ABSTRACT
Background
Gut microbiota profiles are closely related to cardiovascular diseases through mechanisms that include the reported deleterious effects of metabolites, such as trimethylamine N-oxide (TMAO), which have been studied as diagnostic and therapeutic targets. Moderate red wine (RW) consumption is reportedly cardioprotective, possibly by affecting the gut microbiota.
Objectives
To investigate the effects of RW consumption on the gut microbiota, plasma TMAO, and the plasma metabolome in men with documented coronary artery disease (CAD) using a multiomics assessment in a crossover trial.
Methods
We conducted a randomized, crossover, controlled trial involving 42 men (average age, 60 y) with documented CAD comparing 3-wk RW consumption (250 mL/d, 5 d/wk) with an equal period of alcohol abstention, both preceded by a 2-wk washout period. The gut microbiota was analyzed via 16S rRNA high-throughput sequencing. Plasma TMAO was evaluated by LC-MS/MS. The plasma metabolome of 20 randomly selected participants was evaluated by ultra-high-performance LC-MS/MS. The effect of RW consumption was assessed by individual comparisons using paired tests during the abstention and RW periods.
Results
Plasma TMAO did not differ between RW intervention and alcohol abstention, and TMAO concentrations showed low intraindividual concordance over time, with an intraclass correlation coefficient of 0.049 during the control period. After RW consumption, there was significant remodeling of the gut microbiota, with a difference in β diversity and predominance of Parasutterella, Ruminococcaceae, several Bacteroides species, and Prevotella. Plasma metabolomic analysis revealed significant changes in metabolites after RW consumption, consistent with improved redox homeostasis.
Conclusions
Modulation of the gut microbiota may contribute to the putative cardiovascular benefits of moderate RW consumption. The low intraindividual concordance of TMAO presents challenges regarding its role as a cardiovascular risk biomarker at the individual level. This study was registered at clinical trials.gov as NCT03232099.
Keywords: gut microbiota, metabolomics, trimethylamine N-oxide (TMAO), coronary artery disease, wine, redox
Introduction
Recognition of the roles of the gut microbiota in the pathophysiology of cardiovascular diseases (CVDs) (1) and of diet in influencing the microbiota's composition, function, and interaction with the human host (2, 3) has increased. Trimethylamine N-oxide (TMAO), a gut microbiota-dependent metabolite of dietary quaternary ammonium compounds, largely choline and carnitine, has been widely reported to correlate with CVD and major adverse cardiovascular events (MACEs). Different taxa within the gut microbiota metabolize these dietary precursors to trimethylamine (TMA), which is converted to TMAO by flavin monooxygenase enzymes in the liver. Plasma TMAO is associated with atherosclerotic burden and is independently predictive of myocardial infarction, stroke, and cardiovascular death (4).
Different interventions have been proposed to modify plasma TMAO concentrations, such as dietary modifications related to choline content (5), through modulation of the gut microbiota with dietary prebiotic supplements (6, 7) and medical interventions that manipulate the gut microbiota. For instance, experimental studies using a structural analogue of choline, 3,3-dimethyl-1-butanol (DMB) (8), and the gut microbiota-targeting inhibitor iodomethylcholine (9) have been shown to nonlethally inhibit the formation of TMA and TMAO and reduce TMAO concentrations.
One potential influencer of the gut microbiota and plasma TMAO is red wine (RW). RW has been proposed to have health-promoting effects. Moderate RW consumption is correlated with a lower incidence of cardiovascular events (10), cancer (11), and overall mortality (10, 12), which is mainly attributed to its polyphenolic components. The main phenolic compounds in wine are flavonoids, particularly flavan-3-ols, flavonols, and anthocyanins (13). Other nonflavonoid compounds, such as hydroxybenzoic and hydroxycinnamic acids, phenolic alcohols, and stilbenes, are also key components in wine. The polyphenol chemical structure, the food matrix, and enterohepatic circulation can influence polyphenol bioavailability and absorption, and a large percentage of polyphenols is not absorbed in the small intestine. Consequently, ∼90% of polyphenols reach the colon intact, where they can exert a regulatory function and probably act as prebiotics; there, they serve as fuel for bacterial fermentation and are accessible to a large proportion of the gut bacteria (14). Therefore, some beneficial effects of RW might occur through alterations of the gut microbiota (15), fecal metabolome (16), and plasma metabolites (17). For instance, the grape polyphenol proanthocyanidin induces intestinal blooms of Akkermansia muciniphila, and this genus is associated with improved metabolic health (18). Furthermore, Le Roy et al. (19) reported increased gut microbiota α diversity associated with RW consumption in 3 independent human cohorts. In addition, RW contains DMB, a structural analogue of choline, which may inhibit TMA formation in the gut and decrease plasma TMAO concentrations, according to a rodent study (20). In this context, the biological activity of polyphenols in the circulatory system may also depend on the ability of the gut microbiota to transform them into bioavailable metabolites. This highlights the importance of assessing both microbiota and plasma metabolites when a given intervention is tested.
However, the relations between the gut microbiota, TMAO, and plasma metabolomics in patients with coronary artery disease (CAD) remain understudied. The present study aimed to assess the effects of short-term, moderate RW consumption on the gut microbiota and plasma TMAO in 42 male patients with CAD compared with those of alcohol abstention in the same individuals. In addition, it also sought to elucidate the effects of RW on the plasma metabolome in a subset of 20 patients.
Methods
Trial design
This was a randomized, controlled (1:1) crossover trial composed of two 3-wk interventions: one involved the consumption of RW, 250 mL a day, 5 d a week, and the other involved alcohol abstention. Each intervention was preceded by a 2-wk washout period, without consumption of any alcoholic beverages, fermented foods, prebiotics, or probiotics. The trial was registered at www.clinicaltrials.gov as NCT03232099 and was conducted from August 2016 to May 2018.
Patients
Patients were recruited from the Heart Institute, University of São Paulo, São Paulo, Brazil, and from advertisements in local newspapers and on local radio stations. Eligible patients with established CAD were men who were aged between 46 and 69 y, had a BMI (in kg/m2) <30, and were stable and asymptomatic. Only men were selected with the intention of homogenizing our sample since both alcohol metabolism (21) and TMAO metabolism may differ by sex; the activity of flavin monooxygenase 3 (FMO3) has been reported to be higher in female mice than in male mice (22). Established CAD was defined as a diagnosis at least 30 d before the beginning of the study of myocardial infarction, angiographic evidence of ≥50% stenosis of 1 or more epicardial vessels, coronary angioplasty, or coronary artery bypass grafting. Importantly, the included patients had no evidence of acute coronary syndrome (cardiac troponin T concentration, <0.1 µg/L), coronary angioplasty, or coronary artery bypass grafting at least 30 d prior to protocol initiation. The exclusion criteria included antibiotic treatments (<2 mo before the start of the study), heart failure (New York Heart Association functional class ≥II), renal failure (creatinine clearance <30 mL/min by the Cockcroft–Gault formula), hepatic failure (thrombocytopenia, reduced serum albumin, and prolonged prothrombin time), digestive tract cancers, inflammatory bowel diseases, obstructive biliary diseases, prior digestive surgeries (cholecystectomy, gastrectomy or colectomy), and diabetes mellitus or the use of antidiabetic drugs. Patients who self-reported to be regular RW drinkers (defined as a previous consumption of at least 100 mL RW a day) were not included in the study. Patients were also required to have an Alcohol Use Disorders Identification Test (AUDIT) score of 7 or less. The AUDIT (23) score is used to identify individuals with hazardous or harmful alcohol consumption behavior; scores up to 7 suggest a low risk of alcohol abuse. The Scientific Committee of the Heart Institute (Instituto do Coração—InCor-HCFMUSP) and the Institutional Ethical Committee of the Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo approved the study protocol (SDC 4257/15/084—CAAE 57379216.0.0000.0068). All patients gave written informed consent before participating in the study.
Sample size
For sample size calculation, we assumed that the TMAO concentration would decrease by 1.0 µM after the RW intervention and that there would be a within-patient standard deviation of 1.5. These assumptions were made according to Tang et al. (24). We assumed a type I error of P < 0.05 and an 80% test power, and it was estimated that 38 patients would be necessary. Thus, considering a 10% loss rate during the intervention, it was found that 42 patients should be included; the calculation was performed with Sample Size Calculators (http://hedwig.mgh.harvard.edu/sample_size/js/js_crossover_quant.html) (25).
Randomization
The random allocation sequence of patients was generated by the Random Integer Generator (random.org) at the time of patient inclusion in the study, as shown in the Consort Flow Diagram in Supplemental Figure 1. The random allocation sequence was implemented using the participant IDs matched with the allocated sequences.
Interventions
The patients were randomly assigned to start either a 3-wk intervention with RW or a 3-wk intervention with alcohol abstention. RW was provided for all patients at the beginning of the respective intervention period. Ibravin (Brazilian Wine Institute) produced and supplied RW made with the Merlot grape variety. Embrapa (Brazilian Agricultural Research Corporation) assessed the quality of the wine and released it for consumption after their technical approval. The wine used was a Merlot from the 2014 vintage, bottled in August 2016 in 250-mL bottles and customized for the study. The Merlot variety was chosen because it is one of the grapes that is best adapted to the soil and climate of Rio Grande do Sul state, Brazil, where the samples were customized. The RW dosage of 250 mL, with ∼24 g of alcohol, was selected because this amount of RW has been shown to be associated with beneficial effects (26) and has been used in several previous studies investigating the effects of RW on the gut microbiota (15, 27). For men, moderate alcohol consumption can be defined as the ingestion of 1–3 standard drinks per day or the equivalent of 10–30 g of alcohol (28).
The RW sample had an alcohol content (% v) of 12.75, a total acidity (mEq/L) of 95.58, and a volatile acidity (mEq/L) of 7.44. It contained 109 mg/L total anthocyanins (DescSO2) and 2155 mg/L total polyphenols in catechin. Patients received 21 labeled bottles and written guidance to drink 250 mL/d, 5 d a week, for 3 wk. They were also given a dietary recall form to record their daily wine consumption. After the RW intervention period, the patients returned the 21 empty RW bottles and completed the consumption diary.
Overview of protocol visits and measures
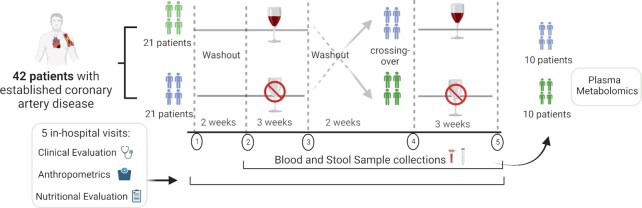
After written informed consent was obtained and randomization was performed, the patients returned 5 times for in-hospital visits consisting of clinical and nutritional assessments, anthropometric measurements, and sample collection. On the first visit, there was no sample collection, and a trained nurse or physician interviewed the patients; recorded their vital signs, weight, height, and waist circumference; and gave instructions about the stool sample collection that would be carried out at home 1 d prior to each of the subsequent 4 visits. In addition, during the first visit, patients underwent nutritional assessment by a trained dietitian, who provided instructions about completing the 3-d dietary recall and advised patients to maintain their diet and physical activity routine. Patients also received written and verbal instructions regarding the 2-wk washout period, during which they were instructed to avoid any alcoholic beverages, fermented products, prebiotics, or probiotics. After the 2 washout periods and the 2 intervention periods, 4 hospital visits were carried out, during which blood sample collection, clinical interviews, and nutritional evaluations were performed. A schematic view of the study design is shown in the Figure 1. The patients brought stool samples that were collected at home 1 d before each visit. The primary endpoint was to assess changes regarding gut microbiota composition and plasma TMAO concentrations between RW consumption and alcohol abstention groups. For biochemical analysis, TMAO analysis, microbiota analysis, and metabolomic analysis, the assessors who collected the outcome data and the data analysts were blinded to the assigned intervention.
FIGURE 1.
Study design. A total of 42 patients with established coronary artery disease were randomly selected for 1 of the 2 groups in the crossover study involving red wine (RW) consumption for 3 wk or alcohol abstention for 3 wk. All patients were evaluated over 5 in-hospital visits for anthropometrics and clinical and nutritional assessments. After an initial evaluation, there was a 2-wk washout period when patients were instructed not to consume alcoholic beverages, fermented foods (yogurt, kombucha, soy lecithin, kefir, sauerkraut, and other fermented veggies), synthetic prebiotics (insulin, fructooligosaccharides), fiber, dairy, food polyphenols (grapes, grape juice, cranberries, strawberries), and probiotics. Samples were collected after the first 2-wk washout period, and patients were randomly assigned for a 3-wk intervention with RW consumption (250 mL/d, 5 d/wk) or 3 wk of alcohol abstention. After these 3 wk, new blood and stool samples were collected, and another 2-wk washout period was implemented. Then, new samples were collected, and patients crossed over: the group that received RW was instructed to abstain from alcohol for 3 wk, and the group that abstained from alcohol in the first 3 wk received RW. After the second 3-wk period, stool and blood samples were collected in both groups. In 20 randomly selected patients (10 from each group), plasma metabolomics was analyzed after the 2 washout periods and after the intervention with RW and abstention.
Dietary intake assessment and compliance
A trained dietitian conducted an in-person interview at every in-hospital visit, focusing on the completion of the dietary diaries and adherence to the dietary guidelines, that is, avoidance of other polyphenol-rich foods, any alcoholic beverages other than the provided RW, prebiotics, probiotics, and fermented products and maintenance of a similar diet pattern throughout the study. In this context, there were written instructions to avoid the consumption of beer, grapes or grape juice, berries or berry juices, yogurt, kombucha, kefir sauerkraut or other fermented veggies, and any products with added fiber or synthetic prebiotics, such as inulin, fructooligosaccharides, or fiber dairy. Patients were also instructed to call or e-mail the dietitian if they had any doubts about the consumption of these products. These measures aimed to decrease the interference of changes in the habitual diet other than RW on the gut microbiota. Patients completed a 3-d dietary recall; they were asked to recall their food consumption in the past 24 h, including all food and drink consumed, for 3 d (2 weekdays and 1 weekend day) at the end of both interventions. The dietary assessment was recorded manually and was dietitian-led reviewed after returning. The daily intake of total energy, macronutrients, and micronutrients was calculated according to the Brazilian Food Composition Table—TACO (29) and the second Brazilian Food Composition Table (http://www.fcf.usp.br/tbca/).
Interruption criteria
Patients were periodically evaluated with respect to safety. In addition to hospital visits, patients were encouraged to contact the study staff by phone or e-mail if they experienced any adverse health-related impacts of the interventions. In the case of RW intolerance—limiting headaches, dizziness, gastrointestinal complaints, or other forms of intolerance—patients were excluded. If there were any MACEs such as myocardial infarction, stroke, heart or digestive tract surgery, or the use of antibiotics or antidiabetic drugs, the protocol was interrupted.
Metagenome profile
Patients received verbal and written instructions regarding the collection of stool samples. They were advised to collect the samples using a collection tube with DNA-stabilizing buffer (STRATEC Biomedical AG) 1 d prior to the visit to the hospital or on the same day of the visit. The samples were immediately stored in provided coolers at 4°C and then transported to the hospital, where they were immediately frozen at –20°C until analysis. Total DNA was extracted from the intestinal microbiota in 200 mg of stool with the PSP Spin Stool Plus DNA kit (STRATEC Biomedical AG). To profile the gut microbiota composition, the hypervariable region (V3–V4) of the bacterial 16S rRNA gene was amplified following the Illumina 16S Metagenomic Sequencing Library Preparation guide (30), which uses the sequences 338F-5′- TCGT CGGC AGCG TCAG ATGT GTAT AAGA GACA GCCT ACGG GNGG CWGC AG-3′ and 785R-5′-GTCT CGTG GGCT CGGA GATG TGTA TAAG AGAC AGGA CTAC HVGG GTAT CTAA TCC-3′ (2 × 300 bp paired-end reads with an insert size of ∼550 bp).
Gut microbiota bioinformatic analysis
The DADA2 tool was used to analyze the fastq sequences to recover single-nucleotide resolved amplicon sequence variants (ASVs) from the amplicon data, according to Magro et al. (31). The total number of ASVs was 1106. To remove the adapter sequences at the 5′ end, the trimLeft option was set to 17 and 21 (forward and reverse reads, respectively). The data were preprocessed, removing possible contaminants (mitochondrial and chloroplast sequences) and filtering infrequent features (features with <10 read counts and present in <2 samples). The taxonomic assignment was performed with the naive Bayesian classifier method implemented in DADA2 using the SILVA database as a reference (version 132). The α diversity was evaluated with the richness, Simpson, and Shannon indexes, and β diversity was assessed with sparse partial least squares regression-discriminant analysis. All gut microbiota data were compared through paired analyses.
Integration analysis of gut microbiota and plasma metabolomics data
Data Integration Analysis for Biomarker discovery using Latent cOmponents (DIABLO) R package (32) version 6.3.2 (http://mixomics.org/mixdiablo/) was used to evaluate and integrate these 2 data sets. DIABLO is a multiomics integrative method that seeks correlated information across different data types by selecting a subset of molecular features while discriminating between multiple phenotypic groups. It maximizes the common or correlated information between multiple omics (multiomics) data sets while identifying the key omics variables. DIABLO is a component-based method (or a dimension reduction technique) that transforms each data set into components maximizing the correlations between components and the phenotype of interest. The first step in DIABLO analysis involves choosing the number of components. The correct number of components can be assessed by evaluating the model performance across all the specified numbers of components.
This analysis aimed to simultaneously identify correlated and discriminant microbiota and plasma metabolomics features able to distinguish the RW and alcohol abstention interventions in the cohort of patients. We evaluated the model across 5 components. The assessment was performed using the perf function, setting the cross-validation method to leave-one-out (validation = “loo”) (32). During this process, the assignment of each new observation to the final predicted class was performed using different prediction distances (maximum, centroids, or Mahalanobis). The performance plot (Supplemental Figure 2) shows that the error rate decreased from 1 to 2 components.
In our data set, we found that the Mahalanobis distance (a multivariate distance metric that measures the distance between a point and a distribution) yielded the best accuracy, indicating that the optimal number of components was 2. The next step was to tune the number of features selected per data set and per component (keepX parameters) using the tune.block.splsda function. The optimal number of variables was selected within a grid of possible keepX variables (from a minimum of 5 to a maximum of 30 features). For each number of keepX variables, the function assesses the model's performance to discriminate between the 2 groups using leave-one-out cross-validation. The model identified 6 taxa for the first component and 5 for the second component, as well as 9 metabolites for the first component and 5 for the second component. These selected features were considered representative of the multiomics biomarkers that better discriminated the RW and alcohol abstention interventions.
TMAO assessment
During the protocol, after a 12-h overnight fast, all 42 patients had blood samples collected at every 1 of 4 examination visits. After collection, the blood samples were stored in the refrigerator, separated into serum and plasma, placed in Eppendorf tubes, and immediately stored at –80°C until analysis. All blood sample analyses were performed in only 2 batches to ensure low variability.
Assessment of TMAO intraindividual variability
To assess TMAO intraindividual variability, we randomly selected 10 patients for 4 additional visits (twice a week) after the protocol was finished. After a 12-h fast, blood samples were collected and analyzed by 3 techniques. Patients were advised not to modify medication, diet, or exercise. The assessments of variability were performed using 3 different collection techniques: 1) blood samples were collected, separated into serum and plasma, placed in Eppendorf tubes, and immediately frozen at –80°C after collection; 2) blood samples were collected, separated into serum and plasma, placed in Eppendorf tubes, and refrigerated for 2 h after collection and then frozen at –80°C; or 3) blood samples from the fingertips were used for dried blood spot (DBS) analysis.
TMAO quantification
The method used was described by Wang et al. (33). TMAO was extracted from a 50-μL plasma sample using 200 μL methanol (containing the internal standard TMAO-d9 at 10 μM) to denature the proteins after mechanical agitation (vortex). The plasma samples were centrifuged to precipitate the proteins, and an aliquot of the supernatant was collected for injection into the LC-MS/MS system. The analytical curve (from 0.25 to 200 μM TMAO) and control samples (0.5, 5, and 100 μM TMAO) were prepared through the addition of TMAO (μM) to a 5% bovine serum albumin (Sigma-Aldrich) aqueous solution containing 0.7% sodium chloride (w/w). TMAO and TMAO analytical standards were purchased from Sigma-Aldrich. The DBS samples were obtained from 2 drops of whole blood collected on Whatman Paper 903 (Protein Saver Card; GE Healthcare) after fingertip pricking. The DBS cards were dried at room temperature for 1 h. To extract TMAO from the DBS samples, two 3.0-mm circular “punches” were obtained from the DBS card and placed in a 1.5-mL Eppendorf tube, and 150 µL 75% methanol/25% water (v/v) solution containing a 1.0-µM concentration of the internal standard TMAO-d9 was added. The tube was mixed with a vortex for 10 s, ultrasonicated for 3 min, vortexed for another 10 s, and then centrifuged at 10,000 × g for 3 min at 4 °C. An aliquot of the extracted solution was transferred to a 96-well plate (or vials) and injected into the LC-MS/MS system. The analytical curve (from 0.25 to 200 μM TMAO) and control samples (0.5, 5, and 100 μM TMAO) were prepared through the addition of TMAO (μM) to whole blood (lyophilized form; ControLab) without the presence of TMAO. The blood was collected on a card following the TMAO extraction procedure and then analyzed. To quantify TMAO and its internal standard TMAO-d9 in the extracted plasma and DBS samples, liquid chromatography (Agilent 1260; Agilent Technologies) coupled to a hybrid triple quadrupole linear ion trap mass spectrometer (3200 QTRAP; SCIEX) was used. The compounds were separated in the LC system using an Atlantis HILIC Silica column (100 × 3.0 mm, 3 μm; Waters Corp) maintained at 35°C and the following mobile phases: (A) aqueous solution containing 10 mM ammonium formate (LC-MS grade; Sigma-Aldrich) and (B) 90% acetonitrile/10% water (v/v) solution containing 10 mM ammonium formate. A 10-µL sample volume was injected, and the compounds were eluted through the column using the following gradient conditions at a flow rate of 500 µL/min: from 0 to 1 min, 0% mobile phase (A); from 1.0 to 5.20 min, 90% (A) to 10% (B); from 5.20 to 7.50 min, maintained at 90% (A); and from 7.50 to 11.5 min, returned to the initial 0% condition (increased the flow to 600 µL/min) and allowed to equilibrate for an extra 1.0 min before reinjection. The TMAO/TMAO-d9 retention time was 6.15 min. After separation, the compounds were ionized by an electrospray ionization (Turbo V; SCIEX) source in positive mode at 5200 V with the nebulizer gas at 45 psi, heater gas at 50 psi, and curtain gas at 15 psi and analyzed in the MS/MS system by multiple reaction mode optimized for the following m/z transitions: 76 (precursor ion) > 58 (product ion) and 85 > 66 for TMAO and TMAO-d9, respectively.
Plasma metabolomics
Using a random integer generator (random.org), 20 of the 42 patients were randomly assigned to have their plasma metabolomic profiles analyzed. The sample size for this subanalysis was determined using the program MetSizeR (34). From the blood samples collected at the 4 visits, 20 patients and 80 samples were selected for untargeted plasma metabolomic analysis. The samples were stored at –80°C and assayed with an untargeted evaluation by ultra-HPLC–MS/MS (Waters ACQUITY) coupled with a HESI-II Thermo Scientific Q-Exactive Orbitrap (35,000 mass resolution) performed by Metabolon. Brief methodologic details are provided in the Supplemental Methods.
Fasting blood sample biochemical analyses
Blood samples of the 42 patients were collected on all 4 examination days after a 12-h fast. Blood sample analyses were performed on the day of collection. At every visit, we analyzed fasting concentrations of plasma glucose, triglycerides, total cholesterol, HDL cholesterol, LDL cholesterol, alanine aminotransferase, aspartate aminotransferase, serum albumin, serum bilirubin, serum lactate dehydrogenase, γ-glutamyl transferase, prothrombin time, high-sensitivity C-reactive protein, IL-6, complete blood count, creatine, urea, and serum lipopolysaccharides. These tests were performed on a Dimension EXL analyzer (Siemens Healthcare), except for troponin I, which was measured using a chemiluminescence immunometric assay (ADVIA Centaur-XP; TnI-Ultra; Siemens Healthineers). Blood counts of total hemoglobin, leukocytes, neutrophils, lymphocytes, and other immune cells (including monocytes, mast cells, basophils, and eosinophils) were obtained using a Sysmex XN-2000 automated hematology analyzer (Sysmex America). Creatinine clearance was estimated using the Cockcroft–Gault equation, and the Friedewald equation was used to estimate LDL cholesterol.
Statistical analysis
All statistical analyses were performed using either SPSS v.25 software for Windows (SPSS, Inc.) or R (R version 4.0.3; R Project for Statistical Computing). Variables were assessed by descriptive measures, according to the type of variable (qualitative or quantitative). For qualitative variables, frequencies and percentages were calculated. Normality of the distribution was assessed through the Shapiro–Wilk test. Data that followed a normal distribution were compared with a 2-sided paired Student t test. Data that did not follow a normal distribution were compared using a paired Wilcoxon test. The level of significance adopted for testing all hypotheses was 5%. The correlations between TMAO measurements and urea, creatinine, glomerular filtration rate, lipid profile measurements, macronutrients, and micronutrients were calculated using the Spearman rank correlation test. The generalized estimating equation method was used to evaluate the pattern of TMAO measurements within an individual longitudinally in the different intervention groups (RW and abstention) and in relation to the use of angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers, and diuretics. This verified the effect of time with an independent variable of interest for the measure assessed over time. We wanted to observe whether there were differences between groups, between times, and for the interaction between the variables and time. Paired comparisons were performed using the Bonferroni test. We investigated the intraindividual concordance of plasma TMAO via repeated-measures analysis with the intraclass correlation coefficient (ICC).
Results
Crossover intervention
Fifty-seven patients were initially included, of whom 15 were excluded. Among the excluded patients, 8 withdrew from the protocol, 2 used antibiotics, 1 had hypertriglyceridemia on the first laboratory examination, and 1 had a leukemia diagnosis and was referred to the Hematology Clinic. One patient used metformin during the protocol, 1 patient had gouty arthritis, and 1 patient had an acute myocardial infarction shortly after randomization. Ultimately, 42 male patients completed the protocol (more details about patient enrollment are displayed in the CONSORT Flow Diagram, Supplemental Figure 1). The patients' baseline characteristics are shown in Table 1. All patients had CAD documented by coronary angiography or a clinical event and were clinically stable, and their ages ranged between 46 and 69 y; they were overweight on average, with a median BMI of 27.1, and they had a mean waist circumference of 98.0 cm. Blood pressure, lipid profiles, and glycemic profiles were well controlled. All the volunteers tolerated RW ingestion well. According to the patients' records of RW consumption, there were no missed dosages, and all the proposed dosages were ingested during the 3-wk intervention period. During the RW period compared with the alcohol abstention period, there were no significant changes in blood pressure, heart rate, or BMI. In addition, there were no significant effects on high-sensitivity C-reactive protein, IL-6, lipopolysaccharides, glucose, or the glomerular filtration rate. However, after RW consumption, there were increases in HDL cholesterol, LDL cholesterol, and total cholesterol concentrations. In addition, liver enzyme concentrations did not change, although decreases in prothrombin time (P = 0.004) and the platelet count (P = 0.02) were observed (Supplemental Tables 1 and 2).
TABLE 1.
Baseline participant demographic and clinical data1
| Variable | All participants (N = 42) |
|---|---|
| Age, y | 60.4 ± 5.4 |
| SBP, mmHg | 119.8 ± 16.0 |
| DBP, mmHg | 72.3 ± 9.3 |
| Heart rate | 68.1 ± 9.3 |
| BMI, kg/m2 | 27.1 (25.2–28.3) |
| Waist circumference, cm | 98.0 ± 8.1 |
| Current smoker | 3 (7.1) |
| Former smoker | 23 (54.7) |
| Hypertension | 21 (50.0) |
| Dyslipidemia | 18 (42.9) |
| Physical activity | 23 (53.8) |
| 5–10 METs.h/wk | 7 (30.4) |
| 10–40 METs.h/wk | 10 (43.5) |
| >40 METs.h/wk | 6 (26.1) |
| Physical inactivity | 19 (45.2) |
| History of myocardial infarction | 28 (66.7) |
| >50% stenosis of epicardial artery | 25 (59.5) |
| CABG | 13 (31.0) |
| PCI | 19 (45.2) |
| Medications | |
| β-Blockers | 30 (71.4) |
| ACE inhibitors | 21 (50.0) |
| ARBs | 10 (23.8) |
| CCBs | 3 (7.1) |
| Aspirin | 37 (88.1) |
| Clopidogrel | 10 (23.8) |
| Statins | 38 (90.5) |
| PPIs | 8 (19.0) |
| Diuretics | |
| Total cholesterol, mg/dL | 143 (127–170) |
| High-density lipoprotein, mg/dL | 46 (39–54) |
| Low-density lipoprotein, mg/dL | 77 (66–97) |
| Triglycerides, mg/dL | 85 (66–111) |
| Apolipoprotein A, g/L | 1.37 (1.22–1.51) |
| Apolipoprotein B, g/L | 0.7 (0.62–0.86) |
| Lipoprotein a, mg/dL | 60.5 (4.3–91.3) |
| Fasting glucose, mg/dL | 90 (86–100) |
| GFR, mL/min/1.73 m2 | 85.5 (72–99) |
| hsCRP, mg/L | 1.01 (0.58–2.77) |
| IL-6, pg/mL | 0.75 (0–4.8) |
| LPS, EU/mL | 0.76 (0.63–0.81) |
Data are presented as the mean ± SD, median (interquartile range), or n (%). ACE, angiotensin-converting enzyme; ARBs, angiotensin-receptor blockers; CABG, previous coronary artery bypass grafting; CCBs, calcium channel blockers; DBP, diastolic blood pressure; GFR, glomerular filtration rate; hsCRP, high-sensitivity C-reactive protein; LPS, lipopolysaccharides; METs, metabolic equivalents; METs.h/wk, METs-h/wk score = (MET level × hours × times/wk); PCI, previous percutaneous coronary intervention; PPIs, proton pump inhibitors; SBP, systolic blood pressure.
There were no significant differences in dietary patterns during the protocol
Patients underwent nutritional evaluation at 5 in-hospital visits and completed a 3-d dietary recall at the end of each intervention period (abstention or RW). Patients were compliant with the nutritional guidance and maintained the same food ingestion pattern, according to both dietary recalls. Except for the inclusion of alcohol and its calories during the RW intervention period, there were no significant differences in other macronutrient or micronutrient consumption between the 2 intervention periods (Table 2). Moreover, the dietitians reviewed all patients' dietary records and confirmed the absence of self-reported intakes of the prohibited study foods according to our dietary guidelines: polyphenol-rich foods, prebiotics, probiotics, and fermented products during the washout and interventions periods. The dietitians reviewed the written recall records for RW consumption and confirmed no consumption of alcoholic beverages during the abstention period and no consumption of other beverages (other than the provided RW) during the RW intervention period. Finally, correlations of nutrients described in the dietary recalls and gut microbiota modifications can be found in the Supplemental Results.
TABLE 2.
Food recall data quantification for the 42 patients during the 2 periods of the study1
| All patients (N = 42) | |||
|---|---|---|---|
| Variable | Abstention | Red wine | P value |
| Macronutrients | |||
| Total energy intake, kcal | 1995.5 ± 608.8 | 2263.5 ± 566.0 | 0.0392 |
| Protein, g | 97.2 ± 34.3 | 99.7 ± 28.1 | 0.6162 |
| Lipids, g | 62.7 ± 24 | 63.6 ± 23.5 | 0.6883 |
| Carbohydrates, g | 258.4 ± 92.6 | 251.9 ± 83.5 | 0.6512 |
| Fiber, g | 23.7 ± 12.8 | 23.2 ± 11.3 | 0.8493 |
| Alcohol, g/kcal | — | 24.0/180 | — |
| Micronutrients | |||
| Cholesterol, g | 326.1 ± 156.7 | 354.9 ± 137.8 | 0.2462 |
| Calcium, g | 611.6 ± 428.8 | 497.5 ± 305.9 | 0.0613 |
| Iron, g | 10.6 ± 9.1 | 17.8 ± 55.0 | 0.4463 |
| Sodium, g | 1603.7 ± 784.1 | 1560.6 ± 761.7 | 0.7782 |
| Potassium, g | 2509.1 ± 904.4 | 2346.6 ± 764.0 | 0.1642 |
| SFA, g | 22.5 ± 9.8 | 22.0 ± 8.5 | 0.4463 |
| MUFA, g | 18.5 ± 7.8 | 18.9 ± 9.3 | 0.7972 |
| PUFA, g | 9 ± 4.8 | 9.7 ± 5.1 | 0.4912 |
Average 3-d dietary recalls were collected at the end of each intervention period. Values are presented as the mean ± SD per day.
Samples followed a normal distribution, according to the Shapiro–Wilk test. Therefore, comparisons were performed with a paired t test.
Samples did not follow a normal distribution, according to the Shapiro–Wilk test. Therefore, comparisons were performed with a paired Wilcoxon test. No differences were observed in macronutrient or micronutrient consumption between periods.
Alterations in the gut microbiota could be used to distinguish RW consumption
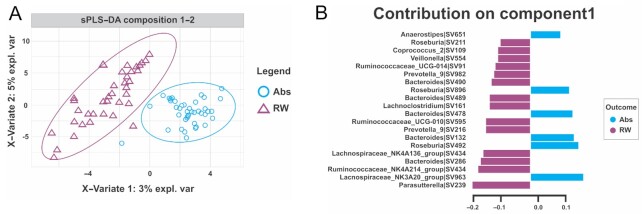
After a 2-wk washout period, the gut microbiota profile observed after RW consumption was modified compared with that after the abstention period. The β diversity showed a clear separation between groups (Figure 2A), and the gut microbiota profile could be used to distinguish RW consumption. In contrast, α diversity did not change after RW consumption (Supplemental Figure 3). After RW consumption, there was a predominance of Bacteroides, Ruminococcaceae, Roseburia, and Prevotella. Parasutterella was the most prominent genus for differentiating the gut microbiota composition during the RW period (Figure 2B). Three stool samples from 3 different patients were removed from the analysis due to low read counts, with <10 reads per sample (31). In addition, we investigated correlations of gut microbiota taxa with clinical, biochemical, and nutritional variables, as shown in Supplemental Figure 4. A full description of these findings can be found in the Supplemental Results.
FIGURE 2.
Sparse partial least squares–discriminant analysis (sPLS-DA). (A) The plot of the 2-component sPLS-DA model showed stool sample clustering according to red wine consumption (RW) or not (Abs). The percentage of variance captured for each principal component (x-axis for the first component and y-axis for the second component) for each study period (RW compared with abstention). The sPLS-DA plot is based on the relative abundance of bacterial taxa in the gut microbiota from the Abs group (blue circle) or RW group (purple triangle) and their 95% confidence ellipses. (B) The contribution plot indicates each genus's contribution to the first component of sPLS-DA. Genus contribution ranked from the bottom (most important) to the top. The colors blue (Abs) and purple (RW) indicate the group in which the genus is most abundant. The horizontal graduation line represents the variance explained by the single genera.
Plasma TMAO analysis
Plasma concentrations of TMAO after the consumption of RW did not differ significantly compared with those following the abstention period (Table 3). There was also no evidence of a carryover effect, as Table 3 shows no significant differences between the beginning of the abstention period and the beginning of the RW period. In addition, TMAO concentrations varied substantially in the samples collected at both the beginning and the end of the abstention period: the ICC between these 2 sets of samples was 0.049 (95% CI: 0.255, 0.345; P = 0.377).
TABLE 3.
Fasting TMAO plasma concentrations at baseline, after the 2-wk washout period, and at the end of each 3-wk intervention period measured in all the patients (N = 42)1
| Intervention | Before intervention | After intervention | P value2 |
|---|---|---|---|
| Abstention | 3.95 (2.64–6.78) | 4.99 (3.08–9.59) | 0.390 |
| Red wine | 4.58 (2.81–8.39) | 3.37 (2.63–7.66) | 0.710 |
| P value3 | 0.258 | 0.600 |
Values are presented as the median (IQR). The data did not follow a normal distribution, according to the Shapiro–Wilk test; therefore, a paired Wilcoxon test was employed to compare groups. TMAO, trimethylamine N-oxide.
Paired Wilcoxon test assessing the effect of the intervention (either red wine consumption or abstention).
Paired Wilcoxon test comparing the abstention period and red wine consumption groups before and after their respective interventions.
In 10 participants selected for assessment of the reproducibility of the plasma TMAO measurements, high concordance was observed among the 3 different sample techniques (Supplemental Table 3), with an ICC of 0.915 (95% CI: 0.861, 0.951; P < 0.001). The highest concordance was between immediate freezing and refrigeration (ICC of 0.993) (Supplemental Table 4). In contrast, there was a low concordance between repeated measures of TMAO (Table 4), indicating significant intermeasurement variability for the same individual over time.
TABLE 4.
Assessment of TMAO intraindividual variability (n = 10)1
| Technique | ICC | 95% CI | P value2 |
|---|---|---|---|
| Freezing | 0.224 | –0.047, 0.627 | 0.060 |
| Refrigeration | 0.208 | –0.058, 0.613 | 0.072 |
| DBS | 0.219 | –0.051, 0.623 | 0.063 |
The intraclass correlation coefficient for each trimethylamine N-oxide (TMAO) sample collection technique in 10 patients among 4 sample collection times showed a similar low concordance of TMAO values. DBS, dried blood spot; ICC, intraclass correlation coefficient.
Under the null hypothesis, the intraclass correlation coefficient is equal to zero.
There was an inverse, weak correlation of plasma TMAO with renal function (Supplemental Results). Regarding nutrient intake (Supplemental Table 5) during the RW period, plasma TMAO was positively correlated with protein intake (ρ: 0.478, P = 0.025) and cholesterol intake (ρ: 0.336, P = 0.045). There was no significant evidence indicating associations between the use of ACE inhibitors or diuretics, TMAO concentrations, and RW intervention (Supplemental Table 6). Regarding the relations between TMAO and gut microbiota, during the abstention period, TMAO was weakly correlated with Roseburia abundance and negatively correlated with Lachnospiraceae abundance. After RW consumption, TMAO plasma concentrations were positively correlated with the abundance of the genus Bacteroides (Supplemental Figure 5C).
Metabolites in several metabolic pathways were modified after RW consumption
In untargeted plasma metabolomics, 39 metabolites changed significantly following the RW intervention (Figure 3B), as listed in Supplemental Table 7 (no indication of carryover effects, Supplemental Table 8). The metabolites with significant changes likely represented changes in the pathways of amino acids, lipids, carbohydrates, and vitamins and cofactors (Figure 3A). Metabolites that significantly changed after RW consumption compared with the abstention period were identified by the paired Wilcoxon signed-rank test for nonparametric data and a paired t test for parametric data.
FIGURE 3.
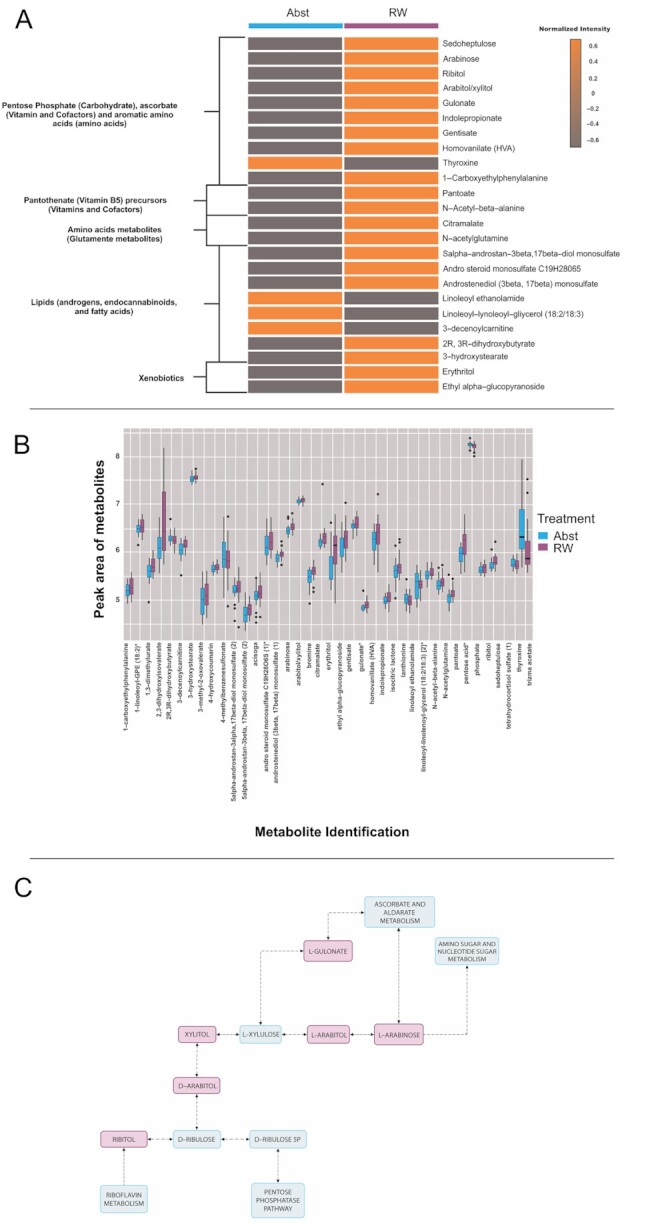
Untargeted plasma metabolome alterations in 20 subjects. (A) Heatmap showing metabolites that were significantly different between the red wine (RW) and abstention (Abst) periods. According to the metabolic pathways and biochemical functions, the graph highlights the most clinically relevant metabolites that were significantly different between the 2 periods of the study (P < 0.05, without adjustments for multiple comparisons). (B) Box-and-whisker plot of the distribution of the discriminating metabolites that were significantly altered after RW consumption compared with the abstention period. (C) Pentose and glucoronate interconversions adapted from Kyoto Encyclopedia of Genes and Genomes pathway analysis (62). In red are the metabolites that were significantly increased after RW, and in blue are the putative pathways these metabolites are involved with. Arrows indicate the direction of the reaction and reversible and irreversible reactions, which are indicated by bidirectional and unidirectional arrows, respectively. Bold lines indicate activation or interaction. Dashed lines indicate an indirect link or unknown reaction.
Pentose phosphate, ascorbate, and aromatic amino acid pathways were modified after RW intervention
The pentose phosphate pathway (PPP), the glucuronate/ascorbate pathway, and aromatic amino acid metabolism exhibited several interconversions (Figure 3C). The concentrations of several metabolites in these pathways increased. After RW, there was a significant increase in the concentrations of sedoheptulose, arabinose, ribitol, arabitol, and xylitol, components of the PPP nonoxidative branch. The increase in the concentration of ribitol, an important metabolite in the PPP and an integral part of riboflavin (vitamin B-2), might indicate modulation of the riboflavin metabolism pathway.
In the glucuronate/ascorbate pathway, there was a significant increase in the concentration of gulonate, which is an obligatory intermediate in ascorbic acid generation.
In addition, there were alterations in microbiota-associated products of aromatic amino acids (i.e., tryptophan, tyrosine, and phenylalanine). After RW consumption, the concentration of indole propionate (IPA), a microbial-derived metabolite of tryptophan, significantly increased. The concentrations of the tyrosine metabolites gentisate and homovanillate also increased, but the concentration of thyroxine decreased. RW consumption also increased the concentration of 1-carboxyethylphenylalanine, which is derived from phenylalanine metabolism.
Amino acid metabolites changed after RW ingestion
After RW consumption, the concentrations of the glutamate metabolites citramalate and N-acetylglutamine were elevated. Concentrations of the branched-chain amino acid metabolites 3-methyl-2-oxovalerate and 2,3-dihydroxyisovalerate also increased. Moreover, the concentrations of lanthionine, a metabolite in cysteine metabolism, and acisoga, a metabolite in polyamine metabolism, were increased after RW consumption.
Lipid metabolites, including androgens, endocannabinoids, and fatty acids, were altered after RW consumption
Compared with abstention, after RW consumption, there were increases in the concentrations of the androgens 5α-androstan-3β, 17β-diol monosulfate (2), androsteroid monosulfate C19H28O6S (1)*, and androstenediol (3β,17β) monosulfate (1). Furthermore, we detected a reduction in the concentrations of the endocannabinoid metabolite linoleoyl ethanolamide and its intermediate linoleoyl-linolenoyl-glycerol (18:2/18:3) [2]* with RW consumption. In addition, after RW consumption, the concentrations of fatty acid metabolites were modified, as shown by a decrease in the 3-decenoylcarnitine concentration and increases in 2R,3R-dihydroxybutyrate and 3-hydroxystearate concentrations.
Xenobiotic metabolites after RW ingestion
After RW consumption, the concentrations of erythritol and ethyl α-glucopyranoside were significantly increased compared with those observed after abstention. In addition, there was an increase in the concentration of 1,3-dimethylurate, a metabolite of theophylline, in the xanthine pathway.
Pantothenate (vitamin B-5) precursor concentrations increased post-RW consumption
After RW consumption, pantoate and N-acetyl-β-alanine concentrations significantly increased compared with those observed after abstention. B complex vitamins are vital for several human metabolic processes and are directly absorbed in the upper digestive tract but are also derived from gut microbiota metabolism.
Integration of plasma metabolomics data and gut microbiota analysis adequately separated the RW period and the abstention period
For 20 randomly selected patients, we used the DIABLO platform for multiomics integrated data to simultaneously identify correlated and discriminant microbiota and plasma metabolomics features able to separate the RW and alcohol abstention interventions in the cohort of patients. The microbiota data set included 586 taxa, while the metabolomic data set consisted of 39 metabolites. The first components from each data set were highly correlated, indicating a high discriminative power of each component to separate the different groups, RW and abstention, with a correlation index of 0.85. Metabolites and taxa in the first component are displayed in Supplemental Figure 5. The optimally selected key predictors included several taxa of gut microbiota (i.e., Streptococcus, Blautia, Ruminococcaceae, Bacteroides, and Prevotella) and metabolites in amino acid pathways, lipid pathways, and cofactor pathways, such as pantoate. The agreement among all data sets is shown in the arrow plot (Figure 4A), which indicates that the 2 omics (microbiota and metabolomics) data sets can distinguish the 2 conditions. The taxa and metabolites varied during the different interventions, and strong correlations among key predictors also contributed to a significant separation between the RW and abstention groups (Figure 4B).
FIGURE 4.
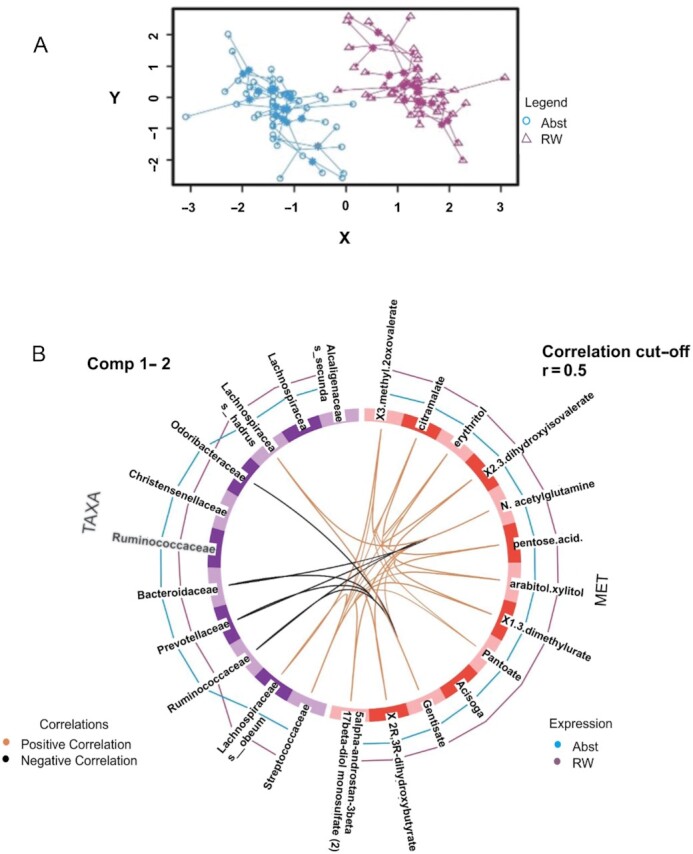
Correlation matrix and multiomics data integration analysis. (A) In the arrow plot, the arrow origin indicates the centroid among all data sets for a given sample, and the tips of the arrows indicate the location of that sample in each block. These graphs highlight the agreement among all data sets at the sample level when modeled with DIABLO. The 2 omics (microbiota taxa and metabolomics) data sets performed well in separating the 2 interventions: red wine (RW) and abstention (Abst). (B) The line outside each circle indicates the group with which each feature is associated [taxa, on the right side of the circle or metabolites (MET), on the left side]. The purple line represents biomarkers associated with the RW period, whereas the blue lines represent those associated with the abstention period. The higher the line is, the higher the discrimination power of the feature. The line inside the circle represents the correlation between the taxa and the metabolites (an orange line indicates a positive correlation, and a black line indicates a negative correlation). The correlation cutoff was set to 0.5.
Discussion
This study showed that in male patients aged between 46 and 69 y (median BMI of 27.1) with stable CAD, short-term, moderate RW consumption did not reduce TMAO concentrations. In parallel, the gut microbiota was modified after RW, as well as the plasma metabolome, particularly pathways affecting amino acids, vitamins and cofactors, lipids, and carbohydrates. In addition, plasma TMAO displayed high intraindividual variability.
Previous studies suggested that dietary modifications could alter plasma TMAO in humans, with mixed results. Higher concentrations of TMAO were found in omnivores than in vegetarians because exposure to dietary L-carnitine promotes the production of TMAO (35). However, in females, a 4-wk diet rich in animal protein (36) did not increase TMAO plasma concentrations. Nevertheless, in a randomized trial with healthy older men, there was an increase in TMAO concentrations with higher dietary protein intake (5). Another dietary modification that could modulate TMAO is RW, which is rich in polyphenols, including resveratrol, which was shown to decrease the plasma TMAO concentration in mice (20); moreover, RW contains DMB, a possible choline competitor for the gut microbiota (37). However, this study did not show a similar response in humans. Similarly, in obese individuals at risk for insulin resistance, consumption of polyphenols (tea or cocoa flavanols) did not reduce fasting TMAO concentrations (38).
Our TMAO analysis was very rigorous, involving a strict method, several measures, and a clear assessment of intraindividual variability that corroborated the analysis. Thus, it is very unlikely that the TMAO variation observed was due to methodologic issues. In addition, the high intraindividual variability of TMAO was unlikely to be explained by medication use, renal function, diet, or the gut microbiota profile. In this context, there was a negative correlation of TMAO plasma concentrations with the abundances of Lachnospiraceae and Blautia (Supplemental Results), gut bacterial taxa that have been implicated in TMAO formation (39, 40), as well as a positive correlation with the abundances of Bacteroides and Roseburia. These results contrast with the literature, as members of Bacteroidetes, such as Roseburia and Bacteroides, would not be capable of converting choline to TMA in the gut (40). Hence, the main factors that could possibly influence TMAO concentrations were investigated and did not explain the observed variations (1, 40–42). Further longitudinal evaluation and additional replicable measurement techniques for TMAO may be required to establish its role as an individual cardiovascular risk biomarker. For instance, Wu et al. (39) found that an oral carnitine challenge test may have better efficacy than fasting plasma TMAO for identifying the TMAO-producing phenotype. Moreover, recent studies highlighted the importance of gut microbiota intraindividual variability in long-term scenarios and revealed that unidentified influences or intrinsic dynamics of the community members of the gut microbiota could be responsible for these variations (43, 44). These overlooked variations could explain the dissimilarities in gut microbiota profiles or gut microbiota-dependent metabolites, such as TMAO.
In addition, analysis of the gut microbiota after the RW intervention, compared with that after the alcohol abstention period, revealed a predominance of Ruminococcaceae, several Bacteroides species, and Prevotella. In a crossover trial, Queipo-Ortuño et al. (15) also documented increases in the abundances of Bacteroides and Prevotella among other genera in 10 healthy volunteers who consumed 272 mL of RW/d for 20 d. In contrast to the work by Chadaideh et al. (45), who found that proanthocyanidins induced A. muciniphila growth in mice fed a high-fat diet, we did not detect the presence of A. muciniphila in our study, possibly because fat consumption was low in our population. Of particular interest, Parasutterella, based on discriminant analysis, was the main genus responsible for alterations in the composition of the gut microbiota after RW consumption. Parasutterella is a genus considered beneficial for fiber digestion, succinate production, salutary aromatic amino acid metabolism, and bile acid metabolism (46). In line with this, the increase in Parasutterella abundance is associated with some of the features found in plasma metabolomic analysis after RW consumption. In particular, there were alterations in tryptophan and tyrosine metabolism because increases in IPA, gentisate, and homovanillate concentrations were observed. Parasutterella is also a member of the Proteobacteria phylum, which includes predicted ascorbate-producing genera that could modify the cellular redox state (47). In accordance with this, an increase in the concentration of gulonate, a key cofactor in the glucoronate/ascorbate pathway, was noted. In addition, the group formed by Ruminococcaceae, Bacteroides, and Prevotella is part of the core microbiota in humans; this group was previously described as one enterotype identifier and is associated with high taxa abundance (48). Taken together, these alterations in gut microbiota taxa suggest changes after RW ingestion, which could possibly be related to some of the alterations observed in the plasma metabolome.
RW has long been proposed to reduce oxidative stress. For example, previous analysis showed that RW could reduce oxidative stress in rodent colonic mucosa (11), and in healthy males, RW increased plasma and LDL polyphenol concentrations and enhanced antioxidant activity (49). Our findings are in line with these proposed redox effects of RW. We found plasma changes that indicate modulations in redox signaling, such as increases in the concentrations of gulonate (50), metabolites in the PPP (51), and ribitol, an integral part of riboflavin, which has recently been studied as an important redox pathway (52). Another putative beneficial influence on redox homeostasis was the increase in the concentrations of the tyrosine metabolites gentisate and homovanillate, derived from polyphenols with redox capability (53). In parallel, we noted metabolite alterations that could be beneficial for insulin resistance and type 2 diabetes mellitus, such as an increase in the concentrations of the tryptophan metabolites IPA (54), glucopyranosides (55), erythritol, and ethyl α-glucopyranoside and a decrease in long-chain acylcarnitine concentrations (56). Furthermore, RW consumption might also beneficially influence energy expenditure and metabolism, as suggested by the decrease in endocannabinoid concentrations (57, 58) and the increase in pantothenate precursor concentrations (59). Importantly, these changes were not significantly correlated with gut microbiota alterations in mediation analysis (DIABLO). Although the mediation analysis of the gut microbiota and the plasma metabolome could clearly separate the 2 intervention periods in this study, we cannot be sure that there was a causal association among these variables.
This study has some limitations. RW intake was not confirmed by direct sample tests but through self-reported consumption and the return of empty bottles. In addition, because we investigated the effects of RW only in men for a short duration, our results may not reflect the long-term effects of RW consumption and thus may not be generalizable to the overall population. In contrast, we minimized any influence of between-subject variability by using a crossover design. Another limitation is that it is not possible to ensure that the energy metabolism changes occurred solely because of polyphenolic or RW influences on the gut microbiota. Alcohol intake may also have influenced energy metabolism, affecting hepatic lipid metabolism, such as fatty acid uptake and de novo lipid synthesis (60, 61). Notably, important confounders that have been frequently mentioned in other investigations of RW, such as genetics and socioeconomic status, were primarily controlled in this design. In addition, in a free-living intervention study, we were able to note no significant differences in dietary consumption between the intervention periods on average. Except for calories added from the alcohol consumption, a similar dietary pattern was present in dietary recalls during the RW and in abstention periods, suggesting that RW consumption was the only dietary change between groups. Moreover, although the number of calories increased during the RW intervention, there were no changes in weight, waist circumference, or glycemic profile. Finally, in contrast to our initial hypothesis, we observed no evidence of decreases in plasma TMAO concentrations, partly because of their intraindividual variability. Further studies with larger sample sizes, longer durations, and additional repeated measures are needed to validate the reported findings, especially with regard to any false-positive findings of metabolite changes.
In summary, our findings indicate that TMAO measurements are highly variable, which may hinder clinical follow-up at the individual level. In addition, TMAO concentrations were not correlated with RW intake. Furthermore, our data indicate that the RW intervention is associated with remodeling of the gut microbiota and significant changes in the plasma metabolome. Although changes in the gut microbiota may have mediated the physiologic effects of RW consumption, these data remain hypothesis-generating and pave the way for future research, possibly with longer follow-ups and different populations.
Supplementary Material
Acknowledgements
We thank Michelle Aparecida Pereira for administrative and secretarial support, Natacia S. P. Rocha for helping in the execution of the protocol, Daniel Lebre for the chromatography coupled with mass spectrometry analysis and for helping with TMAO analysis, Metabolon for the high-quality plasma metabolomic analysis, Mitsue Izosaki and Anna Carolina di Creddo Alves for their dietary and nutritional supervision, and Rossana Veronica Mendez for extensive statistical analysis.
The authors’ responsibilities were as follows—PLDL, FRML, and PL: conceived the trial; EAH: conducted the trial supervised by PLDL and participated actively in writing the manuscript; DF: contributed to the trial design and supervised the statistical analysis; MJAS and AS: contributed to microbiota profiling of stool samples, were involved in the generation of microbiota data, and contributed to data interpretation; NV and WJFL: performed the gut microbiota bioinformatics analyses; NV: integrated gut microbiota information and results of the plasma metabolomic analysis; AMAM and CRCP: performed metabolomic quality control analyses and statistical analyses for metabolomics; RSG: performed statistical analysis of the TMAO data and supervised other statistical work; DOM: performed nutritional analysis and interpretation; EAH, PLDL, FRML, and DF: performed the integrative data analyses and overall data interpretation; and all authors: revised and approved the final manuscript.
Author disclosures: Dr. Libby is an unpaid consultant to, or involved in clinical trials for Amgen, AstraZeneca, Baim Institute, Beren Therapeutics, Esperion Therapeutics, Genentech, Kancera, Kowa Pharmaceuticals, Medimmune, Merck, Norvo Nordisk, Novartis, Pfizer, and Sanofi-Regeneron. Dr. Libby is a member of the scientific advisory board for Amgen, Caristo Diagnostics, Cartesian Therapeutics, CSL Behring, DalCor Pharmaceuticals, Dewpoint Therapeutics, Eulicid Bioimaging, Kancera, Kowa Pharmaceuticals, Olatec Therapeutics, Medimmune, Moderna, Novartis, PlaqueTec, TenSixteen Bio, Soley Thereapeutics, and XBiotech, Inc.
Dr. Libby’s laboratory has received research funding in the last 2 years from Novartis. Dr. Libby is on the Board of Directors of XBiotech, Inc. Dr. Libby has a financial interest in Xbiotech, a company developing therapeutic human antibodies, in TenSixteen Bio, a company targeting somatic mosaicism and clonal hematopoiesis of indeterminate potential (CHIP) to discover and develop novel therapeutics to treat age-related diseases, and in Soley Therapeutics, a biotechnology company that is combining artificial intelligence with molecular and cellular response detection for discovering and developing new drugs, currently focusing on cancer therapeutics.
Dr. Libby’s interests were reviewed and are managed by Brigham and Women’s Hospital and Mass General Brigham in accordance with their conflict-of-interest policies. The other authors report no conflicts of interest.
Notes
This work was supported by the São Paulo Research Foundation (FAPESP) Grant number 2015/212606. There were also contributions from the National Institute of Science and Technology for Diabetes and Obesity (INCT)—CNPq grant number 465693/2014-8 and FAPESP Grant number 2014/50907-5. Support: The Bradesco Bank Foundation, the São Paulo Research Foundation (FAPESP), and the Brazilian Institute of Wine (IBRAVIN) offered financial support for this project. IBRAVIN also provided the special red wine used in the Wine Flora Study. The sponsors had no influence on the design, applied methods, data generation and analysis, or decision to publish.
Dr. Libby receives funding support from the National Heart, Lung, and Blood Institute (1R01HL134892, 1R01HL163099-01), the American Heart Association (18CSA34080399), the RRM Charitable Fund, and the Simard Fund.
The raw Illumina read data for all stool samples have been deposited in the NCBI SRA repository under the BioProject ID PRJNA726242. The metabolomic data sets for this study can be found in the MetaboLights repository under the public URL https://www.ebi.ac.uk/metabolights/editor/study/MTBLS310/. Other data supporting the findings of the study are available in this article and its Supplemental Information files or from the corresponding authors upon request.
Supplemental Figures 1–5, Supplemental Methods, Supplemental Tables 1–8, and Supplemental Results are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: ACE, angiotensin-converting enzyme; ASV, amplicon sequence variant; AUDIT, Alcohol Use Disorders Identification Test; CAD, coronary artery disease; CVD, cardiovascular diseases; DBS, dried blood spot; DMB, 3,3-dimethyl-1-butanol; ICC, intraclass correlation coefficient; IPA, indole propionate; MACE, major adverse cardiovascular event; PPP, pentose phosphate pathway; RW, red wine; TMA, trimethylamine; TMAO, trimethylamine N-oxide.
Contributor Information
Elisa A Haas, Instituto do Coracao (InCor), Hospital das Clinicas HCFMUSP, Faculdade de Medicina, Universidade de São Paulo, São Paulo, SP, Brazil.
Mario J A Saad, Department of Internal Medicine, State University of Campinas (UNICAMP), Campinas, SP, Brazil.
Andrey Santos, Department of Internal Medicine, State University of Campinas (UNICAMP), Campinas, SP, Brazil.
Nicola Vitulo, Department of Biotechnology, Verona University, Verona, Italy.
Wilson J F Lemos, Jr, Bioresources Unit, Center for Health & Bioresources, AIT Austrian Institute of Technology GmbH, Tulln, Austria.
Aline M A Martins, Department of Medical Science, University of Brasília (UnB), Brasília, Brazil.
Carolina R C Picossi, Institute of Chemistry, University of São Paulo, São Paulo, SP, Brazil.
Desidério Favarato, Instituto do Coracao (InCor), Hospital das Clinicas HCFMUSP, Faculdade de Medicina, Universidade de São Paulo, São Paulo, SP, Brazil.
Renato S Gaspar, Instituto do Coracao (InCor), Hospital das Clinicas HCFMUSP, Faculdade de Medicina, Universidade de São Paulo, São Paulo, SP, Brazil.
Daniéla O Magro, Department of Internal Medicine, State University of Campinas (UNICAMP), Campinas, SP, Brazil.
Peter Libby, Division of Cardiovascular Medicine, Department of Medicine, Brigham and Women's Hospital, Harvard Medical School, Boston, MA, USA.
Francisco R M Laurindo, Instituto do Coracao (InCor), Hospital das Clinicas HCFMUSP, Faculdade de Medicina, Universidade de São Paulo, São Paulo, SP, Brazil.
Protasio L Da Luz, Instituto do Coracao (InCor), Hospital das Clinicas HCFMUSP, Faculdade de Medicina, Universidade de São Paulo, São Paulo, SP, Brazil.
References
- 1. Tang WHW, Bäckhed F, Landmesser U, Hazen SL. Intestinal microbiota in cardiovascular health and disease: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73(16):2089–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chadaideh KS, Carmody RN. Host-microbial interactions in the metabolism of different dietary fats. Cell Metab. 2021;33(5):857–72. [DOI] [PubMed] [Google Scholar]
- 3. Alexander M, Turnbaugh PJ. Deconstructing mechanisms of diet-microbiome-immune interactions. Immunity. 2020;53(2):264–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Senthong V, Li XS, Hudec T, Coughlin J, Wu Y, Levison Bet al. . Plasma trimethylamine N-oxide, a gut microbe–generated phosphatidylcholine metabolite, is associated with atherosclerotic burden. J Am Coll Cardiol. 2016;67(22):2620–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mitchell SM, Milan AM, Mitchell CJ, Gillies NA, D'Souza RF, Zeng Net al. . Protein intake at twice the RDA in older men increases circulatory concentrations of the microbiome metabolite trimethylamine-N-oxide (TMAO). Nutrients. 2019;11(9):2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baugh ME, Steele CN, Angiletta CJ, Mitchell CM, Neilson AP, Davy BMet al. . Inulin supplementation does not reduce plasma trimethylamine N-oxide concentrations in individuals at risk for type 2 diabetes. Nutrients. 2018;10(6):793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Maier TV, Lucio M, Lee LH, VerBerkmoes NC, Brislawn CJ, Bernhardt Jet al. . Impact of dietary resistant starch on the human gut microbiome, metaproteome, and metabolome. MBio. 2017;8(5):e01343–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang Z, Roberts AB, Buffa JA, Levison BS, Zhu W, Org Eet al. . Non-lethal inhibition of gut microbial trimethylamine production for the treatment of atherosclerosis. Cell. 2015;163(7):1585–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gupta N, Buffa JA, Roberts AB, Sangwan N, Skye SM, Li Let al. . Targeted inhibition of gut microbial trimethylamine N-oxide production reduces renal tubulointerstitial fibrosis and functional impairment in a murine model of chronic kidney disease. Arterioscler Thromb Vasc Biol. 2020;40(5):1239–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Levantesi G, Marfisi R, Mozaffarian D, Franzosi MG, Maggioni A, Nicolosi GLet al. . Wine consumption and risk of cardiovascular events after myocardial infarction: results from the GISSI-Prevenzione trial. Int J Cardiol. 2013;; 163(3):282–7. [DOI] [PubMed] [Google Scholar]
- 11. Dolara P, Luceri C, De Filippo C, Pietro FA, Giovannelli L, Caderni Get al. . Red wine polyphenols influence carcinogenesis, intestinal microflora, oxidative damage and gene expression profiles of colonic mucosa in F344 rats. Mut Res. 2005;591(1–2):237–46. [DOI] [PubMed] [Google Scholar]
- 12. Gronbaek M, Deis A, Sorensen TIA, Becker U, Schnohr P, Jensen G. Mortality associated with moderate intakes of wine, beer, or spirits. BMJ. 1995;310:1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zorraquín I, Sánchez-Hernández E, Ayuda-Durán B, Silva M, González-Paramás AM, Santos-Buelga Cet al. . Current and future experimental approaches in the study of grape and wine polyphenols interacting gut microbiota. J Sci Food Agric. 2020;100(10):3789–802. [DOI] [PubMed] [Google Scholar]
- 14. Cueva C, Gil-Sánchez I, Ayuda-Durán B, González-Manzano S, González-Paramás AM, Santos-Buelga Cet al. . An integrated view of the effects of wine polyphenols and their relevant metabolites on gut and host health. Molecules. 2017;22(1):99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Queipo-Ortuño MI, Boto-Ordóñez M, Murri M, Gomez-Zumaquero JM, Clemente-Postigo M, Estruch Ret al. . Influence of red wine polyphenols and ethanol on the gut microbiota ecology and biochemical biomarkers. Am J Clin Nutr. 2012;95(6):1323–34. [DOI] [PubMed] [Google Scholar]
- 16. Muñoz-González I, Jiménez-Girón A, Martín-Álvarez PJ, Bartolomé B, Moreno-Arribas MV. Profiling of microbial-derived phenolic metabolites in human feces after moderate red wine intake. J Agric Food Chem. 2013;61(39):9470–9. [DOI] [PubMed] [Google Scholar]
- 17. Jacobs DM, Fuhrmann JC, Van Dorsten FA, Rein D, Peters S, Van Velzen EJJet al. . Impact of short-term intake of red wine and grape polyphenol extract on the human metabolome. J Agric Food Chem. 2012;60(12):3078–85. [DOI] [PubMed] [Google Scholar]
- 18. Zhang L, Carmody RN, Kalariya HM, Duran RM, Moskal K, Poulev Aet al. . Grape proanthocyanidin-induced intestinal bloom of Akkermansia muciniphila is dependent on its baseline abundance and precedes activation of host genes related to metabolic health. J Nutr Biochem. 2018;56:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Le Roy CI, Wells PM, Si J, Raes J, Bell JT, Spector TD. Red wine consumption associated with increased gut microbiota α-diversity in 3 independent cohorts. Gastroenterology. 2020;158(1):270–272.e2. [DOI] [PubMed] [Google Scholar]
- 20. Chen M, Yi L, Zhang Y, Zhou X, Ran L, Yang Jet al. . Resveratrol attenuates trimethylamine-N-oxide (TMAO)-induced atherosclerosis by regulating TMAO synthesis and bile acid metabolism via remodeling of the gut microbiota. MBio. 2016;7(2):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Frezza M, di Padova C, Pozzato G, Terpin M, Baraona E, Lieber CS. High blood alcohol levels in women. N Engl J Med. 1990;322(2):95–9. [DOI] [PubMed] [Google Scholar]
- 22. Fennema D, Phillips IR, Shephard EA. Trimethylamine and trimethylamine N-oxide, a flavin-containing monooxygenase 3 (FMO3)-mediated host-microbiome metabolic axis implicated in health and disease. Drug Metab Dispos. 2016;44(11):1839–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bohn MJ, Babor TF, Kranzler HR. The Alcohol Use Disorders Identification Test (AUDIT): validation of a screening instrument for use in medical settings. J Stud Alcohol. 1995;56(4):423–32. [DOI] [PubMed] [Google Scholar]
- 24. Tang WHW, Wang Z, Levison BS, Koeth RA, Britt EB, Fu Xet al. . Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368(17):1575–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kohn M, Senyak J. Sample size calculators [Internet]. [cited 2022 Jan 31]. Available from: https://sample-size.net/
- 26. Haseeb S, Alexander B, Baranchuk A. Wine and cardiovascular health: a comprehensive review. Circulation2017; ;136:1434–48. [DOI] [PubMed] [Google Scholar]
- 27. Jiménez-Girón A, Muñoz-González I, Martín-Alvarez PJ, Moreno-Arribas MV, Bartolomé B. Towards the fecal metabolome derived from moderate red wine intake. Metabolites. 2014;4(4):1101–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Voskoboinik A, Prabhu S, Ling L-H, Kalman JM, Kistler PM. Alcohol and atrial fibrillation: a sobering review. J Am Coll Cardiol. 2016;68(23):2567–76. [DOI] [PubMed] [Google Scholar]
- 29. Barros Filho A A, Lima DM, Salay E, Siliprandi E, Veris LKM, Bagnato MHSet al. . Tabela brasileira de composição de alimentos—TACO 4a edição revisada e ampliada [Internet]. Universidade Estadual De Campinas—UNICAMP; 2011; [cited 2018 Sep 28]. Available from: http://www.nepa.unicamp.br/taco/contar/taco_4_edicao_ampliada_e_revisada.pdf?arquivo=taco_4_versao_ampliada_e_revisada.pdf [Google Scholar]
- 30.Illumina. Illumina 16S metagenomic sequencing library preparation guide [Internet]. Illumina Technical Note 15044223. 2013; [cited 2022 May 27]. Available from: https://support.illumina.com/content/dam/illumina-support/documents/documentation/chemistry_documentation/16 s/16s-metagenomic-library-prep-guide-15044223-b.pdf
- 31. Magro DO, Santos A, Guadagnini D, Godoy FM, Silva SHM, Lemos WJFet al. . Remission in Crohn's disease is accompanied by alterations in the gut microbiota and mucins production. Sci Reports. 2019;9:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Singh A, Shannon CP, Gautier B, Rohart F, Vacher M, Tebbutt SJet al. . DIABLO: an integrative approach for identifying key molecular drivers from multi-omics assays. Bioinformatics. 2019;35(17):3055–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang Z, Levison BS, Hazen JE, Donahue L, Li XM, Hazen SL. Measurement of trimethylamine-N-oxide by stable isotope dilution liquid chromatography tandem mass spectrometry. Anal Biochem. 2014;455:35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nyamundanda G, Gormley IC, Fan Y, Gallagher WM, Brennan L. MetSizeR: selecting the optimal sample size for metabolomic studies using an analysis based approach. BMC Bioinformatics2013;14:338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BTet al. . Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19(5):576–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Genoni A, Lo J, Lyons-Wall P, Boyce MC, Christophersen CT, Bird Aet al. . A paleolithic diet lowers resistant starch intake but does not affect serum trimethylamine-N-oxide concentrations in healthy women. Br J Nutr. 2019;121(3):322–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jonsson AL, Bäckhed F. Drug the bug! Cell. 2015;163:1565–6. [DOI] [PubMed] [Google Scholar]
- 38. Angiletta CJ, Griffin LE, Steele CN, Baer DJ, Novotny JA, Davy KPet al. . Impact of short-term flavanol supplementation on fasting plasma trimethylamine N-oxide concentrations in obese adults. Food Function. 2018;9(10):5350–61. [DOI] [PubMed] [Google Scholar]
- 39. Wu W-K, Chen C-C, Liu P-Y, Panyod S, Liao B-Y, Chen P-Cet al. . Identification of TMAO-producer phenotype and host–diet–gut dysbiosis by carnitine challenge test in human and germ-free mice. Gut. 2019;68(8):1439–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Skye SM, Zhu W, Romano KA, Guo C-J, Wang Z, Jia Xet al. . Microbial transplantation with human gut commensals containing CutC is sufficient to transmit enhanced platelet reactivity and thrombosis potential. Circ Res. 2018;123(10):1164–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Konop M, Radkowski M, Grochowska M, Perlejewski K, Samborowska E, Ufnal M. Enalapril decreases rat plasma concentration of TMAO, gut bacteria-derived cardiovascular marker. Biomarkers. 2018;23: 380–5. [DOI] [PubMed] [Google Scholar]
- 42. Latkovskis G, Makarova E, Mazule M, Bondare L, Hartmane D, Cirule Het al. . Loop diuretics decrease the renal elimination rate and increase the plasma levels of trimethylamine-N-oxide. Br J Clin Pharmacol. 2018;84(11):2634–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Vandeputte D, De Commer L, Tito RY, Kathagen G, Sabino J, Vermeire Set al. . Temporal variability in quantitative human gut microbiome profiles and implications for clinical research. Nat Commun. 2021;12:6740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Frost F, Kacprowski T, Rühlemann M, Pietzner M, Bang C, Franke Aet al. . Long-term instability of the intestinal microbiome is associated with metabolic liver disease, low microbiota diversity, diabetes mellitus and impaired exocrine pancreatic function. Gut. 2021;70(3):522–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chadaideh KS, Eappen KE, Moore BE, Carmody RN. Common proanthocyanidin-rich foods modulate gastrointestinal blooms of Akkermansia muciniphila in a diet-dependent manner. 2021;1–32. Available from: 10.1101/2021.11.07.466338. [DOI] [Google Scholar]
- 46. Ju T, Kong JY, Stothard P, Willing BP. Defining the role of Parasutterella, a previously uncharacterized member of the core gut microbiota. ISME J2019;13:1520–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chang Y-L, Rossetti M, Vlamakis H, Casero D, Sunga G, Harre Net al. . A screen of Crohn's disease-associated microbial metabolites identifies ascorbate as a novel metabolic inhibitor of activated human T cells. Mucosal Immunol. 2019;12(2):457–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Falony G, Joossens M, Vieira-Silva S, Wang J, Darzi Y, Faust Ket al. . Population-level analysis of gut microbiome variation. Science. 2016;352(6285):560–4. [DOI] [PubMed] [Google Scholar]
- 49. Nigdikar SV, Williams NR, Griffin BA, Howard AN. Consumption of red wine polyphenols reduces the susceptibility of low-density lipoproteins to oxidation in vivo. Am J Clin Nutr. 1998;68(2):258–65. [DOI] [PubMed] [Google Scholar]
- 50. Linster CL, Van Schaftingen E, Vitamin C. Biosynthesis, recycling and degradation in mammals. FEBS J. 2007;274(1):1–22. [DOI] [PubMed] [Google Scholar]
- 51. Ge T, Yang J, Zhou S, Wang Y, Li Y, Tong X. The role of the pentose phosphate pathway in diabetes and cancer. Front Endocrinol. 2020;11:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ashoori M, Saedisomeolia A. Riboflavin (vitamin b 2) and oxidative stress: a review. Br J Nutr. 2014;111(11):1985–91. [DOI] [PubMed] [Google Scholar]
- 53. Abedi F, Razavi BM, Hosseinzadeh H. A review on gentisic acid as a plant derived phenolic acid and metabolite of aspirin: comprehensive pharmacology, toxicology, and some pharmaceutical aspects. Phytother Res. 2020;34(4):729–41. [DOI] [PubMed] [Google Scholar]
- 54. Koh A, Bäckhed F. From association to causality: the role of the gut microbiota and its functional products on host metabolism. Mol Cell. 2020;78(4):584–96. [DOI] [PubMed] [Google Scholar]
- 55. Zhu W, Sun S, Yang F, Zhou K. UHPLC/MS identifying potent α-glucosidase inhibitors of grape pomace via enzyme immobilized method. J Food Sci. 2018;83(4):1131–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Schooneman MG, Vaz FM, Houten SM, Soeters MR. Acylcarnitines. Diabetes. 2013;62(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Friedman AN, Kim J, Kaiser S, Pedersen TL, Newman JW, Watkins BA. Association between plasma endocannabinoids and appetite in hemodialysis patients: a pilot study. Nutr Res. 2016;36(7):658–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Muccioli GG, Naslain D, Bäckhed F, Reigstad CS, Lambert DM, Delzenne NMet al. . The endocannabinoid system links gut microbiota to adipogenesis. Mol Syst Biol. 2010;6(1):392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rodionov DA, Arzamasov AA, Khoroshkin MS, Iablokov SN, Leyn SA, Peterson SNet al. . Micronutrient requirements and sharing capabilities of the human gut microbiome. Front Microbiol. 2019;10:1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Jeon S, Carr R. Alcohol effects on hepatic lipid metabolism. J Lipid Res. 2020;61(4):470–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. You M, Arteel GE. Effect of ethanol on lipid metabolism. J Hepatol. 2019;70(2):237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kanehisa M. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000;28(1):27–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.