Graphical abstract
Keywords: Remdesivir, BPB, Spectrophotometry, Computational
Abstract
Remdesivir was approved by the Food and Drug Administration for the treatment of COVID −19 in hospitalized adult and pediatric patients. Application of computational calculations for choosing the sensitive reagent in spectrophotometric quantitative analysis is very limited. Computational and theoretical studies were used for choosing the best acid dye for selective visible spectrophotometric quantitative analysis of remdesivir. The calculations were performed using Gaussian 03 software with the density functional theory method using B3LYP/6-31G(d) basis set. The theoretical studies revealed that bromophenol blue is a better match for remdesivir than other acid dyes due to the higher calculated interaction energy. The proposed method was based on the reaction of remdesivir with the computationally selected acid dye bromophenol blue to form a yellow ion-pair complex. The spectra showed absorption peaks at 418 nm. Various factors affecting the reaction were optimized. The method was successfully applied for the determination of remdesivir in the pharmaceutical preparation with good accuracy and precision. Beer's law was observed in the concentration range of 2–12 μg/mL of remdesivir. The proposed reaction was used as a basis for the spectrophotometric determination of remdesivir in pure form and in the pharmaceutical preparation.
1. Introduction
The new global coronavirus disease 2019, [COVD-19] is mainly caused by the severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2]. COVD-19 caused severe catastrophic impact on the world's population, resulting in more than 3.8 million deaths worldwide. SARS-CoV-2 is spreading rapidly across the world after the first case of this viral disease was demonstrated in Wuhan, Hubei Province, China, in late December 2019. This promoted the WHO to declare a global pandemic on March 11, 2020 [1], [2]. The rapid spread and devastating effects of COVID-19 promoted researchers around the world to discover antiviral drugs that could control the spread of the virus and help patients recover more quickly. Because the process to approve a novel drug for human use is lengthy and involves numerous phases to collect safety data and identify potential hazards, the easiest and fastest option was to use FDA-approved drugs such as favipiravir, remdesivir and lopinavir/ritonavir [3].
Remdesivir [RV], Fig. 1 , is a nucleotide prodrug of an adenosine analog. It binds to viral RNA-dependent RNA polymerase and inhibits viral replication by premature termination of RNA transcription. RV has shown activity against SARS-CoV-2 in vitro. Intravenous RV is approved by the FDA for the treatment of COVID −19 in hospitalized adult and pediatric patients (aged ≥ 12 years and weighing ≥ 40 kg). It is also available via European emergency approval for the treatment of COVID −19 in hospitalized pediatric patients weighing 3.5 kg to < 40 kg or aged < 12 years and weighing ≥ 3.5 kg. It should be administered in a hospital or health care facility that can provide a similar level of care as an inpatient hospital [4], [5], [6].
Fig. 1.
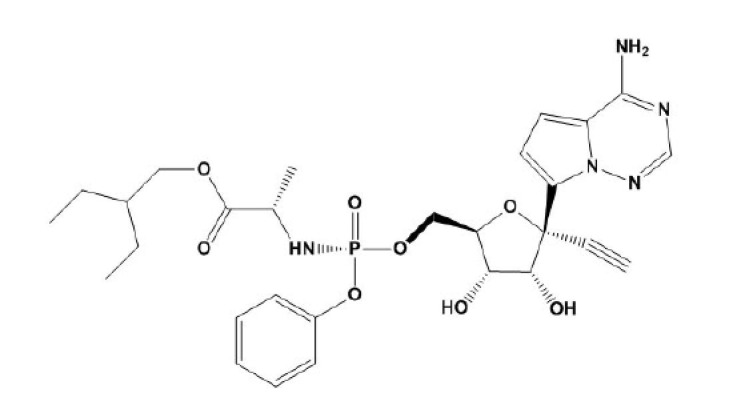
Chemical structure of RV.
Quantitative analysis of compounds based on the structural properties remains an attractive approach for researchers and chemists. In general, many pharmaceutical compounds have different functional groups which can enable selective quantitative analysis [7]. In recent decades, many color-based spectrophotometric methods have been developed for the quantitative analysis of pharmaceutical products. Extractive spectrophotometric methods using various acid dyes are commonly used for the determination of many drugs. There are universally different acid dyes, bromothymol blue [BTB], bromophenol blue [BPB] and bromocresol green [BCG] [8], [9], [10]. Despite the popularity of this technique, the selection of the most suitable acid dye requires many experimental trials, as already published in many articles. Moreover, this recommends the consumption of many chemicals, high cost and long analysis time.
Computational chemistry offers good approach for accurate predictions before the actual experiments are performed. In general, computational calculations provide complementary data to the experimental data [11], [12]. With the progress of computational studies, the authors believed to introduce specific computational and theoretical studies for choosing the sensitive acid dye for a more precise color-based spectrophotometric method.
Since RV is new to the market, there are few published quantitative analytical methods [14], [15]. This work introduces computer-aided studies to select the best acid dye for selective visible spectrophotometric quantitative analysis of RV. The proposed method was based on the reaction of RV with BPB to form a yellow ion-pair complex. The reaction was used as a basis for the spectrophotometric determination of RV in the pure and pharmaceutical preparations.
2. Experimental
2.1. Materials and solvents
Pure pharmaceutical powder of RV [98.36%] and Remdesivir® vials of 100 mg RV were kindly provided by Pharmakeda Health Company, Cairo, Egypt. BPB, [Brixworth northants, U.K.], was prepared as 0.1% and 1.37x10-3 M by dissolving BPB in a small amount of ethanol and making up with water. Ethanol, methanol, sodium hydroxide, potassium acid phthalate, hydrochloric acid, chloroform, carbon tetrachloride, methylene chloride and dichloroethane were kindly provided by El-Nasr Company, Cairo, Egypt. Potassium acid phthalate buffer, pH [1.2–5] prepared by mixing 50 mL of 0.2 M potassium hydrogen phthalate with different volumes of 0.2 M hydrochloric acid and diluting to 200 mL with water.
2.2. Apparatus
Shimadzu UV–Visible 1650 Spectrophotometer, Tokyo, Japan.
2.3. Standard solutions
A standard stock solution of RV [500 μg/mL] was prepared by dissolving 50 mg of the drug powder in 50 mL methanol, and the volume was made up to 100 mL with methanol.
2.4. Procedures
(a)Computational and energy calculations procedures.
Computational approach was done to decrease the number of experimental trials and select the sensitive acid dye. Calculations were performed using Gaussian 03 software with the density functional theory method based on B3LYP/6-31G(d). The structure of RV, BCG, BPB and BTB was drawn individually in Gauss-view software. The compounds were computationally optimized and the energy in the optimized conformations was calculated. Also, the binding energy of interaction between RV and various acid dyes, ΔE, was calculated using the following equation [11]:
Where A is RV calculated optimized energy and B is the acid dye calculated optimized energy. The binding energy of the RV with the cited acid dyes is calculated as a measure of their interaction.
(b)Spectrophotometric procedure.
Aliquots of RV [500 μg/mL] corresponding to [50–300 μg] were transferred to series of 125-mL separator funnels. Then, 5 mL of phthalate buffer, pH 2.4, and then 4 mL of 0.1% BPB solutions were added sequentially. The aqueous layer was adjusted to 15 mL with water and extracted with 25 mL of chloroform by shaking for about 30 sec. The extracts were collected in 25 mL volumetric flasks and the volumes were adjusted to 25 mL with chloroform. The absorbance values of the developed yellow color were measured against a reagent blank at 418 nm.
(c)Procedure for quantitative spectrophotometric analysis of the tablets.
Five Remdesivir® vials were weighed and appropriately mixed. An appropriate weight of powder equivalent to two vials was accurately weighed, transferred to 100-mL volumetric flask and the volume made up to 50 mL with methanol. The solution was shaken vigorously for 30 min and filtered. The volume was made up to 100 mL with methanol to prepare a stock solution containing 2 mg/mL RV. Various concentrations were prepared and quantitatively analyzed using the method described.
3. Results and discussion
Computational calculations help to drastically reduce the number of possible experimental trials for color-based spectrophotometric determination of a given compound. RV is a pharmaceutical compound with a proposed structural properties that could be analyzed colorimetrically by an acid dye complex reaction. In the present work, computational studies were carried out to select the sensitive acid dye for RV. Then, the selected acid dye was used for the color-based spectrophotometric determination of RV in pure form and in the pharmaceutical preparation. The method described was based on the formation of a colored ion pair complex upon the reaction of RV with BPB. The yellow ion pair complex was extracted with chloroform and the absorption spectra were recorded. The reaction was used as a basis for spectrophotometric quantitative analysis of RV in various forms.
3.1. Computational studies for selection of the best acid dye
Experimentally trying out the various reagents involved in a chemical reaction is both time consuming and expensive. It also requires the consumption of many chemicals, high cost and long analysis time. Computational calculations have the ability to generate theoretical data complement the experimental data. Computational calculations help researcher to get predictions before running the actual experiments. The calculations are based on many theories including study of quantum and classical mechanics, molecular dynamics, statistical theory and semi empirical structure-properties relationships [16]. The density functional theory [DFT] is considered the most successful and the promising tool to calculate the electronic structure of matter. DFT predicts a great variety of molecular properties including molecular structures, vibrational frequencies, electrostatic interaction energies, electric and magnetic properties. DFT enables the facility to derive the real properties of the molecule based on a determination of the electron density. Unlike the wave function, which is not a physical reality but a mathematical construct. It was believed that if we can determine the electron density of a molecule, we can discover numerous things about the molecule [17], [18].
To evaluate the strength of an interaction of RV with different acid dyes, BTB, BPB and BCG, calculations were performed to select the highly interactive acid dye recommended for color-based spectrophotometric quantitative analysis of RV. The calculations were performed using the DFT method at the B3LYP/6-31G (d) level. To obtain good estimates of the interaction energy, basis sets with polarization functions on all the atoms must be used. The calculations rapidly increase with the size of the basis set. For this reason, the medium size 6-31G basis sets are most frequently used with biomolecules. is considered to be standard model chemistry for many applications, particular good for organic molecules and clearly provide better results for reaction chemistry calculations [19].
Geometric optimization is the most important step in calculating the energy of the studied compounds. The optimization of RV, BTB, BPB and BCG as well as the formed complexes was carried out by calculating the minimized energy of the respective structures. The interaction energy for the complexes formed between RV and the acid dyes under study was calculated, Table 1 . The results confirmed that the higher stability existed for the complex formed between RV and BPB. Based on the proposed theoretical calculation, BPB could possibly be used as a selective acid dye and provide the highest efficiency for color-based spectrophotometric quantitative analysis of RV.
Table 1.
Computational study of the interaction energy between different acid dyes and RV.
| Name of complex | Interaction energy |
|
|---|---|---|
| ΔE(Hartree) * |
ΔE (KJ/mol) |
|
| RV-BPB | −0.00803 | −21.11 |
| RVS-BCG | −0.00462 | −12.13 |
| RV-BTB | −0.00282 | −7.41 |
*1 Hartree = 2625.5 KJ / mol.
3.2. Spectral characteristics
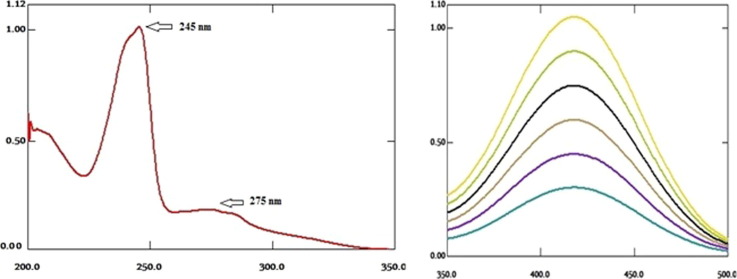
Most drug molecules have UV absorbance activity and exhibit defined UV spectra. Direct UV absorption spectra of RV was done. The spectra showed two maximum absorption peaks at 245 and 275 nm, Fig. 2 . RV reacted with BPB, Fig. 3 , and formed ion-pair complex. The formed yellow ion-pair complex was extracted and quantitatively analyzed. The absorption spectra of the formed complex, Fig. 4 , displayed absorption peaks at 418 nm. Neither RV nor the BPB exhibited any significant absorption at the described wavelength under the same conditions.
Fig. 2.
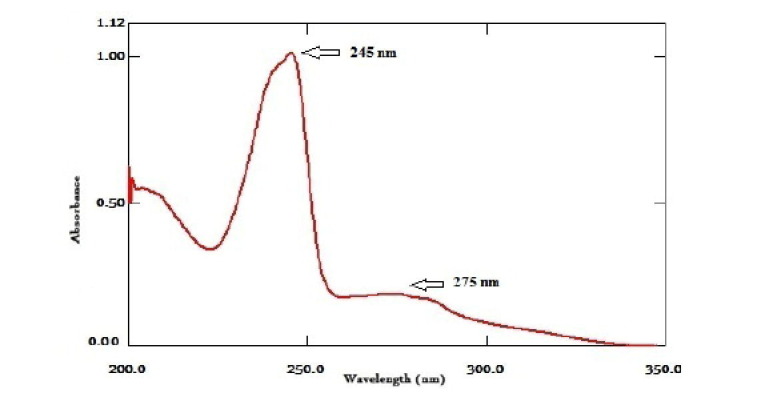
The absorption spectra of 12 µg/mL RV with two absorbance peaks at 245 and 275 nm.
Fig. 3.
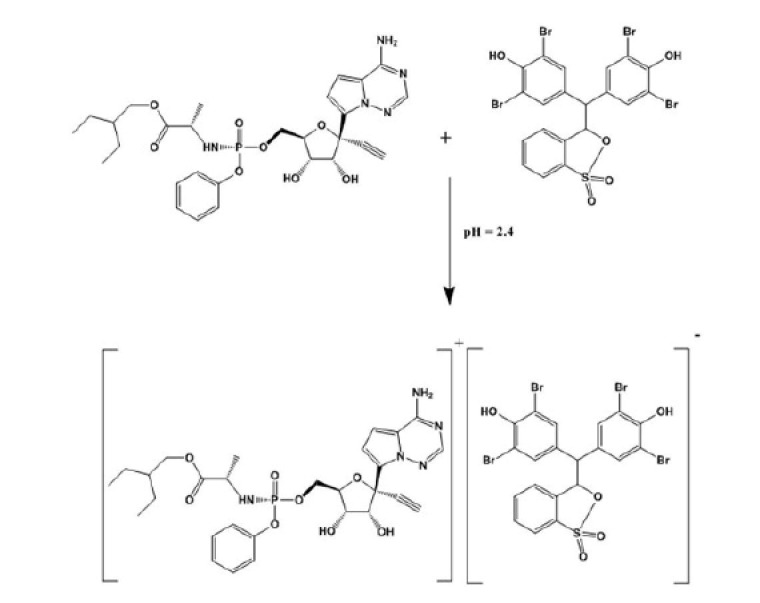
The schematic diagram for the reaction between RV and BPB.
Fig. 4.
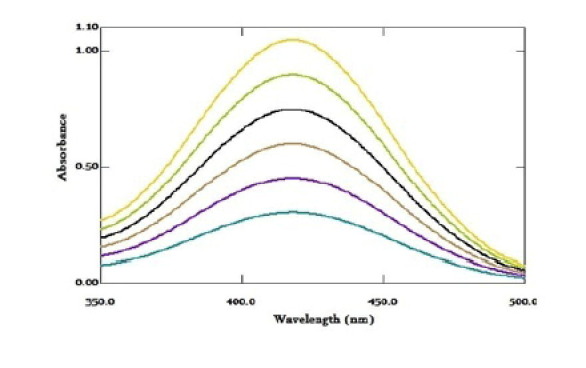
Absorption spectra for the formed yellow ion-pair complex between RV (2–12 µg/mL) and BPP acid dye.
3.3. Optimization of the experimental conditions
The factors affecting the sensitivity of the method described were optimized. During these studies, fixed concentrations of RV were used and each factor was changed separately while the others were held constant. Various factors were tested. Buffer was required for efficient extraction of the cited drug. Addition of 5 mL of phthalate buffer with pH 2.4 was optimum for complete color development, Fig. 5 (a) and 5 (b). Moreover, the volume of BPB should be sufficient to achieve maximum absorption. It was found that 4 mL of 0.1% BPB was optimum for enhancing the extraction of RV, Fig. 5 (c). The effect of different extraction solvents was tried; chloroform was found to be the ideal solvent for extraction of the complexes formed, Fig. 5 (d). Extraction with 25 mL of chloroform was necessary to obtain quantitative recoveries of the complex formed.
Fig. 5.
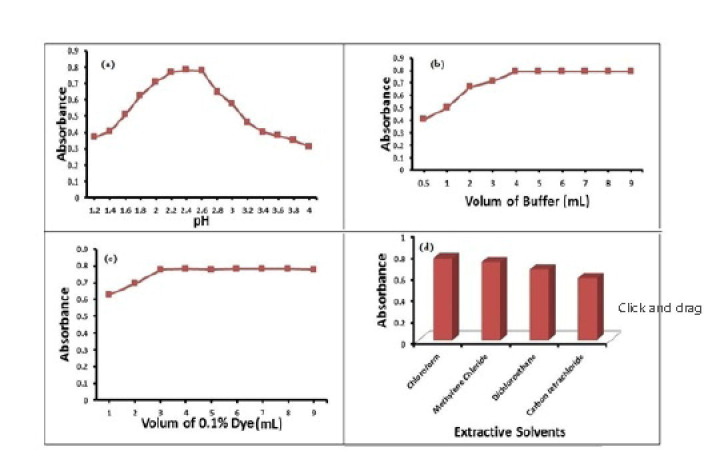
Optimization for different factors affecting the reaction of RV with BPB.
3.4. Determination of stoichiometric ratio of the reaction by using molar ratio method
Molar ratio method [20] was applied to assess the stoichiometric ratio for the reaction between RV and BPB. The general procedure was applied using 1 mL of RV solution [1.37 × 10-3 M] and different volumes [0.5–6 mL] BPB solutions [1.37 × 10-3 M]. By applying the molar ratio. The results indicate that, RV reacts with BPB in ratio 1: 1, Fig. 6 .
Fig. 6.
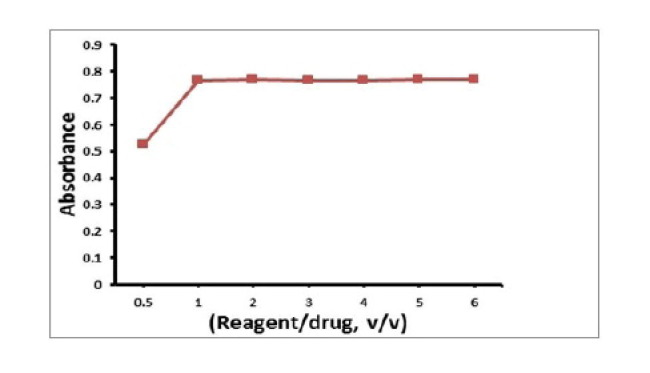
Stoichiometry of the reaction of [1.37 X 10-3 M] RV with [1.37 X 10-3 M] BPB by molar ratio method.
3.5. Validation of the method
The proposed method was validated according to the guidelines of ICH. The evaluation of the regression parameters was presented in Table 2 . The accuracy, expressed as mean percentage recovery [%R], was determined by applying the method for triplicate quantitative analysis of [4, 8, 12 µg/mL] RV. The %R was calculated and presented in Table 2. Precision, expressed as percent relative standard deviation [%RSD], was determined by quantitative analysis of [4, 8, 12 µg/mL] RV. For repeatability, it was performed within one day, and for mean precision, it was performed on three consecutive days. The calculated value of %RSD indicates high precision of the method as shown in Table 2.
Table 2.
Regression and validation parameters for the proposed spectrophotometric quantitative analysis of RV.
| Parameters | RV |
|---|---|
| Wavelength (nm) | 418 |
| Linearity range (μg/mL) | 2─ 12 |
| Slope | 0.0421 |
| Intercept | 0.0012 |
| Coefficient of determination (r2) | 0.9998 |
| Accuracy (%R)a | 99.08 |
| Repeatability precision (RSD)b | 0.752 |
| Intermediate precision (RSD)b | 0.813 |
Average of 9 determinations (3 concentrations repeated 3 times).
RSD of 9 determinations (3 concentrations repeated 3 times).
3.6. Application of the proposed method for quantitative analysis of pharmaceutical preparation
The proposed method was applied to the color-based spectrophotometric quantitative analysis of RV in Remdesivir® vials. The results were statistically compared with those obtained by the reported method [13]. By applying t-test and F-test, no significant differences were found at 95% confidence level, indicating the acceptability of the described methods for color-based spectrophotometric quantitative determination of RV in its pharmaceutical dosage form, Table 3 .
Table 3.
Application of the proposed method for spectrophotometric quantitative analysis of RV with statistical analysis of the data obtained with other data obtained by published method in the pharmaceutical preparation.
| Parameter | Proposed method | Reported method [13] |
|---|---|---|
| Meana | 100.13 | 100.33 |
| SDa | 1.064 | 1.399 |
| Variancea | 1.1236 | 1.959 |
| Student’s t-test (2.306)b | 0.249 | |
| F-test (6.388)b | 1.729 |
Measurements for five samples.
The values in the parenthesis are the corresponding theoretical values of t and F at (P = 0.05).
4. Conclusion
In this paper, computational and theoretical studies were done to select preferred acid dye reagent expected to yield the highest efficiency for the color based spectrophotometric quantitative analysis of remdesivir. The results revealed that bromophenol blue fits better with remdesivir than other acid dye reagents based on the high calculated interaction energy. The described method was based on the reaction of remdesivir with the computationally selected bromophenol blue acid dye to form yellow ion-pair complex. The reaction was utilized as a basis for the spectrophotometric determination of remdesivir in the pure and in the pharmaceutical preparation.
CRediT authorship contribution statement
Ahmed H. Abdelazim: Conceptualization, Methodology, Writing – original draft. Sherif Ramzy: Methodology.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.K. Yuki, M. Fujiogi, S. Koutsogiannaki, COVID-19 pathophysiology: A review, Clinical immunology, 215 (2020) 108427. [DOI] [PMC free article] [PubMed]
- 2.Hafeez A., Ahmad S., Siddqui S.A., Ahmad M., Mishra S. A review of COVID-19 (Coronavirus Disease-2019) diagnosis, treatments and prevention. EJMO. 2020;4:116–125. [Google Scholar]
- 3.Yavuz S., Ünal S. Antiviral treatment of COVID-19. Turkish J. Medical Sci. 2020;50:611–619. doi: 10.3906/sag-2004-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.R.E. Ferner, J.K. Aronson, Remdesivir in covid-19, in, British Medical J. Publishing Group, 2020.
- 5.Norrie J.D. Remdesivir for COVID-19: challenges of underpowered studies. The Lancet. 2020;395(10236):1525–1527. doi: 10.1016/S0140-6736(20)31023-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCreary E.K., Angus D.C. Efficacy of remdesivir in COVID-19. JAMA. 2020;324:1041–1042. doi: 10.1001/jama.2020.16337. [DOI] [PubMed] [Google Scholar]
- 7.Bonfilio R., De Araujo M.B., Salgado H.R.N. Recent applications of analytical techniques for quantitative pharmaceutical analysis: a review. WSEAS Trans. Biology Biomedicine. 2010;7:316–338. [Google Scholar]
- 8.Erk N. Extractive spectrophotometric determination of atorvastatin in bulk and pharmaceutical formulations. Anal. Lett. 2003;36:2699–2711. [Google Scholar]
- 9.Rahman N., Khan N.A., Azmi S.N.H. Extractive spectrophotometric methods for the determination of nifedipine in pharmaceutical formulations using bromocresol green, bromophenol blue, bromothymol blue and eriochrome black T. Il Farmaco. 2004;59:47–54. doi: 10.1016/j.farmac.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen T.D., Le H.B., Dong T.O., Pham T.D. Determination of fluoroquinolones in pharmaceutical formulations by extractive spectrophotometric methods using ion-pair complex formation with bromothymol blue. J. Analytical Methods Chemistry. 2018;2018:1–11. doi: 10.1155/2018/8436948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Attia K.A.M., El-Abasawi N.M., Abdelazim A.H. Colorimetric estimation of alfuzosin hydrochloride in pharmaceutical preparation based on computational studies. Anal. Methods. 2016;8(8):1798–1805. [Google Scholar]
- 12.E. Lewars, Computational chemistry, Introduction to the theory and applications of molecular and quantum mechanics, (2011) 318.
- 13.Du P., Wang G., Yang S., Li P., Liu L. Quantitative HPLC-MS/MS determination of Nuc, the active metabolite of remdesivir, and its pharmacokinetics in rat. Anal. Bioanal. Chem. 2021;413(23):5811–5820. doi: 10.1007/s00216-021-03561-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bulduk I., Akbel E. A comparative study of HPLC and UV spectrophotometric methods for remdesivir quantification in pharmaceutical formulations. J. Taibah Univ. Sci. 2021;15(1):507–513. [Google Scholar]
- 15.Pashaei Y. Analytical methods for the determination of remdesivir as a promising antiviral candidate drug for the COVID-19 pandemic. Drug Discoveries & Therapeutics. 2020;14(6):273–281. doi: 10.5582/ddt.2020.03097. [DOI] [PubMed] [Google Scholar]
- 16.Cramer C.J. John Wiley & Sons; 2013. Essentials of computational chemistry: theories and models. [Google Scholar]
- 17.Gross E.K., Dreizler R.M. Springer Science & Business Media; 2013. Density functional theory. [Google Scholar]
- 18.Geerlings P., De Proft F., Langenaeker W. Conceptual density functional theory. Chem. Rev. 2003;103(5):1793–1874. doi: 10.1021/cr990029p. [DOI] [PubMed] [Google Scholar]
- 19.Tirado-Rives J., Jorgensen W.L. Performance of B3LYP density functional methods for a large set of organic molecules. J. Chem. Theory Comput. 2008;4(2):297–306. doi: 10.1021/ct700248k. [DOI] [PubMed] [Google Scholar]
- 20.Momoki K., Sekino J., Sato H., Yamaguchi N. Theory of curved molar ratio plots and a new linear plotting method. Anal. Chem. 1969;41(10):1286–1299. [Google Scholar]