ABSTRACT
Background
Choline and betaine intakes have been related to cardiovascular health.
Objectives
We aimed to explore the relation between 1-y changes in dietary intake of choline or betaine and 1-y changes in cardiometabolic and renal function traits within the frame of the PREDIMED (PREvención con DIeta MEDiterránea)-Plus trial.
Methods
We used baseline and 1-y follow-up data from 5613 participants (48.2% female and 51.8% male; mean ± SD age: 65.01 ± 4.91 y) to assess cardiometabolic traits, and 3367 participants to assess renal function, of the Spanish PREDIMED-Plus trial. Participants met ≥3 criteria of metabolic syndrome and had overweight or obesity [BMI (in kg/m2) ≥27 and ≤40]. These criteria were similar to those of the PREDIMED parent study. Dietary intakes of choline and betaine were estimated from the FFQ.
Results
The greatest 1-y increase in dietary choline or betaine intake (quartile 4) was associated with improved serum glucose concentrations (−3.39 and −2.72 mg/dL for choline and betaine, respectively) and HbA1c levels (−0.10% for quartile 4 of either choline or betaine intake increase). Other significant changes associated with the greatest increase in choline or betaine intake were reduced body weight (−2.93 and −2.78 kg, respectively), BMI (−1.05 and −0.99, respectively), waist circumference (−3.37 and −3.26 cm, respectively), total cholesterol (−4.74 and −4.52 mg/dL, respectively), and LDL cholesterol (−4.30 and −4.16 mg/dL, respectively). Urine creatinine was reduced in quartile 4 of 1-y increase in choline or betaine intake (−5.42 and −5.74 mg/dL, respectively).
Conclusions
Increases in dietary choline or betaine intakes were longitudinally related to improvements in cardiometabolic parameters. Markers of renal function were also slightly improved, and they require further investigation.
This trial was registered at https://www.isrctn.com/ as ISRCTN89898870.
Keywords: choline, betaine, Mediterranean diet, cardiovascular disease risk, cardiometabolic parameters, renal variables
Introduction
Choline has been recognized as an essential nutrient since 1998 (1). Still, the average consumption in the population is below the recommended intake (2, 3). High-choline-content foods include fish, eggs, and meat, and it also can be found in dairy products, cruciferous vegetables, legumes, and nuts (3).
Choline can be metabolized by 4 main pathways (4). One involves the synthesis of the neurotransmitter acetylcholine in the cytosol of presynaptic cholinergic neurons. The second is the production of phosphatidylcholine, the most abundant phospholipid in the body. The third involves its oxidation to generate betaine. Betaine is a methyl group donor involved in the remethylation of homocysteine to produce methionine. Lastly, choline can be metabolized in the intestine before being absorbed. Gut microbiota can produce trimethylamine (TMA) from choline. TMA is then absorbed, and it is metabolized to trimethylamine-N-oxide (TMAO) in the liver by flavin monooxygenase (FMO) enzymes, particularly FMO3. This same pathway is also followed by dietary betaine, contributing to TMA production in the gut by the microbiota (5).
High plasma TMAO concentrations may predict cardiovascular events, even when other cardiovascular biomarkers are not altered (6). Although several studies show an association between greater plasma TMAO concentrations and CVD risk (7–9), greater plasma TMAO concentrations can also occur when following a healthy diet, rich in foods known to be high in choline and betaine (10) and protective against CVD.
Different studies have observed that greater choline and betaine intakes were associated with lower type 2 diabetes (T2D) risk (11–14). However, this relation has not been confirmed in other studies (15).
The Mediterranean diet (MedDiet) is rich in fiber (from fruits, vegetables, and legumes) and has been shown to reduce CVD risk (16). Furthermore, it has been observed that choline intake is the lowest in Mediterranean countries, compared with northern European countries, where a meat-rich diet such as the Western diet is usually consumed (17). Furthermore, consumption of a MedDiet has been associated with lower TMAO urine concentrations (18, 19) after 3 y of follow-up in the PREDIMED (PREvención con DIeta MEDiterránea; PREvention with MEDiterranean Diet) cohort.
The association between choline and betaine intakes and cardiometabolic health is still under debate. Previous longitudinal studies have examined the association of choline or betaine intake with body composition (20, 21), T2D (15), DNA methylation (22), and TMAO production (23). However, these were prospective and observational studies in infants and adult populations and did not look at cardiometabolic health traits in elder populations with metabolic syndrome (MetS). For this reason, we aimed to analyze the association between choline or betaine intake and cardiometabolic and anthropometric parameters in a longitudinal 1-y follow-up interventional study, within the frame of the PREDIMED-Plus study.
Methods
Study population
The PREDIMED-Plus trial (ISRCTN89898870) is an ongoing 6-y, multicenter, controlled, randomized, parallel-group, and primary prevention trial conducted in 23 research centers of Spain that started in October 2013. The trial aims to assess the effect of an intensive weight loss intervention program based on an energy-restricted traditional MedDiet (er-MedDiet), physical activity (PA) promotion, and behavioral support on hard cardiovascular events (cardiovascular mortality, acute myocardial infarction, or stroke), in comparison with usual dietary counseling with an energy-unrestricted MedDiet without PA promotion (control group). The study protocol, including study design and data collection, has been published (24, 25) and can be found at the PREDIMED-Plus website (https://www.predimedplus.com/en/). Eligible participants were community-dwelling males (55–75 y old) and females (60–75 y old) with overweight or obesity (BMI ≥27 and ≤40 kg/m2) and MetS, according to the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity criteria (26). History of prevalent CVD, cancer, immunodeficiency or HIV-positive status, cirrhosis or liver failure, psychiatric disorders, any disease with <24 mo life expectancy, or acute infection or inflammation were exclusion criteria. Participants with T2D were recruited with a limit of 25% of the total population.
The trial was approved according to the ethical standards of the Declaration of Helsinki by the Institutional Review Boards (IRBs) of all participating centers. All participants provided written informed consent for their participation in the study. The trial was retrospectively registered at the International Standard Randomized Controlled Trial Registry as ISRCTN89898870 on 24 July, 2014.
Study sample
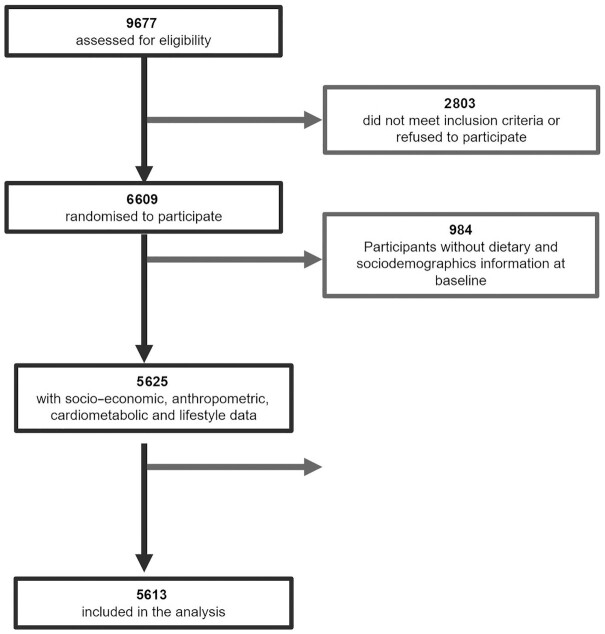
The PREDIMED-Plus trial recruited 6874 participants between October 2013 and December 2016. Baseline and 1-y data from the PREDIMED-Plus study data set dated 22 December, 2020 were used. Participants with missing values for energy intake and dietary intake at baseline and after 1 y of follow-up were excluded (n = 265 and n = 696 participants excluded for missing values at baseline and after 1 y, respectively). We also excluded participants with missing values for lifestyle variables at baseline (n = 288). Then, we imputed missing values for these covariates at 1 y and used the remaining sample. Missing values for covariates were imputed using a multivariable imputation model based on a regression using baseline data for the imputed variables as well as sociodemographic variables as imputing variables. Participants with implausible energy intakes (<500 or >3500 kcal for females and <800 or >4000 kcal for males) at baseline (n = 0) and after 1 y (n = 12) were also excluded (27). The final analytic sample included data from 5613 participants (48.2% females, mean ± SD age: 65.01 ± 4.91 y) (Figure 1) and 30.4% of participants had T2D. For renal function analysis, 3367 participants were included, after excluding participants with urine albumin:creatinine ratio (UACR) >500 to avoid influences for outliers and participants with diminished renal function, as previously described by Díaz-López et al. (28).
FIGURE 1.
Participant flowchart.
Assessment of choline/betaine intake
Food intake was assessed with a 143-item FFQ validated for the Spanish population administered by a trained dietitian in face-to-face interviews (29). Participants were asked to define the frequency of consumption of each food item within a usual year. Nine options for frequency of consumption were given, ranging from “never or hardly ever” to “more than six times a day.” Food intake (g/d) was then estimated by multiplying the portion size by the frequency of consumption and dividing by 7 or by 30 for weekly or monthly consumed food items, respectively. Nutrient estimates were calculated from all items in the FFQ responses that contributed to nutrient intake, by using Spanish food composition tables (30).
To estimate choline/betaine intake, we used USDA databases from 2015–2018 and the website https://nutritiondata.self.com/ and multiplied grams per day of each food item by the amount of choline/betaine per gram of food. Assessment of choline intake through an FFQ has been previously validated in the Framingham Offspring Study (31).
Cardiometabolic, renal function, and anthropometric parameters
Cardiometabolic parameters analyzed included plasma total cholesterol, LDL cholesterol, HDL cholesterol, TGs, and glucose concentrations, and HbA1c levels. The renal function parameters analyzed included serum creatinine and uric acid and urine creatinine and albumin:creatinine ratio. These metabolites were measured with standard analytic procedures in accredited biochemistry analytics laboratories after an overnight fast. LDL cholesterol was calculated with the Friedewald formula. Estimated glomerular filtration rate (eGFR) was calculated following the formula previously described (32). Briefly, eGFR was indirectly estimated by using serum creatinine values and implementing them in the Chronic Kidney Disease Epidemiology Collaboration equation for Caucasian individuals.
Weight and height were measured with standard calibrated scales and a wall-mounted stadiometer, respectively. BMI was calculated as the weight in kilograms divided by the square of height in meters (kg/m2). Waist and hip perimeters were measured with an anthropometric tape. Waist circumference was measured midway between the lowest rib and the iliac crest. Trained dietitians collected anthropometric measures with the participants in light clothing with no shoes or accessories and in duplicate. The mean of the 2 measurements was used in all cases.
Blood pressure (BP) was measured in triplicate with an Omron HEM‐705CP with participants in a seated position after 5 min rest and with a 1-min rest between measurements.
Covariate variables included lifestyle and sociodemographic variables. Trained dietitians administered a general questionnaire in face-to-face visits to collect data about sex; age (continuous); civil status (married or living alone); years of education (continuous); smoking habits (current smoker, former smoker, or never smoker); prevalence of hypertension, T2D, and depression; and regular use of medication.
Lifestyle parameters included the adherence to an er-MedDiet assessed by the 17-item questionnaire developed and validated for the PREDIMED-Plus study (33), PA level assessed by the REGICOR questionnaire (34), and sedentary lifestyle assessed by the Nurses’ Health Study (NHS) questionnaire (35). Participants were classified according to their PA level as sedentary, moderately active, or active.
Statistical analysis
Data analysis was conducted using R version 4.04 (R Foundation for Statistical Computing). The database used for this analysis was 202012220958_PREDIMEDplus_2anys_2020-12-22. Participants were classified according to quartiles of choline or betaine intake, adjusted by residuals of energy intake.
For each variable, data shown in tables are presented as mean ± SD or percentages. Missing values for covariates were imputed using a multivariable regression imputation method with the mice R package. Statistically significant differences in general characteristics at baseline (P < 0.05) among quartiles of choline/betaine consumption were compared using a 1-factor ANOVA or chi-square test. Significant differences in cardiometabolic, renal, and anthropometric parameters among choline/betaine consumption quartiles at baseline were compared by 1-factor ANCOVA test adjusted by age, sex, study center, energy intake, smoking habits, adherence to an er-MedDiet, sedentary lifestyle, T2D prevalence, and medication for diabetes, hypertension, high cholesterol concentrations, heart conditions, and depression.
For the longitudinal analysis, participants were classified according to quartiles of 1-y changes in choline/betaine intake adjusted by energy residuals. Statistically significant differences between quartiles of 1-y changes in choline/betaine consumption for 1-y changes in cardiometabolic, renal, and anthropometric parameters were assessed by 1-factor ANCOVA test. Three adjustment models were applied. The minimal adjustment model 1 included age, sex, and center as covariates. Adjustment model 2 included those covariates from model 1 plus civil status, education, smoking habits, sedentary lifestyle, and choline or betaine intake at baseline. Finally, adjustment model 3 included all covariates from model 2 plus energy intake and changes in energy intake, adherence to an er-MedDiet and changes in adherence to an er-MedDiet, and PA and changes in PA. We also calculated the P for linear trend using the quartile median values of changes in choline or betaine intake as a continuous independent variable in a linear regression test adjusted by age, sex, center, baseline BMI, and baseline energy intake. Sensitivity analyses in addition adjusting for consumption of food groups (baseline consumption of fruits, vegetables, animal protein, fish, eggs, red meat, and dairy products and change in the consumption of these food groups after 1 y of follow-up), nutrients (baseline intake of carbohydrates, fiber, saturated fats, and unsaturated fats and changes in these nutrients’ intake after 1 y of follow-up), medication (lipid-lowering drugs, antihypertensive drugs, antidiabetic medication, and heart medication), and body weight and BMI (baseline and 1-y change) were carried out. Each food group, nutrient, and medication was added alone to the full adjustment model and 1 adjustment was made including all foods, nutrients, or medications.
Sensitivity analyses were made for renal function parameters by excluding participants with T2D.
The association between a 20-mg (approximately the mean choline content of 60 g dry legumes) change in choline/betaine consumption (independent or “predictor” variable) and cardiometabolic, renal, and anthropometric variables (dependent or “outcome” variables) was analyzed with linear regression models using 2 adjustments. The minimal adjustment model or model 1 included age, gender, choline/betaine intake at baseline, and study center as cofactors. The complete adjustment model or model 2 included schooling (y), civil status, smoking habits, sedentary lifestyle, PA and difference in PA, adherence to an er-MedDiet and difference in adherence to an er-MedDiet, and energy intake and difference in energy intake (1 y–baseline), in addition to the cofactors included in model 1.
To determine to what extent different food sources contributed to the individual variability in the differences in choline or betaine intake after 1-y follow-up, stepwise regression was performed. Dietary choline and betaine intakes were adjusted by energy residuals.
Results
Baseline characteristics
The baseline characteristics of the 5613 participants are shown in Tables 1 and 2 according to quartiles of baseline choline (Table 1) and betaine (Table 2) intake, adjusted by energy residuals. Based on the US Institute of Medicine guidelines, choline intake was considered low when the consumption of choline was <400 mg/d, adequate for 400–600 mg/d, and high when intake was >600 mg/d (1). Average choline intake in quartile 1 was 359.95 ± 37.66 mg/d, and the intake range in this quartile was 128.0–404.7 mg/d, meaning that most of the participants in quartile 1 did not meet the choline adequate intake (Table 1) and the rest of the participants presented an adequate intake. Subjects with greater choline intake were mainly females, older, and nonsmokers. They showed greater adherence to an er-MedDiet. Furthermore, there were more participants with T2D among the greatest choline intake group, and consequently, there were more participants in quartile 4 of choline intake receiving antidiabetic medication. Although depression was not more frequent in this quartile, there were more participants receiving medication for depression in quartiles 3 and 4 of choline intake (Table 1). Whereas, participants with greater betaine intake showed greater hypertension and T2D prevalence but lower adherence to an er-MedDiet (Table 2). Medication for T2D was also more frequent in quartile 4, but not medication for hypertension or depression (Table 2).
TABLE 1.
Baseline sociodemographic and lifestyle characteristics of participants according to quartiles of dietary choline intake1
| Characteristics | Q1 (128.0–404.7 mg/d) (n = 1404) | Q2 (404.8–457.7 mg/d) (n = 1403) | Q3 (457.8–509.4 mg/d) (n = 1403) | Q4 (509.5–914.9 mg/d) (n = 1403) | P value |
|---|---|---|---|---|---|
| Choline intake, mg/d | 359.95 ± 37.66 | 431.64 ± 15.38 | 482.30 ± 14.92 | 563.81 ± 53.32 | <0.001 |
| Sex, female | 476 (33.9) | 639 (45.5) | 744 (53.0) | 848 (60.4) | <0.001 |
| Age, y | 64.40 ± 4.95 | 64.96 ± 5.12 | 65.37 ± 4.79 | 65.30 ± 4.70 | <0.001 |
| Civil status, married | 1088 (77.5) | 1095 (78.0) | 1074 (76.6) | 1070 (76.3) | 0.653 |
| Education, y | 11.77 ± 7.50 | 11.39 ± 7.49 | 11.73 ± 8.71 | 11.83 ± 8.77 | 0.483 |
| Smoking habits | <0.001 | ||||
| Smoker | 215 (15.3) | 189 (13.5) | 172 (12.3) | 129 (9.2) | |
| Former smoker | 683 (48.6) | 608 (43.3) | 581 (41.4) | 549 (39.1) | |
| Never smoker | 506 (36.0) | 606 (43.2) | 650 (46.3) | 725 (51.7) | |
| Body weight, kg | 87.68 ± 12.79 | 86.60 ± 12.77 | 85.51 ± 13.18 | 85.50 ± 12.88 | <0.001 |
| BMI, kg/m2 | 32.30 ± 3.35 | 32.54 ± 3.44 | 32.42 ± 3.49 | 32.72 ± 3.47 | 0.009 |
| Energy intake, kcal | 2390.99 ± 582.36 | 2344.24 ± 556.95 | 2326.94 ± 533.18 | 2385.85 ± 516.88 | 0.003 |
| Adherence to er-MedDiet (max: 17 points) | 7.76 ± 2.64 | 8.22 ± 2.65 | 8.73 ± 2.63 | 9.25 ± 2.57 | <0.001 |
| PA, intensity | 0.531 | ||||
| Sedentary | 842 (60.0) | 853 (60.8) | 818 (58.3) | 859 (61.2) | |
| Moderately active | 268 (19.1) | 249 (17.7) | 273 (19.5) | 270 (19.2) | |
| Active | 294 (20.9) | 301 (21.5) | 312 (22.2) | 274 (19.5) | |
| PA, MET · min/wk | 704.66 ± 475.33 | 713.19 ± 465.10 | 716.29 ± 450.69 | 736.69 ± 454.42 | 0.303 |
| Sedentary lifestyle, yes | 638 (45.4) | 650 (46.3) | 648 (46.2) | 587 (41.8) | 0.056 |
| Diabetes prevalence, yes | 375 (26.7) | 400 (28.5) | 450 (32.1) | 483 (34.4) | <0.001 |
| Diabetes medication, yes | 327 (23.3) | 353 (25.2) | 383 (27.3) | 430 (30.6) | <0.001 |
| Hypertension prevalence, yes | 1174 (83.6) | 1184 (84.4) | 1189 (84.7) | 1179 (84.0) | 0.864 |
| Hypertension medication, yes | 1091 (77.7) | 1112 (79.3) | 1095 (78.0) | 1109 (79.0) | 0.630 |
| Cholesterol medication, yes | 695 (49.5) | 720 (51.3) | 730 (52.0) | 732 (52.2) | 0.415 |
| Heart medication, yes | 116 (8.3) | 111 (7.9) | 106 (7.6) | 107 (7.6) | 0.465 |
| Depression prevalence, yes | 286 (20.4) | 276 (19.7) | 281 (20.0) | 312 (22.2) | 0.338 |
| Depression medication, yes | 304 (21.7) | 309 (22.0) | 359 (25.6) | 360 (25.7) | 0.027 |
Total n = 5613. Values are mean ± SD or n (%) unless otherwise indicated. Choline intake was adjusted for residuals of energy intake. Characteristics among quartiles were compared by using 1-factor ANOVA or chi-square test for continuous and categoric variables, respectively. er-MedDiet, energy-restricted traditional Mediterranean diet; MET, metabolic equivalent of task; PA, physical activity; Q, quartile.
TABLE 2.
Baseline sociodemographic and lifestyle characteristics of participants according to quartiles of dietary betaine intake1
| Characteristics | Q1 (0–104.3 mg/d) (n = 1404) | Q2 (104.4–129.9 mg/d) (n = 1403) | Q3 (130.0–160.6 mg/d) (n = 1403) | Q4 (160.7–390.0 mg/d) (n = 1403) | P value |
|---|---|---|---|---|---|
| Betaine intake, mg/d | 83.64 ± 17.18 | 117.36 ± 7.22 | 144.19 ± 8.66 | 191.71 ± 30.18 | <0.001 |
| Sex, female | 583 (41.5) | 737 (52.5) | 694 (49.5) | 693 (49.4) | <0.001 |
| Age, y | 64.71 ± 4.98 | 65.06 ± 4.80 | 65.16 ± 4.99 | 65.11 ± 4.84 | 0.058 |
| Civil status, married | 1101 (78.4) | 1058 (75.4) | 1080 (77.0) | 1088 (77.5) | 0.281 |
| Education, y | 11.96 ± 8.79 | 11.43 ± 8.21 | 11.63 ± 8.09 | 11.71 ± 7.41 | 0.380 |
| Smoking habits | 0.008 | ||||
| Smoker | 208 (14.8) | 170 (12.1) | 165 (11.8) | 162 (11.5) | |
| Former smoker | 627 (44.7) | 578 (41.2) | 595 (42.4) | 621 (44.3) | |
| Never smoker | 569 (40.5) | 655 (46.7) | 643 (45.8) | 620 (44.2) | |
| Body weight, kg | 87.04 ± 12.95 | 85.50 ± 12.72 | 86.21 ± 13.19 | 86.55 ± 12.84 | 0.015 |
| BMI, kg/m2 | 32.35 ± 3.41 | 32.47 ± 3.39 | 32.47 ± 3.46 | 32.68 ± 3.51 | 0.084 |
| Energy intake, kcal | 2458.01 ± 526.04 | 2246.19 ± 557.55 | 2335.37 ± 543.81 | 2408.39 ± 543.11 | <0.001 |
| Adherence to er-MedDiet (max: 17 points) | 8.64 ± 2.75 | 8.70 ± 2.71 | 8.35 ± 2.63 | 8.28 ± 2.61 | <0.001 |
| PA, intensity | 0.159 | ||||
| Sedentary | 859 (61.2) | 841 (59.9) | 859 (61.2) | 813 (57.9) | |
| Moderately active | 262 (18.7) | 243 (17.3) | 268 (19.1) | 287 (20.5) | |
| Active | 283 (20.2) | 319 (22.7) | 276 (19.7) | 303 (21.6) | |
| PA, MET · min/wk | 741.27 ± 464.89 | 714.59 ± 462.78 | 708.74 ± 460.20 | 706.19 ± 457.80 | 0.163 |
| Sedentary lifestyle, yes | 616 (43.9) | 608 (43.3) | 647 (46.1) | 652 (46.5) | 0.239 |
| Diabetes prevalence, yes | 390 (27.8) | 412 (29.4) | 435 (31.0) | 471 (33.6) | 0.007 |
| Diabetes medication, yes | 337 (24.0) | 367 (26.2) | 369 (26.3) | 420 (29.9) | 0.005 |
| Hypertension prevalence, yes | 1137 (81.0) | 1196 (85.2) | 1188 (84.7) | 1205 (85.9) | 0.002 |
| Hypertension medication, yes | 1078 (76.8) | 1099 (78.3) | 1107 (78.9) | 1123 (80.0) | 0.27 |
| Cholesterol medication, yes | 720 (51.3) | 706 (50.3) | 722 (51.5) | 722 (51.5) | 0.622 |
| Heart medication, yes | 125 (8.9) | 112 (8.0) | 111 (7.9) | 92 (6.6) | 0.105 |
| Depression prevalence, yes | 297 (21.2) | 321 (22.9) | 273 (19.5) | 264 (18.8) | 0.036 |
| Depression medication, yes | 329 (23.4) | 335 (23.9) | 317 (22.6) | 351 (25.0) | 0.292 |
Total n = 5613. Values are mean ± SD or n (%) unless otherwise indicated. Betaine intake was adjusted for residuals of energy intake. Characteristics among quartiles were compared using 1-factor ANOVA or chi-square test for continuous and categoric variables, respectively. er-MedDiet, energy-restricted traditional Mediterranean diet; MET, metabolic equivalent of task; PA, physical activity; Q, quartile.
Supplemental Tables 1 and 2 show the dietary intake of participants according to quartiles of choline (Supplemental Table 1) and betaine (Supplemental Table 2) intake. Participants in quartile 4 of choline intake consumed greater quantities of all food groups except for dietary fats and processed foods. Regarding betaine intake, participants in quartile 4 of betaine intake showed greater consumption of all food groups except for fruits and eggs.
Cross-sectional baseline and 1-y follow-up associations between choline or betaine intake and cardiometabolic parameters
Participants with greater choline intake at baseline (Table 1) exhibited greater BMI but lower body weight and waist circumference. However, participants with greater choline intake after 1 y of follow-up showed greater weight at that follow-up time (Supplemental Figure 1A). Baseline greater choline intake was associated with greater glucose, total cholesterol, and HDL cholesterol concentrations and HbA1c levels, but with lower TGs. This trend was maintained after 1 y of follow-up (Supplemental Figure 1A).
Participants with a greater intake of betaine at baseline showed greater BMI, glucose concentrations, and HbA1c levels. After 1 y of follow-up, differences in BMI among quartiles of betaine intake were not significant (Supplemental Figure 1B).
Cross-sectional baseline and 1-y follow-up associations between choline or betaine intake and renal function variables
Participants with greater choline intake at baseline exhibited greater plasma urea and lower plasma creatinine, plasma uric acid, and urine creatinine concentrations (Supplemental Figure 2A). Similar trends were found in the cross-sectional analysis of 1-y follow-up data (Supplemental Figure 2A).
Participants with greater baseline betaine intake showed significantly greater plasma urea concentrations (Supplemental Figure 2B). Plasma urea concentrations were also greater in quartile 4 of betaine intake after 1 y of follow-up (Supplemental Figure 2B). After 1 y of follow-up, plasma uric acid decreased with greater betaine intake (Supplemental Figure 2B).
Longitudinal association between 1-y changes in choline or betaine intake and changes in cardiometabolic parameters
Participants with the greatest increase in their choline intake showed the greatest increase in fruits, nuts, vegetables, saturated fats, and salty and sweet-processed foods consumption. However, they showed the smallest increase in fish, legumes, and whole-grain cereals intakes. These participants also showed a greater decrease in intakes of dairy and meat products (both red and white meat), and eggs, cereals, refined-grain cereals, dietary fats, and processed food (Supplemental Table 3). We found similar trends for participants with the greatest increase in betaine intake (Supplemental Table 4). Regarding the food sources that explained the individual variability in the change of choline or betaine intake after 1-y follow-up, the stepwise regression analysis showed that choline intake reduction in quartile 1 participants was mainly due to a change in the consumption of dairy products, nuts, cereals (both refined- and whole-grain cereals), sweet-processed foods, and vegetables (Supplemental Table 5). There was an increase in the consumption of these foods in quartile 1 of change of choline intake over 1 year, except for refined-grain cereals which showed a decrease in consumption (Supplemental Table 3). The increase in choline intake after 1 y of follow-up in quartile 4 of change in choline intake was mainly due to changes in the consumption of refined-grain cereals, sweet-processed foods, fruits, and red meat. Participants in quartile 4 of change in choline intake reduced red meat and refined-grain cereals intake, whereas they increased fruits and sweet-processed food intake (Supplemental Table 3).
Changes in betaine intake after 1 y of follow-up were mainly due to changes in the consumption of refined-grain cereals in both extreme quartiles of change in betaine intake over 1 year (Supplemental Table 6). Quartile 1 decreased consumption of refined-grain cereals by 4.19 g/d, whereas quartile 4 decreased its consumption by 56.71 g/d (Supplemental Table 4).
Those participants with the greatest increase in choline intake showed the greatest reductions in energy intake, body weight, waist circumference, BMI, HbA1c level, TG, total cholesterol, and LDL-cholesterol concentrations (Table 3), independently of choline intake at baseline. This quartile also showed the greatest increase in PA and adherence to an er-MedDiet. There was a significant linear association between 1-y increase in choline intake and 1-y decrease in energy intake, body weight, waist circumference, HbA1c, and TGs and 1-y increase in PA and HDL cholesterol (Table 3). These same trends were observed in participants with the greatest increase in betaine intake (Table 4).
TABLE 3.
Changes in cardiometabolic and anthropometric parameters according to quartiles of 1-y changes in choline intake1
| Variables | Total n | Q1 (−203.5 to −39.7 mg/d) (n = 1404) | Q2 (−39.6 to −4.3 mg/d) (n = 1403) | Q3 (−4.2 to 35.1 mg/d) (n = 1403) | Q4 (35.2–233.8 mg/d) (n = 1403) | P model 1 | P model 2 | P model 3 | P-linear trend |
|---|---|---|---|---|---|---|---|---|---|
| Δ Choline intake, mg/d | 5613 | −70.46 ± 26.31 | −21.56 ± 10.22 | 14.61 ± 11.42 | 77.46 ± 34.05 | <0.001 | <0.001 | <0.001 | <0.001 |
| Δ Energy intake, kcal/d | 5613 | 438.01 ± 305.64 | 12.61 ± 184.77 | −267.17 ± 184.80 | −783.34 ± 325.17 | <0.001 | <0.001 | <0.001 | <0.001 |
| Δ Adherence to er-MedDiet (max: 17 points) | 5591 | 2.78 ± 3.03 | 3.05 ± 3.19 | 3.35 ± 3.23 | 3.77 ± 3.38 | <0.001 | <0.001 | <0.001 | 0.126 |
| Δ PA, MET · min/wk | 5613 | 432.82 ± 2250.30 | 512.42 ± 2410.53 | 481.80 ± 2454.52 | 712.03 ± 2587.82 | 0.005 | 0.005 | <0.001 | 0.017 |
| Δ BMI, kg/m2 | 5601 | −0.58 ± 1.52 | −0.76 ± 1.58 | −0.92 ± 1.50 | −1.05 ± 1.61 | <0.001 | <0.001 | <0.001 | 0.293 |
| Δ Body weight, kg | 5609 | −1.55 ± 3.88 | −2.01 ± 4.10 | −2.49 ± 4.02 | −2.93 ± 4.41 | <0.001 | <0.001 | <0.001 | <0.001 |
| Δ Waist circumference, cm | 5593 | −1.72 ± 5.34 | −2.44 ± 4.92 | −3.06 ± 5.06 | −3.37 ± 5.09 | <0.001 | <0.001 | <0.001 | <0.001 |
| Δ Glucose, mg/dL | 5492 | −0.59 ± 21.67 | −2.95 ± 22.65 | −3.39 ± 24.65 | −3.39 ± 20.76 | 0.001 | 0.001 | 0.001 | 0.162 |
| Δ HbA1c, % | 4718 | −0.03 ± 0.50 | −0.08 ± 0.57 | −0.09 ± 0.60 | −0.10 ± 0.50 | <0.001 | <0.001 | <0.001 | 0.047 |
| Δ TG, mg/dL | 5472 | −4.06 ± 59.80 | −6.49 ± 64.08 | −7.30 ± 78.98 | −11.88 ± 71.38 | 0.003 | 0.003 | 0.002 | 0.07 |
| Δ Total cholesterol, mg/dL | 5483 | −0.65 ± 30.51 | −1.88 ± 32.08 | −2.48 ± 34.52 | −4.74 ± 32.16 | 0.001 | 0.001 | 0.001 | 0.88 |
| Δ LDL cholesterol, mg/dL | 5088 | −1.19 ± 26.92 | −2.00 ± 28.30 | −2.71 ± 25.91 | −4.30 ± 27.87 | 0.003 | 0.003 | 0.003 | 0.762 |
| Δ HDL cholesterol, mg/dL | 5427 | 1.32 ± 7.71 | 1.54 ± 7.24 | 1.47 ± 7.36 | 1.41 ± 7.22 | 0.806 | 0.806 | 0.827 | <0.001 |
| Δ Systolic BP, mm Hg | 5613 | −3.27 ± 23.66 | −5.37 ± 23.73 | −4.93 ± 25.88 | −4.53 ± 22.80 | 0.234 | 0.234 | 0.211 | 0.247 |
| Δ Diastolic BP, mm Hg | 5613 | −2.18 ± 13.52 | −3.32 ± 13.57 | −3.08 ± 15.26 | −2.94 ± 12.96 | 0.203 | 0.203 | 0.187 | 0.921 |
Total n = 5613. Values are mean ± SD unless otherwise indicated. Choline intake was adjusted by energy residuals. Characteristics among quartiles were compared using 1-factor ANOVA. Model 1: adjusted by sex, age, and center. Model 2: adjusted by sex, age, center, civil status, education, smoking habits, sedentary lifestyle, and choline intake at baseline. Model 3: adjusted by sex, age, center, civil status, education, smoking habits, sedentary lifestyle, choline intake at baseline, adherence to an er-MedDiet and changes in adherence to an er-MedDiet, energy intake and changes in energy intake, and PA and changes in PA. P-linear trend: adjusted by age, sex, center, BMI, and energy intake at baseline. BP, blood pressure; er-MedDiet, energy-restricted traditional Mediterranean diet; MET, metabolic equivalent of task; PA, physical activity; Q, quartile.
TABLE 4.
Changes in cardiometabolic and anthropometric parameters according to quartiles of 1-y changes in betaine intake1
| Variables | Total n | Q1 (−87.8 to −18.2 mg/d) (n = 1404) | Q2 (−18.1 to −2.1 mg/d) (n = 1403) | Q3 (−2.0 to 16.3 mg/d) (n = 1403) | Q4 (16.4–99.0 mg/d) (n = 1403) | P model 1 | P model 2 | P model 3 | P-linear trend |
|---|---|---|---|---|---|---|---|---|---|
| Δ Betaine intake, mg/d | 5613 | −31.83 ± 11.15 | −9.99 ± 4.60 | 6.71 ± 5.29 | 35.13 ± 15.21 | <0.001 | <0.001 | <0.001 | <0.001 |
| Δ Energy intake, kcal/d | 5613 | 392.43 ± 335.55 | 12.89 ± 242.54 | −261.31 ± 242.52 | −743.87 ± 372.17 | <0.001 | <0.001 | <0.001 | <0.001 |
| Δ Adherence to er-MedDiet (max: 17 points) | 5591 | 2.89 ± 3.04 | 3.07 ± 3.16 | 3.31 ± 3.27 | 3.69 ± 3.38 | <0.001 | <0.001 | <0.001 | 0.127 |
| Δ PA, MET · min/wk | 5613 | 416.68 ± 2229.72 | 561.58 ± 2374.00 | 486.55 ± 2530.31 | 674.29 ± 2568.02 | 0.015 | 0.015 | <0.001 | 0.017 |
| Δ BMI, kg/m2 | 5601 | −0.68 ± 1.58 | −0.72 ± 1.51 | −0.93 ± 1.55 | −0.99 ± 1.58 | <0.001 | <0.001 | <0.001 | 0.269 |
| Δ Body weight, kg | 5609 | −1.79 ± 4.07 | −1.90 ± 3.90 | −2.52 ± 4.14 | −2.78 ± 4.35 | <0.001 | <0.001 | <0.001 | <0.001 |
| Δ Waist circumference, cm | 5593 | −1.83 ± 5.48 | −2.34 ± 4.80 | −3.16 ± 5.09 | −3.26 ± 5.05 | <0.001 | <0.001 | <0.001 | <0.001 |
| Δ Glucose, mg/dL | 5492 | −1.14 ± 21.14 | −2.22 ± 22.69 | −4.23 ± 25.28 | −2.72 ± 20.50 | 0.013 | 0.013 | 0.010 | 0.155 |
| Δ HbA1c, % | 4718 | −0.04 ± 0.50 | −0.06 ± 0.55 | −0.10 ± 0.62 | −0.10 ± 0.50 | 0.002 | 0.002 | 0.002 | 0.017 |
| Δ TG, mg/dL | 5472 | −4.16 ± 59.03 | −7.01 ± 67.49 | −7.34 ± 75.63 | −11.21 ± 72.49 | 0.010 | 0.010 | 0.007 | 0.028 |
| Δ Total cholesterol, mg/dL | 5483 | −1.30 ± 29.99 | −1.42 ± 32.53 | −2.50 ± 34.56 | −4.52 ± 32.17 | 0.006 | 0.006 | 0.005 | 0.861 |
| Δ LDL cholesterol, mg/dL | 5088 | −1.64 ± 26.59 | −1.43 ± 28.65 | −2.97 ± 26.45 | −4.16 ± 27.31 | 0.008 | 0.008 | 0.007 | 0.742 |
| Δ HDL cholesterol, mg/dL | 5427 | 1.32 ± 7.69 | 1.51 ± 7.30 | 1.44 ± 7.35 | 1.47 ± 7.18 | 0.672 | 0.672 | 0.707 | <0.001 |
| Δ Systolic BP, mm Hg | 5613 | −3.83 ± 24.29 | −5.14 ± 23.33 | −4.69 ± 24.90 | −4.43 ± 23.66 | 0.624 | 0.624 | 0.593 | 0.379 |
| Δ Diastolic BP, mm Hg | 5613 | −2.51 ± 13.68 | −3.13 ± 13.74 | −3.26 ± 14.34 | −2.63 ± 13.65 | 0.746 | 0.746 | 0.717 | 0.879 |
Total n = 5613. Values are mean ± SD unless otherwise indicated. Betaine intake was adjusted by energy residuals. Characteristics among quartiles were compared using 1-factor ANOVA. Model 1: adjusted by sex, age, and center. Model 2: adjusted by sex, age, center, civil status, education, smoking habits, sedentary lifestyle, and choline intake at baseline. Model 3: adjusted by sex, age, center, civil status, education, smoking habits, sedentary lifestyle, choline intake at baseline, adherence to an er-MedDiet and changes in adherence to an er-MedDiet, energy intake and changes in energy intake, and PA and changes in PA. P-linear trend: adjusted by age, sex, center, BMI, and energy intake at baseline. BP, blood pressure; er-MedDiet, energy-restricted traditional Mediterranean diet; MET, metabolic equivalent of task; PA, physical activity; Q, quartile.
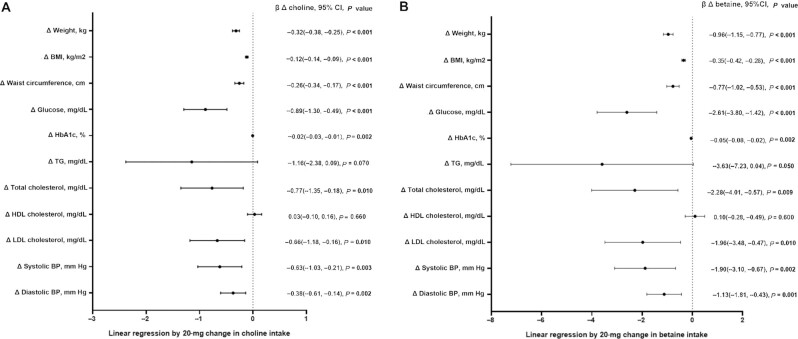
To check if medication had an impact on the effect of increase in choline or betaine intake on cardiometabolic traits, we in addition adjusted by prevalent medication for hypertension, hypercholesterolemia, T2D, and depression. Observed associations did not change (data not shown). To test if the change in consumption of certain food groups or changes in the intake of different nutrients modified these longitudinal associations, we in addition adjusted by food groups (baseline consumption of fruits, vegetables, animal protein, fish, eggs, red meat, and dairy products and change in the consumption of these food groups after 1 y of follow-up) and by nutrient intakes (baseline intakes of carbohydrates, fiber, saturated fats, and unsaturated fats and changes in these nutrients’ intakes after 1 y of follow-up). Associations did not change (data not shown). To test if changes in body weight or BMI could affect changes in cardiometabolic parameters far beyond changes in choline or betaine intake, we carried out a sensitivity analysis by further adjusting by baseline body weight or BMI and by 1-y changes in these parameters. The association between changes in choline or betaine intake and changes in cardiometabolic parameters did not change. We also added both traits (BMI and weight), both at baseline and 1-y changes, and association results did not change (data not shown). Postmenopausal females are less capable of synthesizing choline de novo, because the S-adenosylmethionine that acts as the methyl donor and needs phosphatidylethanolamine-N-methyltransferase is activated by estrogen, and therefore their choline requirements are higher (36). Thus, to test if hormonal replacement therapy in females could affect the association between changes in choline intake and changes in cardiometabolic parameters, we carried out a sensitivity analysis by removing females receiving hormone replacement therapy (n = 115 at baseline and n = 127 at 1 y). The association between changes in choline or betaine intake and changes in cardiometabolic parameters did not change (data not shown). A linear regression was performed to assess changes in anthropometric and cardiometabolic parameters for every 20-mg increase in choline or betaine intake (Figure 2). For every increase in 20 mg of choline intake, body weight, BMI, waist circumference, glucose, HbA1c, total cholesterol, LDL cholesterol, and systolic and diastolic BP decreased (Figure 2A). A trend toward a decrease in TGs was also observed although this association was not significant. The same results were obtained for every 20-mg increase in betaine intake. In this case, the association between increase in betaine intake and decrease in TG concentrations was significant (P = 0.05) (Figure 2B).
FIGURE 2.
Forest plots showing the association of 20-mg increments in dietary choline intake (A) and dietary betaine intake (B) with changes in cardiometabolic parameters, after 1 y of follow-up (n = 5613). Dietary choline and betaine intakes were adjusted by energy residuals. The model was adjusted by age, sex, center, years of schooling, civil status, smoking habits, sedentary lifestyle, choline and betaine intake at baseline, PA and difference in PA, adherence to an er-MedDiet and difference in adherence to an er-MedDiet, and energy intake and difference in energy intake (1 y–baseline). Values are represented as β ± 95% CI, and P value. BP, blood pressure; er-MedDiet, energy-restricted traditional Mediterranean diet; PA, physical activity.
Automatic procedures for category formation in quartiles or quintiles, although very commonly used, can be suboptimal in some applications and can sometimes be harmful to power, precision, and confounder control. Therefore, quartile categories might blindly lump together disparate subgroups of subjects and may thereby hide important effects. For this reason, we repeated our longitudinal analyses by categorizing participants into groups according to validated thresholds for adequate choline intake after 1 y of follow-up (Supplemental Table 7). Participants in the low intake group at 1 y reduced their choline intake (−39.22 ± 49.26 mg/d), whereas intake of choline increased significantly in the other groups (P < 0.001). We found similar associations between greater intake of choline after 1 y of follow-up and greater reduction in total energy intake, BMI, body weight, and waist circumference. However, the significance in the association between choline intake at 1 y and changes in glucose, HbA1c, and lipid traits was lost.
Longitudinal association between 1-y changes in choline or betaine intake and changes in renal function parameters
Participants with the greatest increase in choline intake showed a greater decrease in urine creatinine (Table 5). A similar result was found in those participants with a greater increase in betaine intake after 1 y (Table 6). Changes in the other renal function parameters were not significant. Among participants that increased their choline or betaine intake the most, a greater number of participants with a ≥10% decrease in eGFR was found. As mentioned, T2D prevalence was greater in participants with greater choline or betaine intake, affecting eGFR values owing to diabetic complications. Therefore, we analyzed renal function parameters in participants without diabetes only and a linear decrease in eGFR was not found (data not shown). Also, although a greater percentage of participants who reduced eGFR by >10% in quartiles of greater increase of betaine and choline intake was found, differences across quartiles were not significant (data not shown). Further adjustment by medications, nutrients, food groups, and weight or BMI did not change results (data not shown). However, when we categorized participants according to 1-y intake of choline using estimated adequate levels, a greater percentage of participants who reduced eGFR by >10% was found in the high intake group (Supplemental Table 7). We carried out sensitivity analyses by removing data from women receiving hormone replacement therapy, and the significance for the association between 1-y change in choline intake and urine creatinine concentrations was lost (P = 0.057). The association with 1-y change in betaine intake remained significant (data not shown).
TABLE 5.
Changes in renal function parameters according to quartiles of 1-y changes in choline intake1
| Variables | Total n | Q1 (−203.5 to −39.7 mg/d) (n = 832) | Q2 (−39.6 to −4.3 mg/d) (n = 840) | Q3 (−4.2 to 35.1 mg/d) (n = 836) | Q4 (35.2–233.8 mg/d) (n = 859) | P model 1 | P model 2 | P model 3 | P-linear trend |
|---|---|---|---|---|---|---|---|---|---|
| Δ Urea, mg/dL | 3046 | 0.88 ± 10.10 | 0.42 ± 9.99 | 1.13 ± 10.71 | 0.99 ± 8.62 | 0.515 | 0.516 | 0.511 | 0.360 |
| Δ Creatinine, mg/dL | 3349 | 0.01 ± 0.34 | 0.00 ± 0.12 | 0.00 ± 0.11 | 0.00 ± 0.11 | 0.690 | 0.690 | 0.653 | 0.293 |
| Δ Uric acid, mg/dL | 3260 | 0.12 ± 3.40 | 0.04 ± 2.94 | 0.11 ± 4.91 | 0.00 ± 2.48 | 0.593 | 0.594 | 0.590 | 0.805 |
| Δ Urine creatinine, mg/dL | 3332 | −1.81 ± 53.21 | −0.82 ± 57.63 | −4.16 ± 53.71 | −5.42 ± 56.24 | 0.094 | 0.094 | 0.049 | 0.004 |
| Δ eGFR, mL · min−1 · 1.73m−2 | 3349 | 0.07 ± 9.32 | 0.12 ± 9.15 | −0.07 ± 8.64 | −0.32 ± 8.86 | 0.316 | 0.316 | 0.367 | 0.617 |
| eGFR, >10% decrease, yes | 3349 | 96 (11.0) | 112 (13.0) | 120 (13.8) | 133 (15.0) | 0.203 | 0.203 | 0.195 | 0.195 |
| Δ UACR, mg/g | 3367 | 3.14 ± 43.58 | 2.41 ± 40.55 | −0.33 ± 40.46 | 1.02 ± 36.01 | 0.144 | 0.144 | 0.129 | 0.300 |
Total n = 3367. Values are mean ± SD or n (%) unless otherwise indicated. Choline intake was adjusted by energy residuals. Characteristics among quartiles were compared using 1-factor ANOVA. Model 1: adjusted by sex, age, and center. Model 2: adjusted by sex, age, center, civil status, education, smoking habits, sedentary lifestyle, and choline intake at baseline. Model 3: adjusted by sex, age, center, civil status, education, smoking habits, sedentary lifestyle, choline intake at baseline, adherence to an er-MedDiet and changes in adherence to an er-MedDiet, energy intake and changes in energy intake, and PA and changes in PA. P-linear trend: adjusted by age, sex, center, BMI, and energy intake at baseline. eGFR, estimated glomerular filtration rate; er-MedDiet, energy-restricted traditional Mediterranean diet; PA, physical activity; Q, quartile; UACR, urine albumin:creatinine ratio.
TABLE 6.
Changes in renal function parameters according to quartiles of 1-y changes in betaine intake1
| Variables | Total n | Q1 (−87.8 to −18.2 mg/d) (n = 812) | Q2 (−18.1 to −2.1 mg/d) (n = 855) | Q3 (−2.0 to 16.3 mg/d) (n = 823) | Q4 (16.4–99.0 mg/d) (n = 877) | P model 1 | P model 2 | P model 3 | P-linear trend |
|---|---|---|---|---|---|---|---|---|---|
| Δ Urea, mg/dL | 3046 | 0.91 ± 10.76 | 0.50 ± 8.99 | 1.16 ± 10.99 | 0.85 ± 8.66 | 0.754 | 0.754 | 0.742 | 0.360 |
| Δ Creatinine, mg/dL | 3349 | 0.01 ± 0.35 | 0.00 ± 0.12 | 0.00 ± 0.12 | 0.00 ± 0.11 | 0.568 | 0.568 | 0.534 | 0.293 |
| Δ Uric acid, mg/dL | 3260 | 0.02 ± 1.96 | 0.33 ± 6.12 | −0.08 ± 1.40 | −0.01 ± 2.45 | 0.364 | 0.365 | 0.361 | 0.805 |
| Δ Urine creatinine, mg/dL | 3332 | −2.49 ± 53.83 | 0.19 ± 57.39 | −4.16 ± 52.67 | −5.74 ± 56.70 | 0.091 | 0.091 | 0.042 | 0.004 |
| Δ eGFR, mL · min−1 · 1.73m−2 | 3349 | 0.12 ± 9.39 | 0.06 ± 9.29 | −0.20 ± 8.83 | −0.17 ± 8.48 | 0.417 | 0.417 | 0.478 | 0.617 |
| eGFR, >10% decrease, yes | 3349 | 90 (10.4) | 118 (13.3) | 120 (14.4) | 133 (14.7) | 0.180 | 0.180 | 0.179 | 0.285 |
| Δ UACR, mg/g | 3367 | 2.83 ± 44.90 | 2.47 ± 42.02 | 0.37 ± 36.70 | 0.61 ± 36.85 | 0.158 | 0.158 | 0.148 | 0.300 |
Total n = 3367. Values are mean ± SD or n (%) unless otherwise indicated. Betaine intake was adjusted by energy residuals. Characteristics among quartiles were compared using 1-factor ANOVA. Model 1: adjusted by sex, age, and center. Model 2: adjusted by sex, age, center, civil status, education, smoking habits, sedentary lifestyle, and choline intake at baseline. Model 3: adjusted by sex, age, center, civil status, education, smoking habits, sedentary lifestyle, choline intake at baseline, adherence to an er-MedDiet and changes in adherence to an er-MedDiet, energy intake and changes in energy intake, and PA and changes in PA. P-linear trend: adjusted by age, sex, center, BMI, and energy intake at baseline. eGFR, estimated glomerular filtration rate; er-MedDiet, energy-restricted traditional Mediterranean diet; PA, physical activity; Q, quartile; UACR, urine albumin:creatinine ratio.
Discussion
In our study, an increase in dietary choline or betaine intake over 1 y was associated with improved serum glucose concentrations and HbA1c levels, body weight, BMI, waist circumference, serum total cholesterol, serum LDL cholesterol, and urine creatinine.
Improvements in serum glucose concentrations and HbA1c levels when dietary choline was increased were among the most striking observations. Age has been shown to increase the risk of insulin resistance (37). Exercise improves insulin sensitivity (38). An increase in the adherence to a MedDiet has been shown to improve disease progression and to prevent it (39). And a decrease in calorie intake has been associated with better insulin sensitivity (40). However, the observed association between increase in choline or betaine intake and improvement in diabetes-related traits was independent of choline or betaine intake at baseline as well as of age, PA, increase in the adherence to an er-MedDiet, and decrease in energy intake.
Studies aiming to define the relation between choline intake and insulin sensitivity are controversial. An observational study reported that serum choline concentrations were inversely associated with diabetes risk and its microangiopathy complications. However, serum betaine concentrations did not associate with diabetes risk, but they did associate with diabetes complications (41). In a large cohort of the CODING (Complex Diseases in the Newfoundland population: Environment and Genetics) study, dietary choline and betaine intakes were assessed and correlated with lower fasting glucose and insulin concentrations and lower insulin resistance (11). A more recent observational study examined dietary choline intake in the Kuopio Ischemic Heart Disease Risk Factor Study cohort. After 19 y of follow-up, the authors concluded that greater dietary choline intake was associated with a lower risk of T2D (12). However, a prospective study that included results from the NHS I and II and from the Health Professionals Follow-up Study concluded that greater phosphatidylcholine intake was associated with increased risk of T2D (42). The PREDIMED trial found that greater plasma TMAO concentrations were associated with lower risk of T2D incidence (43), whereas in China, the opposite was found (44). Taking these findings together, it could be suggested that greater amounts of dietary choline intake, within the adequate intake estimated limits, might help to reduce insulin resistance and to improve glucose concentrations and HbA1c levels and, thus, to prevent T2D development.
The mechanisms by which dietary choline may reduce insulin resistance remain to be elucidated. Dietary choline intake reduces inflammatory and oxidative stress markers, which play a role in T2D development (45, 46). In addition, betaine supplementation in mice has been shown to improve carbohydrate metabolism by increasing insulin receptor substrate phosphorylation in the liver (47).
A greater increase in choline or betaine intake was also associated with improvement in lipid and cardiometabolic traits. Observed improvements in cardiometabolic variables could be due to greater adherence to a MedDiet and reduced energy intake (25). HDL increase could also be due to an increase in PA performance (48). Nevertheless, these factors were considered in the analyses. Therefore, we suggest that choline or betaine intake could improve cardiometabolic traits irrespective of improvements in other lifestyle factors. Dietary choline and betaine food sources could have an impact on cardiometabolic variables. In this regard, after 1 y of follow-up, those participants who increased by more their choline and/or betaine intake increased consumption of fruits and salty and sweet-processed foods and decreased consumption of refined-grain cereals, red meat, and processed foods. The decreases in red meat and refined-grain cereals were the main contributors to the change in choline and betaine intakes. Meat consumption has been proven to increase plasma TMAO concentrations, and, consequently, CVD risk (49–51). However, egg consumption, also high in choline, has been reported to improve insulin sensitivity and lipoprotein profile (13, 14, 52–56). Fish, whole-grain cereals, and vegetables have been shown to increase plasma TMAO concentrations (10); however, they have also been proven to improve lipid profile and cardiometabolic parameters (16). Taking all these points together, it could be suggested that improvements in blood lipid profile and cardiometabolic parameters may be due to a change in the main food source of choline and betaine that shows a shift toward a healthier diet, with an increase in consumption of fiber, omega-3 fatty acids, and polyphenols, which may act synergistically with choline and betaine.
Greater betaine concentrations in coronary artery disease subjects have been found to be correlated with lower BP and HbA1c levels, and glucose, insulin, and TG concentrations (57). This is consistent with our results showing that those participants with a greater increase in betaine intake reduced HbA1c levels by more. Besides, we carried out sensitivity analysis by further adjustment for baseline and 1-y changes in consumption of different foods and nutrients and the associations between changes in choline or betaine intake and cardiometabolic and lipid parameters did not lose statistical significance. Taking these findings together, it could be suggested that betaine intake could be beneficial to prevent CVD and T2D.
Lastly, a slight improvement in renal function was observed in those participants with the greatest increase in choline intake. Although an increase in choline or betaine intake was associated with a decrease in urine creatinine, it was also associated with an increase in the percentage of participants who decreased eGFR by >10%. Although this difference was not significant, this could be due to the greater prevalence of participants with T2D in quartiles 3 and 4 of changes in choline and betaine intake, thus increasing the risk of renal failure due to diabetic complications. In fact, a decrease in eGFR >7.5% in the course of 1 y has been proposed as a good indicator of prognosis for renal failure (58). When we analyzed data without participants with T2D, a linear increase of participants that decreased their eGFR was not observed (data not shown). Therefore, it could be suggested that an increase in choline or betaine intake could lead to a decrease in eGFR in those participants with diabetic complications.
The relation between choline and betaine intake and renal function has not been deeply studied yet. Some studies have concluded that there are both positive and negative effects (59). For instance, an er-MedDiet intervention has been reported to preserve kidney function and could delay chronic kidney disease progression (28). That study also reported a decrease in UACR, similar to our results. Thus, we could suggest that a Mediterranean dietary pattern with an adequate intake of choline and high intake of betaine, obtained from fish, eggs, vegetables, nuts, and whole-grain cereals, could improve renal parameters and, in turn, could help to reduce CVD risk.
In summary, here we demonstrate that an increase in choline or betaine intake was related to improvements in lipoprotein profile, cardiometabolic parameters, glucose concentrations, and HbA1c levels, independently of age, sex, sedentary lifestyle, PA, adherence to an er-MedDiet, and smoking habits. However, the impact of choline or betaine intake on renal function deserves further investigation because we found a decrease in urine creatinine with an increase in the intake of these compounds, but we also found an increase in the percentage of participants who decreased eGFR by >10% after 1 y of follow-up.
The present study has some strengths, such as the large sample size and its longitudinal design. In addition, we used validated questionnaires to assess nutritional characteristics and lifestyle and we considered multiple confounders in our analyses.
Nevertheless, our study has some limitations. First, it is based on elder subjects with overweight/obesity and with MetS; therefore, these findings cannot be considered for the general population. Furthermore, the nutritional characteristics and lifestyle parameters are based on self-reported data, implying an information bias. Food and nutrient intakes have been calculated from an FFQ, which has limited sensitivity and specificity to reliably measure food consumption and nutrient intake. In this regard, we have not considered that choline and betaine intakes are consumed as part of a diet in a complex mixture of foods. The specific combination of different foods providing both nutrients could have an impact on our primary outcomes. Moreover, other micronutrients, such as other vitamins and minerals, could have similar effects on cardiometabolic and renal traits. This highlights the fact that the isolated analysis of 1 nutrient is challenging because nutrients are consumed in combination as part of a diet. However, to dampen this limitation, we in addition adjusted by baseline and 1-y changes in intakes of other foods and nutrients, and our observed longitudinal associations were not affected. Another limitation is the regression-to-the-mean method used for our longitudinal analyses. This means that participants with both low and high choline or betaine intake at baseline can contribute to all these quartiles and that quartiles of baseline choline or betaine intake could contribute differently to quartiles of 1-y change in the intake of these nutrients, because we did not account for achievement of absolute intakes. To account for this limitation, we adjusted for baseline choline or betaine intake. Nevertheless, this limitation makes it challenging to interpret the results. Further studies are needed that measure plasma choline and betaine concentrations (or their derived metabolites) and associate these concentrations with health traits. Although analyses were adjusted by several strong confounders, there may be some other, unmeasured confounders that could alter the overall results. Lastly, urine measurements were made on spot morning urine and not on 24-h urine collection. These biomarkers, especially concerning renal parameters, are known for their high biological variability, thus some of the values could be misleading.
Supplementary Material
Acknowledgements
The authors’ responsibilities were as follows—MÁM-G, DC, JS-S, AG, JAM, ÁMA-G, JW, J Vioque, DR, JL-M, RE, FJT, JL, LS-M, AB-C, JAT, VMS, XP, JJG, PM-M, J Vidal, SMF, ER, ZV-R, CO-A, JFG-G, M Malcampo, DM-U, LT-S, AGR, NG-B, AC, AG-R, RMB-L, JMS-L, JB-G, JVS, M Murphy, GG, VM, IS-L, EGO, NB, XH, JMO, RS-C, LD-R, and LD: designed and conducted the research; LD and LD-R: conceived the study idea and the analysis design; LD: supervised the research; LD-R: carried out the analysis procedures, bibliographic research, data preparation, statistical analysis, and wrote the initial drafts; RS-C: assisted with statistical analysis and R programming; MJC: collaborated with data collection and analysis; JMO and MJC: participated in the scientific discussion of experimental results; and all authors: assisted in manuscript revision for intellectual content and read and approved the final manuscript.
Notes
Supported by Ministerio de Ciencia e Innovación through Proyectos de I+D+i “Retos Investigación” grant RTI2018-095569-B-I00 (to JMO) and “Programación Conjunta Internacional” grant PCI2018-093009 (to JMO).
The PREDIMED-Plus (Prevención con Dieta Mediterránea-Plus) trial was supported by European Research Council Advanced Research grant 2014–2019, agreement #340918 (to MÁM-G); the official Spanish institutions for funding scientific biomedical research, CIBER Fisiopatología de la Obesidad y Nutrición (CIBEROBN) and Instituto de Salud Carlos III (ISCIII), through the Fondo de Investigación para la Salud (FIS) which is cofunded by the European Regional Development Fund (coordinated FIS projects led by JS-S and J Vidal, including the following projects: PI13/00673, PI13/00492, PI13/00272, PI13/01123, PI13/00462, PI13/00233, PI13/02184, PI13/00728, PI13/01090, PI13/01056, PI14/01722, PI14/00636, PI14/00618, PI14/00696, PI14/01206, PI14/01919, PI14/00853, PI14/01374, PI14/00972, PI14/00728, PI14/01471, PI16/00473, PI16/00662, PI16/01873, PI16/01094, PI16/00501, PI16/00533, PI16/00381, PI16/00366, PI16/01522, PI16/01120, PI17/00764, PI17/01183, PI17/00855, PI17/01347, PI17/00525, PI17/01827, PI17/00532, PI17/00215, PI17/01441, PI17/00508, PI17/01732, PI17/00926, PI19/00957, PI19/00386, PI19/00309, PI19/01032, PI19/00576, PI19/00017, PI19/01226, PI19/00781, PI19/01560, PI19/01332, PI20/01802, PI20/00138, PI20/01532, PI20/00456, PI20/00339, PI20/00557, PI20/00886, and PI20/01158), and the Especial Action Project “Implementación y evaluación de una intervención intensiva sobre la actividad física Cohorte PREDIMED-Plus” (to JS-S); and Recercaixa grant 2013ACUP00194 (to JS-S). Moreover, JS-S acknowledges the financial support by ICREA (Catalan Institution for Research and Advanced Studies) under the ICREA Academia program; the SEMERGEN grant (to JL); Government of Navarra Department of Health grant 61/2015 (to MÁM-G); Fundació La Marató de TV3 grant 201630.10; the AstraZeneca Young Investigators Award in Category of Obesity and T2D 2017 (to DC); Consejería de Salud de la Junta de Andalucía grants PI0458/2013, PS0358/2016, and PI0137/2018; Generalitat Valenciana grant PROMETEO/2017/017 (to DC); and Balearic Islands Government grant of support to research groups 35/2011 (FEDER funds) (to JAT). RS-C was supported by Spanish Ministerio de Ciencia e Innovación y Ministerio de Universidades Spanish State Research Agency Juan de la Cierva Program training grant FJC2018-038168-I.
Author disclosures: J.S-S reports grants from CIBEROBN, ISCIII (Spain), during the conduct of the study; non-financial support from Nut and Dried Fruit Foundation, personal fees from Instituto Danone Spain, grants from Nut and Dried Fruit Foundation to this Field Code Changed institution, grants from Eroski Distributors, personal fees from Nut and Dried Fruit Foundation, outside the submitted work. E.R reports grants, personal fees, non-financial support and other from California Walnut Commission, grants, personal fees, non-financial support and other from Alexion, personal fees, non-financial support and other from Ferrer International, personal fees from Amarin, personal fees, non-financial support and other from Danone, outside the submitted work. J. L-M reports personal fees and non-financial support from AMGEN, personal fees and non-financial support from SANOFI, personal fees from MSD, personal fees from Laboratorios Dr. Esteve, personal fees from NOVO-NORDISK, outside the submitted work. The other authors declare no conflict of interests.
Supplemental Tables 1–7 and Supplemental Figures 1 and 2 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: BP, blood pressure; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; er-MedDiet, energy-restricted traditional Mediterranean diet; FMO, flavin monooxygenase; Hb1Ac, glycated haemoglobin; MedDiet, Mediterranean diet; MetS, metabolic syndrome; NHS, Nurses’ Health Study; PA, physical activity; PREDIMED, PREvención con DIeta MEDiterránea; TMA, trimethylamine; TMAO, trimethylamine-N-oxide; T2D, type 2 diabetes; UACR, urine albumin:creatinine ratio.
Contributor Information
Laura Díez-Ricote, Nutritional Control of the Epigenome Group, Precision Nutrition and Obesity Program, IMDEA Food, CEI UAM + CSIC, Madrid, Spain.
Rodrigo San-Cristobal, Cardiometabolic Health Group, Precision Nutrition and Cardiometabolic Health Program, IMDEA Food, CEI UAM + CSIC, Madrid, Spain.
M José Concejo, Primary Care Center Ibiza, SERMAS, Madrid, Spain.
Miguel Á Martínez-González, Centro de Investigación Biomédica en Red Fisiopatología de la Obesidad y la Nutrición (CIBEROBN), Institute of Health Carlos III, Madrid, Spain; Department of Preventive Medicine and Public Health, Navarra Institute of Health Research (IdISNA), University of Navarra, Pamplona, Spain; Department of Nutrition, Harvard TH Chan School of Public Health, Boston, MA, USA.
Dolores Corella, Centro de Investigación Biomédica en Red Fisiopatología de la Obesidad y la Nutrición (CIBEROBN), Institute of Health Carlos III, Madrid, Spain; Department of Preventive Medicine, University of Valencia, Valencia, Spain.
Jordi Salas-Salvadó, Centro de Investigación Biomédica en Red Fisiopatología de la Obesidad y la Nutrición (CIBEROBN), Institute of Health Carlos III, Madrid, Spain; Universitat Rovira i Virgili, Departament of Biochemistry and Biotechnology, Human Nutrition Unit, Reus, Spain; Institut d'Investigació Sanitària Pere Virgili (IISPV), Reus, Spain; Nutrition Unit, University Hospital of Sant Joan de Reus, Reus, Spain; Unit of Preventive Medicine & Public Health, Faculty of Medicine & Health Sciences, Universitat Rovira i Virgili, Reus, Spain.
Albert Goday, Centro de Investigación Biomédica en Red Fisiopatología de la Obesidad y la Nutrición (CIBEROBN), Institute of Health Carlos III, Madrid, Spain; Unit of Cardiovascular Risk and Nutrition, Institut Hospital del Mar d`Investigació Médica (IMIM), Barcelona, Spain; Department of Medicine, Universitat Autonoma de Barcelona, Barcelona, Spain.
J Alfredo Martínez, Cardiometabolic Health Group, Precision Nutrition and Cardiometabolic Health Program, IMDEA Food, CEI UAM + CSIC, Madrid, Spain; Centro de Investigación Biomédica en Red Fisiopatología de la Obesidad y la Nutrición (CIBEROBN), Institute of Health Carlos III, Madrid, Spain; Center for Nutrition Research, Department of Nutrition, Food Sciences, and Physiology, University of Navarra, Pamplona, Spain.
Ángel M Alonso-Gómez, Centro de Investigación Biomédica en Red Fisiopatología de la Obesidad y la Nutrición (CIBEROBN), Institute of Health Carlos III, Madrid, Spain; Cardiovascular, Respiratory and Metabolic Area, Bioaraba Health Research Institute; Araba University Hospital, Osakidetza Basque Health Service; University of the Basque Country UPV/EHU, Vitoria-Gasteiz, Spain.
Julia Wärnberg, Centro de Investigación Biomédica en Red Fisiopatología de la Obesidad y la Nutrición (CIBEROBN), Institute of Health Carlos III, Madrid, Spain; Department of Nursing, University of Málaga, Institute of Biomedical Research in Malaga (IBIMA), Málaga, Spain.
Jesús Vioque, CIBER de Epidemiología y Salud Pública (CIBERESP), Carlos III Institute of Health (ISCIII), Madrid, Spain; Institute of Health and Biomedical Research of Alicante, Miguel Hernández University (ISABIAL-UMH), Alicante, Spain.
Dora Romaguera, Centro de Investigación Biomédica en Red Fisiopatología de la Obesidad y la Nutrición (CIBEROBN), Institute of Health Carlos III, Madrid, Spain; Research Group on Nutritional Epidemiology & Cardiovascular Physiopathology, Health Research Institute of the Balearic Islands (IdISBa), Palma de Mallorca, Spain.
José López-Miranda, Centro de Investigación Biomédica en Red Fisiopatología de la Obesidad y la Nutrición (CIBEROBN), Institute of Health Carlos III, Madrid, Spain; Department of Internal Medicine, Maimonides Biomedical Research Institute of Cordoba (IMIBIC), Reina Sofia University Hospital, University of Cordoba, Cordoba, Spain.
Ramon Estruch, Centro de Investigación Biomédica en Red Fisiopatología de la Obesidad y la Nutrición (CIBEROBN), Institute of Health Carlos III, Madrid, Spain; Department of Internal Medicine, Institut d’Investigacions Biomèdiques August Pi Sunyer (IDIBAPS), Hospital Clinic, University of Barcelona, Barcelona, Spain.
Francisco J Tinahones, Centro de Investigación Biomédica en Red Fisiopatología de la Obesidad y la Nutrición (CIBEROBN), Institute of Health Carlos III, Madrid, Spain; Department of Endocrinology, Virgen de la Victoria Hospital, Málaga Biomedical Research Institute (IBIMA), University of Málaga, Málaga, Spain.
José Lapetra, Centro de Investigación Biomédica en Red Fisiopatología de la Obesidad y la Nutrición (CIBEROBN), Institute of Health Carlos III, Madrid, Spain; Research Unit, Department of Family Medicine, Sevilla Primary Care Health District, Sevilla, Spain.
Lluís Serra-Majem, Centro de Investigación Biomédica en Red Fisiopatología de la Obesidad y la Nutrición (CIBEROBN), Institute of Health Carlos III, Madrid, Spain; Research Institute of Biomedical and Health Sciences (IUIBS), University of Las Palmas de Gran Canaria and Maternal and Child Insular University Hospital Center (CHUIMI), Canarian Health Service, Las Palmas de Gran Canaria, Spain.
Aurora Bueno-Cavanillas, CIBER de Epidemiología y Salud Pública (CIBERESP), Carlos III Institute of Health (ISCIII), Madrid, Spain; Department of Preventive Medicine and Public Health, University of Granada, Granada, Spain.
Josep A Tur, Centro de Investigación Biomédica en Red Fisiopatología de la Obesidad y la Nutrición (CIBEROBN), Institute of Health Carlos III, Madrid, Spain; Research Group on Community Nutrition & Oxidative Stress, University of the Balearic Islands, Palma de Mallorca, Spain.
Vicente Martín Sánchez, CIBER de Epidemiología y Salud Pública (CIBERESP), Carlos III Institute of Health (ISCIII), Madrid, Spain; Institute of Biomedicine (IBIOMED), University of León, León, Spain.
Xavier Pintó, Centro de Investigación Biomédica en Red Fisiopatología de la Obesidad y la Nutrición (CIBEROBN), Institute of Health Carlos III, Madrid, Spain; Lipids and Vascular Risk Unit, Internal Medicine, Bellvitge University Hospital, Barcelona, Spain.
José J Gaforio, CIBER de Epidemiología y Salud Pública (CIBERESP), Carlos III Institute of Health (ISCIII), Madrid, Spain; Department of Health Sciences, University Institute for Research on Olives and Olive Oils, University of Jaén, Jaén, Spain.
Pilar Matía-Martín, Department of Endocrinology and Nutrition, San Carlos Clinical Hospital Institute of Health Research (IdISSC), Madrid, Spain.
Josep Vidal, CIBER Diabetes y Enfermedades Metabólicas (CIBERDEM), Carlos III Institute of Health (ISCIII), Madrid, Spain; Department of Endocrinology, Institut d` Investigacions Biomédiques August Pi Sunyer (IDIBAPS), Hospital Clinic, University of Barcelona, Barcelona, Spain.
Sebastián Mas Fontao, Centro de Investigación Biomédica en Red Fisiopatología de la Obesidad y la Nutrición (CIBEROBN), Institute of Health Carlos III, Madrid, Spain; Department of Endocrinology and Nutrition, Jimenez Díaz Foundation Hospital Biomedical Research Institute (IISFJD), Autonomous University of Madrid, Madrid, Spain.
Emilio Ros, Centro de Investigación Biomédica en Red Fisiopatología de la Obesidad y la Nutrición (CIBEROBN), Institute of Health Carlos III, Madrid, Spain; Lipid Clinic, Department of Endocrinology and Nutrition, Institut d’Investigacions Biomèdiques August Pi Sunyer (IDIBAPS), Hospital Clínic, Barcelona, Spain.
Zenaida Vázquez-Ruiz, Centro de Investigación Biomédica en Red Fisiopatología de la Obesidad y la Nutrición (CIBEROBN), Institute of Health Carlos III, Madrid, Spain; Department of Preventive Medicine and Public Health, Navarra Institute of Health Research (IdISNA), University of Navarra, Pamplona, Spain; Endocrinology Service, Navarra Hospital Complex, Osasunbidea, Navarro Health Service, Pamplona, Spain.
Carolina Ortega-Azorín, Centro de Investigación Biomédica en Red Fisiopatología de la Obesidad y la Nutrición (CIBEROBN), Institute of Health Carlos III, Madrid, Spain; Department of Preventive Medicine, University of Valencia, Valencia, Spain.
Jesús F García-Gavilán, Centro de Investigación Biomédica en Red Fisiopatología de la Obesidad y la Nutrición (CIBEROBN), Institute of Health Carlos III, Madrid, Spain; Universitat Rovira i Virgili, Departament of Biochemistry and Biotechnology, Human Nutrition Unit, Reus, Spain; Institut d'Investigació Sanitària Pere Virgili (IISPV), Reus, Spain; Nutrition Unit, University Hospital of Sant Joan de Reus, Reus, Spain.
Mireia Malcampo, Centro de Investigación Biomédica en Red Fisiopatología de la Obesidad y la Nutrición (CIBEROBN), Institute of Health Carlos III, Madrid, Spain; Unit of Cardiovascular Risk and Nutrition, Institut Hospital del Mar d`Investigació Médica (IMIM), Barcelona, Spain.
Diego Martínez-Urbistondo, Internal Medicine Department, HM Sanchinarro Hospital, HM Hospitals, Madrid, Spain.
Lucas Tojal-Sierra, Cardiovascular, Respiratory and Metabolic Area, Bioaraba Health Research Institute; Araba University Hospital, Osakidetza Basque Health Service; University of the Basque Country UPV/EHU, Vitoria-Gasteiz, Spain.
Antonio García Rodríguez, Division of Preventive Medicine and Public Health, University of Malaga, Institute of Biomedical Research in Málaga (IBIMA-University of Malaga), Málaga, Spain.
Nuria Gómez-Bellvert, San Vicent del Raspeig Health Center, Alicante, Spain.
Alice Chaplin, Centro de Investigación Biomédica en Red Fisiopatología de la Obesidad y la Nutrición (CIBEROBN), Institute of Health Carlos III, Madrid, Spain; Research Group on Nutritional Epidemiology & Cardiovascular Physiopathology, Health Research Institute of the Balearic Islands (IdISBa), Palma de Mallorca, Spain.
Antonio García-Ríos, Department of Internal Medicine, Maimonides Biomedical Research Institute of Cordoba (IMIBIC), Reina Sofia University Hospital, University of Cordoba, Cordoba, Spain.
Rosa M Bernal-López, Centro de Investigación Biomédica en Red Fisiopatología de la Obesidad y la Nutrición (CIBEROBN), Institute of Health Carlos III, Madrid, Spain; Department of Endocrinology, Virgen de la Victoria Hospital, Málaga Biomedical Research Institute (IBIMA), University of Málaga, Málaga, Spain.
José M Santos-Lozano, Centro de Investigación Biomédica en Red Fisiopatología de la Obesidad y la Nutrición (CIBEROBN), Institute of Health Carlos III, Madrid, Spain; Research Unit, Department of Family Medicine, Sevilla Primary Care Health District, Sevilla, Spain.
Javier Basterra-Gortari, Centro de Investigación Biomédica en Red Fisiopatología de la Obesidad y la Nutrición (CIBEROBN), Institute of Health Carlos III, Madrid, Spain; Department of Preventive Medicine and Public Health, Navarra Institute of Health Research (IdISNA), University of Navarra, Pamplona, Spain; Endocrinology Service, Navarra Hospital Complex, Osasunbidea, Navarro Health Service, Pamplona, Spain.
José V Sorlí, Centro de Investigación Biomédica en Red Fisiopatología de la Obesidad y la Nutrición (CIBEROBN), Institute of Health Carlos III, Madrid, Spain; Department of Preventive Medicine, University of Valencia, Valencia, Spain.
Michelle Murphy, Centro de Investigación Biomédica en Red Fisiopatología de la Obesidad y la Nutrición (CIBEROBN), Institute of Health Carlos III, Madrid, Spain; Universitat Rovira i Virgili, Departament of Biochemistry and Biotechnology, Human Nutrition Unit, Reus, Spain; Institut d'Investigació Sanitària Pere Virgili (IISPV), Reus, Spain; Nutrition Unit, University Hospital of Sant Joan de Reus, Reus, Spain.
Griselda Gasulla, Centro de Investigación Biomédica en Red Fisiopatología de la Obesidad y la Nutrición (CIBEROBN), Institute of Health Carlos III, Madrid, Spain; Unit of Cardiovascular Risk and Nutrition, Institut Hospital del Mar d`Investigació Médica (IMIM), Barcelona, Spain.
Víctor Micó, Cardiometabolic Health Group, Precision Nutrition and Cardiometabolic Health Program, IMDEA Food, CEI UAM + CSIC, Madrid, Spain.
Itziar Salaverria-Lete, Centro de Investigación Biomédica en Red Fisiopatología de la Obesidad y la Nutrición (CIBEROBN), Institute of Health Carlos III, Madrid, Spain; Cardiovascular, Respiratory and Metabolic Area, Bioaraba Health Research Institute; Araba University Hospital, Osakidetza Basque Health Service; University of the Basque Country UPV/EHU, Vitoria-Gasteiz, Spain.
Estibaliz Goñi Ochandorena, Centro de Investigación Biomédica en Red Fisiopatología de la Obesidad y la Nutrición (CIBEROBN), Institute of Health Carlos III, Madrid, Spain; Department of Preventive Medicine and Public Health, Navarra Institute of Health Research (IdISNA), University of Navarra, Pamplona, Spain.
Nancy Babio, Centro de Investigación Biomédica en Red Fisiopatología de la Obesidad y la Nutrición (CIBEROBN), Institute of Health Carlos III, Madrid, Spain; Universitat Rovira i Virgili, Departament of Biochemistry and Biotechnology, Human Nutrition Unit, Reus, Spain; Institut d'Investigació Sanitària Pere Virgili (IISPV), Reus, Spain; Nutrition Unit, University Hospital of Sant Joan de Reus, Reus, Spain.
Xavier Herraiz, Centro de Investigación Biomédica en Red Fisiopatología de la Obesidad y la Nutrición (CIBEROBN), Institute of Health Carlos III, Madrid, Spain; Unit of Cardiovascular Risk and Nutrition, Institut Hospital del Mar d`Investigació Médica (IMIM), Barcelona, Spain.
José M Ordovás, Centro de Investigación Biomédica en Red Fisiopatología de la Obesidad y la Nutrición (CIBEROBN), Institute of Health Carlos III, Madrid, Spain; Nutritional Genomics and Epigenomics Group, Precision Nutrition and Obesity Program, IMDEA Food, CEI UAM + CSIC, Madrid, Spain; Nutrition and Genomics Laboratory, Jean Mayer USDA Human Nutrition Research Center on Aging, Tufts University, Boston, MA, USA.
Lidia Daimiel, Nutritional Control of the Epigenome Group, Precision Nutrition and Obesity Program, IMDEA Food, CEI UAM + CSIC, Madrid, Spain; Centro de Investigación Biomédica en Red Fisiopatología de la Obesidad y la Nutrición (CIBEROBN), Institute of Health Carlos III, Madrid, Spain.
Data availability
The PREDIMED-Plus trial imposed restrictions on data availability because of the signed consent agreements around data sharing. These only enable access to external researchers for studies following the project purposes. To request access to PREDIMED-Plus trial data used in this study, make a request to the PREDIMED-Plus trial Steering Committee chair at: jordi.salas@urv.cat. The request will then be passed to members of the PREDIMED-Plus Steering Committee for deliberation.
References
- 1. Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and its Panel on Folate, Other B Vitamins, and Choline . Dietary Reference Intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. Washington (DC): National Academies Press (US); 1998. [PubMed] [Google Scholar]
- 2. Zeisel SH, da Costa KA. Choline: an essential nutrient for public health. Nutr Rev. 2009;67(11):615–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wallace TC, Blusztajn JK, Caudill MA, Klatt KC, Natker E, Zeisel SHet al. . Choline: the underconsumed and underappreciated essential nutrient. Nutr Today. 2018;53(6):240–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wiedeman AM, Barr SI, Green TJ, Xu Z, Innis SM, Kitts DD. Dietary choline intake: current state of knowledge across the life cycle. Nutrients. 2018;10(10):1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yang S, Li X, Yang F, Zhao R, Pan X, Liang Jet al. . Gut microbiota-dependent marker TMAO in promoting cardiovascular disease: inflammation mechanism, clinical prognostic, and potential as a therapeutic target. Front Pharmacol. 2019;10:1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tang WHW, Wang Z, Levison BS, Koeth RA, Britt EB, Fu Xet al. . Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368(17):1575–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Senthong V, Wang Z, Li XS, Fan Y, Wu Y, Tang WHWet al. . Intestinal microbiota-generated metabolite trimethylamine-N-oxide and 5-year mortality risk in stable coronary artery disease: the contributory role of intestinal microbiota in a COURAGE-like patient cohort. J Am Heart Assoc. 2016;5(6):e002816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Senthong V, Li XS, Hudec T, Coughlin J, Wu Y, Levison Bet al. . Plasma trimethylamine N-oxide, a gut microbe–generated phosphatidylcholine metabolite, is associated with atherosclerotic burden. J Am Coll Cardiol. 2016;67(22):2620–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tang WHW, Wang Z, Shrestha K, Borowski AG, Wu Y, Troughton RWet al. . Intestinal microbiota-dependent phosphatidylcholine metabolites, diastolic dysfunction, and adverse clinical outcomes in chronic systolic heart failure. J Card Fail. 2015;21(2):91–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Costabile G, Vetrani C, Bozzetto L, Giacco R, Bresciani L, Del Rio Det al. . Plasma TMAO increase after healthy diets: results from 2 randomized controlled trials with dietary fish, polyphenols, and whole-grain cereals. Am J Clin Nutr. 2021;114(4):1342–50. [DOI] [PubMed] [Google Scholar]
- 11. Gao X, Wang Y, Sun G. High dietary choline and betaine intake is associated with low insulin resistance in the Newfoundland population. Nutrition. 2017;33:28–34. [DOI] [PubMed] [Google Scholar]
- 12. Virtanen JK, Tuomainen T-P, Voutilainen S. Dietary intake of choline and phosphatidylcholine and risk of type 2 diabetes in men: the Kuopio Ischaemic Heart Disease Risk Factor Study. Eur J Nutr. 2020;59(8):3857–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Noerman S, Kärkkäinen O, Mattsson A, Paananen J, Lehtonen M, Nurmi Tet al. . Metabolic profiling of high egg consumption and the associated lower risk of type 2 diabetes in middle-aged Finnish men. Mol Nutr Food Res. 2019;63(5):e1800605. [DOI] [PubMed] [Google Scholar]
- 14. Pourafshar S, Akhavan NS, George KS, Foley EM, Johnson SA, Keshavarz Bet al. . Egg consumption may improve factors associated with glycemic control and insulin sensitivity in adults with pre- and type II diabetes. Food Funct. 2018;9(8):4469–79. [DOI] [PubMed] [Google Scholar]
- 15. Dibaba DT, Johnson KC, Kucharska-Newton AM, Meyer K, Zeisel SH, Bidulescu A. The association of dietary choline and betaine with the risk of type 2 diabetes: the Atherosclerosis Risk in Communities (ARIC) study. Diabetes Care. 2020;43(11):2840–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Estruch R, Ros E, Salas-Salvadó J, Covas M-I, Corella D, Arós F, Estruch R, Ros E, Salas-Salvadó J, Covas M-I, Corella D, Arós Fet al. . Retraction and republication: primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med. 2013;368(14):1279–90. Retraction in:et al. N Engl J Med 2018;378(25):2441–2.23432189 [Google Scholar]
- 17. Vennemann FB, Ioannidou S, Valsta LM, Dumas C, Ocké MC, Mensink GBet al. . Dietary intake and food sources of choline in European populations. Br J Nutr. 2015;114(12):2046–55. [DOI] [PubMed] [Google Scholar]
- 18. Vázquez-Fresno R, Llorach R, Urpi-Sarda M, Lupianez-Barbero A, Estruch R, Corella Det al. . Metabolomic pattern analysis after Mediterranean diet intervention in a nondiabetic population: a 1- and 3-year follow-up in the PREDIMED study. J Proteome Res. 2015;14(1):531–40. [DOI] [PubMed] [Google Scholar]
- 19. Barrea L, Annunziata G, Muscogiuri G, Laudisio D, Di Somma C, Maisto Met al. . Trimethylamine N-oxide, Mediterranean diet, and nutrition in healthy, normal-weight adults: also a matter of sex?. Nutrition. 2019;62:7–17. [DOI] [PubMed] [Google Scholar]
- 20. Long J-A, Zhong R-H, Chen S, Wang F, Luo Y, Lu X-Tet al. . Dietary betaine intake is associated with skeletal muscle mass change over 3 years in middle-aged adults: the Guangzhou Nutrition and Health Study. Br J Nutr. 2021;125(4):440–7. [DOI] [PubMed] [Google Scholar]
- 21. Zhong R-H, Long J-A, Wang F, Chen S, Luo Y, Lu X-Tet al. . Association between serum choline and betaine concentrations and longitudinal changes of body composition in community-dwelling middle-aged and older Chinese adults. Appl Physiol Nutr Metab. 2020;45(7):737–44. [DOI] [PubMed] [Google Scholar]
- 22. Taylor RM, Smith R, Collins CE, Mossman D, Wong-Brown MW, Chan E-Cet al. . Methyl-donor and cofactor nutrient intakes in the first 2–3 years and global DNA methylation at age 4: a prospective cohort study. Nutrients. 2018;10(3):273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Miller CA, Corbin KD, da Costa KA, Zhang S, Zhao X, Galanko JAet al. . Effect of egg ingestion on trimethylamine-N-oxide production in humans: a randomized, controlled, dose-response study. Am J Clin Nutr. 2014;100(3):778–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Martínez-González MA, Buil-Cosiales P, Corella D, Bulló M, Fitó M, Vioque Jet al. . Cohort profile: design and methods of the PREDIMED-Plus randomized trial. Int J Epidemiol. 2019;48(2):387–388o. [DOI] [PubMed] [Google Scholar]
- 25. Salas-Salvadó J, Díaz-López A, Ruiz-Canela M, Basora J, Fitó M, Corella Det al. . Effect of a lifestyle intervention program with energy-restricted Mediterranean diet and exercise on weight loss and cardiovascular risk factors: one-year results of the PREDIMED-Plus trial. Diabetes Care. 2019;42(5):777–88. [DOI] [PubMed] [Google Scholar]
- 26. Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KAet al. . Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–5. [DOI] [PubMed] [Google Scholar]
- 27. Willett W. Nutritional epidemiology. 3rd ed. Oxford (United Kingdom): Oxford University Press; 2012. [Google Scholar]
- 28. Díaz-López A, Becerra-Tomás N, Ruiz V, Toledo E, Babio N, Corella Det al. . Effect of an intensive weight-loss lifestyle intervention on kidney function: a randomized controlled trial. Am J Nephrol. 2021;52(1):45–58. [DOI] [PubMed] [Google Scholar]
- 29. Fernández-Ballart JD, Piñol JL, Zazpe I, Corella D, Carrasco P, Toledo Eet al. . Relative validity of a semi-quantitative food-frequency questionnaire in an elderly Mediterranean population of Spain. Br J Nutr. 2010;103(12):1808–16. [DOI] [PubMed] [Google Scholar]
- 30. Moreiras O, Carbajal A, Cabrera L, Cuadrado C. Tablas de composición de alimentos (Spanish food composition tables). 19th ed. Madrid (Spain): Pirámide; 2018. [Google Scholar]
- 31. Cho E, Zeisel SH, Jacques P, Selhub J, Dougherty L, Colditz GAet al. . Dietary choline and betaine assessed by food-frequency questionnaire in relation to plasma total homocysteine concentration in the Framingham Offspring Study. Am J Clin Nutr. 2006;83(4):905–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HIet al. . A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schröder H, Zomeño MD, Martínez-González MA, Salas-Salvadó J, Corella D, Vioque Jet al. . Validity of the energy-restricted Mediterranean Diet Adherence Screener. Clin Nutr. 2021;40(8):4971–9. [DOI] [PubMed] [Google Scholar]
- 34. Molina L, Sarmiento M, Peñafiel J, Donaire D, Garcia-Aymerich J, Gomez Met al. . Validation of the Regicor short physical activity questionnaire for the adult population. PLoS One. 2017;12(1):e0168148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Martínez-González MA, López-Fontana C, Varo JJ, Sánchez-Villegas A, Martinez JA. Validation of the Spanish version of the physical activity questionnaire used in the Nurses’ Health Study and the Health Professionals’ Follow-up Study. Public Health Nutr. 2005;8(7):920–7. [DOI] [PubMed] [Google Scholar]
- 36. Wallace JM, McCormack JM, McNulty H, Walsh PM, Robson PJ, Bonham MPet al. . Choline supplementation and measures of choline and betaine status: a randomised, controlled trial in postmenopausal women. Br J Nutr. 2012;108(7):1264–71. [DOI] [PubMed] [Google Scholar]
- 37. Selvin E, Parrinello CM. Age-related differences in glycaemic control in diabetes. Diabetologia. 2013;56(12):2549–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bird SR, Hawley JA. Update on the effects of physical activity on insulin sensitivity in humans. BMJ Open Sport Exerc Med. 2017;2(1):e000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Martín-Peláez S, Fito M, Castaner O. Mediterranean diet effects on type 2 diabetes prevention, disease progression, and related mechanisms. A review. Nutrients. 2020;12(8):2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chen Z, Watanabe RM, Stram DO, Buchanan TA, Xiang AH. High calorie intake is associated with worsening insulin resistance and β-cell function in Hispanic women after gestational diabetes mellitus. Diabetes Care. 2014;37(12):3294–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chen L, Chen Y-m, Wang L-j, Wei J, Tan Y-z, Zhou J-yet al. . Higher homocysteine and lower betaine increase the risk of microangiopathy in patients with diabetes mellitus carrying the GG genotype of PEMT G774C. Diabetes Metab Res Rev. 2013;29(8):607–17. [DOI] [PubMed] [Google Scholar]
- 42. Li Y, Wang DD, Chiuve SE, Manson JE, Willett WC, Hu FBet al. . Dietary phosphatidylcholine intake and type 2 diabetes in men and women. Diabetes Care. 2015;38(2):e13–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Papandreou C, Bulló M, Zheng Y, Ruiz-Canela M, Yu E, Guasch-Ferré Met al. . Plasma trimethylamine-N-oxide and related metabolites are associated with type 2 diabetes risk in the Prevención con Dieta Mediterránea (PREDIMED) trial. Am J Clin Nutr. 2018;108(1):163–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shan Z, Sun T, Huang H, Chen S, Chen L, Luo Cet al. . Association between microbiota-dependent metabolite trimethylamine-N-oxide and type 2 diabetes. Am J Clin Nutr. 2017;106(3):888–94. [DOI] [PubMed] [Google Scholar]
- 45. Detopoulou P, Panagiotakos DB, Antonopoulou S, Pitsavos C, Stefanadis C. Dietary choline and betaine intakes in relation to concentrations of inflammatory markers in healthy adults: the ATTICA study. Am J Clin Nutr. 2008;87(2):424–30. [DOI] [PubMed] [Google Scholar]
- 46. Rains JL, Jain SK. Oxidative stress, insulin signaling, and diabetes. Free Radic Biol Med. 2011;50(5):567–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kathirvel E, Morgan K, Nandgiri G, Sandoval BC, Caudill MA, Bottiglieri Tet al. . Betaine improves nonalcoholic fatty liver and associated hepatic insulin resistance: a potential mechanism for hepatoprotection by betaine. Am J Physiol Gastrointest Liver Physiol. 2010;299(5):G1068–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Palazón-Bru A, Hernández-Lozano D, Gil-Guillén VF. Which physical exercise interventions increase HDL-cholesterol levels? A systematic review of meta-analyses of randomized controlled trials. Sports Med. 2021;51(2):243–53. [DOI] [PubMed] [Google Scholar]
- 49. Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BTet al. . Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19(5):576–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar Bet al. . Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472(7341):57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Roncal C, Martínez-Aguilar E, Orbe J, Ravassa S, Fernandez-Montero A, Saenz-Pipaon Get al. . Trimethylamine-N-oxide (TMAO) predicts cardiovascular mortality in peripheral artery disease. Sci Rep. 2019;9(1):15580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Blesso CN, Andersen CJ, Barona J, Volek JS, Fernandez ML. Whole egg consumption improves lipoprotein profiles and insulin sensitivity to a greater extent than yolk-free egg substitute in individuals with metabolic syndrome. Metabolism. 2013;62(3):400–10. [DOI] [PubMed] [Google Scholar]
- 53. DiBella M, Thomas MS, Alyousef H, Millar C, Blesso C, Malysheva Oet al. . Choline intake as supplement or as a component of eggs increases plasma choline and reduces interleukin-6 without modifying plasma cholesterol in participants with metabolic syndrome. Nutrients. 2020;12(10):3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. DiMarco DM, Missimer A, Murillo AG, Lemos BS, Malysheva OV, Caudill MAet al. . Intake of up to 3 eggs/day increases HDL cholesterol and plasma choline while plasma trimethylamine-N-oxide is unchanged in a healthy population. Lipids. 2017;52(3):255–63. [DOI] [PubMed] [Google Scholar]
- 55. Lemos BS, Medina-Vera I, Malysheva OV, Caudill MA, Fernandez ML. Effects of egg consumption and choline supplementation on plasma choline and trimethylamine-N-oxide in a young population. J Am Coll Nutr. 2018;37(8):716–23. [DOI] [PubMed] [Google Scholar]
- 56. Ratliff JC, Mutungi G, Puglisi MJ, Volek JS, Fernandez ML. Eggs modulate the inflammatory response to carbohydrate restricted diets in overweight men. Nutr Metab. 2008;5(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Guo F, Qiu X, Zhu Y, Tan Z, Li Z, Ouyang D. Association between plasma betaine levels and dysglycemia in patients with coronary artery disease. Biosci Rep. 2020;40(8):BSR20200676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Nojima J, Meguro S, Ohkawa N, Furukoshi M, Kawai T, Itoh H. One-year eGFR decline rate is a good predictor of prognosis of renal failure in patients with type 2 diabetes. Proc Jpn Acad Ser B Phys Biol Sci. 2017;93(9):746–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tallman DA, Sahathevan S, Karupaiah T, Khosla P. Egg intake in chronic kidney disease. Nutrients. 2018;10(12):1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The PREDIMED-Plus trial imposed restrictions on data availability because of the signed consent agreements around data sharing. These only enable access to external researchers for studies following the project purposes. To request access to PREDIMED-Plus trial data used in this study, make a request to the PREDIMED-Plus trial Steering Committee chair at: jordi.salas@urv.cat. The request will then be passed to members of the PREDIMED-Plus Steering Committee for deliberation.