Abstract
Introduction
In patients with NSCLC harboring oncogenic ALK or ROS1 rearrangements, tyrosine kinase inhibitors have yielded high response rates and improvements in progression-free survival compared with cytotoxic chemotherapy; however, acquired resistance eventually develops. In preclinical models, ALK and MEK coinhibition was able to overcome ALK inhibitor resistance.
Methods
A phase 1 study of the ALK/ROS1 inhibitor ceritinib and the MEK inhibitor trametinib in patients with refractory NSCLC harboring ALK or ROS1 fusions was initiated. A three plus three dose-escalation scheme was used. Two dose levels were investigated. The primary end point was to determine the safety and tolerability of the combination.
Results
Nine patients (n = 8 ALK+, n = 1 ROS1+) were enrolled in the study and completed at least one cycle of therapy. The most common adverse events (all grades) were diarrhea (n = 9; 100%), rash (n = 8; 89%), abdominal pain (n = 5; 56%), and elevated aspartate transaminase/alanine transaminase level (n = 4; 44%). The overall response rate was 22%, whereas disease control rate was 56%. Median duration of response was 7.85 months. The median progression-free survival was 3.0 months (95% confidence interval: 1.5–7.0 mo). The median overall survival was 8.9 months (95% confidence interval: 2.0–not reached)
Conclusions
Data from this trial indicate that the combination of ceritinib and trametinib had no unexpected toxicities and that a tolerable dose could be identified. A subset of patients seemed to obtain clinical benefit from this treatment after progression on prior ALK/ROS1 inhibitor treatment.
ClinicalTrials.gov Identifier: NCT03087448.
Keywords: Ceritinib, Trametinib, ALK-rearrangement, ROS1-rearrangment, Non–small cell lung cancer, MEK inhibition
Introduction
Oncogenic ALK rearrangements are present in approximately 3% to 7% of NSCLC adenocarcinomas.1 Oncogenic rearrangements in ROS1, which shares structural similarity with ALK, occur in approximately 2% to 3% of NSCLC adenocarcinomas.1 Tumors harboring ALK or ROS1 rearrangements are dependent on these molecular alterations for growth and survival. As a result, monotherapy with ALK/ROS1 tyrosine kinase inhibitors often leads to marked initial response in ALK- or ROS1-rearranged NSCLC. Unfortunately, resistance to ALK- or ROS1-directed monotherapy ultimately develops.2, 3, 4 Furthermore, up to 30% of these patients do not experience an initial response to therapy. Identifying and targeting potential mechanisms of resistance to ALK or ROS1 inhibition may lead to longer disease control and patient survival.
Preclinical studies in EML4-ALK NSCLC models revealed that EML4-ALK–driven lung cancers are dependent on RAS MAPK signaling.5 Furthermore, combined ALK and MEK inhibition with ceritinib and trametinib resulted in decreased tumor cell proliferation and survival and increased depth and duration of tumor regression compared with monotherapy treatment both in vitro and in vivo.5 ROS1 fusions have also been found to activate the mitogen-activated protein kinase signaling pathway, and a constitutively active form of MEK (MEK-DD) was sufficient to rescue ROS1-expressing lung cancer cells from crizotinib treatment, suggesting that co-inhibition of ROS1 and MEK may overcome ROS1 inhibitor resistance.6
We sought to evaluate the combination of the second-generation ALK/ROS1 inhibitor ceritinib7 with the MEK1/2 inhibitor trametinib8 in a phase 1 study of patients with advanced ALK- or ROS1-rearranged NSCLC who had progressed on prior treatment with at least one prior ALK/ROS1 inhibitor.
Materials and Methods
This was an investigator-initiated phase 1 trial of ceritinib plus trametinib in patients with ALK- or ROS1-rearranged NSCLC who had progressed on prior ALK/ROS1-targeted therapy. Institutional review boards at University of California Davis and University of California San Francisco approved the study, and all patients provided written informed consent. Eligible patients were above or equal to 18 years of age with histologically or cytologically confirmed stage IIIB or IV NSCLC (American Joint Committee on Cancer version 7) and documented ALK or ROS1 rearrangement by the Clinical Laboratory Improvement Amendments–approved next-generation sequencing or fluorescence in situ hybridization test. Disease progression after treatment with more than or equal to one prior ALK/ROS1 inhibitor was required. Patients with a history of pneumonitis, clinically significant heart disease, impaired gastrointestinal function, or prior systemic treatment within 3 weeks were excluded.
The primary end point of the study was to determine the safety, tolerability, and recommended phase 2 dose (RP2D) of the combination. Secondary end points included objective response rate determined by investigator assessment, disease control rate (DCR), duration of response, progression-free survival (PFS), and overall survival. Adverse events (AEs) were assessed by the Common Terminology Criteria for Adverse Events version 5. A standard three plus three dose-escalation scheme was used. Two dose levels (DLs) were investigated (level 1: ceritinib 300 mg orally daily with food + trametinib 1.5 mg orally daily without food; level 2: ceritinib 450 mg orally daily with food + trametinib 1.5 mg orally daily without food). A level three cohort of ceritinib 450 mg orally daily with food plus trametinib 2.0 mg orally daily without food was planned, but it was not enrolled owing to closure of the trial. Cycle length was defined as 28 days. Dose-limiting toxicities (DLTs) were defined as any treatment-related toxicity occurring within the first cycle of therapy as grade three or four clinically evident nonhematologic toxicity and grade four neutropenia or thrombocytopenia lasting more than 7 days or febrile neutropenia. Patients were followed for safety evaluation until 30 days after their last dose of the study drugs. A minimum of six and a maximum of 18 patients were planned to be enrolled in the phase 1 study.
A phase 1b study was planned on the basis of the RP2D determined after dose escalation was completed; however, the study was stopped early owing to low accrual. Here, we report the safety and efficacy data from the phase 1 dose-escalation portion of the trial.
Results
A total of nine patients were enrolled. Six patients were enrolled and treated at DL 1 and three patients were enrolled and treated at DL 2. The baseline characteristics of the patients treated are summarized in Table 1. Median age was 55 (range: 45–77) years. There were four males and five females. Eight ALK rearrangement-positive and one ROS1 rearrangement-positive patients were enrolled (fusion partners indicated in Table 1). The median number of prior lines of therapy was four, and the median number of prior lines of ALK/ROS1 inhibitor therapy was two.
Table 1.
Baseline Characteristics of Patients
| Patient Characteristics | Number of Patients (%) |
|---|---|
| Age, y, median (range) | 55 (45–77) |
| Sex | |
| M | 4 (44) |
| F | 5 (56) |
| Race | |
| White | 5 (56) |
| Asian | 3 (33) |
| Unknown | 1 (11) |
| History of smoking | |
| Current/former | 1 |
| Never | 8 |
| Prior lines of therapy | |
| 1 | 2 (22) |
| 2 | 1 (11) |
| 3 | 1 (11) |
| 4 | 2 (22) |
| 5 | 2 (22) |
| 6 | 1 (11) |
| Prior lines of ALK/ROS1 inhibitor therapy | |
| 1 | 4 (44) |
| 2 | 1 (11) |
| 3 | 1 (11) |
| 4 | 2 (22) |
| 5 | 1 (11) |
| ALK rearrangement | 8 (88) |
| EML4-ALK V1 | 2 (25) |
| EML4-ALK V3a/b | 2(25) |
| ALK intron 19 rearrangement | 1 (12.5) |
| FISH+ only (no NGS) | 3 (37.5) |
| ROS1 rearrangement | 1 (11) |
| LRIG2-ROS1 | 1 (100) |
F, female; FISH, fluorescence in situ hybridization; M, male; NGS, next-generation sequencing.
The most common AEs and grade 2 or higher AEs are summarized in Table 2. The most common AEs of any grade were diarrhea (n = 9; 100%), rash (n = 8; 89%), nausea (n = 6; 67%), abdominal pain (n = 5; 56%), and elevated aspartate transaminase (AST)/alanine transaminase (ALT) level (n = 4; 44%). The most common study-attributable grade three or higher AE was elevated AST/ALT level (n = 3; 33%). There were no significant differences in the frequency or grade of AEs between DL 1 and DL 2, nor were there any significant differences in AEs on the basis of line of therapy at the time of enrollment. We observed only one DLT (grade three rash) in DL 1. There were no treatment-related deaths.
Table 2.
Summary of Most Common Toxicities Observed
| Toxicity Observed | Grade 1 (%) | Grade 2 (%) | Grade 3 (%) | Total, Any Grade (%) |
|---|---|---|---|---|
| Diarrhea | 6 (67) | 2 (22) | 1 (11) | 9 (100) |
| Rash | 3 (33) | 4 (44) | 1 (11)a | 8 (89) |
| Nausea | 5 (56) | 1 (11) | 6 (67) | |
| Abdominal pain | 4 (44) | 1 (11) | 5 (56) | |
| Elevated AST/ALT | 1 (11) | 3 (33) | 4 (44) | |
| Vomiting | 3 (33) | 3 (33) | ||
| Dizziness | 3 (33) | 3 (33) | ||
| Fatigue | 2 (22) | 2 (22) | ||
| GERD | 2 (22) | 2 (22) | ||
| Weight loss | 2 (22) | 2 (22) | ||
| Pneumonia | 1 (11) | 1 (11) | 2 (22) | |
| Mouth sores | 1 (11) | 1 (11) | ||
| Serum amylase elevation | 1 (11) | 1 (11) | ||
| Encephalopathy | 1 (11) | 1 (11) | ||
| Dyspnea | 1 (11) | 1 (11) | ||
| Mouth sores | 1 (11) | 1 (11) | ||
| Sinusitis | 1 (11) | 1 (11) | ||
| Encephalopathy | 1 (11) | 1 (11) | ||
| Serum amylase increased | 1 (11) | 1 (11) | ||
| Bilirubin increased | 1 (11) | 1 (11) | ||
| Proteinuria | 1 (11) | 1 (11) | ||
| Malaise | 1 (11) | 1 (11) | ||
| Edema | 1 (11) | 1 (11) | ||
| Hypertension | 1 (11) | 1 (11) | ||
| Hypoalbuminemia | 1 (11) | 1 (11) |
ALT, alanine transaminase; AST, aspartate transaminase; GERD, gastroesophageal reflux disease.
Rash was the only treatment emergent greater than grade three adverse event observed (dose level 1).
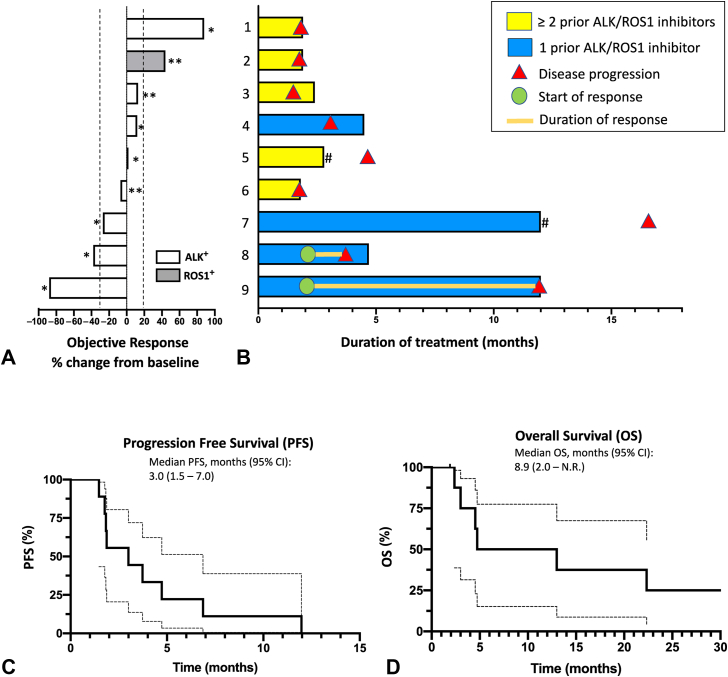
Two patients with ALK+ NSCLC experienced confirmed partial response (PR) for an overall response rate of 22%. One responder experienced an 88% reduction in tumor size by the Response Evaluation Criteria in Solid Tumors version 1.1 (Fig. 1A). Both responders were in DL 1, and both had received only one prior line of ALK inhibitor therapy (Fig. 1B). Three patients (33%, all in DL 1) experienced stable disease (SD) for an overall DCR (PR + SD) of 56%. Four of the five patients with either PR or SD had received only one prior ALK inhibitor (Fig. 1B). The remaining four patients (44%, one in DL 1 and three in DL 2) experienced progression of their disease, including the sole ROS1+ patient. All patients who experienced disease progression had been treated with three or more prior ALK/ROS1 inhibitors (Fig. 1B). The median progression PFS was 3.0 months (95% confidence interval: 1.5–7.0 mo) (Fig. 1C). The median overall survival was 8.9 months (95% confidence interval: 2.0–not reached) (Fig. 1D). The median duration of response among the two patients who achieved a PR was 7.85 months.
Figure 1.
Efficacy of ceritinib plus trametinib in ALK- and ROS1-rearranged NSCLC. (A) Waterfall plot for best percentage change in target lesion size by RECIST version 1.1 criteria by investigator assessment is revealed for patients who received ceritinib plus trametinib. ∗Indicates patients who received DL 1 of ceritinib 300 mg orally daily with food plus trametinib 1.5 mg orally daily without food. ∗∗Indicates patients who received DL 2 of ceritinib 450 mg orally daily with food plus trametinib 1.5 mg orally daily without food. (B) Swimmer’s plot for patients treated with ceritinib plus trametinib with the genotype and DL as indicated in (A). Prior ALK/ROS1 inhibitor therapies for each patient were as follows: (1) crizotinib, ceritinib, alectinib, and brigatinib; (2) crizotinib, ceritinib, and lorlatinib; (3) crizotinib, ceritinib, alectinib, brigatinib, and lorlatinib; (4) alectinib; (5) crizotinib and alectinib; (6) alectinib, brigatinib, and lorlatinib; (7) alectinib; (8) crizotinib; and (9) alectinib. # Indicates patients who discontinued treatment due to toxicity. (C) PFS and (D) OS of patients treated with ceritinib plus trametinib. Kaplan-Meier analysis of investigator-assessed progression with 95% CIs is indicated by the dashed lines. CI, confidence interval; DL, dose level; N.R., not reached; OS, overall survival; PFS, progression-free survival; RECIST, Response Evaluation Criteria in Solid Tumors.
Discussion
This study sought to characterize the safety and efficacy of the combination of the ALK/ROS1 inhibitor ceritinib with the MEK inhibitor trametinib in patients with pretreated ALK+ or ROS1+ NSCLC. The most common AEs observed were rash, diarrhea, and elevated AST and ALT levels, none of which were unexpected, with only rash being a DLT. These results suggest that this treatment regimen—particularly the regimen defined by DL 1 of ceritinib 300 mg (with food) plus trametinib 1.5 mg (without food) both administered daily—may be safe for further clinical investigation. Although we did not observe any DLTs at DL 2, we only enrolled three patients to this cohort; thus, the safety of this DL remains incompletely explored.
We explored the efficacy of the ceritinib plus trametinib combination as a secondary end point of the study. Two responses were observed of eight patients treated with ALK+ disease. We also observed a DCR of 56%. One patient with ALK+ disease had substantial clinical benefit with an 88% reduction in tumor size by the Response Evaluation Criteria in Solid Tumors and a 12-month PFS; however, tissue was not available for next-generation sequencing or other analyses. The number of lines of prior ALK/ROS1 inhibitor therapy may correlate with response, as the two patients with a PR had only received one prior ALK/ROS1 inhibitor, whereas the four patients with disease progression as their best response had received three to five prior ALK/ROS1 inhibitors. This observation suggests that ALK- and MEK-targeted co-treatment has the potential to be a therapeutic option for a select subset of patients with ALK–driven NSCLC but would likely only be effective if used as an earlier line of therapy. Several other studies are underway assessing the safety and efficacy of this approach with different ALK/ROS1 inhibitor and MEK inhibitor combinations (alectinib + cobimetinib, NCT03202940; brigatinib + binimetinib, NCT04005144; and lorlatinib + binimetinib, NCT04292119).
The limitations of this trial include its early closure that ultimately led to a small sample size and the inability to explore further DLs or expansion cohorts in specific patient subsets. Pretreatment biopsies were also unavailable to perform correlative studies that may have provided insight into the molecular mechanisms underlying response or resistance to treatment. This study was closed to accrual before completion owing to the changing landscape of ALK+ NSCLC treatment and availability of newer Food and Drug Administration–approved agents during the study period (e.g., alectinib, lorlatinib, and brigatinib). Further studies would be required to determine the maximum tolerated dose of the combination, although it would be reasonable to consider ceritinib 300 mg plus trametinib 1.5 mg orally daily as the RP2D given its tolerability and efficacy profile.
In summary, co-targeting of ALK/ROS1 and MEK remains a rational strategy to overcome resistance to ALK-targeted therapy. The combination of ALK/ROS1 and MEK inhibition in the limited experience reported here suggests that such a strategy is clinically feasible and may be effective in patients with limited prior exposure to ALK/ROS1 inhibitors or in combination with next-generation ALK/ROS1 inhibitors in clinical development.
CRediT Authorship Contribution Statement
Matthew S. Lara: Data curation, Formal analysis, Writing—original draft preparation.
Matthew A. Gubens: Investigation, Writing—reviewing and editing.
Bianca Bacaltos: Data curation, Project administration.
Lea Daran: Data curation.
Steffany L. Lim: Data curation, Project administration.
Tianhong Li: Investigation.
David R. Gandara: Investigation, Writing—reviewing and editing.
Trever G. Bivona: Conceptualization, Writing—reviewing and editing.
Jonathan W. Riess: Data curation, Formal analysis, Conceptualization, Methodology, Investigation, Supervision, Writing—reviewing and editing.
Collin M. Blakely: Data curation, Formal analysis, Conceptualization, Funding acquisition, Investigation, Supervision, Methodology, Writing—reviewing and editing.
Acknowledgments
This work is supported in part by Novartis Pharmaceuticals and National Cancer Institute P30 CCSG (University of California Davis and University of California San Francisco).
Footnotes
Disclosure: Dr. Gubens reports receiving institutional grants unrelated to this manuscript from Amgen, Celgene, Johnson & Johnson, Merck, Novartis, OncoMed, and Trizell; consulting fees from AstraZeneca, Bristol-Myers Squibb, Cardinal Health, Genzyme, Guardant, iTeos, Genentech, Sanofi, and Surface. Dr. Li reports receiving institutional grants unrelated to this manuscript from Merck, OncoC4, LabyRx Immuno-Oncology, Genentech, Novartis, AbbVie Inc., Astellas, Atlas Medx, AstraZeneca, EMD Serono, RasCal Therapeutics, Jounce, F. Hoffmann-La Roche, Nvigen, and Tempus. Dr. Gandara reports receiving institutional grants unrelated to this manuscript from Amgen, AstraZeneca, Genentech, and Merck; consulting fees from AdaGene, AstraZeneca, Genentech, Guardant Health, IO Biotech, Oncocyte, and OncoHost; honoraria from Lilly, Merck, and Novartis; travel funds from Guardant Health; and advisory board compensation from Lilly, Merck, and Novartis. Dr. Bivona reports receiving institutional grants unrelated to this manuscript from Strategia, Kinnate, and Revolution Medicines; consulting fees from: Engine, Turning Point, Clain, EcoR1, and Granule Therapeutics; and advisory board compensation from Revolution Medicines, Relay, and Rain. Dr. Riess reports receiving institutional grants unrelated to this manuscript from AstraZeneca, Boehringer Ingelheim, Merck, Novartis, Revolution Medicines, and Spectrum; consulting fees from Blueprint Medicines, Boehringer Ingelheim, EMD Serono, and Novartis; and advisory board compensation from Bayer, Beigene, Biodesix, Regeneron, Turning Point, Bristol-Myers Squibb, Daiichi Sankyo, Genentech, Janssen, Jazz Pharmaceuticals, and Sanofi. Dr. Blakely reports receiving an institutional grant in support of this manuscript from Novartis; institutional grants unrelated to this manuscript from AstraZeneca, Genentech, Takeda, Spectrum, Mirati, and Erasca; consulting fees from Blueprint Medicines; honoraria from Amgen and Oncocyte; and advisory board compensation from Bayer and Janssen. Mr. Lara and Ms. Lim declare no conflict of interest.
Cite this article as: Lara MS, Gubens MA, Bacaltos B, et al. Phase 1 study of ceritinib combined with trametinib in patients with advanced ALK- or ROS1-positive NSCLC. JTO Clin Res Rep. 2022;3:100436.
Contributor Information
Jonathan W. Riess, Email: jwriess@ucdavis.edu.
Collin M. Blakely, Email: Collin.Blakely@ucsf.edu.
References
- 1.Tan A.C., Tan D.S.W. Targeted therapies for lung cancer patients with oncogenic driver molecular alterations. J Clin Oncol. 2022;40:611–625. doi: 10.1200/JCO.21.01626. [DOI] [PubMed] [Google Scholar]
- 2.Gainor J.F., Dardaei L., Yoda S., et al. Molecular mechanisms of resistance to first- and second-generation ALK inhibitors in ALK-rearranged lung cancer. Cancer Discov. 2016;6:1118–1133. doi: 10.1158/2159-8290.CD-16-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin J.J., Choudhury N.J., Yoda S., et al. Spectrum of mechanisms of resistance to crizotinib and lorlatinib in. Clin Cancer Res. 2021;27:2899–2909. doi: 10.1158/1078-0432.CCR-21-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rotow J., Bivona T.G. Understanding and targeting resistance mechanisms in NSCLC. Nat Rev Cancer. 2017;17:637–658. doi: 10.1038/nrc.2017.84. [DOI] [PubMed] [Google Scholar]
- 5.Hrustanovic G., Olivas V., Pazarentzos E., et al. RAS-MAPK dependence underlies a rational polytherapy strategy in EML4-ALK-positive lung cancer. Nat Med. 2015;21:1038–1047. doi: 10.1038/nm.3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neel D.S., Allegakoen D.V., Olivas V., et al. Differential subcellular localization regulates oncogenic signaling by ROS1 kinase fusion proteins. Cancer Res. 2019;79:546–556. doi: 10.1158/0008-5472.CAN-18-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blakely C.M., Riess J.W. Interpretation of ceritinib clinical trial results and future combination therapy strategies for ALK-rearranged NSCLC. Expert Rev Anticancer Ther. 2019;19:1061–1075. doi: 10.1080/14737140.2019.1699792. [DOI] [PubMed] [Google Scholar]
- 8.Zeiser R., Andrlová H., Meiss F. Trametinib (GSK1120212) Recent Results Cancer Res. 2018;211:91–100. doi: 10.1007/978-3-319-91442-8_7. [DOI] [PubMed] [Google Scholar]