ABSTRACT
Background
Previous studies on alcohol drinking and health largely have ignored the potential impact of the timing of drinking.
Objectives
We aimed to investigate the joint associations of the timing of alcohol intake with respect to meals (i.e., with meals or outside of meals) and the amount of alcohol consumed with the risk of type 2 diabetes (T2D).
Methods
A total of 312,388 current drinkers from the UK Biobank without T2D at baseline were included. Cox proportional hazards models were used to examine the association between the timing of alcohol intake with respect to meals and the risk of T2D.
Results
During a median of 10.9 y of follow-up, 8598 incident cases of T2D were documented. After adjustment for covariates and the amount of alcohol consumed, consuming alcohol with meals was significantly associated with a 12% lower risk of T2D (HR: 0.88; 95% CI: 0.83, 0.93) than was consuming alcohol outside of meals. In addition, we found that the timing of alcohol intake with respect to meals significantly modified the relations between the amount of alcohol consumed and risk of T2D (P-interaction = 0.017); the beneficial association of moderate drinking with T2D risk was only observed in participants who consumed alcohol with meals, but not in others. Further analyses on various types of alcoholic beverages indicated that the beneficial associations between alcohol drinking with meals and T2D were mainly driven by wine consumption. Moreover, we found that when consumed together with meals, drinking more wine, rather than other alcoholic beverages, was related to lower concentrations of C-reactive protein.
Conclusions
In current drinkers, moderate drinking of alcohol, especially wine, with meals is associated with a lower risk of T2D.
Keywords: alcohol consumption, wine, timing, meals, type 2 diabetes
See corresponding editorial on page 1460.
Introduction
Alcohol drinking has both adverse and beneficial effects on health. Undoubtedly, the harmful use of alcohol is a leading cause of death and disability worldwide (1), whereas moderate drinking when alcohol is consumed in an appropriate way may be beneficial (2). The benefits of moderate drinking on glucose metabolism have been documented in several well-designed clinical trials (3–7). A long-term (2-y), large-scale clinical trial indicated that initiating moderate wine intake as part of a dinner significantly decreased fasting glucose concentrations and improved insulin resistance in well-controlled diabetics (3). Another randomized clinical trial showed that consumption of 30 g alcohol/d with meals had significant beneficial effects on insulin and insulin sensitivity in nondiabetic postmenopausal women (4). However, it is still unclear whether such benefits on glucose metabolism will translate into a reduction in type 2 diabetes (T2D). The relation between moderate drinking and T2D is controversial. Some (8–10), but not all (11–13), previous prospective studies have shown that moderate drinkers have a lower risk of T2D than nondrinkers and heavy drinkers. Intriguingly, we noted that the alcoholic beverages were usually served with meals in those clinical trials that have detected beneficial effects of moderate alcohol intake on glucose metabolism (3–7). Therefore, we hypothesized that the association between alcohol intake and risk of T2D might differ by the timing of alcohol intake with respect to meals. Prior studies have largely ignored the potential impact of the timing of drinking.
In this study, we aimed to investigate the joint associations of the timing of alcohol intake with respect to meals and the amount of alcohol intake with the risk of T2D and T2D-related biomarkers. Notably, to minimize the systematic differences between drinkers and nondrinkers (14), which might lead to an overestimate of protective effects from moderate drinking, all the analyses were restricted to healthy current drinkers.
Methods
Study population
The UK Biobank Study is a population-based cohort study; the study design and methods have been described in detail previously (15). In brief, >0.5 million participants aged 37–73 y were recruited at 22 assessment centers throughout England, Wales, and Scotland from 2006 to 2010. In this study, our analyses were restricted to current drinkers at baseline (current drinkers were defined by indication of drinking alcohol based on an alcohol intake frequency questionnaire and alcohol consumed >0 g/wk) (n = 377,608). We excluded participants with T2D, cardiovascular diseases, or cancers at baseline (n = 57,785); participants with incomplete data on the timing of alcohol intake with respect to meals (n = 204); participants who had reduced their alcohol consumption owing to illness or doctor's advice (n = 6998); and pregnant women (n = 233). A total of 312,388 participants were included in the final analysis (Supplemental Figure 1).
Assessment of exposure
A touch-screen questionnaire at baseline was used to collect information on the frequency of alcohol intake, the usual average consumption of each type of alcoholic beverage, the timing of alcohol intake with respect to meals, approximate changes in alcohol consumption, and the reason for reducing the amount of alcohol drunk. Alcohol intake (g/wk) was calculated by the quantity of each type of drink (red wine, white wine, beer/cider, fortified wine, and spirits) multiplied by its standard drink size and reference alcohol content (1 unit-equivalent described as containing 8 g of pure alcohol; 125 mL wine = 1.6 unit-equivalents, 1 pint of beer (586 mL) = 2.6 unit-equivalents, 25 mL spirits = 1 unit-equivalent, 62.5 mL fortified wine = 1 unit-equivalent) (2). In the main analysis, we categorized weekly alcohol consumption (total, wine, and beer) in grams into 4 categories: >0 to <50 (reference), ≥50 to <100, ≥100 to <200, and ≥200 g/wk. Because of the relatively narrow range for liquor consumption, we categorized weekly ethanol intake from liquor into 3 categories: >0 to <50 (reference), ≥50 to <100, and ≥100 g/wk.
Information on the timing of alcohol intake with respect to meals was collected with the following question. After identifying the participant was a current drinker, each participant was asked, “When you drink alcohol is it usually with meals?” Participants selected either “yes,” “no,” “it varies,” “do not know,” or “prefer not to answer.” Participants who responded “do not know” or “prefer not to answer” were considered to have missing information and then excluded from the analysis.
Assessment of outcome
Information on the diagnosis of T2D was ascertained using the first occurrence variables in the UK Biobank (data field 130709). In brief, information on diagnosis of T2D was predominantly based on primary care data, hospital admission data, and to a lesser extent on self-reported data. Incident T2D was defined by International Classification of Diseases (ICD), Tenth Revision code E11 (the Read codes of primary care have been translated to ICD codes: https://biobank.ndph.ox.ac.uk/showcase/ukb/docs/first_occurrences_outcomes.pdf). Follow-up time was counted from the date of the assessment center visit until the date of diagnosis of T2D (until 1 February, 2020), the date of loss to follow-up, or the date of death, whichever came first.
According to previous studies (3, 5), several T2D-related biomarkers of interest [C-reactive protein (CRP), HDL cholesterol, and glycated hemoglobin (HbA1c)] were selected in this study. We chose CRP as the research target because CRP is a marker of low-grade systemic inflammation, and the anti-inflammatory effect is one of the important biological mechanisms underlying the beneficial association of alcohol consumption with risk of T2D (16, 17). Because the dose–response relation of alcohol intake with HDL cholesterol has been well established in previous studies, we chose HDL cholesterol for comparison with other analyses in this study. HbA1c is an important indicator of long-term glucose metabolism. Blood samples were collected at baseline (2006–2010). CRP (mg/L) and HDL cholesterol (mmol/L) were measured by immunoturbidimetric assay on a Beckman Coulter AU5800; HbA1c (mmol/mol) was measured by HPLC analysis on a Bio-Rad VARIANT II Turbo. Calibration and quality control were conducted according to the manufacturer's recommendations. Details of these measurements can be found on the UK Biobank website (https://biobank.ctsu.ox.ac.uk/showcase). Because nearly half of CRP values fell between 0 and 1 and the distribution was right-skewed, log (units + 1) of CRP values were used in all analyses.
Assessment of covariates
Age, sex, race and ethnicity (self-identified), Townsend deprivation index (TDI; a composite measure of deprivation based on unemployment, non–car ownership, non–home ownership, and household overcrowding; a negative value represents high socioeconomic status), smoking status (current, past, never), physical activity, diet, and family history of diabetes were obtained from touch-screen questionnaires. Height was measured by a Seca 202 height measure. Weight was measured to the nearest 0.1 kg by the Tanita BC-418 MA body composition analyzer. BMI (in kg/m2) was calculated as weight (kg) divided by height in meters squared (m2). Physical activity [metabolic equivalents (METS) min/wk] was calculated according to the International Physical Activity Questionnaire short form: 1 min walking = 3.3 METS, 1 min moderate physical activity = 4 METS, and 1 min vigorous physical activity = 8 METS. Healthy diet score was evaluated by red meat intake (below median), vegetable intake (median or above), fruit intake (median or above), fish intake (median or above), and processed meat intake (below median); 1 point was given for each favorable diet factor and the total diet score ranged from 0 to 5; a healthy diet was defined as a diet score ≥3 (18, 19). Hypertension was defined as a self-reported history of hypertension, a systolic blood pressure ≥140 mm Hg, a diastolic blood pressure ≥90 mm Hg, or taking antihypertensive medications. High cholesterol was defined as a self-reported history of high cholesterol or taking
Statistical analysis
Baseline characteristics were described as means ± SDs or median [IQR] for continuous variables and n (%) for categoric variables across the categories of the timing of alcohol intake with respect to meals. Cox proportional hazards models were used to evaluate the association between the timing of alcohol intake with respect to meals and the risk of T2D. The proportional hazards assumption was tested by the Kaplan–Meier method and Schoenfeld residuals method. A general linear model was used to evaluate the associations between the timing of alcohol intake with respect to meals and CRP concentrations, HDL cholesterol concentrations, and HbA1c levels. We adjusted for several confounders including age (y), sex, race and ethnicity (white European, mixed, Asian, black, others), TDI (continuous), BMI (continuous), smoking status (current, past, never), physical activity (METS min/wk), healthy diet score, hypertension (no or yes), high cholesterol (no or yes), and family history of diabetes (no or yes). For analyses which included HDL cholesterol and CRP concentrations, we also adjusted for fasting time. To evaluate interactions between the timing of alcohol intake with respect to meals and alcohol consumption, multiplicative interaction was assessed by adding interaction terms to the Cox models or general linear model. To test the trend of alcohol consumption within each group, the linear trend was evaluated by assigning the median of the amount and modeling this variable continuously. To test departure from linearity, a linear and a quadratic term were included in the model. Several stratified analyses were performed to evaluate potential modification effects by the following factors: sex (women or men), age (<60 or ≥60 y), race (whites and nonwhites), current smoking (no or yes), BMI (<30 or ≥30), physical activity (<600 MET min/wk or ≥600 MET min/wk), healthy diet (no or yes), TDI (quintile 1, quintile 2 to quintile 4, and quintile 5), hypertension (no or yes), high cholesterol (no or yes), and drinking frequency (<3 or ≥3 times/wk).
All P values were 2-sided and P < 0.05 was considered statistically significant. All statistical analyses were conducted using SAS version 9.4 (SAS Institute Inc.).
Results
Baseline characteristics of the study participants
Table 1 shows participants’ baseline characteristics according to the timing of alcohol intake with respect to meals. Compared with participants who consumed alcohol outside of meals or those who had varying patterns, participants who consumed alcohol with meals were more likely to be older and female; be non–current smokers; have a higher healthy diet score; have lower BMI, physical activity levels, and TDI; as well as have lower prevalence of hypertension and high cholesterol. In addition, participants who consumed alcohol with meals drank less alcohol overall but were more likely to be a regular drinker (frequency of alcohol drinking ≥3 times/wk) and prefer wine than those who consumed alcohol outside of meals or those who had varying patterns.
TABLE 1.
Baseline characteristics according to the timing of alcohol intake with respect to meals1
| No | It varies | Yes | |
|---|---|---|---|
| n (%) | 63,038 (20.2) | 112,178 (35.9) | 137,172 (43.9) |
| Age, y | 55.0 ± 8.2 | 54.9 ± 8.0 | 57.1 ± 7.8 |
| Female | 23,770 (37.7) | 54,378 (48.5) | 81,822 (59.6) |
| Whites | 60,739 (96.4) | 108,127 (96.4) | 131,601 (95.9) |
| BMI, kg/m2 | 27.6 ± 4.5 | 27.2 ± 4.3 | 26.5 ± 4.3 |
| Physical activity, METS min/wk | 3211.6 ± 4424.4 | 2779.5 ± 3609.8 | 2681.0 ± 3282.3 |
| Current smoker | 11,393 (18.1) | 11,982 (10.7) | 8350 (6.1) |
| Healthy diet score | 2.5 ± 1.3 | 2.8 ± 1.2 | 3.0 ± 1.2 |
| TDI | −0.9 ± 3.2 | −1.6 ± 2.9 | −1.9 ± 2.7 |
| Hypertension | 35,606 (56.5) | 58,769 (52.4) | 70,997 (51.8) |
| High cholesterol | 8820 (14.0) | 13,475 (12.0) | 17,084 (12.5) |
| Family history of diabetes | 13,014 (20.6) | 22,578 (20.1) | 26,792 (19.5) |
| Regular drinking (≥3 times/wk) | 32,457 (51.5) | 68,854 (61.4) | 80,497 (58.7) |
| Total alcohol intake, g/wk, median (5th–95th percentiles) | 129.6 (15.2–728.0) | 129.6 (20.0–598.4) | 89.6 (12.8–422.4) |
| Proportion of wine, % | 29.5 ± 37.4 | 56.9 ± 34.4 | 78.3 ± 27.5 |
| Proportion of beer, % | 54.0 ± 41.8 | 32.0 ± 33.7 | 15.4 ± 25.0 |
| Proportion of liquor, % | 16.5 ± 30.3 | 11.1 ± 20.1 | 6.3 ± 13.9 |
Values are n (%) or mean ± SD unless otherwise indicated. METS, metabolic equivalents; TDI, Townsend deprivation index.
The joint associations of timing of alcohol intake with respect to meals and amounts of alcohol intake on the risk of T2D
During a median follow-up of 10.9 y, we documented 8598 incident cases of T2D. After adjustment for age, sex, race, BMI, physical activity, smoking, healthy diet, TDI, hypertension, high cholesterol, and family history of diabetes, a higher proportion of consuming alcohol with meals was significantly associated with a lower risk of T2D (P-trend < 0.001). Compared with participants who consumed alcohol outside of meals, the adjusted HRs were 0.92 (95% CI: 0.87, 0.97) for participants who had varying patterns and 0.88 (95% CI: 0.83, 0.93) for participants who habitually consumed their alcohol with meals. These results did not change appreciably after further adjustment for the amount of alcohol intake and frequency of drinking (Table 2). Because the inverse association might be due to a greater proportion of women, a healthier lifestyle, a higher socioeconomic level, a higher frequency of drinking, or a lower prevalence of other chronic diseases in participants who consumed alcohol with meals than in others, we also performed stratified analyses according to the foregoing confounding factors. We did not find significant interactions between habitually consuming alcohol with meals and these risk factors on the risk of incident T2D (Supplemental Table 1).
TABLE 2.
The association between timing of alcohol intake with respect to meals and risk of type 2 diabetes1
| No | It varies | Yes | P-trend | |
|---|---|---|---|---|
| Cases, n (%) | 2328 (3.7) | 3033 (2.7) | 3237 (2.4) | |
| Model 1, HR (95% CI) | 1 (reference) | 0.92 (0.87, 0.97) | 0.88 (0.83, 0.93) | <0.001 |
| Model 1 + alcohol consumption, HR (95% CI) | 1 (reference) | 0.93 (0.88, 0.98) | 0.86 (0.82, 0.91) | <0.001 |
| Model 1 + frequency of drinking, HR (95% CI) | 1 (reference) | 0.94 (0.89, 0.99) | 0.89 (0.84, 0.94) | <0.001 |
| Model 2, HR (95% CI) | 1 (reference) | 0.94 (0.89, 0.99) | 0.88 (0.83, 0.93) | <0.001 |
Model 1 adjusted for age, sex, race, BMI, physical activity, smoking, healthy diet score, Townsend deprivation index, hypertension, high cholesterol, and family history of diabetes. Model 2 further adjusted for alcohol intake level and frequency of drinking on the basis of model 1.
For total alcohol consumption, we observed a U-shaped association between the total amount of alcohol intake and risk of T2D (P-quadratic trend = 0.005); the “moderate drinking” group (≥100 to <200 g/wk) was associated with the lowest risk of T2D (HR: 0.89; 95% CI: 0.83, 0.96) as compared with the lowest level of total alcohol amount (>0 to <50 g/wk) (Supplemental Figure 2A). Further analyses on various types of alcoholic beverages indicated that wine, beer, and liquor had differential associations with the risk of T2D. Higher amount of wine intake was associated with a lower risk of T2D (P-trend < 0.001), whereas higher amount of beer or liquor intake was associated with a higher risk of T2D (P-trend = 0.034 and 0.034, respectively) (Supplemental Figure 2C, D). For the frequency of alcohol intake, participants drinking >3–4 times/wk was associated with a lower risk of T2D than for those who drank <1 time/wk (Supplemental Table 2).
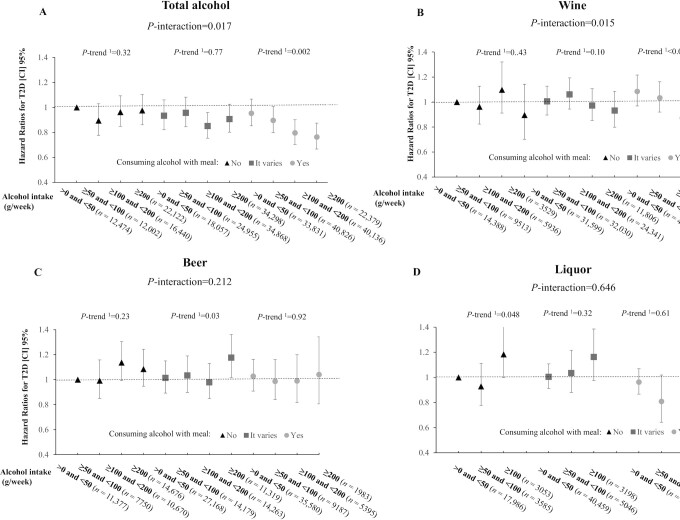
The joint analysis indicated that total alcohol consumption was differently related to the risk of T2D according to the timing of alcohol intake with respect to meals (P-interaction = 0.017) (Figure 1A). Total alcohol consumption was not associated with the risk of T2D in participants who consumed alcohol outside of meals and those who had varying patterns (P-trend = 0.32 and 0.77, respectively), whereas an inverse association was observed in participants who habitually consumed alcohol with meals (P-trend = 0.002). Similar results were also observed after including never drinkers and past drinkers and using never drinkers as the reference group (Supplemental Figure 3). When analyzing various types of alcoholic beverages, a similar significant interaction pattern was observed for wine (P-interaction = 0.015) (Figure 1B), but not for beer or liquor (Figure 1C, D).
FIGURE 1.
Joint association of alcohol consumption and the timing of alcohol intake with respect to meals in relation to risk of T2D. (A) Total alcohol, (B) wine, (C) beer, (D) liquor. Results were adjusted for age, sex, race, BMI, physical activity, smoking (never, past, current), healthy diet score, Townsend deprivation index, hypertension, high cholesterol, family history of diabetes, and frequency of alcohol intake. 1Linear trend; 2quadratic trend.
The joint associations of timing of alcohol intake with respect to meals and alcohol consumption on T2D-related biomarkers
As expected, we observed that a higher proportion of consuming alcohol with meals was associated with lower concentrations of CRP, lower levels of HbA1c, and higher concentrations of HDL cholesterol independent of the amount of alcohol intake and the frequency of drinking (all P-trend < 0.001) (Supplemental Table 3). Higher frequency of alcohol intake was also associated with favorable concentrations of CRP, levels of HbA1c, and concentrations of HDL cholesterol (Supplemental Figure 4).
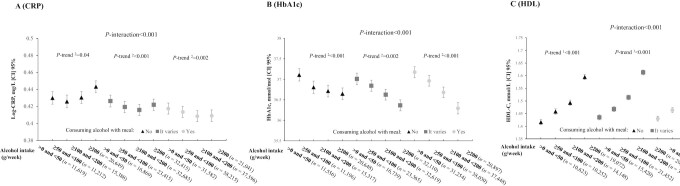
We observed a U-shaped association between the total amount of alcohol intake and CRP concentrations; the lowest concentration of CRP was observed in the “moderate drinking” group (≥100 to <200 g/wk) (P-quadratic trend < 0.001). Further analyses on various types of alcoholic beverages indicated that wine, beer, and liquor had differential associations with CRP concentrations; higher wine consumption was associated with lower concentrations of CRP (P-quadratic trend < 0.001), whereas higher beer or liquor consumption was significantly associated with higher concentrations of CRP (both P-trend < 0.001, respectively) (Supplemental Figure 5A). Intriguingly, the joint analyses indicated that the association of alcohol consumption with CRP concentrations appeared to be different according to the timing of alcohol intake with respect to meals. For total alcohol consumption, a J-shaped association was observed in participants who consumed alcohol outside of meals, whereas a reverse J-shaped association was observed in participants who consumed alcohol with meals (P-interaction < 0.001) (Figure 2A). When analyzing various types of alcoholic beverages, a similar significant interaction pattern was observed for wine (P-interaction = 0.01), but not for liquor (P-interaction = 0.988). The positive associations of beer consumption with CRP concentrations also appeared to be attenuated as the proportion of alcohol consumed with meals increased, although the P value of interaction was not statistically significant (P-interaction = 0.057) (Supplemental Figure 6A).
FIGURE 2.
Joint association of alcohol consumption and the timing of alcohol intake with respect to meals in relation to T2D-related biomarkers. (A) CRP, (B) HbA1c, (C) HDL-C. Results were adjusted for age, sex, race, BMI, physical activity, smoking (never, past, current), healthy diet score, Townsend deprivation index, hypertension, high cholesterol, family history of score, frequency of alcohol intake, and fasting time (for CRP and HDL-C analyses). For analyses on HDL-C, we also further excluded participants with high cholesterol. 1Linear trend; 2quadratic trend. CRP, C-reactive protein; HbA1c, glycated hemoglobin; HDL-C, high-density lipoprotein cholesterol.
Moreover, higher amount of total alcohol intake was associated with lower levels of HbA1c and higher concentrations of HDL cholesterol in a dose–response fashion, respectively. Similar associations were observed for various types of alcoholic beverages (Supplemental Figure 5B, C). The joint analyses showed that the favorable associations of total amount of alcohol intake with HbA1c levels were stronger in participants consuming their alcohol with meals than in those consuming alcohol outside of meals (P-interaction < 0.001) (Figure 2B). When analyzing various types of alcoholic beverages, similar interaction patterns were observed for wine but not for other alcoholic beverages (Supplemental Figure 6B). The favorable associations of alcohol intake with HDL cholesterol also appeared to be stronger in participants who consumed their alcohol with meals than in others (Figure 2C, Supplemental Figure 6C).
Discussion
In this large population-based cohort study in 312,388 healthy current drinkers from the UK Biobank, we found that moderate alcohol intake with meals was significantly associated with a lower risk of T2D than drinking outside of meals, independent of the amount and frequency of alcohol intake. The beneficial associations between alcohol drinking with meals and T2D were mainly driven by wine consumption.
Emerging evidence has indicated that the timing of alcohol intake with respect to meals is related to health outcomes, independent of the amount of alcohol consumed. Our previous study showed that consuming alcohol with meals was associated with lower risks of all-cause mortality, CVD mortality, and cancer mortality than was consuming alcohol outside of meals (2). A large cohort study from the United Kingdom showed that habitually consuming alcohol with meals was related to a 31% lower risk of liver cirrhosis than was consuming alcohol outside of meals (20). For T2D, only a subgroup analysis in the Health Professionals Follow-Up Study reported a null association between the timing of alcohol intake with respect to meals and risk of T2D, probably due to the relatively small sample size (21,511 participants) and short period of follow-up (4 y) (21). In the present study, we took advantage of the large sample size of the UK Biobank and long period of follow-up to show that consuming alcohol with meals was significantly associated with a lower risk of T2D than was consuming alcohol outside of meals. The consistent results of stratified analyses of confounding factors also ensured the robustness of our findings.
A novel finding in this study was that alcohol consumption was differently related to risks of T2D according to the timing of alcohol intake with respect to meals. The observed favorable association of moderate drinking with T2D risk in participants who habitually consumed alcohol with meals was supported by several long-term well-designed feeding studies (3–7). In all these studies, the alcoholic beverages used for testing were usually served with meals. For instance, the longest (2-y) intervention trial to date showed that moderate wine intake, as part of a dinner, significantly decreased HbA1c levels, fasting glucose concentrations, and improved insulin resistance in well-controlled diabetic patients (3). Moreover, a previous study indicated that co-ingestion of alcoholic beverages with white bread significantly reduced the glycemic response of white bread (postprandial blood glucose) (22). On the other hand, potential adverse effects of consuming alcohol outside of meals were also documented in a previous study; a short-term clinical trial found that participants in the fasting state consuming a glucose solution with alcohol significantly increased the 2-h glucose response by 18% in comparison with the pure glucose solution (23). Taken together, our findings highlight the importance of considering the timing of alcohol intake with respect to meals when investigating the relation between the amount of alcohol intake and the risk of T2D.
For T2D-related biomarkers, we found that the beneficial associations between the amount of alcohol intake and levels of HbA1c were stronger in participants who consumed alcohol with meals than in those who did not. Because HbA1c is an important indicator of long-term glycemic control, the results further support our findings on T2D. Intriguingly, distinct relations between the amount of alcohol intake and CRP concentrations were observed in participants with different timing of alcohol intake with respect to meals. In participants who consumed alcohol with meals, a higher amount of alcohol intake was associated with lower concentrations of CRP, but it was associated with higher concentrations of CRP in participants who consumed alcohol outside of meals. The precise mechanisms underlying the observed interactions remain unclear. In addition to slowing the absorption of alcohol, 3 previous clinical trials indicated that consuming alcohol with meals might more quickly and effectively reduce the oxidative stress caused by meals than does consuming alcohol outside of meals, and it is known that oxidative stress is closely related to glucose metabolism, lipid metabolism, and inflammation (24–26). More experimental studies are needed to explore how the timing of alcohol intake affects the association of alcohol consumption and health outcomes.
Moreover, further analyses on various types of alcoholic beverages indicated that the beneficial associations between alcohol drinking with meals and T2D or T2D-related biomarkers were mainly driven by wine consumption. It is unclear whether such unique beneficial associations related to wine consumption are due to the nonalcoholic components (mainly polyphenols) in wine or the healthier lifestyle profiles in wine drinkers than in non–wine drinkers (27, 28). Previous clinical trials indicated that wine seemed to confer greater anti-inflammatory effects than other alcoholic beverages owing to the anti-inflammatory properties of polyphenols (29, 30). However, the systemic bioavailability of polyphenols in wine is also argued to be low and not enough to produce health benefits (31).
The major strengths of our study include the large sample size, the wealth of information on alcohol consumption, and the covariates. In addition, to minimize the selection bias and reverse causality, which may lead to an overestimate of protective effects from moderate drinking (14), we limited our study participants to healthy current drinkers and excluded participants who reduced their alcohol intake owing to health-related problems. We also acknowledged that our study had several potential limitations. First, the specific ingredients of the meals ingested with the alcoholic beverages were not collected in this study. A previous study showed that the inverse association of alcohol consumption and T2D appeared to be stronger in participants with a higher-glycemic-load food intake (32). Second, the specific time of the meals was not collected in this study, either. Two previous studies showed that consuming alcohol at dinnertime might provide additional benefits through improving sleep quality (3, 5). Third, the majority of our participants are white European and it is unknown whether our findings could be generalized to other populations (33). Fourth, T2D cases were ascertained based on national health data sets (primary care, hospital admissions, and the death register data) in this study, which may not fully capture all T2D cases.
In conclusion, our study indicates that moderate drinking of alcohol, especially wine, with meals was associated with a lower risk of T2D in healthy current drinkers. Our findings emphasize the importance of considering the timing of alcohol intake in the relation between alcohol consumption and risk of T2D.
Supplementary Material
Acknowledgements
This research has been conducted using the UK Biobank Resource under Application Number 29256.
The authors’ responsibilities were as follows—HM and LQ: designed the research, provided statistical support, wrote the paper, and had primary responsibility for the final content; HM and XW: analyzed the data; and all authors: conducted the research, contributed to the revision of the manuscript, and read and approved the final manuscript.
Notes
Supported by National Heart, Lung, and Blood Institute grants HL071981, HL034594, and HL126024 (to LQ); and National Institute of Diabetes and Digestive and Kidney Diseases grants DK115679, DK091718, DK100383, and DK078616 (to LQ).
Author disclosures: The authors report no conflicts of interest.
Supplemental Figures 1–6 and Supplemental Tables 1–3 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
HM and XW contributed equally to this work.
Abbreviations used: CRP, C-reactive protein; HbA1c, glycated hemoglobin; ICD, International Classification of Diseases; METS, metabolic equivalents; TDI, Townsend deprivation index; T2D, type 2 diabetes.
Contributor Information
Hao Ma, Department of Epidemiology, School of Public Health and Tropical Medicine, Tulane University, New Orleans, LA, USA.
Xuan Wang, Department of Epidemiology, School of Public Health and Tropical Medicine, Tulane University, New Orleans, LA, USA.
Xiang Li, Department of Epidemiology, School of Public Health and Tropical Medicine, Tulane University, New Orleans, LA, USA.
Yoriko Heianza, Department of Epidemiology, School of Public Health and Tropical Medicine, Tulane University, New Orleans, LA, USA.
Lu Qi, Department of Epidemiology, School of Public Health and Tropical Medicine, Tulane University, New Orleans, LA, USA; Department of Nutrition, Harvard TH Chan School of Public Health, Boston, MA, USA.
Data Availability
Data described in the article and codebook will be made available upon request to the UK Biobank (https://www.ukbiobank.ac.uk/researchers/). Analytic code will be available from the corresponding author on reasonable request.
References
- 1. WHO . Global status report on alcohol and health 2018. Geneva (Switzerland): World Health Organization; 2019. [Google Scholar]
- 2. Ma H, Li X, Zhou T, Sun D, Shai I, Heianza Yet al. Alcohol consumption levels as compared with drinking habits in predicting all-cause mortality and cause-specific mortality in current drinkers. Mayo Clinic Proc. 2021;96(7):1758–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gepner Y, Golan R, Harman-Boehm I, Henkin Y, Schwarzfuchs D, Shelef Iet al. Effects of initiating moderate alcohol intake on cardiometabolic risk in adults with type 2 diabetes: a 2-year randomized, controlled trial. Ann Intern Med. 2015;163(8):569–79. [DOI] [PubMed] [Google Scholar]
- 4. Davies MJ, Baer DJ, Judd JT, Brown ED, Campbell WS, Taylor PR. Effects of moderate alcohol intake on fasting insulin and glucose concentrations and insulin sensitivity in postmenopausal women: a randomized controlled trial. JAMA. 2002;287(19):2559–62. [DOI] [PubMed] [Google Scholar]
- 5. Shai I, Wainstein J, Harman-Boehm I, Raz I, Fraser D, Rudich Aet al. Glycemic effects of moderate alcohol intake among patients with type 2 diabetes: a multicenter, randomized, clinical intervention trial. Diabetes Care. 2007;30(12):3011–6. [DOI] [PubMed] [Google Scholar]
- 6. Sierksma A, Patel H, Ouchi N, Kihara S, Funahashi T, Heine RJet al. Effect of moderate alcohol consumption on adiponectin, tumor necrosis factor-α, and insulin sensitivity. Diabetes Care. 2004;27(1):184–9. [DOI] [PubMed] [Google Scholar]
- 7. Napoli R, Cozzolino D, Guardasole V, Angelini V, Zarra E, Matarazzo Met al. Red wine consumption improves insulin resistance but not endothelial function in type 2 diabetic patients. Metabolism. 2005;54(3):306–13. [DOI] [PubMed] [Google Scholar]
- 8. Koppes LLJ, Dekker JM, Hendriks HFJ, Bouter LM, Heine RJ. Moderate alcohol consumption lowers the risk of type 2 diabetes: a meta-analysis of prospective observational studies. Diabetes Care. 2005;28(3):719–25. [DOI] [PubMed] [Google Scholar]
- 9. Knott C, Bell S, Britton A. Alcohol consumption and the risk of type 2 diabetes: a systematic review and dose-response meta-analysis of more than 1.9 million individuals from 38 observational studies. Diabetes Care. 2015;38(9):1804–12. [DOI] [PubMed] [Google Scholar]
- 10. Rasouli B, Ahlbom A, Andersson T, Grill V, Midthjell K, Olsson Let al. Alcohol consumption is associated with reduced risk of type 2 diabetes and autoimmune diabetes in adults: results from the Nord-Trøndelag health study. Diabet Med. 2013;30(1):56–64. [DOI] [PubMed] [Google Scholar]
- 11. Xu L, Xie J, Chen S, Chen Y, Yang H, Miao Met al. Light-to-moderate alcohol consumption is associated with increased risk of type 2 diabetes in individuals with nonalcoholic fatty liver disease: a nine-year cohort study. Am J Gastroenterol. 2020;115(6):876–84. [DOI] [PubMed] [Google Scholar]
- 12. Kao WHL, Puddey IB, Boland LL, Watson RL, Brancati FL. Alcohol consumption and the risk of type 2 diabetes mellitus: Atherosclerosis Risk in Communities Study. Am J Epidemiol. 2001;154(8):748–57. [DOI] [PubMed] [Google Scholar]
- 13. Han T, Zhang S, Duan W, Ren X, Wei C, Sun Cet al. Eighteen-year alcohol consumption trajectories and their association with risk of type 2 diabetes and its related factors: the China Health and Nutrition Survey. Diabetologia. 2019;62(6):970–80. [DOI] [PubMed] [Google Scholar]
- 14. Naimi TS, Stockwell T, Zhao J, Xuan Z, Dangardt F, Saitz Ret al. Selection biases in observational studies affect associations between ‘moderate’ alcohol consumption and mortality. Addiction. 2017;112(2):207–14. [DOI] [PubMed] [Google Scholar]
- 15. Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh Jet al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12(3):e1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Serino A, Salazar G. Protective role of polyphenols against vascular inflammation, aging and cardiovascular disease. Nutrients. 2019;11(1):53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Akash MSH, Rehman K, Chen S. Role of inflammatory mechanisms in pathogenesis of type 2 diabetes mellitus. J Cell Biochem. 2013;114(3):525–31. [DOI] [PubMed] [Google Scholar]
- 18. Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn Let al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic Impact Goal through 2020 and beyond. Circulation. 2010;121(4):586–613. [DOI] [PubMed] [Google Scholar]
- 19. Ma H, Li X, Zhou T, Sun D, Liang Z, Li Yet al. Glucosamine use, inflammation, and genetic susceptibility, and incidence of type 2 diabetes: a prospective study in UK Biobank. Diabetes Care. 2020;43(4):719–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Simpson RF, Hermon C, Liu B, Green J, Reeves GK, Beral Vet al. Alcohol drinking patterns and liver cirrhosis risk: analysis of the prospective UK Million Women Study. Lancet Public Health. 2019;4(1):e41–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Conigrave KM, Hu BF, Camargo CA, Stampfer MJ, Willett WC, Rimm EB. A prospective study of drinking patterns in relation to risk of type 2 diabetes among men. Diabetes. 2001;50(10):2390–5. [DOI] [PubMed] [Google Scholar]
- 22. Brand-Miller JC, Fatema K, Middlemiss C, Bare M, Liu V, Atkinson Fet al. Effect of alcoholic beverages on postprandial glycemia and insulinemia in lean, young, healthy adults. Am J Clin Nutr. 2007;85(6):1545–51. [DOI] [PubMed] [Google Scholar]
- 23. Hätönen KA, Virtamo J, Eriksson JG, Perälä M-M, Sinkko HK, Leiviskä Jet al. Modifying effects of alcohol on the postprandial glucose and insulin responses in healthy subjects. Am J Clin Nutr. 2012;96(1):44–9. [DOI] [PubMed] [Google Scholar]
- 24. Robertson RP. Red wine and diabetes health: getting skin in the game. Diabetes. 2014;63(1):31–8. [DOI] [PubMed] [Google Scholar]
- 25. Marfella R, Cacciapuoti F, Siniscalchi M, Sasso FC, Marchese F, Cinone Fet al. Effect of moderate red wine intake on cardiac prognosis after recent acute myocardial infarction of subjects with type 2 diabetes mellitus. Diabet Med. 2006;23(9):974–81. [DOI] [PubMed] [Google Scholar]
- 26. Noguer MA, Cerezo AB, Navarro ED, Garcia-Parrilla MC. Intake of alcohol-free red wine modulates antioxidant enzyme activities in a human intervention study. Pharmacol Res. 2012;65(6):609–14. [DOI] [PubMed] [Google Scholar]
- 27. Rimm EB. Wine, beer, and spirits: are they really horses of a different color?. Circulation. 2002;105(24):2806–7. [DOI] [PubMed] [Google Scholar]
- 28. Rimm EB. Invited commentary—alcohol consumption and coronary heart disease: good habits may be more important than just good wine. Am J Epidemiol. 1996;143(11):1094–8. [DOI] [PubMed] [Google Scholar]
- 29. Estruch R, Sacanella E, Badia E, Antúnez E, Nicolás JM, Fernández-Solá Jet al. Different effects of red wine and gin consumption on inflammatory biomarkers of atherosclerosis: a prospective randomized crossover trial: effects of wine on inflammatory markers. Atherosclerosis. 2004;175(1):117–23. [DOI] [PubMed] [Google Scholar]
- 30. Chiva-Blanch G, Urpi-Sarda M, Llorach R, Rotches-Ribalta M, Guillén M, Casas Ret al. Differential effects of polyphenols and alcohol of red wine on the expression of adhesion molecules and inflammatory cytokines related to atherosclerosis: a randomized clinical trial. Am J Clin Nutr. 2012;95(2):326–34. [DOI] [PubMed] [Google Scholar]
- 31. Kroon PA, Clifford MN, Crozier A, Day AJ, Donovan JL, Manach Cet al. How should we assess the effects of exposure to dietary polyphenols in vitro?. Am J Clin Nutr. 2004;80(1):15–21. [DOI] [PubMed] [Google Scholar]
- 32. Mekary RA, Rimm EB, Giovannucci E, Stampfer MJ, Willett WC, Ludwig DSet al. Joint association of glycemic load and alcohol intake with type 2 diabetes incidence in women. Am J Clin Nutr. 2011;94(6):1525–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dakeishi M, Murata K, Sasaki M, Tamura A, Iwata T. Association of alcohol dehydrogenase 2 and aldehyde dehydrogenase 2 genotypes with fasting plasma glucose levels in Japanese male and female workers. Alcohol Alcohol. 2008;43(2):143–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the article and codebook will be made available upon request to the UK Biobank (https://www.ukbiobank.ac.uk/researchers/). Analytic code will be available from the corresponding author on reasonable request.