Abstract
Whereas the autonomic nervous system (ANS) and the immune system used to be assigned separate functions, it has now become clear that the ANS and the immune system (and thereby inflammatory cascades) work closely together. During an acute immune response (e. g., in viral infection like Covid-19) the ANS and the immune system establish a fast interaction resulting in “physiological” inflammation. Based on our knowledge of the modulation of inflammation by the ANS we propose that a reflectory malfunction of the ANS with hyperactivity of the sympathetic nervous system (SNS) may be involved in the generation of acute hyperinflammation. We believe that sympathetic hyperactivity triggers a hyperresponsiveness of the immune system (“cytokine storm”) with consecutive tissue damage. These reflectory neuroimmunological and inflammatory cascades constitute a general reaction principle of the organism under the leadership of the ANS and does not only occur in viral infections, although Covid-19 is a typical current example therefore. Within the overreaction several interdependent pathological positive feedback loops can be detected in which the SNS plays an important part. Consequently, there is a chance to regulate the hyperinflammation by influencing the SNS. This can be achieved by a stellate ganglion block (SGB) with local anesthetics, temporarily disrupting the pathological positive feedback loops. Thereafter, the complex neuroimmune system has the chance to reorganize itself. Previous clinical and experimental data have confirmed a favorable outcome in hyperinflammation (including pneumonia) after SGB (measurable e. g. by a reduction in proinflammatory cytokines).
Abbreviations: ANS, autonomic nervous system; ARDS, acute respiratory distress syndrome; CNS, central nervous system; CRPS, complex regional pain syndrome; HPA, hypothalamic-pituitary-adrenal (axis); IL, interleukin; LA, local anesthetic; N., nervus; NE, norepinephrine; NK, natural killer (cells); NO, nitric oxide; NTS, nucleus tractus solitarius; RVLM, rostral ventrolateral medulla; SG, stellate ganglion; SGB, stellate ganglion block; SIRS, systemic inflammatory response syndrome; SNS, sympathetic nervous system; SP, substance P; TBI, traumatic brain injury; TNF-α, tumor necrosis factor alpha; VNS, vagus nerve stimulation
Keywords: Autonomic nervous system, Hyperinflammation, Cytokine storm, Covid-19, Stellate ganglion block, Local anesthetics
1. Introduction
In viral infections, currently seen in the Covid-19 pandemic caused by the coronavirus SARS-CoV-2, the course is often mild, but some patients experience severe disease due to hyperresponsiveness of the immune system with excessive acute inflammation (hyperinflammation, cytokine storm) and consecutive tissue damage. This can lead to acute respiratory distress syndrome (ARDS), multi-organ involvement, microcirculatory disturbances, and microthrombosis (Dreher et al., 2020; Merad and Martin, 2020; Varga et al., 2020). In lab tests the so-called “cytokine storm”, a strong increase of cytokines (including TNF-α, IL-1, IL-6 and chemokines) is found (Clark and Vissel, 2017; Coperchini et al., 2020; Huang et al., 2020; Liu et al., 2020; Merad and Martin, 2020; Qin et al., 2020; Ruan et al., 2020; Velavan and Meyer, 2020; Zhu et al., 2020). Therefore, tissue damages are not solely due to direct virus-induced cytopathic effects.
The immune and inflammatory processes are – also in the case of a virus infection –reflectorily controlled by the autonomic nervous system (ANS) (Elenkov et al., 2000; Tracey, 2002; Tracey, 2009; Grebe et al., 2010; Jänig, 2014a; Steenblock et al., 2020) which may thus be also responsible for the overshooting of these processes (Steenblock et al., 2020). In our opinion, ARDS, endothelial dysfunction, microcirculatory disorder, coagulopathy, are related entities during a viral infection, e. g. Covid-19 (Fig. 1 ); they are the expression of an excessive, reactive malfunction of the ANS, especially sympathetic hyperactivity (Bellinvia et al., 2020; Steenblock et al., 2020). We postulate that overreacting positive feedback loops on different levels of the nerve-immune-inflammation cascade are involved in this malfunction. Since these positive feedback loops are interdependent, with an important part of the SNS, they can be interrupted simultaneously by means of a short term SGB (“reset”). Interdependent positive feedback loops have the fundamental ability to reorganize themselves after changes of state (autoregulation) (Lorenz, 1972; Kluge and Neugebauer, 1994; Fischer, 1998; Fischer, 2017). Our review shows the influence of SGB on (hyper)inflammation, and new aspects in the neuroimmune system based on positive feedback loops.
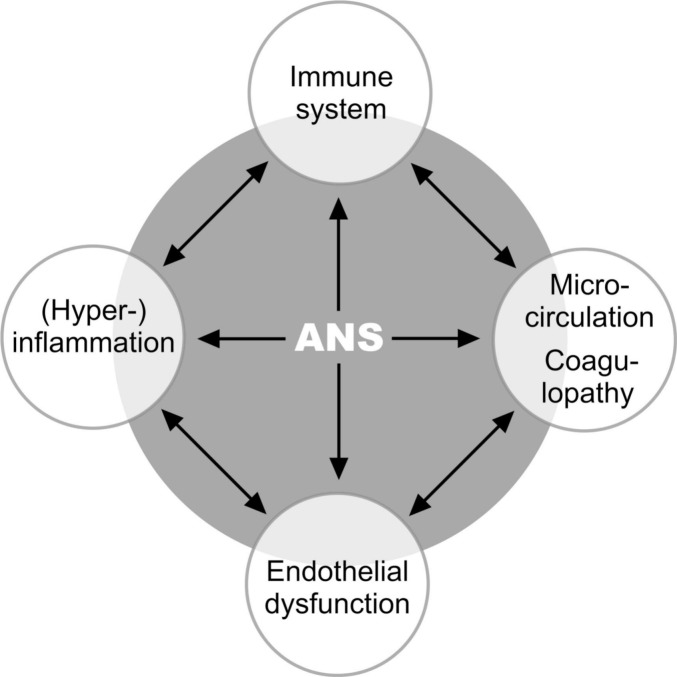
Fig. 1.
Overview of affected systems, e. g. following a virus infection like Covid-19.
The grey area symbolizes that the autonomic nervous system (ANS) is integrated in the communication of all systems and has a coordinating function. The black arrows symbolize that the systems are connected to each other via neuronal and non-neuronal feedback loops and are therefore interdependent. The same neuronal and non-neuronal substances can have both activating and inhibiting effects, depending on the current state of the system as well as on the point in time. (For literature, see text). Interestingly, the reactions in this system take place in principle in the same way, regardless of whether the initial stimulus was a viral infection, mechanical trauma, psychological stress, or other cause. The clinical picture – the disease – is then determined by the organ or organ system mainly affected (see text). In our opinion, the consequences of acute hyperinflammation such as ARDS, endothelial dysfunction, microcirculation disturbance, and coagulopathy are not different entities in a viral infection, but an expression of a malfunction of the ANS with sympathetic hyperactivity.
2. Autonomic nervous system and immune system
2.1. Anatomy and physiology of the autonomic nervous system
The ANS (sympathetic and parasympathetic nervous system) consists of a central (brain and spinal cord) and peripheral part. It maintains an internal environment adapted to various factors. In addition, it significantly contributes to the control of inflammatory and immune processes as well as to the regulation of (micro)circulation (Elenkov et al., 2000; Tracey, 2002; Jänig, 2006; Jänig, 2014a).
The ANS, by virtue of its largely ubiquitous distribution as a feedback autoregulatory system, influences the functions of cells and organs, including the vascular system, endocrine system, and immune system. The SNS is distributed efferently from the sympathetic centers in the brain, including the hypothalamus, rostral ventrolateral medulla (RVLM) (Ogundele et al., 2017; Paton and Spyer, 2013), through the spinal nucleus of the spinal cord (nucleus intermediolateralis), via the para- and prevertebral ganglia. The postganglionic nerve fibers run into the interstitium, with influence to mast cells, macrophages, fibrocytes, leukocytes (van der Zypen, 1967; Pischinger, 2010; Heine, 2011; Benias et al., 2018), and to the organs and organ systems. In addition, the hypothalamic-pituitary-adrenal axis (HPA) stimulates the adrenal cortex, and the sympatho-adrenal (SA) system influences the adrenal medulla (Jänig, 2014a). Sensory nerve fibers in the truncus sympathicus project from the periphery (including the interstitium) via pre- and paravertebral ganglia and dorsal root ganglia to the spinal cord and from there to the centers in the brain. A second way into the brain is via cervical ganglia and the vascular plexus.
The efferent parasympathetic fibers have their origin in the core areas of the vagus nerve (nucleus ambiguus and nucleus dorsalis n. vagi) and form various plexuses together with the SNS (plexus pulmonalis, plexus cardiacus, and plexus coeliacus). The sensory fibers in the vagus nerve project into areas in the brain stem (nucleus tractus solitarius [NTS]). There, the NTS establishes connections to efferent parasympathetic and sympathetic core areas and to integration centers such as the hypothalamus (Ross et al., 1985; Affleck et al., 2012) (Fig. 2, Fig. 3 ).
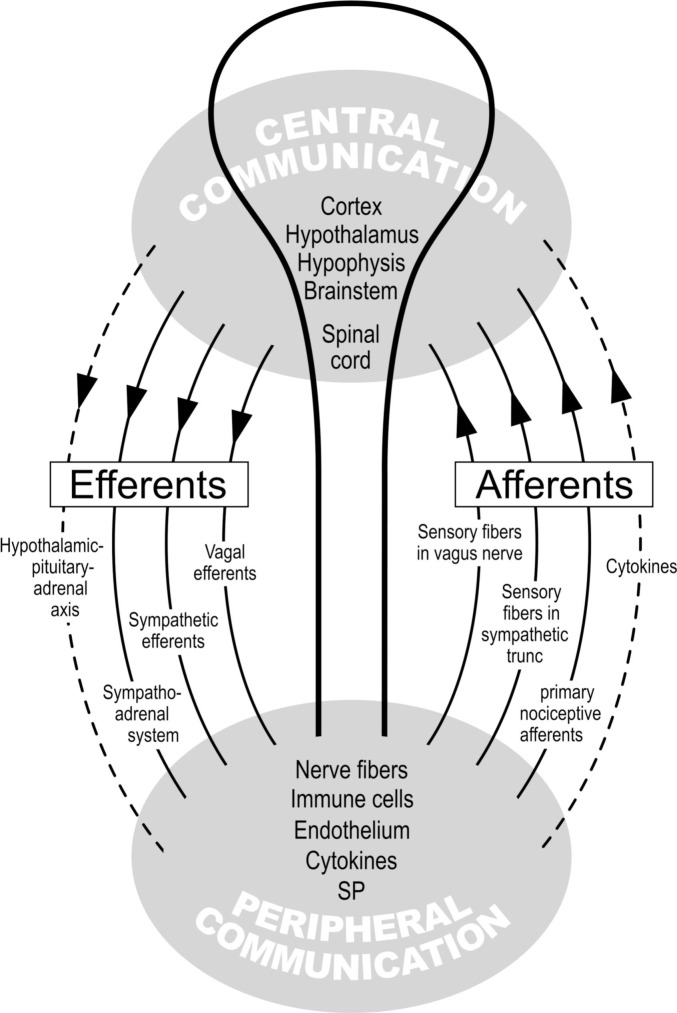
Fig. 2.
Autonomic nervous system and immune system: central and peripheral communication (more details in Fig. 3).
Positive feedback loops exist between AND within the central and peripheral communication. Example: Elements of peripheral communication sensitize each other: Thus, immune cells AND nerve fibers produce cytokines and substance P (SP), which in turn induce the immune cells and nerve fibers to produce even more cytokines and SP (positive feedback loop). The brain is then informed of the cytokine and SP concentration; it knows via the afferent nerve fibers the site of tissue damage and the incipient inflammation. Now, this information is processed (central communication) and the resulting output is then communicated to the periphery by means of the efferents shown here (communication between center and periphery).
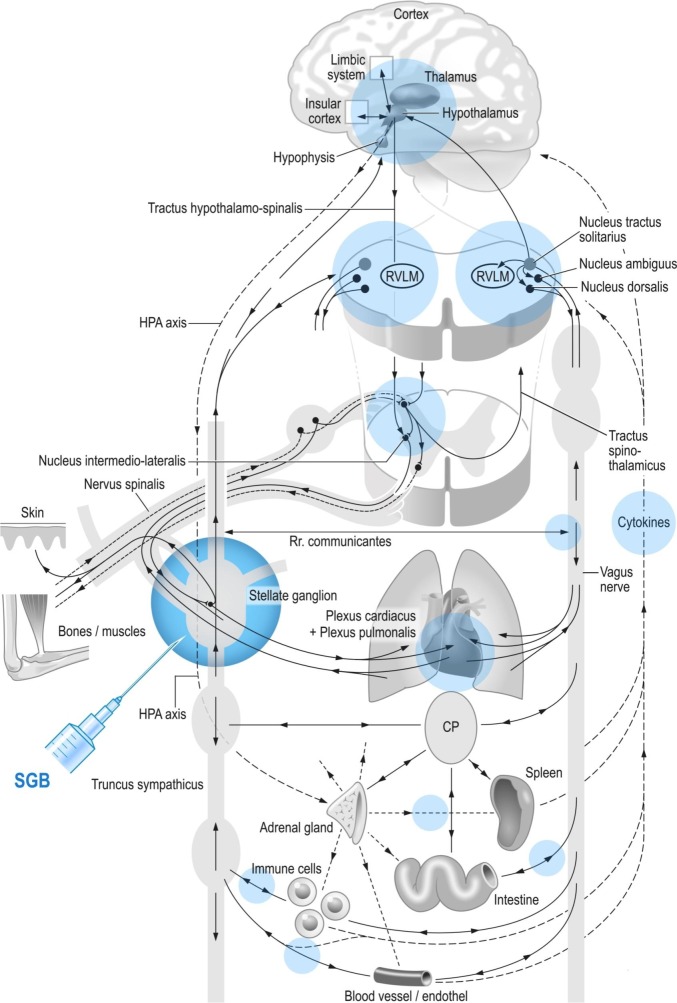
Fig. 3.
Schematic and simplified overview of the autonomic nervous system and immune system and influence of the stellate ganglion block (direct and indirect) on several systems simultaneously (blue fields; see Section 3).
Representation of different levels of the “crosstalk” between nervous system and immune system (described step by step in the text). Each “part” is dependent on the whole system, and the function of the “parts” can hardly be considered in isolation. In addition to negative feedback loops, several positive feedback loops (iterations) are formed so that the system gets a high complexity.
The “reset” by means of stellate ganglion block not only affects the fibers of the autonomic nervous system, but also the subsequent reactions triggered by it, such as the regulation of the immune and inflammatory system and thus, cytokine production, too (see Table 1).
CP: Celiac plexus; HPA: hypothalamic-pituitary-adrenal; RVLM: rostral ventrolateral medulla; SGB: stellate ganglion block. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
2.2. Neuroimmune interactions
Whereas the nervous and the immune system were traditionally thought to be involved in separate functions, it has become clear since the works of Elenkov et al. in 2000 and Tracey in 2002 that the ANS and the immune system (and thereby inflammatory cascades) are in fact inseparable. Central networks of the ANS are informed about the peripheral inflammation and immune status (Tracey, 2002) by sensory nerve fibers (nociceptive neurons, sensory nerve fibers in the truncus sympathicus and in the vagal nerve) as well as by the humoral pathway – especially cytokines, which (among others) originate from the inflamed areas and lymphoid organs (Jänig, 2014a) (Fig. 2, Fig. 3). Humoral and afferent neuronal signals co-determine the strength of the efferent “response” of the vagus and especially of the SNS from the autonomous centers of the brain (Kenney and Ganta, 2014). The ANS can control not only a general inflammatory and immune response, but also local processes in the damaged tissue (with sensory feedback to the brain) (Fig. 2, Fig. 3). Thus, immunological homeostasis is a continuous, homeodynamic process controlled by the ANS which allows throttling or stimulation in effector tissue including microcirculation and immune cells (Jänig, 2011; Jänig, 2014a; Kenney and Ganta, 2014).
During an immune response (e. g. in a viral infection) the brain and the immune system “talk to each other” (Elenkov et al., 2000; Tracey, 2002; O'Connor et al., 2004; Jänig, 2014a; Ji et al., 2016) via afferent and efferent pathways and know about their mutual state (Fig. 2, Fig. 3). Immune cells express adrenoceptors (Bellinger and Lorton, 2014), and, conversely, nerve endings of sensory neurons register changes in the local concentration of immune mediators (cytokines, chemokines) and transmit this information to the brain (Tracey, 2002; Schaible, 2014). Autonomous centers in the brain localize the tissue damage at a specific location in the organism, which would not be possible via the bloodstream. Inflammatory mediators such as cytokines including chemokines are actually neuromodulators (Schaible et al., 2011; Chiu et al., 2013; Ji et al., 2016). In this localized neuroinflammation, also glial cells in the dorsal root ganglia (DRG), the spinal cord and the brain are activated.
Interestingly, not only immune cells (macrophages, monocytes, lymphocytes) can produce cytokines, but also neurons of the central and peripheral nervous system, microglia and astrocytes, and vascular endothelial cells (Galic et al., 2012). Peripheral nerves have partly the same molecular recognition pathways (such as toll-like receptors) as the immune cells (Chiu et al., 2012; Chiu et al., 2013), allowing a very direct and fast communication (interaction). During noxious stimulation (trauma, toxins, viruses, bacteria, etc.) nerve fibers themselves release proinflammatory neuropeptides such as substance P (SP) and calcitonin gene-related peptides (CGRP). These are also released by immune cells such as macrophages, lymphocytes and mast cells (Stanisz et al., 1986). This results in neurogenic inflammation with plasma extravasation and edema (Chiu et al., 2012; Chiu et al., 2013; Jänig, 2014a; Ji et al., 2016; Matsuda et al., 2019). Neuropeptides secreted by nerve fibers can in turn induce cytokine production in immune cells (Ansel et al., 1993; Hosoi et al., 1993; Stanisz, 2001; Veres et al., 2007) and generate in this way a positive feedback loop. Neurogenic inflammation also occurs in virus-associated respiratory infections (King et al., 2001), and viral infections can also activate toll-like receptors in nociceptors thus allowing a modulation of pain and local neuroinflammation (Ji et al., 2016).
3. Why a stellate ganglion block (SGB)?
Surprisingly, the basic neuroimmune interactions in heterogenous clinical pictures (“diagnosis”) with overreaction of the immune and inflammatory system do not seem to differ (see Section 3.5), and thereby allow principally to be influenced (peripherally and centrally) by SGB (Fig. 4 ) (see Section 3.5). In the past, we have gained experience with SGB in heterogeneous clinical pictures which were accompanied by inflammations, immune disorders, circulatory disorders, and pain (Barop, 2017; Fischer, 2020). Therefore we thought of a common pathogenetic mechanism and a “cooperation” between the ANS and the immune system in inflammations, and that their central mechanisms are also influenced by SGB.
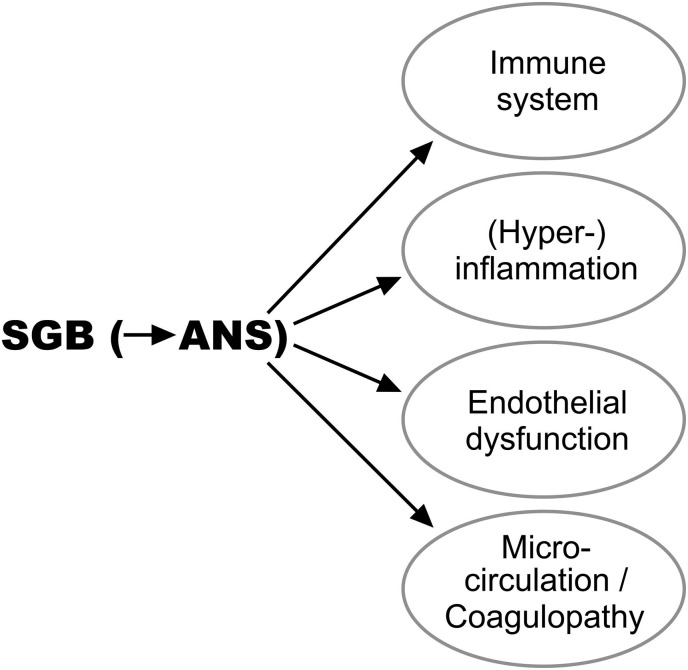
Fig. 4.
The stellate ganglion block (SGB) simultaneously regulates the systems linked to the autonomic nervous system (ANS).
3.1. Anatomy and physiology of the stellate ganglion
The sympathetic SG is located at the level of the 1st rib head in the lamina praevertebralis fasciae cervicalis. The neurons of the ganglion receive their impulses via the rami communicantes albi from the spinal core areas of the nucleus intermediolateralis from C8 to Th5. After switching via divergence principle (McLachlan, 2003) to postganglionic neurons in the SG, the latter supply the upper body quadrant.
The SG modulates the functions in its immediate area of supply: brain, neck, lung, heart, upper extremity, vessels including microcirculation and endothelial function, interstitium, and immune system. The SG does not only influence the immune processing in the periphery but also brain areas involved in the control of the immune system. Therefore, the influence of the SG is not limited to pathophysiological processes in its immediate peripheral supply area (e. g. viral pneumonia, myocarditis) of the upper body quadrant, but also modifies inflammation remote from the SG, e. g. of the intestine. The reason for this is that neuronal and non-neuronal (cytokines, etc.) afferent signals from the intestine are received and integrated in the vagal and sympathetic brain centers which in turn influence the intestine (and its immune system) by feedback (Fig. 3). The autonomous centers in the brain are also under the influence of the SG (Westerhaus and Loewy, 2001), explaining why the brain (supplied by the SG) can provide ubiquitous immune responses and influence inflammation via the sympathetic and parasympathetic system (Elenkov et al., 2000; Tracey, 2002; Tracey, 2009; Jänig, 2014b; Lipov and Candido, 2017). In a review of the literature of the anatomy of SG we noted that the sympathetic and vagal fibers are intertwined by a continuous commingling and via rami communicantes (Puente de la Vega Costa et al., 2016). Therefore, SGB is not a purely sympathetic block; vagal fibers are always influenced by the injection, partly because of their anatomical proximity, partly through the rami communicantes. This is desirable because both sympathetic and vagal efferents and afferents are important for the temporary interruption of the immune and inflammatory reflex paths (Puente de la Vega Costa et al., 2016).
3.2. Regulation of the acute neurogenic hyperinflammation (cytokine storm) by SGB
In the literature, the terms “proinflammatory”, “hyperinflammation” and “cytokine storm” are not exactly defined. We use the terms analogous to the following descriptions.
Proinflammatory: by this we mean substances or processes that have a proinflammatory effect, such as substance P, proinflammatory cytokines, etc. Here, the proinflammatory effect may well be physiological for defense against infectious agents, in trauma, etc. (Esch and Stefano, 2002).
Hyperinflammation: this goes beyond the physiological inflammatory response and causes tissue damage. Scientific publications and the media often use the terms “hyperinflammation” and “cytokine storm” analogously, especially in connection with Covid-19. This is practicable but not very precise. We think the term “acute hyperinflammation” is more precise.
Cytokine storm has no precise definition (Sinha et al., 2020) and “no single definition of cytokine storm as an umbrella term is widely accepted” (Fajgenbaum and June, 2020). Cytokine storm is a hyperactive immune response with excessive increased cytokines and other mediators. This is more than a physiological innate immune answer of an infection; cytokine storm causes via hyperinflammation cell and organ damage and often death (Burke et al., 2020; Sinha et al., 2020; Kim et al., 2021).
In the following, we postulate that in many tissue damages, e. g. after virus infection, the overreaction of the immune system (e. g. cytokine storm) with hyperinflammation is the consequence of an SNS overreaction, and that SGB is a logical possibility to counteract it (Fig. 2, Fig. 3). The physiological balance between the SNS and the vagus nerve is biased towards SNS dominance (Alston et al., 2011; Puente de la Vega Costa et al., 2016; Koopman et al., 2017). Speranski, a student of Pavlov, was able to show in 1950 in animal experiments that after experimental preload of the SNS (“first strike”) (e. g. chronic artificial hypothalamus and pituitary gland irritation), a subsequent, additional stimulus (“second strike”) caused various overshooting, sympathetically triggered, reflectoric inflammations (such as arthritis). For Speranski (1950), excessive inflammation was the result of an overreaction of the preloaded SNS.
The imbalance in the ANS can also promote inflammation when the SNS is dominant (Alston et al., 2011; Puente de la Vega Costa et al., 2016; Koopman et al., 2017). Generally (with exceptions), the hyperactivated SNS has a proinflammatory effect (Schaible and Straub, 2014) while the activated parasympathetic fibers of the vagus nerve have an anti-inflammatory effect. Nevertheless, the sympathetic and parasympathetic nervous systems cannot be considered in isolation; they are more than the sum of their parts (Fischer, 1998; Tracey, 2002), since they are not only centrally but also peripherally more closely intertwined than previously assumed (Puente de la Vega Costa et al., 2016) (see above). Although the pathophysiology was not known in detail at the time of Speranski's experiments, later research has confirmed the “leadership” of the ANS, especially of the SNS, in (excessive) immune reactions and inflammation (Piedimonte et al., 1999; King et al., 2001). Tracey, one of the pioneers of seeing inflammation and immune processes as part of a neuronal reflex involving the SNS and the vagus (Tracey, 2002; Tracey, 2009), proposed neuronal different “setpoints” for the immune response to a stimulus such as infection: A normal “setpoint” results in a protective response (normal inflammation and tissue repair), a decreased “setpoint” in immunosuppression with increased infection, and an increased “setpoint” in hyperinflammation and tissue damage. Similar as in Speranski's (1950) experiments, a direct (hypertension [Kumagai et al., 2012], obesity, or stress) or indirect (organ damages) preload on the SNS may shift Tracey's “setpoint” towards an increase (corresponding to Speranski's “first strike”), so that the additional stimulus – such as an infection (Speranski's “second strike”) – leads to an overreaction of the SNS which disturbes the balance in the ANS (Speranski, 1950; Fischer, 2002; Tracey, 2009; Barop, 2017; Fischer, 2019). This hyperactivity would then be measurable by a cytokine storm and other laboratory parameters as well as tissue damage. In such situations, various authors have proposed to stimulate the vagus nerve in order to improve the balance between the sympathetic system and the vagus nerve (Tracey, 2002; Tracey, 2009; Pavlov and Tracey, 2015; Breit et al., 2018; Pavlov et al., 2020; Tsaava et al., 2020; Caravaca et al., 2019). Effects of vagus nerve stimulation (reduction of inflammation, decreased cytokine production) correspond in principle to SGB which we are promoting here (see Section 5.3). Even though the Tracey model focuses predominantly on systemic autoimmune diseases and not on viral infections, there is striking evidence that the pathogenetic reflex mechanisms might be the same (uniform pathogenesis) with different initial (also viral) stimuli (see Section 3.5.2).
The severity of inflammatory disease is also closely related to the activity of sensory TRPV1-expressing neurons (transient receptor potential channel, formerly known as vanilloid receptor 1 or capsaicin receptor) in the truncus sympathicus and in the vagus nerve, as well as to the activity of sympathetic and vagal efferents in the lungs which modulate inflammation and immune system (Baral et al., 2018; Nahama et al., 2020). These neurons are in cross talk with the immune system (Tränkner et al., 2014; Chavan et al., 2017) and can release more proinflammatory molecules such as SP and cytokines once activated. In order to reduce overproduction of cytokines, the authors suggest to block TRPV1-positive fibers which produce SP. Nahama et al. (2020) propose a periganglionic block corresponding to SGB. Baral et al. (2018) confirmed this approach in animal studies (ablation of TRPV1).
Numerous authors stress the importance of the nervous system in excessive inflammation: Neuronal reflex pathways can increase the secretion of proinflammatory cytokines and inflammation (Heijnen et al., 2002; Tracey, 2002; Perez et al., 2009; Jänig and Baron, 2011; Bellinger and Lorton, 2014). A virus-induced exaggerated immune response (cytokine storm) is considered a consequence of excessive neurogenic mechanisms (prolonged airway hyperresponsiveness) (Perianin et al., 1989; Mejías et al., 2005). Cytokine overproduction seems to be important in tissue damage, e. g. in the lung (Wang et al., 2020). In order to regulate the cytokine storm, we believe it is crucial to regulate the overshooting neurogenic mechanisms, e. g. by SGB, to achieve a different “setpoint” (Tracey, 2009).
Ligand (virus)-receptor interactions, too, activate the secretion of proinflammatory cytokines. The latter activate sensory nerve fibers (Fig. 2) and evoke a reflex response via the SNS and vagus (Tracey, 2009). If this response is overshooting and sympathetic-dominant, electrical vagus nerve stimulation can be applied therapeutically (Tracey, 2002; Tracey, 2009), which corresponds functionally to SGB in the case of a shift towards sympathetic nerve activity. SGB reduces the production of TNF-α, IL-1, IL6, and IL-8 (Liu et al., 2013; Yang et al., 2015; Chen et al., 2018) and increases the production of IL-10, which is anti-inflammatory (Table 1 ) (Liu et al., 2013; Yang et al., 2015; Chen et al., 2018). Chemical sympathectomy showed basically the same results (Grebe et al., 2010). Stimulation of the vagus nerve also reduces the production of proinflammatory cytokines in the spleen by unknown mechanisms (Tracey, 2009), stressing the communication (interaction) between the nervous and the immune system (see above). If even the nucleus tractus solitarius is affected by virus invasion, as described. e. g. in some cases of Covid-19 (Li et al., 2020), it can lead to a sympathetic (catecholamine) storm, which then induces a cytokine storm leading to neurogenic pneumonia (Ur and Verma, 2020a; Ur and Verma, 2020b). Early treatment of the nervous system is – as the authors stress – crucial in this situation (Ur and Verma, 2020b). We believe that SGB is the right choice because it reduces hyperinflammatory mechanisms in the CNS as well as in lung and heart area etc.
Table 1.
Effects of the stellate ganglion block.
Regulation of the immune system/hyperinflammation
|
| Regulation of the endothelial dysfunction (Chen et al., 2012) |
| Regulation of microcirculation and coagulopathy (Levy and Lorch, 1996; Hanamatsu et al., 2002; Pfister and Fischer, 2009; Yamazaki et al., 2012; Zhou et al., 2014; Punj, 2019) |
| Reduction of (neurogenic) pulmonary edema (Pierach and Stotz, 1952; Zhang et al., 2013; Liu et al., 2017; Chen et al., 2018) |
| Reduction of pulmonary arterial hypertension (Myers et al., 1998) |
| Reduction of pathological positive feedback loops (Fischer, 2003; Jänig, 2011; Fischer, 2019) |
As we could show in our previous works, the spinal cord is also strongly involved in the development of peripheral inflammation, with indications that the effects are mediated via the SNS (Boettger et al., 2010b, Boettger et al., 2010a). SGB – as early as possible (Strong et al., 2018) – is a logical intervention to interrupt feedback loops.
We assume that a neuroplastically altered SG intensifies pneumonia towards ARDS, consistent with the neural hypothesis of lung edema (Ur and Verma, 2020b). Neuroimmunologically controlled hyperinflammation also involves functional and structural changes in the endothelium and microcirculation and possibly also coagulopathy (see 3.3, 3.4). This results in further feedback loops (Fig. 1).
Since SGB shuts down the mentioned feedback loops simultaneously (because they are interdependent), the neuroimmune system has now the chance to reorganize itself (autoregulation). This reorganization is therefore not tied to the duration of action of the LA (procaine); rather, it far outlasts the short-term SGB (Puente de la Vega Costa et al., 2016; Barop, 2017; Fischer, 2019).
3.3. Regulation of the endothelial dysfunction by SGB
The ANS influences endothelial functions from outside the vessels (Amiya et al., 2014). Blood vessels are innervated by sympathetic and – only in a few places in the body – parasympathetic nerve fibers. Endothelial dysfunction and inflammation are the consequence of pathologically increased sympathetic nerve activity (Sheng and Zhu, 2018). On the other hand, the endothelium can influence the SNS via vasoactive factors. This results in complex interactions involving positive feedback loops. Inflammation impairs the endothelial function (Sinisalo et al., 2000). Even anxiety or sympathetic hyperactivity, e. g. with Takotsubo syndrome (Naegele et al., 2016) is associated with endothelial dysfunction (Narita et al., 2007) further stressing the connection between ANS, immune system, and endothelium (Mousa et al., 2010).
Endothelial cells can also produce vasoactive substances such as nitric oxide (NO) and endothelin. Endothelin maintains local and generalized processes. It can stimulate the peripheral and central SNS and can also be released by postganglionic sympathetic neurons and control catecholamine release and vascular tone (Yamaguchi, 1997; Schiffrin, 2012). In cooperation with the SNS, endothelin has a pathophysiological role in arterial hypertension including pulmonary hypertension, atherosclerosis, vascular inflammation with fibrosis, and pulmonary fibrosis (Schiffrin, 2012). Increased sympathetic tone combined with infiltration of the blood vessel wall can lead to hypertension (Zhang et al., 2020). Chen et al. (2012) observed a decrease in endothelin (and hypertension) in rats after applying SGB. Like endothelin, NO is involved in the complex regulatory circuits of endothelium–inflammation–ANS and has been favorably influenced by SGB in animal experiments (Chen et al., 2012). Leptin, released from adipocytes, also plays a role in arterial hypertension in collaboration with the SNS, and it causes endothelial dysfunction and increased blood pressure (Wang et al., 2013). Both pathologies are mediated by SNS activation (Wang et al., 2013). These effects of leptin were neutralized by sympathetic denervation (ganglionectomy) (Wang et al., 2013). That obesity and hypertension are risk factors for severe courses of Covid-19 is also understandable from this point of view (see also Section 5.2).
3.4. Regulation of microcirculation – disturbance and coagulopathy by SGB
The severity of an infectious disease is not only determined by pathogenic damage and reflectory (hyper-)inflammation of the organs (e. g. in pneumonia), but also by microcirculatory disturbance with hypercoagulopathy, as with Covid-19. Inflammation is the predominantly sympathetically controlled vascular response to irritants such as viruses and others (Ricker, 1924; Marvar and Harrison, 2012). The response includes vasodilation with disturbed blood flow in irritated tissue, increased vascular permeability, production of cytokines and chemokines, and mobilization of immune cells. In a feedback loop, the brain modulates the intensity of inflammation after being informed by sensory nerve fibers from the irritated tissue (Fig. 2, Fig. 3) (Sternberg, 2006; Marvar and Harrison, 2012). In 1994 already, Greiff et al. reported a microvascular exudative hyperresponsiveness in human coronavirus-induced common cold. Salgado et al. (2010) described disturbed microcirculatory blood flow in patients with severe influenza A (H1N1). The importance (and the link) of microvascular alterations and coagulopathy with microthrombi are also discussed in the pathogenesis of Covid-19 (Damiani et al., 2020; Magro et al., 2020; Varga et al., 2020; Spiezia et al., 2020). In our opinion, the SNS should be more considered. Overactivity of the SNS including the hypothalamic-pituitary-adrenal axis can lead to hypercoagulability with thrombus formation (von Känel and Dimsdale, 2000; Pagano et al., 2014). Microthrombi of the lungs and other organs are mainly mediated by cytokines (Levi et al., 2008; Merad and Martin, 2020), and therefore can be triggered by a pathologic ANS hyperactivity. Wang et al. (2019) also described the role of sympathetic activity on the pathogenesis of pulmonary embolism. Crucial is sympathetic hyperactivity which influences coagulation and fibrinolysis pathways and causes microthrombi with increased plasma D-dimers (Ur and Verma, 2020b). SGB also has an effect on pulmonary arterial hypertension and thus on microcirculation (Myers et al., 1998; Zhao et al., 2019) and is therefore a logical intervention for microcirculation disorders and coagulopathy (Murakawa, 1993; Levy and Lorch, 1996; Hanamatsu et al., 2002; Pfister and Fischer, 2009; Yamazaki et al., 2012; Zhou et al., 2014; Punj, 2019) (Fig. 4).
3.5. Further clinical and experimental data
3.5.1. SGB in pneumonias
The SNS increases proinflammatory cytokines and exacerbates influenza A virus pathogenesis (Grebe et al., 2010). The authors showed that peripheral chemical sympathectomy in mice reduced morbidity and mortality from influenza A virus-induced pneumonia. After sympathectomy they observed a reduced cytokine response and a reduced inflammatory influx of monocytes, neutrophils, and natural killer (NK) cells. In the old works by the surgeon Leriche, it is suggested that sympathectomy for inflammation corresponds in effect to repeated SGB with procaine (Leriche and Fontaine, 1934).
The theoretical background of hyperinflammation due to disequilibrium of the ANS with a dominance of the sympathetic part in acute lung injury stimulated the animal experiments of Liu et al. (2017). They showed that the regulation of the ANS, especially the sympathetic part, by SGB reduced inflammation and improved lung function in rabbits. In principle, vagus nerve stimulation produces a better balance in the ANS in case of overstimulated SNS. Vagus nerve stimulation also had an anti-inflammatory effect, as did the administration of cholinesterase inhibitors, but both were less potent than SGB (Liu et al., 2017). SGB not only reduced proinflammatory cytokines, but also the swelling of microvascular endothelial cells and epithelial type I cells in the lung (Liu et al., 2017).
Chen et al. (2018) showed in septic rats with acute lung injury that SGB reduces TNF-α and IL-6, increases the anti-inflammatory IL-10, and inhibits the infiltration of inflammatory cells into lung tissue.
Baral et al. (2018) produced a methicillin-resistant staphylococcus aureus-pneumonia in mice: TRPV1-expressing sensory neurons in the truncus sympathicus and the vagus nerve were chemically ablated (this corresponds in our opinion to the repeated SGBs in this circuit). Survival was improved and cytokine storm significantly reduced. Thus reduced activity of TRPV1-positive nerve fibers therefore probably prevents the transition of mild pneumonia into ARDS. In our opinion, this is a further plausible reason for SGB in such situations, supported by the fact that the TRPV1 channels can be influenced by LA such as procaine (Yanagidate and Strichartz, 2007). This even raises the question of the additional benefit of an intravenous procaine infusion (Papathanasiou, 2020), especially since procaine is anti-infectious, anti-inflammatory and antithrombotic (Cassuto et al., 2006; Konrad et al., 2006).
Okada et al. (1996) found a reduction in the activity of NK-cells in the spleen after resection of the cervical border strand, and Sugimoto et al. (1999) found that SGB causes a short-term reduction in cytotoxic T-cell activation, i. e. in NK-cells. Yokoyama et al. (2000), also found a modified distribution of lymphocyte subsets and NK cell activity following SGB. SGB also regulates the increased hormone release of the HPA axis, especially the norepinephrine levels (Yokoyama et al., 2000).
3.5.2. SGB in other situations of inflammation or acute hyperinflammation (cytokine storm)
The following studies and experiments come to uniform basic statements about the effect of SGB – although different organ systems such as the lungs, vessels, etc. or a trauma, a postoperative state, an autoimmune disease are affected. In principle, the mechanisms of predominantly sympathetic-triggered inflammation or hyperinflammation are always the same since the body has just a few reaction patterns to respond to stimuli such as infection, trauma, etc. The neuroimmunological and inflammatory cascade seem to be in motion regardless of the initial stimulus. The affected end organ determines the specific clinical appearance. Some examples: Analogous to the overreaction (cytokine storm) of the neuro-endocrine-immune network in Covid-19, the same processes are found in the early phase of severe trauma. It is called systemic inflammatory response syndrome (SIRS) (Liu et al., 2013) and is one of the major factors in complications, organ failure and death. SIRS, too, is associated with an overproduction of proinflammatory cytokines such as IL-1β, IL-6 and TNF-α. SGB regulates the early systemic inflammatory response in severe trauma patients (Liu et al., 2013). The authors found that the concentrations of IL-β, IL-6 and TNF-α (but not IL-4 and IL-10) are significantly lower after SGB than in the control group without SGB. SGB regulates the hyperactive neuroendocrine-immune response to allow homeostasis and is recommended as an effective therapy for impending SIRS.
Yang et al. (2015) found that SGB can regulate cellular and humoral immune functions in patients with traumatic brain injury (TBI); likewise, it can improve an overreacting nerve-endocrine-immune system, excessive vasoconstriction, abnormal blood rheology, and thus restore the balance between the sympathetic (excessive sympathetic tone) and parasympathetic nervous systems. In 50 patients with TBI, Yang et al. (2015) showed that the excessive inflammatory response after TBI is an important reason that aggravates the secondary brain injury. IL-1β, IL-6 and TNF-α were significantly reduced after SGB compared to the control group. The authors also suspect a positive effect of SGB on central and peripheral vasoconstriction.
Thus, severe trauma can cause an overreactive stress response involving the complex feedback in the neuro-endocrine-immune system (Molina, 2005; Liu et al., 2013; Yang et al., 2015). Normally, an adequate stress response is protective. The SNS plays the most important role in these interactions but the balance with the parasympathetic nervous system is important (Puente de la Vega Costa et al., 2016). So, even in severe trauma, one of the most urgent goals is to prevent e. g. a cytokine storm, just as with Covid-19. Thus, the question also arises whether the term SIRS should be defined as a general principle (uniform pathogenesis with different initial stimuli, i. e. causes) in different diseases with pathological hyperinflammation (as with Covid-19). Notably, the principal – physiological or overshooting and therefore damaging – reactive neuroimmunological mechanisms with physiological or hyperinflammation are the same, regardless of whether their trigger is trauma, toxins, viruses, bacteria, etc. or psychological stress.
There are further examples: In a prospective, double blinded randomized parallel study SGB applied before laparoscopic gynecologic surgery decreased pain significantly immediately after surgery (Rahimzadeh et al., 2020). Postoperative pain always has an inflammatory component. Another randomized, double blind, placebo-controlled study (Kumar et al., 2014) showed that SGB had a significant analgesic effect on orthopedic upper limb surgery, additionally measured by opioid consumption. García-Morán et al. (2013) and Guo et al. (2014) demonstrated a favorable effect on perioperative stress-related cytokine production. Sympathetic hyperactivity is regulated not only peripherally, but also centrally via the hypothalamus (Amino et al., 2007; Taneyama and Goto, 2009), and therefore SGB also improved chronic inflammatory bowel disease (ulcerative colitis), as shown as an additional intervention with sulfasalazine medication. In addition to a better clinical course, a reduction in inflammatory mediators (IL-8), faster mucosal regeneration, less pain and fewer complications were observed (Zhao et al., 2017). The inflammatory response and mortality of mice with combined radiation and burn injury can be decreased by SGB (Lu et al., 2006). SGB can prevent or reduce the development of neurogenic edema in the lungs (Zhang et al., 2013) and possibly in the brain (Fischer, 1995). An anti-inflammatory effect of SGB in pulmonary edema is also suggested by Pierach and Stotz (1952), Liu et al. (2017), Chen et al. (2018), Ur and Verma, 2020a, Ur and Verma, 2020b. Activation of sympathetic fibers with nuclei in the upper thoracic spinal cord can cause pneumonitis (Lee, 2009; Lee and Yu, 2014), speaking for their regulation (temporary deactivation of the nerve fibers) by SGB. However, the success depends on the time of inflammation at which the intervention is carried out. It seems most successful in the early stages, as we could show (Schaible and Straub, 2014; Strong et al., 2018). Other studies have also shown a time-dependent effect of SGB (Rahimzadeh et al., 2020).
On the question of the timing of the SGB: Much is still unknown. One of the explanations may be that similar to the pain memory, an inflammation memory may develop under the described positive feedback loops. In other words, sensitization processes lead to neuroplastic changes over time. These may be reversible in some cases, but the timing of the intervention is logically important – we also see the best results clinically when we intervene early with repeated SGB in the sense of “desensitization” (typical for e. g. CRPS [Pfister and Fischer, 2009]). In CRPS for example, central neuroplastic changes also result over time, which are even visible on imaging in the cortex (Maihöfner et al., 2003). It is logical that in the late stage a SGB has less effect. We can try to argue analogously for the neuroimmune system. However, we are only at the beginning of a new idea with the mechanisms of action.
In Covid-19 analysis of cytokine levels (“inflammatory phenotyping”) can identify patients at risk of deterioration (Burke et al., 2020; Caricchio et al., 2021). This can – together with the clinical presentation – help to find the right timing for SGB.
3.6. Regulation of acute hyperinflammation (cytokine storm) without deterioration of immunity or increase in viral load by short-term SGB
In cases of imminent or manifest cytokine storm e. g. in Covid-19, we rather suggest regulation of the neuroimmune system by short-lasting SGB than blockade of immune functions by selective drugs, since a “selective” drug-induced blockage of parts of the neuroimmunological processes may be associated with serious side effects including deterioration of immunity and subsequent secondary infection or increase in viral load (Sanders et al., 2020; Velavan and Meyer, 2020). The administration of corticosteroids is also controversially discussed (Das et al., 2020). They should not be administered routinely in cases of cytokine storm, but only when severe edema and hyaline membranes occur (Xu et al., 2020). This is easy to understand from pathophysiology: mediated by the HPA axis, the serum cortisol concentration is already elevated under disease stress. An “artificial” increase in cortisol concentration results in a significant increase in mortality from Covid-19 (Tan et al., 2020).
Since (despite the term SGB) we do not block but regulate, we do not interfere with the body's natural ability to develop an immunological memory (adaptive immune system) and therefore there is no greater risk of subsequent re-exposure. It is true that the SGB modifies the distribution of the lymphocyte subsets and the NK cell activity (increase in the proportion of B- and T-cells, and a decrease in NK-cells) (see Section 3.5.1), but these effects were transient (Yokoyama et al., 2000). The transient SGB serves mainly to regulate an excessive immune response to a physiological immune response.
3.7. Properties of procaine
In contrast to amide-structured LA (lidocaine, bupivacaine), procaine has a direct vasodilating (regulating) effect in addition to the sympatholytic effect. Furthermore, procaine has an antiinflammatory effect (Hollmann and Durieux, 2000; Cassuto et al., 2006). LA also act on non-neuronal cells such as mast cells, neutrophils and monocytes as well as on cytokine production and also have antibacterial and antiviral effects (Hollmann et al., 2001; Cassuto et al., 2006). LA can also modulate an excessive inflammatory response that is no longer protecting, but destroying tissue (Brown et al., 1995; Groeben et al., 1996; Groeben et al., 1999; Hollmann and Durieux, 2000). In addition, LA can reduce inflammation in the microglia (Zheng et al., 2019).
Procaine has no significant side effects (Fischer et al., 2005; Fischer et al., 2010; Barop, 2017) and no interactions with other drugs because it is not broken down by the liver but by pseudocholinesterase, which is ubiquitously present. This is also an advantage in Covid-19, since most of the time, severely ill patients are elderly, receiving polypharmaceutical treatment.
4. Applying SGB using a minimally invasive technique
In 2016 we presented a modified technique (Puente de la Vega Costa et al., 2016) based on Leriche and Fontaine (1934), Dosch (1986), Fischer (1998), and Barop (2017). Technical details were described in “Autonomic Neuroscience” (Puente de la Vega Costa et al., 2016). We recommend the bilateral SGB with a time interval of ca 1 h (disappearing Horner complex) twice daily for 2 or 3 days.
4.1. Material
Procaine 1% 5 ml each side without any additives. Needle: 20 × 0.4 mm or 12 × 0.3 mm, depending on body type (short needles are possible because of the remaining finger on Chassaignacs tubercle).
This technique allows a very shallow depth of the injection, so that very thin needles can be used, which allows the stellate injection even in anticoagulated patients. However, with extremely thin needles, aspiration is more difficult and must be performed slowly and continuously. For safety reasons, anticoagulated patients must be given several minutes of compression at the injection site.
4.2. Possible complications
Paresis of the recurrent or phrenic nerve on the opposite side can lead to breathing problems. Accidental injection of a LA into a brain-bound blood vessel or into the cranial area of the cerebrospinal fluid space (cave: cervical cerebrospinal fluid cyst) can cause cramps, unconsciousness, cardiac and respiratory arrest.
5. Discussion
5.1. The neuroimmune system – complex and nonlinear?
There is evidence that an imbalance in the ANS (in particular an excessive sympathetic tone) due to pre-existing conditions is at the beginning of the overshooting reactions in Covid-19 and other diseases. Even small changes in non-physiologically balanced complex systems can therefore have a major effect. This is typical in a system with interdepending positive feedback loops (iterations) as described in the previous chapters. This gives the neuroimmune system a complex nonlinear character, analogous the mathematical chaos theory as it was described for nature and biology (Mandelbrot, 1967; Lorenz, 1972; Nicolis and Prigogine, 1977; Feigenbaum, 1978; Kluge and Neugebauer, 1994). We postulate several positive feedback loops dependent on the ANS which play a role in the overreaction of the neuroimmune system, in which neural and humoral factors reinforce each other. In positive feedback loops it is often difficult to find the “starting points”. We will try to illustrate this with some examples: Since neurons express receptors for inflammatory mediators (Schaible, 2014; Ji et al., 2016), SP or cytokines activate sensory nerve fibers to increase the synthesis (White et al., 2005; Zelenka et al., 2005; Xu et al., 2018) and release of even more SP (O'Connor et al., 2004; Matsuda et al., 2019) and cytokines (Galic et al., 2012). This positive feedback loop is a very fast way to reach high concentrations of SP and cytokines, but there is also the danger of overreaction when the system is already preloaded. This could be one of the mechanisms of a cytokine storm. SP can additionally stimulate the production of cytokines by macrophages (e. g. IL-1, IL-6 and TNF-α) (Bill et al., 1979) and induce chemotaxis and degranulation of human neutrophils (Serra et al., 1988; O'Connor et al., 2004). Furthermore, immune cells express adrenoceptors (Bellinger and Lorton, 2014), induced by proinflammatory cytokines (Heijnen et al., 2002; Perez et al., 2009). Thus, the SNS can induce their cytokine production – and cytokines are also produced by nerve fibers themselves (White et al., 2005; Zelenka et al., 2005). Furthermore, certain cytokines (IL-1) increase the synthesis of SP in sympathetic ganglia (Freidin and Kessler, 1991) and stimulate their secretion in afferent neurons (Inoue et al., 1999). The production and secretion of SP by lymphocytes and macrophages (De Giorgio et al., 1998; Lai et al., 1998) causes further positive feedback with expansion and intensification of neurogenic inflammation. Positive feedback loops are strongly interconnected, typical for a complex, iterative, chaotic system.
The question arises if positive feedback loops make sense in physiology. In our opinion these iterations are the quickest way to generate a physiological inflammation. In case of an infection, cytokines and SP can be provided very quickly. However, there is also the disadvantage that such systems are unstable, and we hypothesize that if the SNS is preloaded (such as hypertension, obesity, stress, negative emotions) or the regulatory capacity reduced, the (positive feedback) system can overreact and lead to a cytokine storm with a consecutive acute hyperinflammation in case of viral infections such as SARS-CoV-2. Thus, we believe, that the severity of e. g. viral pneumonia or even ARDS does not depend linearly on the initial stimulus (amount of virus). Depending on time and the current state of the system, the same parts of the ANS can have either an activating or inhibiting effect. For example, the SNS can have a pro- or anti-inflammatory (late phase) effect (Pongratz and Straub, 2014; Strong et al., 2018). Therefore, neuronal pathways can use the same neurotransmitters either synergistically or antagonistically (Tracey, 2009). These observations also show the complexity of the system. In our opinion, the therapeutic manipulation of only one cell type (e. g. by receptor blockers) as a linear, reductionist intervention can only be associated with disadvantages and side effects (as experience has shown), since the cell type in question is protective under physiological conditions and only causes tissue damage in the case of dysregulation (hyperresponsiveness) (Ji et al., 2016). By contrast, the regulation of the ANS by means of short term SGB has an influence on cytokines and chemokines, for example, and – since regulation is used instead of suppression – has no adverse effects on the “physiological” performance of the inflammatory and immune system.
For this reason, the temporary shutdown by means of SGB is a logical intervention, since SGB has an influence at several places at the same time, and the system has the chance to reorganize itself afterwards (autoregulation) which is typical for a complex, nonlinear system.
5.2. Pre-existing conditions strain the neuroimmune system
We put forward the idea that with the hyperresponsiveness of the immune and inflammatory system, an overreaction of the already preloaded ANS plays an important role, specifically, by activity-enhancing positive feedback loops (see Section 3.2). So, the “cytokine storm” is caused by a “sympathetic storm” (Lemke, 2004) in the pre-loaded ANS; the “setpoint” (Tracey, 2002, Tracey, 2009) is shifted, corresponding to Speranski's (1950) “first strike” (see Section 3.2).
Thus, pre-existing sympathetic hyperactivity (including hypertension, obesity, psychological stress, multimorbidity, polymedication, metabolic syndrome, diabetes, intestinal diseases) and the associated central and peripheral neuroimmunological imbalance can lead to severe, sometimes fatal processes in case of a “second strike” (see Section 3.2) as in a viral infection with Covid-19 (Bellinvia et al., 2020) especially in older people, in whom the neuroimmonological system appears to be less adaptable.
Hypertension and obesity are two major recognized risk factors for a severe course of Covid-19, both of which are characterized by sympathetic hyperactivity and imbalance of the SNS and vagus, respectively. As a result, the release of leptins from adipocytes is increased (Wang et al., 2013). Leptins cause endothelial dysfunction with increased release of endothelin, and further increase in blood pressure via the SNS (Wang et al., 2013) (see Section 3.3). Also, central mechanisms play a role: Kumagai et al. (2012) describe the importance of RVLM (rostral ventrolateral medulla) in determining efferent sympathetic nerve activity and blood pressure. In this way, analogous to chaos theory, further interdependent positive feedback loops, mediated via the SNS, are created. These effects are neutralized by sympathetic denervation (Wang et al., 2013) (which corresponds to repeatedly administered SGBs [Leriche and Fontaine, 1934]).
5.3. Vagus nerve stimulation or stellate ganglion block?
The imbalance between the SNS and the vagus with sympathetic hyperactivity may be an important factor in hyperinflammation with cytokine storm. The balance can in principle be improved by vagus nerve stimulation (VNS) or by SGB. Piedimonte et al. (1999) and King et al. (2001) considered early on in their therapeutic considerations the neuronal regulation of neurogenic inflammation in viral infections. Neuromodulation by VNS in reflex inflammation is proposed by various authors (Borovikova et al., 2000; Tracey, 2002; Tracey, 2009; Ji et al., 2016; Breit et al., 2018; Caravaca et al., 2019; Pavlov and Tracey, 2015; Pavlov et al., 2020; Tsaava et al., 2020). Tracey, 2002, Tracey, 2009 suspects that in the future, further neuroimmunological reflex pathways will be discovered which can produce homeostasis in the innate and the adaptive immune system through therapy of the ANS. In general, according to these authors, it should be possible in the future to treat the immune response and the progression of the inflammation therapeutically via the neuronal networks. Caravaca et al. (2019) see the therapeutic potential of the VNS also for autoimmune diseases with hyperinflammation such as chronic inflammatory intestinal diseases, rheumatoid arthritis, etc.
By applying SGB we regulate not only the SNS, but also the vagus nerve because, on the one hand, of their close connections within the periphery (anatomical and rami communicantes), as we described in our review (Puente de la Vega Costa et al., 2016), on the other hand, indirectly, because of the involvement of the sensory vagus “receiving site” in the brain (nucleus tractus solitarius). In addition, SGB enables us to reach the central sympathetic “output” centers such as the hypothalamus, pituitary gland, RVLM, etc. as well as peripheral sympathetic efferents and sensory fibers in the truncus sympathicus. Therefore, from a pathophysiological point of view, we consider SGB more advantageous than VNS, also confirmed experimentally by Liu et al. (2017).
5.4. Why has the SGB not been used more frequently in medicine?
The most common uses of SGB are in CRPS, circulatory disorders, and sympathetically maintained pain. The fear of hemodynamic problems has been a topic for decades. However, we could show that the hemodynamic parameters hardly change (Puente de la Vega Costa et al., 2016). There is a respect for other complications. They mainly occurred when applying old techniques with significantly larger amounts of long-acting LA with a high diffusion capacity (undesired diffusion to neighboring structures). With the short-acting, only slightly diffusing procaine in lower amounts, we have not seen any complications; only a few have been noted in the literature (Fischer et al., 2005; Fischer et al., 2010) – with the reservation that certainly not all complications have been reported and published.
The decades-long fear of the allergic potential of procaine has not been confirmed in recent years (Fischer et al., 2005; Barop, 2017). In the past, mainly the additives triggered allergies. Procaine even has an anti-allergic potency (Barop, 2017; Fischer, 2019).
Another reason for the limited breadth of its application is that SGB is not considered guideline-compliant for most indications – and usually only specially trained anesthetists perform SGBs.
In addition, the range of indications is not known to many clinically active physicians due to decades of neglect of the ANS in pain, immune and inflammatory processes.
Oyama and Node (2014) put it aptly: “Most of the previous studies lacked careful consideration of the impact of the ANS. The main reason is the difficulty to evaluate sympathetic nerve activity directly and easily.”
5.5. Limitations and factors that may impair the efficacy of a stellate ganglion block
Whether the injection can be performed during anticoagulation has to be decided individually (risk-benefit assessment, lack of other therapeutic options, or these have been exhausted). However, the area of injection can well be compressed manually after the (atraumatic) injection, preventing hematoma. Nevertheless, we principally advise against SGB in anticoagulated patients. This is another reason why early recognition of a threatening course of Covid-19 is important; thus, SGB can be performed shortly before anticoagulation is started. Therefore, clinical and laboratory monitoring of high-risk patients should be close-meshed.
The timing of SGB plays a major role: it should be as early as possible, if laboratory or clinical evidence (e. g. beginning respiratory insufficiency) shows that the neuroimmunological networks are overreacting (Burke et al., 2020; Caricchio et al., 2021). In general, we have seen the best results in acute or acutely exacerbated inflammation (Pfister and Fischer, 2009; Schaible and Straub, 2014; Strong et al., 2018) (for “timing” see also Section 3.5.2).
In our experience, corticosteroids or other inhibitory drugs as well as old age can impede the self-organization expected after SGB (Barop, 2017; Fischer, 2019).
The correct technique plays an important role: not only the equilateral Horner symptom complex should be positively checked, but also a temperature increase of the equilateral upper extremity of >0.5 °C; because if the SNS is blocked cranially the SG, a Horner symptom complex is though created, but the postganglionic fibers for the lungs and heart are not directly affected by the block.
In previous studies and experiments, SGB was usually only conducted unilaterally. However, we propose the bilateral SGB with a time interval (see Section 4).
Another limiting factor could be old age (reduced capacity for self-organization) – we got this impression during our clinical work – but this would still have to be verified by means of studies.
6. Conclusions
The sympathetically maintained interdependent positive feedback loops give the neuroimmune system an iterative, nonlinear character (compatible with the mathematical chaos theory). Such systems have the fundamental ability to reorganize themselves (autoregulation) after changes of state e. g. by means of SGB. With this regulation the nonlinearity of the system is respected.
Previous clinical and experimental data show that the repeated, temporary SGB with LA procaine, is capable to regulate the sympathetic-triggered neurogenic inflammation processes.
Because of the very low material costs, this therapy is also suitable for countries with low financial resources in the health system.
Despite the existence of clinical and experimental data on the effect of SGB and its logical mechanisms of action, larger studies are necessary. We hope that this work will serve as a basis for applications to the ethics committees.
Because of the lack of direct measurements of the ANS especially in pain, immune and inflammatory processes, the ANS was not considered enough in the past in our opinion. We hope that our conceptual view with partially new pathophysiological inputs may help to show how important the careful consideration of the ANS is. We also think that the therapy by the ANS is just at the beginning and that there is still a great potential here.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
None.
Acknowledgment
We would like to thank Professor Wilfried Jänig, University Kiel, Germany, many of whose papers we cited, for very constructive discussions. We thank our illustrator, Hans Holzherr, for the remarkable precision of his drawings and for his important hints.
References
- Affleck V.S., Coote J.H., Pyner S. The projection and synaptic organisation of NTS afferent connections with presympathetic neurons, GABA and nNOS neurons in the paraventricular nucleus of the hypothalamus. Neuroscience. 2012;219(1–2):48–61. doi: 10.1016/j.neuroscience.2012.05.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alston E.N., Parrish D.C., Hasan W., Tharp K., Pahlmeyer L., Habecker B.A. Cardiac ischemia-reperfusion regulates sympathetic neuropeptide expression through gp130-dependent and independent mechanisms. Neuropeptides. 2011;45(1):33–42. doi: 10.1016/j.npep.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amino M., Yoshioka K., Morita S., et al. Is the combination therapy of IKr-channel blocker and left stellate ganglion block effective for intractable ventricular arrhythmia in a cardiopulmonary arrest patient? Cardiol. J. 2007;14(4):355–365. [PubMed] [Google Scholar]
- Amiya E., Watanabe M., Komuro I. The relationship between vascular function and the autonomic nervous system. Ann. Vasc. Dis. 2014;7(2):109–119. doi: 10.3400/avd.ra.14-00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansel J.C., Brown J.R., Payan D.G., Brown M.A. Substance P selectively activates TNF-alpha gene expression in murine mast cells. J. Immunol. 1993;150(10):4478–4485. [PubMed] [Google Scholar]
- Baral P., Umans B.D., Li L., et al. Nociceptor sensory neurons suppress neutrophil and γδ T cell responses in bacterial lung infections and lethal pneumonia. Nat. Med. 2018;24(4):417–426. doi: 10.1038/nm.4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barop H. Thieme; Stuttgart, New York: 2017. Textbook an Atlas of Neural Therapy. Diagnosis and Therapy With Local Anesthetics. [Google Scholar]
- Bellinger D.L., Lorton D. Autonomic regulation of cellular immune function. Auton. Neurosci. 2014;182:15–41. doi: 10.1016/j.autneu.2014.01.006. [DOI] [PubMed] [Google Scholar]
- Bellinvia S., Edwards C.J., Schisano M., Banfi P., Fallico M., Murabito P. The unleashing of the immune system in COVID-19 and sepsis: the calm before the storm? Inflamm. Res. 2020;69(8):757–763. doi: 10.1007/s00011-020-01366-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benias P.C., Wells R.G., Sackey-Aboagye B., et al. Structure and distribution of an unrecognized interstitium in human tissues. Sci. Rep. 2018;8(1):4947. doi: 10.1038/s41598-018-23062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bill A., Stjernschantz J., Mandahl A., Brodin E., Nilsson G. Substance P: release on trigeminal nerve stimulation, effects in the eye. Acta Physiol. Scand. 1979;106(3):371–373. doi: 10.1111/j.1748-1716.1979.tb06412.x. [DOI] [PubMed] [Google Scholar]
- Boettger M.K., Weber K., Gajda M., Bräuer R., Schaible H.G. Spinally applied ketamine or morphine attenuate peripheral inflammation and hyperalgesia in acute and chronic phases of experimental arthritis. Brain Behav. Immun. 2010;24(3):474–485. doi: 10.1016/j.bbi.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Boettger M.K., Weber K., Grossmann D., Gajda M., Bauer R., Bär K.J., et al. Spinal tumor necrosis factor alpha neutralization reduces peripheral inflammation and hyperalgesia and suppresses autonomic responses in experimental arthritis: a role for spinal tumor necrosis factor alpha during induction and maintenance of peripheral inflammation. Arthritis Rheum. 2010;62(5):1308–1318. doi: 10.1002/art.27380. PMID: 20213802. [DOI] [PubMed] [Google Scholar]
- Borovikova L.V., Ivanova S., Zhang M., et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405(6785):458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- Breit S., Kupferberg A., Rogler G., Hasler G. Vagus nerve as modulator of the brain-gut axis in psychiatric and inflammatory disorders. Front. Psych. 2018;9:44. doi: 10.3389/fpsyt.2018.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R.H., Robbins W., Staats P., Hirshman C. Prevention of bronchoconstriction by an orally active local anesthetic. Am. J. Respir. Crit. Care Med. 1995;151(4):1239–1243. doi: 10.1164/ajrccm/151.4.1239. [DOI] [PubMed] [Google Scholar]
- Burke H., Freeman A., Cellura D.C., et al. REACT COVID investigators: inflammatory phenotyping predicts clinical outcome in COVID-19. Respir. Res. 2020;21(1):245. doi: 10.1186/s12931-020-01511-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caravaca A.S., Gallina A.L., Tarnawski L., et al. An effective method for acute vagus nerve stimulation in experimental inflammation. Front. Neurosci. 2019;13:877. doi: 10.3389/fnins.2019.00877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caricchio R., Gallucci M., Dass C., et al. Temple University COVID-19 research group. Preliminary predictive criteria for COVID-19 cytokine storm. Ann. Rheum. Dis. 2021;80(1):88–95. doi: 10.1136/annrheumdis-2020-218323. [DOI] [PubMed] [Google Scholar]
- Cassuto J., Sinclair R., Bonderovic M. Anti-inflammatory properties of local anesthetics and their present and potential clinical implications. Acta Anaesthesiol. Scand. 2006;50(3):265–282. doi: 10.1111/j.1399-6576.2006.00936.x. [DOI] [PubMed] [Google Scholar]
- Chavan S.S., Pavlov V.A., Tracey K.J. Mechanisms and therapeutic relevance of neuro-immune communication. Immunity. 2017;46(6):927–942. doi: 10.1016/j.immuni.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.Q., Hu G.X., Fu Q., Jin X.J. Effects of stellate ganglion block on blood pressure in spontaneously hypertensive rats. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2012;41(1):65–68. doi: 10.3785/j.issn.1008-9292.2012.01.011. [DOI] [PubMed] [Google Scholar]
- Chen Y., Guo L., Lang H., et al. Effect of a stellate ganglion block on acute lung injury in septic rats. Inflammation. 2018;41(5):1601–1609. doi: 10.1007/s10753-018-0803-x. [DOI] [PubMed] [Google Scholar]
- Chiu I.M., von Hehn C.A., Woolf C.J. Neurogenic inflammation and the peripheral nervous system in host defense and immunopathology. Nat. Neurosci. 2012;15(8):1063–1067. doi: 10.1038/nn.3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu I.M., Heesters B.A., Ghasemlou N., et al. Bacteria activate sensory neurons that modulate pain and inflammation. Nature. 2013;501(7465):52–57. doi: 10.1038/nature12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark I.A., Vissel B. The meteorology of cytokine storms, and the clinical usefulness of this knowledge. Semin. Immunopathol. 2017;39(5):505–516. doi: 10.1007/s00281-017-0628-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coperchini F., Chiovato L., Croce L., Magri F., Rotondi M. The cytokine storm in COVID-19: an overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020;53:25–32. doi: 10.1016/j.cytogfr.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damiani E., Carsetti A., Casarotta E., et al. Microvascular alterations in patients with SARS-COV-2 severe pneumonia. Ann. Intensive Care. 2020;10(1):60. doi: 10.1186/s13613-020-00680-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das G., Mukherjee N., Ghosh S. Neurological insights of COVID-19 pandemic. ACS Chem. Neurosci. 2020;11(9):1206–1209. doi: 10.1021/acschemneuro.0c00201. [DOI] [PubMed] [Google Scholar]
- De Giorgio R., Tazzari P.L., Barbara G., Stanghellini V., Corinaldesi R. Detection of substance P immunoreactivity in human peripheral leukocytes. J. Neuroimmunol. 1998;82(2):175–181. doi: 10.1016/s0165-5728(97)00201-4. [DOI] [PubMed] [Google Scholar]
- Dosch P. 12. Aufl. Haug; Stuttgart: 1986. Lehrbuch der Neuraltherapie nach Huneke. [Google Scholar]
- Dreher M., Kersten A., Bickenbach J., Balfanz P., Hartmann B., Cornelissen C., et al. The characteristics of 50 hospitalized COVID-19 patients with and without ARDS. Dtsch. Arztebl. Int. 2020;117:271–278. doi: 10.3238/arztebl.2020.0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elenkov I.J., Wilder R.L., Chrousos G.P., Vizi E.S. The sympathetic nerve–an integrative interface between two supersystems: the brain and the immune system. Pharmacol. Rev. 2000;52(4):595–638. [PubMed] [Google Scholar]
- Esch T., Stefano G. Proinflammation: a common denominator or initiator of different pathophysiological disease processes. Med. Sci. Monit. 2002;8(5) [PubMed] [Google Scholar]
- Fajgenbaum D.C., June C.H. Cytokine storm. N. Engl. J. Med. 2020;383(23):2255–2273. doi: 10.1056/NEJMra2026131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigenbaum M.J. Quantitative universality for a class of nonlinear transformations. J. Stat. Phys. 1978;19:25–52. [Google Scholar]
- Fischer L. Neuraltherapie in der notfallmedizin. Ärztez f NHV. 1995;9:676–685. [Google Scholar]
- Fischer L. 1. Aufl. Hippokrates; Stuttgart: 1998. Neuraltherapie. Neurophysiologie, Injektionstechnik, Umsetzung in die Praxis. [Google Scholar]
- Fischer L. In: Neuraltherapie nach Huneke. Dosch P., Barop H., Hahn-Goddefroy J.D., editors. Haug; Stuttgart: 2002. Der Zweitschlag nach Speranski – Parallelen zu Klinik und zu neuen Wissenschaftstheorien; pp. 81–87. (Hrsg.) [Google Scholar]
- Fischer L. Pathophysiology of pain and neural therapy. Praxis. 2003;92(48):2051–2059. doi: 10.1024/0369-8394.92.48.2051. [DOI] [PubMed] [Google Scholar]
- Fischer L. In: Foundations of Morphodynamics in Osteopathy. Liem T., van den Heede P., editors. Handspring Publishing; Edinbourgh: 2017. Physical and neurobiological principles; pp. 67–96. [Google Scholar]
- Fischer L. 5. Aufl. Thieme; Stuttgart: 2019. Neuraltherapie: Neurophysiologie, Injektionstechnik und Therapievorschläge. [Google Scholar]
- Fischer L. In: Handbuch Neuraltherapie – Diagnostik und Therapie mit Lokalanästhetika. Weinschenk S., editor. Thieme; Stuttgart: 2020. Stellate ganglion; pp. 413–416. [Google Scholar]
- Fischer L., Barop H., Maxion-Bergemann S. On behalf of the Swiss Federal Office of Public Health; 2005. Health Technology Assessment HTA Neural therapy according to Huneke. Program Evaluation Complementary Medicine (PEK) [Google Scholar]
- Fischer L., Ludin S.M., Thommen D., Hausammann R. Submitted to the Swiss Federal Office of Public Health; 2010. Application for the Adoption of Interference Field Therapy (Neural Therapy According to Huneke) Into the Health Benefit Basket of the Compulsory Health Insurance. [Google Scholar]
- Freidin M., Kessler J.A. Cytokine regulation of substance P expression in sympathetic neurons. Proc. Natl. Acad. Sci. U. S. A. 1991;88(8):3200–3203. doi: 10.1073/pnas.88.8.3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galic M.A., Riazi K., Pittman Q.J. Cytokines and brain excitability. Front. Neuroendocrinol. 2012;33(1):116–125. doi: 10.1016/j.yfrne.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Morán E., Sandín-Fuentes M.G., Álvarez López J.C., Duro-Aguado I., Urueña-Martínez N., Hernández-Luis C. Electrical storm secondary to acute myocardial infarction and heart failure treated with left stellate ganglion block. Rev. Esp. Cardiol. 2013;66(7):595–597. doi: 10.1016/j.rec.2013.01.015. [DOI] [PubMed] [Google Scholar]
- Grebe K.M., Takeda K., Hickman H.D., et al. Cutting edge: sympathetic nervous system increases proinflammatory cytokines and exacerbates influenza a virus pathogenesis. J. Immunol. 2010;184(2):540–544. doi: 10.4049/jimmunol.0903395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greiff L., Andersson M., Akerlund A., et al. Microvascular exudative hyperresponsiveness in human coronavirus-induced common cold. Thorax. 1994;49(2):121–127. doi: 10.1136/thx.49.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groeben H., Foster W.M., Brown R.H. Intravenous lidocaine and oral mexiletine block reflex bronchoconstriction in asthmatic subjects. Am. J. Respir. Crit. Care Med. 1996;154(4 Pt 1):885–888. doi: 10.1164/ajrccm.154.4.8887580. [DOI] [PubMed] [Google Scholar]
- Groeben H., Silvanus M.T., Beste M., Peters J. Both intravenous and inhaled lidocaine attenuate reflex bronchoconstriction but at different plasma concentrations. Am. J. Respir. Crit. Care Med. 1999;159(2):530–535. doi: 10.1164/ajrccm.159.2.9806102. [DOI] [PubMed] [Google Scholar]
- Guo W., Jin X.J., Yu J., Liu Y., Zhang J.P., Yang D.W., et al. Effects of stellate ganglion block on the peri-operative vasomotor cytokine content and intrapulmonary shunt in patients with esophagus cancer. Asian Pac. J. Cancer Prev. 2014;15(21):9505–9509. doi: 10.7314/apjcp.2014.15.21.9505. PMID: 25422247. [DOI] [PubMed] [Google Scholar]
- Hanamatsu N., Yamashiro M., Sumitomo M., Furuya H. Effectiveness of cervical sympathetic ganglia block on regeneration of the trigeminal nerve following transection in rats. Reg. Anesth. Pain Med. 2002;27(3):268–276. doi: 10.1053/rapm.2002.31206. [DOI] [PubMed] [Google Scholar]
- Heijnen C.J., Rouppe van der Voort C., van de Pol M., Kavelaars A. Cytokines regulate alpha(1)-adrenergic receptor mRNA expression in human monocytic cells and endothelial cells. J. Neuroimmunol. 2002;125(1-2):66–72. doi: 10.1016/s0165-5728(02)00034-6. [DOI] [PubMed] [Google Scholar]
- Heine H. In: Lehrbuch der integrativen Schmerztherapie. Fischer L., Peuker E.T., editors. Haug; Stuttgart: 2011. Grundregulation; pp. 30–34. (Hrsg.) [Google Scholar]
- Hollmann M.W., Durieux M.E. Local anesthetics and the inflammatory response: a new therapeutic indication? Anesthesiology. 2000;93(3):858–875. doi: 10.1097/00000542-200009000-00038. [DOI] [PubMed] [Google Scholar]
- Hollmann M.W., Gross A., Jelacin N., Durieux M.E. Local anesthetic effects on priming and activation of human neutrophils. Anesthesiology. 2001;95(1):113–122. doi: 10.1097/00000542-200107000-00021. [DOI] [PubMed] [Google Scholar]
- Hosoi J., Murphy G.F., Egan C.L., et al. Regulation of langerhans cell function by nerves containing calcitonin gene-related peptide. Nature. 1993;363(6425):159–163. doi: 10.1038/363159a0. [DOI] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue A., Ikoma K., Morioka N., et al. Interleukin-1beta induces substance P release from primary afferent neurons through the cyclooxygenase-2 system. J. Neurochem. 1999;73(5):2206–2213. [PubMed] [Google Scholar]
- Jänig W. Cambridge University Press; Cambridge: 2006. The Integrative Action of the Autonomic Nervous System. [Google Scholar]
- Jänig W. In: Lehrbuch Integrative Schmerztherapie. Fischer L., Peuker E.T., editors. Haug; Stuttgart: 2011. Rolle von motorischen Rückkopplungsmechanismen in der Erzeugung von Schmerzen; pp. 81–89. [Google Scholar]
- Jänig W. Autonomic nervous system and inflammation. Auton. Neurosci. 2014;182:1–3. doi: 10.1016/j.autneu.2014.02.002. [DOI] [PubMed] [Google Scholar]
- Jänig W. Sympathetic nervous system and inflammation: a conceptual view. Auton. Neurosci. 2014;182:4–14. doi: 10.1016/j.autneu.2014.01.004. [DOI] [PubMed] [Google Scholar]
- Jänig W., Baron R. In: Lehrbuch der integrativen Schmerztherapie. Fischer L., Peuker E.T., editors. Haug; Stuttgart: 2011. Pathophysiologie des Schmerzes. (Hrsg.) [Google Scholar]
- Ji R.R., Chamessian A., Zhang Y.Q. Pain regulation by non-neuronal cells and inflammation. Science. 2016;354(6312):572–577. doi: 10.1126/science.aaf8924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Känel R., Dimsdale J.E. Effects of sympathetic activation by adrenergic infusions on hemostasis in vivo. Eur. J. Haematol. 2000;65(6):357–369. doi: 10.1034/j.1600-0609.2000.065006357.x. [DOI] [PubMed] [Google Scholar]
- Kenney M.J., Ganta C.K. Autonomic nervous system and immune system interactions. Compr. Physiol. 2014;4(3):1177–1200. doi: 10.1002/cphy.c130051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.S., Lee J.Y., Yang J.W., et al. Immunopathogenesis and treatment of cytokine storm in COVID-19. Theranostics. 2021;11(1):316–329. doi: 10.7150/thno.49713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King K.A., Hu C., Rodriguez M.M., Romaguera R., Jiang X., Piedimonte G. Exaggerated neurogenic inflammation and substance P receptor upregulation in RSV-infected weanling rats. Am. J. Respir. Cell Mol. Biol. 2001;24(2):101–107. doi: 10.1165/ajrcmb.24.2.4264. [DOI] [PubMed] [Google Scholar]
- Kluge G., Neugebauer G. Oxford; Heidelberg, Berlin: 1994. Grundlagen der Thermodynamik. Spektrum. [Google Scholar]
- Konrad C.J., Schuepfer G.K., Neuburger M., Schley M., Schmelz M., Schmeck J. Reduction of pulmonary edema by short-acting local anesthetics. Reg. Anesth. Pain Med. 2006;31(3):254–259. doi: 10.1016/j.rapm.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Koopman F.A., van Maanen M.A., Vervoordeldonk M.J., Tak P.P. Balancing the autonomic nervous system to reduce inflammation in rheumatoid arthritis. J. Intern. Med. 2017;282(1):64–75. doi: 10.1111/joim.12626. [DOI] [PubMed] [Google Scholar]
- Kumagai H., Oshima N., Matsuura T., et al. Importance of rostral ventrolateral medulla neurons in determining efferent sympathetic nerve activity and blood pressure. Hypertens. Res. 2012;35(2):132–141. doi: 10.1038/hr.2011.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar N., Thapa D., Gombar S., Ahuja V., Gupta R. Analgesic efficacy of pre-operative stellate ganglion block on postoperative pain relief: a randomised controlled trial. Anaesthesia. 2014;69(9) doi: 10.1111/anae.12774. 954-660. [DOI] [PubMed] [Google Scholar]
- Lai J.P., Douglas S.D., Ho W.Z. Human lymphocytes express substance P and its receptor. J. Neuroimmunol. 1998;86(1):80–86. doi: 10.1016/s0165-5728(98)00025-3. [DOI] [PubMed] [Google Scholar]
- Lee L.Y. Respiratory sensations evoked by activation of bronchopulmonary C-fibers. Respir. Physiol. Neurobiol. 2009;167(1):26–35. doi: 10.1016/j.resp.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee L.Y., Yu J. Sensory nerves in lung and airways. Compr. Physiol. 2014;4(1):287–324. doi: 10.1002/cphy.c130020. [DOI] [PubMed] [Google Scholar]
- Lemke D.M. Riding out the storm: sympathetic storming after traumatic brain injury. J Neurosci Nurs. 2004;36(1):4–9. [PubMed] [Google Scholar]
- Leriche R., Fontaine R. L'anesthésie du ganglion étoile: sa technique, ses indications, ses résultats. Presse Med. 1934;42:849–850. [Google Scholar]
- Levi M., Nieuwdorp M., van der Poll T., Stroes E. Metabolic modulation of inflammation-induced activation of coagulation. Semin. Thromb. Hemost. 2008;34(1):26–32. doi: 10.1055/s-2008-1066020. [DOI] [PubMed] [Google Scholar]
- Levy C.E., Lorch F. Recovery of upper limb motor function in tetraplegia with stellate ganglion block treatment of reflex sympathetic dystrophy: a case report. Am. J. Phys. Med. Rehabil. 1996;75(6):479–482. doi: 10.1097/00002060-199611000-00018. [DOI] [PubMed] [Google Scholar]
- Li Y.C., Bai W.Z., Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J. Med. Virol. 2020;92(6):552–555. doi: 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipov E., Candido K. Efficacy and safety of stellate ganglion block in chronic ulcerative colitis. World J. Gastroenterol. 2017;23(17):3193–3194. doi: 10.3748/wjg.v23.i17.3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M.H., Tian J., Su Y.P., Wang T., Xiang Q., Wen L. Cervical sympathetic block regulates early systemic inflammatory response in severe trauma patients. Med. Sci. Monit. 2013;19:194–201. doi: 10.12659/MSM.883833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Tao T., Li W., Bo Y. Regulating autonomic nervous system homeostasis improves pulmonary function in rabbits with acute lung injury. BMC Pulm. Med. 2017;17(1):98. doi: 10.1186/s12890-017-0436-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Zheng X., Tong Q., et al. Overlapping and discrete aspects of the pathology and pathogenesis of the emerging human pathogenic coronaviruses SARS-CoV, MERS-CoV, and 2019-nCoV. J. Med. Virol. 2020;92(5):491–494. doi: 10.1002/jmv.25709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz E.N. American Association for the Advancement of Science, 139th Meeting. 1972. Predictability: does the flap of a butterfly’s wings in Brazil set off a tornado in Texas? [Google Scholar]
- Lu J., Shi Z., Su Y., et al. Effect of cervical sympathetic ganglia block on the mortality of mice with combined radiation and burn injury and its possible mechanism. Chin. J. Clin. Rehab. 2006;10(34):177–181. [Google Scholar]
- Magro C., Mulvey J.J., Berlin D., et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl. Res. 2020;S1931–5244(20):30070. doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maihöfner C., Handwerker H.O., Neundörfer B., Birklein F. Patterns of cortical reorganization in complex regional pain syndrome. Neurology. 2003;61(12):1707–1715. doi: 10.1212/01.wnl.0000098939.02752.8e. [DOI] [PubMed] [Google Scholar]
- Mandelbrot B.B. How long is the cost of britain? Science. 1967;156:636–638. doi: 10.1126/science.156.3775.636. [DOI] [PubMed] [Google Scholar]
- Marvar P.J., Harrison D.G. In: Primer on the Autonomic Nervous System. Third edition. Robertson D., Biaggioni I., Burnstock G., Low P.A., Paton J.F.R., editors. Academic Press; San Diego: 2012. Inflammation, immunity and the autonomic nervous system; pp. 325–329. [DOI] [Google Scholar]
- Matsuda M., Huh Y., Ji R.R. Roles of inflammation, neurogenic inflammation, and neuroinflammation in pain. J. Anesth. 2019;33(1):131–139. doi: 10.1007/s00540-018-2579-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan E.M. Transmission of signals through sympathetic ganglia–modulation, integration or simply distribution? Acta Physiol. Scand. 2003;177(3):227–235. doi: 10.1046/j.1365-201X.2003.01075.x. [DOI] [PubMed] [Google Scholar]
- Mejías A., Chávez-Bueno S., Ramilo O. Respiratory syncytial virus pneumonia: mechanisms of inflammation and prolonged airway hyperresponsiveness. Curr. Opin. Infect. Dis. 2005;18(3):199–204. doi: 10.1097/01.qco.0000168378.07110.72. [DOI] [PubMed] [Google Scholar]
- Merad M., Martin J.C. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat. Rev. Immunol. 2020:1–8. doi: 10.1038/s41577-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina P.E. Neurobiology of the stress response: contribution of the sympathetic nervous system to the neuroimmune axis in traumatic injury. Shock. 2005;24(1):3–10. doi: 10.1097/01.shk.0000167112.18871.5c. [DOI] [PubMed] [Google Scholar]
- Mousa S.A., Shaqura M., Brendl U., Al-Khrasani M., Fürst S., Schäfer M. Involvement of the peripheral sensory and sympathetic nervous system in the vascular endothelial expression of ICAM-1 and the recruitment of opioid-containing immune cells to inhibit inflammatory pain. Brain Behav. Immun. 2010;24(8):1310–1323. doi: 10.1016/j.bbi.2010.06.008. [DOI] [PubMed] [Google Scholar]
- Murakawa K. Changes in microcirculation in the facial nerve with electrical stimulation and chemical blockade of cervical sympathetic trunks. Masui. 1993;42(5):646–652. [PubMed] [Google Scholar]
- Myers J.L., Domkowski P.W., Wang Y., Hopkins R.A. Sympathetic blockade blunts hypercapnic pulmonary arterial vasoconstriction in newborn piglets. Eur. J. Cardiothorac. Surg. 1998;13(3):298–305. doi: 10.1016/s1010-7940(98)00007-4. [DOI] [PubMed] [Google Scholar]
- Naegele M., Flammer A.J., Enseleit F., et al. Endothelial function and sympathetic nervous system activity in patients with takotsubo syndrome. Int. J. Cardiol. 2016;224:226–230. doi: 10.1016/j.ijcard.2016.09.008. [DOI] [PubMed] [Google Scholar]
- Nahama A., Ramachandran R., Cisternas A.F., Ji H. The role of afferent pulmonary innervation in poor prognosis of acute respiratory distress syndrome in COVID-19 patients and proposed use of resiniferatoxin (RTX) to improve patient outcomes in advanced disease state: a review. Med. Drug Discov. 2020;5 doi: 10.1016/j.medidd.2020.100033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita K., Murata T., Hamada T., et al. Interactions among higher trait anxiety, sympathetic activity, and endothelial function in the elderly. J. Psychiatr. Res. 2007;41(5):418–427. doi: 10.1016/j.jpsychires.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Nicolis G., Prigogine I. J. Wiley & Sons; New York, London, Sydney, Toronto: 1977. Self-organization in Nonequilibrium System: From Dissipative Structures to Order Through Fluctuations. [Google Scholar]
- O'Connor T.M., O'Connell J., O'Brien D.I., Goode T., Bredin C.P., Shanahan F. The role of substance P in inflammatory disease. J. Cell. Physiol. 2004;201(2):167–180. doi: 10.1002/jcp.20061. [DOI] [PubMed] [Google Scholar]
- Ogundele O.M., Lee C.C., Francis J. Thalamic dopaminergic neurons projects to the paraventricular nucleus-rostral ventrolateral medulla/C1 neural circuit. Anat. Rec. (Hoboken) 2017;300(7):1307–1314. doi: 10.1002/ar.23528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada M., Guo S.Y., Hisamitsu T. Denervation of the cervical sympathetic nerve inhibited the splenic natural killer cell activity in rats. Masui. 1996;45(5):582–585. [PubMed] [Google Scholar]
- Oyama J., Node K. Sympathetic nerve activity and endothelial function. Hypertens. Res. 2014;37(12):1035–1036. doi: 10.1038/hr.2014.142. [DOI] [PubMed] [Google Scholar]
- Pagano G., Corbi G., Ferrara N. Adrenergic nervous system and hemostasis. J. Hematol. Thromb. Dis. 2014;2:2. doi: 10.4172/2329-8790.1000e108. [DOI] [Google Scholar]
- Papathanasiou G.S. Local anesthetics and Covid19 associated acute respiratory distress syndrome: a new therapeutic indication? Clin. Res. Open Access. 2020;6 doi: 10.16966/2469-6714.156. [DOI] [Google Scholar]
- Paton J.F.R., Spyer K.M. In: Autonomic Failure: A Textbook of Clinical Disorders of the Autonomic Nervous System. 5th ed. Mathias C.J., Bannister R., editors. Oxford University Press; Oxford, New York: 2013. Central nervous control of the cardiovascular system. [Google Scholar]
- Pavlov V.A., Tracey K.J. Neural circuitry and immunity. Immunol. Res. 2015;63(1–3):38–57. doi: 10.1007/s12026-015-8718-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov V.A., Chavan S.S., Tracey K.J. Bioelectronic medicine: from preclinical studies on the inflammatory reflex to new approaches in disease diagnosis and treatment. Cold Spring Harb. Perspect. Med. 2020;10(3) doi: 10.1101/cshperspect.a034140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez D.M., Papay R.S., Shi T. Alpha1-adrenergic receptor stimulates interleukin-6 expression and secretion through both mRNA stability and transcriptional regulation: involvement of p38 mitogen-activated protein kinase and nuclear factor-kappaB. Mol. Pharmacol. 2009;76(1):144–152. doi: 10.1124/mol.108.054320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perianin A., Snyderman R., Malfroy B. Substance P primes human neutrophil activation: a mechanism for neurological regulation of inflammation. Biochem. Biophys. Res. Commun. 1989;161(2):520–524. doi: 10.1016/0006-291x(89)92630-2. [DOI] [PubMed] [Google Scholar]
- Pfister M., Fischer L. The treatment of the complex regional pain syndrome (CRPS 1 and CRPS 2) of the upper limb with repeated local anaesthesia to the stellate ganglion. Praxis. 2009;98(5):247–257. doi: 10.1024/1661-8157.98.5.247. [DOI] [PubMed] [Google Scholar]
- Piedimonte G., Rodriguez M.M., King K.A., McLean S., Jiang X. Respiratory syncytial virus upregulates expression of the substance P receptor in rat lungs. Am. J. Phys. 1999;277(4):L831–L840. doi: 10.1152/ajplung.1999.277.4.L831. [DOI] [PubMed] [Google Scholar]
- Pierach A., Stotz K. Therapy of pulmonary edema with procaine block of the right stellate ganglion. Dtsch. Med. Wochenschr. 1952;77(44):1344–1346. doi: 10.1055/s-0028-1117238. [DOI] [PubMed] [Google Scholar]
- Pischinger A. 11. Aufl. Haug; Stuttgart: 2010. Das System der Grundregulation. [Google Scholar]
- Pongratz G., Straub R.H. The sympathetic nervous response in inflammation. Arthritis Res. Ther. 2014;16(6):504. doi: 10.1186/s13075-014-0504-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puente de la Vega Costa K., Gómez Perez M.A., Roqueta C., Fischer L. Effects on hemodynamic variables and echocardiographic parameters after a stellate ganglion block in 15 healthy volunteers. Auton. Neurosci. 2016;197:46–55. doi: 10.1016/j.autneu.2016.04.002. [DOI] [PubMed] [Google Scholar]
- Punj J. Multiple bilateral ultrasound-guided stellate ganglion blocks to treat acute vasculitis in a recently diagnosed patient of systemic lupus erythematosus. Indian J. Anaesth. 2019;63(10):851–855. doi: 10.4103/ija.IJA_331_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin C., Zhou L., Hu Z., et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimzadeh P., Mahmoudi K., Khodaverdi M., Faiz S.H.R. Effects of ultrasound guided ganglion stellate blockade on intraoperative and postoperative hemodynamic responses in laparoscopic gynecologic surgery. Videosurgery Miniinv. 2020;15(2):351–357. doi: 10.5114/wiitm.2019.89653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricker G. Springer; Berlin: 1924. Pathologie als Naturwissenschaft - Relationspathologie. [Google Scholar]
- Ross C.A., Ruggiero D.A., Reis D.J. Projections from the nucleus tractus solitarii to the rostral ventrolateral medulla. J. Comp. Neurol. 1985;242(4):511–534. doi: 10.1002/cne.902420405. [DOI] [PubMed] [Google Scholar]
- Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46(5):846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salgado D.R., Ortiz J.A., Favory R., Creteur J., Vincent J.L., De Backer D. Microcirculatory abnormalities in patients with severe influenza a (H1N1) infection. Can. J. Anaesth. 2010;57(10):940–946. doi: 10.1007/s12630-010-9365-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders J.M., Monogue M.L., Jodlowski T.Z., Cutrell J.B. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. 2020 doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- Schaible H.G. Nociceptive neurons detect cytokines in arthritis. Arthritis Res. Ther. 2014;16(5):470. doi: 10.1186/s13075-014-0470-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaible H.G., Straub R.H. Function of the sympathetic supply in acute and chronic experimental joint inflammation. Auton. Neurosci. 2014;182:55–64. doi: 10.1016/j.autneu.2013.12.004. May. [DOI] [PubMed] [Google Scholar]
- Schaible H.G., Ebersberger A., Natura G. Update on peripheral mechanisms of pain: beyond prostaglandins and cytokines. Arthritis Res. Ther. 2011;13(2):210. doi: 10.1186/ar3305. Apr 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffrin E.L. In: Primer on the Autonomic Nervous System. 3rd ed. Robertson D., Biaggioni I., Burnstock G., Low P.A., Paton J.F.R., editors. Academic Press; San Diego: 2012. The endothelin system; pp. 135–139. [DOI] [Google Scholar]
- Serra M.C., Bazzoni F., Della Bianca V., Greskowiak M., Rossi F. Activation of human neutrophils by substance P. Effect on oxidative metabolism, exocytosis, cytosolic Ca2+ concentration and inositol phosphate formation. J. Immunol. 1988;141(6):2118–2124. [PubMed] [Google Scholar]
- Sheng Y., Zhu L. The crosstalk between autonomic nervous system and blood vessels. Int. J. Physiol. Pathophysiol. Pharmacol. 2018;10(1):17–28. [PMC free article] [PubMed] [Google Scholar]
- Sinha P., Matthay M.A., Calfee C.S. Is a “Cytokine storm” relevant to COVID-19? JAMA Intern. Med. 2020;180(9):1152–1154. doi: 10.1001/jamainternmed.2020.3313. [DOI] [PubMed] [Google Scholar]
- Sinisalo J., Paronen J., Mattila K.J., et al. Relation of inflammation to vascular function in patients with coronary heart disease. Atherosclerosis. 2000;149(2):403–411. doi: 10.1016/s0021-9150(99)00333-0. [DOI] [PubMed] [Google Scholar]
- Speranski A.P. 2nd ed. International Publishers; New York: 1950. A Basis for the Theory of Medicine. [Google Scholar]
- Spiezia L., Boscolo A., Poletto F., et al. COVID-19-related severe hypercoagulability in patients admitted to intensive care unit for acute respiratory failure. Thromb. Haemost. 2020 doi: 10.1055/s-0040-1710018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanisz A.M. In: NeuroImmune Biology. Vol. 1: New Foundation of Biology. Berczi I., Gorczynski R.M., editors. Elsevier; Amsterdam: 2001. Neurogenic inflammation: role of substance; pp. 373–378. doi:10.1001/jama.2020.6019. [DOI] [Google Scholar]
- Stanisz A.M., Befus D., Bienenstock J. Differential effects of vasoactive intestinal peptide, substance P, and somatostatin on immunoglobulin synthesis and proliferations by lymphocytes from Peyer's patches, mesenteric lymph nodes, and spleen. J. Immunol. 1986;136(1):152–156. [PubMed] [Google Scholar]
- Steenblock C., Todorov V., Kanczkowski W., et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the neuroendocrine stress axis. Mol. Psychiatry. 2020:1–7. doi: 10.1038/s41380-020-0758-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg E.M. Neural regulation of innate immunity: a coordinated nonspecific host response to pathogens. Nat. Rev. Immunol. 2006;6(4):318–328. doi: 10.1038/nri1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong J.A., Zhang J.M., Schaible H.G. In: The Oxford Handbook of the Neurobiology of Pain. Wood J.N., editor. 2018. The sympathetic nervous system and pain. [DOI] [Google Scholar]
- Sugimoto M., Shimaoka M., Taenaka N., Kiyono H., Yoshiya I. Lymphocyte activation is attenuated by stellate ganglion block. Reg. Anesth. Pain Med. 1999;24(1):30–35. doi: 10.1016/s1098-7339(99)90162-1. [DOI] [PubMed] [Google Scholar]
- Tan T., Khoo B., Mills E.G. Association between high serum total cortisol concentrations and mortality from COVID-19. Lancet Diabetes Endocrinol. 2020 doi: 10.1016/S2213-8587(20)30216-3. S2213-8587(20)30216-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taneyama C., Goto H. Fractal cardiovascular dynamics and baroreflex sensitivity after stellate ganglion block. Anesth. Analg. 2009;109(4):1335–1340. doi: 10.1213/ane.0b013e3181b018d8. [DOI] [PubMed] [Google Scholar]
- Tracey K.J. The inflammatory reflex. Nature. 2002;420(6917):853–859. doi: 10.1038/nature01321. PMID: 12490958. [DOI] [PubMed] [Google Scholar]
- Tracey K.J. Reflex control of immunity. Nat. Rev. Immunol. 2009;9(6):418–428. doi: 10.1038/nri2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tränkner D., Hahne N., Sugino K., Hoon M.A., Zuker C. Population of sensory neurons essential for asthmatic hyperreactivity of inflamed airways. Proc. Natl. Acad. Sci. U. S. A. 2014;111(31):11515–11520. doi: 10.1073/pnas.1411032111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsaava T., Datta-Chaudhuri T., Addorisio M.E., Battinelli Masi E., Silverman H.A., Newman J.E. bioRxiv; 2020. Serum Cytokine Levels are Modulated by Specific Frequencies, Amplitudes, and Pulse Widths of Vagus Nerve Stimulation. 01.08.898890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ur A., Verma K. Cytokine storm in COVID19: a neural hypothesis. ACS Chem. Neurosci. 2020;11(13):1868–1870. doi: 10.1021/acschemneuro.0c00346. [DOI] [PubMed] [Google Scholar]
- Ur A., Verma K. Pulmonary edema in COVID19-a neural hypothesis. ACS Chem. Neurosci. 2020;11(14):2048–2050. doi: 10.1021/acschemneuro.0c00370. [DOI] [PubMed] [Google Scholar]
- Varga Z., Flammer A.J., Steiger P., et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velavan T.P., Meyer C.G. Mild versus severe COVID-19: laboratory markers. Int. J. Infect. Dis. 2020;95:304–307. doi: 10.1016/j.ijid.2020.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veres T.Z., Rochlitzer S., Shevchenko M., et al. Spatial interactions between dendritic cells and sensory nerves in allergic airway inflammation. Am. J. Respir. Cell Mol. Biol. 2007;37(5):553–561. doi: 10.1165/rcmb.2007-0087OC. [DOI] [PubMed] [Google Scholar]
- Wang J., Wang H., Luo W., et al. Leptin-induced endothelial dysfunction is mediated by sympathetic nervous system activity. J. Am. Heart Assoc. 2013;2(5) doi: 10.1161/JAHA.113.000299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Yu D., Yu Y., et al. Potential role of sympathetic activity on the pathogenesis of massive pulmonary embolism with circulatory shock in rabbits. Respir. Res. 2019;20(1):97. doi: 10.1186/s12931-019-1069-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Hu B., Hu C., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerhaus M.J., Loewy A.D. Central representation of the sympathetic nervous system in the cerebral cortex. Brain Res. 2001;903(1–2):117–127. doi: 10.1016/s0006-8993(01)02453-2. [DOI] [PubMed] [Google Scholar]
- White F.A., Sun J., Waters S.M., et al. Excitatory monocyte chemoattractant protein-1 signaling is up-regulated in sensory neurons after chronic compression of the dorsal root ganglion. Proc. Natl. Acad. Sci. U. S. A. 2005;102(39):14092–14097. doi: 10.1073/pnas.0503496102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W., Wang T., Zhou H. Substance P and its role in viral infection. Int. J. Clin. Exp. Med. 2018;11(12):12946–12955. [Google Scholar]
- Xu Z., Shi L., Wang Y., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi N. Role of ET(A) and ET(B) receptors in endothelin-1-induced adrenal catecholamine secretion in vivo. Am. J. Phys. 1997;272(4 Pt 2):R1290–R1297. doi: 10.1152/ajpregu.1997.272.4.R1290. [DOI] [PubMed] [Google Scholar]
- Yamazaki H., Nishiyama J., Suzuki T. Use of perfusion index from pulse oximetry to determine efficacy of stellate ganglion block. Local Reg. Anesth. 2012;5:9–14. doi: 10.2147/LRA.S30257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagidate F., Strichartz G.R. Local anesthetics. Handb. Exp. Pharmacol. 2007;177:95–127. doi: 10.1007/978-3-540-33823-9_4. [DOI] [PubMed] [Google Scholar]
- Yang X., Shi Z., Li X., Li J. Impacts of stellate ganglion block on plasma NF-κB and inflammatory factors of TBI patients. Int. J. Clin. Exp. Med. 2015;8(9):15630–15638. [PMC free article] [PubMed] [Google Scholar]
- Yokoyama M., Nakatsuka H., Itano Y., Hirakawa M. Stellate ganglion block modifies the distribution of lymphocyte subsets and natural-killer cell activity. Anesthesiology. 2000;92(1):109–115. doi: 10.1097/00000542-200001000-00021. [DOI] [PubMed] [Google Scholar]
- Zelenka M., Schäfers M., Sommer C. Intraneural injection of interleukin-1beta and tumor necrosis factor-alpha into rat sciatic nerve at physiological doses induces signs of neuropathic pain. Pain. 2005;116(3):257–263. doi: 10.1016/j.pain.2005.04.018. [DOI] [PubMed] [Google Scholar]
- Zhang L., Yao J., Zhang T., Jin J., Zeng X., Yue Z. Stellate ganglion block may prevent the development of neurogenic pulmonary edema and improve the outcome. Med. Hypotheses. 2013;80(2):158–161. doi: 10.1016/j.mehy.2012.11.017. [DOI] [PubMed] [Google Scholar]
- Zhang R.M., McNerney K., Riek A.E., Bernal-Mizrachi C. Immunity and hypertension. Acta Physiol (Oxford) 2020 doi: 10.1111/apha.13487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H.Y., Yang G.T., Sun N.N., Kong Y., Liu Y.F. Efficacy and safety of stellate ganglion block in chronic ulcerative colitis. World J. Gastroenterol. 2017;23(3):533–539. doi: 10.3748/wjg.v23.i3.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Xiang R., Peng X., et al. Transection of the cervical sympathetic trunk inhibits the progression of pulmonary arterial hypertension via ERK-1/2 signalling. Respir. Res. 2019;20(1):121. doi: 10.1186/s12931-019-1090-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y., Hou X., Yang S. Lidocaine potentiates SOCS3 to attenuate inflammation in microglia and suppress neuropathic pain. Cell. Mol. Neurobiol. 2019;39(8):1081–1092. doi: 10.1007/s10571-019-00703-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Yi X., Xing W., Hu S., Maslov K.I., Wang L.V. Microcirculatory changes identified by photoacoustic microscopy in patients with complex regional pain syndrome type I after stellate ganglion blocks. J. Biomed. Opt. 2014;19(8) doi: 10.1117/1.JBO.19.8.086017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z., Cai T., Fan L., et al. Clinical value of immune-inflammatory parameters to assess the severity of coronavirus disease 2019. Int. J. Infect. Dis. 2020;95:332–339. doi: 10.1016/j.ijid.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Zypen E. Elektronenmikroskopische befunde an der endausbreitung des vegetativen nervensystems und ihre deutung. Acta Anat. (Basel) 1967;67(4):481–515. [PubMed] [Google Scholar]