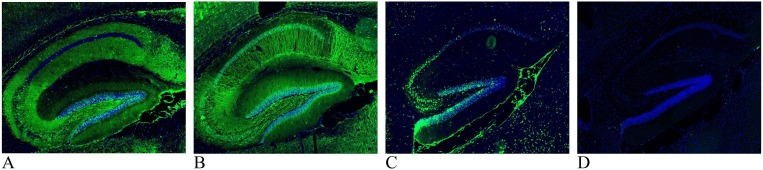
We read the article by Franke et al (Franke et al., 2020) with great interest. Using indirect immunofluorescence on perfused mouse brain tissue, we have also detected unexpected high frequency of strong immunoglobulin G (IgG) immunoreactivity in several mouse brain regions when analyzing cerebrospinal fluid (CSF) from two previously described cases of COVID-19 associated encephalitis (Virhammar et al., 2020, Svedung Wettervik et al., 2020) and have recently reported immunoreactivity against brain tissue in another case of post-COVID-19 acute encephalopathy that presented as malignant catatonia with autonomic instability (Mulder et al., 2020). Common denominators for these three cases were that the initial standard CSF work-up showed only weak indications of an inflammatory processes and tested negative, for both serum and CSF, in a standard autoimmunity screening panel including anti-neuronal autoantibodies against N-methyl-D-aspartate receptor (NMDA-R) (Euroimmun, Lübeck, Germany). While autoimmunity was still suspected in these cases, due to the timing between Covid-19 debut and neurological symptoms, other putative disease mechanisms including focal damage to the blood–brain barrier caused by the virus and/or immune system were also considered. Anti-neuronal autoantibodies in our three cases all target the hippocampus and other brain structures but differed in staining pattern indicating different antigens (see Fig. 1 ). Interestingly, all three cases showed distinct clinical response to immunotherapy including plasma exchange (Virhammar et al., 2020, Svedung Wettervik et al., 2020, Mulder et al., 2020).
Fig. 1.
Images are of 16 μm paraformaldehyde (4%) fixed sagittal cryosections of mouse hippocampus. Immunohistochemistry was performed using cerebrospinal fluid (CSF) diluted 1:4 to detect anti-neuronal antibodies (green; cell nuclei marker DAPI is blue). A: CSF from a patient with COVID-19 associated acute necrotizing encephalopathy where neuropil staining is found in the subiculum, stratum oriens and stratum radiatum in all cornu ammonis (CA) subfields, dentate gyrus polymorph layer and faint in the molecular layer. The pyramidal layer of CA1 and stratum lancosum-moleculare are both negative. B: CSF from a patient presenting with COVID-19 associated acute hemorrhagic leukoencephalitis. In the hippocampus and the cortex (see top left corner) there is a general dendritic staining. Staining can also be observed in some specific cell somas, especially in the subiculum and cortex (not shown). The signal is fainter in the CA1 stratum oriens, CA1 stratum lancosum and the dentate gyrus polymorph layer compared to the rest of the area. C: CSF from patient with COVID-19 associated malignant catatonia shows membrane staining of cells in the pyramidal layer, subiculum and granule cell layer of the dentate gyrus. D: Reference CSF sample, from a donor with bipolar disease without suspected autoimmunity.
Franke et al. (2020) and our findings, demonstrate that screening methods to identify patients with anti-neuronal autoimmunity may be valuable in the clinic in cases with severe encephalopathy and the few available standard commercial assays are negative. Multiplex arrays for screening proteins and other molecules, mass spectrometry, proximal ligation assay and other methods are emerging for antigen identification. Simultaneously efforts for fine mapping of protein expression and distribution in the human and mouse brain by us (Sjostedt et al., 2020) and other efforts, including the Allen brain atlas, provide a reference dataset that can be used to short list potential autoimmunity targets based on regional and cellular patterns in the autoimmunity profile. Antigen identification and the establishment of validated assays is still a time-consuming process. We believe that autoimmunity profiling is a more inclusive and potentially powerful screening method, however, it is not widely implemented and several limitations influence its clinical application. The evaluation of tissue slides requires extensive training in rodent brain anatomy. Many variables in the technique, such as the type and specific strain of animal, methods and drug administration for euthanasia, sample preparation and type of fixation, staining procedures including dilutions, specificity of secondary antibodies and image analysis, may also greatly influence the results. Antibody based methods are frequently referred to as methods with diagnostic implications. Joint efforts are urgently needed to compare, validate and calibrate current and future methods, to standardize the evaluation of autoimmunity to brain proteins.
Our results demonstrate the potential for detecting autoimmune involvement in some patients with COVID-19-associated encephalopathy. Standardization of indirect immunofluorescence and other autoimmunity profiling methods may have a much broader application.
Financial disclosures
The authors report no disclosure.
Funding
This study was funded by the Swedish Research Council, The Medical Training and Research Agreement from Uppsala University and SciLifeLab, Uppsala University.
Ethics
This study was approved by the Swedish Ethical Board. Written informed consent for this case report was obtained from the patient’s guardian. All animal experiments conformed to the European Communities Council Directive (86/609/EEC) and in accordance with Swedish laws and regulations, and all animal experiments were approved by the local ethical committee (Stockholms Djurförsöksetiska Nämnd N183/14).
References
- Franke C., Ferse C., Kreye J., Reincke S.M., Sanchez-Sendin E., Rocco A., Steinbrenner M., Angermair S., Treskatsch S., Zickler D., Eckardt K.-U., Dersch R., Hosp J., Audebert H.J., Endres M., Ploner J.C., Prüß H. High frequency of cerebrospinal fluid autoantibodies in COVID-19 patients with neurological symptoms. Brain Behav. Immun. 2020 doi: 10.1016/j.bbi.2020.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virhammar J., Kumlien E., Fällmar D., Frithiof R., Jackmann S., Sköld M.K., Kadir M., Frick J., Lindeberg J., Olivero-Reinius H., Ryttlefors M., Cunningham J.L., Wikström J., Grabowska A., Bondeson K., Bergquist J., Zetterberg H., Rostami E. Acute necrotizing encephalopathy with SARS-CoV-2 RNA confirmed in cerebrospinal fluid. Neurology. 2020;95(10):445–449. doi: 10.1212/WNL.0000000000010250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svedung Wettervik, T., Kumlien, E., Rostami, E., et al. Intracranial Pressure Dynamics and Cerebral Vasomotor Reactivity in Coronavirus Disease 2019 Patient With Acute Encephalitis. Crit. Care Explor. 2020;2(8):e0197. doi: 10.1097/CCE.0000000000000197. [DOI] [PMC free article] [PubMed]
- Mulder J., Feresiadou A., Fallmar D., Frithiof R., Virhammar J., Rasmusson J.A., Rostami E., Kumlien E., Cunningham L.J. Autoimmune encephalitis presenting with acute excited catatonia in a 40-year-old male patient with Covid-19. Am. J. Psychiatry. 2020 doi: 10.1176/appi.ajp.2020.20081236. In press. [DOI] [PubMed] [Google Scholar]
- Sjostedt, E., Zhong, W., Fagerberg, L., et al. An atlas of the protein-coding genes in the human, pig, and mouse brain. Science. 2020;367(6482). doi: 10.1126/science.aay5947. [DOI] [PubMed]