Abstract
Context
Due to its rare incidence, molecular features of primary aldosteronism (PA) in young adults are largely unknown. Recently developed targeted mutational analysis identified aldosterone-driver somatic mutations in aldosterone-producing lesions, including aldosterone-producing adenomas (APAs), aldosterone-producing nodules (APNs), and aldosterone-producing micronodules, formerly known as aldosterone-producing cell clusters.
Objective
To investigate histologic and genetic characteristics of lateralized PA in young adults.
Methods
Formalin-fixed, paraffin-embedded adrenal tissue sections from 74 young patients with lateralized PA (<35 years old) were used for this study. Immunohistochemistry (IHC) for aldosterone synthase (CYP11B2) was performed to define the histopathologic diagnosis. Somatic mutations in aldosterone-producing lesions were further determined by CYP11B2 IHC-guided DNA sequencing.
Results
Based on the CYP11B2 IHC results, histopathologic classification was made as follows: 48 APAs, 20 APNs, 2 multiple aldosterone-producing nodules (MAPN), 1 double APN, 1 APA with MAPN, and 2 nonfunctioning adenomas (NFAs). Of 45 APAs with successful sequencing, 43 (96%) had somatic mutations, with KCNJ5 mutations being the most common genetic cause of young-onset APA (35/45, 78%). Of 18 APNs with successful sequencing, all of them harbored somatic mutations, with CACNA1D mutations being the most frequent genetic alteration in young-onset APN (8/18, 44%). Multiple CYP11B2-expressing lesions in patients with MAPN showed several aldosterone-driver mutations. No somatic mutations were identified in NFAs.
Conclusion
APA is the most common histologic feature of lateralized PA in young adults. Somatic KCNJ5 mutations are common in APAs, whereas CACNA1D mutations are often seen in APNs in this young PA population.
Keywords: primary aldosteronism, aldosterone-producing adenoma, CYP11B2, somatic mutation, young adults
Primary aldosteronism (PA), which is characterized by renin-independent (autonomous) adrenal aldosterone production, is the most common cause of endocrine-related hypertension. The prevalence of PA is 6% of hypertensive patients in the primary care setting (1) and up to 20% of patients with resistant hypertension (2). The major subtypes of PA are sporadic aldosterone-producing adenoma (APA) and idiopathic hyperaldosteronism (IHA). While APA can be surgically curable, patients with IHA require life-long medical therapy due to the bilateral nature of the disease. As imaging-identified adrenal lesions are not always the cause of aldosterone excess (3), accurate subtype classification is an essential step in management of PA. Adrenal venous sampling (AVS) is recommended by several clinical practice guidelines as the gold standard procedure for subtype classification (4-6). However, the availability of AVS can be limiting because of its technical difficulties. The Endocrine Society clinical practice guideline suggests that a case can be made to proceed directly to unilateral adrenalectomy without the need for AVS in young patients (<35 years old) with marked PA (eg, spontaneous hypokalemia; plasma aldosterone concentration >30 ng/dL, 831 pmol/L) and solitary unilateral apparent adenoma on computed tomography scan (4).
In the past decade, somatic mutations that cause inappropriate aldosterone production (aldosterone-driver somatic mutations) have been identified in APAs through the application of next-generation sequencing (NGS) (7-12). Previous studies indicate the impact of age on the type of somatic mutation, such as somatic KCNJ5 mutations, is common in APAs from young populations (13-15). However, the rare nature of PA in young adults has hindered comprehensive studies to determine histologic and genetic characteristics of APAs in young patients with PA. In this multicenter collaborative study, we investigated histologic and genetic characteristics of adrenal lesions from young patients with PA. Our study hypothesis was that somatic mutations causing PA in young adults exhibit a different prevalence than the mutations causing PA in older patients.
Materials and Methods
Patients
Seventy-four patients with PA younger than 35 years old who underwent unilateral adrenalectomy at the participating centers (University of Michigan, University of Pennsylvania, Ludwig Maximilian University, and Mayo Clinic) were studied. The patients were included based on the availability of archival formalin-fixed, paraffin-embedded (FFPE) tumor blocks. The diagnosis of PA was made according to the institutional consensus available at the time or the Endocrine Society clinical practice guideline (4). Lateralization of aldosterone excess was determined on the basis of the results of AVS in 62 patients (84%). The use of archival FFPE specimens in this study was approved by Institutional Review Boards at each participating center.
Immunohistochemistry
To determine the site of pathologic aldosterone production, immunohistochemistry (IHC) for aldosterone synthase (CYP11B2), which is required for the final steps of aldosterone biosynthesis, was performed on FFPE adrenal tumor sections as described previously (16). A specific monoclonal antibody against human CYP11B2 (clone 41-17B, kindly provided by Dr. Celso E. Gomez-Sanchez, RRID:AB_2650562) was used for CYP11B2 IHC (17). IHC for 17α-hydroxylase/17, 20 lyase (CYP17A1), which is a steroidogenic enzyme required for cortisol production, was performed using a polyclonal antibody against human CYP17A1 (LSBio, LS-B14227, RRID:AB_2857939). Based on the findings of CYP11B2 IHC and hematoxylin and eosin (H&E) staining, we determined histopathologic diagnosis using the criteria of the HISTALDO (histopathology of primary aldosteronism) consensus (18). In brief, a well-circumscribed CYP11B2-expressing tumor with a diameter of 10 mm or lager was considered an APA. If a CYP11B2-expressing lesion that was morphologically identifiable by H&E was smaller than 10 mm but larger than 1 mm in a diameter, the lesion was considered to be an aldosterone-producing nodule (APN).
Sequencing Analysis
Genomic DNA from the aldosterone-producing lesions on the basis of CYP11B2 IHC was isolated from serial unstained FFPE sections using the AllPrep DNA/RNA FFPE kit (QIAGEN) as described previously (16). Aldosterone-driver somatic mutations were identified by direct Sanger sequencing for hotspots of the KCNJ5 gene (19) or Ion Torrent™–based targeted NGS (Thermo Fisher Scientific). The custom Ion AmpliSeq™ panel utilized for targeted NGS included the full coding regions of PA-associated genes (KCNJ5, ATP1A1, ATP2B3, CACNA1D, CACNA1H, and CLCN2) and oncogene hotspots of CTNNB1 and GNAS. The methods for targeted NGS, including library preparation, sequencing, and variant calling were performed as described previously (16). Nomenclature for the identified variants was made as per the Human Genome Variation Society guidelines.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism 8.0.0. Clinical characteristics are shown as medians with interquartile ranges or percentages with numbers. The Mann–Whitney U test and Fisher’s exact test were used for comparison of 2 groups. Differences were considered statistically significant when P < .05.
Results
Clinical and Histologic Characteristics of Young Adults With PA
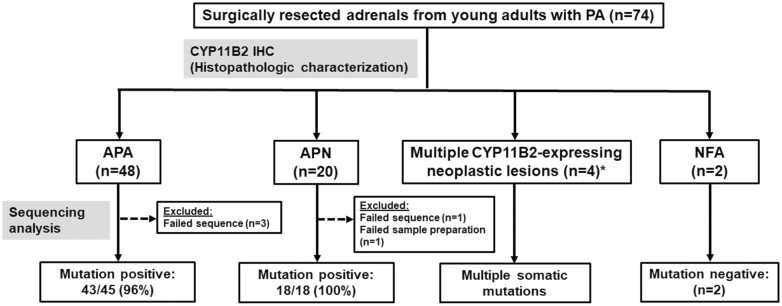
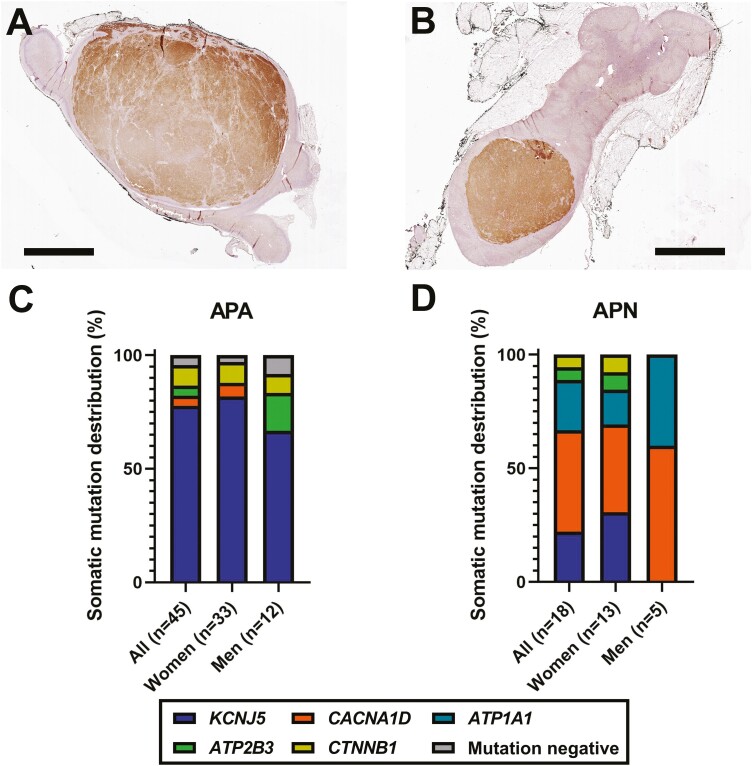
Clinical characteristics of studied patients are shown in Table 1. CYP11B2 IHC revealed CYP11B2-expressing neoplastic lesions in 72 out of 74 (97%) resected adrenals. The remaining 2 CYP11B2-negative tumors were considered as nonfunctioning adrenocortical adenomas (NFAs). Based on the HISTALDO consensus criteria (18), 48, 20, 2, and 1 adrenal(s) were classified as an APA, APN, multiple APNs (MAPN), and double APNs, respectively. There was also a unique case of a dominant APA with MAPN (Fig. 1). Except for tumor diameter on cross-sectional imaging, there was no significant difference in any of the clinical parameters between APA and APN (Table 1). Representative CYP11B2 IHC scanned images of APA and APN are shown in Fig. 2A and 2B. Detailed clinical data of patients with MAPN, double APNs, and 1 with APA and MAPN are shown in Table 2.
Table 1.
Clinical characteristics of studied patients
| All (n = 74) | APA (n = 48) | APN (n = 20) |
P value (APA vs APN) |
|
|---|---|---|---|---|
| Race (White/Black/Asian/unknown) | 45/10/2/17 | 33/6/0/9 | 9/4/1/6 | — |
| Age | 31 (28-33) | 31 (28-33) | 31 (29-34) | .53 |
| Sex (women), %/N | 73, 54/74 | 73, 35/48 | 70, 14/20 | .77 |
| Systolic blood pressure (mmHg) | 145 (136-162)a | 143 (132-163)g | 155 (139-164)l | .32 |
| Diastolic blood pressure (mmHg) | 94 (87-103)a | 92 (85-103)g | 97 (92-108)l | .26 |
| Number of antihypertensive medications | 2 (1-3)b | 2 (1-3)g | 3 (1-3)l | .26 |
| Prevalence of hypokalemia, %/N | 75 (51/68) | 74 (34/46) | 75 (12/16) | >.99 |
| PAC (ng/dL) | 25.0 (15.0-33.8)c | 26.0 (15.0-35.0)h | 22.5 (15.8-26.2)m | .42 |
| PRA (ng/mL/hour) | 0.6 (0.1-0.6)d | 0.2 (0.1-0.6)i | 0.6 (0.2-0.6)n | .60 |
| DRC (mU/L) | 2.0 (2.0-3.7)e | 2.0 (2.0-4.7)j | 2.0 (2.0-3.4)o | .92 |
| ARR (PAC/PRA) | 76.0 (26.5-164.5)d | 113.0 (42.5-229.5)i | 43.0 (25.0-153.0)n | .31 |
| ARR (PAC/DRC) | 11.4 (6.1-27.5)e | 8.9 (4.9-32.2)j | 11.8 (8.5-20.8)o | .68 |
| Tumor diameter on imaging study (mm) | 15.0 (12.0-20.0)f | 16.5 (15.0-22.0)k | 10.0 (7.0-12.0)p | <.0001 |
Medians with interquartile ranges or percentages with counts are presented. Hypokalemia was defined if the patient’s serum potassium level was lower than 3.5 mEq/L or potassium supplementation was administered.
Abbreviations: ARR, aldosterone-to-renin ratio; DRC, direct renin concentration; PAC, plasma aldosterone concentration; PRA, plasma renin activity.
a n = 67;
b n = 68;
c n = 65;
d n = 49;
e n = 13;
f n = 63;
g n = 45;
h n = 43;
i n = 33;
j n = 8;
k n = 44;
l n = 17;
m n = 16;
n n = 11;
o n = 4;
p n = 15.
Figure 1.
Histopathologic characterization and genetic analysis of adrenals from young adults with PA. Histopathologic characterization was done based on the HISTALDO consensus criteria. APA, aldosterone-producing adenoma; APN, aldosterone-producing nodule; MAPN, multiple aldosterone-producing nodules; NFA, nonfunctioning adenoma. *Includes 2 MAPN, 1 double APN, 1 APA with MAPN.
Figure 2.
Histology and somatic mutation spectrum of APA and APN in young adults. (A,B) Histologic characteristics of young-onset aldosterone-producing adenoma (APA) (A) and aldosterone-producing nodule (APN) (B). Scans of CYP11B2 immunohistochemistry are shown (brown, CYP11B2). Scale bars, 5 mm. (C) Somatic mutation distribution of young-onset APA. (D) Somatic mutation distribution of young-onset APN.
Table 2.
Clinical characteristics of patients with unique adrenal histology
| Histology | MAPN | Double APN | APA with MAPN | NFA | NFA with MAPM | |
|---|---|---|---|---|---|---|
| Study ID | ELA50 | ELA146 | ELA130 | ELA160 | ELA120 | ELA128 |
| Basic clinical characteristics | ||||||
| Age | 15 | 22 | 28 | 25 | 34 | 30 |
| Sex | Woman | Man | Woman | Woman | Woman | Woman |
| Race | Unknown | White | Asian | White | Unknown | White |
| Blood pressure (mmHg) | 138/77 | 136/94 | 138/92 | 140/90 | NA | 136/62 |
| Number of antihypertensive medications | 2 | 4 | 1 | 3 | 3 | 5 |
| Serum potassium (mEq/L) | 2.3 | 3.8 | 3.9 | 3.0 | 2.9 | 3.0 |
| Potassium supplementation | No | Yes | No | Yes | Yes | Yes |
| PAC (ng/dL) | 25.1 | 36.0 | 11.0 | 30.8 | 17.0 | 53.8 |
| PRA (ng/mL/h) | NA | 0.6 | 0.6 | 0.7 | 0.6 | 2.8 |
| DRC (mU/L) | 0.8 | NA | NA | NA | NA | NA |
| Serum cortisol post-1-mg DST (µg/dL) | NA | 1.6 | NA | 10.7 | NA | NA |
| CT findings of adrenal glands | Hyperplasia | Bilateral thickening | Left adrenal mass (5 mm) | Bilateral adrenal mass (right 20 mm, left 14 mm) | Left adrenal mass (16 mm) | Left adrenal mass (10 mm) |
| AVS | ||||||
| Cosyntropin stimulation | No | Yes | Yes | Yes | Yes | Yes |
| Right AV aldosterone (ng/dL) | 188.5 | 4250 | 470 | 13 500 | 462 | 947 |
| Right AV cortisol (µg/dL) | 16.5 | 236 | 620 | 1101 | 1020 | 730 |
| Left AV aldosterone (ng/dL) | 3510 | 39 400 | 2280 | 660 | 8810 | 11 411 |
| Left AV cortisol (µg/dL) | 42.5 | 586 | 390 | 372 | 462 | 880 |
| IVC aldosterone (ng/dL) | 23.6 | 314 | 232 | 66.1 | 129 | 57 |
| IVC cortisol (µg/dL) | 5.0 | 12 | 26 | 25.9 | 25 | 24 |
| Selectivity index (right) | 3.3 | 19.7 | 23.8 | 42.5 | 40.8 | 30.4 |
| Selectivity index (left) | 8.5 | 48.8 | 15.0 | 14.4 | 18.5 | 36.7 |
| A/C (right) | 11.4 | 18.0 | 0.8 | 12.3 | 0.5 | 1.3 |
| A/C (left) | 82.6 | 67.2 | 5.8 | 1.8 | 19.1 | 13.0 |
| A/C (IVC) | 4.7 | 26.2 | 8.9 | 2.6 | 5.2 | 2.4 |
| Lateralized ratio | 7.2 | 3.7 | 7.7 | 6.9 | 42.1 | 10.0 |
| Contralateral ratio | 2.4 | 0.7 | 0.1 | 0.7 | 0.1 | 0.5 |
| Side of aldosterone excess by AVS | Left | Left | Left | Right | Left | Left |
| Side of adrenalectomy | Left | Left | Left | Right | Left | Left |
| Postsurgical clinical data | ||||||
| Follow-up timing (months) | 6-12 | 3 | 2 | 1 | NA | 0.5 |
| Blood pressure (mmHg) | 122/80 | 131/91 | NA | 132/92 | NA | 152/94 |
| Number of antihypertensive medications | 1 | 3 | 0 | 0 | NA | 1 |
| Serum potassium (mEq/L) | 4.6 | 4.5 | 4.3 | 4.0 | NA | 4.7 |
| PAC (ng/dL) | 10.2 | NAa | NA | 11.7 | NA | NA |
| PRA (ng/mL/h) | NA | NA | NA | NA | NA | NA |
| DRC (mU/L) | 16.2 | NA | NA | NA | NA | NA |
| DRC (pg/mL) | NA | NA | NA | 11.3 | NA | NA |
Abbreviations: A/C, aldosterone/cortisol; APA, aldosterone-producing adenoma; AV, adrenal vein; AVS, adrenal venous sampling; CT, computed tomography; DRC, direct renin concentration; DST, dexamethasone suppression test; IVC, inferior vena cava; MAPM, multiple aldosterone-producing micronodules; MAPN, multiple aldosterone-producing nodules; NA, not available; NFA, nonfunctioning adenoma; PAC, plasma aldosterone concentration; PRA, plasma renin activity.
a Postsurgical PAC on day 1 after surgery was 7.1 ng/dL.
In our cohort, 14 patients met the criteria of possibly sparing AVS suggested by the Endocrine Society Clinical Practice Guideline, namely young patients (<35 years old) with marked PA (eg, spontaneous hypokalemia; plasma aldosterone concentration >30 ng/dL) and solitary unilateral apparent adenoma on computed tomography scan (4). Of them, 11 underwent AVS and all had concordant results between the imaging study and AVS. Of note, 2 of them underwent AVS before the guideline was published (20). CYP11B2 IHC revealed APA in all but 1 case that had an NFA with multiple aldosterone-producing micronodules (MAPM). All 3 patients who did not undergo AVS had a solitary APA.
Of the patients studied, 21 underwent a 1-mg dexamethasone suppression test prior to surgery (15 APAs, 4 APNs, 1 MAPN, 1 APA with MAPN). Among them, 3 (2 APAs and 1 APA with MAPN) had post-1-mg dexamethasone suppression test cortisol values above 1.8 µg/dL, suggesting excess cortisol secretion. None of the 3 patients had Cushingoid features, indicating mild autonomous cortisol excess. Of the 2 patients with APA, 1 had a solitary APA that had CYP17A1 expression within the tumor, indicating the capacity of tumor cells to produce cortisol and aldosterone. The other had 2 distinct but touching tumors (collision tumors): 1 APA without CYP17A1 expression and the other a CYP11B2-negative but CYP17A1-positive tumor (Fig. 3A-3F), suggesting that the latter could be the source of cortisol excess. In the patient with APA and MAPN, the dominant APA had diffuse expression of CYP17A1 (Fig. 4A-4C). APNs in the adjacent adrenal tissue also showed some degree of CYP17A1 expression (data not shown). Any of these CYP17A1-expressing lesions could contribute to the elevated cortisol levels.
Figure 3.
Histopathologic findings of adrenal tumors from a patient with PA and mild autonomous cortisol excess. (A,B) Scanned images of immunohistochemistry (IHC) of adrenal tumor tissue. Scale bars, 5 mm. (A) CYP11B2 IHC. *Aldosterone-producing adenoma (APA). **CYP11B2-negative tumor. (B) CYP17A1 IHC. (C,D) High-magnification microphotographs of APA (*). Scale bars, 100 µm. (E,F) High magnification microphotographs of the CYP11B2-negative tumor (**). Scale bars, 100 µm. (C, E) CYP11B2 IHC. (D, F) CYP17A1 IHC.
Figure 4.
Histopathologic findings of an adrenal containing multiple types of aldosterone-producing lesions. (A) Scanned image of aldosterone-producing adenoma (APA) with CYP11B2 immunohistochemistry (IHC). Scale bar, 5 mm. (B,C) High-magnification micrographs of APA. Scale bars, 100 µm. (B) CYP11B2 IHC. (C) CYP17A1 IHC. (D,E) Scanned images of adrenal tissue with CYP11B2 IHC (multiple blocks were examined). Scale bars, 5 mm. Single (*) and double (**) asterisks in D indicate aldosterone-producing nodules (APNs). Somatic KCNJ5 c.451G>C and c.451G>A (both result in p.G151R) mutations were identified in the single (*) and double (**) asterisks, respectively. (F) High-magnification micrograph of an aldosterone-producing micronodule (APM) that harbors a somatic ATP1A1 c.995T>G, p.V332G mutation (arrow in E).
Two patients with NFAs underwent unilateral adrenalectomy based on the results of AVS. Detailed clinical data of patients with NFA are demonstrated in Table 2. Histologically, 1 of them had MAPM in the adjacent adrenal and the other had no positive expression of CYP11B2. In the patient with MAPM, normalization of serum potassium and reduction in the number of antihypertensive medications were observed at 2 weeks after surgery. No postsurgical follow-up data were available in the other patient.
Genetic Spectrum of Young-onset PA
Sequencing analysis was successfully done in 45 APAs, 18 APNs, 2 MAPN, 1 double APN, 1 APA with MAPN, and 2 NFAs (Fig. 1). Of note, DNA from individual APNs was sequenced in MAPN (3 APNs from each) and double APN cases. For APA with MAPN cases, DNA from 1 APA, 4 APNs, and 9 aldosterone-producing micronodules (APMs) was sequenced. APM DNA from an NFA case was also sequenced. Of 45 APAs, somatic mutations in genes that are known to be associated with PA or adrenocortical adenomas were identified in 43 out of 45 APAs (96%) (Fig. 1). Somatic KCNJ5 mutations were the most common genetic alteration in young-onset APA (35/45, 78%) (Table 3). Although somatic KCNJ5 mutations have been documented more frequently in APAs from women than from men in previous studies (15, 21-25), sex differences in KCNJ5 mutation prevalence were not observed in this study population (82% [27/33] in women vs 67% [8/12] in men, P = .42) (Fig. 2C). Interestingly, there was a sex difference for the KCNJ5 p.L168R mutation, which was more commonly observed in APAs from women (67% [18/27] compared with 25% [2/8] in men, P = .05). Of the 2 patients with APA and mild autonomous cortisol excess, 1 had a KCNJ5 p.L168R (c.503T>G) mutation and the other had no mutation. The latter had a histopathology suggesting the occurrence of a collision CYP11B2-negative tumor that was mutation negative by targeted NGS.
Table 3.
Somatic mutations in young adults with APA and APN
| Somatic mutation | APA | APN | ||
|---|---|---|---|---|
| Women(n = 33) | Men (n = 12) | Women (n = 13) | Men (n = 5) | |
| KCNJ5 | ||||
| p.T149delinsMA | 0 | 1 | 0 | 0 |
| p.G151R | 8 | 4 | 2 | 0 |
| p.G151_Y152del | 1 | 0 | 0 | 0 |
| p.T158A | 0 | 1 | 0 | 0 |
| p.L168R | 18 | 2 | 2 | 0 |
| CACNA1D | ||||
| p.D201N | 0 | 0 | 1 | 0 |
| p.L272H | 0 | 0 | 1 | 0 |
| p.G403R (exon 8B) | 0 | 0 | 2 | 2 |
| p.F747L | 0 | 0 | 1 | 0 |
| p.R990H | 1 | 0 | 0 | 0 |
| p.V1151A | 0 | 0 | 0 | 1 |
| p.V1338M | 1 | 0 | 0 | 0 |
| ATP1A1 | ||||
| p.L104R | 0 | 0 | 2 | 1 |
| p.E960_A963del | 0 | 0 | 0 | 1 |
| ATP2B3 | ||||
| p.L425_V426del | 0 | 1 | 1 | 0 |
| p.V426_V427del | 0 | 1 | 0 | 0 |
| CTNNB1 | ||||
| p.I35_G38del | 1 | 0 | 0 | 0 |
| p.S37C | 0 | 0 | 1 | 0 |
| p.T41A | 1 | 0 | 0 | 0 |
| p.S45P | 1 | 1 | 0 | 0 |
| Mutation negative | 1 | 1 | 0 | 0 |
In contrast to APAs, the most frequently mutated gene in APNs was CACNA1D (8/18, 44%), which encodes voltage-dependent L-type calcium channel subunit alpha-1D, whereas the prevalence of the KCNJ5 somatic mutation was relatively low (4/18, 22%) (Table 3 and Fig. 2D). There were no significant sex differences in CACNA1D mutation prevalence (38% [5/13] in women vs 60% [3/5] in men, P = .61). There were 2 previously unreported mutations in aldosterone-driver genes in APNs, including ATP1A1 p.E960_A963del (c.2878_2889del) and CACNA1D p.D201N (c.601G>A). These mutations were confirmed by 2 different NGS panels and were not identified in adjacent adrenocortical tissue DNA, suggesting their somatic origin. As for the ATP1A1 p.E960_A963del mutation, a similar mutation (c.2877_2888del) that results in the same amino acid change was reported previously (26). No somatic mutations in NFAs (n = 2) or an APM in the adjacent adrenal to the NFA were detected.
Multiple Aldosterone-producing Lesions With Somatic Mutations
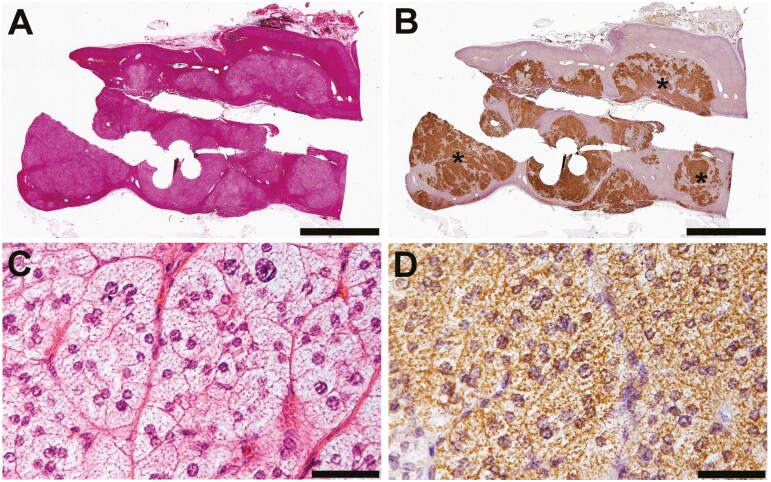
In this young-onset PA cohort, there were 4 patients with unique histologic characteristics: 2 MAPN, 1 double APN, and 1 APA with MAPN. Histologic findings of the resected adrenal from the patient with MAPN are shown in Fig. 5A and 5B. Both patients had the typical clinical phenotype of PA, including hypertension, hypokalemia, and elevated plasma aldosterone concentration with suppressed renin. Adrenal imaging studies documented hyperplasic adrenals with no clear tumor. Unilateral adrenalectomy was performed for both patients based on the AVS findings. Postoperatively, normalization of serum potassium and improvement in blood pressure were observed in both patients. Histologically, the CYP11B2-expressing nodular lesions were composed of lipid-rich clear cells, which are often seen in KCNJ5-mutated APAs (Fig. 5C and 5D) (27-29). Interestingly, all sequenced CYP11B2-expressing lesions harbored identical KCNJ5 mutations (c.451G>A, p.G151R) but there was no evidence of this variant in the adjacent adrenocortical tissue DNA.
Figure 5.
Histopathologic characteristics of multiple aldosterone-producing nodules. (A) Hematoxylin and eosin (H&E) staining. (B) CYP11B2 immunohistochemistry (IHC). Multiple CYP11B2-expressing nodules were observed. All 3 sequenced nodules (*) shared an identical somatic KCNJ5 mutation (c.451G>A, p.G151R). Scale bars, 5 mm. (C,D) High-magnification micrographs of APN. Scale bars, 50 μm. (C) H&E. (D) CYP11B2 IHC.
The patient with double APNs had a relatively mild clinical phenotype of PA (Table 2). The APNs harbored distinct somatic KCNJ5 mutations: 1 had KCNJ5 p.G151R (c.451G>A) mutation and the other had KCNJ5 p.L168R (c.503T>G) mutation. The patient with a dominant APA and MAPN had a typical clinical phenotype of PA (hypokalemia and high aldosterone-to-renin ratio) and concomitant cortisol secretion (Table 2). The imaging study identified bilateral adrenal masses. On the basis of the results of AVS, right adrenalectomy was performed. The resected adrenal demonstrated multiple types of aldosterone-producing lesions (APAs, APNs, and APMs) (Fig. 4A-4F). Interestingly, different aldosterone-driver somatic mutations were identified in these lesions. The NGS results are summarized in Table 4.
Table 4.
Next-generation sequencing results of CYP11B2-expressing lesions in APA with MAPN case
| Sample | Gene | Nucleotide change | Amino Acid change | FDP | VF (%) | Reference sequence |
|---|---|---|---|---|---|---|
| APA | KCNJ5 | c.G451A | p.G151R | 1996 | 41 | NM_000890 |
| APN1 | KCNJ5 | c.G451C | p.G151R | 1991 | 34 | NM_000890 |
| APN2 | KCNJ5 | c.G451C | p.G151R | 1638 | 27 | NM_000890 |
| APN3 | KCNJ5 | c.G451A | p.G151R | 1985 | 12 | NM_000890 |
| APN4 | KCNJ5 | c.T461G | p.F154C | 1999 | 28 | NM_000890 |
| APN5 | CACNA1D | c.C4007G | p.P1336R | 67 | 31 | NM_001128839 |
| APN6 | KCNJ5 | c.A472G | p.T158A | 2000 | 27 | NM_000890 |
| APN7 | KCNJ5 | c.440_442del | p.E147_T148delinsAa | 1990 | 14 | NM_000890 |
| APM1 | — | — | — | — | — | — |
| APM2 | — | — | — | — | — | — |
| APM3 | — | — | — | — | — | — |
| APM4 | — | — | — | — | — | — |
| APM5 | ATP1A1 | c.T995G | p.V332G | 423 | 17 | NM_000701 |
| APM6 | — | — | — | — | — | — |
| APM7 | CACNA1D | c.T827G | p.L276Ra | 1521 | 15 | NM_001128839 |
| APM8 | CACNA1D | c.G1207C | p.G403R | 1993 | 12 | NM_000720 |
| APM9 | — | — | — | — | — | — |
Abbreviations: FDP, flow-corrected read depth; VF, variant allele frequency; APA, aldosterone-producing adenoma; APM, aldosterone-producing micronodule; APN, aldosterone-producing nodule.
a Previously unreported variant.
Discussion
The combination of CYP11B2 IHC and NGS has significantly improved our understanding of the pathophysiology of PA. However, comprehensive histologic and genetic analysis has not been adequately performed in young-onset PA cases due to its rarity. This multicenter collaborative study allowed us to investigate molecular characteristics of PA in young adults.
CYP11B2 IHC revealed that APA is the most common histologic feature of young-onset PA, followed by APN. Combined, these histopathologic forms account for 92% (68/74) of our young adult cohort of PA. Two tumors were classified as NFAs. In 1 of the patients with NFA, adjacent adrenal tissue had MAPM, which may have been the source of aldosterone excess in this patient. Another patient with NFA did not have any CYP11B2-expressing lesions in the adjacent adrenal sections that were studied. Multiple block examination with CYP11B2 IHC may allow identification of CYP11B2-expressing lesions such as APMs in these patients. A recent prospective study of 60 unselected consecutive patients with unilateral PA (mean age of 51 ± 13 years) categorized 45 patients (75%) as having classical histopathologic forms that include APA and APN (30). The authors found that classical histopathologic forms (APA and APN) were associated with better postsurgical biochemical outcome than that of nonclassical forms (MAPN, MAPM, or aldosterone-producing diffuse hyperplasia) (30).
In our study, 78% of young-onset APAs harbored somatic KCNJ5 mutations. The high prevalence of somatic KCNJ5 mutations in young-onset APAs is consistent with the previous findings demonstrating disease onset at a young age as a unique characteristic of KCNJ5-mutated APA (13-15). Predominant prevalence in women is a feature of APA with a somatic KCNJ5 mutation (31, 32). Although no significant sex differences in the prevalence of somatic KCNJ5 mutation were found in APA of this age population, the sex-specific trend in KCNJ5 genotypes (ie, p.L168R vs other mutations) was an interesting observation despite not being statistically significant. Activating CTNNB1 mutations have been reported in 2% to 5% of APAs (22, 33, 34). In our young-onset APA cohort, a slightly higher prevalence of somatic CTNNB1 mutations was observed (4/45, 9%) with no significant sex differences (P > .99).
In contrast to APAs, somatic CACNA1D mutations appear to be common in young-onset APNs, which are smaller CYP11B2-expressing lesions than APAs. In agreement with this, somatic CACNA1D mutations have often been observed in small APAs (10, 15) or in APMs, which were formerly known as aldosterone-producing cell clusters (35, 36). Of note, the histopathologic definition of APN has only recently been determined (18). Therefore, most of the previous studies did not distinguish APN from small APA.
In our cohort, 2 adrenals had MAPN according to the HISTALDO consensus (Fig. 5) (18). Intriguingly, in both MAPN cases, the CYP11B2-expressing lesions shared the identical somatic KCNJ5 mutation (c.451G>A, p.G151R) that is known as 1 of the major hotspots for KCNJ5 mutation in APA. Two similar cases with multiple somatic KCNJ5-mutated (c.451G>A, p.G151R) nodular lesions were recently reported from 2 different groups (37, 38). Maria et al (38) performed ultra-deep sequencing with an average read depth of 1 433 999 and documented very low levels of the KCNJ5 variant at the germline level, suggesting low-level mosaicism of the KCNJ5 mutation. While this may provide an explanation for the results from the MAPN cases in our cohort, the amplification-based NGS method we utilized is unable to adequately distinguish between low-level somatic mosaicism and FFPE artifact—particularly for G>A variants.
There was a rare but interesting case of APA with MAPN in our cohort. As summarized in Table 4, multiple CYP11B2-expressing lesions had distinct aldosterone-driver mutations. Unlike the MAPN cases, APNs in this case showed variable KCNJ5 mutations and 1 had a CACNA1D mutation. Similarly, distinct somatic mutations have been documented in multiple aldosterone-producing nodules within the same adrenal gland (16, 39, 40). Histologic and genetic heterogeneity indicate the possibility of multiple mechanisms underlying the development of PA.
The major findings of our study include the following: (1) APA is the most common histologic feature of young-onset lateralized PA, (2) somatic KCNJ5 mutation is very common in young-onset APA regardless of sex, (3) somatic CACNA1D mutation is the most frequent genetic alteration in young-onset APN, (4) an adrenal with multiple CYP11B2-expressing lesions, a rare histologic form of young-onset PA, can show multiple aldosterone-driver mutations, and (5) NFAs are found in a minority of young adult cases. Our study also demonstrated concordant results among imaging, AVS, and CYP11B2 IHC in most of the young patients with severe PA and unilateral solitary adenoma. However, it is important to note that young adult patients can show heterogeneous clinical presentation, such as bilateral nodules, relatively low plasma aldosterone concentration, and normal potassium levels. In fact, a recent study showed a significant number of young patients with PA (age 40 or younger) had discordant results between an imaging study and AVS (41). Therefore, in young patients with PA, case-specific application of AVS could be beneficial. The major limitations of this study are the retrospective study design, which can lead to institutional selection bias, and our limited ability to assess genotype–clinical outcome correlations. A larger prospective study would more accurately determine histologic subtypes and somatic mutation prevalence and perform further assessment with clinical outcomes.
Acknowledgments
We thank Sarah Brand at the University of Michigan for an organizational role in the American-Australian-Asian Adrenal Alliance (A5) study group, which was supported by the Hirair and Anna Hovnanian Foundation.
Glossary
Abbreviations
- APA
aldosterone-producing adenoma
- APM
aldosterone-producing micronodule
- APN
aldosterone-producing nodule
- AVS
adrenal venous sampling
- FFPE
formalin-fixed, paraffin-embedded
- IHA
idiopathic hyperaldosteronism
- IHC
immunohistochemistry
- H&E
hematoxylin and eosin
- MAPM
multiple aldosterone-producing micronodules
- MAPN
multiple aldosterone-producing nodules
- NFA
nonfunctioning adenoma
- NGS
next-generation sequencing
- PA
primary aldosteronism
Contributor Information
Kazutaka Nanba, Department of Molecular and Integrative Physiology, University of Michigan, Ann Arbor, MI, 48109, USA; Department of Endocrinology and Metabolism, National Hospital Organization Kyoto Medical Center, Kyoto, 612-8555, Japan.
Jessica E Baker, Department of Molecular and Integrative Physiology, University of Michigan, Ann Arbor, MI, 48109, USA.
Amy R Blinder, Department of Molecular and Integrative Physiology, University of Michigan, Ann Arbor, MI, 48109, USA.
Nolan R Bick, Department of Pathology, University of Michigan, Ann Arbor, MI, 48109, USA.
Chia-Jen Liu, Department of Pathology, University of Michigan, Ann Arbor, MI, 48109, USA.
Jung Soo Lim, Department of Molecular and Integrative Physiology, University of Michigan, Ann Arbor, MI, 48109, USA.
Heather Wachtel, Division of Endocrine and Oncologic Surgery, Department of Surgery, Hospital of the University of Pennsylvania, Philadelphia, PA, 19104, USA.
Debbie L Cohen, Division of Renal, Electrolyte and Hypertension, Department of Medicine, Hospital of the University of Pennsylvania, Philadelphia, PA, 19104, USA.
Tracy Ann Williams, Medizinische Klinik und Poliklinik IV, Klinikum der Universität München, Ludwig-Maximilians-Universität München, München, 80336, Germany; Division of Internal Medicine and Hypertension, Department of Medical Sciences, University of Turin, Turin, 10126, Italy.
Martin Reincke, Medizinische Klinik und Poliklinik IV, Klinikum der Universität München, Ludwig-Maximilians-Universität München, München, 80336, Germany.
Melanie L Lyden, Department of Surgery, Mayo Clinic, Rochester, MN, 55905, USA.
Irina Bancos, Division of Endocrinology, Diabetes, Metabolism, and Nutrition, Mayo Clinic, Rochester, MN, 55905, USA.
William F Young, Division of Endocrinology, Diabetes, Metabolism, and Nutrition, Mayo Clinic, Rochester, MN, 55905, USA.
Tobias Else, Division of Metabolism, Endocrine, and Diabetes, Department of Internal Medicine, University of Michigan, Ann Arbor, MI, 48109, USA.
Thomas J Giordano, Department of Pathology, University of Michigan, Ann Arbor, MI, 48109, USA; Division of Metabolism, Endocrine, and Diabetes, Department of Internal Medicine, University of Michigan, Ann Arbor, MI, 48109, USA; Rogel Cancer Center, University of Michigan, Ann Arbor, MI, 48109, USA.
Aaron M Udager, Department of Pathology, University of Michigan, Ann Arbor, MI, 48109, USA; Rogel Cancer Center, University of Michigan, Ann Arbor, MI, 48109, USA; Michigan Center for Translational Pathology, University of Michigan, Ann Arbor, MI, 48109, USA.
William E Rainey, Department of Molecular and Integrative Physiology, University of Michigan, Ann Arbor, MI, 48109, USA; Division of Metabolism, Endocrine, and Diabetes, Department of Internal Medicine, University of Michigan, Ann Arbor, MI, 48109, USA.
Financial Support
This work was supported by grants from American Heart Association (17SDG33660447 to K. Nanba), the Humboldt Foundation (W.E. Rainey), the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (DK106618 and DK043140 to W.E. Rainey), National Center for Advancing Translational Sciences (KL2TR001879 to H. Wachtel), and National Heart, Lung and Blood institute (R01HL130106 to T. Else.). K. Nanba is supported by a Japan Heart Foundation Research Grant. T.A. Williams and M. Reincke receive funding from the Deutsche Forschungsgemeinschaft (DFG) project number 444776998 (WI 5359/2-1 and RE 752/31-1) and project number 314061271-TRR 205. M. Reincke is also supported by the Else Kröner-Fresenius Stiftung in support of the German Conn’s Registry-Else-Kröner Hyperaldosteronism Registry (2013_A182, 2015_A171, and 2019_A104). This research was also partly supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under award K23DK121888 (to I. Bancos). The views expressed are those of the author(s) and not necessarily those of the National Institutes of Health, USA.
Disclosures
I.Bancos reports advisory board participation and/or consulting with Sparrow Pharmaceutics, Adrenas Therapeutics, Corcept, Recordati, and HRA Pharma outside the submitted work.
Data Availability
The datasets generated during and/or analyzed during the present study are not publicly available but are available from the corresponding authors on reasonable request.
References
- 1. Monticone S, Burrello J, Tizzani D, et al. Prevalence and clinical manifestations of primary aldosteronism encountered in primary care practice. J Am Coll Cardiol. 2017;69(14):1811-1820. [DOI] [PubMed] [Google Scholar]
- 2. Calhoun DA, Nishizaka MK, Zaman MA, et al. Hyperaldosteronism among black and white subjects with resistant hypertension. Hypertension. 2002;40(6):892-896. [DOI] [PubMed] [Google Scholar]
- 3. Young WF, Stanson AW, Thompson GB, et al. Role for adrenal venous sampling in primary aldosteronism. Surgery. 2004;136(6):1227-1235. [DOI] [PubMed] [Google Scholar]
- 4. Funder JW, Carey RM, Mantero F, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016;101(5):1889-1916. [DOI] [PubMed] [Google Scholar]
- 5. Rossi GP, Dalla Ca A; Italian Society of H. Clinical management of primary aldosteronism: 2013 practical recommendations of the Italian Society of Hypertension (SIIA). High Blood Press Cardiovasc Prev. 2014;21(1):71-75. [DOI] [PubMed] [Google Scholar]
- 6. Nishikawa T, Omura M, Satoh F, et al. ; Task Force Committee on Primary Aldosteronism The Japan Endocrine Society. Guidelines for the diagnosis and treatment of primary aldosteronism—the Japan Endocrine Society 2009. Endocr J. 2011;58(9):711-721. [DOI] [PubMed] [Google Scholar]
- 7. Choi M, Scholl UI, Yue P, et al. K+ channel mutations in adrenal aldosterone-producing adenomas and hereditary hypertension. Science. 2011;331(6018):768-772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Beuschlein F, Boulkroun S, Osswald A, et al. Somatic mutations in ATP1A1 and ATP2B3 lead to aldosterone-producing adenomas and secondary hypertension. Nat Genet. 2013;45(4):440-4, 444e1-2. [DOI] [PubMed] [Google Scholar]
- 9. Scholl UI, Goh G, Stolting G, et al. Somatic and germline CACNA1D calcium channel mutations in aldosterone-producing adenomas and primary aldosteronism. Nat Genet. 2013;45(9):1050-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Azizan EA, Poulsen H, Tuluc P, et al. Somatic mutations in ATP1A1 and CACNA1D underlie a common subtype of adrenal hypertension. Nat Genet. 2013;45(9):1055-1060. [DOI] [PubMed] [Google Scholar]
- 11. Nanba K, Blinder AR, Rege J, et al. Somatic CACNA1H mutation as a cause of aldosterone-producing adenoma. Hypertension. 2020;75(3):645-649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dutta RK, Arnesen T, Heie A, et al. A somatic mutation in CLCN2 identified in a sporadic aldosterone-producing adenoma. Eur J Endocrinol. 2019;181(5):K37-K41. [DOI] [PubMed] [Google Scholar]
- 13. Boulkroun S, Beuschlein F, Rossi GP, et al. Prevalence, clinical, and molecular correlates of KCNJ5 mutations in primary aldosteronism. Hypertension. 2012;59(3):592-598. [DOI] [PubMed] [Google Scholar]
- 14. Lenzini L, Rossitto G, Maiolino G, et al. A meta-analysis of somatic KCNJ5 K(+) channel mutations in 1636 patients with an aldosterone-producing adenoma. J Clin Endocrinol Metab. 2015;100(8):E1089-E1095. [DOI] [PubMed] [Google Scholar]
- 15. Fernandes-Rosa FL, Williams TA, Riester A, et al. Genetic spectrum and clinical correlates of somatic mutations in aldosterone-producing adenoma. Hypertension. 2014;64(2):354-361. [DOI] [PubMed] [Google Scholar]
- 16. Nanba K, Chen AX, Omata K, et al. Molecular heterogeneity in aldosterone-producing adenomas. J Clin Endocrinol Metab. 2016;101(3):999-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gomez-Sanchez CE, Qi X, Velarde-Miranda C, et al. Development of monoclonal antibodies against human CYP11B1 and CYP11B2. Mol Cell Endocrinol. 2014;383(1-2):111-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Williams TA, Gomez-Sanchez CE, Rainey WE, et al. International histopathology consensus for unilateral primary aldosteronism. J Clin Endocrinol Metab. 2021;106(1):42-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nanba K, Omata K, Tomlins SA, et al. Double adrenocortical adenomas harboring independent KCNJ5 and PRKACA somatic mutations. Eur J Endocrinol. 2016;175(2):K1-K6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Funder JW, Carey RM, Fardella C, et al. Case detection, diagnosis, and treatment of patients with primary aldosteronism: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2008;93(9):3266-3281. [DOI] [PubMed] [Google Scholar]
- 21. Akerstrom T, Crona J, Delgado Verdugo A, et al. Comprehensive re-sequencing of adrenal aldosterone producing lesions reveal three somatic mutations near the KCNJ5 potassium channel selectivity filter. PLoS One. 2012;7(7):e41926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Scholl UI, Healy JM, Thiel A, et al. Novel somatic mutations in primary hyperaldosteronism are related to the clinical, radiological and pathological phenotype. Clin Endocrinol (Oxf). 2015;83(6):779-789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nanba K, Omata K, Else T, et al. Targeted molecular characterization of aldosterone-producing adenomas in White Americans. J Clin Endocrinol Metab. 2018;103(10):3869-3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nanba K, Omata K, Gomez-Sanchez CE, et al. Genetic characteristics of aldosterone-producing adenomas in blacks. Hypertension. 2019;73(4):885-892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nanba K, Yamazaki Y, Bick N, et al. Prevalence of somatic mutations in aldosterone-producing adenomas in Japanese patients. J Clin Endocrinol Metab. 2020;105(11):e4066-e4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Guo Z, Nanba K, Udager A, et al. Biochemical, histopathological, and genetic characterization of posture-responsive and unresponsive APAs. J Clin Endocrinol Metab. 2020;105(9): e3224-e3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Azizan EA, Lam BY, Newhouse SJ, et al. Microarray, qPCR, and KCNJ5 sequencing of aldosterone-producing adenomas reveal differences in genotype and phenotype between zona glomerulosa- and zona fasciculata-like tumors. J Clin Endocrinol Metab. 2012;97(5):E819-E829. [DOI] [PubMed] [Google Scholar]
- 28. Monticone S, Castellano I, Versace K, et al. Immunohistochemical, genetic and clinical characterization of sporadic aldosterone-producing adenomas. Mol Cell Endocrinol. 2015;411:146-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yamazaki Y, Omata K, Tezuka Y, et al. Tumor cell subtypes based on the intracellular hormonal activity in KCNJ5-mutated aldosterone-producing adenoma. Hypertension. 2018;72(3):632-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Meyer LS, Handgriff L, Lim JS, et al. Single-center prospective cohort study on the histopathology, genotype, and postsurgical outcomes of patients with primary aldosteronism. Hypertension. 2021;78(3):738-746. [DOI] [PubMed] [Google Scholar]
- 31. Williams TA, Lenders JW, Burrello J, et al. KCNJ5 mutations: sex, salt and selection. Horm Metab Res. 2015;47(13):953-958. [DOI] [PubMed] [Google Scholar]
- 32. Nanba K, Rainey WE. Genetics in endocrinology: impact of race and sex on genetic causes of aldosterone-producing adenomas. Eur J Endocrinol. 2021;185(1):R1-R11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Akerstrom T, Maharjan R, Sven Willenberg H, et al. Activating mutations in CTNNB1 in aldosterone producing adenomas. Sci Rep. 2016;6:19546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wu VC, Wang SM, Chueh SJ, et al. The prevalence of CTNNB1 mutations in primary aldosteronism and consequences for clinical outcomes. Sci Rep. 2017;7:39121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nishimoto K, Tomlins SA, Kuick R, et al. Aldosterone-stimulating somatic gene mutations are common in normal adrenal glands. Proc Natl Acad Sci USA. 2015;112(33):E4591-E4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Omata K, Anand SK, Hovelson DH, et al. Aldosterone-producing cell clusters frequently harbor somatic mutations and accumulate with age in normal adrenals. J Endocr Soc. 2017;1(7):787-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tamura A, Nishimoto K, Seki T, et al. Somatic KCNJ5 mutation occurring early in adrenal development may cause a novel form of juvenile primary aldosteronism. Mol Cell Endocrinol. 2017;441:134-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Maria AG, Suzuki M, Berthon A, et al. Mosaicism for KCNJ5 causing early-onset primary aldosteronism due to bilateral adrenocortical hyperplasia. Am J Hypertens. 2020;33(2):124-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fernandes-Rosa FL, Giscos-Douriez I, Amar L, et al. Different somatic mutations in multinodular adrenals with aldosterone-producing adenoma. Hypertension. 2015;66(5):1014-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. De Sousa K, Boulkroun S, Baron S, et al. Genetic, cellular, and molecular heterogeneity in adrenals with aldosterone-producing adenoma. Hypertension. 2020;75(4):1034-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gkaniatsa E, Sakinis A, Palmer M, et al. Adrenal venous sampling in young patients with primary aldosteronism. extravagance or irreplaceable? J Clin Endocrinol Metab. 2021;106(5):e2087- e2095. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the present study are not publicly available but are available from the corresponding authors on reasonable request.