Abstract
In schistosomiasis mansoni, hepatic granulomatous inflammation surrounding parasite eggs is mediated by CD4+ T helper (Th) cells sensitized to schistosomal egg antigens (SEA). We previously showed that a prominent lymphoproliferative response of CD4+ Th cells from schistosome-infected C57BL/6 (BL/6) mice was directed against a 62-kDa component of SEA. A partial amino acid sequence of the 62-kDa component was found to be identical with one present in the enzyme phosphoenolpyruvate carboxykinase (PEPCK). Based on this sequence, a cDNA clone containing the entire coding region of PEPCK was identified, and the full recombinant Schistosoma mansoni PEPCK (rSm-PEPCK) of 626 amino acids was purified from a prokaryotic expression system. rSm-PEPCK strongly stimulated a specific T-cell hybridoma, 4E6, as well as CD4+ Th cells from SEA-immunized BL/6 mice and from infected BL/6, CBA, and BALB/c mice. In the infected mice, rSm-PEPCK elicited significant gamma interferon production as well as, to a lesser extent, production of interleukin-2 and -5. In BL/6 and BALB/c mice, the CD4+ Th cell response to rSm-PEPCK was greater than that directed against the egg antigen Sm-p40; conversely, CBA mice responded better to Sm-p40 than to Sm-PEPCK. A 12-amino-acid region (residues 398 to 409: DKSKDPKAHPNS) was demonstrated to contain a T-cell epitope; synthetic peptides containing this epitope significantly stimulated specific hybridoma 4E6 and polyclonal CD4+ Th cells. The identification and characterization of immunogenic egg components will contribute to the understanding and possible control of T-cell-mediated schistosomal disease.
The main immunopathological damage in Schistosoma mansoni infection consists of granulomatous inflammation around parasite eggs in the liver and intestines, which may lead to scarring, portal hypertension, hemorrhage, and death (4, 39). There is considerable variation in the overall severity of this disease, both in humans and in mice. Mice of the C3H and CBA strains develop significantly larger egg granulomas than do those of the C57BL/6 (BL/6) strain (9, 15).
Granulomatous inflammation is a complex hypersensitivity reaction that involves the recruitment of various cells, the activation of numerous mediators, including cytokines, and the synthesis of matrix proteins. Granuloma formation is known to be strictly dependent on CD4+ T helper (Th) cells specific for schistosomal egg antigens (SEA) (18, 27), and there is strong evidence that it can be mediated by Th-1- and Th-2-type CD4+ Th cells (11, 17, 33, 40, 48). The CD4+ Th cells become activated following specific recognition of accessory cell-bound major histocompatibility complex (MHC) class II molecules harboring selected SEA-derived peptides. We previously investigated the nature of the anti-SEA T-cell repertoire by using CD4+ Th cells from infected mice as well as panels of specific T-cell hybridomas. C3H and CBA mice display strong polyclonal T-cell responses to Sm-p40, a demonstrated major egg immunogen in H-2k mice (7, 19, 20). By comparison, in BL/6 mice the response to Sm-p40 is weak, and the relevant sensitizing egg antigens are unknown (1, 19, 20).
We recently showed that a prominent lymphoproliferative response of the CD4+ Th cells from BL/6 mice was directed against a 62-kDa component of SEA (1). A partial amino acid sequence of the 62-kDa component (AGFFGVAPGT) was found to be identical with one present in the enzyme phosphoenolpyruvate carboxykinase (PEPCK) of Caenorhabditis elegans and Treponema pallidum (1). Based on this partial sequence, a cDNA containing the entire coding region of S. mansoni PEPCK (Sm-PEPCK) was identified and the full recombinant Sm-PEPCK (rSm-PEPCK) of 626 amino acids was purified from a prokaryotic expression system. We present further studies on the immunological properties of the rSm-PEPCK, including the identification of a 12-mer peptide that contains a T-cell epitope.
MATERIALS AND METHODS
Infection and immunization of mice.
Six- to eight-week-old female BL/6 (H-2b), CBA (H-2k), and BALB/c (H-2d) mice were purchased from the Jackson Laboratory (Bar Harbor, Maine). Mice were infected intraperitoneally with 70 cercariae of S. mansoni (Puerto Rico strain) obtained from infected Biomphalaria glabrata snails, provided to us by the Biomedical Research Institute, Rockville, Md. Some BL/6 mice were immunized by footpad injection with 50 μg of SEA or 15 μg of rSm-PEPCK emulsified in complete Freund's adjuvant (CFA).
Antigens.
SEA was prepared as described previously (3) at the Biomedical Research Institute. The native 62-kDa antigen was prepared from SEA as previously described (1). The recombinant antigen Sm-p40 (rSm-p40) (amino acids 94 to 341) was prepared as previously described (18). Protein concentrations were determined by a modification of the Bradford method using Coomassie Plus protein assay reagent (Pierce, Rockford, Ill.).
Cloning of Sm-PEPCK.
Search of the expressed sequence tag (EST) database identified an EST clone, SME0108 (accession number AA140576). The clone, provided by G. R. Franco and S. D. J. Pena, Laboratorio de Genetica Bioquimica, Belo Horizonte, Brazil, was radiolabeled with [32P]dCTP by random priming and used to screen an S. mansoni lambda ZAP cDNA library. Three positive clones were identified. The phagemids were excised and sequenced. Clone PEPCK17 was identified as the complete cDNA by sequence analysis. The 626-amino-acid sequence of PEPCK17 was derived from this sequence using the SeqEd program from the Wisconsin Genetics Computer Group Package (version 9) and shown to be related to other PEPCKs by search analysis using the Basic Local Alignment Search Tool from the National Center for Biotechnology Information. Amino acid comparison of Sm-PEPCK (PEPCK17) and the cytosolic and mitochondrial forms of human PEPCK was performed using the Pile-Up program from the Wisconsin Genetics Computer Group Package (version 9).
Expression of the rSm-PEPCK protein.
The coding region of Sm-PEPCK was amplified from the phagemid pBluescript SK(+) (Stratagene, La Jolla, Calif.) by PCR using primers specific for Sm-PEPCK. The PCR product was cloned into TOPO TA vector (Invitrogen, Carlsbad, Calif.). The insert was then excised from the TOPO TA vector by restriction digestion with KpnI and SacI and next subcloned into pGEX-4T-1 vector (Pharmacia Biotech, Piscataway, N.J.). Sm-PEPCK was overexpressed as a fusion protein with 26-kDa S. japonicum glutathione-S-transferase (Sj26). The Sj26-Sm-PEPCK fusion protein was then purified by passage over a glutathione-Sepharose 4B column (Pharmacia Biotech) and eluted by addition of 10 mM reduced glutathione. The fusion protein was cleaved with human thrombin (Sigma, St. Louis, Mo.) and then reloaded onto the glutathione-Sepharose column to purify the rSm-PEPCK (38). Purity of the rSm-PEPCK was determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
Synthesis of Sm-PEPCK peptides.
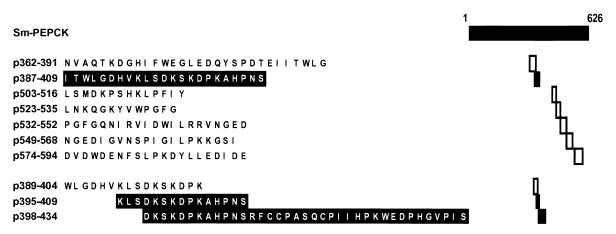
Ten synthetic peptides were prepared by 9-fluorenylmethoxy carbonyl (Fmoc) synthesis technology at the Tufts Core Facility, Department of Physiology, Tufts University School of Medicine. The various peptides used are as follows: p362–391, p387–409, p389–404, p395–409, p398–434, p503–516, p523–535, p532–552, p549–568, and p574–594. For the amino acid sequence of the various peptides see Fig. 6 (the amino acid numbering in the text and figures pertains to Sm-PEPCK). The peptides were analyzed for purity by reverse-phase high-performance liquid chromatography and lyophilized. Stock solutions of 2 mg of protein/ml were prepared in phosphate-buffered saline (PBS).
FIG. 6.
Listing of synthetic peptides of Sm-PEPCK with location and amino acid sequence. The stimulatory peptides are shown in black type; the nonstimulatory peptides are shown in white type.
CD4+ Th-cell responses.
CD4+ Th cells were isolated from mesenteric lymph node cells of mice after 8.5 weeks of infection, or from popliteal and/or axillary lymph nodes of mice immunized 10 days earlier with 50 μg of SEA or 15 μg of rSm-PEPCK in CFA. The cells were purified by negative selection as previously described (19). CD4+ Th cells (1.5 × 105) were incubated in the presence of 4 × 105 irradiated syngeneic splenic antigen-presenting cells (APC) and the indicated antigens for a total of 114 h, and cell proliferation was assessed by 3H-labeled thymidine [3H]dThd) incorporation as previously described (1).
Determination of antigen-specific cytokine production.
Purified CD4+ Th cells (106) from 8.5-week-infected mice were incubated for 24 to 48 h together with the indicated antigens in the presence of 4 × 106 irradiated syngeneic splenic APC. The cytokines gamma interferon (IFN-γ), interleukin-2 (IL-2), IL-4, and IL-5 were measured in culture supernatants by enzyme-linked immunosorbent assay, using cytokine-specific capture monoclonal antibodies, detection monoclonal antibodies, standard cytokines, and protocols from PharMingen (San Diego, Calif.) as previously described (1).
T-cell hybridoma 4E6.
The T-cell hybridoma 4E6 was derived from BL/6 mice (1, 19). Its specific reactivity was assessed by culturing 106 hybridoma cells together with 3 × 106 irradiated (3,000 rads) normal syngeneic splenocytes and graded concentrations of antigens or peptides for 24 h. Positive responses were identified by measuring the proliferation of 9 × 103 HT-2 indicator cells incubated for 22 h in the presence of the hybridoma culture supernatants (46).
SDS-PAGE and electroelution.
SEA was boiled in reducing sample buffer containing 2% SDS and 1% 2-mercaptoethanol and then separated by reduced SDS-PAGE (1). Protein fractions separated by SDS-PAGE were electroeluted. Purity and molecular weight of proteins isolated from gels were assessed on silver-stained gels by using Molecular Analyst 2.1 (Bio-Rad Laboratories, Hercules, Calif.).
Nucleotide sequence accession number.
The cDNA and amino acid sequences for PEPCK17 were submitted to GenBank (accession number AAD24794).
RESULTS
Cloning, sequence analysis, and expression of Sm-PEPCK.
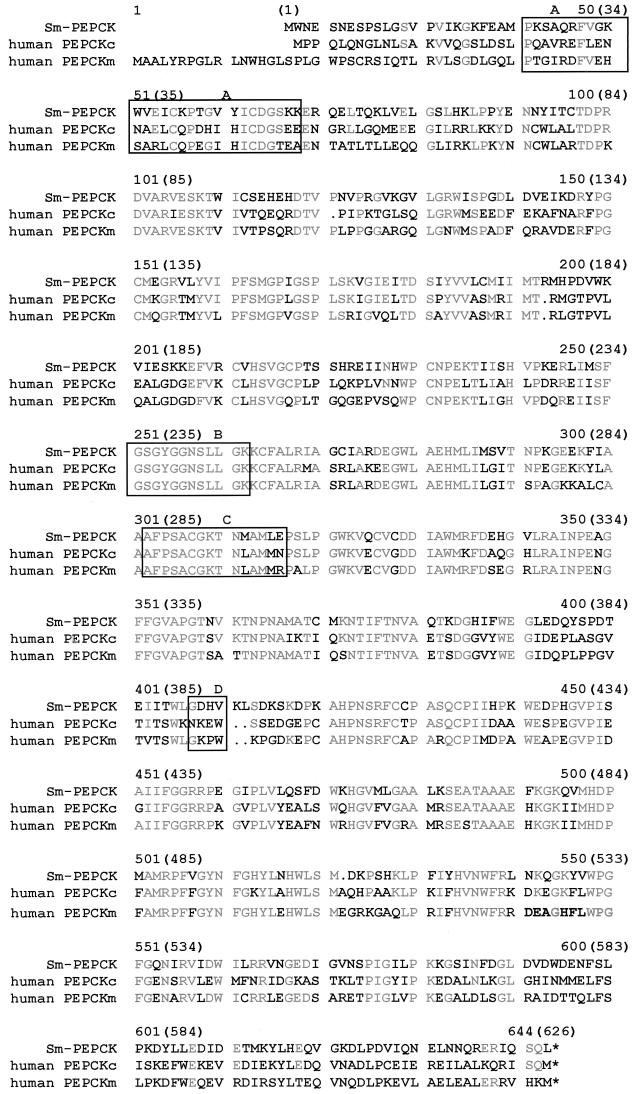
An Sm-PEPCK cDNA clone was identified by microsequence analysis of an endoproteinase fragment of the 62-kDa 4E6 T-cell hybridoma-stimulating peptide and a search of the schistosome EST database. Sequence analysis of the cDNA clone demonstrated 55% identity and 67% similarity to PEPCK from humans. Sm-PEPCK contains 626 amino acids (Fig. 1). Sm-PEPCK exhibits several conserved motifs that positively identify it as PEPCK, such as a phosphoenolpyruvate-binding site, a GTP-binding site, a thiol-binding site, and a guanine-binding site (Fig. 1) (24, 29). Sm-PEPCK was overexpressed in bacteria as a fusion protein and purified, the fusion partner was removed, and the purified rSm-PEPCK was used in subsequent experiments (data not shown) (see Materials and Methods for details).
FIG. 1.
Amino acid Pile-Up diagram of Sm-PEPCK and human PEPCKm and PEPCKc. The amino acid numbering for Sm-PEPCK appears in parentheses. Grey lettering represents amino acid sequence homology between S. mansoni and human sequences. Box A, phosphoenolpyruvate-binding site; box B, GTP-binding site; box C, thiol-binding site; box D, guanine-binding site; ∗, stop codon.
Response of 4E6 T-cell hybridoma and CD4+ Th cells from SEA-immunized BL/6 mice to rSm-PEPCK.
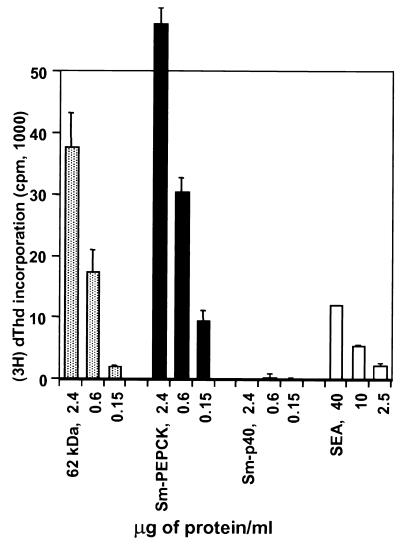
The stimulatory ability of rSm-PEPCK was initially tested on the 4E6 hybridoma cells. Figure 2 shows a representative experiment in which the hybridoma cells responded in a dose-response fashion equally well to both the rSm-PEPCK and the native 62-kDa component from SEA and also, to a lesser degree, to crude SEA but not to the control antigen rSm-p40. These findings indicate that both the recombinant and native PEPCK contain the same T-cell epitope. The stimulatory ability of rSm-PEPCK was also examined using CD4+ Th cells from BL/6 mice immunized with SEA. Results from a representative experiment (Fig. 3) show that rSm-PEPCK elicited a significant proliferative response which was slightly higher than that elicited by equivalent amounts of rSm-p40.
FIG. 2.
Stimulation of 4E6 T-cell hybridoma with the native 62-kDa component of SEA and rSm-PEPCK. Hybridoma cells were cultured with the indicated antigens in the presence of APC, and their stimulation was assessed by incorporation of [3H]dThd into HT-2 indicator cells as described in Materials and Methods. Data are expressed as mean counts per minute (error bars, 1 standard deviation). Background counts per minute from hybridoma cells cultured in the absence of antigen were subtracted. Also shown for comparison are the responses to rSm-p40 and SEA. This experiment was repeated three times with similar results.
FIG. 3.
Proliferation of polyclonal CD4+ Th cells from SEA-immunized BL/6 mice in response to rSm-PEPCK. CD4+ Th cells were isolated from popliteal and axillary lymph nodes of mice immunized subcutaneously with 50 μg of SEA in CFA 10 days earlier. The cells were incubated with the indicated antigens in the presence of APC as described in Materials and Methods. [3H]dThd incorporation was assessed by liquid scintillation spectroscopy. Data are expressed as mean counts per minute (error bars, 1 standard deviation). Background counts per minute from cells cultured in the absence of antigen were subtracted. Also shown for comparison are the responses to rSm-p40 and SEA. This experiment was repeated three times with similar results.
Proliferative and cytokine responses of CD4+ Th cells from S. mansoni-infected BL/6, CBA, and BALB/c mice to rSm-PEPCK.
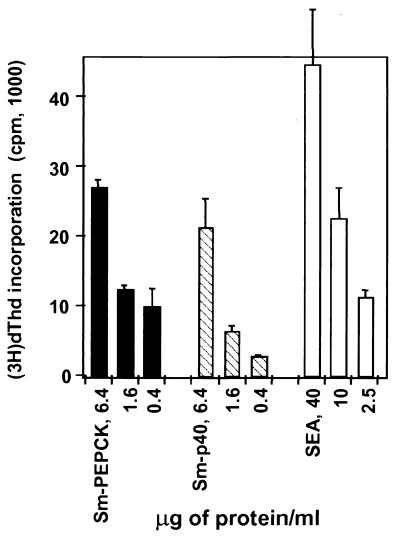
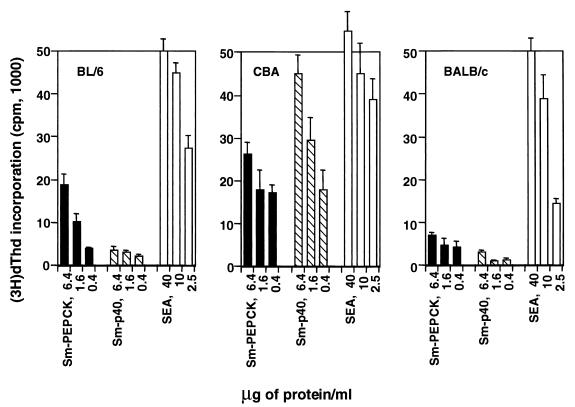
Various strains of mice have been shown to develop dissimilar forms of granulomatous inflammation in response to egg antigens and also to recognize antigens differentially (1, 19). To ascertain its relative antigenicity, rSm-PEPCK was used to stimulate proliferative and cytokine responses in polyclonal CD4+ Th cells from infected mice of these three strains. In all cases, rSm-PEPCK elicited a dose-dependent proliferative response (Fig. 4). In BL/6 and BALB/c mice, this response was stronger than that induced by rSm-p40; conversely, in CBA mice the response to rSm-p40 was predominant.
FIG. 4.
Proliferative responses of CD4+ Th cells from infected BL/6, CBA, and BALB/c mice to rSm-PEPCK. CD4+ Th cells were isolated from mesenteric lymph nodes of 8.5-week-infected mice and incubated with the indicated antigens in the presence of APC as described in Materials and Methods. [3H]dThd incorporation was assessed by liquid scintillation spectroscopy. Data are expressed as mean counts per minute (error bars, 1 standard deviation). Background counts per minute from cells cultured in the absence of antigen were subtracted. Also shown for comparison are the responses to rSm-p40 and SEA. This experiment was repeated three times for BL/6 and CBA and twice for BALB/c, with similar results.
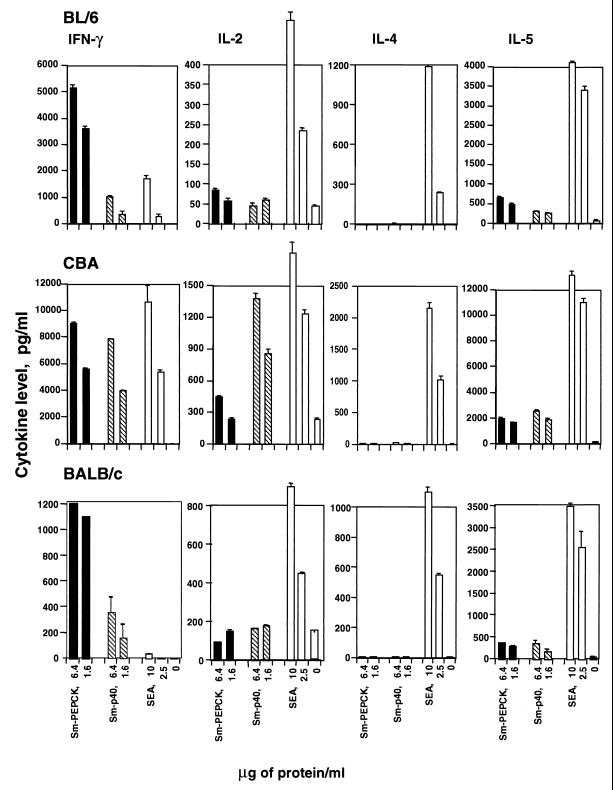
Examination of culture supernatants from CD4+ Th cells revealed that stimulation with rSm-PEPCK elicited in all cases significant IFN-γ production and relatively lower amounts of IL-2 and IL-5 (Fig. 5). In BL/6 and BALB/c mice, after 8.5 weeks of infection, the IFN-γ response to Sm-PEPCK was substantially higher than that elicited by rSm-p40 or SEA, but SEA elicited a higher IL-2 and IL-5 response than either recombinant antigen. In contrast, in similarly infected CBA mice, which display a markedly higher overall cytokine response, the IFN-γ and IL-2 responses to Sm-p40 and to SEA were equal to or stronger than that elicited by Sm-PEPCK. Strikingly, neither rSm-PEPCK nor rSm-p40 elicited any detectable IL-4 production in any of the strains, even though a significant amount of IL-4 was produced in response to SEA.
FIG. 5.
Cytokine production by CD4+ Th cells from BL/6, CBA, and BALB/c mice stimulated with rSm-PEPCK. CD4+ Th cells were isolated from mesenteric lymph nodes of 8.5-week-infected mice and cultured with the indicated antigens in the presence of APC as described in Materials and Methods. The cytokines were measured in culture supernatants by enzyme-linked immunosorbent assay. Data are expressed as means of triplicate determinations (error bars, 1 standard deviation). Also shown for comparison are responses to rSm-p40 and SEA. This experiment was repeated three times for BL/6 and CBA and twice for BALB/c, with similar results.
Localization of T-cell epitope in Sm-PEPCK.
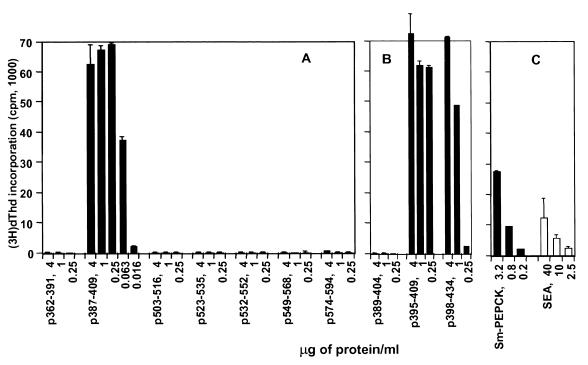
A series of peptides of Sm-PEPCK were prepared based on its primary sequence and the relative probability of encountering a T-cell epitope(s). The peptides' lengths, amino acid compositions, and locations within Sm-PEPCK are shown in Fig. 6. A first series of seven peptides clearly revealed 23-mer p387-409 to contain the epitope recognized by T-cell hybridoma 4E6 (Fig. 7A). Overlapping peptides p389–404, p395–409, and p398–434 were subsequently synthesized and used to stimulate the hybridoma in order to further narrow down the location of the epitope. Strong stimulation with p395–409 and p398–434, but not with p389–404 (Fig. 7B), placed the T-cell epitope within the 12-mer DKSKDPKAHPNS. The stimulatory peptides, the nonstimulatory peptides, and the deduced minimal stimulatory 12-mer segment are shown in Fig. 6.
FIG. 7.
4E6 T-cell hybridoma responses to synthetic peptides. Hybridoma cells were cultured with the indicated peptides in the presence of APC, and their stimulation was assessed by incorporation of [3H]dThd into HT-2 indicator cells as described in Materials and Methods. Data are expressed as mean counts per minute (error bar, 1 standard deviation). Background counts per minute from hybridoma cells cultured in the absence of antigen were subtracted. Also shown for comparison are responses to rSm-PEPCK and SEA. The peptides were tested at least four times with similar results. (A) First set of peptides; (B) second set of peptides; (C) control antigens.
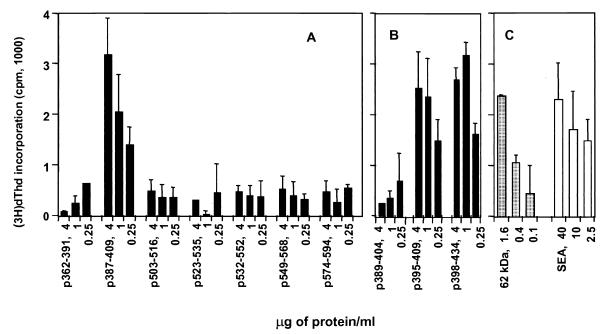
To determine if CD4+ Th cells could similarly respond to the identified T-cell epitope, BL/6 mice were immunized with rSm-PEPCK and the CD4+ Th cells were stimulated with the same peptides. Results (Fig. 8A and B) show that polyclonal CD4+ Th cells exhibited a response pattern analogous to that of the T-cell hybridoma, indicative of the fact that they recognize the same epitope.
FIG. 8.
Proliferation of polyclonal CD4+ Th cells from rSm-PEPCK-immunized BL/6 mice in response to synthetic peptides. CD4+ Th cells were isolated from popliteal and/or axillary lymph nodes of mice immunized subcutaneously with 15 μg of rSm-PEPCK in CFA 10 days earlier. The cells were incubated with the indicated peptides in the presence of APC, as described in Materials and Methods. [3H]dThd incorporation was assessed by liquid scintillation spectroscopy. Data are expressed as mean counts per minute (error bars, 1 standard deviation). Background counts per minute from cells cultured in the absence of antigen were subtracted. Also shown for comparison are the responses to the native 62-kDa antigen and SEA. This experiment was repeated three times with similar results. (A) First set of peptides; (B) second set of peptides; (C) control antigens.
DISCUSSION
Immunopathology in schistosomiasis mansoni is strictly dependent on and mediated by CD4+ Th cells sensitized to egg antigens. Several T-cell-stimulatory activities from schistosome eggs have been described (5, 16, 26), but in contrast to an impressive number of cloned S. mansoni worm antigens (GenBank), the precise identity of most sensitizing egg-stage antigens still remains to be elucidated. Of several potential immunogenic egg components (21, 30, 42), the most abundant and extensively studied is the Sm-p40 antigen, which is a small heat shock protein with homologies to alpha-crystallins (32). Sm-p40 is a potent egg immunogen which elicits a strong I-Ak restricted Th-1-type response in C3H and CBA mice (7, 19, 20). Sm-p40 has been found to have at least three T-cell epitopes, of which one has been found to be dominant (10, 20).
Our laboratory has developed T-cell hybridomas from SEA-sensitized mice as probes to identify novel egg components (1, 19). The reasoning behind this approach is the likelihood that the majority of such clones will have specificities for the most immunogenic egg components, which then could be identified. Using this approach, we demonstrated that a prominent lymphoproliferative response in infected BL/6 mice was directed against a 62-kDa component of SEA (1). Limited proteolytic digestion of the 62-kDa component facilitated partial amino acid sequencing of a peptide identical to one contained in PEPCKs of various species. PEPCK is an important enzyme in carbohydrate synthesis, catalyzing the reaction from oxaloacetate to phosphoenolpyruvate. Although not previously recognized as an egg antigen, PEPCK had been identified in adult worms and sporocysts of S. mansoni (44, 45). Moreover, an EST from a cDNA library of S. mansoni had been partially sequenced (bases 1 to 304) and shown to be homologous with PEPCK (E. M. L. Rabelo, G. R. Franco, V. Azevedo, H. B. Pena, T. M. Santos, W. S. F. Maria, N. A. Rodrigues, J. M. Ortega, and S. D. J. Pena, GenBank DNA sequence a140576). We have now obtained the entire 626-amino-acid region of Sm-PEPCK, expressed the full protein, demonstrated its immunological properties, and identified an epitope recognized by T cells from BL/6 mice.
The deduced amino acid sequence of Sm-PEPCK was compared with the mitochondrial and cytosolic forms of human PEPCK (human PEPCKm and PEPCKc, respectively) as Sm-PEPCK is recognized during human infection (unpublished data) (Fig. 1). Sm-PEPCK has an identity of 55% and a similarity of 67% to both human isoenzymes. Only vertebrate animals appear to have two isoenzyme forms (47), and there is a 17- to 18-amino-acid sequence at the 5′ end of human PEPCKm that is not found on Sm-PEPCK or human PEPCKc. Studies performed on avian PEPCKm have demonstrated a precursor polypeptide with 10 to 14 more amino acids at the N terminus which is processed to the smaller, mature form (47). This precursor sequence may serve as a mitochondrial target signal (29).
The phosphoenolpyruvate-binding site (Fig. 1, box A) is present only in GTP-dependent enzymes and is found in vertebrates, insects, helminths, and fungi (25). Protozoa, yeast, and bacteria contain an ATP-dependent PEPCK (13, 25), and in Trypanosoma and Leishmania species, this enzyme functions differently (25). The binding site consensus sequence PX10AX4CDGSXE (25) is conserved in all GTP-dependent cytosolic PEPCK enzymes studied to date. Sm-PEPCK is unique as it diverges from this sequence at the A and E positions.
All three of the enzymes in Fig. 1 also contain the consensus sequence GX5GK (box B) associated with many GTP-binding domains (24, 25, 29, 47). This sequence is found in a number of GTP-utilizing proteins such as EF-Tu in E. coli, Ras in humans, and several eukaryotic PEPCK enzymes (25). The entire sequence GNSLLGK (in box B) is conserved in all GTP-binding PEPCK enzymes studied thus far (GenBank; also see reference 25). The preceding sequence GSGYG (in box B) is a glycine-rich region often found in ATP-utilizing enzymes (24, 47) and is conserved in both isoforms of human and chicken PEPCK, rat PEPCKc, and now Sm-PEPCK (29).
Box C in Fig. 1 contains the putative catalytic site containing a conserved cysteine found only in GTP-dependent PEPCK enzymes (25). Studies with chicken PEPCK have shown that the enzyme is inactivated when this residue is modified. While this domain's specific function remains unknown, this cysteine residue is hyperreactive and essential (24).
PEPCK proteins should contain binding sites for a GTP-phosphoryl group, for phosphoenolpyruvate, and for a guanine group (29). The consensus sequence for guanine binding of several eukaryotic PEPCK enzymes is divergent: NKEW for human and rat cytosolic forms, GKPW for human mitochondrial form, and GEKW for Haemonchus forms (25) are a few examples. Sm-PEPCK, however, appears the most divergent, sharing only the first conserved G with human PEPCKm (Fig. 1, box D).
Analogous to the native Sm-PEPCK (the 62-kDa antigen), rSm-PEPCK elicited a dominant CD4+ Th-cell proliferative response in schistosome-infected BL/6 mice. By comparison, CBA mice, which displayed the highest overall responses, also reacted strongly to rSm-PEPCK, although this response was relatively weaker than that directed against rSm-p40. BALB/c mice displayed the lowest response to both antigens. These findings support the notion that epitopes on these egg antigens stimulate T cells as a function of the host's MHC and therefore may vary considerably in immunogenicity (and pathogenicity) from strain to strain.
To map the described T-cell epitope on Sm-PEPCK, we took advantage of the 4E6 T-cell hybridoma that was employed previously to identify the 62-kDa antigen, in addition to a series of 10 synthetic peptides spanning the primary sequence of Sm-PEPCK between residues 333 and 594. We focused on this sequence because an epitope stimulating the 4E6 T-cell hybridoma was previously demonstrated in this region (1). We also made use of the I-Ab specific peptide motifs elaborated by Rudensky et al. (36, 37) and listed by Rammensee et al. (35) to identify a candidate CD4+ Th-cell epitope(s) of Sm-PEPCK. This led to the demonstration of an I-Ab-restricted T-cell epitope located within p398–409 (DKSKDPKAHPNS) on Sm-PEPCK. While this strategy was helpful for readily spotting the T-cell epitope, it does not necessarily include all peptide epitopes listed in the MHCPEP database (6). Moreover, the power of predicting MHC class II-binding epitopes is substantially more limited than the well-established ability of class I motifs to predict T-cell epitopes in antigenic proteins (2, 14, 22, 23). For this reason, additional T-cell epitopes are likely to exist in Sm-PEPCK, and their identification as well as assessment of their relative immunodominance and role in immunopathology must await future studies. Furthermore, given its >50% homology (Fig. 1), it will be of interest to determine whether Sm-PEPCK peptide 398–409 also contains a T-cell epitope in humans.
Of considerable interest is the observation that rSm-PEPCK elicited in all mouse strains a predominantly Th-1-type cytokine response characterized by abundant IFN-γ and substantial IL-2 but less IL-5 and virtually no IL-4. This profile contrasts with the cytokines elicited by the native Sm-PEPCK (the 62-kDa antigen), which, at least in the BL/6 mouse, is of a mixed Th-1 and Th-2 type (1). Furthermore, PEPCK elicited a markedly stronger IFN-γ response by itself than when included in SEA, particularly at a time of infection (8.5 weeks) when the overall anti-SEA cytokine response in BL/6 mice shifts entirely to the Th-2 type (33, 40). A similar difference between natural and recombinant antigen has been reported with the major surface glycoprotein (MSG) of Pneumocystis carinii (43), in that CD4+ Th cells from infected rats responded to native MSG with a mixed IFN-γ and IL-4 secretion, whereas the response to the recombinant form of MSG elicited IFN-γ production, but no detectable levels of IL-4. While there may be several possible explanations for the different cytokine profiles elicited by native and recombinant antigens, one obvious reason seems to be the fact that native antigens are glycosylated, whereas recombinant antigens obtained from bacterial expression systems are not. In particular, schistosomal egg components, as a whole, are heavily glycosylated (12), although the level of glycosylation of any particular one is not yet known. Possible inappropriate folding of the recombinant molecule may be less important for the determination of T-cell cytokine production than for the absence of detectable specific enzymatic activity (data not shown).
In more general terms, the pathways leading to preferential Th-1- versus Th-2-type cytokine secretion by CD4+ Th cells have not yet been fully elucidated. Indeed, patterns of susceptibility and resistance to protozoan, helminth, and fungal infections in experimental models have been associated with distinct, mutually exclusive profiles of cytokine production (8, 28, 33). In schistosomiasis, granuloma formation can proceed in the presence of Th-1- and/or Th-2-type environments (11, 17, 33, 40, 48), although in the latter it is more controlled and host-protective than in the former (31, 41). In essence, the gradual elucidation of critical parasite antigens may increase our ability to specifically target and manipulate the CD4+ Th-cell responses to pathogens for the purpose of inducing enhanced resistance at the expense of manageable pathology.
ACKNOWLEDGMENTS
This work was supported in part by Public Health Service grants AI 18919 (to M.J.S.) and U01 AI 45451 (to P.T.L.) and by the UNDP/World Bank WHO Special Program for Research and Training in Tropical Diseases.
Infected B. glabrata snails were provided by the Biomedical Research Institute under NIH-NIAID contract NO1 AI-55260.
REFERENCES
- 1.Asahi H, Hernandez H J, Stadecker M J. A novel 62-kilodalton egg antigen from Schistosoma mansoni induces a potent CD4+ T helper cell response in the C57BL/6 mouse. Infect Immun. 1999;67:1729–1735. doi: 10.1128/iai.67.4.1729-1735.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonneau R H, Salvucci L A, Johnson D C, Tevethia S S. Epitope specificity of H-2Kb-restricted, HSV-1-, and HSV-2-cross-reactive cytotoxic T lymphocyte clones. Virology. 1993;195:62–70. doi: 10.1006/viro.1993.1346. [DOI] [PubMed] [Google Scholar]
- 3.Boros D L, Warren K S. Delayed hypersensitivity-type granuloma formation and dermal reaction induced and elicited by a soluble factor isolated from Schistosoma mansoni eggs. J Exp Med. 1970;132:488–507. doi: 10.1084/jem.132.3.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boros D L. Immunopathology of Schistosoma mansoni infection. Clin Microbiol Rev. 1989;2:250–269. doi: 10.1128/cmr.2.3.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown A P, Remold H G, Warren K S, David J R. Partial purification of antigens from eggs of Schistosoma mansoni that elicit delayed hypersensitivity. J Immunol. 1977;119:1275–1278. [PubMed] [Google Scholar]
- 6.Brusic V, Rudy G, Harrison L C. MHCPEP: a database of MHC-binding peptides. Nucleic Acids Res. 1994;22:3663–3665. doi: 10.1093/nar/22.17.3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cai Y, Langley J G, Smith D I, Boros D L. A cloned major Schistosoma mansoni egg antigen with homologies to small heat shock proteins elicits Th1 responsiveness. Infect Immun. 1996;64:1750–1755. doi: 10.1128/iai.64.5.1750-1755.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carvalho E M, Bacellar O, Brownell C, Regis T, Coffman R L, Reed S G. Restoration of IFN-gamma production and lymphocyte proliferation in visceral leishmaniasis. J Immunol. 1994;152:5949–5956. [PubMed] [Google Scholar]
- 9.Cheever A W, Duvall R H, Hallack T A, Jr, Minker R G, Malley J D, Malley K G. Variation of hepatic fibrosis and granuloma size among mouse strains infected with Schistosoma mansoni. Am J Trop Med Hyg. 1987;37:85–97. doi: 10.4269/ajtmh.1987.37.85. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y, Boros D L. Identification of the immunodominant T cell epitope of p38, a major egg antigen, and characterization of the epitope-specific Th responsiveness during murine schistosomiasis mansoni. J Immunol. 1998;160:5420–5427. [PubMed] [Google Scholar]
- 11.Chikunguwo S M, Kanazawa T, Dayal Y, Stadecker M J. The cell-mediated response to schistosomal antigens at the clonal level. In vivo functions of cloned murine egg antigen-specific CD4+ T helper type 1 lymphocytes. J Immunol. 1991;147:3921–3925. [PubMed] [Google Scholar]
- 12.Cummings R D, Nyame A K. Schistosome glycoconjugates. Biochim Biophys Acta. 1999;1455:363–374. doi: 10.1016/s0925-4439(99)00063-0. [DOI] [PubMed] [Google Scholar]
- 13.Cymeryng C, Cazzulo J J, Cannata J J. Phosphoenolpyruvate carboxykinase from Trypanosoma cruzi. Purification and physicochemical and kinetic properties. Mol Biochem Parasitol. 1995;73:91–101. doi: 10.1016/0166-6851(95)00099-m. [DOI] [PubMed] [Google Scholar]
- 14.DiBrino M, Tsuchida T, Turner R V, Parker K C, Coligan J E, Biddison W E. HLA-A1 and HLA-A3 T cell epitopes derived from influenza virus proteins predicted from peptide binding motifs. J Immunol. 1993;151:5930–5935. [PubMed] [Google Scholar]
- 15.Fanning M M, Peters P A, Davis R S, Kazura J W, Mahmoud A A. Immunopathology of murine infection with Schistosoma mansoni: relationship of genetic background to hepatosplenic disease and modulation. J Infect Dis. 1981;144:148–153. doi: 10.1093/infdis/144.2.148. [DOI] [PubMed] [Google Scholar]
- 16.Harn D A, Danko K, Quinn J J, Stadecker M J. Schistosoma mansoni: the host immune response to egg antigens. I. Partial characterization of cellular and humoral responses to pI fractions of soluble egg antigens. J Immunol. 1989;142:2061–2066. [PubMed] [Google Scholar]
- 17.Henderson G S, Lu X, McCurley T L, Colley D G. In vivo molecular analysis of lymphokines involved in the murine immune response during Schistosoma mansoni infection. II. Quantification of IL-4 mRNA, IFN-gamma mRNA, and IL-2 mRNA levels in the granulomatous livers, mesenteric lymph nodes, and spleens during the course of modulation. J Immunol. 1992;148:2261–2269. [PubMed] [Google Scholar]
- 18.Hernandez H J, Wang Y, Tzellas N, Stadecker M J. Expression of class II, but not class I, major histocompatibility complex molecules is required for granuloma formation in infection with Schistosoma mansoni. Eur J Immunol. 1997;27:1170–1176. doi: 10.1002/eji.1830270518. [DOI] [PubMed] [Google Scholar]
- 19.Hernandez H J, Trzyna W C, Cordingley J S, Brodeur P H, Stadecker M J. Differential antigen recognition by T cell populations from strains of mice developing polar forms of granulomatous inflammation in response to eggs of Schistosoma mansoni. Eur J Immunol. 1997;27:666–670. doi: 10.1002/eji.1830270314. [DOI] [PubMed] [Google Scholar]
- 20.Hernandez H J, Edson C M, Harn D A, Ianelli C J, Stadecker M J. Schistosoma mansoni: genetic restriction and cytokine profile of the CD4+ T helper cell response to dominant epitope peptide of major egg antigen Sm-p40. Exp Parasitol. 1998;90:122–130. doi: 10.1006/expr.1998.4309. [DOI] [PubMed] [Google Scholar]
- 21.Hirsch C, Almeida C A, Doughty B L, Goes A M. Characterization of Schistosoma mansoni 44.7/56.8 kDa egg antigens recognized by human monoclonal antibodies which induce protection against experimental infection and proliferation of peripheral blood mononuclear cells from schistosomiasis patients. Vaccine. 1997;15:948–954. doi: 10.1016/s0264-410x(96)00305-2. [DOI] [PubMed] [Google Scholar]
- 22.Kulkarni A B, Morse III H C, Bennink J R, Yewdell J W, Murphy B R. Immunization of mice with vaccinia virus-M2 recombinant induces epitope-specific and cross-reactive Kd-restricted CD8+ cytotoxic T cells. J Virol. 1993;67:4086–4092. doi: 10.1128/jvi.67.7.4086-4092.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kutubuddin M, Simons J, Chow M. Poliovirus-specific major histocompatibility complex class I-restricted cytolytic T-cell epitopes in mice localize to neutralizing antigenic regions. J Virol. 1992;66:5967–5974. doi: 10.1128/jvi.66.10.5967-5974.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lewis C T, Seyer J M, Carlson G M. Cysteine 288: an essential hyperreactive thiol of cytosolic phosphoenolpyruvate carboxykinase (GTP) J Biol Chem. 1989;264:27–33. [PubMed] [Google Scholar]
- 25.Linss J, Goldenberg S, Urbina J A, Amzel L M. Cloning and characterization of the gene encoding ATP-dependent phosphoenolpyruvate carboxykinase in Trypanosoma cruzi: comparison of primary and predicted secondary structure with host GTP-dependent enzyme. Gene. 1993;136:69–77. doi: 10.1016/0378-1119(93)90449-d. [DOI] [PubMed] [Google Scholar]
- 26.Lukacs N W, Boros D L. Splenic and granuloma T-lymphocyte responses to fractionated soluble egg antigens of Schistosoma mansoni-infected mice. Infect Immun. 1991;59:941–948. doi: 10.1128/iai.59.3.941-948.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mathew R C, Boros D L. Anti-L3T4 antibody treatment suppresses hepatic granuloma formation and abrogates antigen-induced interleukin-2 production in Schistosoma mansoni infection. Infect Immun. 1986;54:820–826. doi: 10.1128/iai.54.3.820-826.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mocci S, Coffman R L. Induction of a Th2 population from a polarized Leishmania-specific Th1 population by in vitro culture with IL-4. J Immunol. 1995;154:3779–3787. [PubMed] [Google Scholar]
- 29.Modaressi S, Christ B, Bratke J, Zahn S, Heise T, Jungermann K. Molecular cloning, sequencing and expression of the cDNA of the mitochondrial form of phosphoenolpyruvate carboxykinase from human liver. Biochem J. 1996;315:807–814. doi: 10.1042/bj3150807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moser D, Doenhoff M J, Klinkert M Q. A stage-specific calcium-binding protein expressed in eggs of Schistosoma mansoni. Mol Biochem Parasitol. 1992;51:229–238. doi: 10.1016/0166-6851(92)90073-s. [DOI] [PubMed] [Google Scholar]
- 31.Mwatha J K, Kimani G, Kamau T, Mbugua G G, Ouma J H, Mumo J, Fulford A J, Jones F M, Butterworth A E, Roberts M B, Dunne D W. High levels of TNF, soluble TNF receptors, soluble ICAM-1, and IFN-gamma, but low levels of IL-5, are associated with hepatosplenic disease in human schistosomiasis mansoni. J Immunol. 1998;160:1992–1999. [PubMed] [Google Scholar]
- 32.Nene V, Dunne D W, Johnson K S, Taylor D W, Cordingley J S. Sequence and expression of a major egg antigen from Schistosoma mansoni. Homologies to heat shock proteins and alpha-crystallins. Mol Biochem Parasitol. 1986;21:179–188. doi: 10.1016/0166-6851(86)90021-6. [DOI] [PubMed] [Google Scholar]
- 33.Pearce E J, Caspar P, Grzych J M, Lewis F A, Sher A. Downregulation of Th1 cytokine production accompanies induction of Th2 responses by a parasitic helminth, Schistosoma mansoni. J Exp Med. 1991;173:159–166. doi: 10.1084/jem.173.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Powrie F, Coffman R L. Cytokine regulation of T-cell function: potential for therapeutic intervention. Immunol Today. 1993;14:270–274. doi: 10.1016/0167-5699(93)90044-L. [DOI] [PubMed] [Google Scholar]
- 35.Rammensee H G, Friede T, Stevanovic S. MHC ligands and peptide motifs: first listing. Immunogenetics. 1995;41:178–228. doi: 10.1007/BF00172063. [DOI] [PubMed] [Google Scholar]
- 36.Rudensky A, Preston-Hurlburt P, Hong S C, Barlow A, Janeway C A., Jr Sequence analysis of peptides bound to MHC class II molecules. Nature. 1991;353:622–627. doi: 10.1038/353622a0. [DOI] [PubMed] [Google Scholar]
- 37.Rudensky A, Preston-Hurlburt P, al-Ramadi B K, Rothbard J, Janeway C A., Jr Truncation variants of peptides isolated from MHC class II molecules suggest sequence motifs. Nature. 1992;359:429–431. doi: 10.1038/359429a0. [DOI] [PubMed] [Google Scholar]
- 38.Smith D B, Johnson K S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988;67:31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- 39.Smithers S R, Doenhoff M J. Schistosomiasis. In: Cohen S, Warren K S, editors. Immunology of parasitic diseases. Oxford, United Kingdom: Blackwell Scientific; 1982. pp. 527–607. [Google Scholar]
- 40.Stadecker M J, Hernandez H J. The immune response and immunopathology in infection with Schistosoma mansoni: a key role of major egg antigen Sm-p40. Parasite Immunol. 1998;20:217–221. doi: 10.1046/j.1365-3024.1998.00150.x. [DOI] [PubMed] [Google Scholar]
- 41.Stadecker M J. The development of granulomas in schistosomiasis: genetic backgrounds, regulatory pathways, and specific egg antigen responses that influence the magnitude of disease. Microbes Infect. 1999;1:505–510. doi: 10.1016/s1286-4579(99)80089-6. [DOI] [PubMed] [Google Scholar]
- 42.Sung C K, Dresden M H. Cysteinyl proteinases of Schistosoma mansoni eggs: purification and partial characterization. J Parasitol. 1986;72:891–900. [PubMed] [Google Scholar]
- 43.Theus S A, Smulian A G, Sullivan D W, Walzer P D. Cytokine responses to the native and recombinant forms of the major surface glycoprotein of Pneumocystis carinii. Clin Exp Immunol. 1997;109:255–260. doi: 10.1046/j.1365-2249.1997.4501348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tielens A G, Van der Meer P, van den Heuvel J M, van den Bergh S G. The enigmatic presence of all gluconeogenic enzymes in Schistosoma mansoni adults. Parasitology. 1991;102:267–276. doi: 10.1017/s0031182000062582. [DOI] [PubMed] [Google Scholar]
- 45.Tielens A G, Horemans A M, Dunnewijk R, van der Meer P, van den Bergh S G. The facultative anaerobic energy metabolism of Schistosoma mansoni sporocysts. Mol Biochem Parasitol. 1992;56:49–57. doi: 10.1016/0166-6851(92)90153-b. [DOI] [PubMed] [Google Scholar]
- 46.Watson J. Continuous proliferation of murine antigen-specific helper T lymphocytes in culture. J Exp Med. 1979;150:1510–1519. doi: 10.1084/jem.150.6.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weldon S L, Rando A, Matathias A S, Hod Y, Kalonick P A, Savon S, Cook J S, Hanson R W. Mitochondrial phosphoenolpyruvate carboxykinase from the chicken. Comparison of the cDNA and protein sequences with the cytosolic isozyme. J Biol Chem. 1990;265:7308–7317. [PubMed] [Google Scholar]
- 48.Wynn T A, Eltoum I, Cheever A W, Lewis F A, Gause W C, Sher A. Analysis of cytokine mRNA expression during primary granuloma formation induced by eggs of Schistosoma mansoni. J Immunol. 1993;151:1430–1440. [PubMed] [Google Scholar]