Abstract
Background
In recent decades, there has been a considerable increase in the number of nanomedicine-based formulations, and their advantages, including controlled/targeted drug delivery with increased efficacy and reduced toxicity, make them ideal candidates for therapeutic delivery in the treatment of complex and difficult-to-treat diseases, such as cancer.
Areas covered
This review focuses on nanomedicine-based formulation development, approved and marketed nanomedicines, and the challenges faced in nanomedicine development as well as their future prospects.
Expert opinion
To date, the Food and Drug Administration and the European Medicines Agency have approved several nanomedicines, which are now commercially available. However, several critical challenges, including reproducibility, proper characterization, and biological evaluation, e.g., via assays, are still associated with their use. Therefore, rigorous studies alongside stringent guidelines for effective and safe nanomedicine development and use are still warranted. In this study, we provide an overview of currently available nanomedicine-based formulations. Thus, the findings here reported may serve as a basis for further studies regarding the use of these formulations for therapeutic purposes in near future.
Keywords: Commercial formulations, Clinical trials, Nanomedicines, Pharmacokinetics
Introduction
Nanotechnology, which is considered a “modern scientific breakthrough” and has been explored and heralded in several scientific studies over the past decades (Bayda et al. 2019), refers to the production and use of materials, systems, and equipment at the nanoscale (Jain 2008). It offers the possibility to provide solutions to persistent problems and unmet needs via the use of interconnected platforms in a plethora of areas, such as chemistry, physics, engineering, biotechnology, and medical sciences. Thus, it expands the possibilities of modern research, especially in the medical field (Mack 2005). Current nanotechnology-based developments in this area include enhanced and precise medicine, the minimization of adverse effects/toxicity, and meeting previously unmet medical needs of patients (Waheed et al. 2022). In recent decades, nanomedicines have been produced, engineered, and industrialized at the chemical, macromolecular, and cellular levels (Mitchell et al. 2021). For example, the use of nanopharmaceuticals, theranostics, and nanoimaging agents in nanomedicine has resulted in significant advancements in disease detection, tomography, prevention, and care (Farjadian et al. 2019).
Poor pharmacokinetic characteristics, such as poor solubility, permeability, and bioavailability limit the therapeutic utility of many potent drug. Thus, the development of formulations with improved pharmacokinetics profiles is necessary (Chenthamara et al. 2019). Nanomedicine-based formulations, either as therapeutic agents or as carriers, can thus be utilized to ensure that drugs target the desired sites and improve the pharmacokinetics and therapeutic profile of drugs (Yetisgin et al. 2020). Notably, the important characteristics of nanomedicines, including nanoscale size (1–100 nm) and large surface area, offer unique possibilities for precise interactions with cells and tissues based on the identification of the appropriate biological targets (Soares et al. 2018). Further, the advantages of nanomedicine-based formulations include decreased undesirable toxicity resulting from non-specific distribution, improved patient adherence, and favorable clinical outcomes (De Jong and Borm 2008).
The availability of funds and the utilization of multidisciplinary technologies in the industry and academia have resulted in the fabrication of some promising nanomedicine-based formulations, such as liposomes, polymer/lipid nanoparticles, and polymer-conjugates (Puri et al. 2009). Several strategies including: (a) the utilization of new materials, fabrication processes, and techniques for the enhancement of drug stability and targeting; (b) the development of nano-sized formulation to provide access through some biological barriers resulting in potent drug targeting, and (c) the loading of desired amounts of drugs, their protection from hostile environments and delivery at a required concentration to the target site without affecting the co-existing healthy tissue, thereby reducing toxicity, have been developed (Zhang et al. 2013).
In this study, we reviewed nanomedicine-based formulations and their considerations as well as their commercialization. We also reviewed the challenges associated with their development, their limitations, and future prospects. Thus, we provide an overview of currently available nanomedicine-based formulations. This may serve as a basis for their use for therapeutic purposes in the near future.
Importance of nanomedicine-based formulations and their ideal properties
Conventional drug delivery systems (CDDSs) are characterized by immediate and burst drug release; thus, their use often requires an increased frequency of administration (Singh et al. 2019). It has also been reported that drug toxicity resulting from the misuse of drugs owing to increased administration frequency, is probable with CDDSs (Wen et al. 2015). Additionally, the low solubility of drugs in CDDSs is a major challenge for pharmaceutical companies as this affects the overall therapeutic efficacy of the drug. Low drug stability is also another common disadvantage of CDDSs as the drug is prone to degradation by the biological fluids/microenvironments in the body (Wen et al. 2015). These formulation-related issues associated with CDDSs can be tactfully overcome via the use of nanomedicine-based formulations (Patra et al. 2018), which have as major advantages: (1) specific delivery of active pharmaceutical agents to the target site. This results in a decreased dosage and the attenuation of associated adverse effects (Choi and Han 2018), (2) enhancement of drug stability. This improves the pharmacokinetic profile and bioavailability of the drug (Onoue et al. 2014), (3) better drug safety and efficacy profiles (Farjadian et al. 2019), (4) the attainment of sustained and controlled drug release profiles (Patra et al. 2018), (5) passive drug targeting via enhanced permeation and retention (EPR) effects and active targeting of tumors and other pathological sites of the body (Golombek et al. 2018), and (6) potentially cheaper formulation (when produced at large scale) as compared with conventional dosage forms (Farjadian et al. 2019). Such advantages are beneficial for improving the quality of life of patients, while reducing the overall quality and cost of healthcare.
Additionally, therapeutic agents can be entrapped, adsorbed, or covalently attached to nanosystems for administration to the body (Yetisgin et al. 2020). A single therapeutic agent or a combination of drugs that provide synergistic therapeutic effects can be delivered using nanomedicine-based formulations (Zhang et al. 2016), which also offer the possibility to realize controlled drug delivery characteristics, resulting in a decrease in dosing frequency and providing huge potential opportunities for designing nanomedicine-based formulations for drugs that go off-patent (Patra et al. 2018). However, different factors (e.g., stability: physical and biological, manufacturing method, scale-up possibility, freeze-drying conditions, and sterilization requirements), which can influence the effectiveness of nanomedicine in drug delivery should be appropriately addressed (Desai 2012). Importantly, the biocompatibility, biodegradability, and non-immunogenicity of nanomedicine-based formulations are characteristics that play important roles in efficient therapeutic delivery, bringing about enhanced bioavailability and reduced adverse/side effects (Chenthamara et al. 2019). Lipids and polymers are the most commonly used materials for preparing biocompatible and biodegradable nanoparticles with higher stabilities, enhanced drug loading capacities, easy surface functionalization for targeting and improving pharmacokinetics profile, and low intrinsic toxicity (Bochicchio et al. 2021).
Considerations in nanomedicine development
Nanomedicine development is a complex process that requires the careful consideration of different aspects, including chemistry, manufacturing, and control aspects as well as economic and regulatory aspects.
The chemistry, manufacturing, and control considerations are a great challenge in the product development and manufacturing scale-up of nanomedicine-based formulations (Desai 2012). Therefore, determining the practicability of developing nanomedicine based on an understanding the composition and structure of the early formulation is necessary. This ensures reproducibility during confirmatory studies as well as safety and efficacy during human clinical trials (Soares et al. 2018). Additionally, for further development as commercial formulations, it is also necessary to determine the physical, chemical, and functional properties of nanomedicines (Mazayen et al. 2022). The appropriate characterization of the chemical properties of each component of nanomedicines using different techniques, such as high-performance liquid chromatography or other chromatography techniques, nuclear magnetic resonance, and mass spectrometry is also necessary (Lin et al. 2014). Furthermore, Lin et al. (2014) reported that nanomedicine characterization should involve the establishment and an understanding of the particle size, zeta potential, purity, viscosity, and pH of the different components. More importantly, for further development, it is necessary to establish acceptable levels of confidence in the biological functions of nanomedicines (Fatehi et al. 2012).
Ensuring the reproducibility of potential commercial-scale manufacturing of nanomedicines, at a reasonable cost, is vital. Therefore, it is crucial to understand the early stages of nanomedicine preparation and determine the feasibility of their industrial-scale chemical handling and processing (Hua et al. 2018). At the laboratory scale, it is possible to process cytotoxic compounds and achieve complex processing. However, realizing such at an industrial scale may be expensive and challenging (Jacquemart et al. 2016). Furthermore, the complex nature of nanomedicine formulation and manufacturing requires close scrutiny to minimize batch-to-batch variations (Sharifi et al. 2022). Therefore, for successful development and commercialization, it is necessary to ensure the purity, potency, safety, and efficacy of the nanomedicines (Desai 2012). Hence, successful industrial-scale manufacturing requires a thorough understanding of the quality of the starting materials as well as the processes involved (Desai 2012).
Furthermore, it is also necessary to consider the investments associated with the development and scaling-up of nanomedicine production (Paliwal et al. 2014). In this regard, it is important to critically analyze proposed nanomedicine development strategies in comparison with other developmental portfolios. The cost of manufacturing equipment, instrumentation, and other facilities also need to be considered when developing investment strategies for nanomedicine development and their subsequent clinical application (Patra et al. 2018).
Regulatory considerations for nanomedicine are also vital. Particularly, consultations with the Food and Drug Administration (FDA) at the early stages of nanomedicine development will aid in clarifying the associated scientific and regulatory issues and in addressing concerns regarding the safety, efficacy, and regulatory status of the formulations (Đorđević et al. 2022). Consequently, an appropriate evaluation framework needs to be used to evaluate the pathways and processes involved in nanomedicine development that are similar to those used in the conventional drug development process. The European Medicines Agency (EMA) has an Expert Working Group that has released some reflection papers for particular nanomedicines to guide marketing authorization applications (Hertig et al. 2021). However, it is still unclear whether the existing regulatory frameworks will pose challenges in future innovative nanomedicine development (Halamoda-Kenzaoui et al. 2022). The uncertainties related to the regulatory processes involving nanomedicines are presented in Fig. 1.
Fig. 1.
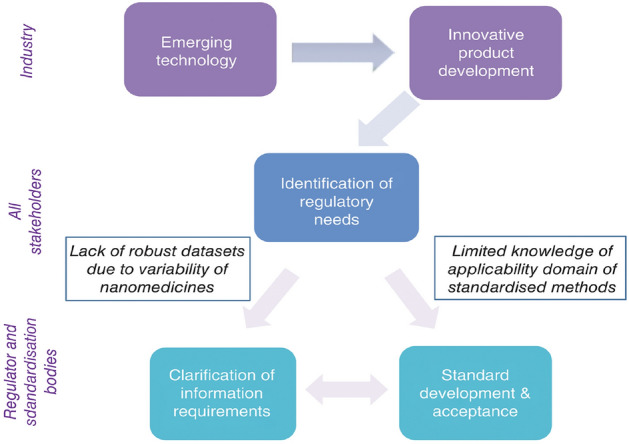
Uncertainties related to the nanomedicine regulatory process [reprinted with permission from Blanka Halamoda-Kenzaoui et al. (2019)]
Clinical trials of nanomedicine-based formulations
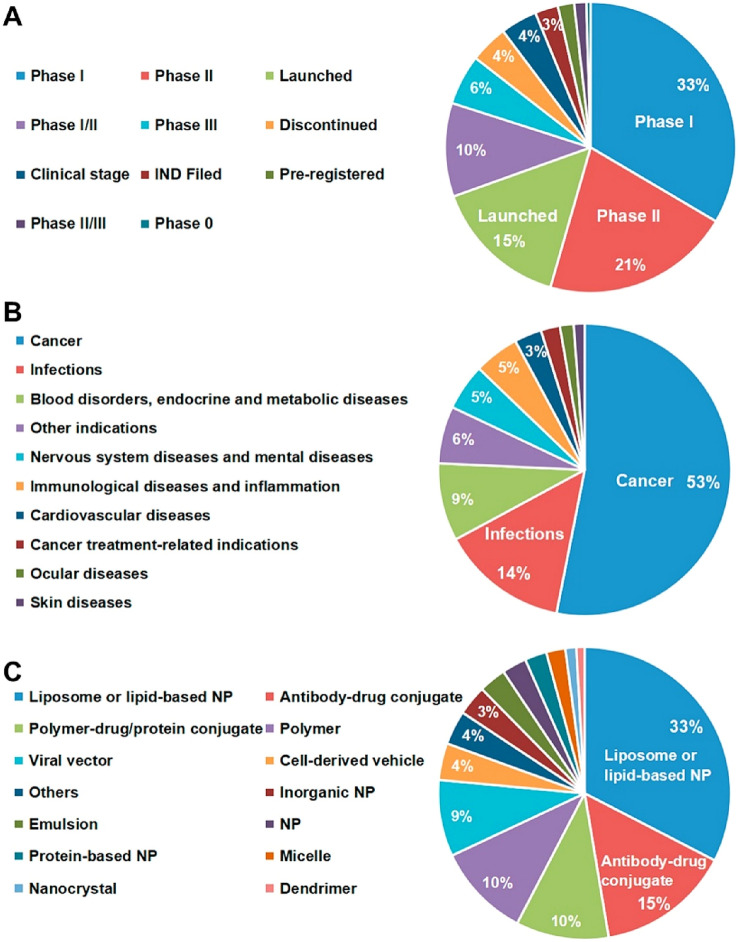
By 2015, a total of 13 nanomedicines had been approved by the US FDA for the treatment of different diseases (Malviya et al. 2021). However, of recent, there has been a rocketing surge in the number of nanomedicine clinical trials. Based on 2021 data, a total of 100 nanomedicines are already being marketed, with another 563 new nanomedicines under clinical trial or other stages (Shan et al. 2022). Further, the majority of nanomedicines under clinical trial are in Phase I (33%) and Phase II (21%), and the prime focus of these nanomedicines is the treatment of cancer (53%) and infections (14%), as well as other diseases, such as blood disorders, endocrine and metabolic diseases, nervous system diseases, immunological diseases, cardiovascular diseases, ocular diseases, and skin diseases (Fig. 2). Additionally, nanomedicines are used in vaccine development and imaging diagnosis. The most prevalent categories of nanomedicines available in the market or in different phases of clinical trials include liposomes or lipid-based nanoparticles (33%), antibody–drug conjugates (15%), polymer-drug/protein conjugates (10%), and polymeric nanoparticles (10%) (Shan et al. 2022).
Fig. 2.
Overview of nanomedicines that are available commercially or in clinical trial. (A) Development status, (B) Indications, and (C) Formulations. NP, nanoparticle [reprinted with permission from Shan et al. (2022)]
Commercial nanomedicine-based formulations
Presently, it has been suggested that nanomedicine-based formulations play a vital role in the global pharmaceutical market and healthcare system. To date, a total of approximately 100 nanomedicine-based formulations have been approved by the FDA and EMA (Shan et al. 2022). Further, several reports have suggested a significant annual increase in the number of nanomedicine-based formulations. Each year, several nanomedicine-based formulations of previously approved drugs enter clinical trial for the investigation of their efficacy relative to conventional dosage forms (Caster et al. 2017). Such nanomedicine-based advancements are attributed to the rapid growth in research and development (R&D) and high market demand. A representative list of FDA/EMA-approved and globally-marketed nanomedicine-based formulations is presented in Table 1.
Table 1.
Representative list of FDA/EMA-approved and globally-marketed nanomedicine-based formulations
| Type of formulation | Nanosystem type | Product name | Active ingredient(s) | Company | Approving organization and approval date | Indication(s) | References |
|---|---|---|---|---|---|---|---|
| Lipid-based nanomedicine | Liposome | Doxil® | Doxorubicin hydrochloride | Johnson and Johnson |
FDA (1995) EMA (1996) |
Metastatic ovarian cancer, HIV-associated Kaposi’s sarcoma, multiple myeloma | James (1995); Barenholz (2012) |
| Lipid complex | Abelcet® | Amphotericin B | Liposome Co | FDA (1995) | Aspergillosis in patients refractory to or intolerant of conventional amphotericin B and for invasive fungal infections | Rust and Jameson (1998) | |
| Liposome | DaunoXome® | Daunorubicin citrate | Galen Ltd |
FDA (1996) EMA (1996) |
HIV-associated Kaposi’s sarcoma | Kaposi’s sarcoma: DaunoXome approved (1996) | |
| PEGylated liposome | Caelyx® | Doxorubicin hydrochloride | Janssen Pharmaceutica NV | EMA (1996) | Metastatic breast cancer, ovarian cancer, AIDS-related Kaposi’s sarcoma | Ranson et al. (2001) | |
| Unilamellar liposome | AmBiosome® | Amphotericin B | NeXstar Pharmaceuticals |
FDA (1997) EMA (2006) |
Fungal infections in febrile neutropenic patients; Aspergillosis, candidiasis, and cryptococcosis infections refractory to amphotericin B | Boswell et al. (1998) | |
| Liposome | Inflexal® V | Inactivated influenza virus vaccine | Crucell (former Berna Biotech Ltd.) | EMA (1997) | Prevents influenza infection | Herzog et al. (2009) | |
| Lipid surfactant | Curosurf® | Pulmonary surfactant | Chiesi Farmaceutici | FDA (1999) | Respiratory Distress Syndrome (RDS) | Ramanathan et al. (2004) | |
| Liposome | Myocet® | Doxorubicin hydrochloride and an anthracycline cytotoxic agent | Teva Pharmaceutical Industries Ltd | EMA (2000) | Metastatic breast cancer | Brucker et al. (2016) | |
| Liposome | Visudyne® | Verteporfin | QLT PhotoTherapeutics | FDA and EMA (2000) | Severe eye conditions like macular degeneration, decreased vision, ocular histoplasmosis, pathologic myopia | Bressler and Bressler (2000) | |
| Liposome | Depocyt® | Cytarabine | Pacira Pharmaceuticals |
FDA (1999) EMA (2001 |
Intrathecal treatment of lymphomatous meningitis | Rizzieri (2016); Glantz et al. (1999) | |
| Liposome | DepoDur® | Morphine sulfate | Endo Pharmaceuticals |
FDA (2004) Disc.* EMA (2006) |
Postoperative analgesia | Pasero and McCaffery (2005) | |
| Liposome | Mepact® | Mifamurtide | Takeda France SAS |
FDA (2001) EMA (2009) |
High grade non-metastatic osteosarcoma and myosarcoma | Frampton (2010) | |
| Liposome | Marqibo® | Vincristine | Talon Therapeutics |
FDA (2012) Disc.* EMA (2012) |
Philadelphia chromosome-negative acute lymphoblastic leukemia in adult patients | Silverman and Deitcher (2013) | |
| Liposome | Lipodox® | Doxorubicin hydrochloride | Sun Pharmaceutical Industries Ltd. (SPIL) | FDA (2013) | Kaposi’s sarcoma, ovarian cancer, multiple myeloma | Chou et al. (2015) | |
| Liposome | Onivyde® | Irinotecan | Merrimack Pharmaceuticals | FDA (2015) | Metastatic pancreatic cancer | Milano et al., (2022) | |
| Liposome | Lipusu® | Paclitaxel | Luye Pgarna | FDA (2016) | Lung squamous cell carcinoma | Zhang et al. (2022) | |
| Liposome | Onpattro® | Patisiran sodium | Alnylam Pharmaceuticals, Inc | FDA and EMA (2018) | Polyneuropathy of hereditary transthyretin-mediated amyloidosis in adults | Akinc et al. (2019) | |
| Liposome | Pfizer-BioNTech vaccine | mRNA vaccine | Pfizer Pharmaceuticals | FDA (2020) | Prevention of COVID-19 infection | Attia et al. (2021) | |
| Liposome | Moderna Vaccine | mRNA vaccine | ModernaTx Inc | FDA (2020) | Prevention of COVID-19 infection | Attia et al. (2021) | |
| Polymer-based nanomedicine | Nanoemulsion | Diprivan® | Propofol | AstraZeneca LP |
FDA (1989) EMA (2001) |
Anesthetic agent for induction and maintenance of anesthesia. For sedation of patient under critical care and those requiring mechanical ventilator | Terblanche and Coetzee (2008) |
| Polymer-protein conjugate | Adagen® | Adenosine deaminase | Enzon Pharmaceuticals Inc | FDA (1990) | Adenosine deaminase-severe combined immunodeficiency disorder | Booth and Gaspar (2009) | |
| Polymer-based | Oncaspar® | L-asparaginase | Enzon Pharmaceuticals Inc |
FDA (1994) EMA (2016) |
Acute lymphoblastic leukemia | Ettinger (1995) | |
| Micelle | PegIntron® | PEGylated interferon alpha-2B | Merck & Co. Inc |
EMA (2000) FDA (2001) Disc.* |
Hepatitis | Tseng et al. (2014) | |
| Polymer-protein conjugate | Neulasta® | Filgrastim | Amgen, Inc | FDA (2002) | Neutropenia | Duncan (2005) | |
| Polymer-protein conjugate | Pegasys® | PEGylated interferon alpha-2A | Genentech, Inc | FDA and EMA (2002) | Hepatitis B and Hepatitis C | Hui and Lau (2005) | |
| Polymer-protein conjugate | Somavert® | Recombinant human growth hormone receptor antagonist | Pfizer, Inc |
EMA (2002) FDA (2003) |
Acromegaly | Parkinson et al. (2003) | |
| Nanoemulsion | Restasis® | Cyclosporine | Allergan | FDA (2003) | Chronic dry eye | Lallemand et al. (2017) | |
| Nanoemulsion | Estrasorb® | Estradiol | Novavax, Inc | FDA (2003) | Treatment of moderate to severe vasomotor symptoms in postmenopausal women | Simon (2006) | |
| Polymer-protein conjugate | Macugen® | Pegaptanib sodium | Pfizer, Inc | FDA (2004) | Wet age-related macular degeneration | Tobin (2006) | |
| Micelle | Genexol-PM® | Paclitaxel | Lupin Ltd | FDA (2007) | Breast cancer | Oerlemans et al. (2010) | |
| Polymer-protein conjugate | Mircera® | Epoetin beta | Vifor pharma | EMA and FDA (2007) | Renal anemia | Bartnicki et al. (2013) | |
| Polymer-peptide conjugate | Cimzia® | Certolizumab pegol | UCB |
FDA (2008) EMA (2009) |
Rheumatoid arthritis, Crohn’s disease, psoriatric arthirits, ankylosing spondylitis | Curtis et al. (2019) | |
| Polymer-protein conjugate | Krystexxa® | Pegloticase | Savient Pharmaceuticals | FDA (2010) | Severe and treatment-refractory chronic gout | Padda et al. (2022) | |
| Polymer-protein conjugate | Plegridy® | Peginterferon beta-1a | Biogene | FDA (2014) | Relapsing remitting multiple sclerosis in adults | Gohil (2014) | |
| Polymer-protein conjugate | Adynovate® | Recombinant antihemophilic factor | Baxalta US Inc | FDA (2015) | Hemophilia A | Dunn et al. (2018) | |
| Polymer-protein conjugate | Rebinyn® | Recombinant coagulation factor IX | Novo Nordisk Inc | FDA (2017) | Hemophilia B | Ezban et al. (2019) | |
| Polymer steroid mixture | Zilretta® | Triamcinolone acetonide | Flexion Therapeutics | FDA (2017) | Knee osteoarthritis | Paik et al. (2019) | |
| Micelle | Apealea® | Paclitaxel | Oasmia Pharmaceutical AB | FDA (2018) | Ovarian cancer, peritoneal cancer, fallopian tube cancer | Borgå et al. (2019) | |
| Nanocrystals | Nanocrystal | Avinza® | Morphine | King Pharma | FDA (2002) | Chronic pain | Semenchuk (2002) |
| Nanocrystal | Ritalin LA® | Methylphenidate hydrochloride | Novartis | FDA (2002) | Attention deficit hyperactivity disorder in children | Driessche et al. (2017) | |
| Nanocrystal | Zanaflex® | Tizanidine hydrochloride | Acorda | FDA (2002) | Muscle relaxant | Kaddar et al. (2012) | |
| Nanocrystal | Emend® | Aprepitant | Merck & Co. Inc | FDA (2003) | Antiemetic | Prommer (2005) | |
| Nanocrystal | Tricor® | Fenofibric aid | Abott Laboratories | FDA (2004) | Antihyperlipidemia | Saurav et al. (2012) | |
| Nanocrystal | NanOss® | Hydroxyapatite | RTI Surgical | FDA (2005) | Bone substitute | Epstein (2015) | |
| Nanocrystal | Megace® ES | Megestrol acetate | Par Pharmaceuticals | FDA (2005) | Anorexia, cachexia and AIDS-related weight loss | Tuca et al. (2013) | |
| Nanocrystal | IVEmend® | Fosaprepitant dimeglumine | Merck & Co. Inc | FDA and EMA (2008) | Antiemetic | Garnock-Jones (2016) | |
| Nanocrystal | Focalin® XR | Dexmethylphenidate hydrochloride | Novartis | FDA (2008) | Attention deficit hyperactivity disorder in children | Moen and Keam (2009) | |
| Nanocrystal | Invega® | Paliperidone palmitate | Janssen Pharmaceuticals | FDA (2009) | Schizophrenia | Nagino et al. (2011) | |
| Inorganic nanoparticles | Iron nanoparticles | Dexferrum® | Iron dextran | American Regent | FDA (1996) | Iron deficiency in chronic kidney disease | Hood et al. (2000) |
| Iron nanoparticles | Venofer® | Iron sucrose | Lutipold Pharmaceuticals, Inc | FDA (2000) | Iron deficiency in chronic kidney disease | Bhandari et al. (2015) | |
| Hafnium oxide nanoparticles | Hensify® | Hafnium oxide | Nanobiotix | EMA (2019) | Locally advanced squamous cell carcinoma | Bonvalot et al. (2019) | |
| Protein based nanoparticles | Engineered fusion protein nanoparticle | Ontak® | Denileukin diftitox | Eisai Co., Ltd | FDA (1999) | Leukemia, T cell lymphoma | Duvic and Talpur (2008) |
| Albumin nanoparticle | Abraxane® | Paclitaxel | Eli Lilly |
FDA (2005) EMA (2008) |
Metastatic breast cancer | Yuan et al. (2020) |
Disc.* Discontinued
Presently, approved and commercially available nanomedicine-based formulations include lipid-based nanomedicines, polymer-based nanomedicines, nanocrystals, inorganic nanoparticles, and protein-based nanoparticles. Among the lipid-based nanomedicines, liposomes are most commonly used for drug delivery. Specifically, liposomes are spherical vesicles (< 200 nm) composed of a lipid bilayer membrane surrounding a hydrophilic core. Hence, they are capable of delivering both hydrophilic and hydrophobic drugs, monoclonal antibodies, siRNA, and other biomolecules (Nakhaei et al. 2021). Further, they can circulate in the bloodstream for extended time periods, providing a longer treatment effect (Sercombe et al. 2015). More importantly, they can accumulate at tumor or infection sites; thus, they naturally locate and deliver drugs to their target sites (Allahou et al. 2021). Additionally, stimuli responsive (temperature- and pH-sensitive) liposomes can be prepared to allow for controlled drug release (Lee and Thompson 2017). Further, polymer-based nanomedicines include polymer-protein conjugates and micelles. The conjugates provide protein drugs with targeting ability and enhanced circulation times (Kiran et al. 2021). It has also been observed that such polymeric nanoparticles facilitate drug release for an extended time period (Kamaly et al. 2016). Moreover, nanocrystals are versatile nanoparticles that can be used for improving the pharmacokinetic-pharmacodynamic properties of poorly soluble drugs (Gigliobianco et al. 2018). Specifically, they enhance the bioavailability and solubility of drugs by increasing surface area at the nanoscale to enhance dissolution (Joshi et al. 2019). Alternatively, inorganic nanoparticles can be used for drug delivery and can also be useful in imaging applications (Luther et al. 2020). In particular, metal oxides, metals, or silica are used as inorganic nanoparticles. In this regard, one of the most widely used inorganic nanomedicines are iron oxide nanodrugs, which have been approved for use in iron replacement therapies (Yadavalli and Shukla 2017; Dadfar et al. 2019). Protein-based nanoparticles include drug-conjugated protein carriers, with the protein itself functioning as the active therapeutic agent, or as a part of a combined complex for targeted delivery (Hong et al. 2020). Albumin protein nanoparticles have gained wider interest in the research community owing to their longer circulation times, ability to accumulate at tumors site via enhanced permeation and retention effects, and their ability to undergo cellular uptake via albumin-receptors (Hassanin and Elzoghby 2020).
Challenges in the development of nanomedicine-based formulations
Based on recent advances in nanomedicine-based formulations, several challenges that need to be considered for nanomedicine development have been identified. For example, the lack of proper methods for the characterization of the safety and efficacy of nanomedicines, is one of the major challenges in nanomedicine development (Desai 2012). In general, a large amount of data has been gathered for nanomedicines like liposomes, polymeric nanomedicines, and micelles. However, varied possibilities for formulation and application require toxicity data for each modification (Patra et al. 2018). Furthermore, to avoid the development of unpredictable side effects, there is a glaring need for an enhanced understanding of nanomedicines prior to them becoming commercially available. However, rigorous research is yet to be conducted to clarify and predict the effect of nanomedicines on biological systems, including the development of new assays that are not affected by nanomedicines (Đorđević et al. 2022).
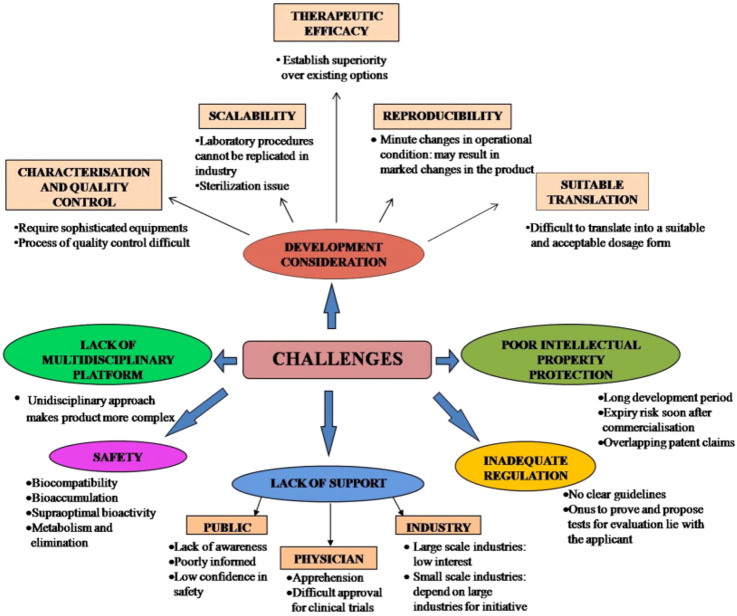
Another challenge in the development of nanomedicine-based formulations is the lack of specific regulatory guidelines (Desai 2012). The FDA approval process for nanomedicines (including preclinical and human clinical studies) are same as those for any other drug or biologic agent (Đorđević et al. 2022). FDA has issued some guidelines for industries regarding the use of nanotechnology. These guidelines encourage manufacturers to consult with the FDA regarding the specific regulatory and scientific issues of relevance for nanomedicines early enough (Mühlebach 2018). Such consultation is also encouraged as it can help in addressing concerns regarding the safety, efficacy, public impact, and regulatory status of the product (Havel et al. 2016). However, separate regulatory guidelines are yet to be established for the development of effective and safe nanomedicine-based formulations. The different challenges associated with the development and commercialization of nanomedicines are presented in Fig. 3.
Fig. 3.
Challenges in the development and commercialization of nanomedicines [reprinted with permission from Kaur et al. (2014)]
Failure of some nanomedicine-based formulations
Pharmaceutical companies developing nanomedicines acquire funding from capital markets, venture capital, and partnerships with other companies; however, the clinical failure of the product often results in the termination of their development and business liquidation (He et al. 2019). One of the most common reasons for the clinical failure of nanomedicines is the nanomedicine showing toxicity in Phase I clinical trials (Fogel 2018). Furthermore, the choice of the drug carrier of the nanomedicine affects its physicochemical properties and the resulting payload, leading to failure (Patra et al. 2018). Moreover, the right selection of patients is a critical factor for ensuring successful clinical trials (Sacristán et al. 2016). High-quality production processes and reproducibility are also critical factors that could bring about the failure of nanomedicines (Soares et al. 2018). Therefore, all the factors that could be responsible for the failure of nanomedicines should be critically considered and addressed to ensure their successful development and subsequent approval for clinical use.
Future prospects of nanomedicine-based formulations
Significant progress has been made in the field of nanomedicine-based formulations in the past decades, with several FDA and EMA approvals. Notably, nanomedicines provide a wide range of avenues for the treatment of complex and difficult-to-treat diseases, such as cancer, lung diseases, and ophthalmic diseases. The most common types of commercially available nanomedicine-based formulations include lipid-based nanomedicines, polymer-based nanomedicines, nanocrystals, inorganic nanoparticles, and protein-based nanomedicines. Such formulations are revolutionizing the treatment of different diseases and have significant impact on the healthcare system. However, the incorporation of a broad range of nanomedicine types is making the formulations more complex. Therefore, it is necessary to adequately address concerns regarding safety and efficacy, while following the guidelines established by agencies such as the FDA and EMA. Further, rigorous studies related to the detailed characterization of nanomedicines, their preclinical and clinical testing, and cost–benefit analyses are urgently needed. Hence, based on the findings reported in previous studies, future rigorous studies, and stringent guidelines to promote safe and effective treatment will make nanomedicines a unique solution for unmet clinical needs.
Acknowledgements
The study was supported by Yeungnam University Research Grant in 2022.
Data availability
Not applicable.
Declarations
Conflict of interest
All authors (R.K. Thapa and J.O. Kim) declare that they have no conflict of interest.
Research involving human and animal rights
This article does not contain any studies with human and animal subjects performed by the author.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Raj Kumar Thapa, Email: rkthapa@gandakiuniversity.edu.np.
Jong Oh Kim, Email: jongohkim@yu.ac.kr.
References
- Akinc A, Maier MA, Manoharan M, Fitzgerald K, Jayaraman M, Barros S, Ansell S, Xinyao D, Hope MJ, Madden TD, Mui BL, Semple SC, Tam YK, Ciufolini M, Witzigmann D, Kulkarni JA, van der Meel R, Cullis PR. The Onpattro story and the clinical translation of nanomedicines containing nucleic acid-based drugs. Nat Nanotechnol. 2019;14(12):1084–1087. doi: 10.1038/s41565-019-0591-y. [DOI] [PubMed] [Google Scholar]
- Allahou LW, Madani SY, Seifalian A. Investigating the application of liposomes as drug delivery systems for the diagnosis and treatment of cancer. Int J Biomater. 2021;2021:3041969. doi: 10.1155/2021/3041969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attia MA, Essa EA, Elebyary TT, Faheem AM, Elkordy AA. Brief on recent application of liposomal vaccines for lower respiratory tract viral infections: from influenza to COVID-19 vaccines. Pharmaceuticals (Basel) 2021;14(11):1173. doi: 10.3390/ph14111173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barenholz Y. Doxil®—the first FDA-approved nano-drug: lessons learned. J Control Release. 2012;160(2):117–134. doi: 10.1016/j.jconrel.2012.03.020. [DOI] [PubMed] [Google Scholar]
- Bartnicki P, Fijałkowski P, Majczyk M, Błaszczyk J, Banach M, Rysz J. Effect of methoxy polyethylene glycol-epoetin beta on oxidative stress in predialysis patients with chronic kidney disease. Med Sci Monit. 2013;19:954–9. doi: 10.12659/msm.884024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayda S, Adeel M, Tuccinardi T, Cordani M, Rizzolio F. The history of nanoscience and nanotechnology: from chemical-physical applications to nanomedicine. Molecules. 2019 doi: 10.3390/molecules25010112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhandari S, Kalra PA, Kothari J, Ambühl PM, Christensen JH, Essaian AM, Thomsen LL, Macdougall IC, Coyne DW. A randomized, open-label trial of iron isomaltoside 1000 (Monofer®) compared with iron sucrose (Venofer®) as maintenance therapy in haemodialysis patients. Nephrol Dial Transplant. 2015;30(9):1577–1589. doi: 10.1093/ndt/gfv096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochicchio S, Lamberti G, Barba AA. Polymer-lipid pharmaceutical nanocarriers: innovations by new formulations and production technologies. Pharmaceutics. 2021 doi: 10.3390/pharmaceutics13020198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonvalot S, Rutkowski PL, Thariat J, Carrère S, Ducassou A, Sunyach MP, Agoston P, Hong A, Mervoyer A, Rastrelli M, Moreno V, Li RK, Tiangco B, Herraez AC, Gronchi A, Mangel L, Sy-Ortin T, Hohenberger P, de Baère T, Le Cesne A, Helfre S, Saada-Bouzid E, Borkowska A, Anghel R, Co A, Gebhart M, Kantor G, Montero A, Loong HH, Vergés R, Lapeire L, Dema S, Kacso G, Austen L, Moureau-Zabotto L, Servois V, Wardelmann E, Terrier P, Lazar AJ, Le Jvmg Bovée C. NBTXR3, a first-in-class radioenhancer hafnium oxide nanoparticle, plus radiotherapy versus radiotherapy alone in patients with locally advanced soft-tissue sarcoma (Act. In.Sarc): a multicentre, phase 2–3, randomised, controlled trial. Lancet Oncol. 2019;20(8):1148–1159. doi: 10.1016/s1470-2045(19)30326-2. [DOI] [PubMed] [Google Scholar]
- Booth C, Gaspar HB. Pegademase bovine (PEG-ADA) for the treatment of infants and children with severe combined immunodeficiency (SCID) Biologics. 2009;3:349–358. [PMC free article] [PubMed] [Google Scholar]
- Borgå O, Lilienberg E, Bjermo H, Hansson F, Heldring N, Dediu R. Pharmacokinetics of total and unbound paclitaxel after administration of paclitaxel micellar or nab-paclitaxel: an open, randomized, cross-over, explorative study in breast cancer patients. Adv Ther. 2019;36(10):2825–2837. doi: 10.1007/s12325-019-01058-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boswell GW, Buell D, Bekersky I. AmBisome (liposomal amphotericin B): a comparative review. J Clin Pharmacol. 1998;38(7):583–592. doi: 10.1002/j.1552-4604.1998.tb04464.x. [DOI] [PubMed] [Google Scholar]
- Bressler NM, Bressler SB. Photodynamic therapy with verteporfin (Visudyne): impact on ophthalmology and visual sciences. Invest Ophthalmol Vis Sci. 2000;41(3):624–628. [PubMed] [Google Scholar]
- Brucker J, Mayer C, Gebauer G, Mallmann P, Belau AK, Schneeweiss A, Sohn C, Eichbaum M. Non-pegylated liposomal doxorubicin for patients with recurrent ovarian cancer: a multicentric phase II trial. Oncol Lett. 2016;12(2):1211–1215. doi: 10.3892/ol.2016.4740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caster JM, Patel AN, Zhang T, Wang A. Investigational nanomedicines in 2016: a review of nanotherapeutics currently undergoing clinical trials. WIREs Nanomed Nanobiotechnol. 2017;9(1):e1416. doi: 10.1002/wnan.1416. [DOI] [PubMed] [Google Scholar]
- Chenthamara D, Subramaniam S, Ramakrishnan SG, Krishnaswamy S, Essa MM, Lin F-H, Walid Qoronfleh M. Therapeutic efficacy of nanoparticles and routes of administration. Biomater Res. 2019;23(1):20. doi: 10.1186/s40824-019-0166-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YH, Han H-K. Nanomedicines: current status and future perspectives in aspect of drug delivery and pharmacokinetics. J Pharm Investig. 2018;48(1):43–60. doi: 10.1007/s40005-017-0370-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou H, Lin H, Liu JM. A tale of the two PEGylated liposomal doxorubicins. Onco Targets Ther. 2015;8:1719–1720. doi: 10.2147/ott.s79089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis JR, Mariette X, Gaujoux-Viala C, Blauvelt A, Kvien TK, Sandborn WJ, Winthrop K, de Longueville M, Huybrechts I, Bykerk VP. Long-term safety of certolizumab pegol in rheumatoid arthritis, axial spondyloarthritis, psoriatic arthritis, psoriasis and Crohn’s disease: a pooled analysis of 11,317 patients across clinical trials. RMD Open. 2019;5(1):e000942. doi: 10.1136/rmdopen-2019-000942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadfar SM, Roemhild K, Drude NI, von Stillfried S, Knüchel R, Kiessling F, Lammers T. Iron oxide nanoparticles: diagnostic, therapeutic and theranostic applications. Adv Drug Deliv Rev. 2019;138:302–325. doi: 10.1016/j.addr.2019.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jong WH, Borm PJ. Drug delivery and nanoparticles: applications and hazards. Int J Nanomed. 2008;3(2):133–149. doi: 10.2147/ijn.s596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai N. Challenges in development of nanoparticle-based therapeutics. Aaps j. 2012;14(2):282–295. doi: 10.1208/s12248-012-9339-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Đorđević S, Gonzalez MM, Conejos-Sánchez I, Carreira B, Pozzi S, Acúrcio RC, Satchi-Fainaro R, Florindo HF, Vicent MJ. Current hurdles to the translation of nanomedicines from bench to the clinic. Drug Deliv Transl Res. 2022;12(3):500–525. doi: 10.1007/s13346-021-01024-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan R. Nanomedicine gets clinical. Mater Today. 2005;8:16–17. doi: 10.1016/S1369-7021(05)71032-4. [DOI] [Google Scholar]
- Dunn AL, Ahuja SP, Mullins ES. Real-world experience with use of antihemophilic factor (Recombinant), PEGylated for prophylaxis in severe haemophilia A. Haemophilia. 2018;24(3):e84–e92. doi: 10.1111/hae.13403. [DOI] [PubMed] [Google Scholar]
- Duvic M, Talpur R. Optimizing denileukin diftitox (Ontak) therapy. Future Oncol. 2008;4(4):457–469. doi: 10.2217/14796694.4.4.457. [DOI] [PubMed] [Google Scholar]
- Epstein NE. Preliminary study showing safety/efficacy of nanoss bioactive versus vitoss as bone graft expanders for lumbar noninstrumented fusions. Surg Neurol Int. 2015;6(Suppl 10):S318–S322. doi: 10.4103/2152-7806.159380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettinger AR. Pegaspargase (Oncaspar) J Pediatr Oncol Nurs. 1995;12(1):46–48. doi: 10.1177/104345429501200110. [DOI] [PubMed] [Google Scholar]
- Ezban M, Hermit MB, Persson E. FIXing postinfusion monitoring: assay experiences with N9-GP (nonacog beta pegol; Refixia(®); Rebinyn(®)) Haemophilia. 2019;25(1):154–161. doi: 10.1111/hae.13671. [DOI] [PubMed] [Google Scholar]
- Farjadian F, Ghasemi A, Gohari O, Roointan A, Karimi M, Hamblin MR. Nanopharmaceuticals and nanomedicines currently on the market: challenges and opportunities. Nanomedicine (lond) 2019;14(1):93–126. doi: 10.2217/nnm-2018-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatehi L, Wolf SM, McCullough J, Hall R, Lawrenz F, Kahn JP, Jones C, Campbell SA, Dresser RS, Erdman AG, Haynes CL, Hoerr RA, Hogle LF, Keane MA, Khushf G, King NM, Kokkoli E, Marchant G, Maynard AD, Philbert M, Ramachandran G, Siegel RA, Wickline S. Recommendations for nanomedicine human subjects research oversight: an evolutionary approach for an emerging field. J Law Med Ethics. 2012;40(4):716–750. doi: 10.1111/j.1748-720X.2012.00703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogel DB. Factors associated with clinical trials that fail and opportunities for improving the likelihood of success: a review. Contemp Clin Trials Commun. 2018;11:156–164. doi: 10.1016/j.conctc.2018.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frampton JE. Mifamurtide. Pediatr Drugs. 2010;12(3):141–153. doi: 10.2165/11204910-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Garnock-Jones KP. Fosaprepitant dimeglumine: a review in the prevention of nausea and vomiting associated with chemotherapy. Drugs. 2016;76(14):1365–1372. doi: 10.1007/s40265-016-0627-7. [DOI] [PubMed] [Google Scholar]
- Gigliobianco MR, Casadidio C, Censi R, Di Martino P. Nanocrystals of poorly soluble drugs: drug bioavailability and physicochemical stability. Pharmaceutics. 2018;10(3):134. doi: 10.3390/pharmaceutics10030134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glantz MJ, Jaeckle KA, Chamberlain MC, Phuphanich S, Recht L, Swinnen LJ, Maria B, LaFollette S, Schumann GB, Cole BF, Howell SB. A randomized controlled trial comparing intrathecal sustained-release cytarabine (DepoCyt) to intrathecal methotrexate in patients with neoplastic meningitis from solid tumors. Clin Cancer Res. 1999;5(11):3394–3402. [PubMed] [Google Scholar]
- Gohil K. Pharmaceutical approval update. P t. 2014;39(10):684–694. [PMC free article] [PubMed] [Google Scholar]
- Golombek SK, May JN, Theek B, Appold L, Drude N, Kiessling F, Lammers T. Tumor targeting via EPR: Strategies to enhance patient responses. Adv Drug Deliv Rev. 2018;130:17–38. doi: 10.1016/j.addr.2018.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halamoda-Kenzaoui B, Holzwarth U, Roebben G, Bogni A, Bremer-Hoffmann S. Mapping of the available standards against the regulatory needs for nanomedicines. WIREs Nanomed Nanobiotechnol. 2019;11(1):e1531. doi: 10.1002/wnan.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halamoda-Kenzaoui B, Geertsma R, Pouw J, Prina-Mello A, Carrer M, Roesslein M, Sips A, Weltring KM, Spring K, Bremer-Hoffmann S. Future perspectives for advancing regulatory science of nanotechnology-enabled health products. Drug Deliv Transl Res. 2022;12(9):2145–2156. doi: 10.1007/s13346-022-01165-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassanin I, Elzoghby A. Albumin-based nanoparticles: a promising strategy to overcome cancer drug resistance. Cancer Drug Resist. 2020;3(4):930–946. doi: 10.20517/cdr.2020.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havel H, Finch G, Strode P, Wolfgang M, Zale S, Bobe I, Youssoufian H, Peterson M, Liu M. Nanomedicines: from bench to bedside and beyond. Aaps j. 2016;18(6):1373–1378. doi: 10.1208/s12248-016-9961-7. [DOI] [PubMed] [Google Scholar]
- He H, Liu L, Morin EE, Liu M, Schwendeman A. Survey of clinical translation of cancer nanomedicines—lessons learned from successes and failures. Acc Chem Res. 2019;52(9):2445–2461. doi: 10.1021/acs.accounts.9b00228. [DOI] [PubMed] [Google Scholar]
- Hertig JB, Shah VP, Flühmann B, Mühlebach S, Stemer G, Surugue J, Moss R, Di Francesco T. Tackling the challenges of nanomedicines: are we ready? Am J Health Syst Pharm. 2021;78(12):1047–1056. doi: 10.1093/ajhp/zxab048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog C, Hartmann K, Künzi V, Kürsteiner O, Mischler R, Lazar H, Glück R. Eleven years of Inflexal V-a virosomal adjuvanted influenza vaccine. Vaccine. 2009;27(33):4381–4387. doi: 10.1016/j.vaccine.2009.05.029. [DOI] [PubMed] [Google Scholar]
- Hong S, Choi DW, Kim HN, Park CG, Lee W, Park HH. Protein-based nanoparticles as drug delivery systems. Pharmaceutics. 2020;12(7):604. doi: 10.3390/pharmaceutics12070604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood SA, O’Brien M, Higgins R. The safety of intravenous iron dextran (Dexferrum) during hemodialysis in patients with end stage renal disease. Nephrol Nurs J. 2000;27(1):41–42. [PubMed] [Google Scholar]
- Hua S, de Matos MBC, Metselaar JM, Storm G. Current trends and challenges in the clinical translation of nanoparticulate nanomedicines: pathways for translational development and commercialization. Front Pharmacol. 2018;9:790. doi: 10.3389/fphar.2018.00790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui CK, Lau GK. Peginterferon-alpha2a (40 kDa) (Pegasys) for hepatitis B. Expert Rev Anti Infect Ther. 2005;3(4):495–504. doi: 10.1586/14787210.3.4.495. [DOI] [PubMed] [Google Scholar]
- Jacquemart R, Vandersluis M, Zhao M, Sukhija K, Sidhu N, Stout J. A single-use strategy to enable manufacturing of affordable biologics. Comput Struct Biotechnol J. 2016;14:309–318. doi: 10.1016/j.csbj.2016.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain KK. Nanomedicine: application of nanobiotechnology in medical practice. Med Princ Pract. 2008;17(2):89–101. doi: 10.1159/000112961. [DOI] [PubMed] [Google Scholar]
- James JS. DOXIL approved for KS. AIDS Treat News. 1995;236:6. [PubMed] [Google Scholar]
- Joshi K, Chandra A, Jain K, Talegaonkar S. Nanocrystalization: an emerging technology to enhance the bioavailability of poorly soluble drugs. Pharm Nanotechnol. 2019;7(4):259–278. doi: 10.2174/2211738507666190405182524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaddar N, Vigneault P, Pilote S, Patoine D, Simard C, Drolet B. Tizanidine (Zanaflex): a muscle relaxant that may prolong the QT interval by blocking IKr. J Cardiovasc Pharmacol Ther. 2012;17(1):102–109. doi: 10.1177/1074248410395020. [DOI] [PubMed] [Google Scholar]
- Kamaly N, Yameen B, Wu J, Farokhzad OC. Degradable controlled-release polymers and polymeric nanoparticles: mechanisms of controlling drug release. Chem Rev. 2016;116(4):2602–2663. doi: 10.1021/acs.chemrev.5b00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur IP, Kakkar V, Deol PK, Yadav M, Singh M, Sharma I. Issues and concerns in nanotech product development and its commercialization. J Control Release. 2014;193:51–62. doi: 10.1016/j.jconrel.2014.06.005. [DOI] [PubMed] [Google Scholar]
- Kiran P, Khan A, Neekhra S, Pallod S, Srivastava R. Nanohybrids as protein-polymer conjugate multimodal therapeutics. Front Med Technol. 2021;3:676025. doi: 10.3389/fmedt.2021.676025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lallemand F, Schmitt M, Bourges J-L, Gurny R, Benita S, Garrigue J-S. Cyclosporine A delivery to the eye: a comprehensive review of academic and industrial efforts. Eur J Pharm Biopharm. 2017;117:14–28. doi: 10.1016/j.ejpb.2017.03.006. [DOI] [PubMed] [Google Scholar]
- Lee Y, Thompson DH. Stimuli-responsive liposomes for drug delivery. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2017 doi: 10.1002/wnan.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin PC, Lin S, Wang PC, Sridhar R. Techniques for physicochemical characterization of nanomaterials. Biotechnol Adv. 2014;32(4):711–726. doi: 10.1016/j.biotechadv.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luther DC, Huang R, Jeon T, Zhang X, Lee YW, Nagaraj H, Rotello VM. Delivery of drugs, proteins, and nucleic acids using inorganic nanoparticles. Adv Drug Deliv Rev. 2020;156:188–213. doi: 10.1016/j.addr.2020.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack J. Nanotechnology: what’s in it for biotech? Biotechnol Healthc. 2005;2(6):29–36. [PMC free article] [PubMed] [Google Scholar]
- Malviya R, Fuloria S, Verma S, Subramaniyan V, Sathasivam KV, Kumarasamy V, Hari Kumar D, Vellasamy S, Meenakshi DU, Yadav S, Sharma A, Fuloria NK. Commercial utilities and future perspective of nanomedicines. PeerJ. 2021;9:e12392. doi: 10.7717/peerj.12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazayen ZM, Ghoneim AM, Elbatanony RS, Basalious EB, Bendas ER. Pharmaceutical nanotechnology: from the bench to the market. Future J Pharm Sci. 2022;8(1):12. doi: 10.1186/s43094-022-00400-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milano G, Innocenti F, Minami H. Liposomal irinotecan (Onivyde): exemplifying the benefits of nanotherapeutic drugs. Cancer Sci. 2022;113(7):2224–2231. doi: 10.1111/cas.15377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, Langer R. Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discovery. 2021;20(2):101–124. doi: 10.1038/s41573-020-0090-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moen MD, Keam SJ. Dexmethylphenidate extended release: a review of its use in the treatment of attention-deficit hyperactivity disorder. CNS Drugs. 2009;23(12):1057–1083. doi: 10.2165/11201140-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Mühlebach S. Regulatory challenges of nanomedicines and their follow-on versions: a generic or similar approach? Adv Drug Delivery Rev. 2018;131:122–131. doi: 10.1016/j.addr.2018.06.024. [DOI] [PubMed] [Google Scholar]
- Nagino K, Koh T, Harada Y. Pharmacological properties of paliperidone ER (INVEGA(®)) and results of its clinical studies. Nihon Yakurigaku Zasshi. 2011;137(6):245–254. doi: 10.1254/fpj.137.245. [DOI] [PubMed] [Google Scholar]
- Nakhaei P, Margiana R, Bokov DO, Abdelbasset WK, Jadidi Kouhbanani MA, Varma RS, Marofi F, Jarahian M, Beheshtkhoo N. Liposomes: structure, biomedical applications, and stability parameters with emphasis on cholesterol. Front Bioeng Biotechnol. 2021;9:705886. doi: 10.3389/fbioe.2021.705886. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Oerlemans C, Bult W, Bos M, Storm G, Nijsen JF, Hennink WE. Polymeric micelles in anticancer therapy: targeting, imaging and triggered release. Pharm Res. 2010;27(12):2569–2589. doi: 10.1007/s11095-010-0233-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onoue S, Yamada S, Chan HK. Nanodrugs: pharmacokinetics and safety. Int J Nanomed. 2014;9:1025–1037. doi: 10.2147/ijn.s38378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padda IS, Bhatt R, Parmar M. StatPearls. Treasure Island: StatPearls Publishing; 2022. Pegloticase. [PubMed] [Google Scholar]
- Paik J, Duggan ST, Keam SJ. Triamcinolone acetonide extended-release: a review in osteoarthritis pain of the knee. Drugs. 2019;79(4):455–462. doi: 10.1007/s40265-019-01083-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paliwal R, Babu RJ, Palakurthi S. Nanomedicine scale-up technologies: feasibilities and challenges. AAPS PharmSciTech. 2014;15(6):1527–1534. doi: 10.1208/s12249-014-0177-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson C, Scarlett JA, Trainer PJ. Pegvisomant in the treatment of acromegaly. Adv Drug Delivery Rev. 2003;55(10):1303–1314. doi: 10.1016/S0169-409X(03)00111-X. [DOI] [PubMed] [Google Scholar]
- Pasero C, McCaffery M. Extended-release epidural morphine (DepoDur™) J PeriAnesthesia Nurs. 2005;20(5):345–350. doi: 10.1016/j.jopan.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Patra JK, Das G, Fraceto LF, Campos EVR, Rodriguez-Torres MDP, Acosta-Torres LS, Diaz-Torres LA, Grillo R, Swamy MK, Sharma S, Habtemariam S, Shin HS. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnology. 2018;16(1):71. doi: 10.1186/s12951-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prommer E. Aprepitant (EMEND): the role of substance P in nausea and vomiting. J Pain Palliat Care Pharmacother. 2005;19(3):31–39. [PubMed] [Google Scholar]
- Puri A, Loomis K, Smith B, Lee JH, Yavlovich A, Heldman E, Blumenthal R. Lipid-based nanoparticles as pharmaceutical drug carriers: from concepts to clinic. Crit Rev Ther Drug Carrier Syst. 2009;26(6):523–580. doi: 10.1615/critrevtherdrugcarriersyst.v26.i6.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan R, Rasmussen MR, Gerstmann DR, Finer N, Sekar K. A randomized, multicenter masked comparison trial of poractant alfa (Curosurf) versus beractant (Survanta) in the treatment of respiratory distress syndrome in preterm infants. Am J Perinatol. 2004;21(3):109–119. doi: 10.1055/s-2004-823779. [DOI] [PubMed] [Google Scholar]
- Ranson MR, Cheeseman S, White S, Margison J. Caelyx (stealth liposomal doxorubicin) in the treatment of advanced breast cancer. Crit Rev Oncol Hematol. 2001;37(2):115–120. doi: 10.1016/s1040-8428(00)00107-4. [DOI] [PubMed] [Google Scholar]
- Rizzieri D. Zevalin(®) (ibritumomab tiuxetan): after more than a decade of treatment experience, what have we learned? Crit Rev Oncol Hematol. 2016;105:5–17. doi: 10.1016/j.critrevonc.2016.07.008. [DOI] [PubMed] [Google Scholar]
- Rust DM, Jameson G. The novel lipid delivery system of amphotericin B: drug profile and relevance to clinical practice. Oncol Nurs Forum. 1998;25(1):35–48. [PubMed] [Google Scholar]
- Sacristán JA, Aguarón A, Avendaño-Solá C, Garrido P, Carrión J, Gutiérrez A, Kroes R, Flores A. Patient involvement in clinical research: why, when, and how. Patient Prefer Adherence. 2016;10:631–640. doi: 10.2147/ppa.s104259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarcoma K. Kaposi’s sarcoma: DaunoXome approved. AIDS Treat News. 1996;246:3–4. [PubMed] [Google Scholar]
- Saurav A, Kaushik M, Mohiuddin SM. Fenofibric acid for hyperlipidemia. Expert Opin Pharmacother. 2012;13(5):717–722. doi: 10.1517/14656566.2012.658774. [DOI] [PubMed] [Google Scholar]
- Semenchuk MR. Avinza Elan. Curr Opin Investig Drugs. 2002;3(9):1369–1372. [PubMed] [Google Scholar]
- Sercombe L, Veerati T, Moheimani F, Wu SY, Sood AK, Hua S. Advances and challenges of liposome assisted drug delivery. Front Pharmacol. 2015;6:286. doi: 10.3389/fphar.2015.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan X, Gong X, Li J, Wen J, Li Y, Zhang Z. Current approaches of nanomedicines in the market and various stage of clinical translation. Acta Pharm Sin B. 2022;12(7):3028–3048. doi: 10.1016/j.apsb.2022.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifi S, Mahmoud NN, Voke E, Landry MP, Mahmoudi M. Importance of standardizing analytical characterization methodology for improved reliability of the nanomedicine literature. Nano-Micro Lett. 2022;14(1):172. doi: 10.1007/s40820-022-00922-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JA, Deitcher SR. Marqibo® (vincristine sulfate liposome injection) improves the pharmacokinetics and pharmacodynamics of vincristine. Cancer Chemother Pharmacol. 2013;71(3):555–564. doi: 10.1007/s00280-012-2042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon JA. Estradiol in micellar nanoparticles: the efficacy and safety of a novel transdermal drug-delivery technology in the management of moderate to severe vasomotor symptoms. Menopause. 2006;13(2):222–231. doi: 10.1097/01.gme.0000174096.56652.4f. [DOI] [PubMed] [Google Scholar]
- Singh AP, Biswas A, Shukla A, Maiti P. Targeted therapy in chronic diseases using nanomaterial-based drug delivery vehicles. Signal Transduct Target Ther. 2019;4(1):33. doi: 10.1038/s41392-019-0068-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares S, Sousa J, Pais A, Vitorino C. Nanomedicine: principles, properties, and regulatory issues. Front Chem. 2018;6:360. doi: 10.3389/fchem.2018.00360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terblanche N, Coetzee JF. A comparison of induction of anaesthesia using two different propofol preparations. South Afr J Anaesth Analg. 2008;14(6):25–29. doi: 10.1080/22201173.2008.10872573. [DOI] [Google Scholar]
- Tobin KA. Macugen treatment for wet age-related macular degeneration. Insight. 2006;31(1):11–14. [PubMed] [Google Scholar]
- Tseng TC, Kao JH, Chen DS. Peginterferon α in the treatment of chronic hepatitis B. Expert Opin Biol Ther. 2014;14(7):995–1006. doi: 10.1517/14712598.2014.907784. [DOI] [PubMed] [Google Scholar]
- Tuca A, Jimenez-Fonseca P, Gascón P. Clinical evaluation and optimal management of cancer cachexia. Crit Rev Oncol Hematol. 2013;88(3):625–636. doi: 10.1016/j.critrevonc.2013.07.015. [DOI] [PubMed] [Google Scholar]
- Van den Driessche C, Bastian M, Peyre H, Stordeur C, Acquaviva É, Bahadori S, Delorme R, Sackur J. Attentional lapses in attention-deficit/hyperactivity disorder: blank rather than wandering thoughts. Psychol Sci. 2017;28(10):1375–1386. doi: 10.1177/0956797617708234. [DOI] [PubMed] [Google Scholar]
- Waheed S, Li Z, Zhang F, Chiarini A, Armato U, Jun W. Engineering nano-drug biointerface to overcome biological barriers toward precision drug delivery. J Nanobiotechnol. 2022;20(1):395. doi: 10.1186/s12951-022-01605-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen H, Jung H, Li X. Drug delivery approaches in addressing clinical pharmacology-related issues: opportunities and challenges. Aaps j. 2015;17(6):1327–1340. doi: 10.1208/s12248-015-9814-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadavalli T, Shukla D. Role of metal and metal oxide nanoparticles as diagnostic and therapeutic tools for highly prevalent viral infections. Nanomedicine. 2017;13(1):219–230. doi: 10.1016/j.nano.2016.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yetisgin AA, Cetinel S, Zuvin M, Kosar A, Kutlu O. Therapeutic nanoparticles and their targeted delivery applications. Molecules. 2020;25(9):2193. doi: 10.3390/molecules25092193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan H, Guo H, Luan X, He M, Li F, Burnett J, Truchan N, Sun D. albumin nanoparticle of paclitaxel (Abraxane) decreases while taxol increases breast cancer stem cells in treatment of triple negative breast cancer. Mol Pharm. 2020;17(7):2275–2286. doi: 10.1021/acs.molpharmaceut.9b01221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Chan HF, Leong KW. Advanced materials and processing for drug delivery: the past and the future. Adv Drug Deliv Rev. 2013;65(1):104–120. doi: 10.1016/j.addr.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang RX, Wong HL, Xue HY, Eoh JY, Wu XY. Nanomedicine of synergistic drug combinations for cancer therapy—strategies and perspectives. J Control Release. 2016;240:489–503. doi: 10.1016/j.jconrel.2016.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Pan Y, Shi Q, Zhang G, Jiang L, Dong X, Gu K, Wang H, Zhang X, Yang N, Li Y, Xiong J, Yi T, Peng M, Song Y, Fan Y, Cui J, Chen G, Tan W, Zang A, Guo Q, Zhao G, Wang Z, He J, Yao W, Wu X, Chen K, Hu X, Hu C, Yue L, Jiang D, Wang G, Liu J, Yu G, Li J, Bai J, Xie W, Zhao W, Wu L, Zhou C. Paclitaxel liposome for injection (Lipusu) plus cisplatin versus gemcitabine plus cisplatin in the first-line treatment of locally advanced or metastatic lung squamous cell carcinoma: a multicenter, randomized, open-label, parallel controlled clinical study. Cancer Commun (lond) 2022;42(1):3–16. doi: 10.1002/cac2.12225. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.