Abstract
Cervical cancer (CC) ranks as the fourth most frequently diagnosed malignancy in females worldwide. Exosomes are a subclass of extracellular vesicles released by nearly all types of cells that act as cargo transport vehicles, carrying proteins, and genetic material (such as miRNAs, long noncoding RNAs, and mRNAs) derived from their parent cells may affect receiving cells and thus have emerged as key players in several biological processes, including inflammatory pathways. In this review, we concentrated on the findings of exosome investigations in CC, particularly their components. They direct the actions of CC cells by inducing surface molecules associated with various biological pathways. We summarized the current knowledge of exosomal RNAs and proteins from CC cells and discussed the feasibility of exosomes as potential biomarkers for CC. We suggest that cancer‐derived exosomes promote metastasis in CC by supporting EMT, controlling the proliferation, invasion, or migration of cancer cells, as well as influencing immune escape and aiding angiogenesis. Overall, cancer‐derived exosomes are critical in the progression of CC, and further studies are necessary to advance our understanding of the clinical value of exosomes in CC.
Keywords: biomarker, cervical cancer, exosomes, microRNA
Cancer‐derived exosomes may promote CC metastasis by boosting cellular epithelial‐mesenchymal transformation (EMT), controlling the proliferation, invasion, or migration of cancer cells, as well as influencing immune escape and aiding angiogenesis.
1. INTRODUCTION
Cervical cancer (CC) is a type of cancer that originates from cells produced at the squamocolumnar junction of the uterine cervix. 1 Cervical cancer ranks as the fourth most common cancer among women globally and is one of the leading causes of cancer deaths in women, especially when discovered at an advanced stage. 2 The development of female CC requires infection with “highly carcinogenic” strains of human papillomavirus (HPV). 3 Human papillomavirus type 16 is the most common form of HPV associated with CC, followed by HPV18, HPV45, and HPV 31. 4 , 5 In the past few years, exosomes have been recognized as a critical factor in several kinds of cancer and other pathologies. They may be used in the clinic as potential biomarkers for detecting and differentiating malignant from noncancerous tissue. 6 , 7 To date, thousands of articles have demonstrated the involvement of exosomes in cancer progression. 8 , 9 This review focuses on CC, emphasizing the progression of exosomal molecules (RNAs and proteins) in CC and the impact on the immune response. In addition, the feasibility of exosomes as potential biomarkers for CC is discussed. Cancer‐derived exosomes may promote CC metastasis through multiple pathways and deserve in‐depth study.
2. STRUCTURE AND FUNCTION OF EXOSOMES
Bonucci and Anderson first reported exosomes in the late 1960s. 10 , 11 These particles are extracellular vesicles with a structure similar to that of cells, ranging from 30 to 150 nm in diameter, and they are present in the same tissue as their associated cells. 12 , 13 , 14 Fluorescence microscopy has been used to detect the presence of exosomes. 15 Nucleic acids, proteins, lipids, and metabolites have been proposed as biomarkers due to their unique molecular properties (8). Exosomes have potential as biomarkers because of their high contents of cytoskeletal proteins, MHC class I and II proteins, adhesion proteins (tetramers, integrins), and encapsulated nucleic acids. 16
Delorme‐Axford et al. demonstrated that exosomal miRNA (chromosome 19 miRNA cluster, C19MC) could significantly prevent infection by inducing autophagy and resistance against viral infections, such as poliovirus, human cytomegalovirus, and herpes simplex virus 1. 17 It has been extensively documented that exosomes play a role in the immunological response. Takahashi et al. demonstrated that human fibroblast exosomes eliminate damaged cytosolic DNA, allowing the cells to maintain their normal state. 18 Exosomes have the potential to influence the immune response by mesenchymal stem cells and signaling pathways in recipient cells, mainly through the transfer of miRNAs. 19 Exosomal miRNAs can also circulate between dendritic cells and suppress gene expression, allowing them to function as antigen receptors. 20 , 21 In general, exosomes are involved in immune responses not only to cancer cells, but also to infectious agents (bacteria, viruses, fungi, and parasites).
3. VARIOUS CARGOES IN EXOSOMES
Exosomes transport various cargoes, including RNAs, proteins, and DNA, which can be picked up directly by other target cells or via biofluids, provoking a variety of phenotypic responses.
3.1. Exosomes as RNA carriers
Exosomes contain a diverse variety of RNA sequences, implying the presence of several RNA biotypes. 22 , 23 Exosomal influence is governed not only by their origin, but also by their cargo composition. Numerous studies have identified the bulk of known ncRNA biotypes, including small nuclear RNAs (snoRNAs), rRNAs, long noncoding RNAs (lncRNAs), PIWI‐interacting RNAs (piRNAs), and transfer RNAs (tRNAs). 24 Exosomes are found in numbers of approximately 2000 trillion in normal human blood and up to 4000 trillion in tumors. 25 In recent years, noncoding RNAs have been extensively studied and have been revealed to function in distant tissues, implicating them in various biological processes via various mechanisms, including epigenetic modifier protein recruitment, mRNA decay regulation, and translation. 26 , 27
3.2. Exosomes as protein carriers
Exosomes are essential protein carriers. They include a varied array of transmembrane proteins, lipid‐anchored membrane proteins, peripherally associated membrane proteins, and exosome lumen‐soluble proteins. 28 Additionally, exosomes carry surface proteins involved in peripheral nervous system communication, the majority of which are engaged in cell‐to‐cell communication. 29 , 30 These surface proteins include tumor necrosis factor (TNF) and wingless (Wnt) proteins. 31 , 32 Thus, exosomes can impact the activity of their target cells directly or indirectly via membrane and surface proteins.
3.3. Exosomes as DNA carriers
Exosomes can transfer large amounts of DNA from cells. 18 Single‐stranded, double‐stranded, and genomic DNA are all types of exosomal DNA. 33 , 34 Due to the encapsulation of exosome serum DNA, it is more stable than unencapsulated DNA in a range of storage conditions. 35 According to Liang Wang et al., exosomal double‐stranded DNA may be used as a biomarker for pheochromocytoma and paraganglioma diagnosis. 34 Thakur et al. found that the capacity of exosomal DNA to identify mutations in parental cancer cells demonstrates its enormous therapeutic potential as a circulating cancer biomarker. 36 However, the amount of exosomal DNA present within the organelle is unclear. Further research will be needed in the future to determine how DNA enters exosomes and how exosomal DNA may be utilized to aid in the identification and treatment of cancer.
4. EXOSOMES IN OTHER CANCERS
4.1. Exosomes in lung cancer
Lung cancer is a diverse illness with various subgroups with pathological and clinical significance. It is one of the China's most common malignant tumors, ranking first in cancer‐related mortality. 37 , 38 Because of inadequate diagnosis and prognosis at an early stage, the mortality rate for lung cancer patients remains high. 39 Therefore, identifying novel targets and biomarkers as practical tools for the management of lung cancer represents a significant challenge.
Numerous studies have focused on the critical role of lung cancer cell exosomes in the occurrence, early detection/diagnosis, and drug resistance of lung cancer. JAE YOUNG KIM et al. discovered that a large amount of COX‐2 loaded on the exosomes of lung cancer cells, which could then be transferred to neighboring or distant cells. 40 Nan Zhang et al. found that the exosome‐derived circSATB2 protein plays a vital role in advancing non‐small cell lung cancer (NSCLC). Through direct binding to miR‐326, CircSATB2 may modulate FSCN1 expression in NSCLC cells, hence boosting cell proliferation, migration, and invasion in the tumor environment. 41 Following the findings of a study conducted by Lingyu Li and colleagues, serum exosomal FECR1 may be a valuable biomarker for diagnosing the progression of small cell lung cancer (SCLC) in patients. 42
4.2. Exosomes in liver cancer
Liver cancer is the second leading cause of cancer‐related death worldwide. 43 Among types of liver cancer, hepatocellular carcinoma (HCC) is the most common. Chronic infection with hepatitis B virus (HBV) is a crucial risk factor for the development and progression of hepatocellular carcinoma (HCC), accounting for more than half of all HCC cases worldwide. 44
Identifying the involvement of exosomal miRNAs and proteins throughout the development of HCC is essential. Many investigations on human samples and several experimental models have been carried out. Research studies have discovered that increased levels of miR‐122 and miR‐99 family expression in exosomes may promote HBV replication. 45 , 46 According to one study, higher miR‐199‐3p and miR‐201 expression levels in exosomes may be associated with decreased HBV replication. 47
Recent research has demonstrated that exosomal miRNAs may be beneficial in the diagnosis and prognosis of HCC. According to the findings of these studies, high levels of let‐7, miR‐122, and miR‐125a‐5p expression were associated with the development of HCC. 48 , 49 , 50 Exosomal miR‐638, miR‐296, miR137, and miR‐940 expression levels were found to be associated with the poor prognosis of HCC patients, and these findings have been confirmed in other cancers. 51 , 52 , 53 , 54 These findings indicate that exosomal miRNAs may contribute to the diagnosis and prognosis of HCC patients.
4.3. Exosomes in gastric cancer
Gastric cancer (GC) is one of the most common malignant tumors globally and has high morbidity and mortality. GC is much more prevalent in Asian countries than in non‐Asian countries. 2 Infection with Helicobacter pylori, high salt intake, and eating fewer fruits and vegetables are risk factors for gastric cancer. 55 Therapy for GC has made significant progress in recent decades, and numerous attempts have been undertaken to develop successful treatment solutions for the condition. However, the morbidity and mortality associated with GC are still high. Therefore, identifying new targets and biomarkers as practical tools for the management of gastric cancer is a significant problem in today's world.
When comparing patients with T4 stage cancer to those with T1 to T3 stage cancer, Tokuhisa et al. discovered that patients with T4 stage cancer had greater exosomal miR‐21 and miR‐1225‐5p expression levels. 56 CD97 proteins are tetraspanins found on the surface of exosomes and in the cell membrane. Exosomes derived from cells with high or low CD97 expression can stimulate the proliferation and migration of carcinoma cells by activating the exosome‐mediated MAPK signaling pathway. 56 According to Chao Li et al., exosomal miRNAs may also play a role in activating this pathway. 57 Furthermore, they discovered that CD97 promotes the proliferation and migration of gastric cancer cell lines in vitro. 57
5. ROLES OF EXOSOMES IN CC
5.1. Exosomes in CC immunity
Exosomes released by both nonimmune and immune cells play a critical role in the immune regulation of CC cells. 58 Buschow SI et al. discovered visible peptide–MHC class II complexes on the surfaces of exosomes, which demonstrated the involvement of exosomes in intercellular antigen transfer. 59 Furthermore, cancer‐derived exosomes have been shown to stimulate immune responses in laboratory animals. 60
Natural killer (NK) cells play critical roles in antiviral and anticarcinogenic immune responses in HPV‐related malignancies. 61 Apart from cytotoxic effects, NK cells are capable of secreting a large and diverse array of signaling molecules in response to the cytokine pattern existing in the tumor environment. 62 Due to the critical function of NK cells in the immune response to HPV, this cell type has been extensively studied in HPV‐related carcinogenesis, particularly in CC. Since the actions of NK cells are dependent on the receptors and ligands linked with them, numerous studies have examined the impact of these molecules on cervical carcinogenesis. Jimenez‐Perez et al. postulated that CC cells might generate decreases in the amounts of NKG2D and NKp46 on the surfaces of NK cells, which were connected with a decrease in cytotoxic effects. 63 According to Wen‐Chun Chang et al., Treg cell function significantly decreased NKG2D expression in CC patients. 64
In both ex vivo and in vivo studies, it has been demonstrated that dendritic cell (DC)‐derived exosomes (Dexo) can induce a specific antitumor immune response. 60 Dexo carries immunologic molecules capable of inducing an intense tumor cytotoxic response. 65 Poly(I: C) was found to have a substantial auxiliary effect on immune responses in some research studies. 66 Shisheng Chen et al. established that incubation with poly(I: C) during exosome synthesis dramatically increased the anti‐CC properties of Dexo(E7 + pIC). 60 They also discovered that Dexo significantly slowed the progression of tumors in mice. 60
In general, exosomes play a key role in the immune response to CC. Exosomes derived from CC may promote cancer progression by impairing NK activities. Exosomes can also produce a targeted antitumor immune response by interacting with dendritic cells.
5.2. Exosomal RNAs in CC
The first report about exosomal RNAs was made by Ratajczak J et al., 67 who claimed that exosomes contain RNAs, which can transfer extracellular RNAs to other cells or organs in a functional form. 67 Small noncoding RNAs (ncRNAs) are particularly abundant in exosomes that contain disproportionate amounts of small nuclear RNAs (snRNAs), microRNAs (miRNAs), Y RNAs, or fragmented ncRNAs. 68 , 69 However, most CC studies have focused on exosomal miRNAs. 29 , 70 , 71 , 72
MiRNAs are single‐stranded RNAs with a length of 22 nucleotides and are found in various organisms. 73 MiRNAs are vital in the regulation of gene transcription. 74 , 75 As guides in posttranscriptional gene silencing, miRNAs can cause snippet mRNA breakage or translational repression, which affects cellular appearance and function. 76 , 77 Several miRNAs have been discovered to regulate B‐cell differentiation, 78 antiviral defense, 79 and carcinogenesis in animals. 80 Therefore, miRNAs are important regulators of cell signaling and homeostasis in the tumor microenvironment. 81 It is possible to assess the expression of miRNAs, which can be utilized as diagnostic or prognostic biomarkers in CC. Lui et al. published the first research to compare the variation of two miRNAs (miR21 and miR‐143) in female CC cell lines and normal cervical tissues. 82 They found that the expression levels of miR‐21 and miR‐143 in CC cells were different from those in normal cervical tissue. 82 Genetic alterations of miRNA loci, such as point mutations and even epigenetic silencing, including DNA methylation or dysregulation of miRNA integrators and transposable elements, have all been linked to anomaly‐induced miRNA expression. 83 The levels of RNA expression in cervical neoplasia biopsy samples and sera from females with CC have been the subject of numerous studies (Table 1).
TABLE 1.
RNAs derived from CC cell exosomes
| Type | Expression level | Clinical value | Cargo | Reference |
|---|---|---|---|---|
| miRNA | Up | Diagnosis/therapy | miR‐221‐3p | 132 |
| miRNA | Down | Diagnosis | miR‐30d‐5p, let‐7d‐3p | 72 |
| lncRNA | Up | Diagnosis | HOTAIR | 135 |
| lncRNA | Up | Predictive | lncRNA DLX6‐AS1 | |
| miRNA | Up | Diagnosis/prognostic | miR‐196a | |
| miRNA | Up | Diagnosis/therapy | miR‐486‐5p | |
| miRNA | Down | Diagnosis | miR‐125a‐5p | |
| lncRNA | Up | Therapy | LINC01305 | |
| miRNA | Up | Diagnosis | miR‐221/222 | |
| miRNA | Up | Diagnosis | miR‐21, miR‐146a | |
| miRNA | Up | Therapy | miR‐22 | |
| lncRNA | Down | Diagnosis/predictive | MEG3, PVT1 | |
| lncRNA | Down | Therapy | GAS5 | |
| lncRNA | Down | Therapy | lncRNA STXBP5‐AS1, TUSC8 | |
| lncRNA | Down | Predictive/prognostic | XLOC_010588 | |
| lncRNA | Up | Predictive | LncRNA SPRY4, ZEB1‐AS1, LINC01305, LOC105374902 | |
| miRNA | Down | Diagnosis/therapy | miR‐34b | |
| miRNA | Up | Therapy | miR‐106a/b | 141 |
| miRNA | Up | Diagnosis | miR‐146a‐5p, miR‐151a‐3p, miR‐2110, miR‐21‐5p | 142 |
| circRNA | Up | Therapy | circRNA‐PVT1 | |
| lncRNA | Up | Therapy | lncRNA HNF1A‐AS1 | 143 |
| miRNA | Up | Therapy | miR‐221‐3p | 130 |
| miRNA | Up | Therapy | miR‐9 | 144 |
| miRNA | Up | Diagnosis | miRNA‐20a, miRNA‐203, miRNA‐21, miRNA‐205, miRNA‐218, miR‐485‐5 | 145 |
| miRNA | Up | Diagnosis / Therapy | miR‐7, miR‐10a, miR‐17‐5p, miR‐135b, miR‐149, miR‐203 | |
| miRNA | Up | Diagnosis/therapy | miR‐877‐3p | 146 |
| miRNA | Up | Diagnosis/therapy | miR‐155‐5p | 147 |
| miRNA | Up | Therapy | miR‐663b | 127 |
| miRNA | Down | Therapy | miR‐5590‐3p, miR‐3156‐3p, miR‐10b | 148 |
| miRNA | Down | Therapy | miR‐34a, miR‐1284, miR‐142 | 58 |
| miRNA | Down | Therapy | miR‐429 | |
| miRNA | Down | Therapy | miR‐101 | |
| miRNA | Down | Therapy | miR‐34a, miR‐1284, miR‐142 | |
| miRNA | Down | Diagnosis/therapy | miR‐24, miR‐451, miR‐125a | |
| miRNA | Up | Diagnosis/therapy | miR‐130a | |
| miRNA | Up | Diagnosis/therapy | miR‐155 |
5.3. Exosomal proteins in CC
There are several types of transmembrane and lipid‐anchored membrane proteins in exosomes. 28 , 84 Exosome proteins are peripherally linked with membrane proteins and soluble proteins in the exosome lumen (Figure 1). This section will provide an overview of many of the more relevant and intriguing proteins consistently identified in CC exosomes.
FIGURE 1.
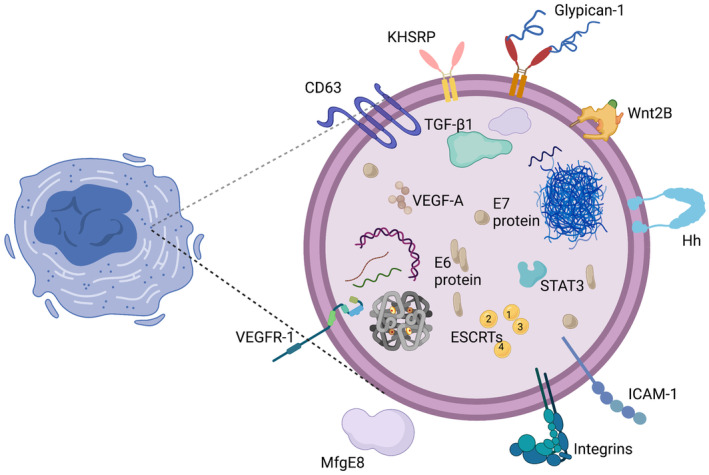
Structural characteristics of CC‐derived exosomes. CC‐derived exosomes are a subtype of extracellular vesicles that range in diameter from 30 to 200 nm and are abundant in certain proteins, lipids, nucleic acids, and glycoconjugates. Objects depicted in the figure are illustrative rather than exhaustive. Abbreviations: KHSRP, KH‐type splicing regulatory protein; Wnt2B, wingless protein 2B; Hh, Hedgehog; TGF‐β1, transforming growth factor‐beta 1; VEGF‐A, vascular endothelial growth factor A; STAT3, signal transducer and activator of transcription 3; E6 protein, a protein encoded by human papillomavirus; E7 protein, a protein encoded by human papillomavirus; VEGFR1, vascular endothelial growth factor receptor 1; MfgE8, milk fat globule protein E8; ESCRTs, endosomal sorting complexes required for transport; ICAM‐1, intracellular adhesion molecule‐1.
HPV pathogenesis was initially reported in 2009, and extracellular vesicles were found to function in this pathogenesis. 85 Following this finding, the same author verified the presence of extracellular survivin within exosomes. 85 Several studies suggest that specific exosomal proteins are involved in the progression of CC, 86 , 87 , 88 including metastasis, invasion, and resistance to chemotherapy. 89
ATF1 is a transcription factor protein that is involved in cell development, survival, and other biochemical processes. 90 Overexpression of ATF1 has been observed in nasopharyngeal carcinoma (NPC), gastrointestinal clear cell sarcoma, and other cancers in various investigations. 91 , 92 , 93 Interestingly, Yanhua Shi et al. discovered that ATF1 levels in the blood exosomes of a CC mouse model were dramatically elevated. 94 Therefore, it should be no surprise that high ATF1 expression in exosomes might be used as a potential diagnostic biomarker for CC.
Small GTPases known as RAS proteins control cell proliferation, survival, and differentiation by acting as downstream effectors of growth factor receptor signaling. 95 When CC mouse blood exosomes were compared to normal mouse tissues in Yanhua Shi's study, the level of RAS protein expression was more than five times higher. 94 The RAS gene confers a substantial positive predictive value for CC. In addition, the RAS oncogene is mutated in approximately 35% to 45% of colorectal cancers, depending on the type. 96 Consequently, it is necessary to determine whether detection of a mutated RAS gene should be part of the diagnostic process.
Numerous studies have examined the protein expression levels in cervical neoplasia tissues and serum from CC patients, and the results are promising (Table 2). Early detection and prevention of CC are essential, and the exosomal detection approach may prove to be an effective tool for the early diagnosis of CC in the future.
TABLE 2.
Proteins derived from CC cell exosomes
| Type | Clinical level | Clinical value | Cargo | Reference |
|---|---|---|---|---|
| Protein | Down | Diagnosis/therapy | THBS2 | 132 |
| Protein | Up | Diagnosis/therapy | P13k, Akt, mTOR | 117, 149 |
| Protein | Up | Diagnosis/therapy/prognosis | Wnt‐2b | 116 |
| Protein | Up | Therapy | MUC16, SIRPA, E7 | 150 |
| Protein | Up | Therapy | CHMP4B, STX‐7, RPL28 | 151 |
| Protein | Up | Therapy | PTCH1, GLI1 | 118 |
| Protein | Down | Therapy | MAPK10 | 136 |
| Protein | Up | Diagnosis | ATF‐1, RAS | 100 |
| Protein | Down | Therapy | HPGD | 152 |
| Protein | Up | Therapy | STAT3 | 153 |
| Protein | Down | Therapy/prognosis | IFNAR1 | 154 |
| Protein | Up | Diagnosis | E6, E7 | 155 |
| Protein | Up | Therapy | KHSRP | 131 |
| Protein | Down | Therapy | ISG15 | 156 |
| Protein | Up | Diagnosis | E2(NF‐κB) | 157 |
| Protein | Up | Therapy | E5(COX‐2) | 158 |
6. EXOSOMES AS A BIOMARKER IN CC
Biological markers (also known as biomarkers) are molecules that are used to diagnose and/or predict the outcome of certain diseases. 97 Since exosomes are relatively stable, they can be found in blood, urine, and other body fluids using inexpensive, straightforward, sensitive, and reliable procedures even after years of sample preservation. 98 In the era of precision medicine, exosomes could be a new class of biomarkers for cancer diagnosis, treatment, and prognosis.
6.1. Exosomes as a diagnostic or therapeutic biomarker
Exosomal miRNA‐containing complexes have the potential to exert various therapeutic effects. In particular, it is possible to envision collecting exosomes from healthy donors and injecting them into patients to treat illness. 99 , 100
Some studies have demonstrated that miR21 enhances cancer propagation, inflammation, and translation. 101 , 102 , 103 , 104 In CC, upregulated miR21 targeted PDCD4, PTEN, RASA1, and TIMP3. 105 Yang et al. discovered that miR181b reduced the generation of cyclic adenosine monophosphate (cAMP) in CC cell lines by downregulating adenylyl cyclase 9 expression to prevent apoptosis and promote cell proliferation. 106 According to the findings of another study, miR143 may affect the death and proliferation of HeLa cells in CC by targeting the transcription factor HIF1. 107 In addition, the overexpression of miR21 has been shown to influence the survival, proliferation, and invasiveness of CC cells, which may be accomplished by targeting tissue inhibitor of metalloproteinase 3 (TIMP3). 108 , 109
As with exosomal ncRNAs, exosomal proteins or transmembrane proteins also play important roles in the diagnosis or therapy of CC. 5 , 110 Liang lj et al. demonstrated that CC‐derived exosomal Wnt2B could drive fibroblast activation into cancer‐associated fibroblasts, and this discovery points the way forward in developing diagnostic and therapeutic targets for CC progression. 111 Zhang W et al. reported that the PI3k/Akt/mTOR signaling pathway might provide possible diagnostic biomarkers or therapeutic targets via exosomes isolated from vaginal secretions. 112 In addition, exosomes can promote cervical angiogenesis via the hedgehog–GLI signaling pathway, identifying exosomes as prospective therapeutic targets for locally progressed metastatic cervical lesions. 113
These studies indicate that exosomes might promote the progression of CC through proteins or RNAs in exosomes, which may play a critical role in the diagnosis or therapy of CC.
6.2. Exosomes as a prognostic biomarker
CC is known to be associated with HPV infection, which is the major risk factor for the disease. The continuous expression of the HPV oncogenic proteins E6 and E7 has been related to the progression of CC through the degradation of the tumor suppressor protein p53 and the deactivation of the retinoblastoma protein (pRB). 114 , 115 , 116 , 117 , 118 Exosomes also play important roles in the development and progression of CC. For example, the overexpression of miRNA‐944 seems to be a biomarker of an adverse prognosis in advanced CC cases. 119 A study conducted in the advanced FIGO stage found that the group with high exosomal miR‐664 expression survived for a shorter period than the group with low exosomal miR‐664 expression. 120 Consequently, exosomes may potentially be used as prognostic biomarkers for CC patients.
Exosomes, which transport biomolecules from one cell to another, are important mediators of cellular communication. Exosomal ncRNAs are engaged in various cellular and biological processes, such as cellular development, cellular differentiation, and migration. 121 Due to their outstanding level of stability, exosomes may be detected in the bloodstream, making serum exosomal ncRNAs an intriguing prospective biomarker in many malignancies, including CC. 122 , 123 According to the findings of one study, the serum exosomal lncRNA DLX6‐AS1 level in CC patients was considerably higher than the levels in CIN abnormal and normal cases. 122 Moreover, DLX6‐AS1 may accelerate the progression of CC by sponging miR‐16‐5p and upregulating ARPP19, which offers novel insight into the prognosis and remedy of CC. 124 In addition, the overexpression of LINC01305 or knockdown significantly increased or decreased the development of CC, 125 which indicated that a higher LINC01305 level was associated with a worse prognosis. Furthermore, BBOX1‐AS1 mediates HOXC6 expression via miR‐361‐3p and HuR, promoting CC development, 126 and BBOX1‐AS1 overexpression predicts a worse prognosis for CC cases.
Although exosomes in the blood of CC patients have not been adequately studied, current experimental evidence supports the notion that these ncRNAs can be used to develop, improve, or strengthen CC prognosis and management strategies.
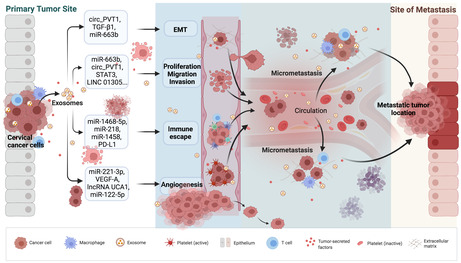
6.3. Cancer‐derived exosomes promote CC metastasis
Since exosomes can carry biomolecules for intercellular transmission, the content of exosomes (such as ncRNAs or proteins) contributes to the metastasis of CC to a certain extent. We can find some evidence of this influence in several recent studies. CC exosomal miRNA‐663b, cicr_PVT1 and TGF‐β1 are related to CC epithelial‐mesenchymal transition (EMT). 89 , 127 In addition, CC‐derived exosomal miRNA‐663b, miRNA‐221‐3p, miRNA‐146b‐3p, miRNA‐125a, cicr_PVT1, wnt‐2b, and miRNA‐744 are critical in the proliferation, invasion, and migration of CC cells. 89 , 111 , 128 , 129 , 130 Additionally, exosomal miRNA‐221, miRNA‐1468‐5p, lincRNA UCA1, and VEGF‐A play crucial roles in CC immune escape and promote angiogenesis. 113 , 130 , 131 , 132 , 133
Based on these studies, we suggest that cancer‐derived exosomes promote metastasis in CC by supporting EMT, controlling the proliferation, invasion, or migration of cancer cells, influencing immune escape, and aiding angiogenesis (Figure 2). Although the roles of exosomes in the most critical and fundamental connections involved in CC metastasis remain unknown, this is a very worthwhile direction for in‐depth research that requires additional investigation.
FIGURE 2.
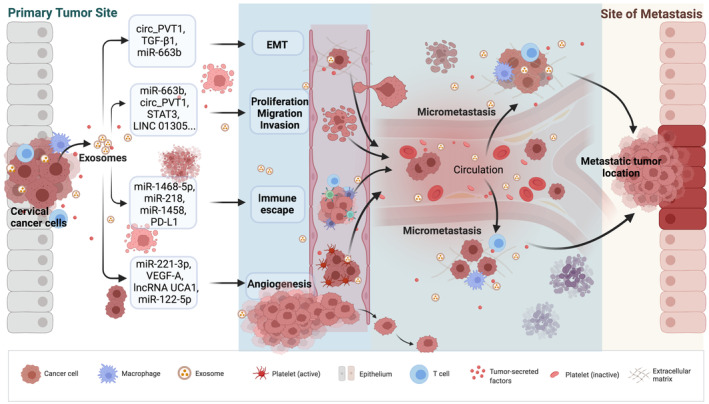
Cancer‐derived exosomes promote CC metastasis. Activated CC‐derived exosome signaling pathways interact with adjacent cells to enhance CC development by boosting cellular epithelial‐mesenchymal transformation (EMT), controlling cell proliferation and migration, and modulating immune escape and angiogenesis.
7. CONCLUSIONS AND PROSPECTS
In recent years, exosomes and their function in cancer have become the subject of increasing research studies. Exosomes derived from cancer can have antagonistic effects on the immune response to malignancies. 134 They can aid tumor cells in evading immune surveillance and developing immunological tolerance, whereas exosomes generated from immune cells have been shown to impede tumor cell growth, proliferation, and metastasis. 58
Exosomes carry many functional components and play roles in a wide range of physiological and pathological processes in the body. In this review, we concentrated on the findings of exosome investigations, specifically on the primary components of exosomes, which include many RNAs and proteins that direct the actions of CC cells by inducing surface molecules linked to various biological pathways. The appropriate transport EVs for transcripts, proteins, and ncRNAs, exosomes may be used as diagnostic, therapeutic, or prognostic biomarkers in CC. Furthermore, cancer‐derived exosomes can influence CC signal transduction pathways by interacting with various mechanisms involved in tumor origin, progression, and metastasis. This capacity enables the development of a plethora of novel methods for cancer detection and therapy. Obtaining liquid biopsies from patients is fairly straightforward. Thus, exosomes will increasingly be studied to aid in early cancer detection. In addition, the ability of exosomes to modulate immune responses in the cancer environment can be used to develop safe and reliable tumor vaccines.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
AUTHOR CONTRIBUTIONS
ZR and SW, the main author of the study, conceived the study and contributed to writing and editing. JY and ZR took part in designing and conducting the study. ZM and XC curated the exosome‐related data in the literature. JL reviewed and revised the manuscript. All of the authors read and approved the final manuscript.
ACKNOWLEDGMENTS
The authors would like to thank The Genius Medicine Consortium (TGMC) for providing the computer cluster and thank BioRender (https://biorender.com/) for providing drawing support.
Ran Z, Wu S, Ma Z, Chen X, Liu J, Yang J. Advances in exosome biomarkers for cervical cancer. Cancer Med. 2022;11:4966‐4978. doi: 10.1002/cam4.4828
Zihan Ran and Shaobo Wu contributed equally to this work.
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
REFERENCES
- 1. Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet (London, England). 2007;370(9590):890‐907. [DOI] [PubMed] [Google Scholar]
- 2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394‐424. [DOI] [PubMed] [Google Scholar]
- 3. Tota JE, Chevarie‐Davis M, Richardson LA, Devries M, Franco EL. Epidemiology and burden of HPV infection and related diseases: implications for prevention strategies. Prev Med. 2011;53(Suppl 1):S12‐S21. [DOI] [PubMed] [Google Scholar]
- 4. Kaufmann AM, Gissmann L, Schneider A. The worldwide perspective on human papillomavirus and cervical cancer. Cancer Epidemiol Biomarkers Prev. 2012;21(9):1400‐1401. [DOI] [PubMed] [Google Scholar]
- 5. Nahand JS, Vandchali NR, Darabi H, et al. Exosomal microRNAs: novel players in cervical cancer. Epigenomics. 2020;12(18):1651‐1660. [DOI] [PubMed] [Google Scholar]
- 6. Razavi ZS, Tajiknia V, Majidi S, et al. Gynecologic cancers and non‐coding RNAs: epigenetic regulators with emerging roles. Crit Rev Oncol Hematol. 2021;157:103192. [DOI] [PubMed] [Google Scholar]
- 7. Rahimian N, Razavi ZS, Aslanbeigi F, et al. Non‐coding RNAs related to angiogenesis in gynecological cancer. Gynecol Oncol. 2021;161(3):896‐912. [DOI] [PubMed] [Google Scholar]
- 8. Becker A, Thakur BK, Weiss JM, Kim HS, Peinado H, Lyden D. Extracellular vesicles in cancer: cell‐to‐cell mediators of metastasis. Cancer Cell. 2016;30(6):836‐848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Xu R, Rai A, Chen M, Suwakulsiri W, Greening DW, Simpson RJ. Extracellular vesicles in cancer ‐ implications for future improvements in cancer care. Nat Rev Clin Oncol. 2018;15(10):617‐638. [DOI] [PubMed] [Google Scholar]
- 10. Anderson HC. Vesicles associated with calcification in the matrix of epiphyseal cartilage. J Cell Biol. 1969;41(1):59‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bonucci E. Fine structure of early cartilage calcification. J Ultrastruct Res. 1967;20(1):33‐50. [DOI] [PubMed] [Google Scholar]
- 12. Chernyshev VS, Rachamadugu R, Tseng YH, et al. Size and shape characterization of hydrated and desiccated exosomes. Anal Bioanal Chem. 2015;407(12):3285‐3301. [DOI] [PubMed] [Google Scholar]
- 13. Gyorgy B, Szabo TG, Pasztoi M, et al. Membrane vesicles, current state‐of‐the‐art: emerging role of extracellular vesicles. Cell Mol Life Sci. 2011;68(16):2667‐2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ghaemmaghami AB, Mahjoubin‐Tehran M, Movahedpour A, et al. Role of exosomes in malignant glioma: microRNAs and proteins in pathogenesis and diagnosis. Cell Communicat Sign. 2020;18(1):120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Booth AM, Fang Y, Fallon JK, Yang JM, Hildreth JE, Gould SJ. Exosomes and HIV gag bud from endosome‐like domains of the T cell plasma membrane. J Cell Biol. 2006;172(6):923‐935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Guenat D, Hermetet F, Pretet JL, Mougin C. Exosomes and other extracellular vesicles in HPV transmission and carcinogenesis. Viruses. 2017;9(8):211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Delorme‐Axford E, Donker RB, Mouillet JF, et al. Human placental trophoblasts confer viral resistance to recipient cells. Proc Natl Acad Sci USA. 2013;110(29):12048‐12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Takahashi A, Okada R, Nagao K, et al. Exosomes maintain cellular homeostasis by excreting harmful DNA from cells. Nat Commun. 2017;8:15287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hashemipour M, Boroumand H, Mollazadeh S, et al. Exosomal microRNAs and exosomal long non‐coding RNAs in gynecologic cancers. Gynecol Oncol. 2021;161(1):314‐327. [DOI] [PubMed] [Google Scholar]
- 20. Montecalvo A, Larregina AT, Shufesky WJ, et al. Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes. Blood. 2012;119(3):756‐766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mori MA, Ludwig RG, Garcia‐Martin R, Brandao BB, Kahn CR. Extracellular miRNAs: from biomarkers to mediators of physiology and disease. Cell Metab. 2019;30(4):656‐673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ekström K, Valadi H, Sjöstrand M, et al. Characterization of mRNA and microRNA in human mast cell‐derived exosomes and their transfer to other mast cells and blood CD34 progenitor cells. J Extracell Ves. 2012;1(1):18389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome‐mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654‐659. [DOI] [PubMed] [Google Scholar]
- 24. Xu YX, Pu SD, Li X, et al. Exosomal ncRNAs: novel therapeutic target and biomarker for diabetic complications. Pharmacol Res. 2022;178:106135. [DOI] [PubMed] [Google Scholar]
- 25. Zhou J, Li XL, Chen ZR, Chng WJ. Tumor‐derived exosomes in colorectal cancer progression and their clinical applications. Oncotarget. 2017;8(59):100781‐100790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dou R, Liu K, Yang C, et al. EMT‐cancer cells‐derived exosomal miR‐27b‐3p promotes circulating tumour cells‐mediated metastasis by modulating vascular permeability in colorectal cancer. Clin Transl Med. 2021;11(12):e595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wei K, Ma Z, Yang F, et al. M2 macrophage‐derived exosomes promote lung adenocarcinoma progression by delivering miR‐942. Cancer Lett. 2022;526:205‐216. [DOI] [PubMed] [Google Scholar]
- 28. Escola JM, Kleijmeer MJ, Stoorvogel W, Griffith JM, Yoshie O, Geuze HJ. Selective enrichment of tetraspan proteins on the internal vesicles of multivesicular endosomes and on exosomes secreted by human B‐lymphocytes. J Biol Chem. 1998;273(32):20121‐20127. [DOI] [PubMed] [Google Scholar]
- 29. Asgarpour K, Shojaei Z, Amiri F, et al. Exosomal microRNAs derived from mesenchymal stem cells: cell‐to‐cell messages. Cell Commun Sign. 2020;18(1):149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yi J, Wang Y, Zhang H, et al. BrucellaInterferon‐inducible transmembrane protein 3‐containing exosome as a new carrier for the cell‐to‐cell transmission of anti‐ activity. Front Veterin Sci. 2021;8:642968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gross JC, Zelarayán LC. The mingle‐mangle of Wnt signaling and extracellular vesicles: functional implications for heart research. Front Cardiovasc Med. 2018;5:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhuang M, Chen X, Du D, et al. SPION decorated exosome delivery of TNF‐α to cancer cell membranes through magnetism. Nanoscale. 2020;12(1):173‐188. [DOI] [PubMed] [Google Scholar]
- 33. Liu Q, Xiao Q, Sun Z, et al. Exosome component 1 cleaves single‐stranded DNA and sensitizes human kidney renal clear cell carcinoma cells to poly(ADP‐ribose) polymerase inhibitor. Elife. 2021;10:e69454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang L, Li Y, Guan X, Zhao J, Shen L, Liu J. Exosomal double‐stranded DNA as a biomarker for the diagnosis and preoperative assessment of pheochromocytoma and paraganglioma. Mol Cancer. 2018;17(1):128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jin Y, Chen K, Wang Z, et al. DNA in serum extracellular vesicles is stable under different storage conditions. BMC Cancer. 2016;16(1):753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Thakur BK, Zhang H, Becker A, et al. Double‐stranded DNA in exosomes: a novel biomarker in cancer detection. Cell Res. 2014;24(6):766‐769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cao M, Chen W. Epidemiology of lung cancer in China. Thorac Cancer. 2019;10(1):3‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fujimoto J, Wistuba II. Current concepts on the molecular pathology of non‐small cell lung carcinoma. Semin Diagn Pathol. 2014;31(4):306‐313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Altintas Z, Uludag Y, Gurbuz Y, Tothill IE. Surface plasmon resonance based immunosensor for the detection of the cancer biomarker carcinoembryonic antigen. Talanta. 2011;86:377‐383. [DOI] [PubMed] [Google Scholar]
- 40. Kim J, Hong SW, Kim S, et al. Cyclooxygenase‐2 expression is induced by celecoxib treatment in lung cancer cells and is transferred to neighbor cells via exosomes. Int J Oncol. 2018;52(2):613‐620. [DOI] [PubMed] [Google Scholar]
- 41. Zhang N, Nan A, Chen L, et al. Circular RNA circSATB2 promotes progression of non‐small cell lung cancer cells. Mol Cancer. 2020;19(1):101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Li L, Li W, Chen N, et al. FLI1 exonic circular RNAs as a novel oncogenic driver to promote tumor metastasis in small cell lung cancer. Clin Cancer Res. 2019;25(4):1302‐1317. [DOI] [PubMed] [Google Scholar]
- 43. Sia D, Villanueva A, Friedman SL, Llovet JM. Liver cancer cell of origin, molecular class, and effects on patient prognosis. Gastroenterology. 2017;152(4):745‐761. [DOI] [PubMed] [Google Scholar]
- 44. Center MM, Jemal A. International trends in liver cancer incidence rates. Cancer Epidemiol Biomark Prevent. 2011;20(11):2362‐2368. [DOI] [PubMed] [Google Scholar]
- 45. Lin Y, Deng W, Pang J, et al. The microRNA‐99 family modulates hepatitis B virus replication by promoting IGF‐1R/PI3K/Akt/mTOR/ULK1 signaling‐induced autophagy. Cell Microbiol. 2017;19(5):e12709. [DOI] [PubMed] [Google Scholar]
- 46. Wang S, Qiu L, Yan X, et al. Loss of microRNA 122 expression in patients with hepatitis B enhances hepatitis B virus replication through cyclin G(1) ‐modulated P53 activity. Hepatology. 2012;55(3):730‐741. [DOI] [PubMed] [Google Scholar]
- 47. Zhang GL, Li YX, Zheng SQ, Liu M, Li X, Tang H. Suppression of hepatitis B virus replication by microRNA‐199a‐3p and microRNA‐210. Antiviral Res. 2010;88(2):169‐175. [DOI] [PubMed] [Google Scholar]
- 48. Giray BG, Emekdas G, Tezcan S, et al. Profiles of serum microRNAs; miR‐125b‐5p and miR223‐3p serve as novel biomarkers for HBV‐positive hepatocellular carcinoma. Mol Biol Rep. 2014;41(7):4513‐4519. [DOI] [PubMed] [Google Scholar]
- 49. Hung CH, Hu TH, Lu SN, et al. Circulating microRNAs as biomarkers for diagnosis of early hepatocellular carcinoma associated with hepatitis B virus. Int J Cancer. 2016;138(3):714‐720. [DOI] [PubMed] [Google Scholar]
- 50. Zekri AN, Youssef AS, El‐Desouky ED, et al. Serum microRNA panels as potential biomarkers for early detection of hepatocellular carcinoma on top of HCV infection. Tumour Biol. 2016;37(9):12273‐12286. [DOI] [PubMed] [Google Scholar]
- 51. Ding D, Zhang Y, Yang R, et al. miR‐940 suppresses tumor cell invasion and migration via regulation of CXCR2 in hepatocellular carcinoma. Biomed Res Int. 2016;2016:7618342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Shi M, Jiang Y, Yang L, Yan S, Wang YG, Lu XJ. Decreased levels of serum exosomal miR‐638 predict poor prognosis in hepatocellular carcinoma. J Cell Biochem. 2018;119(6):4711‐4716. [DOI] [PubMed] [Google Scholar]
- 53. Wang L, Bo X, Zheng Q, Xiao X, Wu L, Li B. miR‐296 inhibits proliferation and induces apoptosis by targeting FGFR1 in human hepatocellular carcinoma. FEBS Lett. 2016;590(23):4252‐4262. [DOI] [PubMed] [Google Scholar]
- 54. Zhao Y, Li F, Zhang X, et al. MicroRNA‐194 acts as a prognostic marker and inhibits proliferation in hepatocellular carcinoma by targeting MAP4K4. Int J Clin Exp Pathol. 2015;8(10):12446‐12454. [PMC free article] [PubMed] [Google Scholar]
- 55. Smyth EC, Nilsson M, Grabsch HI, van Grieken NCT, Lordick F. Gastric cancer. Lancet. 2020;396(10251):635‐648. [DOI] [PubMed] [Google Scholar]
- 56. Tokuhisa M, Ichikawa Y, Kosaka N, et al. Exosomal miRNAs from peritoneum lavage fluid as potential prognostic biomarkers of peritoneal metastasis in gastric cancer. PLoS One. 2015;10(7):e0130472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Li C, Liu DR, Li GG, et al. CD97 promotes gastric cancer cell proliferation and invasion through exosome‐mediated MAPK signaling pathway. World J Gastroenterol. 2015;21(20):6215‐6228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sadri Nahand J, Moghoofei M, Salmaninejad A, et al. Pathogenic role of exosomes and microRNAs in HPV‐mediated inflammation and cervical cancer: a review. Int J Cancer. 2020;146(2):305‐320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Buschow SI, van Balkom BW, Aalberts M, Heck AJ, Wauben M, Stoorvogel W. MHC class II‐associated proteins in B‐cell exosomes and potential functional implications for exosome biogenesis. Immunol Cell Biol. 2010;88(8):851‐856. [DOI] [PubMed] [Google Scholar]
- 60. Chen S, Lv M, Fang S, Ye W, Gao Y, Xu Y. Poly(I:C) enhanced anti‐cervical cancer immunities induced by dendritic cells‐derived exosomes. Int J Biol Macromol. 2018;113:1182‐1187. [DOI] [PubMed] [Google Scholar]
- 61. Amador‐Molina A, Hernández‐Valencia JF, Lamoyi E, Contreras‐Paredes A, Lizano M. Role of innate immunity against human papillomavirus (HPV) infections and effect of adjuvants in promoting specific immune response. Viruses. 2013;5(11):2624‐2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Garcia‐Iglesias T, Del Toro‐Arreola A, Albarran‐Somoza B, et al. Low NKp30, NKp46 and NKG2D expression and reduced cytotoxic activity on NK cells in cervical cancer and precursor lesions. BMC Cancer. 2009;9:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Jimenez‐Perez MI, Jave‐Suarez LF, Ortiz‐Lazareno PC, et al. Cervical cancer cell lines expressing NKG2D‐ligands are able to down‐modulate the NKG2D receptor on NKL cells with functional implications. BMC Immunol. 2012;13:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Chang WC, Li CH, Chu LH, Huang PS, Sheu BC, Huang SC. Regulatory T cells suppress natural killer cell immunity in patients with human cervical carcinoma. Intl J Gynecol Cancer. 2016;26(1):156‐162. [DOI] [PubMed] [Google Scholar]
- 65. Pitt JM, Andre F, Amigorena S, et al. Dendritic cell‐derived exosomes for cancer therapy. J Clin Invest. 2016;126(4):1224‐1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Gupta SK, Tiwari AK, Gandham RK, Sahoo AP. Combined administration of the apoptin gene and poly (I:C) induces potent anti‐tumor immune response and inhibits growth of mouse mammary tumors. Int Immunopharmacol. 2016;35:163‐173. [DOI] [PubMed] [Google Scholar]
- 67. Ratajczak J, Miekus K, Kucia M, et al. Embryonic stem cell‐derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia. 2006;20(5):847‐856. [DOI] [PubMed] [Google Scholar]
- 68. Shurtleff MJ, Yao J, Qin Y, et al. Broad role for YBX1 in defining the small noncoding RNA composition of exosomes. Proc Natl Acad Sci USA. 2017;114(43):E8987‐E8995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wei Z, Batagov AO, Schinelli S, et al. Coding and noncoding landscape of extracellular RNA released by human glioma stem cells. Nat Commun. 2017;8(1):1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Honegger A, Schilling D, Bastian S, et al. Dependence of intracellular and exosomal microRNAs on viral E6/E7 oncogene expression in HPV‐positive tumor cells. PLoS Pathog. 2015;11(3):e1004712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Schageman J, Zeringer E, Li M, et al. The complete exosome workflow solution: from isolation to characterization of RNA cargo. Biomed Res Int. 2013;2013:253957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Zheng M, Hou L, Ma Y, et al. Exosomal let‐7d‐3p and miR‐30d‐5p as diagnostic biomarkers for non‐invasive screening of cervical cancer and its precursors. Mol Cancer. 2019;18(1):76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post‐transcriptional regulation by microRNAs: are the answers in sight? Nat Rev Genet. 2008;9(2):102‐114. [DOI] [PubMed] [Google Scholar]
- 74. Bartel D. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281‐297. [DOI] [PubMed] [Google Scholar]
- 75. Kim VN. MicroRNA biogenesis: coordinated cropping and dicing. Nat Rev Mol Cell Biol. 2005;6(5):376‐385. [DOI] [PubMed] [Google Scholar]
- 76. Ambros V. microRNAs: tiny regulators with great potential. Cell. 2001;107(7):823‐826. [DOI] [PubMed] [Google Scholar]
- 77. Filipowicz W, Jaskiewicz L, Kolb FA, Pillai RS. Post‐transcriptional gene silencing by siRNAs and miRNAs. Curr Opin Struct Biol. 2005;15(3):331‐341. [DOI] [PubMed] [Google Scholar]
- 78. Chen CZ, Li L, Lodish HF, Bartel DP. MicroRNAs modulate hematopoietic lineage differentiation. Science. 2004;303(5654):83‐86. [DOI] [PubMed] [Google Scholar]
- 79. Lecellier CH, Dunoyer P, Arar K, et al. A cellular microRNA mediates antiviral defense in human cells. Science (New York, NY). 2005;308(5721):557‐560. [DOI] [PubMed] [Google Scholar]
- 80. He L, Thomson JM, Hemann MT, et al. A microRNA polycistron as a potential human oncogene. Nature. 2005;435(7043):828‐833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Rupaimoole R, Calin GA, Lopez‐Berestein G, Sood AK. miRNA deregulation in cancer cells and the tumor microenvironment. Cancer Discov. 2016;6(3):235‐246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Lui WO, Pourmand N, Patterson BK, Fire A. Patterns of known and novel small RNAs in human cervical cancer. Cancer Res. 2007;67(13):6031‐6043. [DOI] [PubMed] [Google Scholar]
- 83. Lin S, Gregory RI. MicroRNA biogenesis pathways in cancer. Nat Rev Cancer. 2015;15(6):321‐333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Skotland T, Sandvig K, Llorente A. Lipids in exosomes: current knowledge and the way forward. Prog Lipid Res. 2017;66:30‐41. [DOI] [PubMed] [Google Scholar]
- 85. Khan S, Aspe JR, Asumen MG, et al. Extracellular, cell‐permeable survivin inhibits apoptosis while promoting proliferative and metastatic potential. Br J Cancer. 2009;100(7):1073‐1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Nosaka K, Suzuki S, Yoshikawa T, et al. Heat shock protein 105 as an immunotherapeutic target for patients with cervical cancer. Anticancer Res. 2021;41(10):4741‐4751. [DOI] [PubMed] [Google Scholar]
- 87. Tavakolian S, Goudarzi H, Moridi A, Faghihloo E. Analysing the HERV‐K env, np9, rec and gag expression in cervical tissues. New Microb New Infect. 2021;44:100936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Zeng J, He SL, Li LJ, Wang C. Hsp90 up‐regulates PD‐L1 to promote HPV‐positive cervical cancer via HER2/PI3K/AKT pathway. Mol Med (Cambridge, MA). 2021;27(1):130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Bhat A, Sharma A, Bharti AC. Upstream hedgehog signaling components are exported in exosomes of cervical cancer cell lines. Nanomedicine (Lond). 2018;13(17):2127‐2138. [DOI] [PubMed] [Google Scholar]
- 90. Xu Y, Zhou W, Zhang C, et al. Long non‐coding RNA RP11‐552M11.4 favors tumorigenesis and development of cervical cancer via modulating miR‐3941/ATF1 signaling. Int J Biol Macromol. 2019;130:24‐33. [DOI] [PubMed] [Google Scholar]
- 91. Huang GL, Liao D, Chen H, et al. The protein level and transcription activity of activating transcription factor 1 is regulated by prolyl isomerase Pin1 in nasopharyngeal carcinoma progression. Cell Death Dis. 2016;7(12):e2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Wang H, Zhu Y, Chen H, et al. Colorectal cancer risk variant rs7017386 modulates two oncogenic lncRNAs expression via ATF1‐mediated long‐range chromatin loop. Cancer Lett. 2021;518:140‐151. [DOI] [PubMed] [Google Scholar]
- 93. Wang WL, Mayordomo E, Zhang W, et al. Detection and characterization of EWSR1/ATF1 and EWSR1/CREB1 chimeric transcripts in clear cell sarcoma (melanoma of soft parts). Mod Pathol. 2009;22(9):1201‐1209. [DOI] [PubMed] [Google Scholar]
- 94. Shi Y, Wang W, Yang B, Tian H. ATF1 and RAS in exosomes are potential clinical diagnostic markers for cervical cancer. Cell Biochem Funct. 2017;35(7):477‐483. [DOI] [PubMed] [Google Scholar]
- 95. Moore AR, Rosenberg SC, McCormick F, Malek S. RAS‐targeted therapies: is the undruggable drugged? Nat Rev Drug Discov. 2020;19(8):533‐552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Tan C, Du X. KRAS mutation testing in metastatic colorectal cancer. World J Gastroenterol. 2012;18(37):5171‐5180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Califf RM. Biomarker definitions and their applications. Exp Biol Med (Maywood). 2018;243(3):213‐221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Weber JA, Baxter DH, Zhang S, et al. The microRNA spectrum in 12 body fluids. Clin Chem. 2010;56(11):1733‐1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Syn NL, Wang L, Chow EK, Lim CT, Goh BC. Exosomes in cancer nanomedicine and immunotherapy: prospects and challenges. Trends Biotechnol. 2017;35(7):665‐676. [DOI] [PubMed] [Google Scholar]
- 100. Yang Y, Hong Y, Cho E, Kim GB, Kim IS. Extracellular vesicles as a platform for membrane‐associated therapeutic protein delivery. J Extracell Ves. 2018;7(1):1440131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Chen M, Liu Y, Varley P, et al. High‐mobility group box 1 promotes hepatocellular carcinoma progression through miR‐21‐mediated matrix metalloproteinase activity. Cancer Res. 2015;75(8):1645‐1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Kalogirou C, Schäfer D, Krebs M, et al. Metformin‐derived growth inhibition in renal cell carcinoma depends on miR‐21‐mediated PTEN expression. Urol Int. 2016;96(1):106‐115. [DOI] [PubMed] [Google Scholar]
- 103. Lin TC, Lin PL, Cheng YW, et al. MicroRNA‐184 deregulated by the MicroRNA‐21 promotes tumor malignancy and poor outcomes in non‐small cell lung cancer via targeting CDC25A and c‐Myc. Ann Surg Oncol. 2015;22:S1532‐S1539. [DOI] [PubMed] [Google Scholar]
- 104. Yang CH, Pfeffer SR, Sims M, et al. The oncogenic microRNA‐21 inhibits the tumor suppressive activity of FBXO11 to promote tumorigenesis. J Biol Chem. 2015;290(10):6037‐6046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Gocze K, Gombos K, Kovacs K, Juhasz K, Gocze P, Kiss I. MicroRNA expressions in HPV‐induced cervical dysplasia and cancer. Anticancer Res. 2015;35(1):523‐530. [PubMed] [Google Scholar]
- 106. Yang L, Wang YL, Liu S, et al. miR‐181b promotes cell proliferation and reduces apoptosis by repressing the expression of adenylyl cyclase 9 (AC9) in cervical cancer cells. FEBS Lett. 2014;588(1):124‐130. [DOI] [PubMed] [Google Scholar]
- 107. Zhao J, Li B, Shu C, Ma Y, Gong Y. Downregulation of miR‐30a is associated with proliferation and invasion via targeting MEF2D in cervical cancer. Oncol Lett. 2017;14(6):7437‐7442. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 108. Xu L, Xu Q, Li X, Zhang X. MicroRNA‐21 regulates the proliferation and apoptosis of cervical cancer cells via tumor necrosis factor‐α. Mol Med Rep. 2017;16(4):4659‐4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Zamani S, Hosseini SM, Sohrabi A. miR‐21 and miR29‐a: potential molecular biomarkers for HPV genotypes and cervical cancer detection. MicroRNA (Shariqah, United Arab Emirates). 2020;9(4):271‐275. [DOI] [PubMed] [Google Scholar]
- 110. Nahand JS, Mahjoubin‐Tehran M, Moghoofei M, et al. Exosomal miRNAs: novel players in viral infection. Epigenomics. 2020;12(4):353‐370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Liang LJ, Yang Y, Wei WF, et al. Tumor‐secreted exosomal Wnt2B activates fibroblasts to promote cervical cancer progression. Oncogenesis. 2021;10(3):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Zhang W, Zhou Q, Wei Y, et al. The exosome‐mediated PI3k/Akt/mTOR signaling pathway in cervical cancer. Int J Clin Exp Pathol. 2019;12(7):2474‐2484. [PMC free article] [PubMed] [Google Scholar]
- 113. Bhat A, Yadav J, Thakur K, et al. Exosomes from cervical cancer cells facilitate pro‐angiogenic endothelial reconditioning through transfer of hedgehog‐GLI signaling components. Cancer Cell Int. 2021;21(1):319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Howie HL, Katzenellenbogen RA, Galloway DA. Papillomavirus E6 proteins. Virology. 2009;384(2):324‐334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Lajer CB, Garnaes E, Friis‐Hansen L, et al. The role of miRNAs in human papilloma virus (HPV)‐associated cancers: bridging between HPV‐related head and neck cancer and cervical cancer. Br J Cancer. 2017;117(5):e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Roman A, Munger K. The papillomavirus E7 proteins. Virology. 2013;445(1–2):138‐168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Wang X, Wang HK, Li Y, et al. microRNAs are biomarkers of oncogenic human papillomavirus infections. Proc Natl Acad Sci USA. 2014;111(11):4262‐4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Zur Hausen H. Papillomaviruses causing cancer: evasion from host‐cell control in early events in carcinogenesis. J Natl Cancer Inst. 2000;92(9):690‐698. [DOI] [PubMed] [Google Scholar]
- 119. Park S, Kim J, Eom K, et al. microRNA‐944 overexpression is a biomarker for poor prognosis of advanced cervical cancer. BMC Cancer. 2019;19(1):419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Zhang YX, Qin LL, Yang SY. Down‐regulation of miR‐664 in cervical cancer is associated with lower overall survival. Eur Rev Med Pharmacol Sci. 2016;20(9):1740‐1744. [PubMed] [Google Scholar]
- 121. Ponting CP, Oliver PL, Reik W. Evolution and functions of long noncoding RNAs. Cell. 2009;136(4):629‐641. [DOI] [PubMed] [Google Scholar]
- 122. Ding XZ, Zhang SQ, Deng XL, Qiang JH. Serum Exosomal lncRNA DLX6‐AS1 is a promising biomarker for prognosis prediction of cervical cancer. Technol Cancer Res Treat. 2021;20:1533033821990060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Lv A, Tu Z, Huang Y, Lu W, Xie B. Circulating exosomal miR‐125a‐5p as a novel biomarker for cervical cancer. Oncol Lett. 2021;21(1):54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Xie F, Xie G, Sun Q. DLX6‐AS1Long noncoding RNA promotes the progression in cervical cancer by targeting Axis. Cancer Biother Radiopharm. 2020;35(2):129‐136. [DOI] [PubMed] [Google Scholar]
- 125. Huang X, Liu X, Du B, et al. LncRNA LINC01305 promotes cervical cancer progression through KHSRP and exosome‐mediated transfer. Aging. 2021;13:19230‐19242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Xu J, Yang B, Wang L, et al. LncRNA BBOX1‐AS1 upregulates HOXC6 expression through miR‐361‐3p and HuR to drive cervical cancer progression. Cell Prolif. 2020;53(7):e12823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. You X, Wang Y, Meng J, et al. Exosomal miR‐663b exposed to TGF‐β1 promotes cervical cancer metastasis and epithelial‐mesenchymal transition by targeting MGAT3. Oncol Rep. 2021;45(4):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Guo Q, Zhang Q, Lu L, Xu Y. RUSC1‐AS1Long noncoding RNA promotes tumorigenesis in cervical cancer by acting AS a competing endogenous RNA of microRNA‐744 and consequently increasing Bcl‐2 expression. Cell Cycle (Georgetown, Tex). 2020;19(10):1222‐1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Wang H, Wei M, Kang Y, Xing J, Zhao Y. Circular RNA circ_PVT1 induces epithelial‐mesenchymal transition to promote metastasis of cervical cancer. Aging. 2020;12(20):20139‐20151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Zhang L, Li H, Yuan M, Li M, Zhang S. Cervical cancer cells‐secreted Exosomal microRNA‐221‐3p promotes invasion, migration and angiogenesis of microvascular endothelial cells in cervical cancer by down‐regulating MAPK10 expression. Cancer Manag Res. 2019;11:10307‐10319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Kok VC. Current understanding of the mechanisms underlying immune evasion from PD‐1/PD‐L1 immune checkpoint blockade in head and neck cancer. Front Oncol. 2020;10:268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Wu XG, Zhou CF, Zhang YM, et al. Cancer‐derived exosomal miR‐221‐3p promotes angiogenesis by targeting THBS2 in cervical squamous cell carcinoma. Angiogenesis. 2019;22(3):397‐410. [DOI] [PubMed] [Google Scholar]
- 133. Zhou C, Wei W, Ma J, et al. Cancer‐secreted exosomal miR‐1468‐5p promotes tumor immune escape via the immunosuppressive reprogramming of lymphatic vessels. Mol Ther. 2021;29(4):1512‐1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Ren G, Wang Y, Yuan S, Wang B. Dendritic cells loaded with HeLa‐derived exosomes simulate an antitumor immune response. Oncol Lett. 2018;15(5):6636‐6640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Cafforio P, Palmirotta R, Lovero D, et al. Liquid biopsy in cervical cancer: hopes and pitfalls. Cancer. 2021;13(16):3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Pan ZX, Zhang XY, Chen SR, Li CZ. Upregulated exosomal miR‐221/222 promotes cervical cancer via repressing methyl‐CpG‐binding domain protein 2. Eur Rev Med Pharmacol Sci. 2019;23(9):3645‐3653. [DOI] [PubMed] [Google Scholar]
- 137. Liu J, Sun H, Wang X, et al. Increased exosomal microRNA‐21 and microRNA‐146a levels in the cervicovaginal lavage specimens of patients with cervical cancer. Int J Mol Sci. 2014;15(1):758‐773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Konishi H, Hayashi M, Taniguchi K, et al. The therapeutic potential of exosomal miR‐22 for cervical cancer radiotherapy. Cancer Biol Ther. 2020;21(12):1128‐1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. He J, Huang B, Zhang K, Liu M, Xu T. Long non‐coding RNA in cervical cancer: from biology to therapeutic opportunity. Biomed Pharmacother. 2020;127:110209. [DOI] [PubMed] [Google Scholar]
- 140. Esfandyari S, Elkafas H, Chugh RM, Park HS, Navarro A, Al‐Hendy A. Exosomes as biomarkers for female reproductive diseases diagnosis and therapy. Int J Mol Sci. 2021;22(4):2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Raji GR, Sruthi TV, Edatt L, Haritha K, Sharath Shankar S, Sameer Kumar VB. Horizontal transfer of miR‐106a/b from cisplatin resistant hepatocarcinoma cells can alter the sensitivity of cervical cancer cells to cisplatin. Cell Sign. 2017;38:146‐158. [DOI] [PubMed] [Google Scholar]
- 142. Ma G, Song G, Zou X, et al. Circulating plasma microRNA signature for the diagnosis of cervical cancer. Cancer Biomark. 2019;26(4):491‐500. [DOI] [PubMed] [Google Scholar]
- 143. Luo X, Wei J, Yang FL, et al. Exosomal lncRNA HNF1A‐AS1 affects cisplatin resistance in cervical cancer cells through regulating microRNA‐34b/TUFT1 axis. Cancer Cell Int. 2019;19:323. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 144. Tong F, Mao X, Zhang S, et al. HPV + HNSCC‐derived exosomal miR‐9 induces macrophage M1 polarization and increases tumor radiosensitivity. Cancer Lett. 2020;478:34‐44. [DOI] [PubMed] [Google Scholar]
- 145. Hasanzadeh M, Movahedi M, Rejali M, et al. The potential prognostic and therapeutic application of tissue and circulating microRNAs in cervical cancer. J Cell Physiol. 2019;234(2):1289‐1294. [DOI] [PubMed] [Google Scholar]
- 146. Mendaza S, Fernández‐Irigoyen J, Santamaría E, et al. Understanding the molecular mechanism of miR‐877‐3p could provide potential biomarkers and therapeutic targets in squamous cell carcinoma of the cervix. Cancer. 2021;13(7):1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Li H, Chi X, Li R, Ouyang J, Chen Y. HIV‐1‐infected cell‐derived exosomes promote the growth and progression of cervical cancer. Int J Biol Sci. 2019;15(11):2438‐2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Wu Y, Wang X, Meng L, et al. Changes of miRNA expression profiles from cervical‐vaginal fluid‐derived exosomes in response to HPV16 infection. Biomed Res Int. 2020;2020:7046894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Pourhanifeh MH, Darvish M, Tabatabaeian J, et al. Therapeutic role of curcumin and its novel formulations in gynecological cancers. J Ovar Res. 2020;13(1):130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Kannan A, Hertweck KL, Philley JV, Wells RB, Dasgupta S. Genetic mutation and exosome signature of human papilloma virus associated oropharyngeal cancer. Sci Rep. 2017;7:46102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Lin Y, Zhang C, Xiang P, Shen J, Sun W, Yu H. Exosomes derived from HeLa cells break down vascular integrity by triggering endoplasmic reticulum stress in endothelial cells. J Extracell Ves. 2020;9(1):1722385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Yao S, Xu J, Zhao K, et al. Down‐regulation of HPGD by miR‐146b‐3p promotes cervical cancer cell proliferation, migration and anchorage‐independent growth through activation of STAT3 and AKT pathways. Cell Death Dis. 2018;9(11):1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Fan Z, Cui H, Xu X, et al. MiR‐125a suppresses tumor growth, invasion and metastasis in cervical cancer by targeting STAT3. Oncotarget. 2015;6(28):25266‐25280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Zhu W, Li L, Li D. Rs11655237 polymorphism of LINC00673 affects the prognosis of cervical cancer by interfering with the interaction between LINC00673 and microRNA‐1231. J Cell Physiol. 2020;235(11):8155‐8166. [DOI] [PubMed] [Google Scholar]
- 155. Harden ME, Munger K. Human papillomavirus 16 E6 and E7 oncoprotein expression alters microRNA expression in extracellular vesicles. Virology. 2017;508:63‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Zhou MJ, Chen FZ, Chen HC, et al. ISG15 inhibits cancer cell growth and promotes apoptosis. Int J Mol Med. 2017;39(2):446‐452. [DOI] [PubMed] [Google Scholar]
- 157. Prabhavathy D, Subramanian CK, Karunagaran D. Re‐expression of HPV16 E2 in SiHa (human cervical cancer) cells potentiates NF‐κB activation induced by TNF‐α concurrently increasing senescence and survival. Biosci Rep. 2015;35(1):e00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Kim SH, Oh JM, No JH, Bang YJ, Juhnn YS, Song YS. Involvement of NF‐kappaB and AP‐1 in COX‐2 upregulation by human papillomavirus 16 E5 oncoprotein. Carcinogenesis. 2009;30(5):753‐757. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.


