Abstract
The inhibins control reproduction by suppressing follicle-stimulating hormone synthesis in pituitary gonadotrope cells. The newly discovered inhibin B coreceptor, TGFBR3L, is selectively and highly expressed in gonadotropes in both mice and humans. Here, we describe our initial characterization of mechanisms controlling cell-specific Tgfbr3l/TGFBR3L transcription. We identified two steroidogenic factor 1 (SF-1 or NR5A1) cis-elements in the proximal Tgfbr3l promoter in mice. SF-1 induction of murine Tgfbr3l promoter–reporter activity was inhibited by mutations in one or both sites in heterologous cells. In homologous cells, mutation of these cis-elements or depletion of endogenous SF-1 similarly decreased reporter activity. We observed nearly identical results when using a human TGFBR3L promoter–reporter. The Tgfbr3l gene was tightly compacted and Tgfbr3l mRNA expression was essentially absent in gonadotropes of SF-1 (Nr5a1) conditional knockout mice. During murine embryonic development, Tgfbr3l precedes Nr5a1 expression, though the two transcripts are fully colocalized by embryonic day 18.5 and thereafter. Collectively, these data indicate that SF-1 directly regulates Tgfbr3l/TGFBR3L transcription and is required for postnatal expression of the gene in gonadotropes.
Keywords: transcription, inhibin, receptor, pituitary, knockout mouse, cell line
Reproduction is controlled by intricate endocrine feedforward/feedback loops between the brain, pituitary gland, and the gonads. Follicle-stimulating hormone (FSH) is a dimeric glycoprotein produced by pituitary gonadotrope cells that regulates ovarian follicle development and estrogen biosynthesis in females and spermatogenesis in males (1, 2). FSH production is stimulated by gonadotropin-releasing hormone (GnRH) from the brain and by transforming growth factor β (TGFβ) ligands, such as the activins, which are currently thought to act in an autocrine/paracrine manner in the pituitary to stimulate transcription of the FSHβ subunit (3).
FSH also stimulates the synthesis and secretion of activin-related TGFβ ligands, known as the inhibins, from the gonads. The ovaries produce inhibins A and B (4–6), whereas the adult testes in most mammalian species produce inhibin B alone (7). Inhibins feed back to gonadotropes to suppress FSH production by competitively binding to activin receptors (8). Inhibin binding to these receptors is enhanced by transmembrane coreceptors. The TGFβ type III receptor (TGFBR3), also known as betaglycan, can mediate the actions of both inhibins (8, 9); however, conditional deletion of the Tgfbr3 gene in gonadotropes principally impairs inhibin A, leaving inhibin B action intact (10). This observation led to the recent discovery of a betaglycan-like protein, TGFBR3L, which functions as a specific inhibin B coreceptor in gonadotropes (11).
Whereas betaglycan is broadly expressed, including in the pituitary, gonads, and adrenal glands (12), Tgfbr3l/TGFBR3L expression appears to be restricted to gonadotropes in adult mice and humans (11, 13–15). The mechanisms conferring this cell-specific expression have not been elucidated. Here, we report that the nuclear receptor, steroidogenic factor 1 (SF-1, product of the Nr5a1 gene), regulates Tgfbr3l/TGFBR3L transcription via conserved regulatory elements in the proximal promoter. In vivo, SF-1 is necessary for the maintenance, but not the initial expression, of Tgfbr3l in gonadotropes.
Materials and Methods
DNA Constructs
The wild-type murine −999/+1 Tgfbr3l and human −996/+1 TGFBR3L luciferase promoter–reporters were produced by polymerase chain reaction (PCR) amplification of genomic DNA (see Table 1 for primers). The PCR products were ligated into pGL3-Basic (Promega, Madison, WI, USA) or pA3-luc (16). The murine SF-1 expression construct has been described previously (17). The mutant promoter–reporters were constructed using the QuikChange protocol with primers described in Table 1. All constructs were confirmed by sequencing (Génome Québec, Montreal, QC, Canada).
Table 1.
Primers
| Murine Tgfbr3l promoter amplification | |
| 5′ RACE gene-specific RT primer | GGACGGACGAGGTATTGTGA |
| 5′ RACE gene-specific outer R | CCTGCGTCCGGTATTCAATG |
| 5′ RACE gene-specific inner R | GGGCGTGAAGAAGGTGTTAC |
| −999/+1 promoter amplification F | AAAACTCGAGGTAGCTGATGCAACCATACGTAG |
| −999/+1 promoter amplification R | AAAAAAGCTTGACCGGCAGCGAGCACCT |
| Human TGFBR3L promoter amplification | |
| −996/+1 promoter amplification F | AAAACTCGAGTAGGCGTAGCATCCCTCTC |
| −996/+1 promoter amplification R | TTTTAAGCTTGGACCAGCTGAGGTCGGA |
| Tgfbr3l/TGFBR3L mutagenesis | |
| Tgfbr3l SF-1 site 1 mut F | ATCTGAGCACATGCTCAATTCCAGCCATGGATAAGGGC |
| Tgfbr3l SF-1 site 1 mut R | GCCCTTATCCATGGCTGGAATTGAGCATGTGCTCAGAT |
| Tgfbr3l SF-1 site 2 mut F | GGTGGCAGCCTCACCAATTCCAGGGCTACC |
| Tgfbr3l SF-1 site 2 mut R | GGTAGCCCTGGAATTGGTGAGGCTGCCACC |
| TGFBR3L SF-1 site 1 mut F | CCAGAGCCAATGCCCAATTCCTGAGGGGATTAAGGG |
| TGFBR3L SF-1 site 1 mut R | CCCTTAATCCCCTCAGGAATTGGGCATTGGCTCTGG |
| TGFBR3L SF-1 site 2 mut F | GGCGGCCCTGTAATTGGCAAGGAGGGAGGCA |
| TGFBR3L SF-1 site 2 mut R | TGCCTCCCTCCTTGCCAATTACAGGGCCGCC |
| qPCR primers | |
| Fshb F | GTGCGGGCTACTGCTACACT |
| Fshb R | CAGGCAATCTTACGGTCTCG |
| Gnrhr F | TTCGCTACCTCCTTTGTCGT |
| Gnrhr R | CACGGGTTTAGGAAAGCAAA |
| Lhb F | ACTGTGCCGGCCTGTCAACG |
| Lhb R | AGCAGCCGGCAGTACTCGGA |
| Nr5a1 F | AGGAGTTCGTCTGTCTCAAGTTCCT |
| Nr5a1 R | ACAAGGTGTAATCCAACAGGGCAG |
| Rpl19 F | CGGGAATCCAAGAAGATTGA |
| Rpl19 R | TTCAGCTTGTGGATGTGCTC |
| Tgfbr3l F | CCTGACACCAGTGCCTTTGA |
| Tgfbr3l R | CTAGGGGACGGACGAGGTAT |
| Tgfbr3l SF-1 ChIP qPCR F | TCAGTACATCAAGAAAGCCC |
| Tgfbr3l SF-1 ChIP qPCR R | GTACCCAGCCCTCTAGGT |
| Gene desert qPCR F | GTCACAGAAACGCAAAGGTTTA |
| Gene desert qPCR R | CCCAAAGTCATGTTGTACTTGATAG |
| Genotyping primers | |
| GRIC F | GGACATGTTCAGGGATCGCCAGGC |
| GRIC R | GCATAACCAGTGAAACAGCATTGCTG |
| Nr5a1 F | AGGAGTTCGTCTGTCTCAAGTTCC |
| Nr5a1 R | ACAAGGTGTAATCCAACAGGGCAG |
| Nr5a1 R (recombinant) | TGCGTGCAATCCATCTTGTTCAAT |
5′ Rapid Amplification of cDNA Ends
5′ Rapid amplification of cDNA ends (RACE) was performed using the FirstChoice RLM-RACE kit (AM1700, Invitrogen, Waltham, MA, USA) following the manufacturer’s protocol. Briefly, 10 µg of intact 5′ capped mRNA from the pituitary of a male C57BL6 mouse was decapped and ligated to the 5′ RACE adaptor. The ligated mRNA was reverse transcribed with Moloney Murine Leukemia Virus reverse transcriptase (MMLV-RT) using a gene-specific primer located in exon 4 (see Table 1). Nested PCR was performed on the resulting cDNA using adaptor-specific forward primers and gene-specific reverse primers (see Table 1). PCR products were ligated into the pGEM-T Easy vector (A1360, Promega) and sequenced (Génome Québec, Montreal, QC, CAN).
Cell Culture and Promoter–Reporter Assays
All cells were cultured at 37°C in a humidified incubator with 5% CO2. Human embryonic kidney (HEK) 293T cells (ATCC CRL-3216; RRID:CVCL_0063; provided by Dr. Terry Hébert, McGill University) were cultured in Dulbecco’s modified Eagle’s medium (319-005-CL, Wisent, St-Bruno, QC, Canada) supplemented with 5% (v/v) fetal bovine serum (098150, Wisent). Immortalized murine gonadotrope-like LβT2 cells (18) (RRID:CVCL_0398; provided by Dr. Pamela Mellon, University of California, San Diego, CA, USA) were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% (v/v) fetal bovine serum.
Promoter–reporter assays were performed as previously described (16). Briefly, HEK293T and LβT2 cells were seeded at densities of 50 000 and 150 000 cells per well, respectively, in 48-well plates. The following day, HEK293T cells were transfected using polyethylenimine (PEI) at a ratio of 1:3 (μg DNA to μg PEI). LβT2 cells were transfected using Lipofectamine 3000 (L3000015, ThermoFisher Scientific, Burlington, ON, Canada) following the manufacturer’s protocol. Control (D-001210-05) and Nr5a1 (D-051262-01) short interfering RNAs (siRNAs) were purchased from Dharmacon (Lafayette, CO, USA). For assays where siRNAs and the murine promoter–reporter were cotransfected, the pA3-luc backbone was used. Twenty-four hours after transfection, cells were serum starved for an additional 24 hours. After starvation, cells were lysed in 50 µL/well passive lysis buffer (25 mM Tris-phosphate [pH 7.8], 10% [v/v] glycerol, 1% [v/v] Triton X-100, 1 mg/mL bovine serum albumin, 2 mM ethylenediaminetetraaceticacid [EDTA]) for 10 minutes at room temperature with agitation. Twenty microliters of cell lysis supernatant was combined with 100 µL of assay buffer (15 mM potassium phosphate [pH 7.8], 25 mM glycylglycine, 15 mM MgSO4, 4 mM EDTA, 2 mM adenosine triphosphate, 1 mM dithiothreitol, 0.04 mM D-luciferin), and luciferase activity was measured on an Orion II microplate luminometer (Berthold Detection Systems, Oak Ridge, TN, USA). All experiments were performed in technical triplicates and the experiments repeated as indicated in the figures.
DNA Affinity Purification Assay and Immunoblot
Thirty microliters of Dynabeads® M-280 (11205D, Dynal, Invitrogen) were washed three times with 2× B&W buffer (10 mM Tris, pH 7.5, 1 mM EDTA, 2 M NaCl), then 10 µM each of wild-type or mutant biotinylated double-stranded probe (see Table 2) was incubated with the beads in 1× B&W buffer at room temperature for 15 minutes. Beads were washed twice with 2× B&W buffer and once with 1× binding buffer (5% [v/v] glycerol, 20 mM Tris, pH 7.5, 1 mM EDTA, 1 mM dithiothreitol, 0.15% Triton X-100, 100 mM NaCl, 4 mM MgCl2), then blocked for 30 minutes at room temperature using 1% (w/v) bovine serum albumin in binding buffer, and lastly resuspended in 50 μL of 1× binding buffer. LβT2 cells were grown until confluent in 10-cm plates and harvested using 1 mL of DNAP lysis buffer (300 mM NaCl, 20 mM Tris-HCl [pH 7.5], 1% [v/v] Triton X-100, 1 mM polymethylsulfonyl fluoride, 2 μg/mL leupeptin and aprotinin). One hundred microliters of clarified lysate was combined in a 500-μL reaction with 100 μL of 5× binding buffer, 10 μL of 0.5 μg/μL salmon sperm DNA (Invitrogen, 15632011), and 50 μL of DNA-bound streptavidin magnetic beads. The reaction was incubated at 4°C for 2 hours with agitation, followed by 5 washes in 1× binding buffer. Bound proteins were eluted in 40 μL of 0.1% sodium dodecyl sulfate (SDS) at 100°C for 5 minutes.
Table 2.
DNAP probes
| Tgfbr3l SF-1 site 1 WT sense | TATCCATGGCTGGCCTTGAGCATGT |
| Tgfbr3l SF-1 site 1 WT antisense | ACATGCTCAAGGCCAGCCATGGATA |
| Tgfbr3l SF-1 site 1 Mut sense | TATCCATGGCTGGAATTGAGCATGT |
| Tgfbr3l SF-1 site 1 Mut antisense | ACATGCTCAATTCCAGCCATGGATA |
| Tgfbr3l SF-1 site 2 WT sense | AGCCTCACCAAGGCCAGGGCTACCT |
| Tgfbr3l SF-1 site 2 WT antisense | AGGTAGCCCTGGCCTTGGTGAGGCT |
| Tgfbr3l SF-1 site 2 Mut sense | AGCCTCACCAATTCCAGGGCTACCT |
| Tgfbr3l SF-1 site 2 Mut antisense | AGGTAGCCCTGGAATTGGTGAGGCT |
| TGFBR3L SF-1 site 1 WT sense | TAATCCCCTCAGGCCTTGGGCATTG |
| TGFBR3L SF-1 site 1 WT antisense | CAATGCCCAAGGCCTGAGGGGATTA |
| TGFBR3L SF-1 site 1 Mut sense | TAATCCCCTCAGGAATTGGGCATTG |
| TGFBR3L SF-1 site 1 Mut antisense | CAATGCCCAATTCCTGAGGGGATTA |
| TGFBR3L SF-1 site 2 WT sense | CTCCTTGCCAAGGACAGGGCCGCCT |
| TGFBR3L SF-1 site 2 WT antisense | AGGCGGCCCTGTCCTTGGCAAGGAG |
| TGFBR3L SF-1 site 2 Mut sense | CTCCTTGCCAATTACAGGGCCGCCT |
| TGFBR3L SF-1 site 2 Mut antisense | AGGCGGCCCTGTAATTGGCAAGGAG |
Ten microliters of 5× Laemmli buffer (250 mM Tris pH 6.8, 10% SDS, 50% glycerol, 0.02% bromophenol blue, and 10% β-mercaptoethanol) was added and eluted proteins were resolved by SDS-polyacrylamide gel electrophoresis on a 10% resolving gel prepared using a 30% (w/w) acrylamide/bis-acrylamide (29:1) solution in running buffer (25 mM Tris, 250 mM glycine, 0.1% SDS, pH 8.3). Proteins were transferred to Protran nitrocellulose membranes (GE 10600001, Millipore Sigma, Oakville, Ontario, Canada) in Towbin buffer (25 mM Tris, 192 mM glycine, pH 8.3, 20% methanol), blocked with 5% milk (w/v) in Tris-buffered saline (TBS; 150 mM NaCl, 10 mM Tris [pH 8.0]) containing 0.05% (v/v) Tween 20 (TBST) and incubated overnight at 4°C with agitation with an antibody against SF-1 diluted in blocking buffer (1:1000; D1Z2A; Cell Signaling Technology, Danvers, MA, USA; RRID:AB_2798030). The next day, membranes were washed in TBST and incubated in horseradish peroxidase–conjugated antirabbit secondary antibody (1:5000; AP182P; Millipore Sigma; RRID:AB_92591) in blocking buffer for 1 hour at room temperature with agitation. Membranes were once again washed in TBST, and bands were visualized using enhanced chemiluminescence substrate (NEL105001, PerkinElmer, Waltham, MA, USA) and an Amersham Imager 600 (GE Healthcare, Chicago, IL, USA).
Chromatin Immunoprecipitation
LβT2 cells were seeded at a density of 1.5 million cells/well in a 6-well plate. After 3 days, formaldehyde was added to a final concentration of 1%, and crosslinking was performed for 10 minutes at room temperature. The reaction was then quenched with 125 mM glycine for 5 minutes at room temperature. Cells were scraped and collected in 1 mL of ice-cold phosphate-buffered saline (PBS) and centrifuged at 800g for 10 minutes at 4°C. The cell pellet was then resuspended in 100 μL of Nuc101 EZ lysis buffer (NUC101, Millipore Sigma) with protease inhibitor cocktail (04693116001, Millipore Sigma) and 1 mM polymethylsulfonyl fluoride for 5 minutes on ice, then centrifuged at 500g for 5 minutes at 4°C. Supernatant was discarded and the pellet washed in Nuc101 EZ lysis buffer as above. The nuclei were then divided into 3 tubes for digestion.
Nuclei (in 50 μL of Nuc101 EZ lysis buffer) were mixed with 6 μL of 10× MNase buffer (10 mM Tris-HCl [pH 7.5], 50 mM NaCl, 1 mM EDTA, 50% glycerol), 0.44 μL of 200 mM dithiothreitol, and 1 μL of diluted MNase (M0247S, New England Biolabs, Ipswich, MA, USA; diluted 1:10 in MNase reaction buffer (50 mM Tris-HCl [pH 7.9], 5 mM CaCl2) in a total reaction volume of 60 μL. Nuclei were digested for 10 minutes at 37°C and the digestion was stopped with 6.6 μL of 100 mM EDTA and 6.6 μL of 1% Triton X-100/1% sodium deoxycholate. Chromatin was incubated on ice for 20 minutes, then 220 μL of complete IP buffer (20 mM Tris-HCl [pH 8], 2 mM EDTA, 150 mM NaCl, 0.1% Triton X-100, 1× protease inhibitor cocktail, 1 mM polymethylsulfonyl fluoride) was added. Ten percent of the chromatin was removed and kept as “input.” NaCl was added to the input chromatin to a final concentration of 0.2 M, and protein:DNA complexes were reverse crosslinked overnight at 65°C. The next day, samples were incubated for 30 minutes at 37°C with 10 µg of RNase A in a total volume of 200 μL followed by 1 hour at 55°C with 10 µg of proteinase K in a total volume of 200 μL. DNA was extracted with phenol:chloroform:isoamyl alcohol and precipitated with sodium acetate and ethanol overnight at −20°C. The DNA was pelleted at 15 000 rpm at 4°C for 20 minutes, washed with 70% ethanol, dried, dissolved in 30 µL of 10 mM Tris-HCl (pH 8.0), and quantified using Nanodrop.
Ten micrograms of chromatin was precleared for 1 hour at 4°C with 10 µL of Dynal protein G beads (10003D, Invitrogen) on an end-over-end rotator, then incubated with protein G beads conjugated to either rabbit IgG (2729, Cell Signaling Technology; RRID:AB_1031062) or rabbit anti-SF-1 (12 800, Cell Signaling Technology; RRID:AB_2798030) overnight. To conjugate the antibodies to the beads, 10 µL of beads (washed 3 times in complete IP buffer) were incubated with 1 µg of antibody in a total volume of 200 µL for 4 hours at 4°C on an end-over-end rotator. The next day, after overnight incubation with chromatin, beads were sequentially washed with complete IP buffer (1 × 5 minutes at 4°C), low salt buffer (20 mM Tris-HCl [pH 8], 2 mM EDTA, 150 mM NaCl, 1% Triton X-100, 0.1% SDS) (2 × 5 minutes at 4°C), and high salt buffer (20 mM Tris-HCl [pH 8], 2 mM EDTA, 500 mM NaCl, 1% Triton X-100, 0.1% SDS) (1 × 5 minutes followed by 1 × 10 minutes at 4°C). Chromatin was eluted for 90 minutes at 65°C in 30 µL of elution buffer (1 M NaHCO3, 1% SDS), then reverse crosslinked, digested with RNase A and proteinase K, and extracted as described above.
Input and immunoprecipitated chromatin were analyzed using quantitative (q)PCR (described below) using primers listed in Table 1.
Generation of Nr5a1 Conditional Knockout Mice
The Nr5a1fl/fl and GnrhrIRES-Cre/IRES-Cre (GRIC) mice have been previously described (19, 20). Cre-mediated recombination occurs in the germline of male GRIC mice (21); thus, the GRIC allele was introduced via the female in all crosses. Nr5a1fl/fl males (Jackson Laboratory, 007041) (22) were crossed with GRIC females to produce Nr5a1fl/+;GnrhrGRIC/+ progeny. Nr5a1fl/fl males were then crossed with Nr5a1fl/+;GnrhrGRIC/+ females to produce Nr5a1fl/fl;Gnrhr+/+ (control) and Nr5a1fl/fl;GnrhrGRIC/+ (conditional knockout; cKO) animals. Genotyping and assessment of genomic recombination were conducted as previously described (23) (primers listed in Table 1). All animals were housed on a 12 hours light:12 hours dark cycle and given access to food and water ad libitum. All animal work was conducted in accordance with federal and institutional guidelines and with the approval of the McGill University Facility Animal Care Committee-DOW-A (protocol 5204).
Organ Collection and Processing
Testes, seminal vesicles, ovaries, and uteri were dissected from control and cKO males at 8-10 weeks of age, and females at 9-10 weeks of age. Control females were collected at random points in the estrous cycle; cKO females were acyclic. All reproductive organs were weighed on an analytical balance. Pituitary glands were snap frozen in liquid nitrogen and stored at −80°C until analysis.
Blood Collection and Hormone Analyses
Blood was collected by cardiac puncture and allowed to coagulate at room temperature for approximately 30 minutes. Whole blood was centrifuged at 3000 rpm for 10 minutes at room temperature. Serum was collected and stored at −20°C until hormone analyses were conducted.
Serum FSH was assessed using a Milliplex kit (Millipore, MPTMAG-49K, custom-made for FSH only) following the manufacturer’s instructions (lower detection limit: 23.7 pg/mL; dynamic range: 61.0 pg/mL to 250 000 pg/mL; limit of quantification: 61.0 pg/mL; intra-assay coefficient of variation <15%). Serum luteinizing hormone (LH) was measured using an in-house sandwich enzyme-linked immunosorbent assay (24) (lower detection limit: 0.117 ng/mL; dynamic range: 0.117 ng/mL to 30 ng/mL; limit of quantification: 0.516 ng/mL; intra-assay coefficient of variation: <10%) (25).
RNA Extraction and Reverse Transcription Quantitative PCR
RNA was extracted from tissues and LβT2 cells using TRIzol Reagent (15596018; Invitrogen, Waltham, MA, USA) following the manufacturer’s protocol. Two hundred nanograms of total RNA (concentration determined using Nanodrop spectrophotometer) was reverse transcribed using random hexamers (C1181, Promega) and MMLV reverse transcriptase (M1701, Promega).
qPCR analysis was performed using EvaGreen (ABMMmix, Diamed, Missisauga, ON, Canada) and primers listed in Table 1 on a Corbett Rotorgene 600 instrument (Corbett Life Science, Sydney, NSW, Australia). mRNA levels were determined using the 2–ΔΔCT method. Gene expression was normalized to ribosomal protein L19 (Rpl19). All primers were validated for efficiency and specificity.
Immunofluorescence
Eleven-week-old mice were perfused with 4% paraformaldehyde in PBS and dissected pituitaries were post-fixed in 4% paraformaldehyde (P6148, Millipore Sigma) at room temperature for 2 hours. Samples were washed in PBS, cryoprotected with 30% sucrose overnight at 4°C, and embedded in Optimal Cutting Temperature (OCT) compound (95057-838, VWR International, Radnor, PA, USA). Frozen tissue was sectioned at a thickness of 10 μm using a Leica CM3050S cryostat, mounted on Fisherbrand Superfrost Plus slides (22-037-246, ThermoFisher Scientific), and stored at −80°C until use. Antigen retrieval was performed by incubating tissue in 0.01 M citric acid–sodium citrate buffer (pH 6.0) with 0.05% Triton X-100 at 90°C to 95°C for 30 minutes and cooled gradually until buffer temperature was between 30°C and 40°C. Sections were washed with PBS with 0.1% Tween (PBST), blocked in 5% bovine serum albumin (in PBST) for 1 hour at room temperature, then incubated overnight at 4°C with an antibody against SF-1 (described above) (1:100, diluted in blocking solution). Slides were washed in PBST, incubated at room temperature with Alexa Fluor 647 goat antirabbit (1:600; A27040, Invitrogen) for 1 hour, washed in PBST, and mounted in Prolong Diamond Antifade Mountant with DAPI (P36966, Invitrogen). Fluorescent images were captured using a Leica SP8 confocal laser scanning microscope with a Leica HC PL CS2 63×/1.4 NA oil objective.
Single-cell Analyses
Pituitaries were collected from 10- to 12-week-old control and cKO males, immediately snap frozen in liquid nitrogen, and stored at −80°C until analysis. Nuclei were isolated from individual pituitaries and single nucleus assay for transposase-accessible chromatin (snATAC-seq) was performed using the Single Cell ATAC reagent kit V1 (10X Genomics) as described (13). snATAC-seq data were processed using the Cell Ranger-ATAC pipeline version 1.2.0 (10X Genomics). Samples processed in multiple wells were combined using the aggr function. Clustering was performed in Signac (26) following standard procedures, and clusters were annotated based on chromatin accessibility at the promoter of known pituitary cell type markers (13). We used igvtools 2.3.32 (27) to generate chromatin accessibility tracks for the gonadotropes using a window size of 400 base pairs per cut-site. Tracks were normalized to both the number of gonadotropes and the median number of fragments per gonadotrope in each sample. snATAC-seq datasets can be found in the GEO data repository under the accession number GSE198907.
Human scRNAseq datasets were previously published (28). The processed dataset was downloaded from the GEO data repository under the accession number GSE142653. Cells were filtered and the dataset was scaled as previously described (28) and count thresholds were adjusted where necessary to account for background. The filtered and scaled gene expression dataset was analyzed using Seurat v.4.0.4 and standard procedures (29, 30). Cell types were assigned as previously published, and clustering was performed on the subset of endocrine cells (28).
RNAscope mRNA In Situ Hybridization
Wild-type CD1 mice (MGI: 5649524) were purchased from Charles River Laboratories. To analyze embryonic stages of pituitary gland development, females and males were time-mated for the generation of embryos. Midday of the day of vaginal plug was considered embryonic day 0.5 (E0.5). Adult pituitary glands were collected from 8-week-old CD-1 males. All animals were housed on a 12 hours light:12 hours dark cycle and given access to food and water ad libitum under compliance of the Animals (Scientific Procedures) Act 1986 and King's College London ethical review.
Dissected embryos and adult pituitaries were fixed in 10% neutral buffered formalin at room temperature for 16 to 24 hours. Samples were then washed in PBS and dehydrated through graded ethanol series before paraffin embedding as previously described (31). Embryo samples were sectioned along the sagittal plane for ages E9.5-16.5 and frontal plane for older embryos and adult pituitaries, at a thickness of 5 μm.
The RNAscope 2.5 HD Duplex assay was used according to the manufacturer’s recommendations, with a combination of the following probes: Mm-Tgfbr3l-C1 (Cat# 1040221-C1), Mm-Nr5a1-C2 (Cat# 445731-C2), Mm-Fshb-C2 (Cat# 445351-C2), Mm-Lhb-C2 (Cat# 478401-C2). All reagents were from Advanced Cell Diagnostics (Newark, CA, USA). All sections were counterstained with Mayer’s hematoxylin (Vector H-3404) and mounted with Vectamount Permanent Mounting Medium (Vector H-5000).
For brightfield images, slides were scanned using an Olympus BX34F Brightfield microscope.
Statistical Analysis
Luciferase assays in HEK93T and LβT2 cells were analyzed by one-way or two-way analysis of variance (ANOVA), followed by post hoc Holm–Sidak multiple comparisons tests. Where indicated, effects of genotype between two groups were assessed by unpaired t-tests with Welch’s correction. Statistical analyses were performed using Prism 9, GraphPad software. Alpha was set at P < .05.
Results
Two SF-1 Binding Sites are Located in the Proximal Tgfbr3l Promoter
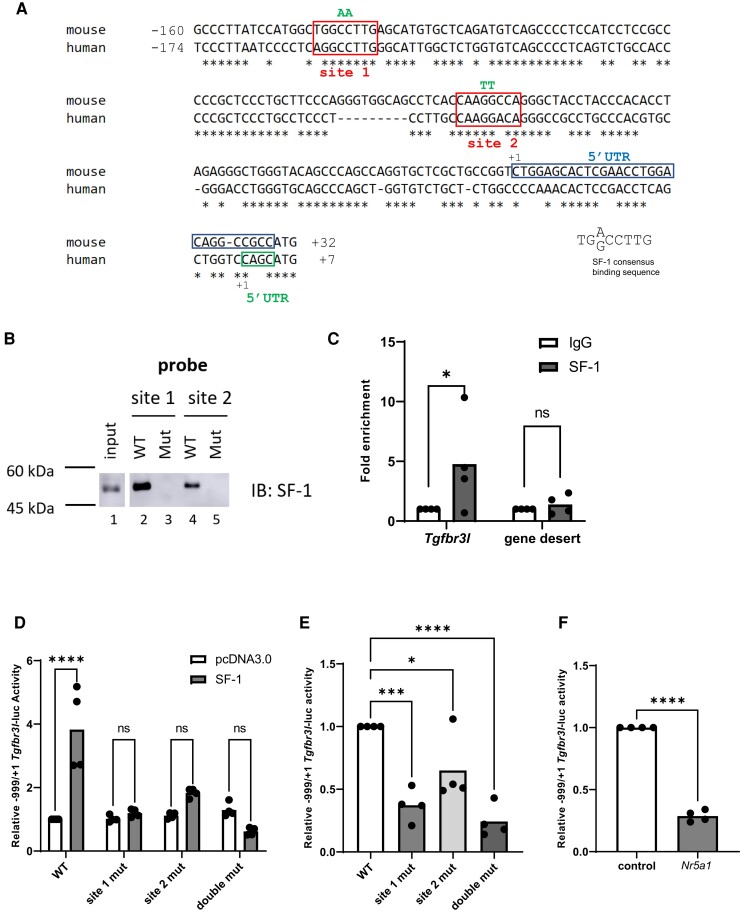
We mapped the Tgfbr3l transcription start site (TSS, +1) in murine pituitary RNA using 5′ RACE. Sequencing of several clones indicated that transcription was initiated at one of two sites: either 29 base pairs (at +1) or 52 base pairs (at −23) upstream of the start codon (Fig. 1A). The 29 base pair untranslated region (UTR) was more common (8 clones out of 11) and was therefore used to define the TSS. Both experimentally determined UTRs differed from the computationally predicted TSS (at −40) in GenBank (NM_001195258.1). We then analyzed the proximal promoter region for potential transcription factor binding sites and identified two candidate SF-1 cis-elements at −145 to −138 (CTGGCCTTG, site 1) and −67 to −60 (CAAGGCCAG, site 2). These two elements were reverse complements of each other and correspond to the consensus SF-1 binding motif (32, 33).
Figure 1.
SF-1 activates murine Tgfbr3l transcription via two cis-elements in the proximal promoter. (A) Alignment of the murine and human Tgfbr3l/TGFBR3L promoters. In both cases, +1 refers to the transcription start site. The most common murine 5′ untranslated region (5′ UTR) is boxed in blue and the human 5′ UTR is boxed in green. The conserved SF-1 binding sites are boxed in red and labeled as site 1 and site 2. Mutated base pairs (in B, C, and D) are indicated above in green. (B) DNAP using probes corresponding to the wild-type and mutant murine SF-1 cis-elements. Whole cell protein lysates from LβT2 cells (input) or proteins interacting with the probes were analyzed via immunoblot (IB) using an SF-1 antibody. (C) Chromatin immunoprecipitation for SF-1 of the indicated genomic regions in LβT2 cells (n = 4). (D) HEK293T cells were transfected with 225 ng/well of the indicated murine −999/+1 Tgfbr3l-luc reporters as well as 3.125 ng/well of either pcDNA3.0 (empty expression vector) or SF-1 expression vector. WT, wild-type; site 1 mut, mutated SF-1 site 1; site 2 mut, mutated SF-1 site 2; double mut, both SF-1 sites mutated. (E) LβT2 cells were transfected with 225 ng/well of the indicated promoter–reporters. (F) LβT2 cells were transfected with 225 ng/well of the −999/+1 Tgfbr3l-luc reporter and 10 nM of control or Nr5a1 siRNA. In D-F, lysates were collected and reporter activity measured by luciferase assay. Data represent the mean of three or more independent experiments performed in triplicate. Data were analyzed by two-way ANOVA followed by Holm–Sidak multiple comparisons test in (D), one-way ANOVA followed by Dunnett’s multiple comparisons test in (E), and two-tailed unpaired t test with Welch’s correction in (C and F). ns, not significant; *P < .05; ***P < .001; ****P < .0001.
To assess SF-1 binding to these sequences, we performed DNA affinity purification assays (DNAP) using biotinylated double-stranded DNA probes corresponding to each candidate cis-element (Fig. 1B). To ascertain specific binding, we also used DNA probes with two base pair mutations in the putative SF-1 cis-elements. Endogenous SF-1 protein from LβT2 cells bound to wild-type (Fig. 1B, lanes 2 and 4) but not mutant probes (lanes 3 and 5) corresponding to both cis-elements. Though the two cis-elements were identical (reverse complements of each other), site 1 appeared to bind SF-1 more strongly than site 2. Finally, we performed chromatin immunoprecipitation in LβT2 cells to assess SF-1 binding in the context of native chromatin. Compared with IgG, we observed enrichment of SF-1 in a region of the Tgfbr3l promoter containing the two cis-elements but not in a negative (gene desert) control (34) (Fig. 1C).
SF-1 Activates Murine Tgfbr3l Promoter–Reporter Activity
To examine Tgfbr3l transcription, we ligated ∼1 kb of the murine Tgfbr3l 5′ flanking sequence (−999/+1) upstream of luciferase in the pGL3-Basic reporter plasmid. In heterologous HEK293T cells, ectopically expressed SF-1 activated Tgfbr3l reporter activity (Fig. 1D). Mutations in either or both SF-1 sites blunted or blocked this stimulatory effect. In homologous murine LβT2 gonadotrope-like cells, mutations in the SF-1 binding sites significantly decreased basal reporter activity relative to wild type (Fig. 1E). Reporter activity was also significantly attenuated following SF-1 knockdown in LβT2 cells using a previously validated siRNA against Nr5a1 (Fig. 1F) (17).
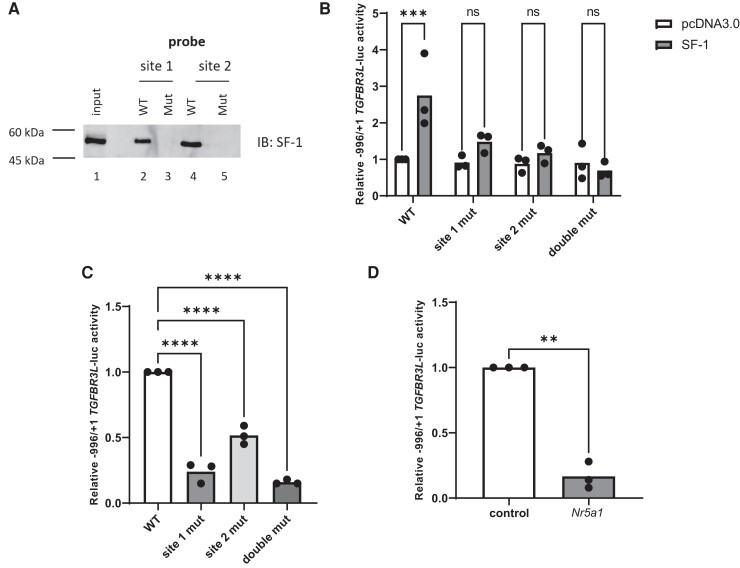
SF-1 also Regulates Human TGFBR3L Promoter–Reporter Activity
The TSS of human TGFBR3L was previously determined (11). Alignment of the murine Tgfbr3l and human TGFBR3L promoters demonstrated a high degree of sequence conservation, including in the two SF-1 cis-elements (Fig. 1A). Using DNAP, we demonstrated SF-1 binding to both cis-elements in the human promoter (Fig. 2A, lanes 2 and 4). Mutations in critical bp in these sites blocked SF-1 binding (lanes 3 and 5). In HEK293T cells, SF-1 overexpression induced human TGFBR3L promoter–reporter activity (Fig. 2B). The mutations that blocked SF-1 binding (Fig. 2A) also abrogated SF-1 induction of reporter activity (Fig. 2B). In LβT2 cells, mutations in these cis-element decreased basal promoter–reporter activity (Fig. 2C), as did siRNA-mediated knockdown of SF-1 (Fig. 2D).
Figure 2.
Mechanisms of human TGFBR3L transcriptional regulation by SF-1 are conserved. (A) DNAP was performed as in Fig. 1B, but with probes corresponding to the wild-type and mutant human SF-1 cis-elements. (B) HEK293T cells were transfected with 225 ng/well of the indicated human −996/+1 TGFBR3L-luc reporters as well as 3.125 ng/well of either pcDNA3.0 (empty expression vector) or SF-1 expression vector. WT, wild-type; site 1 mut, mutated SF-1 site 1; site 2 mut, mutated SF-1 site 2; double mut, both sites mutated. (C) LβT2 cells were transfected with 225 ng/well of the indicated promoter–reporters. (D) LβT2 cells were transfected with 225 ng/well of the −996/+1 TGFBR3L-luc reporter and 10 nM of control or Nr5a1 siRNA. In B-D, luciferase assays were performed as in Fig. 1. Data represent the mean of three independent experiments performed in triplicate and were analyzed by two-way analysis of variance followed by Holm–Sidak multiple comparisons test (B), one-way ANOVA followed by Dunnett’s multiple comparisons test (C), or by a two-tailed unpaired t test with Welch’s correction (D). ns, not significant; **P < .01; ***P < .001; ****P < .0001.
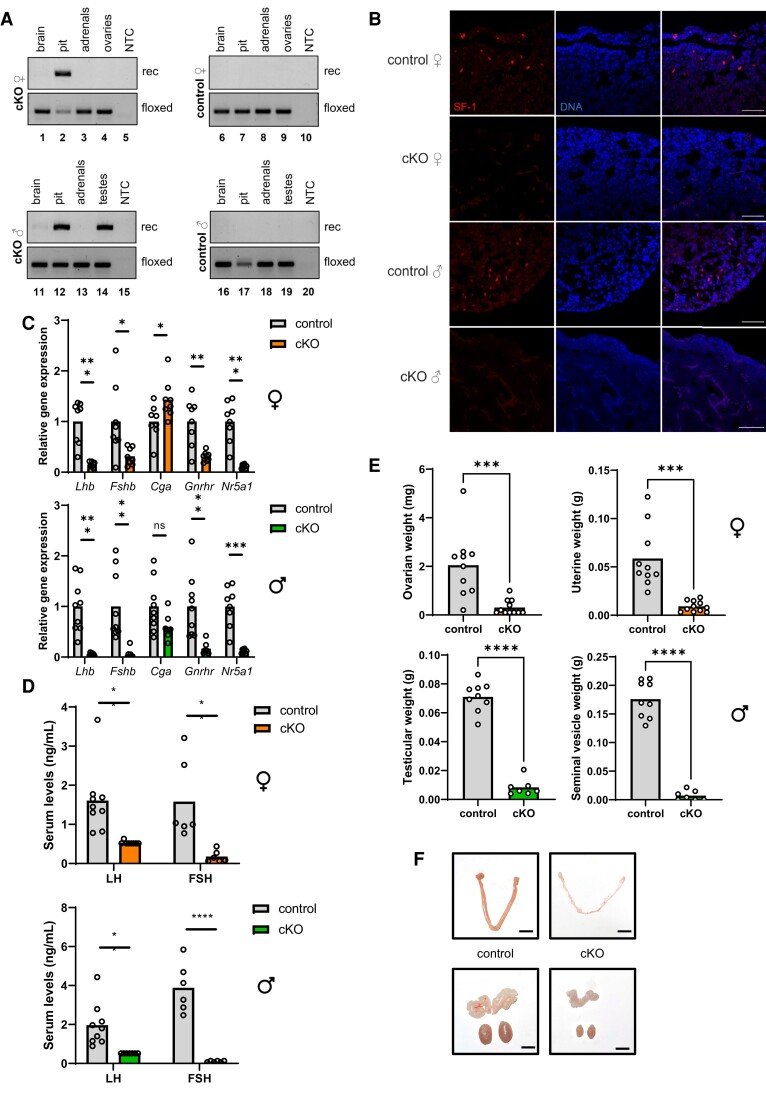
Tgfbr3l Expression is SF-1-Dependent In Vivo
To determine whether SF-1 regulates Tgfbr3l expression in vivo, we generated gonadotrope-specific Nr5a1 knockout mice by crossing GnrhrGRIC and Nr5a1fl/fl animals. Cre is expressed in gonadotropes and in the male germline with the GRIC Cre-driver line (19). We observed recombination of the floxed Nr5a1 allele in pituitaries (both sexes, lanes 2 and 12) and in testis (lane 14, Fig. 3A) (21). There was no evidence of recombination in control mice, which harbored floxed alleles but no Cre (Fig. 3A, lanes 6-9 and 16-19). Ablation of SF-1 protein (Fig. 3B) and Nr5a1 mRNA expression (Fig. 3C) was demonstrated by immunofluorescence and RT-qPCR on pituitaries of cKOs (Nr5a1fl/fl; GnrhrGRIC/+, cKO) compared with controls (Nr5a1fl/fl).
Figure 3.
Gonadotrope-specific SF-1 knockout mice exhibit hypogonadotropic hypogonadism. (A) Genomic DNA was extracted from the indicated tissues of Nr5a1fl/fl; GnrhrGRIC/+ (cKO; left panels) and Nr5a1fl/fl (control; right panels) mice and analyzed by PCR for the presence of the floxed or recombined (rec) Nr5a1 alleles. (B) Pituitary sections from 11-week-old control and cKO mice were analyzed for SF-1 by immunofluorescence (red); DAPI (blue) was used to stain nuclei. Scale bars: 50 µm. (C) cDNA was prepared from total RNA isolated from individual pituitary glands of 8- to 10-week-old control and cKO female (top) and male (bottom) mice and analyzed by RT-qPCR for expression of Lhb, Fshb, Cga, Gnrhr, and Nr5a1. (D) Serum LH and FSH levels in female (top) and male (bottom) control and cKO mice. (E) Ovarian, uterine, testicular, and seminal vesicle weights and (F) representative images of gonads and accessory sex organs from control and cKO females (top) and males (bottom). Scale bars: 5 mm. Female data in all panels represent randomly cycling females. Data were analyzed by two-tailed unpaired t tests with Welch’s correlation. ns, not significant; *P < .05; **P < .01; ***P < .001; ****P < .0001.
SF-1 is required for gonadotropin synthesis and fertility in vivo (20, 35). Here, we observed profound impairments in gonadotropin β subunit (Lhb and Fshb) and Gnrhr, but not Cga, mRNA levels in pituitaries of female and male cKOs (Fig. 3C). Serum LH and FSH levels were correspondingly reduced in cKO mice (Fig. 3D). Both female and male cKOs were infertile, with severely hypoplastic gonads and accessory sex organs (ovaries and uteri in females; testes and seminal vesicles in males; Fig. 3E and 3F). Collectively, the data demonstrate that loss of SF-1 expression and function was complete in our model.
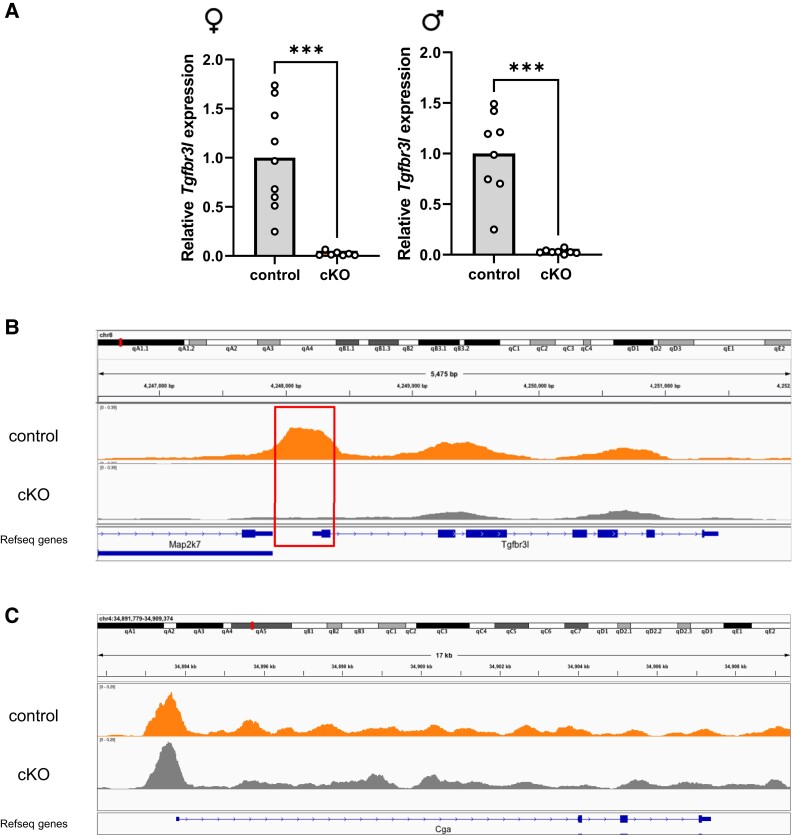
Consistent with the in vitro reporter data (Fig. 1), pituitary Tgfbr3l expression was abrogated in both female and male cKO mice (Fig. 4A). Next, we performed single-nucleus ATAC-sequencing on pituitaries of male control and cKO mice. In the controls, we identified open chromatin around the Tgfbr3l promoter in gonadotropes (upper track in Fig. 4B). In contrast, the corresponding region was closed in cKO mice (lower track). The Cga promoter was open in gonadotropes of both genotypes (Fig. 4C), consistent with their equivalent expression of the gene (Fig. 3C).
Figure 4.
SF-1 is required for Tgfbr3l expression in adult murine pituitary glands. (A) Pituitary cDNA from control and cKO female and male mice (described in Fig. 3) were analyzed for Tgfbr3l expression by RT-qPCR. Data were analyzed by two-tailed unpaired t tests with Welch’s correlation. ***P < .001. Chromatin accessibility, as measured with single-nucleus ATAC-seq, over the (B) Tgfbr3l and (C) Cga genes in gonadotropes of 10- to 12-week-old control (orange) and cKO (grey) males. Exon 1 and the promoter of Tgfbr3l are boxed in red in B. Shown are representative tracks from a control and a cKO animal.
Tgfbr3l Precedes Nr5a1 Expression in the Embryonic Murine Pituitary
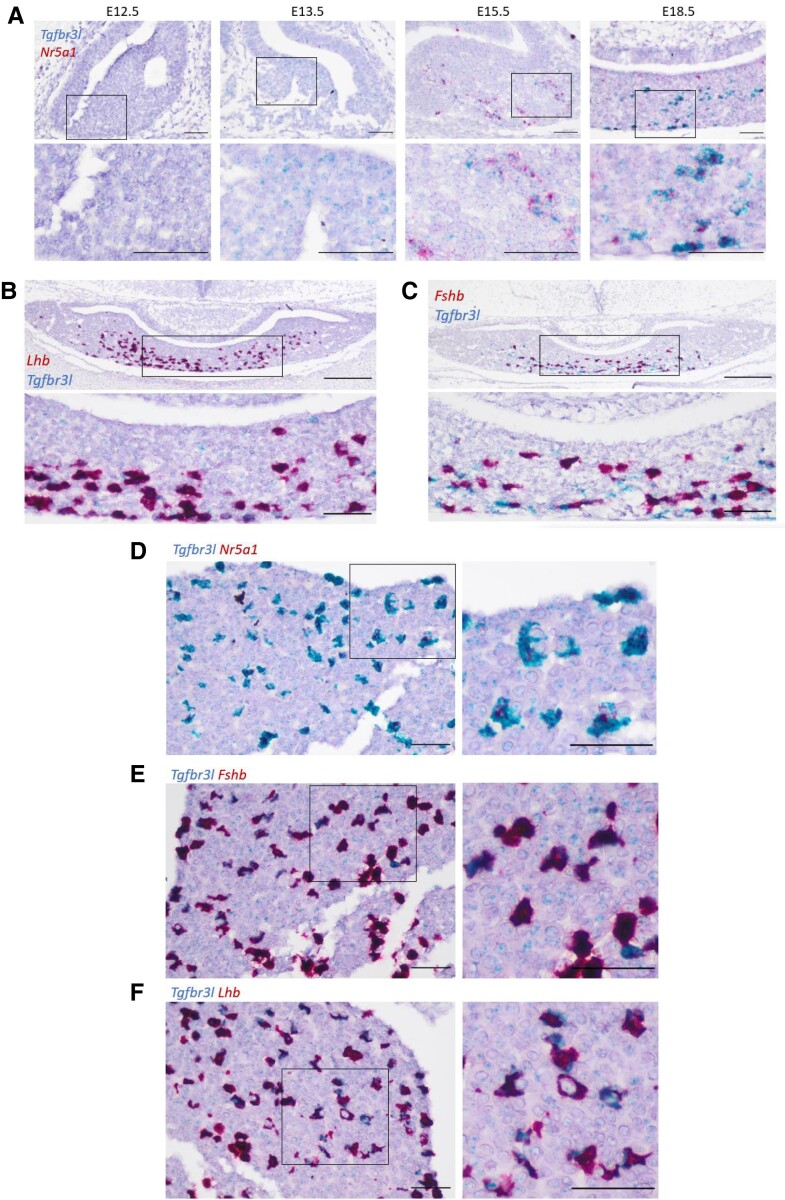
Next, we examined Tgfbr3l expression during murine pituitary development. Using mRNA in situ hybridization (RNAscope) on CD1 mouse embryos, we observed a low level of Tgfbr3l expression as early as E13.5 (Fig. 5A). In contrast, Nr5a1 mRNA was first detected at E15.5 (Fig. 5A). Tgfbr3l colocalized with Nr5a1 (Fig. 5A) and Lhb by E18.5 (Fig. 5B). Tgfbr3l colocalization with Fshb was not complete at E18.5 (Fig. 5C). In adult (8-week-old) males, Tgfbr3l colocalized with Nr5a1 (Fig. 5D), Fshb (Fig. 5E), and Lhb (Fig. 5F).
Figure 5.
Nr5a1 and Tgfbr3l expression in fetal and adult murine pituitaries. (A) mRNA in situ hybridization (RNAscope) for Tgfbr3l (blue) and Nr5a1 (red) on heads of wild-type CD1 embryos at the indicated ages. Boxed regions in the top panels are magnified in the bottom panels. Scale bars: 50 μm. Duplex RNAscope for Tgfbr3l (blue) and (B) Lhb or (C) Fshb (red) on heads of CD1 embryos at E18.5. Scale bars: 200 μm (top) and 50 μm (bottom). Duplex RNAscope for Tgfbr3l (blue) and (D) Nr5a1, (E) Fshb, or (F) Lhb (red) on pituitaries of 8-week-old CD1 males. Scale bars: 50 μm.
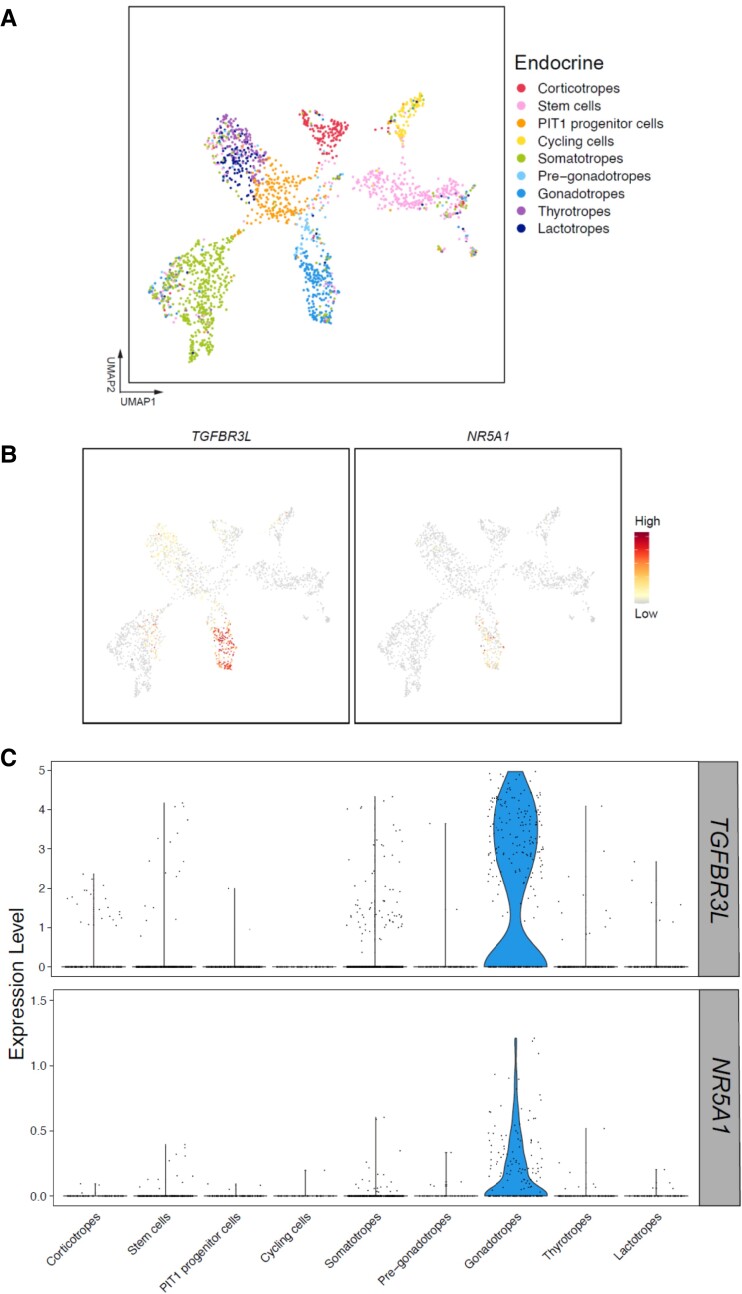
Finally, to gain insight into TGFBR3L regulation in human development, we analyzed a human embryonic pituitary single-cell RNA sequencing database (28) (Fig. 6A). TGFBR3L and NR5A1 expression were enriched in gonadotropes 7 to 25 weeks postfertilization (Fig. 6B and 6C).
Figure 6.
TGFBR3L and NR5A1 expression is enriched in gonadotropes of human fetal pituitaries. (A) UMAP plots of scRNAseq of 21 human embryonic pituitaries collected 7-25 weeks post-fertilization. Different colored clusters represent different pituitary cell types. (B) Feature plots of TGFBR3L and NR5A1 expression. (C) Violin plots of TGFBR3L and NR5A1 expression in the defined cell lineages.
Discussion
The novel inhibin B coreceptor, TGFBR3L, appears to be uniquely or principally expressed in pituitary gonadotropes (11, 13–15). Here, we demonstrate that the gonadotrope-specific transcription factor SF-1 regulates murine Tgfbr3l expression, at least in part, via two cis-elements in the proximal promoter. The data also suggest that this mechanism is conserved in humans. Beyond binding to these elements, how SF-1 regulates Tgfbr3l is not yet clear. However, the closed chromatin state of the Tgfbr3l locus in gonadotropes of cKO mice suggests that SF-1 promotes chromatin accessibility through the recruitment of histone modifying enzymes (36–38).
Whereas SF-1 is necessary for Tgfbr3l expression, it is likely not sufficient. Though restricted to gonadotropes among pituitary cell types, SF-1 is expressed in other tissues that do not express Tgfbr3l (11), including the ventromedial hypothalamus, adrenal glands, and gonads (39). Within gonadotropes, SF-1 physically and functionally interacts with early growth response 1 and paired-like homeodomain transcription factors to regulate gonadotrope-specific expression of Lhb (17). In contrast, SF-1 cooperates with LIM homeodomain proteins to regulate Gnrhr promoter activity (40). It is possible that SF-1 might interact with similar or distinct transcription factors to confer gonadotrope-specific Tgfbr3l expression. Notably, there is a candidate early growth response 1 binding site between the two SF-1 cis-elements in the Tgfbr3l promoter, which is the subject of our ongoing investigations.
Importantly, the dependence of Tgfbr3l expression on SF-1 may be an emergent property. Nr5a1 mRNA is reliably detected in developing murine pituitary on embryonic day 14.5, but not at E13.5 (35). Using RNAscope, Tgfbr3l mRNA was first detected at E13.5, when Nr5a1 mRNA was absent. These data indicate that the initial expression of Tgfbr3l is SF-1 independent. Nevertheless, Nr5a1 and Tgfbr3l are coexpressed at least as early as E15.5 and continue to be thereafter. The loss of Tgfbr3l mRNA in Nr5a1 cKO mice demonstrates that, at least postnatally, the gene is uniquely/preferentially expressed in gonadotropes in an SF-1–dependent manner. Though we have not yet established what initially drives Tgfbr3l expression, this mechanism does not compensate for the loss of SF-1 in adulthood.
NR5A1 and TGFBR3L are also coexpressed in human embryonic pituitary development. However, based on the available data, we cannot determine precisely when these transcripts first emerge relative to one another. Regardless, as in mouse, SF-1 and TGFBR3L are coexpressed in adult human gonadotropes, as well as in gonadotrope tumors (14, 28).
Gnrhr and the gonadotropin β subunits (Lhb and Fshb) are canonical markers of the gonadotrope lineage. The expression of all three depends on SF-1 (35). Recent single-cell and single-nucleus RNA-sequencing analyses of murine, rat, and human pituitaries similarly establish Tgfbr3l/TGFBR3L as another gonadotrope-specific gene (13, 41, 42). It is therefore notable that its expression is also SF-1 dependent. These data demonstrate SF-1’s role as a master regulator of gonadotrope identity. Nevertheless, as the expression of two of these genes, Gnrhr (21) and Tgfbr3l, precedes Nr5a1 developmentally, gonadotrope-lineage specification appears to be SF-1 independent. Indeed, treatment of Nr5a1 knockout mice with exogenous GnRH is sufficient to increase gonadotropin production (43), indicating that gonadotropes are present even in the absence of SF-1. It will be interesting to determine whether GnRH treatment similarly induces Tgfbr3l. Collectively, the data indicate that SF-1 is required for the full expression of the gonadotrope-specific transcriptome, but is not required for gonadotrope specification per se.
Finally, we should note that deciphering mechanisms controlling Tgfbr3l/TGFBR3L expression may have translational relevance. Female Tgfbr3l knockout mice have elevated FSH levels and enhanced fertility due to impaired inhibin B negative feedback (11). Therefore, either blocking inhibin B binding to TGFBR3L or reducing TGFBR3L expression would provide means to increase endogenous FSH levels. Such an outcome could be favorable in the context of assisted reproduction. Though SF-1 itself may not be an ideal therapeutic target, delineation of the Tgfbr3l/TGFBR3L transcriptional regulatory machinery may uncover a more suitable and selective approach to decrease gene expression. The results reported here provide an important first step in this direction.
Acknowledgments
The thank the following individuals: Dr. Bruce Murphy (Université de Montréal) for floxed SF-1 mice; Drs. Terry Hébert (HEK293T; McGill University) and Pamela Mellon (LβT2; UCSD) for immortalized cell lines; Dr. Keith Parker (UT Southwestern Medical Center) for the SF-1 expression vector; and Dr. Caroline David for feedback on the manuscript. We acknowledge the New York Genome Center for sequencing. This work was supported in part through the computational and data resources and staff expertise provided by Scientific Computing at the Icahn School of Medicine at Mount Sinai.
Abbreviations
- ANOVA
analysis of variance
- cKO
conditional knockout
- EDTA
ethylenediaminetetraaceticacid
- FSH
follicle-stimulating hormone
- GnRH
gonadotropin-releasing hormone
- HEK
Human embryonic kidney
- LH
luteinizing hormone
- PBS
phosphate-buffered saline
- PBST
PBS with Tween
- PCR
polymerase chain reaction
- RACE
rapid amplification of cDNA ends
- SDS
sodium dodecyl sulfate
- SF-1
steroidogenic factor 1
- siRNA
short interfering RNA
- TBS
Tris-buffered saline
- TGF
transforming growth factor
- TGFBR3
TGFβ type III receptor
- TSS
transcription start site
- UTR
untranslated region
Contributor Information
Yeu-Farn Lin, Department of Pharmacology and Therapeutics, McGill University, Montreal, Quebec H3G 1Y6, Canada.
Gauthier Schang, Department of Pharmacology and Therapeutics, McGill University, Montreal, Quebec H3G 1Y6, Canada.
Evan R S Buddle, Department of Pharmacology and Therapeutics, McGill University, Montreal, Quebec H3G 1Y6, Canada.
Hailey Schultz, Department of Anatomy and Cell Biology, McGill University, Montreal, Quebec H3A 0C7, Canada.
Thea L Willis, Centre for Craniofacial and Regenerative Biology, King’s College London, London SE1 1UL, UK.
Frederique Ruf-Zamojski, Department of Neurology, Center for Advanced Research on Diagnostic Assays, Icahn School of Medicine at Mount Sinai, New York, NY 10029, USA.
Michel Zamojski, Department of Neurology, Center for Advanced Research on Diagnostic Assays, Icahn School of Medicine at Mount Sinai, New York, NY 10029, USA.
Natalia Mendelev, Department of Neurology, Center for Advanced Research on Diagnostic Assays, Icahn School of Medicine at Mount Sinai, New York, NY 10029, USA.
Ulrich Boehm, Department of Experimental Pharmacology, Center for Molecular Signaling, Saarland University School of Medicine, Homburg 66421, Germany.
Stuart C Sealfon, Department of Neurology, Center for Advanced Research on Diagnostic Assays, Icahn School of Medicine at Mount Sinai, New York, NY 10029, USA.
Cynthia L Andoniadou, Centre for Craniofacial and Regenerative Biology, King’s College London, London SE1 1UL, UK.
Daniel J Bernard, Department of Pharmacology and Therapeutics, McGill University, Montreal, Quebec H3G 1Y6, Canada; Department of Anatomy and Cell Biology, McGill University, Montreal, Quebec H3A 0C7, Canada.
Funding
Canadian Institutes of Health Research project grant PJT-162343 (D.J.B.); National Institutes of Health Grant DK46943 (S.C.S.); Medical Research Council (M.R.C.) project grant MR/T012153/1 (C.L.A.); Dr. Samuel Solomon Fellowship in Endocrinology (McGill University Health Centre) (Y.F.L. and G.S.); Canadian Institutes of Health Research Master’s Graduate Scholarship (Y.F.L. and H.S.); Canadian Institutes of Health Research Doctoral Research Award (G.S.); Fonds de Recherche du Québec – Santé, Master’s Scholarship (G.S.); “Cell Therapies and Regenerative Medicine” Four-Year Welcome Trust PhD Training Programme (King’s College London) (T.L.W.).
Author Contributions
Y.F.L. and D.J.B. were responsible for the experimental design, data analyses, and manuscript preparation. Y.F.L. and E.R.S.B. conducted the in vitro experiments. G.S., H.S., and Y.F. were responsible for tissue collection, mouse colony management, and analyses from the SF-1 strain. T.L.W. performed the RNAscope. U.B. provided the GRIC strain. N.M. isolated nuclei from individual pituitaries. F.R.Z. performed the snATAC-seq experiments and M.Z. analyzed the snATAC-seq datasets. H.S. analyzed the human scRNA-seq datasets. D.J.B., C.L.A., and S.C.S. secured the grant funding for the research. All authors approved the final version of the manuscript.
Disclosures
The authors have nothing to disclose. The authors declare no competing interests.
Data Availability
Most data are available in the manuscript. ATAC-seq data were deposited in GEO: GSE198907.
References
- 1. Edson MA, Nagaraja AK, Matzuk MM. The mammalian ovary from genesis to revelation. Endocr Rev. 2009;30(6):624‐712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Simoni M, Weinbauer GF, Gromoll J, Nieschlag E. Role of FSH in male gonadal function. Ann Endocrinol (Paris). 1999;60(2):102‐106. [PubMed] [Google Scholar]
- 3. Fortin J, Ongaro L, Li Y, et al. Minireview: activin signaling in gonadotropes: what does the FOX say… to the SMAD? Mol Endocrinol. 2015;29(7):963‐977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Woodruff TK, D’Agostino J, Schwartz NB, Mayo KE. Dynamic changes in inhibin messenger RNAs in rat ovarian follicles during the reproductive cycle. Science. 1988;239(4845):1296‐1299. [DOI] [PubMed] [Google Scholar]
- 5. Woodruff TK, Meunier H, Jones PB, Hsueh AJ, Mayo KE. Rat inhibin: molecular cloning of alpha- and beta-subunit complementary deoxyribonucleic acids and expression in the ovary. Mol Endocrinol. 1987;1(8):561‐568. [DOI] [PubMed] [Google Scholar]
- 6. Davis SR, Krozowski Z, McLachlan RI, Burger HG. Inhibin gene expression in the human corpus luteum. J Endocrinol. 1987;115(3):R21‐R23. [DOI] [PubMed] [Google Scholar]
- 7. Anawalt BD, Bebb RA, Matsumoto AM, et al. Serum inhibin B levels reflect Sertoli cell function in normal men and men with testicular dysfunction. J Clin Endocrinol Metab. 1996;81(9):3341‐3345. [DOI] [PubMed] [Google Scholar]
- 8. Lewis KA, Gray PC, Blount AL, et al. Betaglycan binds inhibin and can mediate functional antagonism of activin signalling. Nature. 2000;404(6776):411‐414. [DOI] [PubMed] [Google Scholar]
- 9. Makanji Y, Temple-Smith PD, Walton KL, Harrison CA, Robertson DM. Inhibin B is a more potent suppressor of rat follicle-stimulating hormone release than inhibin A in vitro and in vivo. Endocrinology. 2009;150(10):4784‐4793. [DOI] [PubMed] [Google Scholar]
- 10. Li Y, Fortin J, Ongaro L, et al. Betaglycan (TGFBR3) Functions fs an inhibin A, but not inhibin B, coreceptor in pituitary gonadotrope cells in mice. Endocrinology. 2018;159(12):4077‐4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brule E, Wang Y, Li Y, et al. TGFBR3L is an inhibin B co-receptor that regulates female fertility. Sci Adv. 2021;7(51):eabl4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bilandzic M, Stenvers KL. Betaglycan: a multifunctional accessory. Mol Cell Endocrinol. 2011;339(1‐2):180‐189. [DOI] [PubMed] [Google Scholar]
- 13. Ruf-Zamojski F, Zhang Z, Zamojski M, et al. Single nucleus multi-omics regulatory landscape of the murine pituitary. Nat Commun. 2021;12(1):2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sjostedt E, Kolnes AJ, Olarescu NC, et al. TGFBR3L – an uncharacterised pituitary specific membrane protein detected in the gonadotroph cells in non-neoplastic and tumour tissue. Cancers (Basel). 2020;13(1):114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Data from 2022 Aug 8: The Human Protein Atlas. http://www.proteinatlas.org/ENSG00000260001-TGFBR3L/tissue.
- 16. Wang Y, Fortin J, Lamba P, et al. Activator protein-1 and smad proteins synergistically regulate human follicle-stimulating hormone beta-promoter activity. Endocrinology. 2008;149(11):5577‐5591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fortin J, Lamba P, Wang Y, Bernard DJ. Conservation of mechanisms mediating gonadotrophin-releasing hormone 1 stimulation of human luteinizing hormone beta subunit transcription. Mol Hum Reprod. 2009;15(2):77‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Alarid ET, Windle JJ, Whyte DB, Mellon PL. Immortalization of pituitary cells at discrete stages of development by directed oncogenesis in transgenic mice. Development. 1996;122(10):3319‐3329. [DOI] [PubMed] [Google Scholar]
- 19. Wen S, Schwarz JR, Niculescu D, et al. Functional characterization of genetically labeled gonadotropes. Endocrinology. 2008;149(6):2701‐2711. [DOI] [PubMed] [Google Scholar]
- 20. Zhao L, Bakke M, Krimkevich Y, et al. Steroidogenic factor 1 (SF1) is essential for pituitary gonadotrope function. Development. 2001;128(2):147‐154. [DOI] [PubMed] [Google Scholar]
- 21. Wen S, Ai W, Alim Z, Boehm U. Embryonic gonadotropin-releasing hormone signaling is necessary for maturation of the male reproductive axis. Proc Natl Acad Sci U S A. 2010;107(37):16372‐16377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Luo X, Ikeda Y, Parker KL. A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell. 1994;77(4):481‐490. [DOI] [PubMed] [Google Scholar]
- 23. Zhou X, Wang Y, Ongaro L, et al. Normal gonadotropin production and fertility in gonadotrope-specific Bmpr1a knockout mice. J Endocrinol. 2016;229(3):331‐341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Steyn FJ, Wan Y, Clarkson J, Veldhuis JD, Herbison AE, Chen C. Development of a methodology for and assessment of pulsatile luteinizing hormone secretion in juvenile and adult male mice. Endocrinology. 2013;154(12):4939‐4945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schang G, Ongaro L, Schultz H, et al. Murine FSH production depends on the activin type II receptors ACVR2A and ACVR2B. Endocrinology 2020;161(7):bqaa056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stuart T, Srivastava A, Madad S, Lareau CA, Satija R. Single-cell chromatin state analysis with Signac. Nat Methods. 2021;18(11):1333‐1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Thorvaldsdottir H, Robinson JT, Mesirov JP. Integrative Genomics Viewer (IGV): high-performance genomics data visualization and exploration. Brief Bioinform. 2013;14(2):178‐192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang S, Cui Y, Ma X, et al. Single-cell transcriptomics identifies divergent developmental lineage trajectories during human pituitary development. Nat Commun. 2020;11(1):5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Butler A, Hoffman P, Smibert P, Papalexi E, Satija R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat Biotechnol. 2018;36(5):411‐420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stuart T, Butler A, Hoffman P, et al. Comprehensive integration of single-cell data. Cell. 2019;177(7):1888‐1902.e21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Russell JP, Lim X, Santambrogio A, et al. Pituitary stem cells produce paracrine WNT signals to control the expansion of their descendant progenitor cells. Elife. 2021;10:e59142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Doghman M, Figueiredo BC, Volante M, Papotti M, Lalli E. Integrative analysis of SF-1 transcription factor dosage impact on genome-wide binding and gene expression regulation. Nucleic Acids Res. 2013;41(19):8896‐8907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Keri RA, Nilson JH. A steroidogenic factor-1 binding site is required for activity of the luteinizing hormone beta subunit promoter in gonadotropes of transgenic mice. J Biol Chem. 1996;271(18):10782‐10785. [DOI] [PubMed] [Google Scholar]
- 34. Bohaczuk SC, Thackray VG, Shen J, Skowronska-Krawczyk D, Mellon PL. FSHB transcription is regulated by a novel 5’ distal enhancer with a fertility-associated single nucleotide polymorphism. Endocrinology. 2021;162(1):bqaa181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ingraham HA, Lala DS, Ikeda Y, et al. The nuclear receptor steroidogenic factor 1 acts at multiple levels of the reproductive axis. Genes Dev. 1994;8(19):2302‐2312. [DOI] [PubMed] [Google Scholar]
- 36. Harris J, Gouhier A, Drouin J. Mechanisms in endocrinology: Pioneer transcription factors in pituitary development and tumorigenesis. Eur J Endocrinol. 2021;184(1):R1‐R15. [DOI] [PubMed] [Google Scholar]
- 37. Mayran A, Sochodolsky K, Khetchoumian K, et al. Pioneer and nonpioneer factor cooperation drives lineage specific chromatin opening. Nat Commun. 2019;10(1):3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zaret KS, Carroll JS. Pioneer transcription factors: establishing competence for gene expression. Genes Dev. 2011;25(21):2227‐2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schimmer BP, White PC. Minireview: steroidogenic factor 1: its roles in differentiation, development, and disease. Mol Endocrinol. 2010;24(7):1322‐1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Granger A, Bleux C, Kottler ML, Rhodes SJ, Counis R, Laverriere JN. The LIM-homeodomain proteins Isl-1 and Lhx3 act with steroidogenic factor 1 to enhance gonadotrope-specific activity of the gonadotropin-releasing hormone receptor gene promoter. Mol Endocrinol. 2006;20(9):2093‐2108. [DOI] [PubMed] [Google Scholar]
- 41. Fletcher PA, Smiljanic K, Maso Prévide R, et al. Cell type- and sex-dependent transcriptome profiles of rat anterior pituitary cells. Front Endocrinol. 2019;10:623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhang Z, Zamojski M, Smith GR, et al. Single nucleus transcriptome and chromatin accessibility of postmortem human pituitaries reveal diverse stem cell regulatory mechanisms. Cell Rep. 2022;38(10):110467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ikeda Y, Luo X, Abbud R, Nilson JH, Parker KL. The nuclear receptor steroidogenic factor 1 is essential for the formation of the ventromedial hypothalamic nucleus. Mol Endocrinol. 1995;9(4):478‐486. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Most data are available in the manuscript. ATAC-seq data were deposited in GEO: GSE198907.