Highlights
-
•
Breast cancer is common and radiation therapy (RT) is crucial in its multimodality management.
-
•
Bibliometrics is a powerful tool to reveal the scientific literature.
-
•
The annual scientific production growth rate is around 7% in the last two decades.
-
•
The primary interests of breast cancer radiation oncology have evolved over time, adding different topics to the portfolio of interest.
Keywords: Breast cancer, Radiotherapy, Radiation therapy, Bibliometric analysis, Bibliometrics
Abstract
Background and purpose
Breast cancer is the most common malignancy in women and radiation therapy (RT) is crucial in its multimodality management. Since bibliometrics is a powerful tool to reveal the scientific literature, we decided to perform a bibliometric analysis of the literature on breast cancer radiotherapy. We explored emerging trends and common patterns in research, tracking collaboration and networks, and foreseeing future directions in this clinical setting.
Material and methods
The electronic Scopus database was searched using the keywords “breast cancer” and “radiotherapy” to include manuscripts published in English, between 2000 and 2021. Data analysis was performed using R-Studio 0.98.1091 software with a machine-learning bibliometric method, based on the bibliometrix R package. The most relevant authors were quantified per number and fractionalized number of authored documents. Author productivity was analysed through Lotka’s law. Bradford’s law was applied to identify the nucleus of journals focused on the addressed topic. Mainstream themes area included isolated topics (niche themes), new topics (emerging themes), hot topics (motor themes) and essential topics (basic themes).
Results
A total of 27 184 documents was found, mainly original articles (76 %). The annual growth rate was 6.98 %, with an increase in scientific production from 485 to 2000 documents between 2000 and 2021. Overall, 2 544 journals published ≥ 1 documents. The most relevant authors were affiliated in the United States. Surgical procedures, cancer type and treatment strategies represented basic themes, while primary systemic therapy and sentinel lymph node biopsy were emerging themes. Health-related quality of life was a niche theme, while RT techniques had high centrality.
Conclusion
The primary interests of breast cancer radiation oncologists have evolved over time, adding safety, health related quality of life, sustainability of treatments and combination to systemic therapies to radiotherapy efficacy and effectiveness and treatment outcomes.
Introduction
Breast cancer (BC) is the most common malignancy in women, comprising around 30 % of female cancers and representing an important issue for public health [1]. The annual incidence and prevalence are geographically scattered, mirroring the distribution of risk factors, economic development and corresponding lifestyle and social factors, and competing causes of death [2], [3]. BC mortality is decreasing, reflecting the increased access to cancer prevention and early detection, together with the improvements in the multidisciplinary management of early and advanced disease, sustained by tailored approaches in the fields of surgical, medical, and radiation oncology [1], [4], [5].
Radiation therapy (RT) has a crucial role in the multimodality management of BC [3]. It is a standard approach for early-stage BC after breast conservation, halving 10-year rates of any BC recurrence and reducing by 1/6th the 15-year breast cancer-related mortality [6]. Post-mastectomy radiation therapy (PMRT) can reduce by more than 10 % the rate of any recurrence at 10 years in node positive women, leading to an 8 % reduction of the rate of 20-year breast-cancer mortality [7].
New possibilities have emerged in breast cancer RT allowing for targeted solutions and personalized approaches. The increased adoption of hypofractionation, the selective use of the boost to the lumpectomy cavity, the reduction in treatment volume with partial breast irradiation, the introduction of volume-based target volume definition, and the integration with primary systemic therapy strategies (hereinafter referred to as neoadjuvant chemotherapy), all enlarged the therapeutic portfolio of the radiation oncologist to provide BC patients with tailored strategies [8], [9], [10], [11], [12], [13], [14].
Since bibliometrics is a powerful tool to reveal the scientific literature on a specific topic within a certain timespan, we decided to perform a bibliometric analysis of the documents published on breast cancer RT in the last two decades [15]. With the present analysis, we aimed at exploring emerging trends and common patterns in research, tracking collaboration and networks, and foreseeing future directions in clinical research in radiation oncology applied to BC.
Materials and methods
A comprehensive search of the Scopus database was performed, using “breast cancer” and “radiotherapy” as primary search strategy, and refining it to include manuscripts i) written in English ii) limited to humans and iii) published between January 1th, 2000 and December 31st, 2021. Search fields included article title, abstract and keywords. A Boolean search was created. After running the search strategy, exact keywords “Prostate Cancer”, “Lung Cancer”, “Melanoma”, “Colorectal Cancer” were excluded to narrow the search scope.
A sensitivity analysis was performed adding the terms ‘’radiation’’ and ‘’radiation therapy’’ to increase the degree of comprehensiveness of the search strategy. Bibliographic metadata were downloaded in BibTex format and exported in R environment (R-Studio 0.98.1091 software). The Bibliometrix R package was used for the bibliometric analysis [16]. A bibliographic data frame was created with cases corresponding to all selected documents. Each document included bibliographic attributes: authors’ names, affiliations, title, keywords, journal, year, volume, issue, pages, editor(s) and citation count.
The “summary ()” function was used to summarize the main results, displaying the principal information about data collection (annual scientific production, average citations per year), sources (most relevant sources, most cited sources, source dynamics), authors (most relevant authors and affiliations, corresponding author’s country, country specific production) and documents (most global cited documents, most frequent keywords, word dynamics). The annual growth rate was used to describe the progression ratio of the scientific production over time.
The most relevant authors were quantified both per number and fractionalized number of authored documents, based on the assumption of a uniform contribution of all co-authors to each document. Author productivity was analysed through Lotka’s law. Bradford’s law was applied to identify the nucleus of journals focused on the addressed topic. Multiple countries production (MCP) indicated the number of documents in which at least one co-author was affiliated in a different country than the first author. Most relevant affiliations (frequency distribution of affiliations of all co-authors for each document) were based on disambiguated affiliation items, applying semantic similarity. The corresponding author’s country was used to list the most relevant countries by corresponding author. Country scientific production referred to authors appearances by country affiliations (number of documents indicates authors appearances by country affiliations). The most productive country was based on the first author’s affiliation. Correspondence analysis and clustering approach were used to identify common shared keywords, authors/institutions relationship in the research themes. A clustering algorithm was applied on the keyword network, plotting a thematic map. Mainstream themes area included isolated topics (niche themes), new topics (emerging themes), hot topics (motor themes) and essential topics (basic themes). Each bubble represented a network cluster. Keywords with the highest occurrence value were used to define the bubble name. The bubble size was proportional to cluster word occurrence and its position was set according to cluster centrality and density. For the thematic evolution map, three cutting points were fixed: 2005, 2010 and 2015 (the colour code indicates different clusters). The function “biblioNetwork ()” generated different network matrices, computing country scientific collaboration [NetMatrix <- biblioNetwork(M, analysis = “collaboration”, network = “countries”, sep = “;”)]. The “Biblioshiny ()” function was used to plot all the results.
Results
Overview
A total of 27 184 documents, mainly articles (as defined by SCOPUS) (n = 20 632; 76 %) and review papers (n = 3 544; 13 %), was collected from 2000 to 2021. Overall, 81 765 authors contributed to these documents with an average of 6 co-authors per publication. The annual growth rate was 6.98 %, leading to a scientific production increment from 485 documents in 2000 up to 2000 documents in 2021. The mean total citations per year revealed a stable range between 2 and 3 citations with two peaks: in 2013 and 2016 with 3.3 and 3.4 citations per year, respectively. The mean total citation per article showed the highest peak in 2001 with 55.1 mean citations and the lowest in 2021 with 1.1 mean citations, reflecting the time available to be referenced as a citation.
Sources
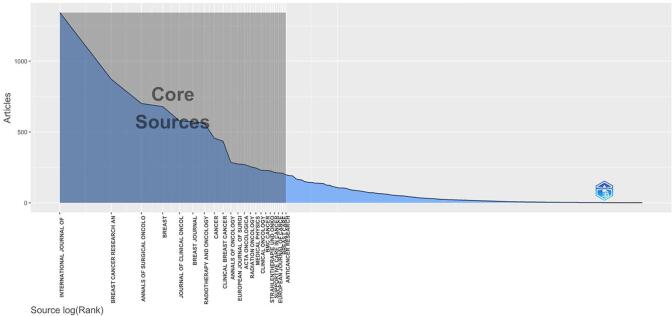
Overall, 2 544 journals published one or more documents. As displayed in Supplementary material (Fig. 1), 21 journals (core sources) published about one third of all the documents retrieved. The most relevant source was “International Journal of Radiation Oncology Biology Physics” with 1 346 articles published between 2000 and 2021, followed by “Breast Cancer Research and Treatment” (n = 872), “Annals of Surgical Oncology” (n = 701) and “The Breast” (n = 679). Dynamics of the top-10 relevant sources are plotted in Supplementary material (Suppl-1). Amongst the top 10 journals, there was a growth in number of publications on the topic in 2010–2021 compared to 2000–2010 (n = 3878 versus n = 2605) The most cited journals (from reference lists) by all 27 184 documents were the “Journal of Clinical Oncology” with 58 720 citations, followed by the “International Journal of Radiation Oncology Biology Physics” with 47 644 citations, “Cancer” with 34 511 citations and the “New England Journal of Medicine” with 23 551 citations.
Fig. 1.
Source clustering through Bradford’s law.
Authors, affiliations, countries
The most relevant authors were Buchholz T.A., Haffty B.G., and Morrow M. with 213 (37.1), 167 (43.3), and 153 (41.2) documents fractionalized frequency, respectively.
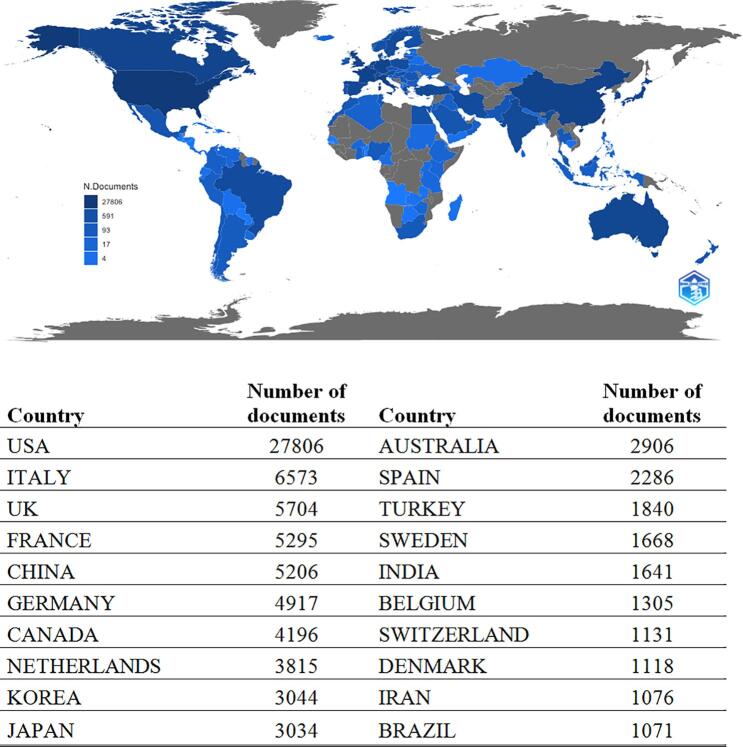
The frequency distribution of the scientific productivity identified several “core” authors (n = 2 315; 2.8 %) who have written at least ten documents and “occasional” authors (n = 56 879; 69.6 %) who published just one paper-Supplementary material (Suppl-2). Top twenty corresponding author’s country is presented in Supplementary material (Suppl-3). Based on the MCP ratio, Turkey, the major countries in East Asia (India, Japan, and Korea) and the United States of America (USA) showed a relatively low international collaboration rate, whereas Germany, the Netherlands, Belgium, and Sweden the highest. Country-specific production is displayed in Fig. 2, with the USA being the most productive country, accounting for 27 806 documents. Most relevant affiliations showed the predominance of North American centres (merging similar affiliation names) followed by the European Institute of Oncology in Italy and the Institut Curie in France-Supplementary material (Suppl-4).
Fig. 2.
Country-specific production (blue intensity is proportional to the number of documents). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Documents
The top twenty most cited articles are listed in Table 1 [6], [7], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34]. The most cited documents on the primary search strategy (“breast cancer” and “radiotherapy”) were maintained in the sensitivity analysis with the addition of the terms ‘’radiation’’ and ‘’radiation therapy’’.
Table 1.
Top twenty most global cited documents as per Scopus.
| Paper | DOI | Total Citations | TC per Year | Normalized TC |
|---|---|---|---|---|
| FISHER B, 2002, NEW ENGL J MED [17] | https://doi.org/10.1056/NEJMoa022152 | 4443 | 211,5714 | 80,3022 |
| ROMOND EH, 2005, NEW ENGL J MED [18] | https://doi.org/10.1056/NEJMoa052122 | 4383 | 243,5 | 82,7022 |
| VERONESI U, 2002, NEW ENGL J MED [19] | https://doi.org/10.1056/NEJMoa020989 | 3118 | 148,4762 | 56,3544 |
| GOLDHIRSCH A, 2011, ANN ONCOL [20] | https://doi.org/10.1093/annonc/mdr304 | 2546 | 212,1667 | 74,0907 |
| DARBY S, 2011, LANCET [6] | 1. https://doi.org/10.1016/S0140-6736(11)61629-2 | 2253 | 187,75 | 65,5641 |
| GOLDHIRSCH A, 2013, ANN ONCOL [21] | https://doi.org/10.1093/annonc/mdt303 | 2119 | 211,9 | 71,1855 |
| GIULIANO AE, 2011, J AM MED ASSOC-a [22] | https://doi.org/10.1001/jama.2011.90 | 2099 | 174,9167 | 61,0826 |
| DARBY SC, 2013, NEW ENGL J MED [23] | https://doi.org/10.1056/NEJMoa1209825 | 2094 | 209,4 | 70,3457 |
| HARRIS L, 2007, J CLIN ONCOL [24] | https://doi.org/10.1200/JCO.2007.14.2364 | 1830 | 114,375 | 43,2204 |
| RASTOGI P, 2008, J CLIN ONCOL [25] | https://doi.org/10.1200/JCO.2007.15.0235 | 1241 | 82,7333 | 32,2834 |
| MCGALE P, 2014, LANCET [7] | https://doi.org/10.1016/S0140-6736(14)60488-8 | 1208 | 134,2222 | 51,1539 |
| MANSEL RE, 2006, J NATL CANCER INST [26] | https://doi.org/10.1093/jnci/djj158 | 1199 | 70,5294 | 28,0748 |
| EBCTCG, 2005, LANCET [27] | https://doi.org/10.1016/S0140-6736(05)66544-0 | 1166 | 50,6957 | 21,8032 |
| COATES AS, 2015, ANN ONCOL [28] | https://doi.org/10.1093/annonc/mdv221 | 1156 | 144,5 | 52,6589 |
| GOLDHIRSCH A, 2009, ANN ONCOL [29] | https://doi.org/10.1093/annonc/mdp322 | 1155 | 825.000 | 322.757 |
| WHELAN TJ, 2010, NEW ENGL J MED [30] | https://doi.org/10.1056/NEJMoa0906260 | 1145 | 880.769 | 316.309 |
| KRAG DN, 2010, LANCET ONCOL [31] | 1. https://doi.org/10.1016/S1470-2045(10)70207-2 | 1112 | 855.385 | 307.193 |
| FISHER B, 2002, NEW ENGL J MED-a [32] | https://doi.org/10.1056/NEJMoa020128 | 1019 | 485.238 | 184.173 |
| VAN DONGEN JA, 2000, J NATL CANCER INST [33] | https://doi.org/10.1093/jnci/92.14.1143 | 1011 | 439.565 | 189.048 |
| BUZDAR AU, 2005, J CLIN ONCOL [34] | https://doi.org/10.1200/JCO.2005.07.032 | 993 | 551.667 | 187.368 |
DOI: digital object identifier; TC: total citations.
Among these twenty papers, twelve are original articles [22], [23], [25], [26], [17], [18], [19], [30], [31], [32], [33], three are meta-analyses/review articles [6], [7], [27], and five clinical practice guidelines/consensus [20], [21], [24], [28], [29]. The first ranked article is the “Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer” [17], with 4 443 global citations.
Most of these articles (n = 13) were published between 2000 and 2010, whereas more recent papers (2011–2021) were limited in number, likely due to the shorter citation time-window. To capture all relevant papers, we also considered separately the distribution of most cited articles during 2000–2010 [17], [18], [19], [24], [25], [26], [27], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41] and 2011–2021 [6], [7], [28], [20], [21], [22], [23], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54]. Details are shown in Supplementary material (Suppl-5 and Suppl-6).
Once having removed the search strategy terms “breast cancer” and “radiotherapy”, the keyword analysis revealed that “chemotherapy”, “prognosis”, and “mastectomy” were the three topmost frequent words, occurring 905, 684, and 662 times, respectively.
Conceptual structure
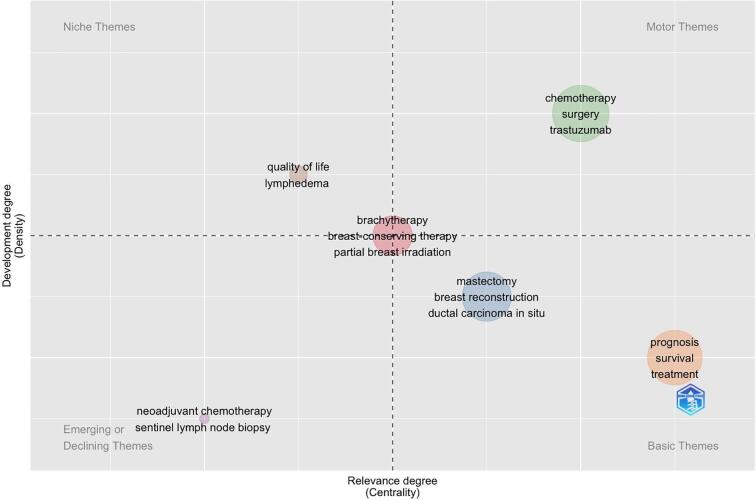
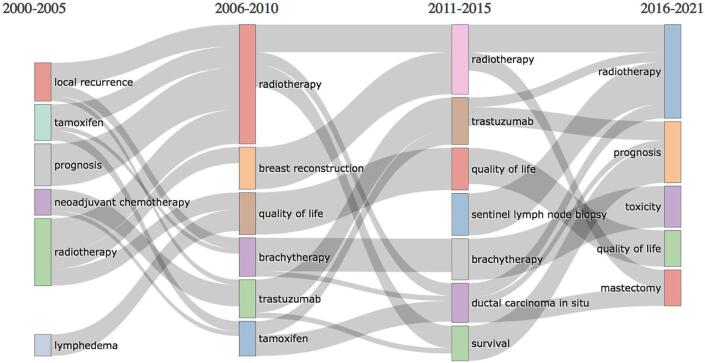
The main themes and trends are showed in Fig. 3 and Fig. 4. The thematic map (Fig. 3) demonstrated that neoadjuvant chemotherapy and sentinel lymph node were emerging themes, while clinical outcomes (prognosis, survival), surgical procedures (mastectomy, breast reconstruction), cancer type (ductal carcinoma in situ) and treatment strategies represented basic and transversal themes. Quality of life and lymphedema were isolated topics. The centrality of RT techniques –brachytherapy and partial breast irradiation – highlighted the theme importance and development over time. Fig. 4 showed the evolution of the main thematic areas and their relationship during four distinct time-periods: 2000–2005, 2006–2010, 2011–2015, and 2016–2021.
Fig. 3.
Thematic map.
Fig. 4.
Thematic evolution.
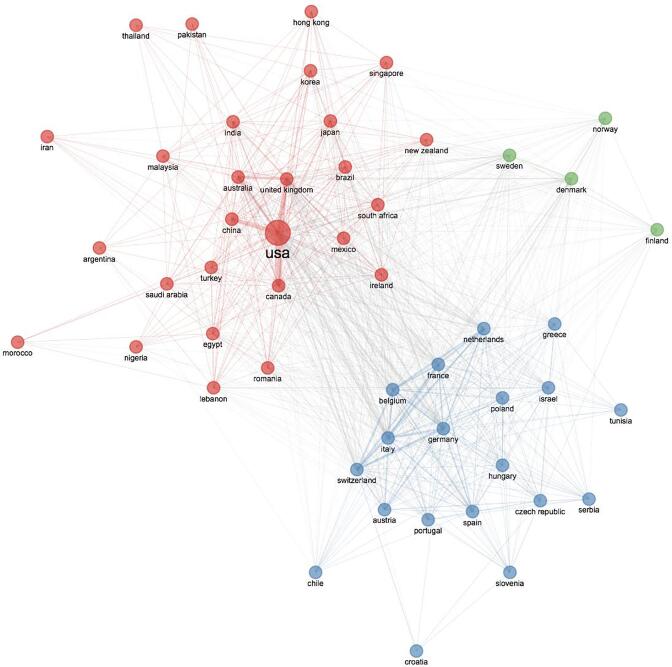
Over time, “radiotherapy” theme connections were diverse, including breast reconstruction, quality of life, ductal carcinoma in situ, survival, and mastectomy. “Local recurrence”, “tamoxifen” and “prognosis” merged into the “radiotherapy” topic in 2006–2010. “Sentinel lymph node biopsy” started as a niche theme and merged in the “radiotherapy” area in 2016–2021. “Brachytherapy” first appeared in 2006–2010, continued to draw attention in the following five years (a slight linkage was evident with “ductal carcinoma in situ”) and then merged into “toxicity” theme. Country collaboration network (Fig. 5) showed country collaboration based on publications. The line thickness indicates collaboration proximity. The USA had a strong connection with Canada, China, and the United Kingdom (UK). The tendency of European countries to cooperate with each other is evident.
Fig. 5.
Collaboration network.
Discussion
Our bibliometric analysis of the scientific literature on breast cancer RT in 2000–2021 revealed an average increase in the annual scientific production of around 7 %, with an absolute number of 2000 papers published in 2021. Amongst the more than 27 000 documents published between 2000 and 2021, 76 % included original articles, highlighting the novelty of the specific scientific production. Around one third of all published documents are edited by a limited number of high-ranked journals, with an impact factor greater than 5, covering the field of clinical, radiation, and medical oncology together with BC. Journals dedicated to BC, had a steep increase in the number of publications on RT in 2010–2021, showing a higher scientific interest for radiation oncology as part of the multidisciplinary management of BC.
The geographical distribution of the scientific publications covers all continents, reflecting the high incidence of BC worldwide. The predominant hubs for scientific production are North America (USA and Canada), Europe (Italy, UK, France, Germany), and Asia (China, Japan, Korea, India). The USA, Canada, UK and China have both a high quantitative scientific throughput and an efficient collaborative networking capacity, which is not scattered but rather selected. Continental European countries can sustain high scientific productivity and tend to implement scientific collaboration at a European level. Nordic European countries have their own specific cooperative networks. Other countries (Iran, India, Korea) can sustain high scientific productivity, with a prevalent ‘stand-alone’ approach, based on national collaborations. The geographical distribution of the scientific production is mirrored by the top-productive academic institutions which are in North America (University of Texas, University of Toronto, Harvard Medical School) and Europe (European Institute of Oncology, Institut Curie, Netherlands Cancer Institute). One limitation of the current analysis is that collaboration was measured using the simple surrogate of co-authorship, which may not reflect an active scientific network, nor the scientific value of the published work. Moreover, the search strategy we used was chosen as a trade-off between comprehensiveness and usability, to limit the background noise potentially derived by the addition of other more allusive keywords (as radiation or radiation therapy).
Amongst the most cited publications in 2000–2010, some have a straightforward RT-oriented research question. The seminal papers by Fisher and Veronesi et al. provided long-term results of the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-06 and Milan trials demonstrating the oncological safety for breast conserving therapy (breast conserving surgery and RT) over mastectomy in ≤ 2 cm early BC [17], [18]. Similarly, the European Organization for Research and Treatment of Cancer (EORTC) 10,801 trial reported on 10-year results of breast conserving therapy for patients with tumours up to 5 cm in diameter, showing non-inferiority over modified radical mastectomy [33]. In the setting of surgical de-escalation, Fisher et al. reported on the 25-year results of the NSABP B-04 trial which investigated the possibility to reduce surgical treatments, comparing Halsted mastectomy to less extensive surgery (total mastectomy with or without RT), showing no advantage from radical mastectomy [32].
In the setting of PMRT, Ragaz et al. reported on the 20-year results of the British Columbia study, which enrolled patients with premenopausal node positive high-risk BC treated with modified radical mastectomy and axillary dissection, showing that PMRT (37.5 Gy/16 fractions) and adjuvant chemotherapy resulted in better survival compared to chemotherapy alone, with good compliance and acceptable long-term toxicity [41].
Whelan et al. reported on the 10-year results of accelerated moderately hypofractionated whole breast irradiation after breast-conserving surgery for invasive BC with clear surgical margins and negative nodes, which was demonstrated to be non-inferior to 2-Gy fractionated radiation [30]. Bartelink et al. reported on the 5-year result of the EORTC ‘boost vs no boost’ trial, investigating the role of an additional radiation dose of 16 Gy to the tumour bed in early breast cancer patients after breast-conserving surgery and 50 Gy whole breast irradiation, showing a local recurrence risk reduction, especially in patients below 50 years [39]. These studies set the standard for breast conserving surgery, provided evidence for PMRT in high-risk patients, extra dose to the lumpectomy cavity in younger women and supported post-operative hypofractionated RT.
Other highly cited documents in 2000–2010 had a strong impact on radiation oncology, even if not directly addressing a RT question. Two trials investigated the role of sentinel lymph node biopsy (SLNB). The Axillary Lymphatic Mapping Against Nodal Axillary Clearance (ALMANAC) trial compared quality-of-life in patients with clinically node-negative invasive BC who received SLNB vs standard axillary treatment, showing reduced arm morbidity and better quality of life for SLNB [26]. The NSABP B-32 trial tested SLNB for equal survival and regional control compared to axillary dissection in BC patients with clinically negative lymph nodes, showing equivalent disease-free and overall survival and regional control between groups [31]. These trials paved the way for de-escalation of the surgical treatment of the axilla, with an impact on the decision-making process of the radiation oncologist, with respect to regional nodal irradiation.
Other trials investigated the role of systemic agents as adjuvant and neoadjuvant treatment strategies. The Early Breast Cancer Trialists’ Cooperative Group (EBCTCG) meta-analysis explored the effect of chemotherapy and hormonal therapy for early BC, showing a reduction in 15-year mortality rates for cyclophosphamide, methotrexate, fluorouracil, anthracycline-based combinations, tamoxifen, or ovarian suppression [27]. Three trials assessed the clinical benefit of trastuzumab in Human Epidermal Growth Factor Receptor (HER)-2-positive breast cancer. The NSABP-B31 and North Central Cancer Treatment Group (NCCTG) N9831 trials compared adjuvant chemotherapy based on doxorubicin and cyclophosphamide with or without trastuzumab combined with paclitaxel in women with surgically removed HER2 positive BC, showing improved outcomes with trastuzumab [18]. Buzdar et al. proved that the addition of trastuzumab to chemotherapy in the setting of neoadjuvant chemotherapy could increase pathologic complete response rate in HER2-positive BC [34]. The NeOAdjuvant Herceptin (NOAH) trial showed that the incorporation of trastuzumab in the neoadjuvant and adjuvant setting improved event-free and overall survival, and clinical and pathological tumour responses in locally advanced or inflammatory HER2-positive BC [36]. Finally, the NSABP B-18 and B-27 trials demonstrated that neoadjuvant and adjuvant chemotherapy are equivalent. The B-27 trials also showed that the addition of preoperative taxanes to doxorubicin, cyclophosphamide improves tumour response. Patients with a pathological complete response had better survival [25]. For the radiation oncology community, those trials stressed the importance of properly combining radiation with systemic therapy, including targeted agents. They also highlighted the need to investigate the role of post-operative RT after neoadjuvant chemotherapy defining indications for PMRT where uncertainty exists regarding the initial axillary nodal status.
The St. Gallen 9th, 10th, and 11th edition papers (2005, 2007, 2009) have a high number of citations. During these meetings, the algorithm for the selection of adjuvant systemic therapy for early BC changed to include risk assessment and endocrine responsiveness, refining, and extending a target-oriented approach based on subgroups defined by predictive markers and menopausal status [29], [35], [40].
In 2011–2021, several highly cited documents had a direct RT-oriented research question. The EBCTCG meta-analysis reported on RT after breast-conserving surgery, which halves the rate of disease recurrence at 10 years and reduces by about a sixth the rate of BC death at 15 years [6]. Another EBCTCG meta-analysis demonstrated that PMRT reduced 10-year recurrence and 20-year BC mortality rates in women with 1–3 positive lymph nodes after mastectomy and axillary surgery, regardless of systemic therapy [7]. Darby et al. showed that heart irradiation increased the risk of ischemic heart disease in BC patients, proportionally to the mean heart dose and increasing over years [23]. The EORTC 10981–22023 AMAROS showed that axillary RT provides comparable axillary control but significantly less morbidity compared to axillary node dissection, in T1-T2 BC with no palpable lymph nodes and positive sentinel lymph node biopsy [44]. Haviland et al. reported on the 10-year results of the UK Standardisation of Breast Radiotherapy (START) A and B trials, showing that hypofractionated RT is safe and effective in early breast cancer patients and supporting the use of 40 Gy in 15 fractions in clinical practice [47]. The Cancer and Leukemia Group B (CALGB) 9343 tested whether the omission of postoperative RT after breast conservation in early BC patients aged ≥ 70 years receiving adjuvant tamoxifen is safe, reporting an excess in loco-regional failure without RT, with no impact on distant-disease free and overall survival [50]. The EORTC 22922/10925 trial demonstrated an improvement in disease-free and distant disease-free survival and a reduction in breast-cancer mortality, with a marginal effect on overall survival, for regional nodal irradiation added to whole-breast or chest wall radiation in early-stage BC patients [51]. Finally, the TARGIT trial reported on the 5-year local recurrence rate for accelerated partial breast irradiation given with single-dose targeted intraoperative RT [53]. These studies reinforced the role of RT after both breast conserving surgery and mastectomy, provided evidence for regional nodal and axillary irradiation and addressed RT de-escalation in low-risk patients. However, their clinical impact was often adjusted to more recent evidence in the years following publication, showing that scientific medical process follows a dynamic course over time.
Other highly cited documents in 2011–2021 were the 2015 European Society of Medical Oncology (ESMO) Guidelines on primary BC, the 4th European School of Oncology (ESO)-ESMO International Consensus Guidelines for Advanced Breast Cancer and two other review papers [42], [43], [44], [52]. The recent consensus paper of the 15th edition of the St. Gallen congress in 2017 had a high number of citations and addressed intensity, duration, and side effects of loco-regional and systemic treatments to shape escalated or de-escalated therapies based on tumour stage and biology [54]. A systematic review and meta-analysis assessed the incidence of unilateral arm lymphoedema after BC, showing extensive surgery and obesity as risk factors [45]. Giuliano et al. demonstrated that the omission of axillary node dissection does not lead to inferior overall survival in T1-T2 BC patients, with no palpable lymph nodes and 1–2 positive sentinel lymph nodes with macro metastases, after breast conservation and whole breast RT [22]. Finally, the KATHERINE trial demonstrated that adjuvant trastuzumab emtansine (TDM-1) can halve the risk of recurrence of invasive BC or death in HER2 positive BC patients with residual disease after neoadjuvant chemotherapy including trastuzumab [49].
In 2000–2021, themes related to treatment modality, prognosis, and surgical topics (mastectomy and breast reconstruction) were constant, highlighting their pervasive nature in BC research. The centrality of the theme breast-conserving therapy together with clinical and technical aspects mostly related to it (partial breast irradiation and brachytherapy) stresses the crucial role of RT as an organ-preserving strategy and the importance of RT optimization in this setting. Neoadjuvant chemotherapy is an emerging trend, probably to gain precedence in the scientific literature soon. Communications between the analysed themes is shown in Fig. 4. Overall, the research field of breast cancer RT presents high cohesion and focuses on multidisciplinary management. The thematic area of RT mostly comprises basic and motor themes across all four 5-year periods. The variety of topics that are covered by research papers (local recurrence, prognosis and survival, target therapies, breast reconstruction and mastectomy, quality of life, ductal carcinoma in situ) suggests that different sub-themes addressing specific clinical and/or technical research questions are being investigated. The diversity of research themes over time and the combination/bifurcation of thematic relations also implies that research in this field is still developing. Brachytherapy research is another highly developed thematic area and in the last period (2016–2021) is linked to the toxicity theme. During 2011–2015, sentinel lymph node biopsy appears as a new additional thematic area. Interestingly, the theme ‘’lymphedema’’ disappears after 2000–2005, but originates the thematic area “quality of life’’. This crucial theme remains isolated over time, suggesting the switch from a disease-oriented model to a patient-oriented research paradigm aimed at optimizing patient pathways during oncological care.
The major limitation of the study is linked to its intrinsic nature. Bibliometric analysis generates quantitative information with a potential result over- or under- estimation. Data quality can be biased by inadequate items standardization, such as per affiliations. We retrieved only documents within the Scopus database, making the present study deficient in reflecting the entire global research activity on BC and RT. Also, quantitative data, particularly the number of citations may be impacted by the number of years in which the publication can collect references. Finally, several studies have been updated and published many times, reporting on different endpoints and at different follow up times.
Conclusion and take-home messages
Radiation therapy research purposes have changed over time, reflecting the evolution of BC oncology worldwide. The management of BC is currently based on a multidisciplinary integration of treatments, including RT, systemic and targeted agents, and surgery. The primary interests of radiation oncologists have evolved over time, adding safety, health related quality of life, sustainability of treatments and combination to systemic therapy to the general efficacy and effectiveness and treatment outcomes. The data presented herein provide a unique window through which to appreciate the eclectic nature of RT as a discipline, emphasizing the fact that technology and clinical management cannot evolve without each other in a virtuous process of constant growth and optimization.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ctro.2022.11.015.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 2.Bray F., Ferlay J., Soerjomataram I., et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Loibl S., Poortmans P., Morrow M., Denkert C., Curigliano G. Breast cancer. Lancet. 2021;397:1750–1769. doi: 10.1016/S0140-6736(20)32381-3. [DOI] [PubMed] [Google Scholar]
- 4.Aristei C., Kaidar-Person O., Arenas M., et al. The 2016 Assisi Think Thank Meeting on breast cancer: white paper. Crit Rev Oncol Hematol. 2016;160:211–221. doi: 10.1007/s10549-016-3998-2. [DOI] [PubMed] [Google Scholar]
- 5.Arenas M., Selek U., Kaidar-Person O., et al. The 2018 assisi think thanks meeting: International expert panel white paper. Crit Rev Oncol Hematol. 2020;151 doi: 10.1016/j.critrevonc.2020.102967. [DOI] [PubMed] [Google Scholar]
- 6.Early Breast Cancer Trialists' Collaborative Group (EBCTCG), Darby S., McGale P., Correa C., Taylor C., Arriagada R., et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378(9804):1707–1716. doi: 10.1016/S0140-6736(11)61629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.EBCTCG (Early Breast Cancer Trialists' Collaborative Group), McGale P., Taylor C., Correa C., Cutter D., Duane F., et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014;383(9935):2127–2135. doi: 10.1016/S0140-6736(14)60488-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haviland J.S., Owen J.R., Dewar J.A., Agrawal R.K., Barrett J., Barrett-Lee P.J., Dobbs H.J., Hopwood P., Lawton P.A., Magee B.J., Mills J., Simmons S., Sydenham M.A., Venables K., Bliss J.M., Yarnold J.R., START Trialists' Group The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol. 2013;14(11):1086–1094. doi: 10.1016/S1470-2045(13)70386-3. [DOI] [PubMed] [Google Scholar]
- 9.Murray Brunt A., Haviland J.S., Whheatley D.A., et al. Hypofractionated breast radiotherapy for 1 week versus 3 weeks (FAST-Forward): 5-year efficacy and late normal tissue effects from a multicentre, non-inferiority, randomized, phase 3 trial. Lancet. 2020;395:1613–1626. doi: 10.1016/S0140-6736(20)30932-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coles C.E., Griffin C.L., Kirby A.M., et al. Partial-breast radiotherapy after breast conservation surgery for patients with early breast cancer (UK IMPORT LOW trial): 5-year results from a multicentre, randomized, controlled, phase 3, non-inferiority trial. Lancet. 2017;390:1048–1060. doi: 10.1016/S0140-6736(17)31145-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bartelink H., Horiot J.C., Poortmans P., Struikmans H., Van den Bogaert W., Barillot I., et al. European Organization for Research and Treatment of Cancer Radiotherapy and Breast Cancer Groups. Recurrence rates after treatment of breast cancer with standard radiotherapy with or without additional radiation. N Engl J Med. 2001;345(19):1378–1387. doi: 10.1056/NEJMoa010874. [DOI] [PubMed] [Google Scholar]
- 12.Strnad V., Otto O.J., Hildebrandt G., et al. 5-year results of accelerated partial breast irradiation using sole interstitial multicatheter brachytherapy versus whole-breast irradiation with boost after breast-conserving surgery for low-risk invasive and in-situ carcinoma of the female breast: a randomized, phase 3, non-inferiority trial. Lancet. 2016;387:229–238. doi: 10.1016/S0140-6736(15)00471-7. [DOI] [PubMed] [Google Scholar]
- 13.Offersen B.V., Boersma L.J., Kirkove C., et al. ESTRO consensus guideline on target volume delineation for elective radiation therapy of early stage breast cancer. Radiother Oncol. 2015;114:3–10. doi: 10.1016/j.radonc.2014.11.030. [DOI] [PubMed] [Google Scholar]
- 14.Meattini I., Becherini C., Boersma L., et al. European Society for Radiotherapy and Oncology Advisory Committee in Radiation Oncology Practice consensus recommendations on patient selection and dose and fractionation for external beam radiotherapy in early breast cancer. Lancet Oncol. 2022;23:e21–e31. doi: 10.1016/S1470-2045(21)00539-8. [DOI] [PubMed] [Google Scholar]
- 15.Franco P., Segelov E., Johnsson A., Riechelmann R., Guren M.G., Das P., et al. A machine-learning-based bibliometric analysis of the scientific literature on anal cancer. Cancers. 2022;14:1697. doi: 10.3390/cancers14071697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aria M., Cuccurullo C. Bibliometrix: An R-tool for comprehensive science mapping analysis. J Informetrics. 2021;11(4):959–975. [Google Scholar]
- 17.Fisher B., Anderson S., Bryant J., Margolese R.G., Deutsch M., Fisher E.R., et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347(16):1233–1241. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 18.Romond E.H., Perez E.A., Bryant J., Suman V.J., Geyer C.E., Jr, Davidson N.E., et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353(16):1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 19.Veronesi U., Cascinelli N., Mariani L., Greco M., Saccozzi R., Luini A., et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347(16):1227–1232. doi: 10.1056/NEJMoa020989. [DOI] [PubMed] [Google Scholar]
- 20.Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thürlimann B, Senn HJ; Panel members. Strategies for subtypes--dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011;22(8):1736-47. [DOI] [PMC free article] [PubMed]
- 21.Goldhirsch A., Winer E.P., Coates A.S., Gelber R.D., Piccart-Gebhart M., Thürlimann B., Senn H.J., Panel members Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol. 2013;24(9):2206–2223. doi: 10.1093/annonc/mdt303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giuliano A.E., Hunt K.K., Ballman K.V., Beitsch P.D., Whitworth P.W., Blumencranz P.W., et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA. 2011;305(6):569–575. doi: 10.1001/jama.2011.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Darby S.C., Ewertz M., McGale P., Bennet A.M., Blom-Goldman U., Brønnum D., et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368(11):987–998. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 24.Harris L., Fritsche H., Mennel R., Norton L., Ravdin P., Taube S., et al. American Society of Clinical Oncology. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol. 2007;25(33):5287–5312. doi: 10.1200/JCO.2007.14.2364. [DOI] [PubMed] [Google Scholar]
- 25.Rastogi P., Anderson S.J., Bear H.D., Geyer C.E., Kahlenberg M.S., Robidoux A., et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. 2008;26(5):778–785. doi: 10.1200/JCO.2007.15.0235. [DOI] [PubMed] [Google Scholar]
- 26.Mansel R.E., Fallowfield L., Kissin M., Goyal A., Newcombe R.G., Dixon J.M., et al. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the ALMANAC Trial. J Natl Cancer Inst. 2006;98(9):599–609. doi: 10.1093/jnci/djj158. [DOI] [PubMed] [Google Scholar]
- 27.Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687-717. [DOI] [PubMed]
- 28.Coates A.S., Winer E.P., Goldhirsch A., Gelber R.D., Gnant M., Piccart-Gebhart M., Thürlimann B., Senn H.J., Panel Members Tailoring therapies–improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann Oncol. 2015;26(8):1533–1546. doi: 10.1093/annonc/mdv221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldhirsch A., Ingle J.N., Gelber R.D., Coates A.S., Thürlimann B., Senn H.J., Panel members Thresholds for therapies: highlights of the St Gallen International Expert Consensus on the primary therapy of early breast cancer 2009. Ann Oncol. 2009;20(8):1319–1329. doi: 10.1093/annonc/mdp322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whelan T.J., Pignol J.P., Levine M.N., Julian J.A., MacKenzie R., Parpia S., et al. Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med. 2010;362(6):513–520. doi: 10.1056/NEJMoa0906260. [DOI] [PubMed] [Google Scholar]
- 31.Krag D.N., Anderson S.J., Julian T.B., Brown A.M., Harlow S.P., Costantino J.P., et al. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol. 2010;11(10):927–933. doi: 10.1016/S1470-2045(10)70207-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fisher B., Jeong J.H., Anderson S., Bryant J., Fisher E.R., Wolmark N. Twenty-five-year follow-up of a randomized trial comparing radical mastectomy, total mastectomy, and total mastectomy followed by irradiation. N Engl J Med. 2002;347(8):567–575. doi: 10.1056/NEJMoa020128. [DOI] [PubMed] [Google Scholar]
- 33.van Dongen J.A., Voogd A.C., Fentiman I.S., Legrand C., Sylvester R.J., Tong D., et al. Long-term results of a randomized trial comparing breast-conserving therapy with mastectomy: European Organization for Research and Treatment of Cancer 10801 trial. J Natl Cancer Inst. 2000;92(14):1143–1150. doi: 10.1093/jnci/92.14.1143. [DOI] [PubMed] [Google Scholar]
- 34.Buzdar A.U., Ibrahim N.K., Francis D., Booser D.J., Thomas E.S., Theriault R.L., et al. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol. 2005;23(16):3676–3685. doi: 10.1200/JCO.2005.07.032. [DOI] [PubMed] [Google Scholar]
- 35.Goldhirsch A., Glick J.H., Gelber R.D., Coates A.S., Thürlimann B., Senn H.J., Panel members Meeting highlights: international expert consensus on the primary therapy of early breast cancer 2005. Ann Oncol. 2005;16(10):1569–1583. doi: 10.1093/annonc/mdi326. [DOI] [PubMed] [Google Scholar]
- 36.Gianni L., Eiermann W., Semiglazov V., Manikhas A., Lluch A., Tjulandin S., et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet. 2010;375(9712):377–384. doi: 10.1016/S0140-6736(09)61964-4. [DOI] [PubMed] [Google Scholar]
- 37.Mauri D., Pavlidis N., Ioannidis J.P. Neoadjuvant versus adjuvant systemic treatment in breast cancer: a meta-analysis. J Natl Cancer Inst. 2005;97(3):188–194. doi: 10.1093/jnci/dji021. [DOI] [PubMed] [Google Scholar]
- 38.Ross J.S., Slodkowska E.A., Symmans W.F., Pusztai L., Ravdin P.M., Hortobagyi G.N. The HER-2 receptor and breast cancer: ten years of targeted anti-HER-2 therapy and personalized medicine. Oncologist. 2009;14(4):320–368. doi: 10.1634/theoncologist.2008-0230. [DOI] [PubMed] [Google Scholar]
- 39.Bartelink H., Horiot J.C., Poortmans P., Struikmans H., Van den Bogaert W., Barillot I., et al. European Organization for Research and Treatment of Cancer Radiotherapy and Breast Cancer Groups. Recurrence rates after treatment of breast cancer with standard radiotherapy with or without additional radiation. N Engl J Med. 2001;345(19):1378–1387. doi: 10.1056/NEJMoa010874. [DOI] [PubMed] [Google Scholar]
- 40.Goldhirsch A., Wood W.C., Gelber R.D., Coates A.S., Thürlimann B., Senn H.J. 10th St. Gallen conference. Progress and promise: highlights of the international expert consensus on the primary therapy of early breast cancer 2007. Ann Oncol. 2007;18(7):1133–1144. doi: 10.1093/annonc/mdm271. [DOI] [PubMed] [Google Scholar]
- 41.Ragaz J., Olivotto I.A., Spinelli J.J., Phillips N., Jackson S.M., Wilson K.S., et al. Locoregional radiation therapy in patients with high-risk breast cancer receiving adjuvant chemotherapy: 20-year results of the British Columbia randomized trial. J Natl Cancer Inst. 2005;97(2):116–126. doi: 10.1093/jnci/djh297. [DOI] [PubMed] [Google Scholar]
- 42.Senkus E., Kyriakides S., Ohno S., Penault-Llorca F., Poortmans P., Rutgers E., et al. ESMO Guidelines Committee. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(Suppl 5):v8–v. [Google Scholar]
- 43.Fan L., Strasser-Weippl K., Li J.J., St Louis J., Finkelstein D.M., Yu K.D., et al. Breast cancer in China. Lancet Oncol. 2014;15(7):e279–e289. doi: 10.1016/S1470-2045(13)70567-9. [DOI] [PubMed] [Google Scholar]
- 44.Donker M., van Tienhoven G., Straver M.E., Meijnen P., van de Velde C.J., Mansel R.E., et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981–22023 AMAROS): a randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol. 2014;15(12):1303–1310. doi: 10.1016/S1470-2045(14)70460-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.DiSipio T., Rye S., Newman B., Hayes S. Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysis. Lancet Oncol. 2013;14(6):500–515. doi: 10.1016/S1470-2045(13)70076-7. [DOI] [PubMed] [Google Scholar]
- 46.Harbeck N., Gnant M. Breast cancer. Lancet. 2017;389(10074):1134–1150. doi: 10.1016/S0140-6736(16)31891-8. [DOI] [PubMed] [Google Scholar]
- 47.Haviland JS, Owen JR, Dewar JA, Agrawal RK, Barrett J, Barrett-Lee PJ, Dobbs HJ, Hopwood P, Lawton PA, Magee BJ, Mills J, Simmons S, Sydenham MA, Venables K, Bliss JM, Yarnold JR; START Trialists' Group. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol. 2013;14(11):1086-1094. [DOI] [PubMed]
- 48.Robert N.J., Diéras V., Glaspy J., Brufsky A.M., Bondarenko I., Lipatov O.N., et al. RIBBON-1: randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2-negative, locally recurrent or metastatic breast cancer. J Clin Oncol. 2011;29(10):1252–1260. doi: 10.1200/JCO.2010.28.0982. [DOI] [PubMed] [Google Scholar]
- 49.von Minckwitz G., Huang C.S., Mano M.S., Loibl S., Mamounas E.P., Untch M., et al. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N Engl J Med. 2019;380(7):617–628. doi: 10.1056/NEJMoa1814017. [DOI] [PubMed] [Google Scholar]
- 50.Hughes K.S., Schnaper L.A., Bellon J.R., Cirrincione C.T., Berry D.A., McCormick B., et al. Lumpectomy plus tamoxifen with or without irradiation in women age 70 years or older with early breast cancer: long-term follow-up of CALGB 9343. J Clin Oncol. 2013;31(19):2382–2387. doi: 10.1200/JCO.2012.45.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Poortmans P.M., Collette S., Kirkove C., Van Limbergen E., Budach V., Struikmans H., et al. EORTC Radiation Oncology and Breast Cancer Groups. Internal Mammary and Medial Supraclavicular Irradiation in Breast Cancer. N Engl J Med. 2015;373(4):317–327. doi: 10.1056/NEJMoa1415369. [DOI] [PubMed] [Google Scholar]
- 52.Cardoso F., Senkus E., Costa A., Papadopoulos E., Aapro M., André F., et al. 4th ESO-ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 4)†. Ann Oncol. 2018;29(8):1634–1657. doi: 10.1093/annonc/mdy192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vaidya J.S., Wenz F., Bulsara M., Tobias J.S., Joseph D.J., Keshtgar M., et al. TARGIT trialists' group. Risk-adapted targeted intraoperative radiotherapy versus whole-breast radiotherapy for breast cancer: 5-year results for local control and overall survival from the TARGIT-A randomised trial. Lancet. 2014;383(9917):603–613. doi: 10.1016/S0140-6736(13)61950-9. [DOI] [PubMed] [Google Scholar]
- 54.Curigliano G, Burstein HJ, Winer EP, Gnant M, Dubsky P, Loibl S, Colleoni M, Regan MM, Piccart-Gebhart M, Senn HJ, Thürlimann B; St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2017, André F, Baselga J, Bergh J, Bonnefoi H, Brucker SY, Cardoso F, Carey L, Ciruelos E, Cuzick J, Denkert C, Di Leo A, Ejlertsen B, Francis P, Galimberti V, Garber J, Gulluoglu B, Goodwin P, Harbeck N, Hayes DF, Huang CS, Huober J, Hussein K, Jassem J, Jiang Z, Karlsson P, Morrow M, Orecchia R, Osborne KC, Pagani O, Partridge AH, Pritchard K, Ro J, Rutgers EJT, Sedlmayer F, Semiglazov V, Shao Z, Smith I, Toi M, Tutt A, Viale G, Watanabe T, Whelan TJ, Xu B. De-escalating and escalating treatments for early-stage breast cancer: the St. Gallen International Expert Consensus Conference on the Primary Therapy of Early Breast Cancer 2017. Ann Oncol. 2017;28(8):1700-1712. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.