Abstract
Background and Purpose
Few reports include volumetric measurements as endpoints after stereotactic radiotherapy (SRT) despite the importance of such measurements. This study aimed to (1) investigate the impact of the volumetric response (specifically, an over 65% and over 90% volume reduction in brain metastases) at 6 months post‐SRT on local control and (2) identify the predictive factors for a volumetric response of over 65% and over 90%.
Materials and Methods
This study included 250 unresected brain metastases (>0.3 cc) treated with SRT. Doses were stratified according to the biological effective dose (BED). The BED was calculated using four models: linear‐quadratic (LQ): α/β = 10; LQ: α/β = 20; LQ cubic: α/β = 12; and LQ linear: α/β = 10. The median prescription dose was 30 Gy/3 fractions (BED20, 45). The median follow‐up time after SRT was 18.6 months (range, 6.4–81.8 months).
Results
In the multivariate analysis, over 65% volume reduction and over 90% volume reduction were prognostic factors for local control (hazard ratio: 2.370, p = 0.011 and hazard ratio: 3.161, p = 0.014, respectively). A dose of 80% of the gross tumor volume (GTV) D80 > BED20 58 was a predictive factor for over 65% and over 90% volume reductions (odds ratio: 1.975, p = 0.023; odds ratio: 3.204, p < 0.001, respectively).
Conclusion
Robust volume reduction of brain metastases at 6 months post‐SRT can predict local control. GTV D80 in the LQ model: α/β = 20 may be warranted for good volume reduction.
Keywords: brain metastasis, stereotactic radiosurgery, stereotactic radiotherapy
Over 65% and over 90% volume reduction of brain metastases at 6 months post‐SRT predicts good local control. Beneficial volume reduction may require increasing the dose to GTV D80 in the LQ model: α/β = 20, and inhomogeneous dose distribution may be required for SRT for brain metastases.
1. INTRODUCTION
Stereotactic radiotherapy (SRT) is one of the most important treatment modes for brain metastases. 1 , 2 It includes both stereotactic radiosurgery and fractionated stereotactic radiotherapy. 1 , 2 , 3 The mass effect of brain metastases and peritumoral edema often causes severe neurological symptoms that worsen the quality of life (QOL). 4 , 5 Reducing the volume of brain metastases reduces the amount of peritumoral edema and leads to fewer neurological symptoms and improved QOL. 4 , 5 Early volumetric reduction after SRT is a prognostic factor for local tumor control. 1 , 5
The criteria used to assess response and progression in brain metastases are heterogeneous. 6 , 7 The Response Assessment in Neuro‐Oncology Brain Metastases (RANO‐BM) guidelines are the first step toward standardizing the criteria. 8 These guidelines suggest including volumetric reduction as a study endpoint because it is more reliable than using the sum of the longest diameter of the tumor. 9 However, few reports include this endpoint because volumetric analysis after SRT takes time and effort. 1 , 2 , 5 , 8
Over 65% volume reduction in brain metastases corresponds to a partial response in the RANO‐BM guidelines. 8 However, in a previous study, over 65% volume reduction at 3 months post‐SRT did not predict local control. 1 Whether over 65% volume reduction at 6 months post‐SRT would do so has yet to be investigated. It is possible that a reduction of over 90% is necessary for the achievement of local control.
This study aimed to (1) investigate the effect of the volumetric response (specifically, over 65% and over 90%) at 6 months post‐SRT on local control and (2) identify the predictive factors for a volumetric response of over 65% and over 90%.
2. MATERIALS AND METHODS
2.1. Patients
This study included 147 patients with 250 unresected brain metastases treated with SRT at the Osaka International Cancer Institute between 2013 and 2020, identified from our electronic database. The exclusion criteria were as follows: no magnetic resonance imaging (MRI) 5.0–8.5 months after SRT, whole‐brain radiotherapy before the evaluation MRI, brain metastases with local tumor progression before the evaluation MRI, and brain metastases <0.3 cc at baseline. The evaluation MRI was the MRI performed nearest to 6 months (median, 6.3 months; range, 5.0–8.5 months) after SRT.
All patients provided written informed consent for the use of their data prior to starting SRT. The institutional review of the board of the Osaka International Cancer Institute approved this study (21150). Table 1 lists the characteristics of the patients and their brain metastases. A total of 107 patients received systemic therapy concurrently (within 1 month before or after SRT). Of 107 patients, 30 patients received immunotherapy including immune checkpoint inhibitors and 58 patients received target therapy including angiogenesis inhibitors, human epidermal growth factor receptor type 2 targeted agents, tyrosine kinase inhibitors, and cyclin‐dependent kinase inhibitors.
TABLE 1.
Patient, brain metastases, and SRT characteristics
| Characteristic | Category | Value | |
|---|---|---|---|
| Patients (n = 147) | |||
| Age (years) | Median (range) | 66 | (22–85) |
| 22–65, n (%) | 70 | (47.6) | |
| >65, n (%) | 77 | (52.4) | |
| Sex, n (%) | Male | 73 | (49.7) |
| Female | 74 | (50.3) | |
| PS, n (%) | 0 | 95 | (64.6) |
| 1 | 35 | (23.8) | |
| 2 | 13 | (8.8) | |
| 3 | 4 | (2.7) | |
| Primary cancer, n (%) | Lung | 97 | (66.0) |
| Breast | 19 | (12.9) | |
| GI | 8 | (5.4) | |
| Kidney | 7 | (4.7) | |
| Melanoma | 5 | (3.4) | |
| Others | 11 | (7.5) | |
| Brain metastases (n = 250) and SRT | |||
| PTV prescription dose, n (%) | 20 Gy/1 fraction | 15 | (6.0) |
| 24 Gy/1 fraction | 90 | (36.0) | |
| 30 Gy/3 fractions | 48 | (19.2) | |
| 30 Gy/5 fractions | 3 | (1.2) | |
| 35 Gy/5 fractions | 94 | (37.6) | |
| GTV (cc) | Median (range) | 1.1 | (0.3–33.1) |
| 0.3–1, n (%) | 118 | (47.2) | |
| 1–4, n (%) | 95 | (38.0) | |
| >4, n (%) | 37 | (14.8) | |
| SRT modality, n (%) | Conformal RT | 12 | (4.8) |
| Manual VMAT | 103 | (41.2) | |
| HA VMAT | 135 | (54.0) | |
Abbreviations: GI, gastrointestinal; GTV, gross tumor volume; HA VMAT, HyperArc volumetric modulated arc therapy; PS, performance status; PTV, planning target volume; RT, radiotherapy; SRT, stereotactic radiotherapy.
2.2. Treatments
The SRT treatment has been previously described. 10 , 11 For simulation, the patient was immobilized using a thermoplastic mask, and planning computed tomography (CT) was performed with a thickness of 1 mm. Planning CT scans were loaded into a treatment planning system (Eclipse; Varian Medical Systems, Palo Alto, CA, USA). The gross tumor volume (GTV) was delineated by referring to a T1‐weighted, gadolinium‐enhanced magnetic resonance image. The planning target volume (PTV) was determined by adding an isotropic margin of 1 mm (range, 1–3 mm) to the GTV.
The median prescription dose was 30 Gy/3 fractions to cover 95% of the volume of the combined PTV. The median isodose (prescription dose/max dose × 100) was 79.2% (range, 43.0–92.8%). The isodose was determined by the physician's preference, and dose inhomogeneity was allowed within the GTV. From 2013 to 2019, we ordinarily prescribed 30 Gy/3 fr or 30–35 Gy/5 fr for GTV >4 cc and 20–24 Gy/1 fr for GTV <4 cc. From 2020, we ordinarily prescribed 35 Gy/5 fr in any case. Doses to the brain tissue were reduced to the minimum in the optimization process. All treatments were performed using a C‐arm linear accelerator (Linac) (Clinac 23Ex, Ture Beam STX, or Edge; Varian Medical Systems, Palo Alto, CA, USA).
Follow‐ups included clinical examination and MRI and were performed at least every 4 months during the first 2 years after SRT initiation and at least every 6 months afterward. The interval was shortened when the tumor volume increased or new symptoms developed. The median follow‐up time after SRT initiation was 18.6 months (range, 6.4–81.8 months).
The evaluation MRI was loaded into the treatment planning system and registered with the SRT plan. The radiation oncologist delineated the tumor after referring to the T1‐weighted, gadolinium‐enhanced MRI. Tumor contouring on evaluation MRI included the treatment effects. The volume of the tumor (cc) on evaluation MRI was evaluated, and the volume reduction rate from GTV (cc) was calculated.
2.3. Definitions
Local control was calculated from the time of the evaluation MRI to the radiological observation of tumor progression of a treated lesion. Tumor progression was defined according to the RANO‐BM guidelines. 8 When a differential diagnosis of tumor progression and brain necrosis was needed, tumor progression was defined as the correspondence between the contrast‐enhanced volume on the T1‐weighted MRI scans and the low signal‐defined lesion margin on the T2‐weighted MRI scans 12 and/or if the maximum standardized uptake value (SUV) within the tumor/SUV within the normal gray matter was over 1.4 on 11C‐methionine positron emission tomography. 13
2.4. Statistical analysis
Local control rates and hazard ratios (HRs) were estimated using the Kaplan–Meier method and Cox proportional hazard models, respectively. A univariate analysis using a logistic regression model was performed to determine odds ratios (ORs) for a set of candidate predictor variables; this analysis showed the raw uncorrected effects of each variable for over 65% and over 90% volume reduction.
For the GTV parameter, GTV D100, D98, D80, D60, D40, D20, D2, and Dmax were analyzed. Doses were stratified according to the biological effective dose (BED). It is a matter of debate which formula should be used to calculate the BED for SRT for brain metastases 2 , 14 , 15 , 16 , 17 , 18 ; hence, we used four models: two linear‐quadratic (LQ) models: α/β = 10 and α/β = 20; the linear‐quadratic cubic (LQC) model: α/β = 12; and the linear‐quadratic linear (LQL) model: α/β = 10. The formulas for these models are as follows:
BED LQ models = nd [1 + d/(α/β)].
BED LQC model = nd [1 + d/(α/β) ‐ d2/(α/γ)].
BED LQL model = nd [1 + d*G(δd)/(α/β)].
where n is the number of fractions, d is the dose per fraction, α/γ is 648 Gy2, δ is 0.14, and G(χ) = (2/χ2)(χ‐1+ e ‐χ). 2 , 14 , 15 , 16 , 17
In the univariate analysis, we used the Pearson correlation coefficient (r) to evaluate the correlation between the GTV parameters themselves, and we used the Spearman's correlation coefficient to evaluate the correlation between the clinical (treatment) parameters and the GTV parameters. When either coefficient for the candidate prognostic factors was >0.40 in the univariate analysis, we selected only one variable for the multivariate analysis. The GTV parameter with the lowest Akaike information criterion (AICc) value was deemed the most predictive. Evidence ratios (EVRs) were calculated; models with an EVR <2.7 were considered to have substantial support. 19 Statistical significance was set at a p‐value of <0.05. Statistical analysis was performed using SPSS version 25 software (SPSS Inc., Chicago, IL, USA).
3. RESULTS
3.1. Local control
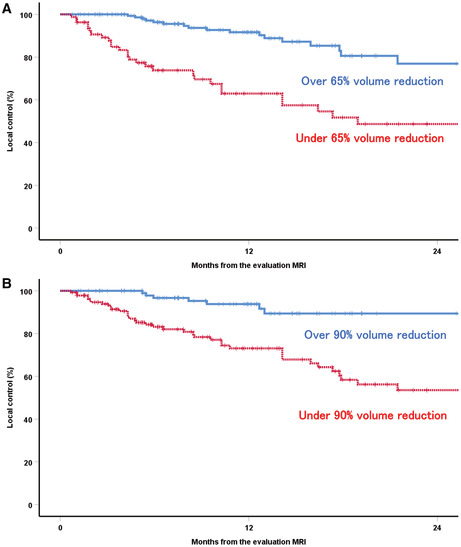
Over 65% and over 90% volume reduction approximately 6 months post‐SRT (i.e., at the evaluation MRI) was achieved for 169 (67.6%) and 111 (44.4%) of the 250 brain metastases, respectively. The overall local control rate at 0.5 and 1.5 years from the evaluation MRI (approximately 1 and 2 years after SRT, respectively) was 89.4% and 71.3%, respectively. The local control rate at 0.5 years from the evaluation MRI was 96.3% vs 73.8% for over vs under 65% volume reduction and 96.7% vs 83.2% for over vs under 90% volume reduction (Figure 1).
FIGURE 1.

Comparison of the local control rates for over vs under 65% volume reduction (A) and over 90% volume reduction (B) at the evaluation MRI. MRI, magnetic resonance imaging.
The results of the univariate and multivariate analyses for local control are shown in Table 2. Over 65% and over 90% volume reduction were prognostic factors for local control in the multivariate analysis (HR: 2.370, p = 0.011 and HR: 3.161, p = 0.014, respectively).
TABLE 2.
Results of the univariate and multivariate analyses of potential prognostic factors for local control
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | p‐value | HR (95% CI) | p‐value | |
| >65% volume reduction at the evaluation MRI | ||||
| Yes | 1 | <0.001 | 1 | 0.011 |
| No | 3.897 (2.181–6.962) | 2.370 (1.219–4.607) | ||
| >90% volume reduction at the evaluation MRI | ||||
| Yes | 1 | <0.001 | 1 | 0.014 |
| No | 5.649 (2.533–12.596) | 3.161 (1.259–7.940) | ||
| GTV (cc) | ||||
| 0.3–1 | 1 | <0.001 | 1 | <0.001 |
| >1 | 5.657 (2.534–2.630) | 5.685 (2.542–12.716) | ||
| Age (years) | ||||
| 22–65 | 1 | 0.194 | ||
| >65 | 1.461 (0.824–2.589) | |||
| PS | ||||
| 0–1 | 1 | 0.650 | ||
| 2–3 | 0.788 (0.281–2.207) | |||
| Primary cancer | ||||
| Lung, breast | 1 | 0.580 | ||
| Others | 0.812 (0.388–1.699) | |||
| # of fractions | ||||
| 1 (SRS) | 1 | 0.323 | ||
| >1 (FSRT) | 1.347 (0.746–2.430) | |||
| SRT modality | ||||
| HA VMAT | 1 | 0.674 | ||
| Others | 1.136 (0.627–2.061) | |||
Abbreviations: CI, confidence interval; FSRT, fractionated stereotactic radiotherapy; GTV, gross tumor volume; HA VMAT, HyperArc volumetric modulated arc therapy; HR, hazard ratio; MRI, magnetic resonance imaging; PS, performance status; SRS, stereotactic radiosurgery; SRT, stereotactic radiotherapy.
3.2. Analysis of over 65% and 90% volume reduction at 6 months post‐SRT
Over 65% volume reduction significantly correlated with improved performance status in the univariate analysis (Table 3). GTV D80 in the LQ model: α/β = 20 had the lowest AICc value for both over 65% and over 90% volume reduction (Table 4). This model was the only model with an EVR of <2.7 for over 65% volume reduction, while GTV D80 and D98 in the LQ model: α/β = 20 was the only model with an EVR of <2.7 for over 95% volume reduction (Table 4).
TABLE 3.
Results of the univariate analysis for prognostic factors for over 65% and over 90% volume reduction at the evaluation MRI
| Variable | Over 65% | Over 90% | ||
|---|---|---|---|---|
| OR (95% CI) | p‐value | OR (95% CI) | p‐value | |
| Age (years) | ||||
| 22–65 | 1 | 0.450 | 1 | 0.488 |
| >65 | 0.815 (0.480–1.385) | 0.838 (0.508–0.381) | ||
| PS | ||||
| 0–1 | 1 | 0.009 | 1 | 0.052 |
| 2–3 | 0.367 (0.173–0.779) | 0.445 (0.197–1.006) | ||
| Primary cancer | ||||
| Lung, breast | 1 | 0.141 | 1 | 0.220 |
| Others | 0.626 (0.336–1.168) | 0.679 (0.365–1.261) | ||
| # of fractions | ||||
| 1 (SRS) | 1 | 0.788 | 1 | 0.922 |
| >1 (FSRT) | 1.076 (0.630–1.839) | 0.975 (0.588–1.616) | ||
| SRT modality | ||||
| HA VMAT | 1 | 0.377 | 1 | 0.810 |
| Others | 1.272 (0.746–2.172) | 1.063 (0.645–1.754) | ||
| GTV (cc) | ||||
| 0.3–1 | 1 | 0.546 | 1 | 0.240 |
| >1 | 0.849 (0.499–1.445) | 0.741 (0.449–1.222) | ||
| Time of the Evaluation MRI (months) | ||||
| 5–6.5 | 1 | 0.181 | 1 | 0.263 |
| 6.5–8.5 | 1.443 (0.843–2.472) | 1.332 (0.806–2.199) | ||
| Each GTV dose for BED LQ model: α/β = 10 | ||||
| D100 | 1.010 (0.992–1.029) | 0.286 | 1.012 (0.994–1.030) | 0.193 |
| D98 | 1.018 (0.998–1.039) | 0.084 | 1.017 (0.997–1.036) | 0.089 |
| D80 | 1.026 (1.005–1.048) | 0.015 | 1.022 (1.002–1.042) | 0.028 |
| D60 | 1.020 (1.002–1.038) | 0.033 | 1.016 (1.000–1.033) | 0.056 |
| D40 | 1.023 (1.006–1.039) | 0.007 | 1.018 (1.003–1.034) | 0.019 |
| D20 | 1.018 (1.005–1.031) | 0.008 | 1.014 (1.002–1.026) | 0.021 |
| D2 | 1.014 (1.003–1.024) | 0.012 | 1.012 (1.002–1.021) | 0.016 |
| Dmax | 1.013 (1.003–1.023) | 0.013 | 1.011 (1.002–1.020) | 0.018 |
| Each GTV dose for BED LQ model: α/β = 20 | ||||
| D100 | 1.037 (0.997–1.080) | 0.071 | 1.040 (1.000–1.082) | 0.050 |
| D98 | 1.062 (1.017–1.109) | 0.007 | 1.056 (1.011–1.102) | 0.013 |
| D80 | 1.067 (1.025–1.110) | 0.001 | 1.054 (1.016–1.094) | 0.006 |
| D60 | 1.041 (1.010–1.073) | 0.010 | 1.032 (1.004–1.061) | 0.027 |
| D40 | 1.037 1.011–1.064) | 0.005 | 1.028 (1.005–1.051) | 0.017 |
| D20 | 1.026 (1.007–1.046) | 0.009 | 1.020 (1.002–1.037) | 0.025 |
| D2 | 1.019 (1.004–1.035) | 0.016 | 1.015 (1.002–1.029) | 0.023 |
| Dmax | 1.018 (1.003–1.032) | 0.018 | 1.014 (1.002–1.027) | 0.025 |
| Each GTV dose for BED LQC model: α/β = 12 | ||||
| D100 | 1.042 (0.998–1.088) | 0.063 | 1.040 (0.996–1.085) | 0.073 |
| D98 | 1.050 (1.008–1.094) | 0.019 | 1.038 (0.999–1.078) | 0.054 |
| D80 | 1.040 (1.007–1.073) | 0.016 | 1.028 (0.999–1.057) | 0.056 |
| D60 | 1.027 (1.002–1.053) | 0.031 | 1.019 (0.997–1.041) | 0.092 |
| D40 | 1.021 (1.001–1.041) | 0.038 | 1.015 (0.997–1.032) | 0.095 |
| D20 | 1.016 (1.000–1.031) | 0.045 | 1.011 (0.998–1.025) | 0.100 |
| D2 | 1.012 (1.000–1.024) | 0.060 | 1.009 (0.999–1.020) | 0.082 |
| Dmax | 1.011 (0.999–1.022) | 0.063 | 1.009 (0.999–1.019) | 0.083 |
| Each GTV dose for BED LQL model: α/β = 10 | ||||
| D100 | 1.041 (0.999–1.086) | 0.056 | 1.037 (0.997–1.080) | 0.072 |
| D98 | 1.046 (1.007–1.087) | 0.020 | 1.035 (0.999–1.072) | 0.058 |
| D80 | 1.038 (1.007–1.069) | 0.016 | 1.027 (0.999–1.055) | 0.057 |
| D60 | 1.026 (1.002–1.051) | 0.034 | 1.018 (0.997–1.040) | 0.096 |
| D40 | 1.021 (1.002–1.041) | 0.032 | 1.015 (0.998–1.033) | 0.087 |
| D20 | 1.016 (1.001–1.032) | 0.038 | 1.012 (0.998–1.026) | 0.090 |
| D2 | 1.013 (1.000–1.025) | 0.050 | 1.010 (0.999–1.021) | 0.074 |
| Dmax | 1.012 (1.000–1.024) | 0.053 | 1.010 (0.999–1.020) | 0.074 |
| Each GTV dose for BED LQ model: α/β = 20 | ||||
| D80 < BED 58 | 1 | 0.007 | 1 | <0.001 |
| D80 > BED 58 | 2.162 (1.237–3.776) | 3.356 (1.861–6.050) | ||
Note: For BED variables OR, increase per 1.
TABLE 4.
AICc and EVR values for over 65% and over 95% volume reduction at the evaluation MRI
| Model | GTV dose | Over 65% | Over 90% | ||
|---|---|---|---|---|---|
| AICc | EVR | AICc | EVR | ||
| BED LQ: α/β = 10 | D100 | 315.797 | 125.721 | 343.734 | 23.976 |
| D98 | 313.906 | 48.841 | 342.520 | 13.067 | |
| D80 | 310.811 | 10.392 | 340.508 | 4.778 | |
| D60 | 312.180 | 20.606 | 341.657 | 8.487 | |
| D40 | 309.301 | 4.884 | 339.752 | 3.274 | |
| D20 | 309.599 | 5.669 | 340.000 | 3.706 | |
| D2 | 310.188 | 7.611 | 339.536 | 2.939 | |
| Dmax | 310.393 | 8.432 | 339.644 | 3.102 | |
| BED LQ: α/β = 20 | D100 | 313.690 | 43.841 | 341.444 | 7.630 |
| D98 | 309.484 | 5.353 | 338.983 | 2.229 | |
| D80 | 306.129 | 1.000 | 337.380 | 1.000 | |
| D60 | 309.909 | 6.620 | 340.392 | 4.509 | |
| D40 | 308.419 | 3.143 | 339.574 | 2.995 | |
| D20 | 309.595 | 5.658 | 340.341 | 4.395 | |
| D2 | 310.636 | 9.522 | 340.197 | 4.090 | |
| Dmax | 310.869 | 10.698 | 340.307 | 4.321 | |
| BED LQC: α/β = 12 | D100 | 313.444 | 38.767 | 342.119 | 10.693 |
| D98 | 311.240 | 12.879 | 341.659 | 8.496 | |
| D80 | 310.816 | 10.418 | 341.746 | 8.873 | |
| D60 | 312.031 | 19.126 | 342.591 | 13.539 | |
| D40 | 312.374 | 22.705 | 342.636 | 13.847 | |
| D20 | 312.708 | 26.831 | 342.724 | 14.470 | |
| D2 | 313.193 | 34.194 | 342.400 | 12.306 | |
| Dmax | 313.298 | 36.038 | 342.418 | 12.417 | |
| BED LQL: α/β = 10 | D100 | 313.218 | 34.625 | 342.112 | 10.655 |
| D98 | 309.355 | 5.018 | 341.760 | 8.935 | |
| D80 | 310.893 | 10.827 | 341.766 | 8.963 | |
| D60 | 312.237 | 21.201 | 342.650 | 13.944 | |
| D40 | 312.110 | 19.897 | 342.483 | 12.827 | |
| D20 | 312.400 | 23.002 | 342.556 | 13.304 | |
| D2 | 312.878 | 29.212 | 342.224 | 11.269 | |
| Dmax | 312.981 | 30.755 | 342.235 | 11.331 | |
Abbreviations: AICc, Akaike Information Criterion; BED, biological effective dose; EVR, evidence ratio; GTV, gross tumor volume; LQ, linear‐quadratic; LQC, linear‐quadratic cubic; LQL, linear‐quadratic linear; MRI, magnetic resonance imaging.
GTV D80 > BED20 58 predicted over 65% and over 90% volume reduction at 6 months post‐SRT in the univariate analysis (Table 3). For GTV D80 > BED20 58, over 65% and 90% volume reductions were achieved in 73.1% and 53.2% (vs 55.7% and 25.3% for GTV D80 < BED20 58) of the brain metastases, respectively. GTV D80 > BED20 58 was also a predictive factor for over 65% and over 90% volume reduction in the multivariate analysis (OR: 1.975, p = 0.023 and OR: 3.204, p < 0.001, respectively) (Table 5).
TABLE 5.
Results of the multivariate analysis for prognostic factors for over 65% and 90% volume reduction at the evaluation MRI
| Variable | Over 65% | Over 90% | ||
|---|---|---|---|---|
| OR (95% CI) | p‐value | OR (95% CI) | p‐value | |
| Each GTV dose for BED LQ model: α/β = 20 | ||||
| D80 < BED 58 | 1 | 0.023 | 1 | <0.001 |
| D80 > BED 58 | 1.975 (1.096–3.557) | 3.204 (1.735–5.918) | ||
| PS | ||||
| 0–1 | 1 | 0.063 | 1 | 0.369 |
| 2–3 | 0.474 (0.216–1.042) | 0.674 (0.284–1.596) | ||
| Primary cancer | ||||
| Lung, breast | 1 | 0.157 | 1 | 0.298 |
| Others | 0.630 (0.333–1.195) | 0.710 (0.373–1.353) | ||
| Each GTV (cc) | ||||
| 0.3–1 | 1 | 0.870 | 1 | 0.580 |
| >1 | 0.955 (0.548–1.664) | 0.861 (0.508–1.460) | ||
| Time of the evaluation MRI | ||||
| 5–6.5 months | 1 | 0.225 | 1 | 0.208 |
| 6.5–8.5 months | 1.417 (0.807–2.487) | 1.407 (0.827–2.396) | ||
Abbreviations: BED, biological effective dose; CI, confidence interval; FSRT, fractionated stereotactic radiotherapy; GTV, gross tumor volume; HA VMAT, HyperArc volumetric modulated arc therapy; LQ, linear‐quadratic; LQC, linear‐quadratic cubic; LQL, linear‐quadratic linear; MRI, magnetic resonance imaging; OR, odds ratio; PS, performance status; SRS, stereotactic radiosurgery.
4. DISCUSSION
This is the first study to report that over 65% and over 90% volume reduction at 6 months post‐SRT were prognostic factors for local control. Our study results agree with similar studies. 1 , 4 , 5 In a previous study, an over 20% volume reduction at 3 months post‐SRT predicted local control. 1 However, an over 65% volume reduction at 3 months post‐SRT did not predict local control. 1 An over 65% volume reduction in brain metastases corresponds to a partial response in the RANO‐BM guidelines. 8 Three months post‐SRT seems early for evaluation, and 6 months post‐SRT may be adequate for evaluation according to the definition of RANO‐BM guidelines of partial response. 8 Maximum volume reduction of brain metastases leads not only to long‐term local control but may also improve peritumoral edema and QOL. 4 Consequently, the patient may be able to receive and endure systemic therapy. 4 Because systemic therapy is advancing, maximum volume reduction of brain metastases is becoming increasingly important these days.
The GTV parameters that correlated with over 65% as well as over 90% volume reduction at 6 months post‐SRT were investigated. GTV D80 in the LQ model: α/β = 20 best predicted both, and thus, is apparently important for favorable volume reduction after SRT. GTV D80 > BED20 58 also predicted over 65% and over 90% volume reductions. When the prescribed dose is 30 Gy/3 fractions (the median dose in this study) in an 80% isodose, achieving GTV D80 > BED20 58 is difficult; an inhomogeneous dose distribution is required. In previous studies, inhomogeneous dose distribution correlated with good local control after Gamma Knife radiotherapy 20 , 21 and resulted in better local control than did homogeneous distribution after Linac‐based SRT. 22 BED20 58 is approximately 41.5 Gy/5 fractions and is similar to BED10 80. Matsuyama et al. reported that BED10 80 for the PTV predicted good local control after SRT for brain metastases. 23 Hence, long‐term local control requires a relatively high dose. Based on these results, we added constraints to the GTV dose in our protocol in 2022. In our new protocol, the prescription dose for the PTV (GTV + 1 mm margin) was 35 Gy/5 fractions (BED20 47), which resulted in a more inhomogeneous dose distribution. For GTV >0.3 cc, GTV D80 > 50 Gy/5 fractions (BED20 75) was used when possible.
It is highly debated which of the following models should be chosen when performing SRT for brain metastases: LQ: α/β = 10; LQ: α/β = 20; LQC: α/β = 12; or LQL: α/β = 10. 2 , 14 , 15 , 16 , 17 , 18 LQ: α/β = 10 is well suited for stereotactic body radiotherapy for early‐stage lung cancer. 14 However, it is not appropriate for late‐stage lung cancer with brain metastases, which are best treated using LQL: α/β = 10. 14 The HyTEC group reported that LQ: α/β = 20 better predicted local control than LQ: α/β = 10 in patients with brain metastases. 18 In the present study, GTV D80 in the LQ model: α/β = 20 predicted over 65% and over 90% volume reduction at 6 months post‐SRT. Various doses and fractions are used in SRT and need to be stratified according to the BED in the future. More studies are needed to determine which model best predicts local control and volume reduction after SRT for brain metastases.
When treating brain metastases via SRT, each GTV is often very small, and the median GTV is often approximately 0.2 cc. 1 , 24 , 25 In a previous study, GTV of >0.2 cc was a risk factor for local recurrence; when the GTV was <0.2 cc, very good local control was obtained after treatment with an 80% isodose line. 25 In very small brain metastases, a homogenous distribution is sufficient for tumor control. Our study excluded brain metastases with a GTV of <0.3 cc because they are not suitable for volumetric measurements after SRT. An inhomogeneous distribution may be more important when the GTV volume was over 0.3 cc.
There were some limitations to our study. First, because it was retrospective, the timing of the evaluation imaging was somewhat variable. The median time for the evaluation MRI was 6.3 months, with a range of 5–8.5 months. However, the time of the evaluation MRI (5–6.5 vs 6.5–8.5 months post‐SRT) was not a predictive factor for an over 65% and 90% response. In a previous study, the median volume reduction rate was 44.2% at 3 months, 69.6% at 6 months, and 75.3% at 12 months after SRT. 1 Tumor volume changes dramatically within the first 3 months after SRT. 1 Hence, our finding that over 65% volume reduction at 6 months post‐SRT predicts local control is reasonable. The volumetric analysis takes time and effort, and even retrospective reports of volumetric measurements are limited. 1 , 2 , 5 , 8 More retrospective studies that include volumetric analysis are needed. Second, our study included a variety of primary cancers, and the effects of systemic therapy were not analyzed. Systemic therapy is rapidly advancing and may be influencing tumor response. More studies of the impact of systemic therapy on brain metastases are required.
In conclusion, over 65% and over 90% volume reduction of brain metastases at 6 months post‐SRT predicts good local control. Beneficial volume reduction may require increasing the dose to GTV D80 in the LQ model: α/β = 20, and inhomogeneous dose distribution may be required for SRT for brain metastases. Further studies are needed to confirm these findings.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Naoyuki Kanayama, Toshiki Ikawa, Takero Hirata. Acquisition of data, analysis, and/or interpretation of data: Naoyuki Kanayama, Toshiki Ikawa, Shingo Ohira, Takero Hirata, Masahiro Morimoto, Teruki Teshima, Koji Konishi. Manuscript writing and final approval of manuscript: All authors.
ETHICAL APPROVAL STATEMENT
The institutional review of the board of the Osaka International Cancer Institute approved this study (21150).
PATIENT CONSENT STATEMENT
All patients provided written informed consent for the use of their data prior to starting SRT.
ACKNOWLEDGMENTS
This work was supported by JSPS KAKENHI Grant No. 21K15857. The funding source had no involvement in the study design, collection, analysis, or interpretation of data.
Kanayama N, Ikawa T, Ohira S, et al. Volumetric reduction of brain metastases after stereotactic radiotherapy: Prognostic factors and effect on local control. Cancer Med. 2022;11:4806‐4815. doi: 10.1002/cam4.4809
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Oft D, Schmidt MA, Weissmann T, et al. Volumetric regression in brain metastases after stereotactic radiotherapy: time course, predictors, and significance. Front Oncol. 2020;10:590980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Putz F, Weissmann T, Oft D, et al. FSRT vs. SRS in brain metastases‐differences in local control and radiation necrosis‐a volumetric study. Front Oncol. 2020;10:559193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Steinmann D, Vordermark D, Gerstenberg W, et al. Quality of life working Group of the German Radiation Oncology Society (DEGRO). Quality of life in patients with limited (1–3) brain metastases undergoing stereotactic or whole brain radiotherapy: a prospective study of the DEGRO QoL working group. Strahlentherapie & Onkologie. 2020;196(1):48‐57. [DOI] [PubMed] [Google Scholar]
- 4. Kim WH, Kim DG, Han JH, et al. Early significant tumor volume reduction after radiosurgery in brain metastases from renal cell carcinoma results in long‐term survival. Int J Radiat Oncol Biol Phys. 2012;82(5):1749‐1755. [DOI] [PubMed] [Google Scholar]
- 5. Sharpton SR, Oermann EK, Moore DT, et al. The volumetric response of brain metastases after stereotactic radiosurgery and its post‐treatment implications. Neurosurgery. 2014;74(1):9‐15. discussion 16; quiz 16. [DOI] [PubMed] [Google Scholar]
- 6. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228‐247. [DOI] [PubMed] [Google Scholar]
- 7. Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47(1):207‐214. [DOI] [PubMed] [Google Scholar]
- 8. Lin NU, Lee EQ, Aoyama H, et al. Response assessment in neuro‐oncology (RANO) group. Response assessment criteria for brain metastases: proposal from the RANO group. The Lancet Oncol. 2015;16(6):e270‐e278. [DOI] [PubMed] [Google Scholar]
- 9. Bauknecht HC, Romano VC, Rogalla P, et al. Intra‐ and interobserver variability of linear and volumetric measurements of brain metastases using contrast‐enhanced magnetic resonance imaging. Invest Radiol. 2010;45(1):49‐56. [DOI] [PubMed] [Google Scholar]
- 10. Ohira S, Ueda Y, Kanayama N, et al. Impact of multileaf collimator width on dose distribution in HyperArc fractionated stereotactic irradiation for multiple (−) brain metastases. Anticancer Res. 2021;41(6):3153‐3159. [DOI] [PubMed] [Google Scholar]
- 11. Komiyama R, Ohira S, Ueda H, et al. Intra‐fractional patient motion when using the Qfix encompass immobilization system during HyperArc treatment of patients with brain metastases. J Appl Clin Med Phys. 2021;22(3):254‐260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kano H, Kondziolka D, Lobato‐Polo J, Zorro O, Flickinger JC, Lunsford LD. T1/T2 matching to differentiate tumor growth from radiation effects after stereotactic radiosurgery. Neurosurgery. 2010;66(3):486‐491. discussion 491–492. [DOI] [PubMed] [Google Scholar]
- 13. Yomo S, Oguchi K. Prospective study of 11C‐methionine PET for distinguishing between recurrent brain metastases and radiation necrosis: limitations of diagnostic accuracy and long‐term results of salvage treatment. BMC Cancer. 2017;17(1):713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gago‐Arias A, Neira S, Pombar M, Gómez‐Caamaño A, Pardo‐Montero J. Evaluation of indirect damage and damage saturation effects in dose–response curves of hypofractionated radiotherapy of early‐stage NSCLC and brain metastases. Radiother Oncol. 2021;161:1‐8. [DOI] [PubMed] [Google Scholar]
- 15. Guerrero M, Carlone M. Mechanistic formulation of a lineal‐quadratic‐linear (LQL) model: split‐dose experiments and exponentially decaying sources. Med Phys. 2010;37(8):4173‐4181. [DOI] [PubMed] [Google Scholar]
- 16. Guerrero M, Li XA. Extending the linear‐quadratic model for large fraction doses pertinent to stereotactic radiotherapy. Phys Med Biol. 2004;49(20):4825‐4835. [DOI] [PubMed] [Google Scholar]
- 17. Wiggenraad R, Verbeek‐de Kanter AV, Kal HB, Taphoorn M, Vissers T, Struikmans H. Dose‐effect relation in stereotactic radiotherapy for brain metastases. A systematic review. Radiother Oncol. 2011;98(3):292‐297. [DOI] [PubMed] [Google Scholar]
- 18. Redmond KJ, Gui C, Benedict S, et al. Tumor control probability of radiosurgery and fractionated stereotactic radiosurgery for brain metastases. Int J Radiat Oncol Biol Phys. 2021;110(1):53‐67. [DOI] [PubMed] [Google Scholar]
- 19. Burnham KP, Anderson DR. Multimodel inference: understanding AIC and BIC in model selection. Sociol Methods Res. 2004;33(2):261‐304. [Google Scholar]
- 20. Abraham C, Garsa A, Badiyan SN, et al. Internal dose escalation is associated with increased local control for non‐small cell lung cancer (NSCLC) brain metastases treated with stereotactic radiosurgery (SRS). Adv Radiat Oncol. 2018;3(2):146‐153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kennedy WR, DeWees TA, Acharya S, et al. Internal dose escalation associated with increased local control for melanoma brain metastases treated with stereotactic radiosurgery. J Neurosurg. 2021;135(3):855‐861. [DOI] [PubMed] [Google Scholar]
- 22. Lucia F, Key S, Dissaux G, et al. Inhomogeneous tumor dose distribution provides better local control than homogeneous distribution in stereotactic radiotherapy for brain metastases. Radiother Oncol. 2019;130:132‐138. [DOI] [PubMed] [Google Scholar]
- 23. Matsuyama T, Kogo K, Oya N. Clinical outcomes of biological effective dose‐based fractionated stereotactic radiation therapy for metastatic brain tumors from non‐small cell lung cancer. Int J Radiat Oncol Biol Phys. 2013;85(4):984‐990. [DOI] [PubMed] [Google Scholar]
- 24. Alongi F, Nicosia L, Figlia V, et al. Long‐term disease outcome and volume‐based decision strategy in a large cohort of multiple brain metastases treated with a mono‐isocentric linac‐based stereotactic radiosurgery technique. Clin Transl Oncol. 2021;23(8):1561‐1570. [DOI] [PubMed] [Google Scholar]
- 25. Kraft J, van Timmeren JE, Mayinger M, et al. Distance to isocenter is not associated with an increased risk for local failure in LINAC‐based single‐isocenter SRS or SRT for multiple brain metastases. Radiother Oncol. 2021;159:168‐175. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


