Abstract
Background
Microbiologic diagnosis of childhood tuberculosis may be difficult. Oral swab specimens are a potential noninvasive alternative to sputum specimens for diagnosis.
Methods
This was a prospective diagnostic accuracy study of oral swab specimens (buccal and tongue) for pulmonary tuberculosis diagnosis in children (aged ≤ 15 years) in 2 South African hospital sites. Children with cough of any duration as well as a positive tuberculin skin test result, tuberculosis contact, loss of weight, or chest radiograph suggestive of pulmonary tuberculosis were enrolled. Two induced sputum specimens were tested with Xpert MTB/RIF (or Xpert MTB/RIF Ultra) assay and liquid culture. Oral swab specimens were obtained before sputum specimens, frozen, and later tested with Xpert MTB/RIF Ultra. Children were classified as microbiologically confirmed tuberculosis, unconfirmed tuberculosis (receipt of tuberculosis treatment), or unlikely tuberculosis according to National Institutes of Health consensus definitions based on sputum microbiologic results.
Results
Among 291 participants (median age [interquartile range], 32 [14–73] months), 57 (20%) had human immunodeficiency virus (HIV), and 87 (30%) were malnourished; 90 (31%) had confirmed pulmonary tuberculosis (rifampicin resistant in 6 [7%] ), 157 (54%), unconfirmed pulmonary tuberculosis, and 44 (15%), unlikely tuberculosis. A single oral swab specimen was obtained from 126 (43%) of the participants (tongue in 96 and buccal in 30) and 2 swab specimens from 165 (57%) (tongue in 110 and buccal in 55). Sensitivity was low (22% [95% confidence interval, 15%–32%]) for all swab specimens combined (with confirmed pulmonary tuberculosis as reference), but specificity was high (100% [91%–100%]). The highest sensitivity was 33% (95% confidence interval, 15%–58%) among participants with HIV. The overall yield was 6.9% with 1 oral swab specimen and 7.2% with 2.
Conclusions
Use of the Xpert MTB/RIF Ultra assay with oral swab specimens provides poor yield for microbiologic pulmonary tuberculosis confirmation in children.
Keywords: tuberculosis, children, pulmonary, oral swab specimen, Xpert MTB/RIF Ultra
Oral swab specimens provide a potential alternative to sputum specimens for diagnosing childhood pulmonary tuberculosis. However, in a prospective diagnostic accuracy study, sensitivity for the Xpert MTB/RIF Ultra assay was poor, 22% overall. Specificity was high, and a second specimen did not increase yield.
Globally, among the 1 million tuberculosis cases estimated to occur in children every year, only a third are reported to be diagnosed and treated [1, 2]. Difficulties in diagnosing tuberculosis clinically and in microbiologic confirmation of tuberculosis in children are major contributors to this large gap [3]. Children are more likely to have paucibacillary disease, and it is more difficult to obtain microbiologic confirmation of pulmonary tuberculosis in children than in adults, particularly in young children [4].
Microbiologic confirmation, however, is important in order to identify drug resistance, initiate rapid treatment, and limit unnecessary overtreatment of children with nonspecific clinical or radiologic signs. Induced sputum (IS) or gastric aspirate specimens tested with culture provide the greatest opportunity for microbiologic confirmation of pulmonary tuberculosis among children, although a significant proportion of cases remain clinically diagnosed [5]. Because sputum induction or gastric aspiration require infrastructure [6] and should be repeated on sequential days for maximum yield, these are not widely used and are often limited to hospital settings, despite demonstrations of feasibility, safety, and efficacy [7].
Oral swab specimens may be a more easily obtained, alternative specimen for microbiologic diagnosis of pulmonary tuberculosis in children [8–10], one that could be subject to rapid testing with the Xpert MTB/RIF Ultra assay, which has higher sensitivity than its predecessor, Xpert MTB/RIF [11, 12]. Our group has previously shown that testing of oral swab specimens with an in-house quantitative polymerase chain (PCR) could be potentially useful to supplement microbiologic diagnosis in children in whom tuberculosis is suspected [13]. The aim of the current study was to investigate the diagnostic accuracy of oral swab specimens, either tongue or buccal (cheek), tested with the Xpert MTB/RIF Ultra assay, for pulmonary tuberculosis in children, as an approach that could be scalable in settings with a high tuberculosis burden.
METHODS
Study Population
This was a prospective diagnostic accuracy study with convenience sampling, nested within a larger childhood tuberculosis diagnosis study, enrolling children (aged <15 years) with presumptive pulmonary tuberculosis in 2 hospital sites: Red Cross Children Hospital in Cape Town and Dora Nginza Hospital in Eastern Cape, South Africa, from September 2017 to October 2020. There was no prespecified sample size. Study staff screened children at the pediatric facilities of both hospitals, daily throughout the week, for those who met inclusion criteria. Written informed consent from a parent and the child’s assent (in those >7 years of age) was obtained after an explanation of the study in the family’s home language.
Children were consecutively enrolled if they presented with cough of any duration and any of the following: household contact with someone with known tuberculosis within the preceding 6 months, loss of weight or failure to gain weight in the last 3 months or positive tuberculin skin test (TST) result (2TU purified protein derivative [PPD]-RT23; Staten Serum Institute) (defined as ≥ 10 mm in those without and ≥ 5 mm in those with human immunodeficiency virus [HIV]), or chest radiograph suggestive of tuberculosis. Children were excluded if they were currently receiving tuberculosis therapy or prophylaxis for longer than 72 hours, if they were not resident in the respective cities, or if informed consent was unavailable. Prior tuberculosis treatment was not an exclusion. The analysis included enrolled participants with available IS results and oral swab specimens. Participants with a diagnosis of only confirmed extrapulmonary tuberculosis were excluded from the analysis. The Human Research Ethics Committee of the Faculty of Health Sciences, University of Cape Town approved the study.
Participants had a chest radiograph, a tuberculin skin test, and HIV testing when HIV status was unknown at enrollment. Children with HIV were classified based on World Health Organization (WHO) clinical staging; CD4 cell count and HIV viral load (Abbott RealTime HIV-1 assay) were determined. Nutritional status was calculated as height-for-age, weight-for-age, and body mass index-for-age z scores using WHO child growth standards 2006. Tuberculosis therapy was initiated at the discretion of the treating physician. Follow-up visits were conducted 1 and 3 months after enrollment for all participants to assess response to therapy and categorize children according to tuberculosis diagnostic category, based on National Institutes of Health consensus diagnostic categories [14], and again at 6 months for those with confirmed or unconfirmed tuberculosis.
Sample Collection and Testing
Paired oral swab and IS specimens were obtained, with the oral swab specimen obtained before IS. Participants refrained from brushing teeth or eating solid food for ≥4 hours before an oral swab specimen was obtained. PurFlock swabs (Puritan Products) or FLOQSwabs (COPAN Diagnostics) were used to swab the tongue or inside of both cheeks. FLOQSwabs were used from May 2019 onward. Swab specimens were immediately placed in sterile saline for storage and frozen at −80°C. IS specimens were obtained using hypertonic saline nebulization and suctioning, as described elsewhere [15]. IS specimens were tested with liquid culture and the Xpert MTB/RIF or MTB/RIF Ultra assay (the assay used was not systematically recorded). The second set of paired samples was obtained the next day or after 4 hours. Other, nonsputum specimens were also tested where presentation suggested extrapulmonary in addition to pulmonary tuberculosis. Results of IS were available to treating clinicians but not those from oral swab specimens, which were batched for testing.
Automated liquid culture (BACTEC MGIT) and the Xpert MTB/RIF Ultra assay were performed according to manufacturer instructions. For testing with the Xpert Ultra assay, oral swab specimens, frozen in 500 µL of saline, were thawed at room temperature, and 1.6 mL of Xpert sample reagent was added. After vortexing and incubation, 2 mL was then added to the Ultra cartridge for testing using standard 4-module GeneXpert instruments. Xpert Ultra assay results on oral swab specimens are reported using the semiquantitative scale. Staff conducting Xpert testing on oral swab specimens were blinded to the results of other study tests.
Data Analysis
Participants were categorized based on National Institutes of Health consensus definitions [14] as having confirmed pulmonary tuberculosis (Xpert MTB/RIF Ultra– or culture-positive respiratory specimen, excluding oral swab specimens), unconfirmed pulmonary tuberculosis (no microbiologic confirmation and receipt of tuberculosis treatment), or unlikely tuberculosis (no tuberculosis treatment and symptoms improved at follow-up visits). Malnutrition and stunting were defined as weight-for-age and height-for-age z scores below −2.0, respectively.
The primary reference standard was confirmed pulmonary tuberculosis based on IS specimens. The sensitivity and specificity (with 95% confidence intervals) of the Xpert MTB/RIF Ultra assay with 1 and/or 2 oral swab specimens were calculated using confirmed pulmonary tuberculosis as the positive and unlikely tuberculosis as the negative reference standard. In addition, sensitivity and specificity were calculated using both confirmed and unconfirmed tuberculosis as the positive reference standard. Stratified analyses were conducted for the following: participants aged <12 months, <5 years and ≥5 years, children with HIV, HIV-negative children, malnourished and stunted children, and for those with tongue versus buccal swab specimens. Indeterminate Xpert MTB/RIF Ultra assay results were excluded from sensitivity and specificity calculations. Continuous data were summarized by medians (with interquartile ranges), while categorical data were summarized by proportions and compared using χ2 tests (IBM SPSS statistical software; version 26).
RESULTS
Study Population
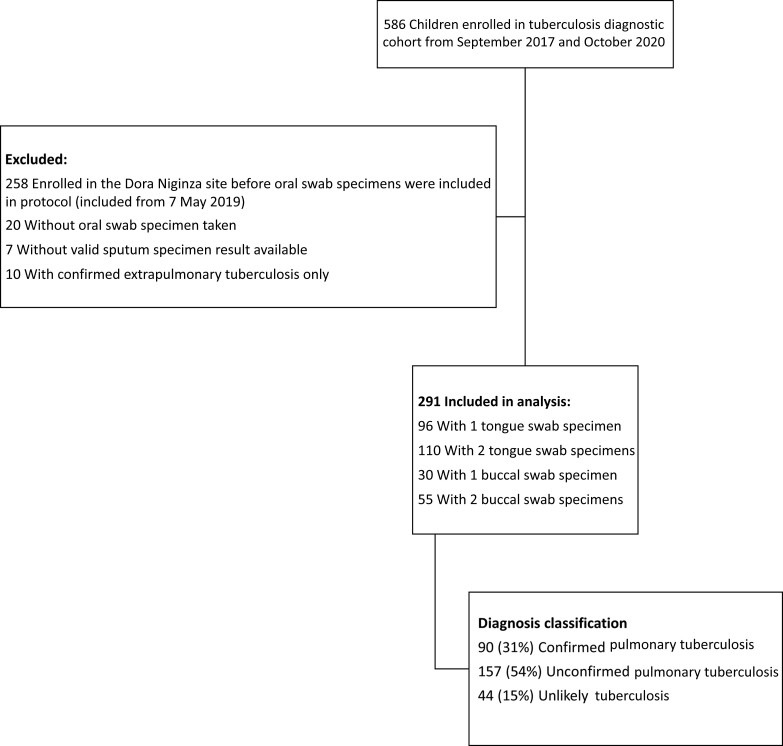
Between 15 September 2017 and 5 October 2020, a total of 586 participants were recruited to the parent tuberculosis diagnosis study from both sites; 295 were excluded, primarily owing to enrollment before oral swab specimens were included (Figure 1). The final analysis included 291 participants. Their median age was 32 months (interquartile range, 14–73 years), and 133 (46%) were female (Table 1). Overall, 57 (20%) had HIV, with a median CD4 cell count of 631/mL3 (interquartile range, 174–1025/ mL3), and 48 (84%) were receiving antiretroviral treatment. Malnutrition occurred in 87 participants (30%), and 108 (37%) were stunted (Table 1).
Figure 1.
Flow diagram showing the numbers of participants enrolled in the prospective childhood tuberculosis diagnosis study, the numbers excluded from analysis, and the reasons for exclusion.
Table 1.
Characteristics of Participants by Pulmonary Tuberculosis Diagnostic Category
| Characteristic | Participants, No. (Column %)a | |||
|---|---|---|---|---|
| Confirmed Pulmonary Tuberculosis (n = 90 [30.9%]) |
Unconfirmed Pulmonary Tuberculosis (n = 157 [54.0%]) |
Unlikely Tuberculosis (n = 44 [15.1%]) |
Total (N = 291) |
|
| Female sex | 42 (46.7) | 69 (43.9) | 22 (50.0) | 133 (45.7) |
| Age, median (IQR), m | 32.4 (13.5–101.6) | 31.0 (14.0–68.6) | 44.5 (16.9–74.0) | 32.0 (14.3–72.6) |
| Previous tuberculosis treatment | 5 (5.6) | 13 (8.3) | 4 (9.1) | 22 (7.6) |
| Household contact with known tuberculosis case | 40 (44.4) | 80 (51.0) | 18 (40.9) | 138 (47.4) |
| BCG vaccination | 76 (84.4) | 149 (94.9) | 38 (86.4) | 263 (90.4) |
| TST positive | 47 (52.2) | 83 (52.9) | 10 (22.7) | 140 (48.1) |
| BMIZ, median (IQR) | 15.1 (13.8–17.0) | 15.5 (14.2–17.0) | 15.8 (14.2–17.4) | 15.4 (14.1–17.0) |
| WAZ, median (IQR) | −1.52 (−2.52 to −0.54) | −1.05 (−2.25 to −0.19) | −0.60 (−1.81–0.29) | −1.23 (−2.37 to −0.14) |
| Malnutrition | 33 (36.7) | 45 (28.7) | 9 (20.5) | 87 (29.9) |
| HAZ, median (IQR) | −1.74 (−2.85 to −0.81) | −1.42 (−2.53 to −0.35) | −1.46 (−2.24 to 0.23) | −1.44 (−2.63 to −0.38) |
| Stunting | 42 (46.7) | 53 (33.8) | 13 (29.5) | 108 (37.1) |
| HIV positive | 15 (16.7) | 37 (23.6) | 5 (11.4) | 57 (19.6) |
| Receiving ART (% of HIV positive) | 15 (100) | 28 (75.7) | 5 (100) | 48 (84.2) |
| CD4 cell count, median (IQR), cells/mL3 | 583 (225–833) | 799 (181–1192) | 321 (95–1135) | 631 (174–1025) |
| Hospitalized | 72 (80.0) | 119 (75.8) | 38 (86.4) | 229 (78.7) |
| No. of tongue swab specimens | ||||
| 1 | 25 (27.8) | 59 (37.6) | 12 (27.3) | 96 (33.0) |
| 2 | 32 (35.6) | 68 (43.3) | 10 (22.7) | 110 (37.8) |
| No. of buccal swab specimens | ||||
| 1 | 9 (10.0) | 9 (5.7) | 12 (27.3) | 30 (10.3) |
| 2 | 24 (26.7) | 21 (13.4) | 10 (22.7) | 55 (18.9) |
Abbreviations: ART, antiretroviral treatment; BMIZ, body mass index z score; HAZ, height for age z score; HIV, human immunodeficiency virus; IQR, interquartile range; TST, tuberculin skin test; WAZ, weight for age z score.
Data represent no. (column %) of participants unless otherwise specified.
Among the 291 participants, 90 (31%) had confirmed pulmonary tuberculosis, 157 (54%) had unconfirmed tuberculosis, and 44 (15%) had unlikely tuberculosis (Table 1). Among the 90 participants with confirmed pulmonary tuberculosis based on IS, 52 (58%) were both Xpert and culture positive, 23 (26%) were positive only with Xpert, and 15 (17%) were culture positive only. Two-thirds (60 of 90) had microbiologic diagnosis based on the first IS specimen, with another 18 diagnoses (20%) based on the second specimen, 9 (10%) on a third specimen, 2 (2%) on a fourth, and 1 on a fifth. This equates to an initial yield of 20.6% (60 of 291), with an incremental yield of 1.7% (5 of 291) when a second specimen was tested with the Xpert MTB/RIF or Xpert MTB/RIF Ultra assay. Overall, 6 participants had microbiologically diagnosed rifampicin resistance (6.7% of those with confirmed pulmonary tuberculosis).
Diagnostic Performance of Oral Swab Specimens for Tuberculosis Diagnosis
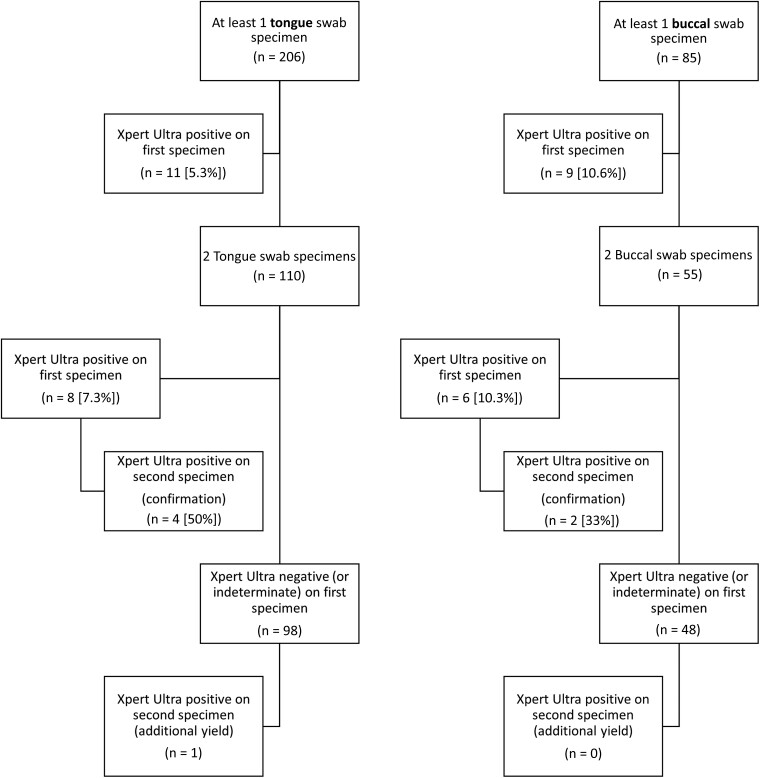
Participants included 96 with 1 tongue and 110 with 2 tongue swab specimens, and 30 with 1 buccal and 55 with 2 buccal swab specimens. Among the 456 oral swab specimens, valid Xpert MTB/RIF Ultra assay results were available for 448 (98.2%), of which only 27 specimens (6.0%) were positive (among 21 participants). Among the 27 positive specimens, most were graded as trace positive (n = 14 [52%]); 4 (15%) were graded as very low and 7 (26%) as low, and only 2 were graded as medium. Among the 13 specimens graded as medium, low, or very low with the Xpert Ultra assay, none had rifampicin resistance detected.
Among the 21 participants with a positive oral swab specimen, 20 (95%) had confirmed and 1 had unconfirmed pulmonary tuberculosis. Among the 206 participants with ≥1 tongue swab specimen, 11 (5.3%) tested positive on the first swab specimen, as did 9 of 85 participants (10.6%) with ≥1 buccal swab specimen (P = .12). With either tongue or buccal swab specimens, yield did not increase with a second specimen (Figure 2). Among the 6 participants with rifampicin-resistant pulmonary tuberculosis diagnosed through IS testing, only 1 had a positive oral swab specimen with the Xpert Ultra assay. This specimen was graded as low positive, with rifampicin resistance not detected.
Figure 2.
Yield of Xpert MTB/RIF Ultra assay (Xpert Ultra)–positive results from oral (tongue [left] and buccal [right]) swab specimens.
Overall sensitivity for the diagnosis of confirmed pulmonary tuberculosis or any pulmonary tuberculosis (confirmed or unconfirmed) was low (Tables 2 and 3). The highest sensitivity was observed among participants with HIV for the diagnosis of confirmed pulmonary tuberculosis (33.3% [95% confidence interval, 15.2%–58.3%]). There was no significant difference in sensitivity between buccal and tongue swab specimens (Table 2), nor by HIV status, age, nutrition, or swab type (Table 3). Specificity was high overall, with no positive results in the unlikely tuberculosis group.
Table 2.
Accuracy of the Xpert MTB/RIF Ultra Assay for Diagnosing Pulmonary Tuberculosis in Children by Number of Oral Swab Specimens
| Swab Specimen Type | Any Oral Swab Specimen | 1 Oral Swab Specimen | 2 Oral Swab Specimens | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Participants, No.a | Sensitivity (95% CI), % | Specificity (95% CI), % | Participants, No.a | Sensitivity (95% CI), % | Specificity (95% CI), % | Participants, No.a | Sensitivity (95% CI), % | Specificity (95% CI), % | |
| Reference standard: confirmed and unconfirmed pulmonary tuberculosis combined | |||||||||
| Tongue and buccal combined | 288 | 8.5 (5.7–12.7) | 100 (91.6–100) | 285 | 8.2 (5.4–12.3) | 100 (91.4–100) | 164 | 10.3 (6.4–16.4) | 100 (83.2–100) |
| Reference standard: confirmed pulmonary tuberculosis only | |||||||||
| Tongue and buccal combined | 132 | 22.2 (14.9–31.9) | 100 (91.6–100) | 130 | 21.4 (14.1–31.0) | 100 (91.4–100) | 75 | 25.0 (15.5–37.7) | 100 (83.2–100) |
| Buccal only | 54 | 27.3 (15.1–44.2) | 100 (84.5–100) | 54 | 27.3 (15.1–44.2) | 100 (84.5–100) | 34 | 25.0 (12.0–44.9) | 100 (72.3–100) |
| Tongue only | 78 | 19.3 (11.1–31.3) | 100 (84.5–100) | 76 | 17.9 (10.0–29.8) | 100 (83.9–100) | 41 | 25.0 (13.3–42.1) | 100 (70.1–100) |
Abbreviation: CI, confidence interval.
Excluding participants with invalid Xpert TB/RIF Ultra assay results.
Table 3.
Accuracy of the Xpert MTB/RIF Ultra Assay With 1 or 2 Oral Swab Specimens for Diagnosing Pulmonary Tuberculosis in Children: Subgroup Analyses
| Subgroup | Participants, No.a | Sensitivity (95% CI), %b | Specificity (95% CI), %b |
|---|---|---|---|
| HIV positive | 19 | 33.3 (15.2–58.3) | 100 (51.0–100) |
| HIV negative | 112 | 20.3 (17.7–30.8) | 100 (90.8–100) |
| Malnutrition | 42 | 27.3 (15.1–44.2) | 100 (70.1–100) |
| Malnutrition and/or stunting | 66 | 25.5 (16.6–38.9) | 100 (79.6–100) |
| Age <12 m | 26 | 22.2 (9.0–45.2) | 100 (67.6–100) |
| Age <60 m | 81 | 18.2 (10.2–30.3) | 100 (87.1–100) |
| Age >60 m | 51 | 28.6 (16.3–45.1) | 100 (80.6–100) |
| PurFlock swabs only | 82 | 24.1 (14.6–37.0) | 100 (88.0–100) |
| COPAN FLOQSwabs only | 50 | 19.4 (9.8–35.0) | 100 (78.5–100) |
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus.
Excluding participants with invalid Xpert MTB/RIF Ultra assay results.
All results are given for tongue and buccal swab specimens combined.
DISCUSSION
This is the first study to report on the use of the Xpert MTB/RIF Ultra assay on oral swab specimens for pulmonary tuberculosis diagnosis in children. Overall, oral swab specimens had very low sensitivity for confirmed and unconfirmed pulmonary tuberculosis although specificity was high. Oral swab specimen testing did not significantly increase the number of participants confirmed with tuberculosis, compared with testing of sputum specimens, as only 1 participant with unconfirmed pulmonary tuberculosis had a positive oral swab specimen. In contrast to the relatively high incremental yield from a second sputum sample, the yield of microbiologically confirmed tuberculosis was not significantly increased with the use of 2 oral swab specimens compared with 1. There was a trend for increased yield with the use of buccal compared with tongue swab specimens, but the yield was low for both.
The sensitivity of 22% for oral swab specimen microbiologic confirmation, or 27% for buccal swab specimens only, is similar to the 30% reported by our group for a single buccal swab specimen but lower than the 43% for 2 swab specimens tested with a manual real-time PCR assay [13]. The prior study is limited by a much smaller sample size and use of an in-house PCR test. There were also several methodologic differences, which are summarized in Table 4. While these differences may have contributed to the higher sensitivity and lower specificity in our prior study, our aim with the current study was to assess the utility of oral swab specimens with Xpert Ultra testing, as would be done programmatically.
Table 4.
Comparison of Methods Used for Oral Swab Collection and Analysis in a Previous Study and the Current Study
| Method | Study by Nicol et al [13] Using In-House PCR Assay | Current Study Using Xpert MTB/RIF Ultra Assaya |
|---|---|---|
| Swab type | GE Healthcare OmniSwabs or Puritan PurFlock swabs | Copan FLOQSwabs or Puritan PurFlock swabs |
| Swab storage buffer | 500 µL of 0-mmol/L Tris, 50-mmol/L EDTA, 50-mmol/L sucrose, 100-mmol/L NaCl, 1% SDS | 500 µL of 0.9% sterile saline |
| Storage period before testing (−80°C) | 6 mo to 2 y | 6 m to 3 y |
| DNA extraction | Qiagen QIAmp DNA mini kit | Cepheid proprietary |
| Volume of eluate used for PCR | Entire eluate (15 µL of resuspended ethanol-precipitated DNA) | Cepheid proprietary |
| DNA target sequences | IS6110 | IS6110, IS1081, rpoB |
| Cycle threshold for positivity | <40 | <37 (IS6110 or IS1081); <40 (rpoB) |
Abbreviations: EDTA, ethylenediaminetetraacetic acid; NaCl, sodium chloride; PCR, polymerase chain reaction; SDS, sodium dodecyl sulfate.
See reference [16] for details of Xpert MTB/RIF Ultra assay.
Consistent with our prior findings, the microbiologic yield from IS specimens was substantially higher than that from oral swab specimens. The yield in the current study is also similar to the 21% sensitivity reported in a study from Peru that used buccal swab specimens and real-time PCR analysis of extracted DNA in children [17]. Among South African adults, the sensitivity of oral swab specimens for pulmonary tuberculosis has been reported to be as high as 83% and 90%, although both studies also used manual quantitative PCR methods for oral swab specimen analysis and ≥2 swab specimens per patient and had high proportions of sputum smear–positive pulmonary tuberculosis [9, 10]. However, the sensitivity of oral swab specimens tested with the Xpert Ultra assay among adults with diagnosed pulmonary tuberculosis has been reported to be about 50% in other studies in different settings [8, 18]. These data suggest that, despite the improved sensitivity of the Xpert MTB/RIF Ultra over the Xpert MTB/RIF assay, the sensitivity among paucibacillary specimens, including oral swab specimens, is insufficient for tuberculosis diagnosis in children.
The highest sensitivity was found among children with HIV, at 33%. While this is still low, rapid tuberculosis diagnosis through Xpert MTB/RIF Ultra analysis of a noninvasive, easily obtainable specimen could be beneficial in this vulnerable patient group at high risk of rapid progression and poor treatment outcomes. This could be particularly relevant in settings with a high risk of tuberculosis drug resistance and high prevalence of HIV coinfection. However, among the 6 participants with diagnosed rifampicin-resistant tuberculosis, only 1 had a positive oral swab specimen result in which rifampicin resistance was not detected. This likely reflects a greater risk of discordant rifampicin resistance results among specimens with low semiquantitative Xpert MTB/RIF test results [19] and suggests a limited benefit of a test such as the Xpert MTB/RIF Ultra assay, which allows simultaneous testing for rifampicin susceptibility. In contrast, urine lipoarabinomannan testing has shown somewhat higher sensitivity for tuberculosis diagnosis in both children with HIV and those without HIV, particularly with use of the newer FujiLAM test, with an overall sensitivity of 42% in our setting, but higher in children with HIV, at 60% [20].
Both tongue and buccal oral swab specimens have been used to assess the utility of oral swab specimens for pulmonary tuberculosis diagnosis [21, 22]. Among adults, tongue swab specimens are reported to increase diagnostic yield relative to cheek or gum swab specimens [10]. While our data suggest that buccal swab specimens may be associated with a higher yield, this finding may be due to differences in swabbing technique and proficiency. To date, there are limited data comparing the utility of tongue versus cheek swab specimens for pulmonary tuberculosis diagnosis in children.
The current study has several limitations. Our participant cohort was recruited based on a strong clinical suspicion of tuberculosis; 85% of participants were classified as either having confirmed or unconfirmed tuberculosis. The relatively small number of participants in the unlikely tuberculosis group likely restricted power in the specificity calculations; nevertheless, specificity was consistently high. We also used 2 swab types across the course of this study, switching from Puritan PurFlock swabs to COPAN FLOQSwabs in May 2019. COPAN FLOQSwabs have been reported to collect significantly more biomass than Puritan swabs [23], but there was no significant difference in yield between these 2 swab types. Finally, the Xpert MTB/RIF Ultra assay has been optimized for testing sputum specimens and may be suboptimal for oral swab specimen analysis [4].
Tuberculosis in children is often paucibacillary and displays a diverse spectrum of disease, complicating diagnosis. In addition, children are less likely to expectorate sputum, particularly the very young [24]. The WHO target product profile for rapid biomarker–based nonsputum diagnostic test for intrathoracic tuberculosis in children specifies an optimal sensitivity of ≥66% and specificity of 98% for microbiologically confirmed tuberculosis [25]. To date, none of available tests conducted on nonsputum specimens, including urine, stool, or oral swab specimens (shown here), reach this target when compared with a reference that includes probable pulmonary tuberculosis.
Encouragingly, a systematic review suggested that Xpert MTB/RIF testing on stool specimens results in a pooled sensitivity of 50%, although there is considerable heterogeneity across studies [26]. However, studies including the yields of Xpert MTB/RIF assays with both stool and IS specimens have consistently shown substantially higher yield from IS specimens [27]. Processing of stool specimens for testing with Xpert MTB/RIF or Xpert MTB/RIF Ultra assays is likely to be more complex than that required for oral swab specimen testing, although more recent work has shown that a relatively simple procedure may be adequate [28]. While no single specimen may provide sufficient sensitivity, potentially a combination of specimens, incorporating noninvasive sampling into an algorithmic approach, may improve pediatric tuberculosis diagnosis overall [5].
Overall, the data shown here suggest that the poor sensitivity of oral swab specimens for pulmonary tuberculosis diagnosis in children may preclude their routine use, but there may be some benefit among children with HIV or in places where other respiratory samples are not obtainable for microbiologic testing. In contrast, the use of culture or rapid molecular diagnostic tools, such as the Xpert Ultra assay, with sputum specimens, either IS or expectorated sputum, offers the greatest yield for microbiologic confirmation of pulmonary tuberculosis diagnosis in children. Given the extremely low rate of tuberculosis case detection, and particularly the low rate of diagnosis for drug-resistant tuberculosis in children in most settings with a high tuberculosis burden, efforts should focus on scaling up sputum induction capacity for children.
Contributor Information
Helen Cox, Division of Medical Microbiology, Department of Pathology, University of Cape Town, Cape Town, South Africa; Wellcome Centre for Infectious Disease Research, and Institute of Infectious Disease and Molecular Medicine, University of Cape Town, Cape Town, South Africa.
Lesley Workman, Department of Paediatrics and Child Health and South African Medical Research Council Unit on Child and Adolescent Health, University of Cape Town and Red Cross War Memorial Children’s Hospital, Cape Town, South Africa.
Lindy Bateman, Department of Paediatrics and Child Health and South African Medical Research Council Unit on Child and Adolescent Health, University of Cape Town and Red Cross War Memorial Children’s Hospital, Cape Town, South Africa.
Zoe Franckling-Smith, Department of Paediatrics and Child Health and South African Medical Research Council Unit on Child and Adolescent Health, University of Cape Town and Red Cross War Memorial Children’s Hospital, Cape Town, South Africa.
Margaretha Prins, Department of Paediatrics and Child Health and South African Medical Research Council Unit on Child and Adolescent Health, University of Cape Town and Red Cross War Memorial Children’s Hospital, Cape Town, South Africa.
Juaneta Luiz, Department of Paediatrics and Child Health and South African Medical Research Council Unit on Child and Adolescent Health, University of Cape Town and Red Cross War Memorial Children’s Hospital, Cape Town, South Africa.
Judi Van Heerden, Division of Medical Microbiology, Department of Pathology, University of Cape Town, Cape Town, South Africa.
Lemese Ah Tow Edries, Division of Medical Microbiology, Department of Pathology, University of Cape Town, Cape Town, South Africa.
Samantha Africa, Division of Medical Microbiology, Department of Pathology, University of Cape Town, Cape Town, South Africa.
Veronica Allen, Division of Medical Microbiology, Department of Pathology, University of Cape Town, Cape Town, South Africa.
Cynthia Baard, Department of Paediatrics and Child Health and South African Medical Research Council Unit on Child and Adolescent Health, University of Cape Town and Red Cross War Memorial Children’s Hospital, Cape Town, South Africa.
Widaad Zemanay, Division of Medical Microbiology, Department of Pathology, University of Cape Town, Cape Town, South Africa.
Mark P Nicol, Division of Medical Microbiology, Department of Pathology, University of Cape Town, Cape Town, South Africa; Division of Infection and Immunity, School of Biomedical Sciences, University of Western Australia, Perth, Australia.
Heather J Zar, Department of Paediatrics and Child Health and South African Medical Research Council Unit on Child and Adolescent Health, University of Cape Town and Red Cross War Memorial Children’s Hospital, Cape Town, South Africa.
Notes
Acknowledgments. The authors thank all the clinical staff at Red Cross Children’s Hospital and Dora Nginza Hospital, as well as the laboratory staff at the National Health Laboratory Service diagnostic microbiology laboratory at Groote Schuur Hospital and at Red Cross Children’s Hospital. They acknowledge the chief executive officer and management teams of Red Cross Children’s Hospital and Dora Nginza Hospital for their support of the study. They thank the children and their caregivers.
Financial support. This work was supported by the Regional Prospective Observational Research in Tuberculosis (RePORT TB) Consortium, which is cofunded by the Medical Research Council of South Africa and the US Office of AIDS Research, National Institutes of Health (grant DAA2-16-62066-1). Additional funding from; the Medical Research Council of South Africa (SA-MRC, funding for the Tuberculosis Collaborating Centre for Child Health and for the MRC Unit on Child and Adolescent Health); the National Institutes of Health (grant RO1HD058971); the Global Health Innovative Technology Fund (grant G2017-207); Australian National Health and Medical Research Council (investigator grant APP1174455 to M. P. N.); the SA-MRC (support to H. J. Z.); and the National Institute of Allergy and Infectious Diseases (support to M. P. N.).
References
- 1. Dodd PJ, Gardiner E, Coghlan R, Seddon JA. Burden of childhood tuberculosis in 22 high-burden countries: a mathematical modelling study. Lancet Glob Health 2014; 2:e453–9. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization . Global tuberculosis report 2021. Geneva, Switzerland: World Health Organization, 2021. [Google Scholar]
- 3. Harries AD, Kumar AMV. Challenges and progress with diagnosing pulmonary tuberculosis in low- and middle-income countries. Diagnostics (Basel) 2018; 8:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Atherton RR, Cresswell FV, Ellis J, Kitaka SB, Boulware DR. Xpert MTB/RIF Ultra for tuberculosis testing in children: a mini-review and commentary. Front Pediatr 2019; 7:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zar HJ, Workman LJ, Prins M, et al. Tuberculosis diagnosis in children using Xpert Ultra on different respiratory specimens. Am J Respir Crit Care Med 2019; 200:1531–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Joel DR, Steenhoff AP, Mullan PC, et al. Diagnosis of paediatric tuberculosis using sputum induction in Botswana: programme description and findings. Int J Tuberc Lung Dis 2014; 18:328–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moore HA, Apolles P, de Villiers PJ, Zar HJ. Sputum induction for microbiological diagnosis of childhood pulmonary tuberculosis in a community setting. Int J Tuberc Lung Dis 2011; 15:1185–90, i. [DOI] [PubMed] [Google Scholar]
- 8. Mesman AW, Calderon R, Soto M, et al. Mycobacterium tuberculosis detection from oral swabs with Xpert MTB/RIF ULTRA: a pilot study. BMC Res Notes 2019; 12:349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wood RC, Luabeya AK, Weigel KM, et al. Detection of Mycobacterium tuberculosis DNA on the oral mucosa of tuberculosis patients. Sci Rep 2015; 5:8668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Luabeya AK, Wood RC, Shenje J, et al. Noninvasive detection of tuberculosis by oral swab analysis. J Clin Microbiol 2019; 57:e01847-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dorman SE, Schumacher SG, Alland D, et al. Xpert MTB/RIF Ultra for detection of Mycobacterium tuberculosis and rifampicin resistance: a prospective multicentre diagnostic accuracy study. Lancet Infect Dis 2018; 18:76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nicol MP, Workman L, Prins M, et al. Accuracy of Xpert Mtb/Rif Ultra for the diagnosis of pulmonary tuberculosis in children. Pediatr Infect Dis J 2018; 37:e261–3. [DOI] [PubMed] [Google Scholar]
- 13. Nicol MP, Wood RC, Workman L, et al. Microbiological diagnosis of pulmonary tuberculosis in children by oral swab polymerase chain reaction. Sci Rep 2019; 9:10789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Graham SM, Cuevas LE, Jean-Philippe P, et al. Clinical case definitions for classification of intrathoracic tuberculosis in children: an update. Clin Infect Dis 2015; 61(suppl 3):S179–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zar HJ, Hanslo D, Apolles P, Swingler G, Hussey G. Induced sputum versus gastric lavage for microbiological confirmation of pulmonary tuberculosis in infants and young children: a prospective study. Lancet 2005; 365:130–4. [DOI] [PubMed] [Google Scholar]
- 16. Chakravorty S, Simmons AM, Rowneki M, et al. The new Xpert MTB/RIF Ultra: improving detection of Mycobacterium tuberculosis and resistance to rifampin in an assay suitable for point-of-care testing. MBio 2017; 8:e00812-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Flores JA, Calderon R, Mesman AW, et al. Detection of Mycobacterium tuberculosis DNA in buccal swab samples from children in Lima, Peru. Pediatr Infect Dis J 2020; 39:e376–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lima F, Santos AS, Oliveira RD, et al. Oral swab testing by Xpert® MTB/RIF Ultra for mass tuberculosis screening in prisons. J Clin Tuberc Other Mycobact Dis 2020; 19:100148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Murithi W, Click ES, McCarthy KD, et al. Need for caution when interpreting Xpert® MTB/RIF results for rifampin resistance among children. Int J Tuberc Lung Dis 2021; 25:911–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nicol MP, Schumacher SG, Workman L, et al. Accuracy of a novel urine test, Fujifilm SILVAMP tuberculosis lipoarabinomannan, for the diagnosis of pulmonary tuberculosis in children. Clin Infect Dis 2021; 72:e280–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mesman AW, Calderon RI, Pollock NR, et al. Molecular detection of Mycobacterium tuberculosis from buccal swabs among adult in Peru. Sci Rep 2020; 10:22231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Song Y, Ma Y, Liu R, et al. Diagnostic yield of oral swab testing by TB-LAMP for diagnosis of pulmonary tuberculosis. Infect Drug Resist 2021; 14:89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wood RC, Andama A, Hermansky G, et al. Characterization of oral swab samples for diagnosis of pulmonary tuberculosis. PLoS One 2021; 16:e0251422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Marais BJ, Schaaf HS. Tuberculosis in children. Cold Spring Harb Perspect Med 2014; 4:a017855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. World Health Organization . Meeting report: high priority target product profiles for new tuberculosis diagnostics, report of a consensus meeting. Geneva, Switzerland, 2014. Available at: https://www.who.int/publications/i/item/WHOHTM-TB-2014.18. Accessed 1 September 2021.
- 26. Gebre M, Cameron LH, Tadesse G, Woldeamanuel Y, Wassie L. Variable diagnostic performance of stool Xpert in pediatric tuberculosis: a systematic review and meta-analysis. Open Forum Infect Dis 2021; 8:ofaa627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nicol MP, Spiers K, Workman L, et al. Xpert MTB/RIF testing of stool samples for the diagnosis of pulmonary tuberculosis in children. Clin Infect Dis 2013; 57:e18–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. de Haas P, Yenew B, Mengesha E, et al. The simple one-step (SOS) stool processing method for use with the Xpert MTB/RIF assay for a child-friendly diagnosis of tuberculosis closer to the point of care. J Clin Microbiol 2021; 59:e0040621. [DOI] [PMC free article] [PubMed] [Google Scholar]