Abstract
Purpose
Many investigations on prognostic factors in lung cancer have been conducted; however, little is known regarding the outcomes of lung cancer cases complicated by deep vein thrombosis (DVT). This study aimed to determine the risk factors and impact on outcomes of lung cancer patients concurrent with DVT.
Methods
Lung cancer patients who underwent lower-extremity venous ultrasound were enrolled in this study. The patients were divided into a DVT group and a non-DVT group. Demographic information, clinical characteristics, and survival were analyzed by t-test, Wilcoxon test, chi-squared test, and logistic regression analysis.
Results
Of the 160 enrolled lung cancer patients, DVT was detected in 30 patients. Among the DVT group, adenocarcinoma was the most common histological type (27/30, 90.00%). Lung cancer complicated with DVT was associated with advanced stage, more severe myocardial injury, and a hypercoagulable state (P < .05). Differences in driver genes between the two groups were not significant. Radiologically, lung cancer patients with DVT were more likely to present with pericardial effusion and pleural effusion than patients without DVT (P < .05). Following multivariable logistic regression analysis, advanced stage (OR 5.368, [95%CI 1.871-18.165], P = .021), NT-proBNP >300 pg/ml (OR 5.575, [95%CI 1.733-3.722], P = .018), D-dimer >5 mg/L (OR 8.449, [95%CI 4.323-18.536], P = .004), CRP >12 mg/L (OR 6.687, [95%CI 1.967-13.617], P = .010), and serum CEA >25 ng/ml (OR 4.755, [95%CI 1.358-3.123], P = .029) were independent risk factors for adenocarcinoma complicated with DVT. Finally, survival analysis revealed that the occurrence of DVT resulted in a poorer prognosis despite anticoagulant therapy (P < .05).
Conclusion
DVT is a potential complication in patients with lung adenocarcinoma and could represent a prognostic marker for unfavorable outcome. It is essential to screen for DVT in high-risk adenocarcinoma patients.
Keywords: DVT, lung cancer, adenocarcinoma, prognosis, risk
Introduction
Lung cancer is a common cause of morbidity and mortality worldwide.1 Many factors have been reported to be significantly associated with prognosis in lung cancer. The clinical outcomes of lung cancer patients mainly depend on the tumor-node-metastasis (TNM) stage. However, patients with the same TNM stage may have different clinical outcomes.2 Evidence on tumor markers is inconsistent, and only a few markers have emerged as clinically useful.3 More clinical markers are needed to complement the assessment of prognosis in lung cancer patients.
The relationship between coagulation and malignancy is well recognized. Lung cancer patients often suffer from blood hypercoagulation and alterations in blood coagulation.4 Cancer can increase coagulability and the release of procoagulant microparticles into the circulation,5 which may lead to an increased incidence of thromboembolic disease. Furthermore, transient risk factors, such as surgery, trauma, acute infection, and hospitalization, can significantly increase the risk of cancer-associated thrombosis.6 It has been reported that activation of the coagulation system and procoagulant changes are relevant to advanced tumor behavior, including tumor invasion and distant metastasis.7,8
Deep vein thrombosis (DVT) has an incidence of 1-2/1000 and is the third most common cardiovascular disease.9 Venous stasis, vascular injury, and hypercoagulability are three factors that contribute to the formation of thrombosis.10 Although the association between DVT and malignancy is well established, the prognostic value of DVT in lung cancer remains unclear. We investigated differences in the clinical features between lung cancer patients with DVT and without DVT to further understand the impact of DVT on lung cancer.
Methods
Study Design and Population
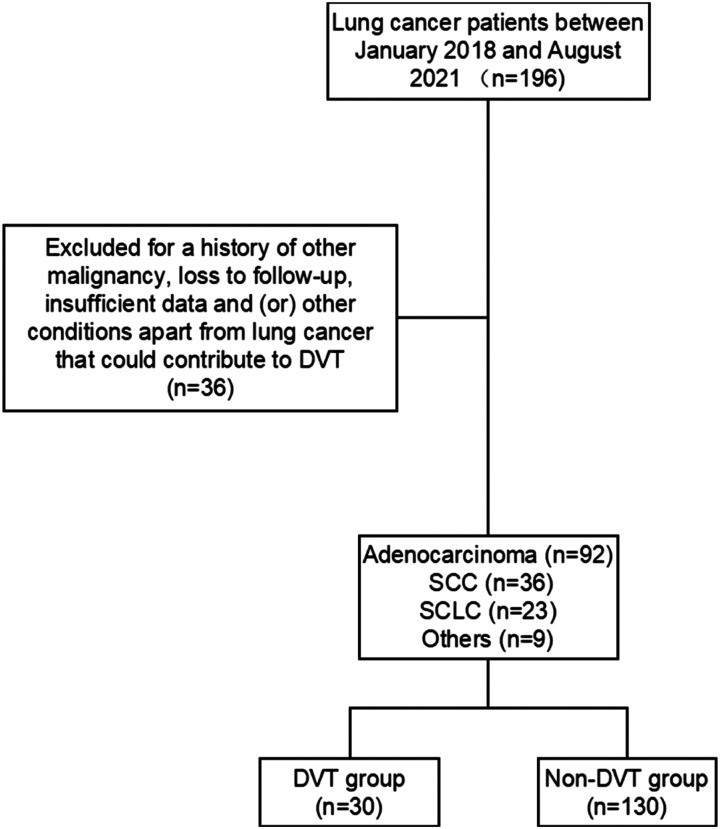
This study was conducted at the First Affiliated Hospital of Soochow University between January 2018 and August 2021. We included 160 patients who had pathologically confirmed lung cancer in this retrospective study and divided them into a DVT group and a non-DVT group according to venous ultrasound imaging. Patients were excluded if they had a history of other malignancy, loss to follow-up, insufficient data, superficial DVTs and/or underlying conditions other than lung cancer that could contribute to DVT. The inclusion and exclusion process is summarized in Figure 1. This study was approved by the Ethics Committee of the First Affiliated Hospital of Soochow University (2022-071).
Figure 1.
Flow chart of the inclusion and exclusion criteria for this study.
Data Acquisition
Demographic Data
Demographic data collected for all study patients included age, gender, and comorbidities.
Tumor Information
Tumor histology was classified as small-cell lung cancer (SCLC) or non-small cell lung cancer (NSCLC) and was further divided into adenocarcinoma, squamous cell carcinoma (SCC), and other NSCLC. Clinical stage was classified as I to IV according to TNM 8th edition. For statistical purposes, we categorized the population into two groups: early stage (I–II) and advanced stage (III–IV).9 Response Evaluation Criteria in Solid Tumors (RECIST) provided the standards for evaluating the response to treatment.
Laboratory Data
Cardiac injury parameters (NT-proBNP, LDH), blood parameters (RBC, RDW, PLT, PDW, WBC, Hb), coagulation parameters (D-dimer, TT, PT, APTT, AT-III, FDP, Fbg), an inflammatory parameter (CRP), and a tumor maker (serum CEA) were recorded by reviewing medical records. Relevant data were obtained postoperatively.
DVT Detection
All patients enrolled were examined with venous ultrasound by a ultrasound expert, then divided into a DVT group and a non-DVT group based on presence/absence of the DVT. The criteria for the diagnosis of DVT were the following: loss of compressibility of the vein, presence of intraluminal echogenicity, and absence of venous flow.
Survival Analysis
Survival information was obtained from the medical record system, outpatient follow-up, or telephone follow-up. The outcome was mortality from any cause within 1 year of diagnosis or until the time of study termination.
Statistical Analysis
Statistical analysis was performed using IBM SPSS Statistics 25.0 and GraphPad Prism 8.0. Normally distributed continuous variables were compared using Student’s t-test and are expressed as means ± standard deviations (x ± S). Non-normally distributed continuous variables were compared using Wilcoxon test and are expressed as medians (25th to 75th percentile range). The chi-square test was used for categorical variables, and categorical variables are presented as percentages and frequencies. Variables associated with risk factors for patients complicated with DVT in the univariate analysis (P < .05) were included in a stepwise multiple logistic regression model. Logistic regression analysis of r was performed. The Kaplan–Meier method and log-rank test were applied to calculate and compare survival probability. P < .05 was considered statistically significant.
Results
Demographic Information
A total of 160 lung cancer patients were included in the study, of which 30 patients presented with DVT and 130 without DVT during follow-up. As shown in Table 1, the average age in the DVT group (62.7 ± 14.6 years) vs. the non-DVT group (65.6 ± 9.1 years) was comparable (P > .05), as was the proportion of males in the DVT group (63.33%) vs. the non-DVT group (78.46%) (P > .05). The cohort comprised patients with adenocarcinoma (92/160, 57.50%), SCC (36/160, 22.50%), SCLC (23/160, 14.38%), and others (9/160, 5.62%).
Table 1.
Demographic information of subjects.
| Total | DVT Group (n = 30) | Non-DVT Group (n = 130) | t/χ2 | P | |
|---|---|---|---|---|---|
| Age(years) | 62.7 ± 14.6 | 65.6 ± 9.1 | .329 | ||
| Sex | |||||
| Male | 121 | 19(63.33%) | 102(78.46%) | .487 | .485 |
| Histology | 160 | 16.961 | .001 | ||
| Adenocarcinoma | 92 | 26 | 66 | ||
| SCC | 36 | 0 | 36 | ||
| SCLC | 23 | 2 | 21 | ||
| Others | 9 | 1 | 8 | ||
Considering adenocarcinoma was the most populous histology among all lung cancer patients with DVT, 96 adenocarcinoma patients were enrolled for further investigation.
An Increased Risk of DVT in Patients With Adenocarcinoma
In terms of histological type of lung cancer, adenocarcinoma was the predominant type and was significantly more common in the DVT group (26/30, 86.67%) than in the non-DVT group (66/130, 50.77%) (P < .05) Table 1. Notably, only 2 of the 22 SCLC patients had DVT, and no SCC patient developed DVT. Patients with adenocarcinoma were more likely to suffer from DVT than patients with other histological types of lung cancer. Based on these data, lung adenocarcinoma patients with DVT (n = 26) or without (n = 66) DVT were included in the following investigation.
DVT Indicated Advanced Stage and Disease Progression
Compared with the non-DVT group, most lung adenocarcinoma patients in the DVT group were in stage III–IV (25/26, 96.15% vs. 50/66, 75.75%, P < .05), with an increased level of CEA (55.78 [2.69-197.60] vs. 5.50 [3.26-15.89] ng/ml, P < .05) Table 2. When screening for DVT, 71 (71/92, 77.17%) lung adenocarcinoma patients had progressive disease (PD) and 21 had either partial response (PR, 12/92, 13.04%) or stable disease (SD, 9/92, 9.78%). Accordingly, the ratio of PD was significantly increased in the DVT group (24/26, 92.31%) compared with the non-DVT group (47/66, 71.21%) (P < .05), and the ratio of PR-SD was significantly decreased in the DVT group (2/21, 9.52%) compared with the non-DVT group (19/21, 90.48%). Our data indicate that DVT is associated with advanced tumor and is a clinic predictor for disease progression.
Table 2.
Clinicopathological features in cohort of lung adenocarcinoma.
| Total | DVT Group (n = 26) | Non-DVT Group (n = 66) | t/χ2 | P | |
|---|---|---|---|---|---|
| Age(years) | 63.7 ± 9.8 | 62.3 ± 13.2 | .594 | ||
| Sex | |||||
| Male | 58 | 17 | 41 | .085 | .770 |
| Comorbidities | |||||
| Diabetes | 10 | 2 | 8 | .378 | .539 |
| Hypertension | 37 | 10 | 27 | .046 | .829 |
| Hepatopathy | 16 | 3 | 13 | .864 | .353 |
| COPD | 4 | 1 | 3 | .022 | .882 |
| Disease status | 4.712 | .030 | |||
| PD | 71 | 24 | 47 | ||
| PR-SD | 21 | 2 | 19 | ||
| Stages | 5.151 | .023 | |||
| I-II | 17 | 1 | 16 | ||
| III-IV | 75 | 25 | 50 | ||
| Cancer-Directed therapy | |||||
| Surgical intervention | 36 | 8 | 28 | 1.064 | .302 |
| Chemotherapy | 70 | 12 | 58 | 3.076 | .076 |
| Radiotherapy | 10 | 1 | 9 | 1.845 | .174 |
| Immunotherapy | 16 | 2 | 14 | 2.373 | .123 |
| Targeted therapy | 37 | 6 | 31 | 2.541 | .111 |
DVT and Tumor Driver Genes
Among the 58 patients who underwent gene detection, the rates of EGFR mutation were 35.71% (5/14) and 31.82% (14/44) in the two groups, respectively (P > .05) Table 3. We investigated the pathology reports of 59 patients. ALK gene rearrangements were observed in 25.00% (4/16) of adenocarcinoma patients with DVT, in contrast with 9.30% (4/43) in the non-DVT group (P > .05). Our research did not support the effect of driver genes on DVT in lung adenocarcinoma patients.
Table 3.
Driver genes for adenocarcinoma patients concurrent with or without DVT.
| Driver genes | Total | DVT Group | Non-DVT Group | χ2 | P |
|---|---|---|---|---|---|
| EGFR-mutant | 19(58) | 5(14) | 14(44) | .013 | .789 |
| ALK-rearranged | 8(59) | 4(16) | 4(43) | 1.295 | .117 |
Myocardial Injury and High Coagulation State in DVT Group
Regarding cardiac injury parameters, DVT compared with no DVT was significantly associated with increased NT-proBNP (367.25 [210.94-659.32] vs. 129.70 [36.83-306.60] pg/ml) and LDH (266.5 [184.7-305.7] vs. 222.0 [169.9-314.5] U/L) (P < .05 for each) Table 4. Regarding coagulation parameters, D-dimer (7.04 [2.66-17.91] vs. 1.03 [.41-1.69] mg/L) and FDP (19.01 [4.84-75.82] vs. 3.34 [1.69-5.55] mg/L) were higher, and AT-III (89 ± 14% vs. 96 ± 12%) was lower in the DVT group compared with the non-DVT group (P < .05 for each). Furthermore, WBC (10.93 [8.19-19.81] vs. 10.23 [7.71-11.42] ×109/L) and CRP (10.46 [2.19-35.16] vs. 2.62 [1.07-7.7] mg/L) levels were significantly elevated in the DVT group compared with the non-DVT group (P < .05 for each). These findings suggest that patients with DVT presented more unfavorable laboratory data.
Table 4.
Laboratory data in cohort of lung adenocarcinoma patients.
| Laboratory Data | DVT Group (n = 26) | Non-DVT Group (n = 66) | P |
|---|---|---|---|
| Cardiacinjury parameters | |||
| NT-pro BNP (pg/ml) | 367.25(210.94-659.32) | 129.70(36.83-306.60) | .000 |
| LDH (U/L) | 266.5(184.7-305.7) | 222.0(169.9-314.5) | .018 |
| Coagulation parameters | |||
| D-dimer (mg/L) | 7.04(2.66-17.91) | 1.03(.41-1.69) | .000 |
| TT (sec) | 16.8 ± 1.5 | 17.0 ± 1.7 | .557 |
| PT (sec) | 12.70(11.10-13.53) | 12.50(11.90-13.15) | .047 |
| APTT (sec) | 34.05 ± 10.17 | 33.84 ± 7.32 | .103 |
| AT-III (%) | 89 ± 14 | 96 ± 12 | .040 |
| FDP (mg/L) | 19.01(4.84-75.82) | 3.34(1.69-5.55) | .000 |
| Fbg (g/L) | 3.85(2.97-4.30) | 3.64(2.99-4.60) | .947 |
| Blood parameters | |||
| RBC (1012/L) | 4.26(3.94-4.60) | 4.22(3.85-4.49) | .097 |
| RDW (%) | 13.9(13.2-15.9) | 14.7(13.2-16.3) | .908 |
| PLT (109/L) | 266(232-329) | 200(153-286) | .544 |
| PDW (%) | 14.6(11.0-15.8) | 16.1(15.6-16.5) | .013 |
| Hb (g/L) | 127 ± 19 | 124 ± 27 | .657 |
| WBC (109/L) | 10.93(8.19-19.81) | 10.23(7.71-11.42) | .023 |
| Inflammation parameter | |||
| CRP (mg/L) | 10.46(2.19-35.16) | 2.62(1.07-7.7) | .001 |
| Tumor maker | |||
| CEA (ng/ml) | 55.78(2.69-197.60) | 5.50(3.26-15.89) | .045 |
Higher Prevalence of Pericardial Effusion and Pleural Effusion in DVT Group
Data on ultrasound, CT, and CTPA were obtained using the PACS system. Pericardial effusion and pleural effusion appeared as echo-lucent spaces on ultrasound or an attenuation value on chest CT.11 Pericardial effusion (50.00%, 13/26 vs. 13.64%, 9/66; P < .05) and pleural effusion (80.77%, 21/26 vs. 24.24%, 16/66; P<.05) were more frequent in the DVT group than in the non-DVT group.
As the gold standard for the diagnosis of acute pulmonary embolism, CTPA was performed on 21 patients: 14 patients in the DVT group and 7 in the non-DVT group. Pulmonary embolism was observed in 13 patients in the DVT group and 5 patients in the non-DVT group (92.85% vs. 71.43%, P > .05). Although lung adenocarcinoma patients with DVT may be at an increased risk of developing pulmonary embolism, patients without DVT should also be carefully monitored.
Risk Factors in Adenocarcinoma Patients Complicated With DVT
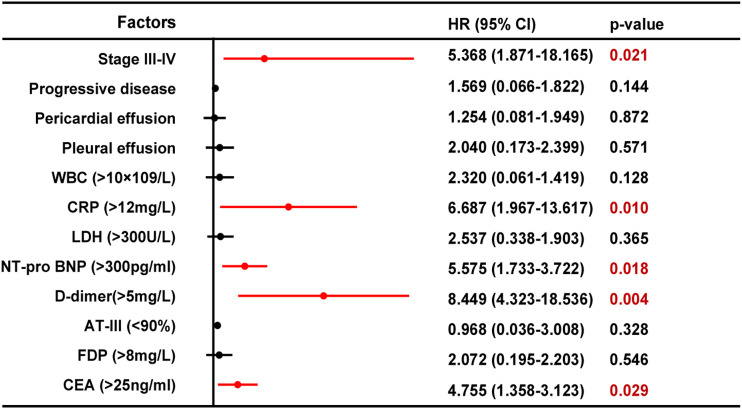
We selected the upper quartile of total sample as cut-off values for each factor. Following univariate analysis, in lung adenocarcinoma patients, advanced stage (P = .029), WBC >9×109/L (P = .014), CRP >12 mg/L (P = .026), NT-proBNP >300 pg/ml (P = .031), D-dimer >5 mg/L (P = .016), and CEA >25 ng/ml (P = .038) were associated with an increased risk of DVT Table 5. Multivariate logistic regression analysis revealed similar results. Advanced stage (OR 5.368, [95%CI 1.871-18.165], P = .021), NT-proBNP >300 pg/ml (OR 5.575, [95%CI 1.733-3.722], P = .018), D-dimer >5 mg/L (OR 8.449 [95%CI 4.323-18.536], P = .004), CRP >12 mg/L (OR 6.687 [95%CI 1.967-13.617], P = .010), and CEA >25 ng/ml (OR 4.755 [95%CI 1.358-3.123], P = .029) were independent risk factors for adenocarcinoma patients complicated with DVT Figure 2.
Table 5.
Univariate analysis and multivariate analysis data of adenocarcinoma patients complicated with DVT.
| Risk factor | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Stage III-IV | 4.776 (1.570-3.914) | .029 | 5.368 (1.871-18.165) | .021 |
| PD | 2.320 (.061-1.419) | .128 | 1.569 (.066-1.822) | .144 |
| Pericardial effusion | 1.088 (.028-2.983) | .297 | 1.254 (.081-1.949) | .872 |
| Pleural effusion | .089 (.160-12.059) | .765 | 2.040 (.173-2.399) | .571 |
| WBC (>9×109/L) | 6.003 (1.095-3.305) | .014 | 2.320 (.061-1.419) | .128 |
| CRP (>12 mg/L) | 4.964 (1.785-2.208) | .026 | 6.687 (1.967-13.617) | .010 |
| LDH (>300U/L) | .554 (.102-3.015) | .494 | 2.537 (.338-1.903) | .365 |
| NT-pro BNP (>300pg/ml) | 4.633 (1.343-5.406) | .031 | 5.575 (1.733-3.722) | .018 |
| D-dimer(>5 mg/L) | 5.841 (2.137-14.469) | .016 | 8.449 (4.323-18.536) | .004 |
| AT-III (<90%) | .710 (.048-3.353) | .400 | .968 (.036-3.008) | .328 |
| FDP (>6 mg/L) | 1.290 (.147-14.469) | .819 | 2.072 (.195-2.203) | .546 |
| CEA (>25 ng/ml) | 4.311 (1.197-5.104) | .038 | 4.755 (1.358-3.123) | .029 |
Figure 2.
Forest plots displayed Hazard Rate (HR) with 95% confidence interval (CI) of multivariate analysis for each risk factor.
DVT has a Negative Impact on Prognosis of Lung Adenocarcinoma
Everyone diagnosed with a DVT get anticoagulation. In the DVT group, 23 patients received subcutaneous injection of low-molecular-weight heparin (LMWH) and 3 patients recieved direct oral anticoagulants. Anticoagulation time and dose were adjusted according to patients’ weight and coagulation.
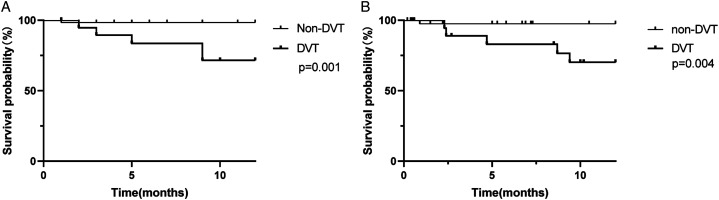
Among the lung adenocarcinoma subjects (n = 92), the 12-month survival rate was 80.77% in the DVT group (n = 26) and 96.97% in the non-DVT group (n = 66). According to Kaplan–Meier analysis, the 12-month survival rate in the DVT group was significantly lower than in the non-DVT group (P < .05) (Figure 3). Then we selected advanced adenocarcinoma (stage III–IV, n = 75) as another cohort to evaluate the prognostic role of DVT independent of tumor stage. As shown in Figure 3, the 12-month survival rate for the DVT group was 80.00%, which was significantly lower than the non-DVT group (96.00%, P < .05). These findings may suggest that DVT is associated with poor prognosis in lung adenocarcinoma regardless of tumor stage.
Figure 3.
Kaplan-Meier survival analysis was used to estimate the 12-month survival. A, all enrolled adenocarcinoma patients with (n = 26) or without (n = 66) DVT (P < .05). B, advanced adenocarcinoma (stage III-IV) with (n = 25) or without (n = 50) DVT (P < .05).
Discussions
Our study showed that, in a cohort of lung cancer patients, adenocarcinoma is the most common histological type in DVT patients, and DVT occurrence in adenocarcinoma may indicate worsening of cancer.
The risk of DVT in lung cancer varies widely and is associated with histological type and stage. Regarding histological type in lung cancer patients, adenocarcinoma is a predictor of DVT. Adenocarcinoma produces mucins, which act as a ligand for selectins on platelets and leukocytes; such interactions between selectins and mucins are involved in generating platelet-rich microthrombi in the microvasculature.12 In lung adenocarcinoma, leukocytosis is frequently present and has the greatest contribution to the development of thrombosis.13 Cancer cells activate neutrophils and monocytes and exert coagulant-promoting effects.14 As a result, adenocarcinoma patients have a higher incidence of thrombosis.
The association between cancer stage and thrombosis risk has been observed in several studies. It has been reported that patients with advanced lung adenocarcinoma are predisposed to DVT, and tumor grade may help identify patients with cancer who are at a high risk of DVT.15 Compared with patients in the primary stage, advanced cancer patients have a relatively shortened clotting time and increased plasminogen activator inhibitor level,4 which may be related to the higher risk of DVT.
In the comparison of imaging data, we demonstrated that adenocarcinoma with DVT was significantly associated with pleural effusion and pericardial effusion. Pleural effusion is a frequent complication of lung cancer, and the presence of pleural effusion in lung adenocarcinoma patients is associated with disease progression, advanced stage, and poor prognosis.16,17 Furthermore, lung cancer is the most common cause of pericardial involvement, and pericardial effusion can progress to cardiac tamponade, which is a life-threatening condition.18,19 It is paramount that clinicians are aware of pleural effusion and pericardial effusion and that they are recognized and managed in a timely manner.
The survival of lung cancer patients has improved owing to important advances in treatment and diagnosis.20 However, DVT led to a decreased survival rate in our study compared with the non-DVT group, which is consistent with a study that reported that DVT was associated with an increased risk of death and indicated lung cancer mortality.21 DVT may predict local or regional disease and biologically aggressive cancer.22 Activation of blood coagulation and hemostatic abnormalities may lead to the recruitment of inflammatory cells, generation of tumor stroma, and angiogenesis, promoting tumor growth, metastasis, and recurrence.23 Clot formation surrounding metastatic cells in the blood stream may allow tumor cells to escape immune surveillance and form distant metastatic deposits.22 These observations suggest that abnormal blood coagulation is correlated with progressive cancer and decreased survival.
In addition, treatment methods for DVT affect the prognosis of lung cancer patients with DVT.24 In this study, all DVT patients received anticoagulation therapy. A previous study highlighted that prophylactic anticoagulation therapy in lung cancer patients could significantly reduce thrombotic events and improve prognosis.25 However, anticoagulation increases the risk of bleeding. The complex interplay between disease- and treatment-related factors also increases the risk of bleeding, such as abnormal clotting mechanisms caused by the tumor and thrombocytopenia caused by chemotherapy.26 Therefore, it is difficult to achieve pharmacological prevention in cancer patients. Unfortunately, our data failed to answer the question of whether cancer patients with VTE should receive more aggressive anticoagulation than other patients with thrombosis. Guidelines regarding the use of anticoagulants in lung cancer patients vary.15 Specific therapeutic studies are needed to determine the optimal duration and dose of anticoagulant treatment in patients with cancer-related DVT.
Compared with previous studies, this analysis had the advantage of exploring the impact of DVT on adenocarcinoma and identifying a predictor of disease progression more comprehensively. Unavoidably, there were some limitations due to the retrospective design and small cohort of patients in this study. The sample size was not very large as the study took place at a single center. Furthermore, unavoidable selection bias due to the retrospective nature of our study limits the interpretation of the results.
Our study presents a novel observation that DVT is a potential complication in lung adenocarcinoma patients, and patients with DVT have a higher risk of progression and poorer prognosis. It is essential to screen for DVT in high-risk adenocarcinoma patients as it will help to improve the management of these patients. Clinicians need to pay attention and implement effective treatments in lung cancer patients with DVT.
Acknowledgments
We thank the patients, the nurses and clinical staff who are providing care for the patient, and staff at the local and state health departments.
Appendix.
Abbreviations
- APTT
activated partial thromboplastin time
- AT-III
antithrombin III
- CEA
carcinoembryonic antigen
- CRP
C-reactive protein
- CT
computed tomography
- CTPA
computed tomography pulmonary angiography
- DVT
deep vein thrombosis
- Fbg
fibrinogen
- FDP
fructose diphosphate
- Hb
hemoglobin
- LC
Lung cancer
- LDH
lactate dehydrogenase
- NSCLC
non-small cell lung cancer
- NT-pro BNP
N-terminal-pro-B-type natriuretic peptide
- PACS
picture archive systems
- PD
progressive disease
- PDW
platelet distribution width
- PLT
platelet count
- PR
partial response
- PT
prothrombin time
- RBC
red blood cell count
- RDW
red blood cell volume distribution width
- SCC
squamous cell carcinoma
- SCLC
small cell lung cancer
- SD
stable disease
- TT
thrombin time
- WBC
White Blood Cells count
Footnotes
Author contributions: All authors contributed to the study conception and design. Cheng Chen and Yi-fan Jin conceived the idea, designed, and supervised the study, drafted the manuscript. Yi-fan Jin, Ye-qiu Ye and Yu-jia Jin collected data and had full access to all of the data and took responsibility for the integrity of the data. Xin-yun Zhu and Ming Sha analyzed data and performed statistical analysis. All authors read and approved the final manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Technology Research and Development Funding of Suzhou city SKY2021034 (to CC). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Ethical Approval: This study was approved by the Ethics Committee of the First Affiliated Hospital of Soochow University (2022-071).
ORCID iD
Cheng Chen https://orcid.org/0000-0002-9583-7991
References
- 1.Xu K, Zhang C, Du T, et al. Progress of exosomes in the diagnosis and treatment of lung cancer[J]. Biomed Pharmacother. 2021;134:111111. [DOI] [PubMed] [Google Scholar]
- 2.Wang Y, Zhou Y, Zhou K, et al. Prognostic value of pre-treatment red blood cell distribution width in lung cancer: a meta-analysis. Biomarkers. 2020;25(3):241-247. [DOI] [PubMed] [Google Scholar]
- 3.McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM. REporting recommendations for tumour MARKer prognostic studies (REMARK). Br J Cancer. 2005;93(4):387-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cui YQ, Tan XM, Liu B, et al. Analysis on risk factors of lung cancer complicated with pulmonary embolism[J]. The Clinical Respiratory Journal. 2021;15(1):65-73. [DOI] [PubMed] [Google Scholar]
- 5.Zhou Y, Guo Y, Cui Q, et al. Application of Thromboelastography to Predict Lung Cancer Stage[J]. Technology in Cancer Research & Treatment, 2020;19:1079219999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Syrigos K, Grapsa D, Sangare R, et al. Prospective Assessment of Clinical Risk Factors and Biomarkers of Hypercoagulability for the Identification of Patients with Lung Adenocarcinoma at Risk for Cancer-Associated Thrombosis: The Observational ROADMAP-CAT Study[J]. The Oncologist. 2018;23(11):1372-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang F, Wang Y, Sun P, et al. Fibrinogen promotes malignant biological tumor behavior involving epithelial –mesenchymal transition via the p-AKT/p-mTOR pathway in esophageal squamous cell carcinoma[J]. J Cancer Res Clin Oncol. 2017;143(12):2413-2424. [DOI] [PubMed] [Google Scholar]
- 8.Fan S, Guan Y, Zhao G, et al. Association between plasma fibrinogen and survival in patients with small-cell lung carcinoma[J]. Thorac Cancer. 2018;9(1):146-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Midulla M, Chevallier O, Comby PO, et al. Endovascular management of the deep venous thrombosis: A new challenging role for the endovascular specialist in 2020[J]. Catheter Cardiovasc Interv. 2021;98(4):748-755. [DOI] [PubMed] [Google Scholar]
- 10.Stone J, Hangge P, Albadawi H, et al. Deep vein thrombosis: pathogenesis, diagnosis, and medical management[J]. Cardiovasc Diagn Ther. 2017;7(S3):S276-S284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Azarbal A, LeWinter MM. Pericardial Effusion[J]. Cardiol Clin. 2017;35(4):515-524. [DOI] [PubMed] [Google Scholar]
- 12.Salla E, Dimakakos EP, Tsagkouli S, et al. Venous Thromboembolism in Patients Diagnosed With Lung Cancer[J]. Angiology. 2016;67(8):709-724. [DOI] [PubMed] [Google Scholar]
- 13.Hisada Y, Mackman N. Cancer-associated pathways and biomarkers of venous thrombosis[J]. Blood. 2017;130(13):1499-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hiraide M, Shiga T, Minowa Y, et al. Identification of risk factors for venous thromboembolism and evaluation of Khorana venous thromboembolism risk assessment in Japanese lung cancer patients[J]. J Cardiol. 2020;75(1):110-114. [DOI] [PubMed] [Google Scholar]
- 15.Chen Y, Tsang YS, Chou X, et al. A lung cancer patient with deep vein thrombosis:a case report and literature review[J]. BMC Cancer. 2019;19(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanayama T, Taniguchi T, Tomita H, et al. ALDH1 and SALL4 Expression in Cell Block Samples from Patients with Lung Adenocarcinoma and Malignant Pleural Effusion[J]. Diagnostics. 2021;11(8):1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng M, Zhu J, Liang L, et al. Diagnostic value of tumor markers for lung adenocarcinoma-associated malignant pleural effusion: a validation study and meta-analysis[J]. Int J Clin Oncol. 2017;22(2):283-290. [DOI] [PubMed] [Google Scholar]
- 18.Vemireddy LP, Jain N, Aqeel A, et al. Lung Adenocarcinoma Presenting as Malignant Pericardial Effusion/Tamponade[J]. Cureus. 2021;2(13). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Imai H, Kaira K, Masubuchi K, et al. Management of Lung Cancer-Associated Malignant Pericardial Effusion with Intrapericardial Administration of Carboplatin: A Retrospective Study[J]. Curr Oncol. 2022;29(1):163-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schabath MB, Cote ML. Cancer Progress and Priorities: Lung Cancer[J]. Cancer epidemiology, biomarkers & prevention. 2019;28(10):1563-1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Howlett J, Benzenine E, Cottenet J, et al. Could venous thromboembolism and major bleeding be indicators of lung cancer mortality? A nationwide database study[J]. BMC Cancer. 2020;20(1):461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rees PA, Clouston HW, Duff S, et al. Colorectal cancer and thrombosis[J]. Int J Colorectal Dis. 2018;33(1):105-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fei X, Wang H, Yuan W, et al. Tissue factor pathway inhibitor-1 is a valuable marker for the prediction of deep venous thrombosis and tumor metastasis in patients with lung cancer. BioMed Res Int. 2017;2017:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang X, Huang J, Bingbing Z, et al. Risk factors, risk assessment, and prognosis in patients with gynecological cancer and thromboembolism[J]. J Int Med Res. 2020;48(4):1410459461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fuentes HE, Oramas DM, Paz LH, et al. Meta-analysis on anticoagulation and prevention of thrombosis and mortality among patients with lung cancer[J]. Thromb Res. 2017;154:28-34. [DOI] [PubMed] [Google Scholar]
- 26.Wang H, Xu X, Pu C, et al. Clinical characteristics and prognosis of cancer patients with venous thromboembolism[J]. J Cancer Res Therapeut. 2019;15(2):344-349. [DOI] [PubMed] [Google Scholar]