Abstract
Context
Leukocyte telomere length (LTL) is a biomarker of biological aging and is associated with metabolic diseases such as type 2 diabetes. Insufficient maternal vitamin D was associated with increased risk for many diseases and adverse later life outcomes.
Objective
This study investigates the relationship between vitamin D levels and offspring LTL at early life.
Methods
This observational, longitudinal, hospital-based cohort study included eligible mother-child pairs from the HAPO Hong Kong Field Centre, with 853 offspring at age 6.96 ± 0.44 (mean ± SD) years. LTL was measured using real-time polymerase chain reaction while serum vitamin D metabolites 25(OH)D2, 25(OH)D3, and 3-epi-25(OH)D3 were measured in maternal blood (at gestation 24-32 weeks) and cord blood by liquid chromatography–mass spectrometry.
Results
LTL at follow-up was significantly shorter in boys compared with girls (P < 0.001) at age 7. Childhood LTL was negatively associated with childhood BMI (β ± SE = -0.016 ± 0.007)(P = 0.02) and HOMA-IR (β ± SE = −0.065 ± 0.021)(P = 0.002). Multiple linear regression was used to evaluate the relationship between 25(OH)D and LTL, with covariate adjustments. Childhood LTL was positively correlated with total maternal 25(OH)D (0.048 ± 0.017) (P = 0.004) and maternal 3-epi-25(OH)D3 (0.05 ± 0.017) (P = 0.003), even after adjustment for covariates. A similar association was also noted for cord 3-epi-25(OH)D3 (0.037 ± 0.018) (P = 0.035) after adjustment for offspring sex and age.
Conclusion
Our findings suggest 25(OH)D3 and 3-epi-25(OH)D3 in utero may impact on childhood LTLs, highlighting a potential link between maternal vitamin D and biological aging.
Keywords: early programming, maternal vitamin D, telomere, 3-epi-25(OH)D3, longitudinal study
Telomeres are the protective caps at the chromosome ends. They are commonly regarded as a biomarker for biological aging. In humans, leukocyte telomere length (LTL) is associated with cancer, type 2 diabetes, and cardiovascular diseases (CVD). Adults with CVD (1) and type 2 diabetes (2) have shorter LTLs, while LTL at a later age is strongly correlated with LTL at an earlier age. Studies of LTL at an early age have also suggested that LTL could be affected by factors such as maternal metabolic conditions (3) and socioeconomical status (4).
Vitamin D, as an important micronutrient, is not only involved in maintaining bone and skeletal health but is also thought to be associated with glucose and insulin action (5). Deficiency of serum vitamin D is associated with the risk of later life metabolic problems such as obesity, insulin resistance, and CVD (6). Maternal deficiency of vitamin D is associated with gestational diabetes, maternal infections, and fetal growth restriction (7) The 2 main forms of vitamin D are 25(OH)D3 and 25(OH)D2. 25(OH)D2 is sourced from a diet like sun-dried Shiitake mushrooms (8), while 25(OH)D3 can be synthesized in the skin by UVB from sunlight or ingested via high oil content fish like salmon. 3-epi-25(OH)D3 is the isomer form of 25(OH)D3. Its presence affects the determination of newborns’ vitamin D sufficiency. Little is known about 3-epi-25(OH)D3 except for its possible association with maturation of neonatal liver (9).
Early-life LTL and vitamin D have been reported to be similarly correlated with newborn conditions. Later life LTL and adulthood vitamin D were also observed to be similarly correlated with adulthood metabolic illnesses and pregnant mothers’ conditions. LTL and vitamin D levels may be correlated with each other from early to later age. In a cross-sectional study of healthy adults from the United States, serum vitamin D was significantly associated with longer LTL, after adjustment for age, race, education, and C-reactive protein (10). Other reports have also suggested a positive association between vitamin D and LTL. We sought to examine the association of maternal and cord vitamin D with both cord blood and childhood LTL at age 7. Vitamin D levels were examined by measuring 25-hydroxyvitamin D [25(OH)D], which is the major vitamin D metabolite. We hypothesized that maternal and cord serum 25(OH)D would be associated with offspring cord blood and childhood LTL at age 7 years.
Methods
Subjects
Subjects from the Hong Kong Field Centre of the Hyperglycaemia and Adverse Pregnancy Outcome (HAPO) Study were used to examine the association between LTL and vitamin D. HAPO was a multinational, multicenter cohort aimed at investigating the risk of adverse outcomes associated with various degrees of maternal glucose intolerance and establishing glucose thresholds associated with increased risk of pregnancy complications (11). From 2009 to 2013, families recruited into the HAPO Study for the Hong Kong field center were recalled for detailed evaluation at approximately 7 years postpartum (12). Subjects who participated in the follow-up evaluation were used in this analysis. All subjects were of southern Han Chinese descent (11) (13). There were 1600 eligible subjects at the start of the HAPO Study at the Hong Kong field center; due to factors such as failure to contact or refusal to participate, a total of 970 mother-child pairs returned for follow-up when the child was approximately age 7. Detailed documentation of each participant’s background, including family history of diabetes mellitus, maternal smoking and drinking habits before pregnancy, as well as childhood lifestyle factors such as the frequency of physical activity, was recorded using standardized questionnaires. Written consent was obtained from the parents or the legal guardians. This study was approved by the Joint Chinese University of Hong Kong-New territories East Cluster Research Ethics Committee. More details can be found in the online supplementary file (14).
Laboratory Measurements
Mid-gestational serum from mothers and cord serum were stored at −80 °C. Serum 25(OH)D2, 25(OH)D3, and 3-epi-25(OH)D3 were extracted from the sera and measured by liquid chromatography with tandem mass spectrometry (LC-MS/MS) at the Biomedical Mass Spectrometry Unit, Department of Chemical Pathology, The Chinese University of Hong Kong as previously described (15, 16). From the maternal blood drawn at the time of the oral glucose tolerance test (OGTT) at around 28 weeks’ gestation and the cord blood at birth, serum 25(OH)D3, 3-epi-25(OH)D3, and 25(OH)D2 were measured. The lowest limit of detection was set at 0.4 nmol/L for all isoforms, with readings less than limit of detection denoted as 0 nmol/L. Inter-batch coefficients of variation (CVs) for 25(OH)D2 and 25(OH)D3 were < 3.8% and that for 3-epi-25(OH)D3 was < 7.6%. Total vitamin D was calculated by the summation of 25(OH)D2 and 25(OH)D3, without including 3-epi-25(OH)D3.
Newborn and childhood DNA was extracted with kits or according to a standardized protocol. LTL was measured with real-time quantitative polymerase chain reaction (qPCR) following a modified protocol first proposed by Cawthon et al (17, 18). In brief, both telomere length and a single copy gene, human βglobin (HBG), were measured in triplicate on 96-well qPCR plates. For each qPCR plate being measured, 1 negative control (with the addition of no-template control) and 1 reference DNA were included to adjust for plate-to-plate variability and quality control. LTL was then calculated with ΔΔCt and reported as telomere-single copy gene ratio (T/S ratio). The overall inter-plate CVs of the telomere and HBG assays were 1.34% and 0.48%, respectively. The overall intra-plate CV was 0.93% for telomere and 0.73% for HBG.
Statistical Analysis
Data are presented as mean ± SD, median (Q1, Q3), or percentage (%). Data distribution having a skewness within ± 1 were considered normally distributed. Cord blood LTL and childhood LTL at age 7 were found to be normally distributed with a skewness of 0.07 and −0.05, respectively. Maternal total vitamin D and 25(OH)D3 were reported to be normally distributed, while maternal 3-epi-25(OH)D3 and cord total vitamin D, 25(OH)D3 and 3-epi-25(OH)D3 were skewed (Supplementary figures 1-8) (14). Comparisons between 2 groups were performed with Student’s unpaired t test. Chi-square (λ2) or Fisher’s exact tests were used, as appropriate, for categorical variables. Bivariate linear regression was conducted to test the relationships between LTL and baseline characteristics. Normal inverse transformation was applied during the regression analysis. Regression models were developed first without adjustment as “unadjusted”, followed by additional analyses “adjusted for sex and age/ gestational age” (model 1), and “adjusted for maternal and offspring characteristics” (model 2). In model 2, the common covariates applied for both LTLs were sex, parity, maternal age at estimated date of confinement (EDC), maternal systolic blood pressure at 28 weeks gestation, current maternal and paternal diabetes mellitus, C-section, maternal pre-pregnancy body mass index (BMI), and maternal glucose area under the curve (GAUC) at 28 weeks. For cord blood LTL, ponderal index was included for additional adjustment, while the childhood LTL was adjusted for child age, BMI, systolic blood pressure, and self-reported exercise level (categorized 0, 1, 2; 0 denotes the lowest exercise frequency). Homeostasis model assessment of insulin resistance (HOMA-IR) was calculated: fasting plasma insulin × fasting plasma glucose)/22.5. GAUC was defined as the plasma glucose level-time curve from 0 to 120 minutes during OGTT.
To check the validity of our associations, additional analyses with vitamin D categorized as “deficient” (< 50 nmol/L), “sufficient” (< 75-50 nmol/L), and “more sufficient” (≥ 75 nmol/L), were conducted according to recommended thresholds. To examine for changes in LTL from birth to age 7, the cord LTL and the childhood LTL were divided into quintiles according to their age LTL group. Their ranks in childhood LTL (1-5) were then subtracted by the subject’s cord LTL rank to examine their LTL rank change. Data analyses were performed with the Statistical Package for the Social Sciences for Windows, version 25.0 (SPSS Inc., Chicago, USA) and R version 4.0.2 (R Core Team, Vienna, Austria). P ≤ 0.05 (2-tailed) was considered statistically significant.
Results
Characteristics for Mother and Offspring at Birth and Age 7
There were 970 mother-offspring pairs who participated in this follow-up study at age 7 (Supplementary figure 9) (14). After excluding subjects born preterm, 926 mother-offspring pairs were eligible for our current analysis, in which 853 offspring at age 7 and a subset of 313 newborns had available DNA and maternal sera for measurement of LTL and vitamin D levels. The mean childhood LTL was 1.49 ± 0.47 ΔΔCt (mean ± SD), whereas mean cord blood LTL was 1.75 ± 0.62 ΔΔCt (Table 1). Maternal and cord sera vitamin D levels were available for offspring with LTL measurements. The mean maternal age was 31.3 ± 4.6 years at the time of pregnancy. The mean age at the follow-up visit for the offspring was at 7.0 ± 0.4 years. Approximately 48% of the offspring were female and the mean BMI was 15.1 ± 2.3 kg/m2 at follow-up.
Table 1.
Baseline characteristics of mothers and children
| Characteristics | Values: mean ± SD, median [Q1, Q3], or number (%) |
|---|---|
| Maternal (total number = 853) | |
| Maternal age EDC (year) | 31.3 ± 4.6 |
| Prenatal smoking (yes) | 14 (1.6) |
| Parity, 0 vs ≥ 1, (nulliparous) | 513 (60.1) |
| Maternal pre-preg BMI (kg/m2) | 20.9 ± 2.9 |
| Maternal fasting glucose (mmol/L) | 4.4 ± 0.3 |
| Maternal 1-hour glucose (mmol/L) | 7.7 ± 1.6 |
| Maternal 2-hour glucose (mmol/L) | 6.6 ± 1.3 |
| Maternal OGTT GAUC (hr) | 13.1 ± 2.2 |
| Maternal SBP (mmhg) | 101.3 ± 9.6 |
| Offspring (presented data are offspring with children’s LTL) | |
| Cord telomere length (ΔΔCt) (n = 313) | 1.75 ± 0.62 |
| Childhood telomere length (ΔΔCt) | 1.49 ± 0.47 |
| Gestational age at delivery (week) | 39.6 ± 1.1 |
| Breastfeeding (yes) | 440 (51.6) |
| C-section (yes) | 212 (24.9) |
| Offspring sex (female) | 414 (48.5) |
| Offspring age (year) | 7.0 ± 0.4 |
| Children’s BMI (kg/m2) | 15.1 ± 2.3 |
| Children’s exercise levels (0, 1, 2) | |
| 0 | 86 (10.1) |
| 1 | 456 (53.5) |
| 2 | 311 (36.5) |
| Children’s mean SBP (mmhg) | 101.9 ± 8.8 |
| Children’s HOMA-IR | 0.4 [0.4, 0.9] |
| Maternal DM status at FU (yes) | 101 (11.8) |
| Paternal DM status at FU (yes) | 97 (10.5) |
Abbreviations: BMI, body mass index; C-section, cesarean section; HOMA-IR, homeostasis model assessment of insulin resistance; LTL, leukocyte telomere length; maternal age EDC, maternal age at estimated date of confinement; maternal/paternal DM status at FU, maternal/paternal diabetes status at follow-up; OGTT GAUC, oral glucose tolerance test glucose area under the curve; SBP, systolic blood pressure.
The mean maternal total 25(OH)D measurement was 57.7 ± 19.9 nmol/L during pregnancy, and concentrations of maternal 25(OH)D3 and 3-epi-25(OH)D3 were 56.6 ± 19.6 nmol/L and 1.9 [1.2, 2.7] nmol/L (median [interquartile range]), respectively. Cord total 25(OH)D was 39.8 [31.6, 50.3] nmol/L, while the concentrations of cord 25(OH)D3 and 3-epi-25(OH)D3 were 39.6 [31.3, 49.9] and 1.6 [1.0, 2.4] nmol/L, respectively. The concentration of 25(OH)D2 was low in both maternal and cord sera, ranging from 0.4 to 26.8 nmol/L and 0.4 to 27.6 nmol/L, respectively, with 25(OH)D2 at undetectable levels in the majority of samples (Supplementary Figures 4 and 8) (14). As a result, total 25(OH)D was used as the trait for primary analyses for association with LTL, with the majority of 25(OH)D comprised of 25(OH)D3. Association between LTL and 25(OH)D3 and 3-epi-25(OH)D3 were conducted as exploratory secondary analyses.
Relationship Between LTL and Maternal and Offspring Factors
For children’s LTL at age 7, both sex and cesarean section as a delivery method (C-section) were examined for their effects on LTL. Childhood LTL was significantly different between males and females, with longer LTL in female offspring (P < 0.001). We did not observe any significant difference in LTL between offspring delivered through C-section vs offspring who were not (P = 0.103).
Univariate association was used to examine the association between childhood LTL and adjusted covariates (Table 2). Childhood LTL was positively associated with maternal age (β ± SE = 0.013 ± 0.003, P < 0.001) and maternal GAUC during the pregnancy OGTT (β ± SE = 0.021 ± 0.008, P = 0.007). Offspring sex was significantly associated with LTL, with female offspring having significantly longer LTL (β ± SE = 0.116 ± 0.032, P < 0.001). Offspring exercise level (0, 1, 2, with 0 denoting the lowest frequency of exercise) was negatively related to childhood LTL (β ± SE = −0.022 ± 0.007, P = 0.004), that is, children with lower self-reported exercise level have longer LTL in childhood. For LTL at age 7, shorter LTL was associated with indices of adiposity: higher current BMI (β ± SE = −0.016 ± 0.007, P = 0.02) and higher HOMA-IR (β ± SE = −0.065 ± 0.021, P = 0.002) (Supplementary Table 1) (14). After applying Bonferroni correction to correct for examining 16 traits, only maternal age EDC, offspring sex, HOMA-IR, and childhood exercise levels remained significantly associated with LTL.
Table 2.
Univariate associations between cord and children’s LTL with their basic characteristics
| Cord blood LTL (n = 313) | 7-year-old LTL (n = 853) | |||||
|---|---|---|---|---|---|---|
| Unstd Beta | SE | P | Unstd Beta | SE | P | |
| Maternal age EDC | 0.008 | 0.009 | 0.333 | 0.013 | 0.003 | <0.001 |
| Maternal pre-pregnancy BMI | -0.005 | 0.013 | 0.703 | 0.001 | 0.006 | 0.822 |
| Maternal SBP | -0.005 | 0.004 | 0.211 | -0.001 | 0.002 | 0.649 |
| Maternal GAUC | 0.022 | 0.018 | 0.221 | 0.021 | 0.008 | 0.007 |
| Ponderal index | 0.032 | 0.018 | 0.068 | -0.007 | 0.007 | 0.366 |
| C-section (yes/no) | 0.171 | 0.095 | 0.074 | 0.062 | 0.039 | 0.109 |
| Gestational age at delivery | 0.007 | 0.033 | 0.827 | -0.005 | 0.014 | 0.718 |
| Parity | 0.107 | 0.062 | 0.083 | 0.035 | 0.026 | 0.17 |
| Offspring sex (female = 1) | 0.037 | 0.074 | 0.62 | 0.116 | 0.032 | <0.001 |
| Follow-up age | N.A. | N.A. | N.A. | -0.047 | 0.037 | 0.2 |
| Childhood Exercise level (0,1, 2 with 0 = lowest) |
N.A. | N.A. | N.A. | -0.022 | 0.007 | <0.001 |
| Childhood BMI at age 7 | N.A. | N.A. | N.A. | -0.016 | 0.007 | 0.02 |
| Childhood SBP at age 7 | N.A. | N.A. | N.A. | -0.0003 | 0.002 | 0.891 |
| Maternal DM status at FU | 0.244 | 0.357 | 0.495 | 0.04 | 0.138 | 0.771 |
| Paternal DM status at FU | 0.103 | 0.112 | 0.355 | 0.004 | 0.052 | 0.942 |
Abbreviations: BMI, body mass index; C-section, cesarean section; LTL, leukocyte telomere length; maternal age EDC, maternal age at estimated date of confinement; maternal/paternal DM status at FU, maternal/paternal diabetes status at follow-up; GAUC, glucose area under the curve; SBP, systolic blood pressure.
Relationship Between Cord Blood LTL and Children’s LTL
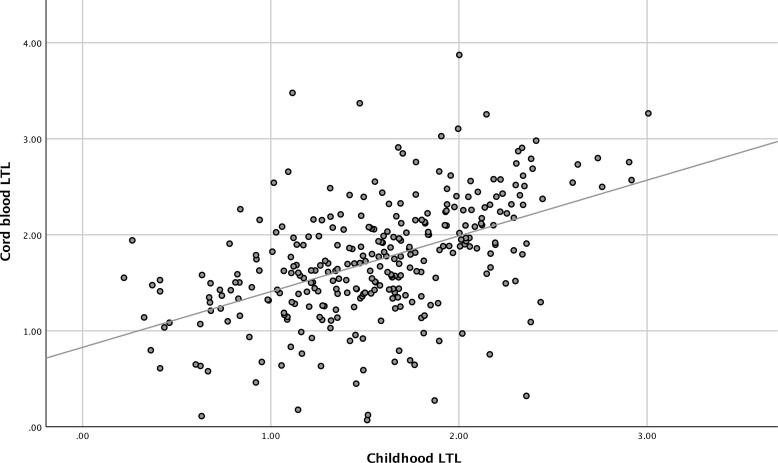
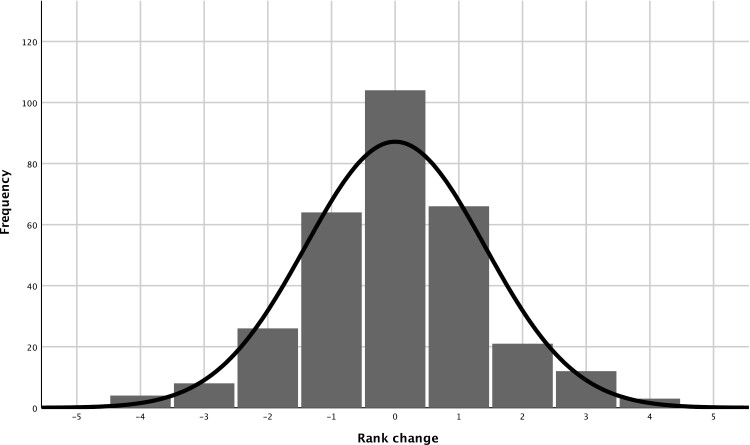
Examination of the relationship between cord blood and childhood LTL revealed a moderate positive association between the LTL at the 2 different time points (r = 0.49, P < 0.001) (Fig. 1). Any change in relative LTL over the period spanned by the 2 time points was also examined using quintile LTL rank change (Fig. 2). Childhood LTL quintile to cord blood LTL quintile rank changes were illustrated with a histogram using data from the 305 offspring with both cord and childhood DNA available. This revealed that 76.4 % of the offspring exhibited either no or only 1 rank change. Most offspring maintained their LTL rank in the cohort, highlighting the stability of the ranking for relative telomere length.
Figure 1.
Association between cord and childhood LTL.
Figure 2.
Offspring LTL quintile rank change.
Relationship Between Maternal 25(OH)D Levels and Offspring LTL
Consistent positive associations were observed between LTL at age 7 and the maternal total 25(OH)D levels, including in the fully adjusted model (β ± SE = 0.048 ± 0.017, P = 0.004) (Table 3). As expected, similar associations were observed between LTL and maternal 25(OH)D3 (β ± SE = 0.046 ± 0.017, P = 0.005), the main isoform of vitamin D. Interestingly, consistent associations between childhood LTL and maternal 3-epi-25(OH)D3 were also observed (β ± SE = 0.05 ± 0.017, P = 0.003), as were associations between childhood LTL and cord blood 25(OH)D. After full adjustment, the same direction but insignificant association was observed between childhood LTL and cord serum total 25(OH)D (β ± SE = 0.031 ± 0.18, P = 0.085). Cord 3-epi-25(OH)D3 (β ± SE = 0.037 ± 0.018, P = 0.035) was also positively associated with childhood LTL after adjustment for sex and age. This association was however no longer significant after full model adjustment (P = 0.1).
Table 3.
Associations between children’s LTL with in utero vitamin D
| Unadjusted | Model 1 | Model 2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Unstd Beta | SE | P | Unstd Beta | SE | P | Unstd Beta | SE | P | |
| Maternal 25(OH)D total | 0.003 | 0.001 | 0.001 | 0.056 | 0.017 | 0.001 | 0.048 | 0.017 | 0.004 |
| Maternal 25(OH)D3 | 0.003 | 0.001 | 0.001 | 0.054 | 0.017 | 0.001 | 0.046 | 0.017 | 0.005 |
| Maternal 3-epi-25(OH)D3 | 0.038 | 0.012 | 0.002 | 0.056 | 0.017 | 0.001 | 0.05 | 0.017 | 0.003 |
| Cord 25(OH)D total | 0.001 | 0.001 | 0.296 | 0.029 | 0.018 | 0.099 | 0.031 | 0.018 | 0.085 |
| Cord 25(OH)D3 | 0.001 | 0.001 | 0.308 | 0.028 | 0.018 | 0.115 | 0.03 | 0.018 | 0.095 |
| Cord 3-epi-25(OH)D3 | 0.034 | 0.014 | 0.017 | 0.037 | 0.018 | 0.035 | 0.029 | 0.018 | 0.100 |
Unadjusted: No adjustment. Model 1 (basic) adjusted: sex and age at childhood. Model 2 (maternal and offspring confounders) adjusted: Model 1 + childhood body mass index (BMI), systolic blood pressure, gestational age, and exercise level parity, maternal age at estimated date of confinement, maternal systolic blood pressure, current maternal and paternal diabetes mellitus, C-section, maternal pre-pregnancy BMI, maternal glucose area under the curve (at 28 weeks).
Abbreviations: C-section, cesarean section; LTL, leukocyte telomere length.
There was no relationship observed between cord blood LTL and cord 25(OH)D levels (Table 4). Consistent with results presented earlier, maternal total 25(OH)D (β ± SE = 0.07 ± 0.035, P = 0.047), as well as 25(OH)D3 (β ± SE = 0.073 ± 0.036, P = 0.041), were positively associated with cord blood LTL, including after adjusting for all covariates. Maternal 3-epi-25(OH)D3 also showed a positive but insignificant association with cord blood LTL in the fully adjusted model (β ± SE = 0.062 ± 0.036, P = 0.087). With the sample size of the childhood blood LTL (n = 853) being more than double to that of the cord blood LTL (n = 313), the difference in sample size may partly explain the differences in associations between the LTL and maternal and cord 25(OH)D levels.
Table 4.
Associations between cord blood LTL with in utero vitamin D
|
|
Unadjusted | Model 1 | Model 2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Unstd Beta | SE | P | Unstd Beta | SE | P | Unstd Beta | SE | P | |
| Maternal 25(OH)D total | 0.004 | 0.002 | 0.023 | 0.075 | 0.036 | 0.036 | 0.07 | 0.035 | 0.047 |
| Maternal 25(OH)D3 | 0.004 | 0.002 | 0.02 | 0.079 | 0.036 | 0.029 | 0.073 | 0.036 | 0.041 |
| Maternal 3-epi-25(OH)D3 | 0.044 | 0.028 | 0.114 | 0.066 | 0.036 | 0.072 | 0.062 | 0.036 | 0.087 |
| Cord 25(OH)D total | -0.003 | 0.002 | 0.180 | -0.024 | 0.035 | 0.487 | -0.018 | 0.035 | 0.604 |
| Cord 25(OH)D3 | -0.003 | 0.002 | 0.166 | -0.026 | 0.035 | 0.462 | -0.019 | 0.035 | 0.593 |
| Cord 3-epi-25(OH)D3 | -0.012 | 0.026 | 0.648 | -0.038 | 0.036 | 0.297 | -0.053 | 0.037 | 0.154 |
Unadjusted: No adjustment. Model 1 (basic) adjusted: sex and age at childhood. Model 2 (maternal and offspring confounders) adjusted: Model 1 + childhood body mass index (BMI), systolic blood pressure, gestational age, and exercise level parity, maternal age at estimated date of confinement, maternal systolic blood pressure, current maternal and paternal diabetes mellitus, C-section, maternal pre-pregnancy BMI, maternal glucose area under the curve (at 28 weeks).
Abbreviations: C-section, cesarean section; LTL, leukocyte telomere length.
LTL and Vitamin D Stratified by Maternal Vitamin D Status
Maternal vitamin D is commonly classified according to its sufficiency status. With reference to the recommended thresholds for defining vitamin D sufficiency according to the Endocrine Society guidelines (8) with a cutoff of 50 nmol/L, maternal 25(OH)D of less than 50 nmol/L was classified as “deficient”, <75-50 nmol/L as “sufficient”, and 75 nmol/L or greater as “more sufficient” (Supplementary Table 2) (14), in order to examine the impact of maternal vitamin D status on offspring LTL. There were a similar number of male and female offspring in each class (P = 0.762). With increasing maternal 25(OH)D in each group, there was a significant association with increasing LTL in cord blood (P = 0.025) and with childhood LTL (P = 0.045).
Discussion
In this longitudinal study of mother-offspring pairs with detailed phenotyping during pregnancy, at birth, and during follow-up, our main findings are (i) offspring LTL in childhood is inversely associated with markers of insulin resistance and obesity; (ii) LTL in childhood and LTL in cord blood are associated; (iii) maternal vitamin D levels, cord blood vitamin D levels, and offspring LTL in childhood are positively associated; (iv) maternal and cord blood levels of the novel 3-epi-25(OH)D3 are positively associated and a similar but insignificant association (P = 0.1) with childhood LTL, respectively. Our results provide a potential novel link between maternal vitamin D levels and the developmental origins of metabolic diseases later in life.
Shorter LTL in adults is associated with various diseases. A meta-analysis of 24 studies including 43 000 people with CVD suggested that, when comparing the tertile with the shortest LTL to those in the tertile with the longest LTL, the shortest LTL tertile was associated with a 54% higher risk of coronary heart disease (1). Another meta-analysis reported a shorter telomere length in both type 1 and type 2 diabetes (standardized mean difference [SMD]: −3.41; 95% CI −4.01, −2.80) when compared with people without diabetes (19). In our study, children’s LTL was positively associated with female sex (20) and higher maternal age (21) and inversely associated with offspring BMI (22), consistent with earlier LTL studies. We also found that childhood HOMA-IR, a marker of insulin resistance, is inversely associated with childhood LTL. In a Danish cohort of teenagers whose mothers had gestational diabetes (GDM) or normal glucose tolerance during pregnancy, LTL was negatively associated with HOMA-IR and fasting insulin levels in 9- to 16-year-old females (20). Our findings are consistent with other LTL studies from both early age and later age that reported an inverse association between LTL and cardiometabolic traits (2).
Apart from observing an association between current cardiometabolic traits and childhood LTL, our study also found a strong association between LTL at birth and later in childhood. The strong association between cord blood and childhood LTL as well as little change in LTL ranking demonstrate that the LTL rank of offspring is relatively stable from birth to childhood, with a high number of offspring maintaining their relative rank. This is similar to what has been observed in other studies, where a high proportion of subjects maintained their LTL rank between early and old age (23, 24). Together, these findings suggest a potential association between early-life exposure and later life outcomes involving LTL.
Previous research has suggested a potential link between maternal vitamin D and telomere length both in women (25) and newborns (26). In our study, childhood LTL was strongly associated with maternal total 25(OH)D. Furthermore, the same relationship was also observed between maternal total 25(OH)D and cord blood LTL. Similar trends were also observed in both cord and childhood LTL when offspring were categorized according to maternal total 25(OH)D as “deficient” (<50 nmol/L), “sufficient” (<75-50 nmol/L) and “more sufficient” (≥75 nmol/L). A small study from Korea noted that maternal vitamin D tertile at late gestation was associated with cord blood LTL (26). A study from Belgium reported a positive association between childhood LTL and childhood 25(OH)D. However, their association was observed only in boys and not in analyses that included both sexes (27). In contrast, other studies have suggested no relationship between maternal vitamin D and cord blood LTL (28). Our findings with maternal and cord blood vitamin D levels measured using mass spectrometry as well as LTL measured at 2 timepoints at birth and during childhood demonstrate a consistent association between maternal vitamin D levels and offspring LTL.
To perform its biological functions, stable blood vitamin D [25(OH)D] must first be hydrolyzed into its active form, calcitriol (1-25DHCC), to increase its affinity for the vitamin D receptor (VDR), although it has been suggested that stable vitamin D may also bind to and activate the VDR (29). The hydrolysis of vitamin D takes place in the kidney, pancreas, placenta, and immune cells. Vitamin D may be linked with telomere length through at least 2 possible mechanisms (26).
First, both stable 25(OH)D and active calcitriol are anti-inflammatory and could lower inflammatory markers in mothers and newborns. Shorter newborn LTL was shown to be associated with higher maternal markers of inflammation markers in early gestation such as the TNF-α/IL-10 ratio (30). Higher vitamin D levels may lower the potential effect of oxidative stress on shortening cord blood LTL.
Second, calcitriol as the active isoform of vitamin D, can upregulate the activity of telomerase. One proposed pathway is the phosphatidylinositol 3-OH kinase (PI3K/Akt) pathway (31) that is upregulated by VDR in the setting of increased calcitriol (32). Phosphorylation of human telomerase reverse transcriptase (hTERT), via the PI3K/Akt pathway, would, in turn, upregulate the expression of telomerase, which maintains and lengthens LTL. This upregulation of telomerase in turn could give rise to longer LTLs in the presence of higher vitamin D levels. Given our findings in both newborns and childhood, further investigation on the potential interaction between vitamin D and telomerase is warranted.
Of note, the first mechanism related to anti-oxidation has been suggested as a mechanism for both active and unhydrolyzed, inactive vitamin D while the latter human telomerase reverse transcriptase (hTERT)-related mechanism has been suggested for the active form of vitamin D. Active vitamin D is found in a dynamic state with inactive, stable vitamin D (33). In our study however, only measurements of the stable 25(OH)D were performed, and we are not able to make inferences about the activity of the vitamin D.
One interesting observation from our study is the potential role of 3-epi-25(OH)D3 in early life. As noted, total vitamin D consisted mainly of 25(OH)D3 in our study. 3-epi-25(OH)D3, when compared with 25(OH)D3, has a similar action on parathyroid hormone secretion, but at a much lower affinity for the vitamin D receptor (9). There is little knowledge regarding the role of 3-epi-25(OH)D3, except that it was reported to have an inverse relationship with the maturity of neonatal liver function (34) and became undetectable in the first year after birth. 3-epi-25(OH)D3 became a concern as the inclusion of 3-epi-25(OH)D3 may overestimate the sufficiency of vitamin D in newborns, leading to the need to analyze total vitamin D separately from its epimer in infants (35). In adult and pregnant women however, 3-epi-25(OH)D3 was found to be present in trace amounts and did not affect the classifications of sufficiency (36). In our study, although the 3-epi-25(OH)D3 to 25(OH)D3 ratio was as high as 18.1% and 15.3% in maternal and cord sera, respectively, the mean ratio was around 4% in maternal and cord sera. A limited effect of 3-epi-25(OH)D3 would be expected, given the low concentration ratio in maternal or cord sera, but with limited information it is hard to propose a firm mechanism to explain the positive association between 3-epi-25(OH)D3 and LTL or to state that the 3-epi-25(OH)D3 had minimal or no effect, given the strong associations that were observed. Some similarities between LTL and vitamin D3 epimer were as follows. Preterm infants were reported to have higher vitamin D3 epimer than term infants (37), which is consistent with the cord blood LTL being longer in preterm infants (38). 3-epi-25(OH)D3 was found to decrease during the first year after birth. The reduction of 3-epi-25(OH)D3 level was similar to the shortening of LTL in relation to age (34). Conceptually, LTL as a marker of age could also be a maturation marker similar to 3-epi-25(OH)D3, as 3-epi-25(OH)D3 was reported to be associated with the development of the neonatal liver. These similarities provide some food for thought related to the associations between LTL and vitamin D3 isomer; further research, however, is warranted to explore their association and potential underlying mechanisms. Taking into consideration that cardiometabolic traits were also associated with LTL in both our study and other studies, the positive association between LTL and maternal vitamin D and 3-epi-25(OH)D3 could suggest that early-life maternal vitamin D levels may be linked to offspring risk of disease later in life.
The potential clinical implications of the vitamin D–LTL association, however, require further study. While we observed a positive correlation between maternal vitamin D levels and offspring LTL, whether vitamin D is causally linked to LTL remains unclear. Future studies, including utilizing Mendelian randomization, can help clarify whether there are potential causal links. Clarifying whether there is a causal link would be important, given that several intervention trials involving vitamin D supplementation in pregnancy or outside pregnancy to improve metabolic health have in general been negative. For example, the Vitamin D and Lifestyle Intervention for Gestational Diabetes Mellitus Prevention (DALI) study included a vitamin D intervention arm (1600 IU vitamin D3 daily) starting at around 20 weeks gestation achieved vitamin D sufficiency, but without significant changes in perinatal outcomes (39). Other studies have also suggested vitamin D intervention during pregnancy was associated with little or unclear benefits to the offspring (40). Further examination of the mechanism between 25(OH)D3, 3-epi-25(OH)D3, and telomere length would be of interest. It would also be interesting to measure LTL in some of these vitamin D intervention trials in which the offspring are being followed up, to examine whether vitamin D supplementation would modify LTL.
Strengths of the study include a detailed examination of the association between vitamin D and LTL at 2 different time points (at birth and 7 years), including consideration of 3 different 25(OH)D isoforms that were measured using the gold-standard of mass spectrometry. A lasting association of maternal 25(OH)D with LTL from birth to age 7 was shown in this cohort. Some limitations regarding the study design should be noted. For example, the maternal vitamin D level was determined at around 28 weeks gestation (24-32 weeks), whereas the cord blood vitamin D was collected at birth, which may reduce the association between the vitamin D levels measured at these 2 different timepoints. Additional time points during pregnancy would be helpful to understand the temporal relationship between changes in maternal vitamin D levels and cord blood vitamin D and their relationship to LTL. LTL itself is known to be confounded by many factors such as paternal age, diet, and socioeconomic status. Although HAPO is a well-characterized cohort, some information was not collected as part of the study, which limited the ability to adjust for potential confounders, and hence the possibility of residual confounding cannot be excluded. Analyses were based on the calculation of relative LTL, and we were not able to compare absolute LTL. Given the observational nature of the study, we can only infer association; determining whether maternal vitamin D is causally associated with offspring LTL would require further studies including mechanistic studies and exploration through Mendelian randomization studies as aforementioned. The mechanisms underlying the association of cord and maternal 3-epi-25(OH)D3 with childhood LTL are also not clear due to the limited understanding and warrant further research both on this epimer and its relationship with LTL.
In summary, this study has demonstrated that childhood LTL is strongly associated with cord blood LTL as well as with current cardiometabolic traits. LTLs at both time points were also shown to be associated with maternal vitamin D and the novel marker 3-epi-25(OH)D3, suggesting a potential link between early maternal vitamin D and metabolic condition later in childhood related to a continuum of LTL. Further studies to examine whether there are causal links between vitamin D and LTL are warranted.
Acknowledgments
The authors acknowledge Dr. Andrzej Januszewski and Dr. Luke Carroll (University of Sydney) for providing assistance for the DNA QC material.
Glossary
Abbreviations
- 25(OH)D
25-hydroxyvitamin D
- BMI
body mass index
- C-section
cesarean section
- CV
coefficient of variation
- CVD
cardiovascular diseases
- EDC
estimated date of confinement
- GAUC
glucose area under the curve
- GDM
gestational diabetes
- HAPO
Hyperglycaemia and Adverse Pregnancy Outcome cohort
- HBG
human βglobin
- HOMA-IR
homeostasis model assessment of insulin resistance
- LTL
leukocyte telomere length
- OGTT
oral glucose tolerance test
- qPCR
quantitative polymerase chain reaction
- VDR
vitamin D receptor
Contributor Information
Kwun Kiu Wong, Department of Medicine and Therapeutics, The Chinese University of Hong Kong, Hong Kong, China.
Feifei Cheng, Department of Medicine and Therapeutics, The Chinese University of Hong Kong, Hong Kong, China.
Di Mao, Department of Obstetrics and Gynaecology, The Chinese University of Hong Kong, Hong Kong, China.
Cadmon K P Lim, Department of Medicine and Therapeutics, The Chinese University of Hong Kong, Hong Kong, China.
Claudia H T Tam, Department of Medicine and Therapeutics, The Chinese University of Hong Kong, Hong Kong, China.
Chi Chiu Wang, Department of Obstetrics and Gynaecology, The Chinese University of Hong Kong, Hong Kong, China; School of Biomedical Sciences, The Chinese University of Hong Kong, Hong Kong, China; Chinese University of Hong Kong–Sichuan University Joint Laboratory in Reproductive Medicine, The Chinese University of Hong Kong, Hong Kong, China; Li Ka Shing Institute of Health Sciences, The Chinese University of Hong Kong, Hong Kong, China.
Lai Yuk Yuen, Department of Obstetrics and Gynaecology, The Chinese University of Hong Kong, Hong Kong, China.
Michael H M Chan, Department of Chemical Pathology, The Chinese University of Hong Kong, Hong Kong, China.
Chung Shun Ho, Department of Chemical Pathology, The Chinese University of Hong Kong, Hong Kong, China.
Mugdha V Joglekar, Diabetes and Islet Biology Group, School of Medicine, Western Sydney University, Australia; NHMRC Clinical Trial Centre, Faculty of Medicine and Health, University of Sydney, Australia.
Anandwardhan A Hardikar, Diabetes and Islet Biology Group, School of Medicine, Western Sydney University, Australia; NHMRC Clinical Trial Centre, Faculty of Medicine and Health, University of Sydney, Australia.
Alicia J Jenkins, Department of Medicine and Therapeutics, The Chinese University of Hong Kong, Hong Kong, China; NHMRC Clinical Trial Centre, Faculty of Medicine and Health, University of Sydney, Australia.
Boyd E Metzger, Northwestern University Feinberg School of Medicine, Chicago, USA.
William L Lowe, Jnr., Northwestern University Feinberg School of Medicine, Chicago, USA
Wing Hung Tam, Department of Obstetrics and Gynaecology, The Chinese University of Hong Kong, Hong Kong, China.
Ronald C W Ma, Department of Medicine and Therapeutics, The Chinese University of Hong Kong, Hong Kong, China; Li Ka Shing Institute of Health Sciences, The Chinese University of Hong Kong, Hong Kong, China; Hong Kong Institute of Diabetes and Obesity, The Chinese University of Hong Kong, Hong Kong, China; Chinese University of Hong Kong-Shanghai Jiao Tong University Joint Research Centre in Diabetes Genomics and Precision Medicine, Hong Kong, China.
Funding Disclosure
This study was supported by the Research Grants Council General Research Fund (ref. 14118718), Research Impact Fund (R4012-18), and the Croucher Foundation Senior Medical Research Fellowship. The HAPO study was funded by the National Institute of Child Health and Human Development and the National Institute of Diabetes and Digestive Diseases (grant nos. R01-HD34243 and R01-HD34242). The HAPO follow-up study at the Hong Kong center was supported by funding from the Research Grants Council of the Hong Kong SAR, China (grants CUHK 473408, 471713, 14118316, and 14102719).
Author Contributions
K.W. measured telomere length, performed statistical analysis, and wrote the manuscript. F.C., D.M., C.H.T.T., and L.Y.Y. analyzed the data and interpreted results. C.K.P.L. contributed to study logistics and prepared samples. D.M., L.Y.Y., C.C.W., M.H.M.C., and C.S.H. measured vitamin D levels. M.V.J., A.J.J., and A.A.H. developed the modified LTL measurement method. W.H.T. and R.C.W.M. recruited subjects, contributed to study logistics and data analysis. B.E.M. and W.L.L.J. initiated the original HAPO study, obtained funding for the original study, and contributed to study logistics and provided samples. W.H.T. and R.C.W.M. designed the research, supervised the research work, performed statistical analysis, and wrote the manuscript. All authors contributed to the writing of the manuscript and approved the final version. R.C.W.M. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict Of Interest
R.C.W.M. has received research grants for clinical trials from AstraZeneca, Bayer, MSD, Novo Nordisk, Sanofi, and Tricida Inc. and honoraria for consultancy or lectures from AstraZeneca, Bayer, Boehringer Ingelheim, and Roche Diagnostics, all used to support diabetes research at the Chinese University of Hong Kong. R.C.W.M. is a co-founder of GemVCare, a technology start-up initiated with support from the Hong Kong Government Innovation and Technology Commission and its Technology Start-up Support Scheme for Universities (TSSSU). A.J.J. has received research grants for clinical trials from Abbott and Sanofi-Aventis, and honoraria for consultancy for Abbott, Amgen, Medtronic, and Sanofi-Aventis. No other potential conflicts of interest relevant to this article were reported.
Prior Presentation
Parts of this work were presented in oral presentation at the 2021 American Diabetes Association’s Virtual 81st Scientific Sessions, June 25-29, 2021.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1. Haycock PC, Heydon EE, Kaptoge S, Butterworth AS, Thompson A, Willeit P. Leucocyte telomere length and risk of cardiovascular disease: systematic review and meta-analysis. BMJ. 2014;349:g4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cheng F, Carroll L, Joglekar MV, et al. Diabetes, metabolic disease, and telomere length. Lancet Diabetes Endocrinol. 2021;9(2):117-126. [DOI] [PubMed] [Google Scholar]
- 3. McAninch D, Bianco-Miotto T, Gatford KL, et al. The metabolic syndrome in pregnancy and its association with child telomere length. Diabetologia. 2020;63(10):2140-2149. [DOI] [PubMed] [Google Scholar]
- 4. Martens DS, Janssen BG, Bijnens EM, et al. Association of parental socioeconomic status and newborn telomere length. JAMA Netw Open. 2020;3(5):e204057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pittas AG, Lau J, Hu FB, Dawson-Hughes B. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab. 2007;92(6):2017-2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Park JE, Pichiah PT, Cha Y-S. Vitamin D and metabolic diseases: growing roles of vitamin D. J Obes Metab Syndr. 2018;27(4):223-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Boyle VT, Thorstensen EB, Mourath D, et al. The relationship between 25-hydroxyvitamin D concentration in early pregnancy and pregnancy outcomes in a large, prospective cohort. Br J Nutr. 2016;116(8):1409-1415. [DOI] [PubMed] [Google Scholar]
- 8. Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911-1930. [DOI] [PubMed] [Google Scholar]
- 9. Bailey D, Perumal N, Yazdanpanah M, et al. Maternal–fetal–infant dynamics of the C3-epimer of 25-hydroxyvitamin D. Clin Biochem. 2014;47(9):816-822. [DOI] [PubMed] [Google Scholar]
- 10. Mazidi M, Michos ED, Banach M. The association of telomere length and serum 25-hydroxyvitamin D levels in US adults: the National Health and Nutrition Examination Survey. Arch Med Sci. 2017;13(1):61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Metzger BE, Lowe LP, Dyer AR, et al. ; HAPO Study Cooperative Research Group. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358:1991-2002. [DOI] [PubMed] [Google Scholar]
- 12. Tam WH, Ma RCW, Ozaki R, et al. In utero exposure to maternal hyperglycemia increases childhood cardiometabolic risk in offspring. Diabetes Care. 2017;40(5):679-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tam CH, Ma RC, Yuen LY, et al. The impact of maternal gestational weight gain on cardiometabolic risk factors in children. Diabetologia. 2018;61(12):2539-2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wong KK, Cheng F, Mao D, et al. Vitamin D levels during pregnancy are associated with offspring telomere length: a longitudinal mother-child study supplementary file. figshare. Deposited April 19, 2022. https://figshare.com/s/1a1818b65f3fd0b42593 [DOI] [PMC free article] [PubMed]
- 15. Ong L, Saw S, Sahabdeen NB, Tey KT, Ho CS, Sethi SK. Current 25-hydroxyvitamin D assays: do they pass the test? Clin Chim Acta. 2012;413(13-14):1127-1134. [DOI] [PubMed] [Google Scholar]
- 16. Mao D, Yuen LY, Ho CS, et al. Maternal and neonatal 3-epi-25-hydroxyvitamin D concentration and factors influencing their concentrations. J Endocr Soc. 2022;6(1):bvab170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30(10):e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Joglekar MV, Satoor SN, Wong WKM, Cheng F, Ma RCW, Hardikar AA.. An Optimised Step-by-Step Protocol for Measuring Relative Telomere Length. Methods Protoc 2020; 3(2):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang J, Dong X, Cao L, et al. Association between telomere length and diabetes mellitus: a meta-analysis. J Int Med Res. 2016;44(6):1156-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hjort L, Vryer R, Grunnet LG, et al. Telomere length is reduced in 9-to 16-year-old girls exposed to gestational diabetes in utero. Diabetologia. 2018;61(4):870-880. [DOI] [PubMed] [Google Scholar]
- 21. Prescott J, Du M, Wong J, Han J, De Vivo I. Paternal age at birth is associated with offspring leukocyte telomere length in the nurses’ health study. Hum Reprod. 2012;27(12):3622-3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lamprokostopoulou A, Moschonis G, Manios Y, et al. Childhood obesity and leucocyte telomere length. Eur J Clin Invest. 2019;49(12):e13178. [DOI] [PubMed] [Google Scholar]
- 23. Wojcicki JM, Shiboski S, Heyman MB, et al. Telomere length change plateaus at 4 years of age in Latino children: associations with baseline length and maternal change. Mol Genet Genomics. 2016;291(3):1379-1389. [DOI] [PubMed] [Google Scholar]
- 24. Farzaneh-Far R, Lin J, Epel E, Lapham K, Blackburn E, Whooley MA. Telomere length trajectory and its determinants in persons with coronary artery disease: longitudinal findings from the heart and soul study. PLoS One. 2010;5(1):e8612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Richards JB, Valdes AM, Gardner JP, et al. Higher serum vitamin D concentrations are associated with longer leukocyte telomere length in women. Am J Clin Nutr. 2007;86(5):1420-1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kim JH, Kim GJ, Lee D, et al. Higher maternal vitamin D concentrations are associated with longer leukocyte telomeres in newborns. Matern Child Nutr. 2018;14(1):e12475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Van Aart C, Michels N, Sioen I, Martens D, Nawrot T, De Henauw S. Vitamin D as predictor of telomere length in the transition from child to adolescent. Rev Epidemiol Sante Publique. 2018;66(Suppl 5):S237. [Google Scholar]
- 28. Vahter M, Broberg K, Harari F. Placental and cord blood telomere length in relation to maternal nutritional status. J Nutr. 2020;150(10):2646-2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tuohimaa P. Vitamin D and aging. J Steroid Biochem Mol Biol. 2009;114(1-2):78-84. [DOI] [PubMed] [Google Scholar]
- 30. Lazarides C, Epel ES, Lin J, et al. Maternal pro-inflammatory state during pregnancy and newborn leukocyte telomere length: a prospective investigation. Brain Behav Immun. 2019;80:419-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kang SS, Kwon T, Do SI. Akt protein kinase enhances human telomerase activity through phosphorylation of telomerase reverse transcriptase subunit. J Biol Chem. 1999;274(19):13085-13090. [DOI] [PubMed] [Google Scholar]
- 32. Buitrago C, Pardo VG, Boland R. Role of VDR in 1α, 25-dihydroxyvitamin D3-dependent non-genomic activation of MAPKs, Src and Akt in skeletal muscle cells. J Steroid Biochem Mol Biol. 2013;136:125-130. [DOI] [PubMed] [Google Scholar]
- 33. Tang JC, Jackson S, Walsh NP, Greeves J, Fraser WD. The dynamic relationships between the active and catabolic vitamin D metabolites, their ratios, and associations with PTH. Sci Rep. 2019;9(1):1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Singh RJ, Taylor RL, Reddy GS, Grebe SK. C-3 epimers can account for a significant proportion of total circulating 25-hydroxyvitamin D in infants, complicating accurate measurement and interpretation of vitamin D status. J Clin Endocrinol Metab. 2006;91(8):3055-3061. [DOI] [PubMed] [Google Scholar]
- 35. Gallo S, Comeau K, Vanstone C, et al. Effect of different dosages of oral vitamin D supplementation on vitamin D status in healthy, breastfed infants: a randomized trial. JAMA. 2013;309(17):1785-1792. [DOI] [PubMed] [Google Scholar]
- 36. Bailey D, Veljkovic K, Yazdanpanah M, Adeli K. Analytical measurement and clinical relevance of vitamin D3 C3-epimer. Clin Biochem. 2013;46(3):190-196. [DOI] [PubMed] [Google Scholar]
- 37. Ooms N, van Daal H, Beijers AM, Gerrits GPJ, Semmekrot BA, van den Ouweland JM. Time-course analysis of 3-epi-25-hydroxyvitamin D 3 shows markedly elevated levels in early life, particularly from vitamin D supplementation in preterm infants. Pediatr Res. 2016;79(4):647-653. [DOI] [PubMed] [Google Scholar]
- 38. Vasu V, Turner KJ, George S, Greenall J, Slijepcevic P, Griffin DK. Preterm infants have significantly longer telomeres than their term born counterparts. PLoS One. 2017;12(6):e0180082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Corcoy R, Mendoza LC, Simmons D, et al. The DALI vitamin D randomized controlled trial for gestational diabetes mellitus prevention: No major benefit shown besides vitamin D sufficiency. Clin Nutr. 2020;39(3):976-984. [DOI] [PubMed] [Google Scholar]
- 40. Bi W, Nuyt A, Weiler H, Leduc L, Santamaria C, Wei S. Association Between Vitamin D Supplementation During Pregnancy and Offspring Growth, Morbidity, and Mortality: A Systematic Review and Meta-analysis. JAMA Pediatr. 2018;172(7):635-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.