-density lipoprotein cholesterol in relation to coronary artery calcification in the Multi-Ethnic Study of Atherosclerosis (MESA) J Clin Lipidol. 2020;14(1):109–121. doi: 10.1016/j.jacl.2019.11.005. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lawler P.R., Akinkuolie A.O., Ridker P.M., Sniderman A.D., Buring J.E., Glynn R.J., et al. Discordance between circulating atherogenic cholesterol mass and lipoprotein particle concentration in relation to future coronary events in women. Clin Chem. 2017;63(4):870–879. doi: 10.1373/clinchem.2016.264515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pencina M.J., D'Agostino R.B., Zdrojewski T., Williams K., Thanassoulis G., Furberg C.D., et al. Apolipoprotein B improves risk assessment of future coronary heart disease in the Framingham Heart Study beyond LDL-C and non-HDL-C. Eur J Prev Cardiol. 2015;22(10):1321–1327. doi: 10.1177/2047487315569411. [DOI] [PubMed] [Google Scholar]
- 32.Sniderman A.D., Islam S., Yusuf S., McQueen M.J. Discordance analysis of Apolipoprotein B and non-high density lipoprotein cholesterol as markers of cardiovascular risk in the INTERHEART study. Atherosclerosis. 2012;225(2):444–449. doi: 10.1016/j.atherosclerosis.2012.08.039. [DOI] [PubMed] [Google Scholar]
- 33.Mora S., Buring J.E., Ridker P.M. Discordance of low-density lipoprotein (LDL) cholesterol with alternative LDL-related measures and future coronary events. Circulation. 2014;129(5):553–561. doi: 10.1161/CIRCULATIONAHA.113.005873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sniderman A.D., Lamarche B., Contois J.H., de Graaf J. Discordance analysis and the Gordian Knot of LDL and non-HDL cholesterol versus apoB. Curr Opin Lipidol. 2014;25(6):461–467. doi: 10.1097/MOL.0000000000000127. [DOI] [PubMed] [Google Scholar]
- 35.Sniderman A.D., St-Pierre A.C., Cantin B., Dagenais G.R., Despres J.P., Lamarche B. Concordance/discordance between plasma apolipoprotein B levels and the cholesterol indexes of atherosclerotic risk. Am J Cardiol. 2003;91(10):1173–1177. doi: 10.1016/s0002-9149(03)00262-5. [DOI] [PubMed] [Google Scholar]
- 36.Martin S.S., Michos E.D. Are we moving towards concordance on the principle that lipid discordance matters? Circulation. 2014;129(5):539–541. doi: 10.1161/CIRCULATIONAHA.113.007207. [DOI] [PMC free article] [PubMed] [Google Scholar]