Abstract
Maximization of life-history traits is under constraints due to both, limitations of resource acquisition and the restricted pathways of resource allocation. Drosophila melanogaster has served as an excellent model organism to not only unravel various trade-offs among life history traits but also numerous aspects of host immune response. Drosophila larvae are semi-aquatic that live, feed and excrete inside the food source-often over-ripe fruits and vegetables that are rich in both commensal and pathogenic microbiota that can impact the larval survival. In this study, we have used six populations of D. melanogaster, three of which are selected for faster pre-adult development and extended adult longevity, and their three ancestral controls, to explore the impact of selection on the basal immune activity in the larval stage. The larvae from selected populations had nearly significantly upregulated plasmatocyte density, significantly higher percent phagocytosis, phagocytic index and higher transcript levels of Tep3, eater and NimC1. Selected populations also had significantly upregulated crystal cell number along with higher transcript of PPO2. Out of seven tested AMPs level, Drosomycin was significantly upregulated in selected populations while Drosocin was significantly higher in control populations. ROS levels were comparable in the selected and control populations. Our results strongly suggest that enhanced basal immune activity during larval stage manages the faster development and could be responsible for comparable larval survival of selected and control populations.
Keywords: Drosophila, Faster development, Phagocytosis, Phenoloxidase, AMPs, Life history trade-offs
Drosophila; faster development; phagocytosis; phenoloxidase, AMPs; life history trade-offs.
1. Introduction
The optimality principles of life-history evolution act to maximize the average fitness of organisms (Charlesworth, 1990a; Nesse and Williams, 1995). Evolution of life-history traits is a result of trade-offs arising due to interaction between the extrinsic factors such as environment and intrinsic factors such as genetics, development and physiology of organisms. Trade-offs are common between functions that regulate survival, longevity, and reproductive success and thus, maximization of fitness by evolutionary forces is under certain constraints (Novoseltsev et al., 2002). The observed life-history traits are an outcome of the constrained optimization of fitness (Parker and Maynard Smith, 1990; Charlesworth, 1990b; Partridge and Sibly, 1991). These constraints are both due to limitations of resource acquisition (Van Noordwijk and de Jong, 1986) and the restricted pathways of resource allocation (Gadgil and Bossert, 1970). Several studies have proposed the presence of a trade-off between maintenance/survival and reproduction (Kirkwood, 1977; Kirkwood and Holliday, 1979) and attempts have been made to outline optimal resource allocation in terms of costs of reproduction (Williams, 1966; Calow, 1977; Bell and Koufopanou, 1985; Reznick, 1985; Partridge and Harvey, 1988; Partridge and Sibly, 1991). Studies on holometabolous insects such as Drosophila melanogaster have indicated that adult size (Robinson and Partridge, 2001), adult physiology (Robinson and Partridge, 2001; Rodrigues et al., 2015) and various other life-history traits (Tu and Tatar, 2003; Aguila et al., 2007; Reis, 2016; Shenoi and Prasad, 2016; Stefana et al., 2017; Klepsatel et al., 2018) are affected by environmental conditions during larval phase by impacting the development duration (Zwaan et al., 1995; Tu and Tatar, 2003; Zwaan, 2003; Baldal et al., 2005, 2006; Brakefield et al., 2005) through altered larval nourishment (Min et al., 2006; Reis, 2016; Stefana et al., 2017). Varying the quality and or quantity of diet during development affects adult body size and associated life-history traits (Tu and Tatar 2003; Grandison et al., 2009; Zajitschek et al., 2019). Selection for faster pre-adult development is reported to result in reduced adult body size as a correlated response (Nunney, 1996; Chippindale et al., 1997; Prasad et al., 2001; Zwaan et al., 2013; Handa et al., 2014; Sharma et al., 2020) primarily due to significantly reduced feeding duration post attainment of critical size (Sharma et al., 2020). The reduced feeding duration post attainment of critical size resulted in significant reduction in lipid and protein content of larvae during the post critical larval stage (Sharma and Shakarad, 2021). Further, the flies from faster developing populations were significantly smaller in size compared to their ancestral controls (Sharma et al., 2020) and had significantly lower life-time fecundity but comparable lifespan post sustained selection for faster pre-adult development and extended lifespan for over 134 generations (Sharma and Shakarad, 2021), although they had significantly higher life-time fecundity after 110 generations of selection when paired with male from their ancestral control populations (Handa et al., 2014). It has been reported that the correlated responses to selection can disappear when selection is continued over long periods (Archer et al., 2003; Phelan et al., 2003). There are strong positive correlations between tolerance to various stresses and longevity (Service et al., 1985; Rose et al., 1992; Force et al., 1995; Zwaan et al., 1995; Chippindale et al., 1998; Norry and Loeschcke, 2003) as opposed to the negative correlations longevity and stress tolerance display with reproductive output (Salmon et al., 2001; Baldal et al., 2006; Singh et al., 2021). Response to various abiotic and biotic stresses by organisms is contingent to exposure to the stressors themselves. However, even in most stable external conditions, the immune activity has to be maintained in order to maintain physiological homeostasis. A recent study on human subjects reported sustained increase in innate immune activity with aging (Franceschi et al., 2007). Similarly, in Drosophila, activation of a single Anti-microbial peptide (AMP)- Drosocin (Dro) has been reported to result in a significant extension of lifespan (Loch et al., 2017). However, another study with D. melanogaster (Kounatidis et al., 2017) reported reduction in lifespan due to overexpression of the AMPs- Dro, Attacin A (AttA) and Cecropin (CecA1) in neurons or glia. Further, Boots (2011) reported an increase in development time as a correlated response to selection for resistance against baculovirus, ‘Plodia interpunctella Granulosis Virus’ (PiGV) in Indian meal moth, Plodia interpunctella. However, reciprocal selection experiments in this species showed opposite results. Populations of P. interpunctella selected for shorter development time showed increased resistance to PiGV, while those selected for longer development time showed decreased resistance to PiGV, thus showing the non-symmetrical nature of trade-offs (Bartlett et al., 2020). Two recent studies, reported that D. melanogaster populations selected for faster pre-adult development and extended reproductive lifespan showed both, larval survival (Sharma et al., 2020; Shrivastava et al., 2022) and adult longevity (Sharma and Shakarad, 2021) to be comparable with control populations despite being under selection for extended reproductive lifespan. However, life-time fecundity was significantly lower (Sharma and Shakarad, 2021). It is likely, that these populations of D. melanogaster that are under selection for faster pre-adult development and extended reproductive lifespan (non-specific selection for longer lifespan) have compromised reproduction-a crucial life-history trait as a consequence of heightened immune function in order to ensure survival during the pre-adult and or adult stages.
In the present study, we explore the immune responses in D. melanogaster populations under simultaneous selection for faster pre-adult development and extended reproductive lifespan. We determined the number of circulating plasmatocytes-the major phagocytic cells in Drosophila and crystal cells-the platelet like cells involved in melanization. We also quantified the level of Reactive Oxygen Species (ROS) that act as messengers in immune signalling. We further explored the expression levels of various genes associated with phagocytosis, melanization as well as AMP genes by quantitative real time PCR. We used a total of six populations for this study. Three of the six were selected for faster pre-adult development and extended adult lifespan, and the other three were their respective ancestral control populations that were on a 21-day discrete generation cycle and were not under any conscious selection for either development time and/or extended adult longevity. We report that the number of circulating plasmatocytes per microliter of hemolymph were non-significantly higher, while the number of crystal cells were significantly higher in the selected populations compared to the controls. The ROS level was comparable among the selected and control populations. However, percent phagocytosis and phagocytic index was significantly higher in the selected populations compared to controls. The transcript levels of the three genes that are responsible for phagocytic activity- Tep3, eater and NimC1 were significantly higher in the selected populations. Of the two genes that are responsible for melanization (of crystal cells), the relative expression of PPO2 was significantly higher while PPO1 was not. The relative transcript levels of antimicrobial peptide genes- Drosomycin (Drs) was significantly higher in selected populations compared to controls, while that of Drosocin (Dro) was significantly higher in control populations. The relative transcript levels of Metchnikowin (Mtk), Diptericin (Dpt), Defensin (Def), Cecropin A1 (CecA1) and Attacin A (AttA) were comparable.
2. Material and methods
2.1. Fly lines and their maintenance
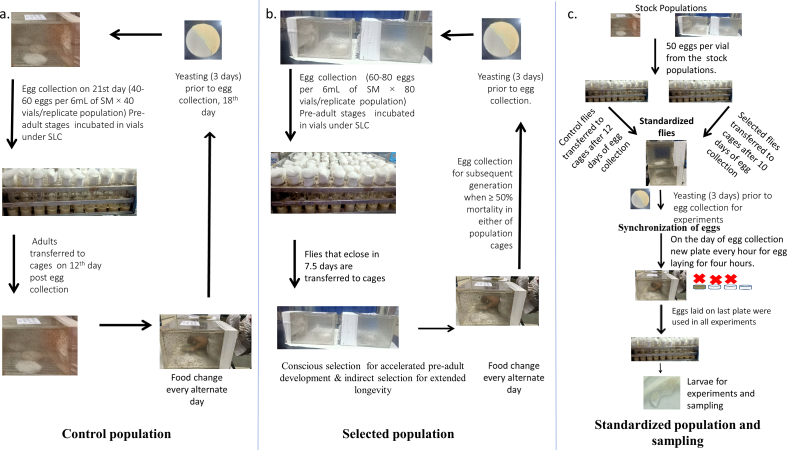
A total of six Drosophila melanogaster populations of two different selection type were used in this study. The two selection types were-the ancestral control (C) and selected (S). Detailed maintenance protocols for the C and S populations are described in our earlier publications (Handa et al., 2014; Chauhan et al., 2020; Sharma and Shakarad, 2021). Briefly, all the growth stages were cultured on banana-jaggery media (hence forth referred to as SM, Chandrashekara and Shakarad, 2011) in Power Scientific Inc., USA environmental chambers at 25 ± 1 °C temperature, 70 ± 5% RH and 24:0 L:D (here after referred to as SLC, Chandrashekara and Shakarad, 2011). The egg, larval and pupal stages were reared in Borosilicate glass vials; while the adult stage in Plexiglass population cages of 25 cm × 20 cm × 15 cm with a cloth sleeve on one side. The adults were provided with fresh SM plate every alternate day till 3 days prior to collecting eggs for starting the next cycle. Three days prior to egg collection the adults were provided with fresh SM plate supplemented with live yeast-acetic acid paste in order to boost the egg production. The notable differences in the maintenance of C and S populations are: (i) the egg density in the C populations was 40–60 eggs per 6 mL SM vial (composition of SM and recipe can be found in Chauhan et al., 2020), while that in S populations it was 60–80 eggs. (ii) Forty vials per population were incubated in C populations, whereas 160 vials per population were incubated in S populations. (iii) All adults from the 40 vials that emerged by day 12 from egg collection day were transferred to pre-labeled population cages in case of C populations (Figure 1a); but in case of S populations, only the earliest emerging 15-20 adults were transferred to pre-labelled populations cages (Figure 1b). The adults of S populations were maintained in two sister-cages in order to avoid crowding, and were allowed to age till 50% adult mortality. (iv) Average effective egg-to-adult development of C populations was 9.5 days, while that of S populations was 7.5 days. (v) The effective adult age at the time of egg collection for starting the next generation was 11.5 and 35 days in C and S populations, respectively. (vi) The eggs from the surviving flies of the two sister cages for each replicate S populations were recombined to avoid independent evolution in them. (vii) The C populations were not on conscious selection for either reduced or delayed development time, while S populations were being selected reduced pre-adult development time and extended adult longevity. The number of breeding adults in both C and S was ∼1600 per population. In the S populations, the selection for faster per-adult development was relaxed post 130 generations of selection as almost all flies started eclosing in 7.5 days. After 130 generations, only 40 vials were collected per sister cage and all the flies that eclosed by seven and half days were transferred to a pre-labeled breeding cages. The S populations had been through a total of 180 generations of selection when this study was initiated. The C and S populations have been referred to as JB (Joshi Baseline) and FLJ (Faster developing Late reproducing JB derived) (Rajamani et al., 2006; Shrivastava et al., 2022), or Genotypically Large and Genotypically Small (Handa et al., 2014); in our earlier publications. Further, our selected populations share their ancestral populations (Control/JB/Genotypically Large) with the Faster developing Early reproducing JB derived (FEJ) populations of Prasad et al. (2001), Dey et al. (2016) and Shrivastava et al. (2022). The C and S populations were passed through one generation of common rearing conditions in order to eliminate all non-genetic effects (Prasad et al., 2001; Sharma et al., 2020; Shrivastava et al., 2022). The egg collection from C and S populations was staggered by the developmental time difference such that the chronological age of the parents of assay flies as well as that of assay flies themselves were comparable (Sharma et al., 2020; Chauhan et al., 2020; Shrivastava et al., 2022), these flies were termed as standardized flies (Figure 1c). All the experiments were done using the progeny of standardized flies. Late L3 female larvae were used in all the experiments being reported here. Data was collected using larvae from three replicate populations each for C and S lines. The maintenance protocol and sampling of larvae for experiments is shown in Figure 1.
Figure 1.
Maintenance of stock populations and synchronization for experiments. (a) Control populations undergo 21-day egg-to-egg discrete cycle. Briefly 40–60 eggs are collected in 40 vials with 6ml media and on 12th day, all the eclosing adults are transferred to cages. Food change is done every alternate day. On 18th day from egg collection day, fresh food plate supplemented with yeast paste is provided. On day 20th food plate was removed and fresh food cut into two half portions so as to increase egg laying surface area are provided at 18:00 h. Egg plates are taken out the following day (day 21) at 09:00 h and eggs collected for starting next generation. (b) Selected populations undergo two selection pressures simultaneously, (i) for faster pre-adult development and (ii) for extended reproductive longevity. Briefly flies are maintained in two sister cages, eggs are collected from two cages and mixed, and distributed into 80 vials (40 vials per sister cage) each containing 60–80 eggs. All the adults eclosing in 7.5 days are transferred to cages and culture vials discarded. Fresh food is provided every alternate day. Flies are aged until 50% mortality is observed in any of the sister cages. On noticing ∼50% mortality, fresh food plate supplemented with yeast is provided for 3 days just as for control flies; following which eggs are collected for starting the next generation. (c) For any experiments that needs to be performed, standardized populations are generated to avoid any non-genetic parental effects. Briefly, eggs are collected in 40 vials/population at a density of 50 eggs/per vial. All emerging adults are transferred to pre-labelled cages on 10th and 12th day for selected and control populations, respectively so as to obtain age matched flies. These are referred to as standardized flies. They are provided with yeast supplemented food plate for three days prior to egg collection. Eggs are collected such that stage matched larvae from selected and control populations for various assays are available on the day of assay.
2.1.1. Synchronization of eggs
In order to obtain eggs of similar developmental stage, the flies were provided with 4 laying plates over 4 successive 1 h windows. The first three plates were of SM and the last plate was of non-nutritive agar. Eggs laid on the agar plate were used in all the experiments, while those laid on the 3 SM plates were discarded (Sharma et al., 2020; Chauhan et al., 2020; Shrivastava et al., 2022). Egg collection from C and S populations were staggered by their developmental time difference so as to obtain larvae of similar physiological state at a given sampling time (Figure 1c).
2.2. Circulating plasmatocyte density
Hemocytes play an important role in shaping the extracellular environment and the immune response. In Drosophila, only three types of mature hemocytes can be distinguished (Crozatier and Meister, 2007). 95% of all Drosophila immune cells prior to infection are plasmatocytes (Ratheesh et al., 2015). Plasmatocytes are phagocytotically active, ingesting cellular debris during normal development, and engulfing microorganisms during the immune response (Grogorian and Hartenstine, 2013); thus they influence development (Bunt et al., 2010; Sears et al., 2003; Zhou et al., 1995) and physiology (Woodcock et al., 2014) besides defending against bacteria (Braun et al., 1998; Vlisidou et al., 2015), fungi (Braun et al., 1998), viruses (Costa et al., 2009), and cancer (Pastor_Pareja et al., 2008; Parisi et al., 2014). Hence, we counted the number of circulating plasmatocytes in the larvae from selected and control populations without exposing them to any infection. Late L3 female larvae were identified using Zeiss stereo-zoom binocular microscope. Five larvae were collected in a Tarsons petri-plate of 5.5cm diameter, washed with reverse osmosis (RO) water so as to remove food particles sticking to the body surface, rolled on a tissue towel to remove excess water and immobilized on an ice block. Larvae were then placed in a cavity block and hemolymph was released into the cavity by tearing the cuticle using a pair of sharp dissection needles under Zeiss stereo-zoom binocular microscope. Utmost care was taken to avoid gut breaking. 1μl of hemolymph was pipetted out into microfuge tube with 4μl of 1X PBS that was placed in ice bucket. Sample was then mixed with 0.4% trypan blue solution (Himedia, TC193) in 1:1 ratio (5μl:5μl) and pipetted several times to ensure a uniform cell suspension. 10 μL of trypan blue-cell suspension was carefully loaded on a hemocytometer by touching the coverslip at its edge with the pipette tip. Hemocyte density was counted using Neubauer hemocytometer under Nikon microscope at 10X magnification. Counts of hemocytes were made in each of the 4 corner squares of hemocytometer per sample. Average cell density was estimated using mobile App Cells Calculator V2.2, using the formula:
Average cell density per microliter of hemolymph = (avg. cell count per square × dilution factor)
Twelve such samples were taken per population. In all 60 late L3 female larvae were used per population, making it a total of 180 larvae per selection type. The average values of 12 samples per population were used in statistical analysis.
2.3. Reactive oxygen species (ROS) estimation
Reactive oxygen species (ROS) play an important role in many homeostasis processes involving metabolism, immunity, growth and differentiation; besides causing oxidative damage to cells and tissues (Shadel and Horvath, 2015). Improved redox homeostasis was suggested to be responsible for enhanced survival of flies under hyperoxia (Zhao et al., 2010). Since plasmatocytes comprises 95% of immune cell population, the ROS produced by these cells under unchallenged conditions could indicate the internal stress that the organism might be experiencing. As ROS have many physiological functions, we assessed the ROS level in plasmatocytes of selected and control population late L3 female larvae. Dihydroethidium (DHE) was used to measure the superoxide radicals in plasmatocytes. A stock solution of 30mM (Invitrogen Molecular Probes, D11347) was reconstituted in anhydrous DMSO (dimethyl sulfoxide, Sigma Aldrich 276855). For staining, dye was diluted to 25mM conc. in 1X PBS. 100μl of hemocyte suspension (≈3 × 104 cells) in Schneider’s media (Sigma-Aldrich S0146) was plated in pre-cleaned slides and was kept in humidified chamber at 25 °C for an hour. Slides were then washed with 1X PBS and incubated with 25 mM DHE for 10 min in a dark chamber at 25 °C. Slides were then washed with 1X PBS and mounted with Vectashield media containing DAPI and images acquired under Nikon eclipse (Ni-E) fluorescence microscope and fluorescence intensity quantification was done using ImageJ software. CTCF (corrected total cell fluorescence) of 30 cells per sample was quantified. CTCF was calculated using formula.
CTCF = Integrated density of cell – (Area of selected cell × Mean fluorescence intensity of background)
Five such samples per replicate population were quantified and the mean of the five samples were used in statistical analysis.
2.4. Phagocytosis assay
Although the selected larvae had non-significantly higher plasmatocytes number, the ROS level was not significantly high. This prompted us to ascertain plasmatocyte function. We assessed the phagocytic activity of plasmatocytes isolated from late L3 female larvae from selected and control populations. We followed the methodology described by Dhankhar et al. (2021) to assess phagocytosis activity. 100μl of hemocyte suspension (≈3 × 104 cells) in Schneider’s media was plated on precleaned slides and incubated in humified chamber at 25 °C for an hour to allow plasmatocytes to adhere to glass slides. Meanwhile, overnight grown culture of Escherichia coli was harvested by centrifugation at 12000 rpm at 4 °C, washed three times with 1X PBS and then heat killed at 90 °C for an hour. Heat killed bacteria was again washed three times and then stained with 10 μg/ml propidium iodide (PI) (Sigma Aldrich, P4170) on a shaker at room temperature. Finally, bacteria were washed with 1X PBS and diluted to 108 cells/ml by measuring O.D. at 600nm. Following incubation for adherence of cells, the slides were washed with 1X PBS to remove non adherent cells, and heat killed E. coli (100μl of 108 cells/ml) stained with PI was added on cells adhered to slide and incubated for 35 min at 25 °C for phagocytosis. Slides were then washed with 1X PBS to remove un-phagocytosed bacteria, fixed with methanol, air dried and mounted with DAPI (Vector lab, H1200). Percentage phagocytosis was calculated by counting the number of cells showing engulfment of PI stained bacteria per 100 plasmatocytes per slide under fluorescence microscope. 3 slides per block per population were counted and population means were used in data analysis and graphical representation. The represented images were captured using fluorescence-microscope at 60X magnification.
Further we assessed phagocytosis index (number of bacteria engulfed by one cell) using GFP tagged Pe (Pseudomonas entomophila), (a kind gift from Prof. N.G. Prasad, IISER, Mohali). The method remained the same except instead of heat killed, PI stained E. coli we used GFP tagged Pe and incubation time was 40 min. Number of bacterial cells engulfed in 30 randomly chosen plasmatocytes per slide were counted in 3 slides per populations. Phagocytosis index was estimated as the average of 3 slide data which were average of 30 plasmatocytes per slide. The represented images were captured using confocal microscope at 60X magnification.
2.5. Crystal cell count
Crystal cells serve as a critical indicator of Drosophila’s stress status and any abnormal change in crystal cell count and their activity can be the first crucial sign for outlining the alteration in immune response (Dhankhar et al., 2021). In order to understand the involvement of immune system in faster pre-adult development we assessed the number of crystal cells in the selected and control populations. Giving heat shock to larvae in PBS at 70 °C causes spontaneous activation of the prophenoloxidase zymogen within crystal cells, leading to their blackening, and consequently making them visible through the cuticle as black puncta (Rizki et al., 1980). To visualize the crystal cells, 10 larvae per block were exposed to heat shock in water bath at 70 °C for 10 min. This leads to auto melanization of crystal cells that appear as black pustules on posterior-most abdominal segments A6, A7 and A8 (Dhankhar et al., 2021). Images of larvae were taken using NIKON SMZ1000 microscope and melanized cells were counted using ImageJ software. Ten larvae were sampled per population per selection and population means were used in statistical analysis.
2.6. Phenoloxidase activity
Phenoloxidase is a key enzyme in the melanization process that catalyzes the oxidation of phenols to quinones, which subsequently polymerize into melanin. Since significantly higher melanized dots were observed on the cuticle of selected larvae, we measured enzymatic Phenoloxidase (PO) activity with a L-DOPA assay in the whole body extract of late L3 female larvae from selected and control populations. For measuring PO activity, 5 larvae were homogenized in homogenization buffer (1X PBS containing protease inhibitor (Sigma-Aldrich, S8820) and centrifuged at 13000rpm for 10 min. Supernatant was collected in pre-labelled pre chilled Eppendorf and protein concentration of each sample was determined using Pierce BCA protein estimation kit (Thermo Scientific 23227). To quantify PO activity 50 μl of supernatant containing 10μg of protein was plated on 96-well plate and 50 μl of 3mM L-3-4-dihydroxyphenylalanine (L-DOPA) was added to it. Phenol-oxidase converts L-DOPA into orange color dopachrome. Absorbance at 492nm was taken as criteria to estimate PO activity. Each sample was plated in duplicate and two such samples per block per population were used for the assay.
2.7. Expression of melanization and anti-microbial peptide genes
Invertebrates lack an adaptive immune system and hence, innate immune system plays an important role in host defense against pathogens. Antimicrobial peptides (AMPs) represent the front lines in the coevolutionary struggle between host and pathogen as they are the main effector molecules of innate immunity in many organisms (Hanson et al., 2019). Drosophila larvae compete with fungi and bacteria for nutrients. We isolated RNA from whole body of 10 late L3 female larvae per sample and measured mRNA transcript levels of anti-microbial peptide genes grouped into three families based on their main biological targets, gram-positive bacteria (Def), gram-negative bacteria (CecA1, Dro, AttA, Dpt), or fungi (Drs, Mtk) (Imler and Bulet, 2005). Further we quantified the transcript level of genes involved in melanization (PPO1 and PPO2). Similarly, we also measured mRNA expression level of eater and NimC1-genes involved in adherence of hemocyte to sessile chambers; and Tep3 which along with eater and NimC1 is involved in phagocytosis.
Total RNA was extracted from each sample using TRI Reagent (Sigma, T9424), and the quality of the RNA was determined using a NanoDrop 2000 spectrophotometer at 260 and 280 nm absorbance; a 260/280 ratio close to 2 was considered as pure RNA. To remove any genomic DNA contamination, RNA aliquot was processed with RQ1 DNase (M6101; Promega, USA) and reverse transcribed for cDNA synthesis using the first strand cDNA synthesis kit (Cat no. K1622; Thermo Fisher Scientific). Real-time quantitative polymerase chain reaction (qPCR) with SYBR Green chemical MasterMix (Cat no. A25742, Applied Biosystems by Thermo Fischer Scientific) was used to quantify the mRNA expression level of the gene of interest on a ViiA7 thermal cycler (Applied Biosystems, Foster City, CA, USA). We used gene specific primer designed using Primer3 and validated in silico on NCBI blast (Table 1). Total reaction mixture of 6 μl had 3 μl SYBR Green PCR MasterMix, 1 μl forward primer (1 μM), 1 μl reverse primer (1 μM), and 1 μl (10 ng/l) cDNA. Both, genes of interest and the reference (Rp49) gene were run in duplicates. The fold change in 2-(Ct) value was used to calculate mRNA expression (Livak and Schmittgen, 2001). In a nutshell, fluorescence exceeding background level gave cycle threshold (Ct). ΔCt was calculated (Ct target gene - Ct reference gene). Ct values were normalized using Ct value of pool sample that consisted of a mix of cDNA of all samples (ΔΔCt) from both populations. Fold change was calculated by negative value of ΔΔCt powered to 2 (2−ΔΔCt) and was plotted.
Table 1.
Primers used in the study.
| Primer name | Primer sequence 5'-3' |
|---|---|
| Tep3 F | TCCAAGGGTCCATGTGATGC |
| Tep3 R | TAATCCCAACCCGTTCACCG |
| Diptericin F | CAGCCTGAACCACTGGCATA |
| Diptericin R | TCGAATCCTTGCTTTGGGCT |
| Drosomycin F | TACTTGTTCGCCCTCTTCGC |
| Drosomycin R | CTCCTCCTTGCACACACGAC |
| Metchnikowin F | GCATCAATCAATTCCCGCCA |
| Metchnikowin R | GCTCTGCCAGCACTGATGTA |
| Defensin F | TCAGCCAGTTTCCGATGTGG |
| Defensin R | CCACTTGGAGAGTAGGTCGC |
| Attacin A F | GCCCAATCGTGCTACTACCTT |
| Attacin A R | AATACGCAGGCTTAGCCGA |
| Cecropin A1 F | CGCTCAGACCTCACTGCAATATC |
| Cecropin A1 R | GCCAGAATGAGAGCGACGAAA |
| Rp49 F | CCGCTTCAAGGGACAGTATC |
| Rp49 R | ATCTCGCCGCAGTAAACG |
| eater F | GGCTTCTGCACGAAACCAAA |
| eater R | ATACAGCGGCCAGGAGATTG |
| PPO1 F | TTTGCCCACGATCCCGATTACC |
| PPO1 R | TGTCGATGAATCCGTGCCACC |
| PPO2 F | TGACCTGCACAACAACGGACAC |
| PPO2 R | TCACCCATCACGCCAAAGGAC |
| NimC1 F | AGGTGTGTGCGAGTGTGAAA |
| NimC1 R | CATAATCCGTTCTCCGGGCA |
| Drosocin F | CCATCGTTTTCCTGCT |
| Drosocin R | CTTGAGTCAGGTGATCC |
2.8. Statistical analyses
When drawing evolutionary inferences (as in selection studies), the relevant unit of replication is the population (Chippindale et al., 1994; Prasad et al., 2000; Chauhan et al., 2020; Sharma and Shakarad, 2021). Hence, in the context of the experimental design, we have blocks (representing common ancestry) which get both selected (FLJ) and control (JB) selection regimes. Therefore, the key question being asked is, ‘whether block means, on an average, differ across the selection regimes, tested as MS Selection Regime/MS Block × Selection Regime’. These MS terms and the F-test for significant main effect of Selection Regime, will not change even if we included individual data within each Block × Selection Regime combination in the analysis. The variation among individuals within Block × Selection Regime combination would be used only to test significant main effects of block and the Block × Selection Regime interaction. Since the blocks represent a random sample of ancestral lineages, we were not interested in assessing the magnitude of block effects or interactions. We were interested in the selection main effect as our biological hypothesis (Kutner et al., 2005). Hence, population means were used as units of analysis with selection as fixed variable and replication as a random variable. Further, according to Central Limit Theorem, population means are expected to be normally distributed even if the data in each population are non-normal (Zar, 2011). Furthermore, although Analysis of Variance (ANOVA) is robust-operating well even with considerable heterogeneity of variances as long as all sample sizes are equal or nearly equal (Zar, 2011; Glass et al., 1972); we subjected the data to Welch’s ANOVA-a test that is not sensitive to unequal variances. We subjected each trait data to Shapiro–Wilk normality test (data shown in supplementary files). Crystal cell count, transcript levels of eater, PPO1, PPO2, Drs, Mtk, Def, Dpt, CecA1 and AttA data were non-normal and hence were subjected to log-transformations. The transformed data was reassessed for normality and found to be following normal distribution (data shown in supplementary files). Data was then subjected to Welch’s ANOVA-a test that is not sensitive to unequal variances. All statistical analyses were performed on Real Statistics Add-Ins in MS Excel (Microsoft Corporation, 2018).
3. Results
3.1. Selection for faster development leads to non-significant increase in circulating plasmatocyte numbers
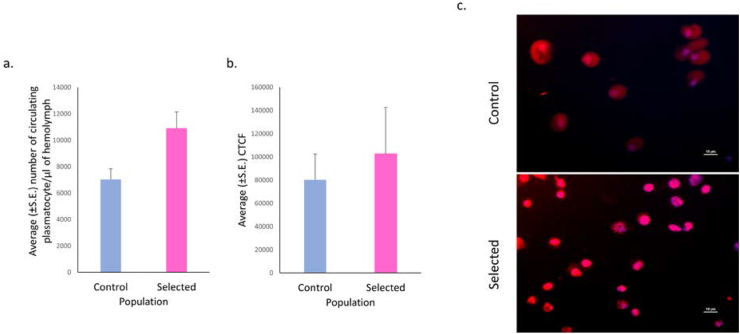
95% of the immune cells in Drosophila are plasmatocytes, which are phagocytic cells that clear cellular debris and ingest foreign microorganisms. We quantified circulating plasmatocytes numbers in control and selected populations and found that they were non-significantly higher in the selected populations (F = 6.908, p = 0.068; Figure 2a) with the effect size, ω2 being 0.496.
Figure 2.
(a) Quantification of plasmatocytes in late L3 female larval hemolymph showed non-significantly higher numbers (F = 6.908, p = 0.068) in selected compared to stage matched control larval hemolymph. However, the effect size was 0.496. (b) Quantification of fluorescence intensity as ‘corrected total cell fluorescence (CTCF)’ revealed that ROS level was comparable (F = 0.243, p = 0.655) in stage matched larval plasmatocytes of selected and control larvae. (c) Images of ROS intensity in circulating plasmatocytes isolated from control and selected larvae. Red = dihydroethidium (DHE) and Blue = DAPI, scale bar represents 10μm. Welch’s ANOVA was performed following Shapiro-Wilks normality test. Data are presented as mean ± SEM.
3.2. Comparable ROS production in plasmatocytes of selected and control populations
Since 95% of immune cells are plasmatocytes, the ROS these cells produce under unchallenged normal conditions may be an indication of any internal stress the organism may be going through. We quantified ROS in plasmatocytes and found there was no significant difference in the ROS levels of selected and control populations (F = 0.243, p = 0.655; Figure 2b, c), thereby implying that the selected populations are not experiencing any internal stress due to faster development.
3.3. Selected populations show higher phagocytosis
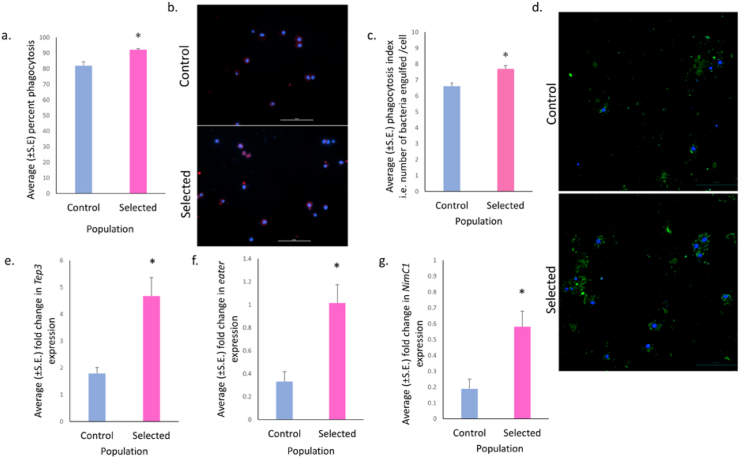
Following the method of Dhankhar et al. (2021) we quantified the ability of plasmatocytes to engulf heat-killed propidium iodide (PI)-stained E. coli by counting the number of cells capable of active phagocytosis. The plasmatocytes isolated from selected larvae showed a significantly higher percentage of phagocytosis compared to larvae from control populations (F = 17.173, p = 0.041; Figure 3a, b) with effect size of selection being large (ω2 = 0.729). Further, the phagocytosis index of the plasmatocytes from the selected populations was also significantly higher than that of the control populations (F = 14.74, p = 0.019; Figure 3c, d). The effect size was large (ω2 = 0.696).
Figure 3.
Increased plasmatocyte activity and mRNA levels of Tep3, eater and NimC1 in selected populations. (a) Quantification of percent phagocytosis (number of cells showing phagocytosis per 100 plasmatocytes) in plasmatocytes isolated from selected population showed significantly higher (F = 17.173, p = 0.041, ω2 = 0.729) phagocytosis than control. The effect size was 0.729. (b) Fluorescence microscope images showing the engulfment of heat killed PI- stained E. coli DH5α by plasmatocytes isolated from control and selected stage matched larvae. Scale bar represents 50μm. (c) Quantification of phagocytosis index (number of bacteria engulfed by one cell) in plasmatocytes isolated from selected population (pink bar) showed significantly higher (F = 14.74, p = 0.019, ω2 = 0.696) phagocytosis index than control (blue bar) stage matched larvae. (d) Confocal microscope images showing engulfment of GFP tagged Pe (Pseudomonas entomophila) by plasmatocytes isolated from control and selected stage matched larvae. Scale bar represents 50μm. (e) Increased expression of Tep3 transcript levels in late L3 selected larvae (F = 15.875, p = 0.042, ω2 = 0.713) than control. (f) eater transcript levels in late L3 selected larvae was significantly higher than in control larvae (F = 18.114, p = 0.019, ω2 = 0.74). (g) Significantly higher NimC1 transcript levels in late L3 selected larvae (F = 11.285, p = 0.037, ω2 = 0.632) than control. Welch’s ANOVA was performed following normality check using Shapiro-Wilks test. Data are presented as mean ± SEM. p-value: ∗p < 0.05 #p ≈ 0.05.
We further analyzed the expression of genes involved in phagocytosis activity. Thioester-containing proteins (TEPs) participate in both humoral and cellular arms of immune response in Drosophila by Toll pathway activation which in turn regulate several antimicrobial peptides expression. They are required for efficient phagocytosis of bacteria, notably gram positive species (Dostálová et al., 2017). The relative transcript levels of Tep3 were significantly higher in the selected populations compared to the controls (F = 15.875, p = 0.042; Figure 3e), and the effect size was also large (ω2 = 0.713).
eater and NimC1 are the two main receptors for phagocytosis of bacteria in Drosophila (Melcarne et al., 2019). The relative transcript levels of both, eater (F = 18.114, p = 0.019) (Figure 3f) and NimC1 (F = 11.285, p = 0.037) (Figure 3g) genes were significantly higher in the selected populations compared to the controls, thereby suggesting possible mechanism for increased phagocytosis. The effect size was large for both, eater (ω2 = 0.74) and NimC1 (ω2 = 0.632).
3.4. Selection for faster pre-adult development and extended reproductive lifespan leads to increased crystal cell numbers but not PO activity
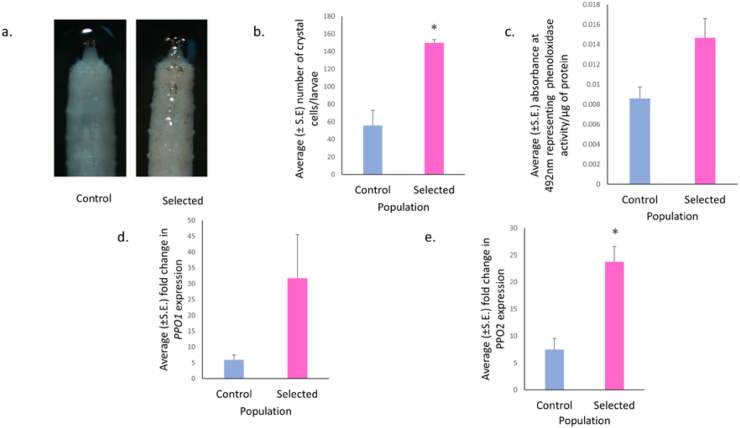
Quantification of crystal cells using ImageJ software showed that the selected populations had significantly higher number of crystal cells compared to their ancestral populations (F = 28.389, p = 0.028, Figure 4a,b). The effect size was large (ω2 = 0.82). Phenoloxidase is a key enzyme in the melanization process that catalyzes the oxidation of phenols to quinones, which subsequently polymerize into melanin. Since significantly higher melanized dots were observed on the cuticle of selected larvae, we measured enzymatic PO activity with an L-DOPA assay in the whole body extract of late L3 female larvae from selected and control populations. The PO activity in the larvae from selected population was nearly significantly higher than the controls (F = 7.115, p = 0.069, Figure 4c). The effect size was large (ω2 = 0.505). Drosophila genome encodes three POs, which are primarily produced as zymogens or prophenoloxidases (PPO). PPO1 and PPO2 produced by the crystal cells are responsible for all the PO activity. PPO1 is involved in the rapid early delivery of PO activity, while PPO2 is accumulated in the crystals of crystal cells and provides a storage form that can be deployed at a later phase (Binggeli et al., 2014). We checked the transcript levels of PPO1 and PPO2 in the late L3 female larvae of selected and control populations and found that the relative transcript levels of PPO1 gene were comparable (F = 2.689, p = 0.238, Figure 4d), while that of PPO2 were significantly higher (F = 24.517, p = 0.027, Figure 4e) in the selected populations compared to the controls. The effect size was large (ω2 = 0.797).
Figure 4.
Increased Crystal cell count and PPO2 transcript levels in selected populations. (a) Crystal cells in the posterior abdominal segments of control and selected late L3 female larvae. (b) Circumferential crystal cells were counted from the three posterior segments A6, A7 and A8 of the heat shock treated control and selected late L3 female larvae. Larvae from selected populations showed significantly higher (F = 28.389, p = 0.028) crystal cell number than control. The effect size, ω2 was 0.82. Crystal cells were counted manually using ImageJ manual counter. (c) Phenoloxidase activity was measured using L-DOPA as a substrate. Phenoloxidase converts L-DOPA into orange color dopachrome. Absorbance at 492nm was taken as criteria to estimate Phenoloxidase activity. Phenoloxidase activity was nearly significantly higher (F = 7.115, p = 0.069) in selected as compared to control, with an effect size of 0.505. (d) PPO1 transcript levels were comparable (F = 2.689, p = 0.238) in control and selected larvae. (e) Increased expression of PPO2 transcript was seen in late L3 selected larvae (F = 24.517, p = 0.027) than control larvae. The effect size was large (ω2 = 0.797). Welch’s ANOVA was performed following normality check using Shapiro-Wilks. Data are presented as mean ± SEM. P-value: ∗p < 0.05.
3.5. Transcript levels of antimicrobial peptides
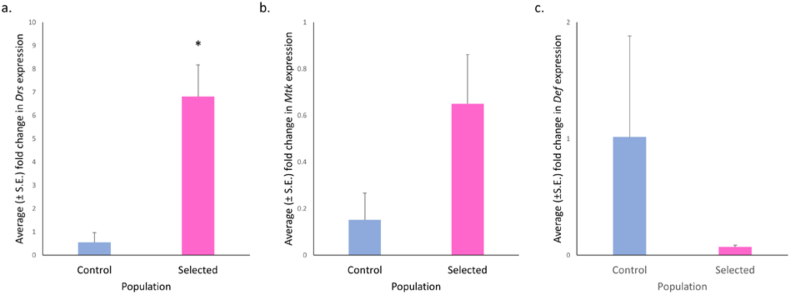
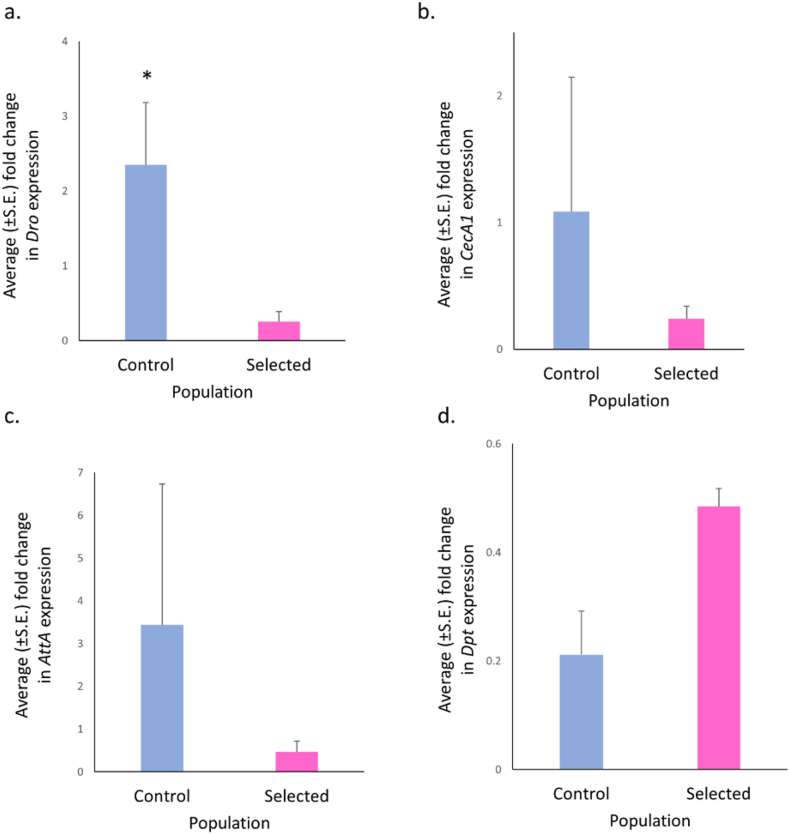
Drs and Mtk are two important anti-fungal peptides produced by D. melanogaster. We ascertained the relative transcript levels of Drs and Mtk genes and found Drs to be significantly higher in the selected populations compared to the controls (F = 37.019, p = 0.014; Figure 5a) with large effect size (ω2 = 0.811); while Mtk levels were comparable (F = 4.875, p = 0.119; Figure 5b). The relative transcript levels of Def-an AMP that targets gram positive bacteria (Imler and Bulet, 2005) and involved in tumor cell death (Parvy et al., 2019) were non significantly lower (F = 4.437, p = 0.259) in the selected compared to control populations (Figure 5c). However, the effect size was medium (ω2 = 0.462). Of the four tested antimicrobial peptides that target gram-negative bacteria, CecA1 (F = 1.784, p = 0.385; Figure 6b), AttA (F = 0.09, p = 0.792; Figure 6c) and Dpt (F = 2.476, p = 0.360; Figure 6d) were comparable. Further, Dro levels were significantly higher (F = 14.181, p = 0.026; Figure 6a) with large effect size (ω2 = 0.625) in the control populations compared to the selected populations.
Figure 5.
Selection for faster pre-adult development and extended reproductive lifespan leads to significantly higher levels of Drs. (a) Significantly increased expression of Drs in late L3 selected larvae than controls (F = 37.019, p = 0.014) with large effect size (ω2 = 0.811). (b) No significant difference (F = 1.03, p = 0.451) in mRNA expression of Mtk was observed in control and selected larvae. (c) No significant difference in Def transcript levels were observed (F = 4.438, p = 0.259). However, the effect size was medium (ω2 = 0.462). Welch’s ANOVA was performed following normality check using Shapiro-Wilks. Data are presented as mean ± SEM. P-value: ∗p < 0.05.
Figure 6.
Higher levels of Dro-a gram-negative targeting antimicrobial peptide in control population. (a) Significantly higher levels of Dro transcript in control than selected larvae (F = 14.181, p = 0.026) with effect size of 0.687. (b) Comparable CecA1 transcript level were seen in control and selected larvae (F = 1.784, p = 0.385). (c) Non-significantly higher (F = 0.092, p = 0.792) expression of AttA was observed in control than selected larvae. (d) Dpt transcript levels in selected and control population larvae were comparable (F = 2.476, p = 0.360). Welch’s ANOVA was performed following Shapiro-Wilks normality check t. Data are presented as mean ± SEM. P-value: ∗p ≈ 0.05.
4. Discussion
Maximization of fitness by evolutionary forces is under certain constraints due to limitations of resource acquisition (Van Noordwijk and de Jong, 1986) and the restricted pathways of resource allocation (Gadgil and Bossert, 1970), leading to trade-off among traits. Most extensively studied trade-offs in organisms are between survival and reproduction. Drosophila melanogaster has served as a wonderful model organism to unravel many details about the trade-offs. In a recently published paper Sharma and Shakarad (2021) reported a significant decline in reproduction among D. melanogaster populations that were under simultaneous selection for faster pre-adult development time and extended adult longevity. Interestingly, the longevity of the selected populations was comparable to that of control populations. The comparable longevity despite being under selection for extended longevity is a surprise in the light of several published studies. However, Kounatidis et al. (2017) reported a reduction in lifespan of D. melanogaster flies that had overexpression of AMPs. We hypothesize that the reduction in the ultimate fitness traits is an outcome of heightened immune activity in our selected populations. In this study, we compared the immune activity in fly populations selected for faster pre-adult development and extended longevity with their ancestral controls under unchallenged conditions. Circulating plasmatocyte density in the selected populations were non significantly higher (Figure 2a) compared to the control populations. Another study that selected populations for faster pre-adult development and early reproduction (FEJ) reported a significantly higher plasmatocyte count in the selected populations compared to their controls (JBs) (Dey et al., 2016). The ancestral control populations used in our study and Dey et al. (2016) are the same populations. The difference in the two selection regimens is in their adult life-history. The FEJs have an effective reproductive longevity of 3 days whereas FLJs have an effective reproductive longevity of ∼35 days. This differential adult longevity might be responsible for the difference in plasmatocyte counts; as trait correlations are suggested to span life-stage boundaries (Chippindale et al., 1996).
Redox homeostasis is crucial in maintaining the physiology in a dynamic yet equilibrium state. ROS are key molecules that are important in this redox homeostasis involving metabolism, growth and differentiation, and immunity (Shadel and Horvath, 2015). The ROS levels in the selected and control populations were comparable (Figure 2b), suggesting that the selected populations are maintaining the redox homeostasis.
Drosophila larvae are semi-aquatic in nature and reside within their food source-often over-ripe fruits and vegetables (and growth media in the laboratory cultures). The larval food source is also ideal growth media for many kinds of microorganisms. Cellular immune defense of Drosophila comprises of plasmatocytes, crystal cells and lamellocytes of which plasmatocytes constitute 95% of the hemocytes and are the primary sites of phagocytosis in the hemolymph. Plasmatocytes isolated from selected larvae showed a significantly higher percentage of phagocytosis compared to control populations (Figure 3a), suggesting that the larvae from selected populations were more efficient in clearing microorganisms and apoptotic cells from the hemolymph; as phagocytosis is a cellular process for ingesting and eliminating particles larger than 0.5μm in diameter, including microorganisms, foreign substances, and apoptotic cells (Uribe-Querol and Rosales, 2020). TEPs are required for efficient phagocytosis of bacteria, notably gram positive species (Dostálová et al., 2017) and eater and NimC1 are the two main receptors for phagocytosis of bacteria in Drosophila (Melcarne et al., 2019). The relative transcript levels of Tep3 (Figure 3c), eater (Figure 3d) and NimC1 (Figure 3e) were significantly higher in the selected populations compared to the controls. Taken together, our results clearly indicate that the selected populations have enhanced phagocytosis through upregulation of key genes so as to effectively maintain tissue homeostasis in the growing larvae. Further, phagocytosis has been suggested to be crucial for clearing the debris during tissue reorganization at the time of metamorphosis (Melcarne et al., 2019). Hence, selection for faster pre-adult development in the selected populations could be providing the selection pressure for increased phagocytosis, leading to a decline in adult lifespan, as trade-offs are suggested to span life-stage boundaries (Chippindale et al., 1996). Although selection for pre-adult development time was relaxed after 130 generations, the flies continued to emerge by 7.5 days; suggesting that the populations had stabilized for development time perhaps as a trade-off to continued selection on extended reproductive lifespan. It is also likely that traits that are preserved by multiple sources of selection could continue to respond even when one source of selection is eliminated, provided the traits under question have evolutionary advantage (Lahti et al., 2009). Increased reproductive longevity and progeny production are two adult life-history traits that are crucial but are consequent to completing pre-adult development and emerging as adults. Longevity is clearly dependent on health of an organism, and health itself is ensured by robust immune system that is energy expensive. While selection for increased reproductive lifespan could enhance the immune function, the enhanced immune function itself could be responsible for the lack of and or reversal of response to selection on extended lifespan; leading to comparable lifespan of selected and control populations (Sharma and Shakarad, 2021).
Crystal cells of Drosophila act as a sensor of physical and physiological stress and are primarily involved in melanization reactions (Dhankhar et al., 2021). A significantly higher crystal cell count was observed in late L3 female larvae of selected populations (Fig. 4a, b). An earlier study suggested a tissue specific regulation of hemocyte proliferation and differentiation by ecdysone through induction of division of hematopoietic progenitors in order to maintain a critical basal population of immature immune effector cells (Sorrentino et al., 2002). We have earlier reported a significant higher ecdysone level in the selected populations throughout the L3 stages (Chauhan et al., 2020). It is likely that the significantly higher crystal cell numbers in the selected populations is perhaps due to increased ecdysone levels. Prophenoloxidase (proPO) in the cytoplasm of crystal cells get converted into active form of phenoloxidase (PO) under stress conditions (Meister and Lagueux, 2003). Female late L3 larvae from selected populations showed marginal significantly higher phenoloxidase (PO) activity (Figure 4c) making us wonder why crystal cell number is upregulated but not PO activity. Contrary to our results, increased PO activity was reported in populations selected for faster pre-adult development and early reproduction (Dey et al., 2016). The difference in the results of the two studies might be due to the differences in the adult selection regime and or the difference in the pre-adult development duration as a consequence of differing number of generations of their respective selection regime. The selected populations used in Dey et al. (2016) study had been through 350 generations of selection for rapid development and early reproduction; while the selected populations used in the present study had been through 180 generations of selection for faster pre-adult development and extended reproductive longevity. The heightened yet mellowed down basal immune function might be helping our selected flies to maintain homeostasis. Corroborating this observation the transcript levels PPO2 were significantly higher in the selected populations compared to the controls (Figure 4d). PPO2 is accumulated in the crystals of crystal cells and provides a storage form for deployment at later stage, while PPO1 is involved in rapid early delivery of PO activity (Binggeli et al., 2014).
In Drosophila, activated PO leads to ROS production during melanin biosynthesis that acts as a signalling molecule for activation of other immune responses like encapsulation, nodule formation, phagocytosis, and AMP production (Cerenius et al., 2008; Tang, 2009; Dhankhar et al., 2021). Relative transcript levels of Drs-an anti-fungal AMP that is specifically expressed in salivary glands and trachea (Tzou et al., 2000) was significantly higher in the selected populations compared to the controls (Figure 5a), while transcript levels of Mtk-another antifungal AMP specifically expressed in antenno-maxillary organ, midgut and trachea (Tzou et al., 2000) were comparable (Figure 5b). Of the four AMPs involved in defense against gram negative bacteria, Dpt that is specifically expressed in the larval digestive tract (Tzou et al., 2000) showed non-significantly higher relative transcript levels in the selected populations (Figure 6d). However, Dro-an AMP that is specific to tracheal epithelia (Tzou et al., 2000) showed significantly higher transcript levels in the control populations (Figure 6a). Relative transcripts levels of two other genes- CecA1 (Figure 6b) and AttA (Figure 6c) that are known to be involved in defense against subset of fungal and gram-negative bacteria (Carboni et al., 2022) were non-significantly lower in the selected populations, and Def (Figure 5c)- an AMP specific to oral epithelia and provides defense against gram positive bacteria showed no significant difference. Several regulatory molecules involved in immune response have been reported to be up-regulated by ecdysone (Meister and Richards, 1996; Dimarcq et al., 1997; Tan et al., 2014). Earlier we have reported that selected populations used in this study had significantly higher ecdysone levels throughout their L3 stage compared to the controls (Chauhan et al., 2020). Indeed, it is likely that increased ecdysone levels are responsible for the heightened immune function in our selected populations. The enhanced immune activity through melanization, phagocytosis, redox homeostasis and upregulation of key AMPs responsible for defense against pathogenic microbes is likely to have contributed to comparable larval survival in the selected and control populations (Sharma et al., 2020; Shrivastava et al., 2022) as against reduced larval survival in the populations selected for faster pre-adult development and early reproduction (Prasad et al., 2000, 2001). In our opinion, this is the first extensive in vivo study that shows selection for faster pre-adult development and extended adult longevity in Drosophila melanogaster leads to enhanced immune activity in the larval stage, leading to comparable larval survival. Further, it is likely that the enhanced immune activity in the larvae is responsible for the reduction in life-time egg production as reported by Sharma and Shakarad (2021) and longevity comparable to control despite being under selection for extended adult longevity. Immune challenge is reported to be energetically costly in white cabbage butterfly- Pieris brassicae L. (Freitak et al., 2003). The immune system is a major physiological system that is an essential component of body maintenance through cellular renewal and repair (McDade, 2005) and hence is expected to trade off with other life-history traits according to theory (Rauw, 2012). In holometabolous insects such as Drosophila melanogaster, selection operating on one phase of the life cycle is likely to impinge on other and lead to trade-offs that span life-stage boundaries (Chippindale et al., 1996). Our selected populations seem to be doing all the running they can to be on par with the control populations, much like Lewis Carroll’s Alice Through the Looking Glass!
Declarations
Author contribution statement
Nidhi Krishna Shrivastava: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Namita Chauhan: Performed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Mallikarjun N. Shakarad: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
Dr. Mallikarjun N. Shakarad was supported by Institute of Eminence (IoE), University of Delhi [IoE/2021/12/FRP]. Nidhi Krishna Shrivastava was supported by University Grants Commission, Government of India [Ref. No. 19/06/2016(i)EU-V-346377]. Namita Chauhan was supported by Council for Scientific and Industrial Research, Government of India [Ref. No. 09/045(1391)-2015-EMR1].
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of interest’s statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgments
We thank Prof. N. G. Prasad, IISER, Mohali for his kind gift of GFP tagged Pe. We thank Prof. Namita Agrawal, Department of Zoology, University of Delhi and Prof. Amitabh Joshi, Jawaharlal Nehru Centre for Advance Scientific Research, Bengaluru for their valuable inputs in improving the manuscript. We would like to thank anonymous reviewers and editors for their valuable comments on earlier versions of the manuscript.
References
- Aguila J.R., Suszko J., Gibbs A.G., Hoshizaki D.K. The role of larval fat cells in adult Drosophila melanogaster. J. Exp. Biol. 2007;210:956–963. doi: 10.1242/jeb.001586. [DOI] [PubMed] [Google Scholar]
- Archer M.A., Phelan J.P., Beckman K.A., Rose M.R. Breakdown in correlations during laboratory evolution. II. Selection on stress resistance in Drosophila populations. Evolution. 2003;57:536–543. doi: 10.1111/j.0014-3820.2003.tb01545.x. [DOI] [PubMed] [Google Scholar]
- Baldal E.A., van der Linde K., van Alphen J.J.M., Brakefield P.M., Zwaan B.J. The effects of larval density on adult life-history traits in three species of Drosophila. Mech. Ageing Dev. 2005;126:407–416. doi: 10.1016/j.mad.2004.09.035. [DOI] [PubMed] [Google Scholar]
- Baldal E.A., Brakefield P.M., Zwaan B.J. Multitrait evolution in lines of Drosophila melanogaster selected for starvation resistance: the role of metabolic rate and implications for the evolution of longevity. Evolution. 2006;60(7):1435–1444. [PubMed] [Google Scholar]
- Bartlett L.J., Visher E., Haro Y., Roberts K.E., Boots M. The target of selection matters: an established resistance-development-time negative genetic trade-off is not found when selecting on development time. J. Evol. Bio. 2020;33:1109–1119. doi: 10.1111/jeb.13639. [DOI] [PubMed] [Google Scholar]
- Bell G., Koufopanou V. The cost of reproduction. Dawkins R., Ridley M., editors. Oxf. Surv. Evol. Biol. 1985;3:83–131. Oxford, U.K. [Google Scholar]
- Binggeli O., Neyen C., Poidevin M., Lemaitre B. Prophenoloxidase activation is required for survival to microbial infections in Drosophila. PLoS Pathog. 2014;10(5) doi: 10.1371/journal.ppat.1004067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boots M. The evolution of resistance to a parasite is determined by resources. Jukka Jokela, Ruth G.Shaw., editors. Am. Nat. 2011;178(2):214–220. doi: 10.1086/660833. [DOI] [PubMed] [Google Scholar]
- Brakefield P.M., Gems D., Cowen T., Christensen K., Grubeck-Loebenstein B., Keller L., Oeppen J., Rodriguez-Pena A., Stazi M.A., Tatar M., Westendorp R.G.J. What are the effects of maternal and pre-adult environments on ageing in humans, and are there lessons from animal models? Mech. Ageing Dev. 2005;126:431–438. doi: 10.1016/j.mad.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Braun A., Hoffmann J.A., Meister M. Analysis of the Drosophila host defense in domino mutant larvae, which are devoid of hemocytes. Proc. Natl. Acad. Sci. U.S.A. 1998;95:14337–14342. doi: 10.1073/pnas.95.24.14337. 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunt S., Hooley C., Hu N., Scahill C., Weavers H., Skaer H. Hemocyte-secreted type IV collagen enhances BMP signaling to guide renal tubule morphogenesis in Drosophila. Dev. Cell. 2010;19:296–306. doi: 10.1016/j.devcel.2010.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calow P. Ecology, evolution, and energetics: a study in metabolic adaptation. Adv. Ecol. Res. 1977;10:1–62. [Google Scholar]
- Carboni A.L., Hanso M.A., Lindsay S.A., Wasserman S.A., Lemaitre B. CecA1s contribute to Drosophila host defense against a subset of fungal and Gram-negative bacterial infection. Genetics. 2022;220(1):1–10. doi: 10.1093/genetics/iyab188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerenius L., Lee B.L., Soderhall K. The proPO-system: pros and cons for its role in invertebrate immunity. Trends Immunol. 2008;29(6):263–271. doi: 10.1016/j.it.2008.02.009. [DOI] [PubMed] [Google Scholar]
- Chandrashekara K.T., Shakarad M.N. Aloe vera or resveratrol supplementation in larval diet delays adult aging in the fruit fly, Drosophila melanogaster. J. Gerentol. A Biol. Sci. Med. Sci. 2011;66(9):965–971. doi: 10.1093/gerona/glr103. [DOI] [PubMed] [Google Scholar]
- Charlesworth B. In: Harrison D.E., editor. II. Telford Press; Caldwell, NJ: 1990. Natural Selection and Life History Patterns; pp. 21–40. Genetic effects on aging. [Google Scholar]
- Charlesworth B. Optimization models, quantitative genetics, and mutations. Evolution. 1990;44:520–538. doi: 10.1111/j.1558-5646.1990.tb05936.x. [DOI] [PubMed] [Google Scholar]
- Chauhan N., Shrivastava N.K., Agrawal N., Shakarad M.N. Wing patterning in faster developing Drosophila is associated with high ecdysone titer and wingless expression. Mech. Dev. 2020;163 doi: 10.1016/j.mod.2020.103626. [DOI] [PubMed] [Google Scholar]
- Chippindale A.K., Chu T.J.F., Rose M.R. Complex trade-offs and the evolution of starvation resistance in Drosophila melanogaster. Evolution. 1996;50(2):753–766. doi: 10.1111/j.1558-5646.1996.tb03885.x. [DOI] [PubMed] [Google Scholar]
- Chippindale A.K., Alipaz J.A., Chen H., Rose M.R. Experimental evolution of accelerated development in Drosophila. 1. Developmental speed and larval survival. Evolution. 1997;51:536–551. doi: 10.1111/j.1558-5646.1997.tb01477.x. [DOI] [PubMed] [Google Scholar]
- Chippindale A.K., Gibbs A.G., Sheik M., Yee K.J., Djawdan M., Bradley T.J., Rose M.R. Resource acquisition and the evolution of stress resistance in Drosophila melanogaster. Evolution. 1998;52:1342–1352. doi: 10.1111/j.1558-5646.1998.tb02016.x. [DOI] [PubMed] [Google Scholar]
- Costa A., Jan E., Sarnow P., Schneider D. The Imd pathway is involved in antiviral immune responses in Drosophila. PLoS One. 2009;4 doi: 10.1371/journal.pone.0007436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crozatier M., Meister M. Drosophila haematopoiesis. Cell Microbiol. 2007 May;9(5):1117–1126. doi: 10.1111/j.1462-5822.2007.00930.x. PMID: 17394559. [DOI] [PubMed] [Google Scholar]
- Dey P., Mendiratta K., Bose J., Joshi A. Enhancement of larval immune system traits as a correlated response to selection for rapid development in Drosophila melanogaster. J. Genet. 2016;95:719–723. doi: 10.1007/s12041-016-0659-5. [DOI] [PubMed] [Google Scholar]
- Dhankhar J., Agrawal N., Shrivastava A. Pan-neuronal expression of human mutant Huntingtin in Drosophila impairs immune response of hemocytes. J. Neuroimmunol. 2021;363 doi: 10.1016/j.jneuroim.2021.577801. [DOI] [PubMed] [Google Scholar]
- Dimarcq J.-L., Imler J.-L., Lanot R., Ezekiwits R.A.B., Hoffmann J.A., Janeway C.A., Lagueux M. Treatment of 1(2)mbn Drosophila tumorous blood cells with the steroid hormone ecdysone amplifies the inducibility of antimicrobial peptide gene expression. Insect Biochem. Mol. Biol. 1997;27(10):877–886. doi: 10.1016/s0965-1748(97)00072-6. [DOI] [PubMed] [Google Scholar]
- Dostalova A., Rommelaere S., Poidevin M., Lemaitre B. Thioester-containing proteins regulate the Toll pathway and play a role in Drosophila defense against microbial pathogens and parasitoid wasps. BMC Biol. 2017;15(1):79. doi: 10.1186/s12915-017-0408-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Force A.G., Staples T., Soliman S., Arking R. Comparative biochemical and stress analysis of genetically selected Drosophila strains with different longevities. Dev. Genet. 1995;17:340–351. doi: 10.1002/dvg.1020170407. [DOI] [PubMed] [Google Scholar]
- Franceschi C., Capri M., Monti D., Giunta S., Olivieri F., Sevini F., Panourgia M.P., Invidia L., Celani L., Scurti M., Cevenini E., Castellani G.C., Salvioli S. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech. Ageing Dev. 2007;128:92–105. doi: 10.1016/j.mad.2006.11.016. [DOI] [PubMed] [Google Scholar]
- Freitak D., Ots I., Vanatoa A., Hõrak P. Immune response is energetically costly in white cabbage butterfly pupae. Proc. Roy. Soc. Lond. B. 2003;270(Suppl.):S220–S222. doi: 10.1098/rsbl.2003.0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadgil M., Bossert W.H. Life historical consequences of natural selection. Am. Nat. 1970;104:1–24. [Google Scholar]
- Glass G.V., Peckham P.D., Sanders J.R. Consequences of failure to meet assumptions underlying the fixed effects analysis of variance and covariance. Rev. Educ. Res. 1972;42(3):237–288. [Google Scholar]
- Grandison R.C., Piper M.D.W., Partridge L. Amino-acid imbalance explains extension of lifespan by dietary restriction in Drosophila. Nature. 2009;462:1061–1064. doi: 10.1038/nature08619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grogorian M., Hartenstine V. Hematopoiesis and hematopoietic organs in arthropods. Dev. Gene. Evol. 2013;223:103–115. doi: 10.1007/s00427-012-0428-2. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa J., Chanderashekhra K.T., Kashyap K., Sageena G., Shakarad M. Gender based disruptive selection maintains body size polymorphism in Drosophila melanogaster. J. Biosci. 2014;39:609–620. doi: 10.1007/s12038-014-9452-x. 2014. [DOI] [PubMed] [Google Scholar]
- Hanson M.A., Lemaître B., Unckless R.L. Dynamic evolution of antimicrobial peptides underscores trade-offs between immunity and ecological fitness. Front. Immunol. 2019;10:2620. doi: 10.3389/fimmu.2019.02620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imler J.L., Bulet P. Antimicrobial peptides in Drosophila: structures, activities and gene regulation. Chem. Immunol. Allergy. 2005;86:1–21. doi: 10.1159/000086648. [DOI] [PubMed] [Google Scholar]
- Kirkwood T.B.L. Evolution of ageing. Nature. 1977;270:301–304. doi: 10.1038/270301a0. [DOI] [PubMed] [Google Scholar]
- Kirkwood T.B.L., Holliday R. The evolution of ageing and longevity. Proc. Roy. Soc. Lond. B. 1979;205:531–546. doi: 10.1098/rspb.1979.0083. [DOI] [PubMed] [Google Scholar]
- Klepsatel P., Prochazka E., Galikova M. Crowding of Drosophila larvae affects lifespan and other life-history traits via reduced availability of dietary yeast. Exp. Gerontol. 2018;110:298–308. doi: 10.1016/j.exger.2018.06.016. [DOI] [PubMed] [Google Scholar]
- Kounatidis I., Chtarbanova S., Cao Y., Hayne M., Jayanth D., Ganetzky B., Ligoxygakis P. NF-ĸB immunity in the brain determines fly lifespan in healthy aging and age-related neurodegeneration. Cell Rep. 2017;19:836–848. doi: 10.1016/j.celrep.2017.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutner M.H., Nachtsheim C.J., Neter J., Li W. Applied Linear Statistical Models. 5th Edition. McGraw-Hill Irwin; Boston, MA, USA: 2005. [Google Scholar]
- Lahti D.C., Johnson N.A., Ajie B.C., Otto S.P., Hendry A.P., Blumstein D.T., Coss R.G., Donohue K., Foster S.A. Relaxed selection in the wild. Trends Ecol. Evol. 2009 Sep;24(9):487–496. doi: 10.1016/j.tree.2009.03.010. [DOI] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real time quantitative PCR and the 2-ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Loch G., Zinke I., Mori T., Carrera P., Schroer J., Takeyama H., Hoch M. Antimicrobial peptides extend lifespan in Drosophila. PLoS One. 2017;12(5) doi: 10.1371/journal.pone.0176689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDade T.W. Lifehistory, maintenance, and the early origins of immune function. Am. J. Hum. Biol. 2005;17:81–94. doi: 10.1002/ajhb.20095. [DOI] [PubMed] [Google Scholar]
- Meister M., Lagueux M. Drosophila blood cells. Cell Microbiol. 2003;5(9):573–580. doi: 10.1046/j.1462-5822.2003.00302.x. [DOI] [PubMed] [Google Scholar]
- Meister M., Richards G. Ecdysone and insect immunity: the maturation of the inducibility of the Dpt gene in Drosophila larvae. Insect Biochem. Mol. Biol. 1996;26(2):155–160. doi: 10.1016/0965-1748(95)00076-3. [DOI] [PubMed] [Google Scholar]
- Melcarne C., Ramond E., Dudzic J., Bretscher A.J., Kurucz É., Andó I., Lemaître B. Two Nimrod receptors, NimC1 and Eater, synergistically contribute to bacterial phagocytosis in Drosophila melanogaster. FEBS J. 2019;286:2670–2691. doi: 10.1111/febs.14857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Microsoft Corporation Microsoft Excel. 2018. https://office.microsoft.com/excel Available at:
- Min K.J., Hogan M.F., Tatar M., O’Brien D.M. Resource allocation to reproduction and soma in Drosophila: a stable isotope analysis of carbon from dietary sugar. J. Insect Physiol. 2006;52:763–770. doi: 10.1016/j.jinsphys.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Nesse M., Williams G.C. In: Evolution and Healing. Nesse M., Williams G.C., editors. Weidenfeld and Nicolson; London: 1995. Evolution by natural selection; pp. 13–25. [Google Scholar]
- Norry F.M., Loeschcke V. Heat-induced expression of a molecular chaperone decreases by selecting for long-lived individuals. Exp. Gerontol. 2003;38:673–681. doi: 10.1016/s0531-5565(03)00057-3. [DOI] [PubMed] [Google Scholar]
- Novoseltsev V.N., Arking R., Novoseltsev J.A., Yashin A.I. Evolutionary optimality applied to Drososphila experiments: hypothesis of constrained reproductive efficiency. Evolution. 2002;56(6):1136–1149. doi: 10.1111/j.0014-3820.2002.tb01427.x. [DOI] [PubMed] [Google Scholar]
- Nunney L. The response to selection for fast larval development in Drosophila melanogaster and its effect on adult weight: an example of a fitness trade-off. Evolution. 1996;50:1193–1204. doi: 10.1111/j.1558-5646.1996.tb02360.x. [DOI] [PubMed] [Google Scholar]
- Parker G.A., Maynard Smith J. Optimality theory in evolutionary biology. Nature. 1990;348:27–33. [Google Scholar]
- Partridge L., Harvey P.H. The ecological context of life history evolution. Science. 1988;241:1449–1454. doi: 10.1126/science.241.4872.1449. [DOI] [PubMed] [Google Scholar]
- Partridge L., Sibly R. Constraints in the evolution of life histories. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1991;332:3–13. [Google Scholar]
- Parvy J.-P., Yu Y., Dostalova A., Kondo S., Kurjan A., Bulet P., Lemaître B., Vidal M., Cordaro J.B. The antimicrobial peptide Def cooperates with tumour necrosis factor to drive tumour cell death in Drosophila. Elife. 2019;8 doi: 10.7554/eLife.45061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor-Pareja J.C., Wu M., Xu T. An innate immune response of blood cells to tumors and tissue damage in Drosophila. Dis Model Mech. 2008;1:144–154. doi: 10.1242/dmm.000950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisi F., Stefanatos R.K., Strathdee K., Yu Y., Vidal M. Transformed epithelia trigger non-tissue-autonomous tumor suppressor response by adipocytes via activation of Toll and Eiger/TNF signaling. Cell Rep. 2014;6:855–867. doi: 10.1016/j.celrep.2014.01.039. [DOI] [PubMed] [Google Scholar]
- Phelan J.P., Archer M.A., Beckman K.A., Chippindale A.K., Nusbaum T.J., Rose M.R. Breakdown in correlations during laboratory evolution. I. Comparative analyses of Drosophila populations. Evolution. 2003;57:527–535. doi: 10.1111/j.0014-3820.2003.tb01544.x. [DOI] [PubMed] [Google Scholar]
- Prasad N.G., Shakarad M., Anitha D., Rajamani M., Joshi A. Correlated responses to selection for faster development and early reproduction in Drosophila: the evolution of larval traits. Evolution. 2001;55:1363–1372. doi: 10.1111/j.0014-3820.2001.tb00658.x. [DOI] [PubMed] [Google Scholar]
- Prasad N.G., Shakarad M., Gohil V.M., Sheeba V., Rajamani M., Joshi A. Evolution of reduced pre-adult viability and larval growth rate in laboratory populations of Drosophila melanogaster selected for shorter development time. Genet. Res. 2000;76:249–259. doi: 10.1017/s0016672300004754. [DOI] [PubMed] [Google Scholar]
- Rajamani M., Raghanvendra N., Prasad N.G., Archana N., Joshi A., Shakarad M. Reduced larval feeding rate is a strong evolutionary correlate of rapid development in Drosophila melanogaster. J. Genet. 2006;85(3):209–212. doi: 10.1007/BF02935333. [DOI] [PubMed] [Google Scholar]
- Ratheesh A., Belyaeva V., Siekhus D.R. Drosophila immune cell migration and adhesion during embryonic development and larval immune responses. Curr. Opin. Cell Biol. 2015;36:71–79. doi: 10.1016/j.ceb.2015.07.003. [DOI] [PubMed] [Google Scholar]
- Rauw W.M. Immune response from a resource allocation prespective. Front. Genet. 2012;3:267. doi: 10.3389/fgene.2012.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis T. Effects of synthetic diets enriched in specific nutrients on Drosophila development, body fat, and lifespan. PLoS One. 2016;11:1–12. doi: 10.1371/journal.pone.0146758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznick D. Costs of reproduction: an evaluation of the empirical evidence. Oikos. 1985;44:257–267. [Google Scholar]
- Rizki T.M., Rizki R.M., Grell E.H. A mutant affecting the crystal cells in Drosophila melanogaster. Wilhelm Roux's Arch. Dev. Biol. 1980;188(2):91–99. doi: 10.1007/BF00848799. [DOI] [PubMed] [Google Scholar]
- Robinson S.J.W., Partridge L. Temperature and clinical variation in larval growth efficiency in Drosophila melanogaster. J. Evol. Biol. 2001;14:14–21. doi: 10.1046/j.1420-9101.2001.00259.x. [DOI] [PubMed] [Google Scholar]
- Rodrigues M.A., Martins N.E., Bakance L.F., Broom L.N., Dias A.J.S., Fernandes A.S.D., Rodrigues F., Sucena E., Mirth C.K. Drosophila melanogaster larvae make nutritional choices that minimize developmental time. J. Insect Physiol. 2015;81:69–80. doi: 10.1016/j.jinsphys.2015.07.002. [DOI] [PubMed] [Google Scholar]
- Rose M.R., Vu L.N., Park S.U., Graves J.L. Selection on stress resistance increases longevity in Drosophila melanogaster. Exp. Gerontol. 1992;27:241–250. doi: 10.1016/0531-5565(92)90048-5. [DOI] [PubMed] [Google Scholar]
- Salmon A.B., Marx D.B., Harshman L.G. A cost of reproduction in Drosophila melanogaster: stress susceptibility. Evolution. 2001;55(8):1600–1608. doi: 10.1111/j.0014-3820.2001.tb00679.x. [DOI] [PubMed] [Google Scholar]
- Sears H.C., Kennedy C.J., Garrity P.A. Macrophage-mediated corpse engulfment is required for normal Drosophila CNS morphogenesis. Development. 2003;130:3557–3565. doi: 10.1242/dev.00586. [DOI] [PubMed] [Google Scholar]
- Service P.M., Hutchinson E.W., MacKinley M.D., Rose M.R. Resistance to environmental stress in Drosophila melanogaster selected for postponed senescence. Physiol. Zool. 1985;58:380–389. [Google Scholar]
- Shadel G.S., Horvath T.L. Mitochondrial ROS signaling in organismal homeostasis. Cell. 2015;163:560–569. doi: 10.1016/j.cell.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma K., Mishra N., Shakarad M.N. Evolution of reduced minimum critical size as a response to selection for rapid pr-adult development in Drosophila melanogaster. R. Soc. Open Sci. 2020;7 doi: 10.1098/rsos.191910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma K., Shakarad M.N. Fitness consequences of biochemical adaptation in Drosophila melanogaster populations under simultaneous selection for faster pre-adult development and extended lifespan. Sci. Rep. 2021;11 doi: 10.1038/s41598-021-95951-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoi V.N., Prasad N.G. Local adaptation to developmental density does not lead to higher mating success in Drosophila melanogaster. J. Evol. Biol. 2016;29(10):2036–2042. doi: 10.1111/jeb.12927. [DOI] [PubMed] [Google Scholar]
- Shrivastava N.K., Farand A.K., Shakarad M.N. Long-term selection for faster development and early reproduction leads to up-regulation of genes involved in redox homeostasis. Adv. Redox Res. 2022 [Google Scholar]
- Singh K., Kochar E., Ghalot P., Bhatt K., Prasad N.G. Evolution of reproductive traits have no apparent life-history associated cost in populations of Drosophila melanogaster selected for cold shock resistance. BMC Ecol. Evol. 2021;21:219. doi: 10.1186/s12862-021-01934-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrentino R.P., Carton Y., Govind S. Cellular immune response to parasite infection in the Drosophila lymph gland is developmentally regulated. Dev. Biol. 2002;243:65–80. doi: 10.1006/dbio.2001.0542. [DOI] [PubMed] [Google Scholar]
- Stefana M.I., Driscoll P.C., Obata F., Pengelly A.R., Newell C.L., MacRae J.I., Gould A.P. Developmental diet regulates Drosophila lifespan via lipid autotoxins. Nat. Commun. 2017;8:1384. doi: 10.1038/s41467-017-01740-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan K.L., Vlisidou I., Wood W. Ecdysone mediates the development of immunity in the Drosophila embryo. Curr. Biol. 2014;24:1145–1152. doi: 10.1016/j.cub.2014.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H. Regulation and function of the melanization reaction in Drosophila. Fly. 2009;3(1):105–111. doi: 10.4161/fly.3.1.7747. [DOI] [PubMed] [Google Scholar]
- Tu M.P., Tatar M. Juvenile diet restriction and the aging and reproduction of adult Drosophila melanogaster. Aging Cell. 2003;2:327–333. doi: 10.1046/j.1474-9728.2003.00064.x. [DOI] [PubMed] [Google Scholar]
- Tzou P., Ohresser S., Ferrandon D., Capovilla M., Reichhart J.M., Lemaitre B., Hoffmann J.A., Imler J.L. Tissue-specific inducible expression of antimicrobial peptide genes in Drosophila surface epithelia. Immunity. 2000;13(5):737—748. doi: 10.1016/s1074-7613(00)00072-8. [DOI] [PubMed] [Google Scholar]
- Uribe-Querol E., Rosales C. Phagocytosis: our current understanding of a universal biological process. Front. Immunol. 2020;11:1066. doi: 10.3389/fimmu.2020.01066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Noordwijk A.J., de Jong G. Acquisition and allocation of resources: their influence on variation in life history tactics. Am. Nat. 1986;128:137–142. [Google Scholar]
- Vlisidou I., Wood W. Drosophila blood cells and their role in immune responses. FEBS J. 2015;2015 doi: 10.1111/febs.13235. [DOI] [PubMed] [Google Scholar]
- Williams G.C. Natural selection, the cost of reproduction and a refinement of Lack’s principle. Am. Nat. 1966;100:687–690. [Google Scholar]
- Woodcock K.J., Kierdorf K., Pouchelon C.A., Vivancos V., Dionne M.S., Geissmann F. Macrophage-derived upd3 cytokine causes impaired glucose homeostasis and reduced lifespan in Drosophila fed a lipid-rich diet. Immunity. 2014 doi: 10.1016/j.immuni.2014.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H.W., Zhou D., Haddad G.G. Antimicrobial peptides increase tolerance to oxidant stress in Drosophila melanogaster. J. Biol. Chem. 2010;286(8):6211–6218. doi: 10.1074/jbc.M110.181206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajitschek F., Georgolopoulos G., Vourlou A., Ericsson M., Zajitschek S.R.K., Friberg U., Maklakov A.A. Evolution under dietary restriction decouples survival from fecundity in Drosophila melanogaster females. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2019;74:1542–1548. doi: 10.1093/gerona/gly070. [DOI] [PubMed] [Google Scholar]
- Zar J.H. fourth ed. Pearson Education, Inc. and Dorling Kindersley Publishing Inc.; Noida, India: 2011. Biostatistical Analysis. [Google Scholar]
- Zhou L., Hashimi H., Schwartz L.M., Nambu J.R. Programmed cell death in the Drosophila central nervous system midline. Curr. Biol. 1995;5:784–790. doi: 10.1016/s0960-9822(95)00155-2. [DOI] [PubMed] [Google Scholar]
- Zwaan B.J. Artificial selection for developmental time in Drosophila melanogaster in relation to the evolution of aging: direct and correlated responses. Evolution. 1995;49:635–648. doi: 10.1111/j.1558-5646.1995.tb02300.x. [DOI] [PubMed] [Google Scholar]
- Zwaan B.J. Linking development and aging. Sci. Aging Knowl. Environ. 2003;2003(47):32. doi: 10.1126/sageke.2003.47.pe32. [DOI] [PubMed] [Google Scholar]
- Zwaan B., Bijlsma R., Hoekstra R.F. Artificial selection for developmental time in D. melanogaster in relation to the evolution of ageing: direct and correlated responses. Evolution. 2013;49:635–648. doi: 10.1111/j.1558-5646.1995.tb02300.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supp. material/referenced in article.