Abstract
The Afa/Dr diffusely adhering Escherichia coli (DAEC) C1845 strain harboring the F1845 fimbrial adhesin interacts with the brush border-associated CD55 molecule and promotes elongation of brush border microvilli resulting from rearrangement of the F-actin network. This phenomenon involves the activation of a cascade of signaling coupled to the glycosylphosphatidylinositol-anchored receptor of the F1845 adhesin. We provide evidence that infection of the polarized human intestinal cell line Caco-2/TC7 by strain C1845 is followed by an increase in the paracellular permeability for [3H]mannitol without a decrease of the transepithelial resistance of the monolayers. Alterations in the distribution of tight-junction (TJ)-associated occludin and ZO-1 protein are observed, whereas the distribution of the zonula adherens-associated E-cadherin is not affected. Using the recombinant E. coli strains HB101(pSSS1) and -(pSSS1C) expressing the F1845 fimbrial adhesin, we demonstrate that the adhesin-CD55 interaction is not sufficient for the induction of structural and functional TJ lesions. Moreover, using the actin filament-stabilizing agent Jasplakinolide, we demonstrate that the C1845-induced functional alterations in TJs are independent of the C1845-induced apical cytoskeleton rearrangements. The results indicated that pathogenic factor(s) other than F1845 adhesin may be operant in Afa/Dr DAEC C1845.
Diffusely adhering Escherichia coli (DAEC) has been recognized as one of the six classes of diarrheagenic E. coli (for a review, see reference 47). DAEC strains, which are defined by their diffuse adherence pattern on cultured epithelial HeLa or Hep-2 cells (17, 57), have been associated with persistent diarrhea in children older than infants (4, 28, 32, 37). In addition, some DAEC strains have been involved in uropathogenic infections (35, 36, 49, 52, 65). The only known virulence factors of DAEC strains are their adhesins. Most of the DAEC adhesins belong to the Afa/Dr family of adhesins, which recognize as a receptor a glycosylphosphatidylinositol-anchored protein, the decay-accelerating factor (DAF or CD55) (48). The Afa/Dr family includes the AfaE-I and AfaE-III adhesins (26, 35, 36), the Dr and DR-II adhesins (42, 49), and the F1845 adhesin (8, 9, 39). A DNA sequence from the F1845 adhesin operon (daaC) (9) has been used as a DAEC probe in several epidemiological studies. However, the lack of a probe common to all DAEC strains has somewhat hindered the relevance of epidemiological studies. The DAEC family appears as a heterogeneous group of E. coli strains which might be evolutionarily close to enteroaggregative E. coli (18, 62). On the other hand, a subset of diffusely adhering strains have been renamed diffusely adhering enteropathogenic E. coli because they contain a homologue of the locus of enterocyte effacement pathogenicity island and exhibit pathogenic properties characteristic of enteropathogenic E. coli strains (5, 61).
Insights into the cellular events occurring after the interaction of Afa/Dr DAEC strains with host cells have been obtained using the strain C1845 harboring the F1845 adhesin, which was isolated from a child with diarrhea (8, 9). It has been observed that this strain promotes elongation of the cell membrane in nonintestinal undifferentiated cells (16). Using human differentiated intestinal cells in culture, we have demonstrated that adhesin F1845 interacts with the brush border-associated CD55 molecule (6, 7) and that cell infection by strain C1845 is followed by elongation of brush border microvilli resulting from rearrangement of the F-actin network (6). Recently, evidence has been provided that the F-actin disorganization results from the activation of a cascade of signaling coupled to the glycosylphosphatidylinositol-anchored receptor CD55 (51).
Although structural alterations following Afa/Dr DAEC adherence have been examined, the changes in epithelial-cell physiology induced by DAEC have not been addressed. In polarized epithelial cells, it is well established that the junctional complex regulates paracellular solute flow and lateral diffusion between apical and basolateral plasma membrane domains (for reviews, see references 21 and 40). Several cytoskeleton-associated proteins play pivotal roles in the architectural organization of the polarized cells. Moreover, it is well established that several gastrointestinal epithelial functions are influenced by the establishment and maintenance of the polarized organization of the epithelial intestinal cells. Indeed, the organization of polarized epithelial cells in monolayers provides a permeability barrier between different environments (1, 14, 19, 20, 42, 60). The junctional complex is a highly developed structure which functions as a “fence” separating the apical and basolateral domains, thereby segregating cell surface proteins and lipids in each domain. The junctional complex also functions as a “gate” to provide a permeability barrier between the mucosal and serosal environments and to enable vectorial transport across the cellular layer. The complexity of regulation of the paracellular pathway by its well-defined structures is now apparent and is permanently in progress.
In the present study, we investigated whether infection by the Afa/Dr DAEC strain C1845, which promotes apical-cytoskeleton disassembly, alters the barrier and transport functions of intestinal epithelial cells. Using culture monolayers of the polarized human intestinal cell line Caco-2/TC7, we show that C1845 infection induces an increase in paracellular permeability to radiolabeled probes and alterations in the distribution of tight junction (TJ)-associated proteins. These structural and functional injuries localized at the TJs in cell-cell junctional complexes provide new insights into the pathophysiological events occurring upon infection by Afa/Dr DAEC strains.
MATERIALS AND METHODS
Reagents.
[2-3H]mannitol (15 to 30 Ci/mM) was from Amersham (Les Ulis, France). [1,2-3H]polyethylene glycol (PEG) 900 (2 Ci/mM) was from NEN (Paris, France). Fluorescein-5 and -6 sulfonic acid (FS) and Jasplakinolide (JAS) were from Molecular Probes (Eugene, Oreg.).
Cell culture.
The cultured human colonic adenocarcinoma Caco-2/TC7 clone cells (13) established from the parental Caco-2 cell line (24, 55) were used. Cells were routinely grown in Dulbecco modified Eagle's minimal essential medium (25 mM glucose) (Eurobio, Paris, France) supplemented with 20% fetal calf serum (Boehringer, Mannheim, Germany) and 1% nonessential amino acids. For maintenance purposes, the cells were passaged weekly using 0.25% trypsin in Ca2+Mg2+-free phosphate-buffered saline (PBS) containing 3 mM EDTA. The cells were seeded in 24-well tissue culture plates (Corning Glass Works, Corning, N.Y.) at a concentration of 2.5 × 104 per well. Maintenance of the cells and all experiments were carried out at 37°C in a 10% CO2–90% air atmosphere. Differentiated cells were used at late postconfluence, i.e., 15 days in culture.
Bacterial strains.
The clinical isolate E. coli C1845 harboring the fimbrial F1845 adhesin (9) and the HB101 laboratory strain carrying high-copy-number plasmid pSSS1 or low-copy-number plasmid pSSS1C, both expressing the F1845 fimbrial adhesin, were grown at 37°C for 18 h in Luria broth (Difco Laboratories, Detroit, Mich.) with the appropriate antibiotic for the recombinant strain. The bacterial cultures were washed in Luria broth before infection.
Spent culture supernatant of an 18-h culture of strain C1845 (C1845-SCS) was obtained by centrifugation at 10,000 × g for 30 min at 4°C. C1845-SCS was sampled and centrifuged at 10,000 × g for 30 min at 4°C. The centrifuged C1845-SCS was passed through a sterile 0.22-μm-pore-size filter unit, Millex GS (Millipore, Molsheim, France). The filtered C1845-SCS was controlled for the absence of C1845 bacteria by plating it on tryptic soy agar to confirm the absence of bacterial colonies.
The Salmonella enterica serovar Typhimurium strain SL1344 (22) was cultured in Luria broth at 37°C.
Cell infection.
The method used for Caco-2/TC7 cell infection has been described elsewhere (6, 7). Briefly, the cell monolayers were washed twice with PBS. Infecting E. coli bacteria were suspended in culture medium, and a total of 108 CFU/well of this suspension was added to each well of the tissue culture plate. The infection assay was conducted in the presence of 1% mannose to prevent type 1 fimbria-mediated binding. The plates were incubated at 37°C in 10% CO2–90% air for 3 h. The monolayers were then washed three times with sterile PBS. Each assay was conducted in triplicate with three successive passages of Caco-2/TC7 cells.
Infection culture medium of DAEC C1845-infected Caco-2/TC7 cells (C1845-ICM) was obtained from 3-h-infected cells. C1845-ICM was sampled and centrifuged at 10,000 × g for 5 min at 4°C. The centrifuged C1845-ICM was passed through a sterile 0.22-μm-pore-size filter unit (Millex GS). The filtered C1845-ICM was controlled for the absence of C1845 bacteria by plating it on tryptic soy agar to confirm the absence of bacterial colonies. C1845-ICM was 20-fold concentrated with an ultrafree centrifugal filter device with a 100-kDa cutoff (Millipore).
Antibodies.
Monoclonal antibodies (MAbs) against ZO-1 protein and E-cadherin were from Biogenesis (Interchim, Montluçon, France). The MAb directed against occludin was obtained from Zymed laboratories (San Francisco, Calif.). Fluorescein isothiocyanate (FITC)-phalloidin was from Molecular Probes Inc. Anti-rabbit and anti-mouse FITC-coupled and rhodamine isothiocyanate-coupled goat antiglobulins were from Institut Pasteur Productions (Paris, France).
Immunofluorescence.
Monolayers of Caco-2/TC7 cells were prepared on glass coverslips, which were placed in 24-well tissue culture plates (Corning Glass Works). The cell monolayers were fixed for 15 min at room temperature in 3.5% paraformaldehyde in PBS, washed three times, and then treated with 50 mM NH4Cl for 10 min. When ZO-1, occludin, and E-cadherin were to be visualized, the coverslips were permeabilized by incubation with 0.2% Triton X-100 in PBS for 4 min, and the coverslips were then washed three times with PBS. The permeabilized cell monolayers were incubated with specific primary antibody (diluted 1:20 to 1:100 in 0.2% gelatin–PBS) for 45 min at room temperature, washed, and then incubated with their respective secondary FITC- or rhodamine isothiocyanate-conjugated antibody. Appropriate secondary antibodies were used at a dilution of 1:20 to 1:200 in 0.2% gelatin–PBS. No fluorescent staining was observed when nonimmune serum was used or when the primary antibody was omitted.
When F-actin was to be visualized, the coverslips were permeabilized by incubation with 0.2% Triton X-100 in PBS for 4 min at room temperature before incubation with fluorescein-phalloidin for 45 min at room temperature. The coverslips were then washed three times with PBS.
Specimens were mounted in Dabco antifadent mounting medium (Citifluor Laboratories, Birmingham, United Kingdom). The specimens were examined by epifluorescence microscopy using a Leitz Aristoplan microscope with epifluorescence coupled to an Image Analyzer Visiolab 1000 (Biocom, Les Ulis, France) with a 100× oil immersion objective. More than 200 individual cells were examined for each assay conducted in triplicate with three successive passages of Caco-2/TC7 cells. All photographs were taken on T-MAX 400 black and white film (Eastman Kodak Co., Rochester, N.Y.).
Confocal analysis was conducted with a confocal laser scanning microscope (model TCS SP; Leica, Heidelberg, Germany), using a UV 100×.4NA 0.1 PL APO 1.4 to 0.7 objective. Photographic images were resized, organized, and labeled with Adobe (San Jose, Calif.) Photoshop software. The printed images are representative of the original data.
LDH release.
Cell integrity was determined by measuring the lactate dehydrogenase (LDH) in the culture medium postinfection using a commercially available kit, Enzyline LDH (Biomérieux, Dardilly, France). The results are expressed as the percentage of LDH released; 100% LDH release was measured in control H2O-lysed cells.
Transepithelial resistance measurements.
Monolayers of Caco-2/TC7 cells grown in filters mounted in chamber culture (Costar culture plate inserts; 0.4-μm porosity; 4.7 cm2; 3 × 104 cells per cm2), which delineates an apical (luminal) and a basolateral (serosal) reservoir. After bacterial infection, the integrity of the confluent polarized monolayers was checked by measuring transepithelial membrane resistance (TER) with a volt-ohmmeter (Millicel ERS; Millipore). TER was calculated as Ω · cm2 by multiplying the measured electrical resistance by the surface area of the filter. The background reading of the free control filter was subtracted.
Permeability measurements.
The permeability of Caco-2/TC7 cell monolayers was determined by measuring the paracellular passage of water-soluble radioactive or fluorescent compounds of various sizes from apical to basolateral compartments of the chamber culture (Costar culture plate inserts; 0.4-μm porosity; 3 × 104 cells per filter).
[3H]mannitol, [3H]PEG, or FS was dissolved in culture medium. To determine flux in the apical-to-basolateral direction, the tracer solution (2.5 μCi/ml for radioactive compounds or 200 μg/ml for FS) was loaded into the apical side of the monolayer, and the cells were incubated for 1 h at 37°C. After the incubation period, the tracer concentrations in the apical and basolateral compartments were assayed. The concentrations of [3H]mannitol and [3H]PEG were determined by measurement in a β-scintillation counter. Values were corrected for the background radioactivity of the medium or PBS, as appropriate. The fluorescence due to FS was determined with a Jobin-Yvon JY3C spectrofluorimeter at an excitation wavelength of 410 nm (slit width, 2 nm) and an emission wavelength of 530 nm (slit width, 10 nm).
JAS treatment.
JAS (1 μM) was added to the culture medium 45 min before infection. Treatment was maintained during the infection time course (3 h). In a preliminary experiment, we confirmed that JAS at the concentration used had no effect on DAEC C1845 binding. Moreover, examination of uninfected cells treated with JAS by measuring LDH release and TER showed no modification in the cell and monolayer integrities.
Analysis.
Results are expressed as means ± standard error of the mean. For statistical comparisons, Student's t test was performed.
RESULTS
DAEC C1845 infection induces dome formation in Caco-2/TC7 cell monolayers, revealing an increase in paracellular permeability.
In polarized epithelial cells forming monolayers, the intercellular junctional complexes are the major intercellular structures that restrict permeation through the paracellular spaces (for reviews, see references 1, 19, and 42). Indeed, epithelia forming barriers regulate the vectorial transport of ions and solutes between different biological compartments separated by these barriers. Caco-2 cells forming monolayers cultured on impermeable support, i.e., on plastic culture dishes, are known to be a constitutively dome-forming cell line. These domes result from fluid accumulation in randomly distributed areas that evolve into the monolayers (29).
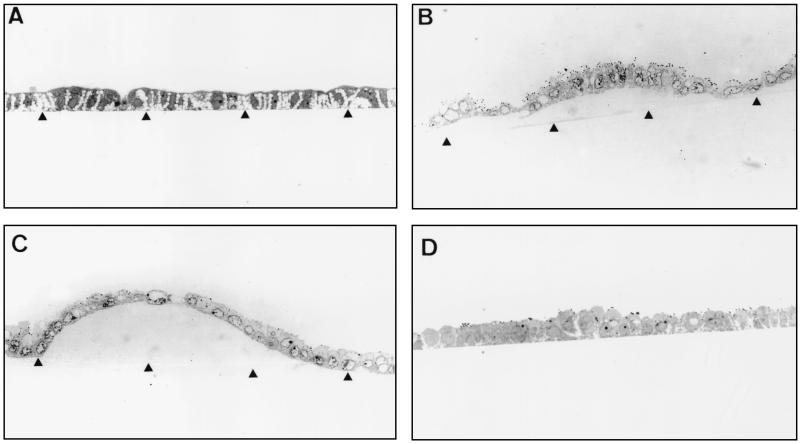
We observed an increase in the level of domes formed in Caco-2/TC7 clone cells upon Afa/Dr DAEC strain C1845 infection. In order to visualize this phenomenon, the infected Caco-2/TC7 cell monolayers were fixed, embedded in Epon, and reembedded in order to make sections perpendicular to the bottom of the flask. Examination by light microscopy of semithin sections of DAEC C1845-infected Caco-2/TC7 monolayers at 3 h postinfection revealed that domes of different sizes were formed (Fig. 1): small domes in which a small number of cells were detached from the bottom of the flask culture (Fig. 1B) and large domes including a large number of cells detached from the bottom of the flask culture without disruption of the monolayer (Fig. 1C). Determination of the release of LDH in the culture medium showing no release of the intracellular enzyme compared with the noninfected cells (control, 16 ± 5 U/liter; infected cells, 17 ± 4 U/liter) demonstrates that cell integrity is maintained in DAEC C1845-infected cells. An increase in areas where fluid accumulates has been observed in Caco-2 cells under different types of physiological stimulation (for a review, see reference 66). Because some enterovirulent pathogens alter TJs, we have conducted additional experiments to determine whether the DAEC C1845-induced dome formation results from alterations induced in the TJs of the Caco-2/TC7 cell monolayers.
FIG. 1.
Dome formation in Caco-2/TC7 cell monolayers upon DAEC C1845 infection. Confluent differentiated Caco-2/TC7 cells were infected apically at 37°C in a 10% CO2–90% air atmosphere for 3 h with C1845 bacteria (108 CFU/well). The cell monolayers were processed for light microscopic examination of sections perpendicular to the bottom of the flask (arrowhead) in semithin sections of cells. (A) Control cells. (B and C) DAEC C1845-infected cells showing different sizes of domes. (D) HB101(pSSS1)-infected cells. Magnification, ×100.
DAEC C1845 infection of Caco-2/TC7 cell monolayers disrupts TJ gate function: nonionic molecules.
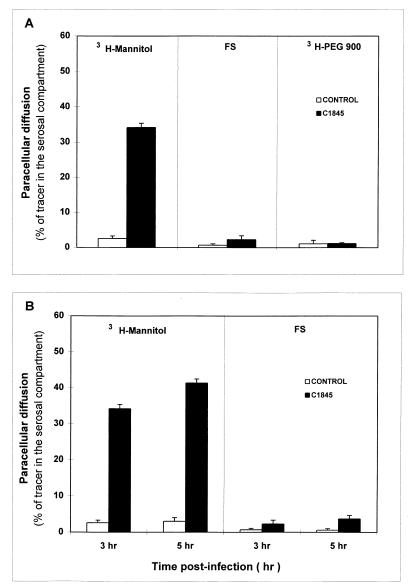
In order to examine the mechanism by which the dome formation occurs, we have conducted a set of experiments. The effect of DAEC C1845 infection in a Caco-2/TC7 intestinal monolayer on the paracellular diffusion of nonionic molecular tracers was determined. Paracellular permeability was measured using defined paracellular markers of different sizes: [3H]mannitol, 182 Da; FS, 478 Da; and [3H]PEG 900, 900 Da. The mucosal-to-serosal flux rate of markers across the filter-grown Caco-2/TC7 monolayers was determined at 3 h postinfection (Fig. 2A). The rate of unidirectional flux of markers was negligibly low in control monolayers. DAEC C1845 infection resulted in a highly significant increase in the paracellular permeability for [3H]mannitol compared with that of control monolayers. In contrast, no change in the paracellular permeability of FS and PEG 900 was found. In order to determine whether the size of the DAEC C1845-induced junctional lesion evolved during DAEC C1845 infection, the mucosal-to-serosal flux rates of [3H]mannitol and FS across the monolayers were determined at 5 h postinfection. No significant change in the paracellular permeability to [3H]mannitol and FS occurred at 5 h postinfection compared with that of 3 h postinfection (Fig. 2B).
FIG. 2.
DAEC C1845 infection promotes an increase in paracellular fluxes of Caco-2/TC7 cell monolayers. Monolayers of Caco-2/TC7 cells were grown in filters mounted in chamber culture (Costar). The cells were infected apically at 37°C in a 10% CO2–90% air atmosphere for 3 h with C1845 bacteria (108 CFU/well). Paracellular fluxes of [3H]mannitol (182 Da), FS (478 Da), and [3H]PEG 900 (900 Da) were measured in the mucosal-to-serosal direction with or without DAEC C1845 infection. The error bars indicate standard errors of the mean.
The effect of DAEC C1845 infection on the TER in Caco-2/TC7 cell monolayers was examined. No change in TER measured on filter-grown Caco-2/TC7 monolayers was found upon DAEC C1845 infection (control, 784 ± 10 Ω · cm2; infected cells, 792 ± 15 Ω · cm2). This result was intriguing, since it has been generally observed that upon bacterial infection increase in diffusion of nonionic molecules is accompanied by a decrease in TER. In consideration of this, we controlled the response of the Caco-2/TC7 monolayers to bacterial infection. For this purpose, Caco-2/TC7 cell monolayers were infected by serovar Typhimurium strain SL1344, known to induce a decrease of TER in Caco-2 cells by altering TJs (22, 33). After a 4-h period of infection, strain SL1344 induced a highly significant decrease of TER in Caco-2/TC7 cell monolayers (control, 800 ± 15 Ω · cm2; infected cells, 412 ± 10 Ω · cm2).
When the pH values of the incubating medium in DAEC C1845-infected Caco-2/TC7 cells at 3 h postinfection were examined, a pH of 5.4 was observed, indicating that acidosis develops during infection. It has been reported that incubation of Caco-2BB2 cell monolayers at a pH of 5.43 during a long-term exposure of 6 to 24 h results in an increase of nonionic molecule permeability (46). A control experiment was conducted by incubating the Caco-2/TC7 cells at a pH of 5.3 during a 3-h period. No significant change in the mucosal-to-serosal passage of [3H]mannitol was found ([3H]mannitol in the serosal compartment was as follows: control, 2.75% ± 0.88%; culture medium at pH 5.3, 4.30% ± 2.47% of the [3H]mannitol applied in the mucosal compartment). Moreover, buffering the culture medium during C1845 infection results in an increase in the mucosal-to-serosal passage of [3H]mannitol ([3H]mannitol in the serosal compartment was as follows: control, 3.15% ± 0.75%; C1845 infection at pH 7.4, 34.45% ± 1.35%) which is not significantly different from that observed during C1845 infection without buffering. These results demonstrate that the increase in the paracellular permeability of Caco-2/TC7 monolayers to [3H]mannitol observed during the 3-h period of Afa/Dr DAEC C1845 infection does not result from the acidosis developed during the cell infection.
DAEC C1845 infection induces selective alterations in distribution of TJ-associated proteins.
The above-described increase in the paracellular permeability to [3H]mannitol upon DAEC C1845 infection suggests a mechanism involving alteration in the distribution of functional proteins associated with the junctional complexes. For polarized epithelial cells forming monolayers, the intercellular junctional complexes include well-defined structures: TJs or zonula occludens (ZO), zonula adherens (ZA), and desmosomes (for reviews, see references 1, 19, and 42).
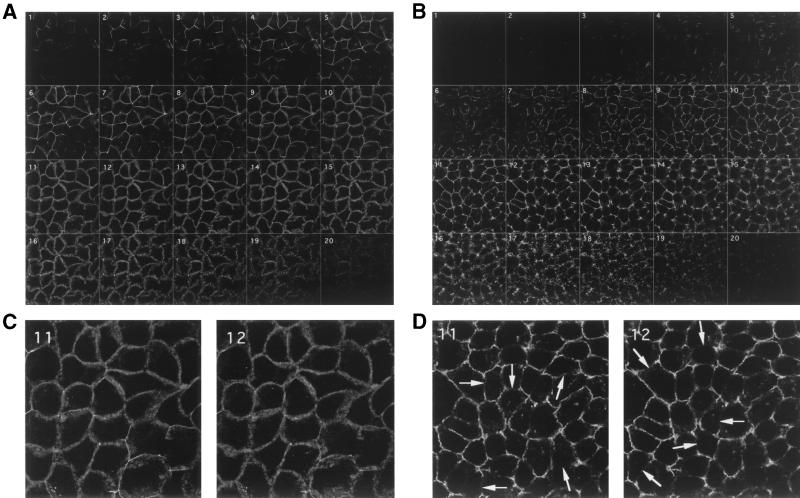
The most apical structures of the junctional complex are the TJs, which function as the major paracellular barrier. Functional proteins have been identified as specifically associated with the TJs. Several cytoplasmic-protein members of the membrane-associated guanylate kinase (MAGuK) family of proteins, including the ZO-1, -2, and -3 proteins (2, 31, 59), interact either directly or indirectly with occludin and may recruit signaling molecules as well as the actin cytoskeleton to the TJs. The functional transmembrane protein occludin localized to TJ strands appears to be involved in TJ gate function (25, 30, 45). We examined whether the distribution of TJ-associated proteins in Caco-2/TC7 cells is modified upon DAEC C1845 infection. Figures 3 and 4 show that occludin and ZO-1 staining were localized to sites of cell-cell boundaries in control uninfected cells. The distributions of both ZO-1 and occludin were characterized by a brightly stained continuous band and displayed sharp, honeycomblike organization. Analyzed by confocal microscopy, the occludin distribution in DAEC C1845-infected cells appeared profoundly modified (Fig. 3). The confocal microscopy analysis conducted from the apical to the basolateral domains of the cells shows that the localization of occludin was lower (Fig. 3B) than that in control cells (Fig. 3A). Moreover, the occludin labeling was disorganized, with formation of large gaps (Fig. 3D) compared with that in control cells (Fig. 3C). In DAEC C1845-infected cells, the ZO-1 staining also appeared disorganized, with marked discontinuities (Fig. 4).
FIG. 3.
Distribution of occludin in confluent monolayers of control and DAEC C1845-infected Caco-2/TC7 cells. Confluent differentiated Caco-2/TC7 cells were infected apically at 37°C in a 10% CO2–90% air atmosphere for 3 h with C1845 bacteria (108 CFU/well). The cells were fixed with 3.5% paraformaldehyde, washed, and processed for indirect-immunofluorescence labeling as described in Materials and Methods. Cells stained with anti-occludin MAb were examined with a confocal laser scanning microscope (model TCS SP; Leica, Heidelberg, Germany). (A and C) Control uninfected cells. (B and D) DAEC C1845-infected cells. (A and B) The samples were analyzed by serial optical horizontal sectioning (one section every 0.36 μm), and xy sections are shown. The sections start at the apical domain of the cells, and the analysis was conducted until the section just beneath the TJ area. The apical domain of the cells was determined by observation of F-actin (not shown). (C) High-magnification micrographs of sequential sections 11 and 12 showing the brightly stained continuous band in control cells displaying the typically honeycomblike organization. (D) High-magnification micrographs of sequential sections 11 and 12 showing the stained discontinuous band in DAEC C1845-infected cells with the remaining honeycomblike organization. The arrows indicate gaps. (C and D) Magnification, ×100.
FIG. 4.
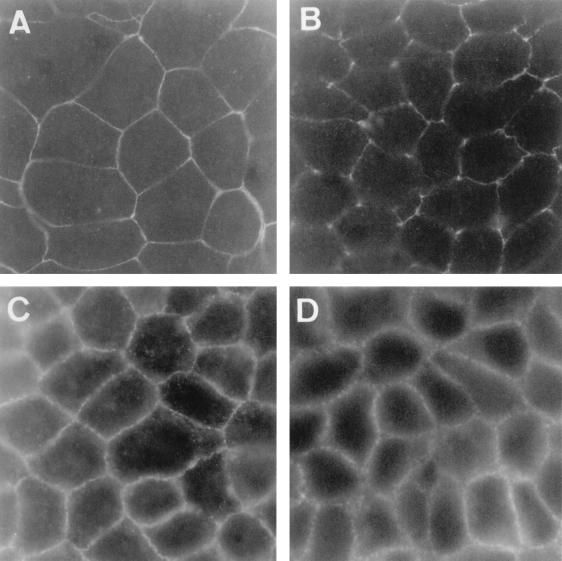
Distribution of ZO-1 and E-cadherin in confluent monolayers of control and DAEC C1845-infected Caco-2/TC7 cells. The cells were processed as described in the legend to Fig. 3. The cells were stained with anti-ZO-1 or anti-E-cadherin MAb and then examined in a Leitz Aristoplan microscope with epifluorescence. (A and C) Control cells. (B and D) DAEC C1845-infected cells. Immunofluorescence labeling of ZO-1 protein (A and B) and E-cadherin (C and D) is shown. Magnification, ×100.
Next to the TJs lies the ZA, also called the adherens junction. The main adhesion receptors in the ZA are the classical cadherins associated with catenins (for reviews, see references 10, 27, and 60). The classic cadherins (e.g., E-, N-, and P-cadherins) are single-transmembrane-domain proteins involved in calcium-dependent homophilic cell-cell recognition and adhesion. Among their functions, cadherins orchestrate the assembly in the intercellular adherens junctions of the electron-dense plaque that anchors microfilaments to the plasma membrane by linking the cadherin-catenin complex to the actin cytoskeleton, thereby playing a pivotal role in the establishment and maintenance of the cell architecture (for a review, see reference 30). We examined whether E-cadherin distribution is modified upon DAEC C1845 infection. As shown in Fig. 4, E-cadherin distribution in control uninfected cells forms a larger continuous band with a honeycomblike organization. No obvious change was found in the E-cadherin distribution in C1845-infected Caco-2/TC7 cells.
Altogether, these results demonstrate that infection by DAEC C1845 bacteria in polarized epithelial intestinal cells forming monolayers is followed by redistribution of TJ-associated ZO-1 and occludin proteins, whereas the ZA-associated E-cadherin is unchanged. These results suggest a specific opening of a functional cell barrier localized at the TJs in the junctional domain.
Characteristics of the DAEC C1845-induced structural and functional alterations in TJs.
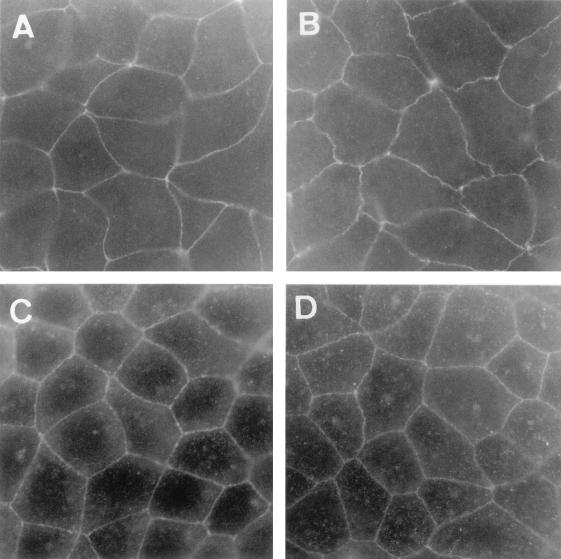
We have previously reported that the DAEC C1845-induced cell injuries in Caco-2 cells result from the F1845 adhesin interaction with CD55 as a receptor (6). To determine whether the DAEC C1845-induced alterations in TJs are dependent on the F1845 adhesin-receptor interaction, we designed experiments using the recombinant E. coli HB101 strains carrying the high-copy-number plasmid pSSS1 or the low-copy-number plasmid pSSS1C, both expressing the fimbrial F1845 adhesin (Table 1 and Fig. 1 and 5). Examination by light microscopy of semithin sections revealed that no dome was observable in the HB101(pSSS1)-infected Caco-2/TC7 monolayers at 3 h postinfection (Fig. 1D). No change in paracellular passage of [3H]mannitol was observed in the Caco-2/TC7 cells infected with the recombinant HB101(pSSS1) and -(pSSS1C) strains (Table 1). Similarly, distribution of TJ-associated ZO-1 and occludin was not altered with the strain HB101(pSSS1) (Fig. 5).
TABLE 1.
DAEC C1845-induced increase in paracellular permeability to [3H]mannitol in Caco-2/TC7 cell monolayers
| Strain | [3H]mannitol paracellular passagea |
|---|---|
| Control | 2.85 ± 1.28 |
| E. coli C1845 | 33.25 ± 1.50d |
| E. coli HB101(pSSS1) (F1845+) | 3.90 ± 2.47 |
| E. coli HB101(pSSS1C) (F1845+) | 4.75 ± 2.58 |
| C1845-SCSb | 3.72 ± 2.65 |
| C1845-ICMb | 3.50 ± 1.30 |
| E. coli C1845 with JASc | 32.72 ± 2.65d |
Cells were processed as described in the legend to Fig. 2. Paracellular fluxes of [3H]mannitol were measured in the mucosal-to-serosal direction with or without infection. The results are expressed as the percentage of [3H]mannitol applied in the mucosal compartment.
SCS, spent culture supernatant of an 18-h culture of strain C1845. ICM, 20-fold-concentrated infection culture medium sampled from 3-h C1845-infected Caco-2/TC7 cells.
JAS (1 μM) was added to the culture medium 45 min before infection and maintained during the infection time course.
Student t test; P < 0.01 compared with control.
FIG. 5.
Lack of alteration in distribution of TJ-associated proteins upon E. coli HB101(pSSS1) (F1845+) infection in Caco-2/TC7 cells. The cells were processed as described in the legend to Fig. 3. Cells stained with anti-ZO1 or anti-occludin MAb were examined in a Leitz Aristoplan microscope with epifluorescence. (A and C) Control cells. (B and D) E. coli HB101(pSSS1)-infected cells. (A and B) Immunofluorescence labeling of ZO-1 protein. (C and D) Immunofluorescence labeling of occludin. Magnification, ×100.
It has been reported that enterovirulent pathogens altered epithelial barrier and transport functions by their secreted toxins (58). In order to examine whether the observed DAEC C1845-induced functional TJ lesions result from secreted components, we conducted an additional experiment. An 18-h spent culture supernatant of the strain (C1845-SCS) and a 3-h infection culture medium of Caco-2/TC7 cells (C1845-ICM) were centrifuged, filtered, and applied to Caco-2/TC7 cell monolayers to determine their effects on the paracellular passage of [3H]mannitol. As shown in Table 1, both C1845-SCS and C1845-ICM failed to promote any change in the passage of mannitol.
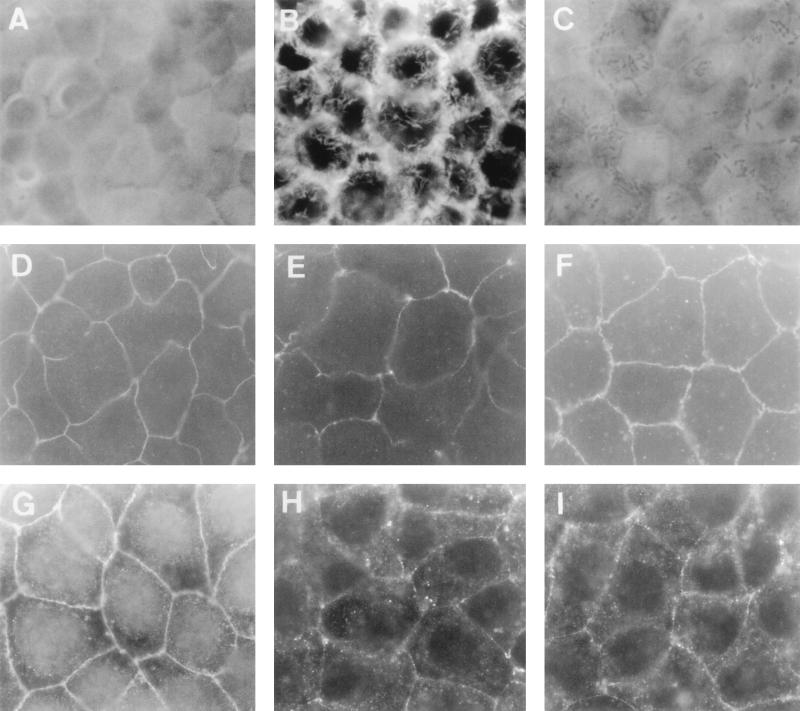
It has previously been reported that alterations in the junctional domain could result from the contraction of the apical cytoskeleton in polarized epithelial cells as a consequence of centrifugal traction of the TJ membrane, thus regulating TJ permeability (43, 50, 63). Stabilization of actin filaments can easily be obtained by treating the cells with JAS, a monocyclic peptide isolated from the sea sponge Jaspis johnstoni (64). The effect of JAS against DAEC C1845-induced increase in paracellular permeability to [3H]mannitol and apical F-actin and TJ-associated protein distribution is shown in Table 1 and Fig. 6. Different results were obtained for F-actin, ZO-1, and occludin distribution. JAS treatment was followed by the reappearance of the apical distribution of F-actin and ZO-1 protein, but not of occludin. F-actin immunolabeling in C1845-infected cells treated with JAS shows the homogenous, fine, and flocculated actin labeling centrally in the cells, representing microvillus-associated F-actin (Fig. 6C). ZO-1 immunolabeling in C1845-infected cells treated with JAS (Fig. 6F) shows the characteristic brightly stained continuous band with honeycomblike organization observed in control uninfected cells (Fig. 6D). In contrast, the immunolabeling of occludin in C1845-infected cells treated with JAS reveals a diffuse immunolabeling (Fig. 6I) without the bands with bright staining observable in the control uninfected cells. Moreover, large gaps showing discontinuities in occludin labeling were still observed. When examining the effects of JAS against the DAEC C1845-induced increase in paracellular permeability of Caco-2/TC7 monolayers to [3H]mannitol, we found that JAS treatment does not modify the increased passage of [3H]mannitol from the mucosal to the serosal compartment (Table 1). Taken together, these results indicated that stabilization of the apical F-actin network is not sufficient to prevent all the DAEC C1845-induced functional TJ injuries.
FIG. 6.
Effects of JAS on the DAEC C1845-induced alteration in distribution of apical F-actin and TJ-associated ZO-1 and occludin proteins in Caco-2/TC7 cells. The cells were processed as described in the legend to Fig. 3. (A, D, and G) Uninfected cells treated with JAS (1 μM). (B, E, and H) DAEC C1845-infected cells. (C, F, and I) DAEC C1845-infected cells treated with JAS (1 μM). (A to C) Immunofluorescence labeling of F-actin. (D to F) Immunofluorescence labeling of ZO-1 protein. (G to I) Immunofluorescence labeling of occludin. Note that in panel C, the adhering bacteria appeared at the cell surface of the DAEC C1845-infected cells treated with JAS. Magnification, ×95.
DISCUSSION
Several enteropathogens target the junctional complex in the gastrointestinal epithelium to develop pathogenicity (for a review, see reference 23). Moreover, the target proteins of several bacterial toxins are localized at the junctional complexes of polarized epithelial cells (for a review, see reference 58). The results presented here show that alterations observed in the junctional domains of monolayers of polarized Caco-2/TC7 cells upon Afa/Dr DAEC C1845 infection are quite different from those produced by other enterovirulent bacteria altering the junctional domain. In particular, we demonstrate that cell infection results in selective lesions in the intestinal epithelial barrier. Indeed, the paracellular permeability to [3H]mannitol was increased with no change in paracellular passage of nonionic molecules having higher molecular masses. This phenomenon was accompanied by a dramatic alteration in distribution of TJ-associated ZO-1 protein and occludin. In contrast, no change in TER was observed. The functional dissociation of paracellular permeability from electrical resistance upon DAEC C1845 infection is surprising, since these two parameters have generally been considered to evolve in parallel. For example, oxidant-induced disruption of epithelial barrier function in Caco-2 and T84 cells promotes a reduction in TER accompanied by an increase in [3H]mannitol permeability (56). An identical situation has been observed upon bacterial infection. Short-term infection of the polarized Madin-Darby canine kidney (MDCK) II monolayers with strain SL1344 resulted in morphological distortions in the intercellular junctions affecting distribution of E-cadherin and ZO-1 protein and a progressive decrease in TER (33). Enteropathogenic E. coli (11, 54) or enterohemorrhagic E. coli (53) infection promotes structural and functional alterations in the junctional domain characterized by changes in the resistance and permeability of the monolayers, accompanied by irregular distribution of the TJ-associated ZO-1 protein. However, the apparent dissociation of changes in TER and transepithelial mannitol flux is not without precedent. Li et al. (38) recently reported that the Shiga toxin-producing E. coli expressing the locus for enterocyte effacement causes disruption of the epithelial-cell function in T84 cells without inducing the typical attaching-effacing lesion and disruption of the actin cytoskeleton. Shiga toxin-producing E. coli infection is characterized by an increase in mannitol permeability accompanied by an unconventional increase in TER. In consideration of recent results, the correlation between TER and the permeability of the monolayers has now been reexamined. A decrease in TER resulting from tumor necrosis factor alpha treatment of Caco-2BBE cells was not accompanied by a change in the transepithelial flux of mannitol (44). When examining the role of RhoA and Rac 1 in TJ structure and function, Jou et al. (34) demonstrated that the TJs behave as a molecular sieve that determines molecule diffusion based on size. Balda et al. (3) reported that in the low-resistance MDCK strain 2 cells expressing terminally truncated chicken occludin (HAoccludinCT3), a discontinuous junctional staining pattern of HAoccludin-CT3 and a disrupted junctional distribution of endogenous occludin were observed. Surprisingly, this alteration was accompanied by an increase in paracellular permeability without a change in the size and ion selectivity of the paracellular pathway, and it does not correlate with a decrease in TER. Moreover, the observed alteration of the fence function separating lipids into apical and basolateral domains demonstrates a failure in TJs. It was interesting to note that the results obtained upon Afa/Dr DAEC C1845 infection in Caco-2/TC7 monolayers resemble the observations obtained with mutant occludin. The status of occludin that functions as regulated forming or activating protein could explain several of the contradictory results observed. A current opinion is that the junctional barrier contains pores or channels which could be selectively opened under different physiological and pathological situations. A model involving the presence of fluctuating aqueous channels embedded in TJ strands modulating one parameter without affecting the other has been proposed by Claude (15) and modified by Cereijido et al. (12). According to this hypothesis, a series of one or more diffusion barriers are not continuously tightly sealed but can be opened and closed in a fluctuating manner upon physiological stimulation or in a pathological situation. Two mechanisms have been proposed to explain these phenomena. The first, adjusting the number of parallel strands, results from modulation of the spacing between the parallel strands and the frequency of cross bridges. The second results from interdigitating TJ strands on the extracellular surfaces of opposing membranes, thus forming a molecular sieve to regulate ion and solute diffusion along the paracellular pathway.
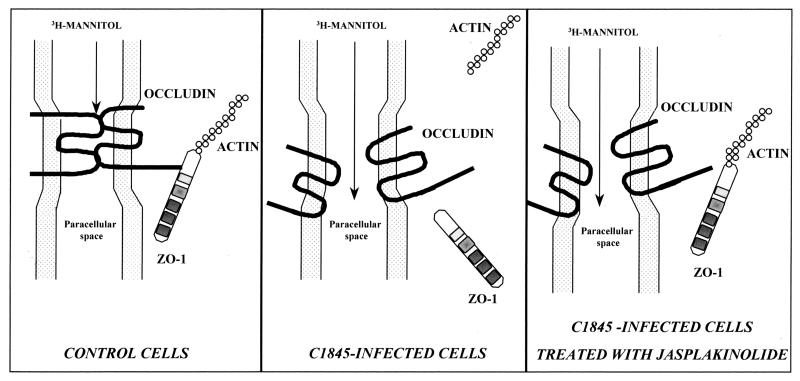
When examining whether the stabilization of the apical F-actin cytoskeleton by JAS treatment could block the DAEC C1845-induced TJ injuries, we found surprising results. Indeed, we showed that JAS treatment leads only to the reappearance of ZO-1 distribution without any change in occludin distribution or in the increase in paracellular permeability to [3H]mannitol. Similar results have recently been reported by Ma et al. (41) in examining the regulation of paracellular permeability in Caco-2 cells. Indeed, using the actin-disassembling drug cytochalasin b, these authors observed that inhibitors of the protein synthesis blocked the cytochalasin b-induced ZO-1 disassembly but failed to block the induced decrease in TER. Taken together, these results suggest that the reappearance of TJ-associated ZO-1 protein, which is an indication of the “retightening” of TJs, is not sufficient to promote the functional “reclosure” of the TJs. This could result from the different status of ZO-1 and occludin proteins. The MAGuK proteins of TJs, called ZO-1, ZO-2, and ZO-3 proteins, are localized in the cytoplasmic plaque domains of TJs (for reviews, see references 1, 14, 19, and 42). The complex of ZO proteins may be the center of a network of protein-protein interactions, such as the recruitment of proteins to establish TJs, which are essential to signal transduction events. Moreover, the cytoplasmic plaque proteins establish a link with the underlying actin-based cytoskeleton. The status of occludin in TJs is different from that of ZO-1 protein. Indeed, occludin, as claudin(s) and JAM, is a membrane-associated molecule with NH2 and COOH termini localized in the cytoplasm, whereas two extracellular loops project into the paracellular space. Through its COOH terminus, occludin interacts with the ZO-1 protein, establishing in turn a link with the cytoskeleton. In parallel, through its extracellular loops occludin behaves as a cell-cell adhesion molecule by interacting with another occludin in the neighboring cell or other membrane-associated molecules projecting loops in the paracellular space. These loop-to-loop interactions in the paracellular space seem to play a pivotal role in the sealing and/or opening of the paracellular space. The observation reported here that treatment with the F-actin-stabilizing agent JAS promotes the reinstallation of the ZO-1 protein without an effect on occludin is of interest in regard to the above-described different statuses of ZO-1 and occludin. In view of the data presented here, it is tempting to speculate that (i) the relocalization of ZO-1 upon JAS treatment in C1845-infected cells results from the stabilization of the cytoskeleton and from the link between F-actin and the ZO-1 protein; (ii) the incomplete relocalization of occludin, which plays a pivotal role in sealing the TJs, upon JAS treatment in C1845-infected cells, could explain how the DAEC C1845-induced increase in paracellular permeability to [3H]mannitol remains unchanged (Fig. 7).
FIG. 7.
Model depicting the proposed mechanism of TJ lesions induced upon Afa/Dr DAEC C1845 infection of Caco-2/TC7 cell monolayers. (Control cells) MAGuK proteins (ZO-1, ZO-2, and ZO-3) function as scaffolds of the TJ plaque to cross-link TJ strands, containing occludin and claudins, to the actin-based cytoskeleton. TJ plaque-associated ZO-1 directly binds the actin filaments. ZO-1 protein cross-links the TJ strand-associated occludin at the cytoplasmic surface to establish specialized membrane domains. Intramembranous particle strands are associated laterally with other TJ strands in opposing membranes of adjacent cells to form paired strands, where the paracellular space is completely obliterated (barrier function). (C1845-infected cells) Apical F-actin network is disassembled (6, 51). Both the TJ-associated proteins ZO-1 and occludin are delocalized. In parallel, the paracellular passage of mannitol is increased. (C1845-infected cells treated with JAS) Stabilization of actin filaments in infected cells treated with JAS allows ZO-1 protein to reinstall as in control cells, whereas occludin does not reinstall. In parallel, the increase in paracellular passage of mannitol remains unchanged. Relinking of the TJ plaque-associated ZO-1 to the actin-based cytoskeleton without re-cross-linking of the TJ strand-associated occludin could explain the lack of TJ sealing, allowing the remaining increased paracellular passage of mannitol.
In summary, our data present evidence for the first time that the Afa/Dr DAEC strain C1845 promotes increases in epithelial permeability through disassembly of TJ-associated proteins. Moreover, we show that DAEC C1845-induced alterations in TJs are not due to an apical cytoskeleton disassembly induced by the F1845-CD55 interaction. The pathophysiological consequences of the DAEC C1845-induced increase in epithelial permeability in vivo could be either an alteration in electrochemical gradients in the intestinal epithelium resulting in diarrhea or the initiation of an inflammatory response. In order to provide new insights into the pathophysiological mechanism by which the Afa/Dr enterovirulent DAEC promotes TJ lesions, we are attempting to identify the DAEC C1845 virulence factor promoting TJ alterations. Moreover, an additional biochemical analysis of the TJ-associated proteins will be required to understand the signal transduction involved.
ACKNOWLEDGMENTS
We thank S. Moseley for the generous gift of recombinant strains. We thank G. Delrue (INSERM SC6) for his skills in producing the drawings.
J. Guignot is supported by a doctoral fellowship from the Ministère de l'Education Nationale, de la Recherche et de la Technologie (MENRT). A.-B. Blanc-Potard is supported by a post-doctoral grant from the Fondation pour la Recherche Médicale (FRM). A. L. Servin is supported for this work by a grant from the Programme de Recherche Fondamentale en Microbiologie et Maladies Infectieuses et Parasitaires (MENRT-PRFMMIP).
REFERENCES
- 1.Anderson J M, van Itallie C M. Tight junctions and the molecular basis for regulation of paracellular permeability. Am J Physiol. 1995;269:G467–G475. doi: 10.1152/ajpgi.1995.269.4.G467. [DOI] [PubMed] [Google Scholar]
- 2.Anderson J M, van Itallie C M, Peterson M D, Stevenson B R, Carew E A, Mooseker M S. ZO-1 mRNA and protein expression during tight junction assembly in Caco-2 cells. J Cell Biol. 1989;109:1047–1056. doi: 10.1083/jcb.109.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balda M S, Whitney J A, Flores C, Gonzales S, Cereijido M, Matter K. Functional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical-basolateral intramembrane diffusion barrier by expression of mutant tight junction membrane protein. J Cell Biol. 1996;134:1031–1049. doi: 10.1083/jcb.134.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baqui A H, Sack R B, Black R E, Haider K, Hossain A, Alim A R, Yunus M, Chowdhury H R, Siddique A K. Enteropathogens associated with acute and persistent diarrhea in Bangladeshi children less than 5 years of age. J Infect Dis. 1992;166:792–796. doi: 10.1093/infdis/166.4.792. [DOI] [PubMed] [Google Scholar]
- 5.Beinke C, Laarmann S, Wachter C, Karch H, Greune L, Schmidt M A. Diffusely adhering Escherichia coli strains induce attaching and effacing phenotypes and secrete homologs of Esp proteins. Infect Immun. 1998;66:528–539. doi: 10.1128/iai.66.2.528-539.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernet-Camard M F, Coconnier M H, Hudault S, Servin A L. Pathogenicity of the diffusely-adhering strain Escherichia coli C1845: F1845 adhesin-decay accelerating factor interaction, brush border microvillus injury, and actin disassembly in cultured human intestinal epithelial cells. Infect Immun. 1996;64:1918–1928. doi: 10.1128/iai.64.6.1918-1928.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bernet-Camard M F, Coconnier M H, Hudault S, Servin A L. Differential expression of complement proteins and regulatory decay accelerating factor in relation to differentiation of cultured human colon adenocarcinoma cell lines. Gut. 1996;38:248–253. doi: 10.1136/gut.38.2.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bilge S S, Apostol J M, Jr, Fullner K J, Moseley S L. Transcriptional organization of the F1845 fimbrial adhesin determinant of Escherichia coli. Mol Microbiol. 1993;7:993–1006. doi: 10.1111/j.1365-2958.1993.tb01191.x. [DOI] [PubMed] [Google Scholar]
- 9.Bilge S S, Clausen C R, Lau W, Moseley S L. Molecular characterization of a fimbrial adhesin, F1845, mediating diffuse adherence of diarrhea-associated Escherichia coli to HEp-2 cells. J Bacteriol. 1989;171:4281–4289. doi: 10.1128/jb.171.8.4281-4289.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buxton R S, Magee A L. Structure and interactions of desmosomal and other cadherins. Semin Cell Biol. 1992;3:157–167. doi: 10.1016/s1043-4682(10)80012-1. [DOI] [PubMed] [Google Scholar]
- 11.Canil C, Rosenshine I, Ruchkowski S, Donnenberg M S, Kaper J B, Finlay B B. Enteropathogenic E. coli decreases the transepithelial electrical resistance of polarized epithelial monolayers. Infect Immun. 1993;61:2755–2762. doi: 10.1128/iai.61.7.2755-2762.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cereijido M, Gonzales-Mariscal L, Contreras G. Tight junction; barrier between higher organisms and environment. New Physiol Sci. 1989;4:72–74. [Google Scholar]
- 13.Chantret I, Rodolosse A, Barbat A, Dussault E, Brot-Laroche E, Zweibaum A, Rousset M. Differential expression of sucrase-isomaltase in clones isolated from early and late passages of the cell line Caco-2: evidence for glucose-dependent negative regulation. J Cell Sci. 1994;107:213–225. doi: 10.1242/jcs.107.1.213. [DOI] [PubMed] [Google Scholar]
- 14.Citi S. The molecular organization of tight junctions. J Cell Biol. 1993;121:485–489. doi: 10.1083/jcb.121.3.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Claude P. Morphological factors influencing transepithelial permeability: a model for the resistance of the zonula occludens. J Membr Biol. 1978;32:219–232. doi: 10.1007/BF01870332. [DOI] [PubMed] [Google Scholar]
- 16.Cookson S T, Nataro J P. Characterization of Hep-2 cell projection formation induced by diffusely adherent Escherichia coli. Microb Pathog. 1996;21:421–434. doi: 10.1006/mpat.1996.0073. [DOI] [PubMed] [Google Scholar]
- 17.Cravioto A, Tello A, Navarro A, Ruiz J, Villafan H, Uribe F, Eslava C. Association of Escherichia coli HEp-2 adherence patterns with type and duration of diarrhoea. Lancet. 1991;337:262–264. doi: 10.1016/0140-6736(91)90868-p. [DOI] [PubMed] [Google Scholar]
- 18.Czezulin J R, Whittam T S, Henderson I R, Navarro-Garcia F, Nataro J P. Phylogenetic analysis of enteroaggregative and diffusely adherent Escherichia coli. Infect Immun. 1999;67:2692–2699. doi: 10.1128/iai.67.6.2692-2699.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Denker B M, Nigam S K. Molecular structure and assembly of the tight junction. Am J Physiol. 1998;274:F1–F9. doi: 10.1152/ajprenal.1998.274.1.F1. [DOI] [PubMed] [Google Scholar]
- 20.Diammond J M. Twenty-first Bowditch lecture: the epithelial junction: bridge, gate, and fence. Physiologist. 1977;20:10–18. [PubMed] [Google Scholar]
- 21.Fath K R, Mamajiwalla S N, Burgess D R. The cytoskeleton in development of epithelial cell polarity. J Cell Sci. 1993;17:65–73. doi: 10.1242/jcs.1993.supplement_17.10. [DOI] [PubMed] [Google Scholar]
- 22.Finlay B B, Falkow S. Salmonella interactions with polarized human intestinal Caco-2 epithelial cells. J Infect Dis. 1990;162:1096–1106. doi: 10.1093/infdis/162.5.1096. [DOI] [PubMed] [Google Scholar]
- 23.Finlay B B, Falkow S. Common themes in microbial pathogenicity revisited. Microbiol Mol Biol Rev. 1997;61:136–169. doi: 10.1128/mmbr.61.2.136-169.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fogh J, Fogh J M, Orfeo T. One hundred and twenty seven cultured human tumor cell lines producing tumors in nude mice. J Natl Cancer Inst. 1977;59:221–226. doi: 10.1093/jnci/59.1.221. [DOI] [PubMed] [Google Scholar]
- 25.Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsusika S, Tsusika S. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123:1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garcia M I, Gounon P, Courcoux P, Labigne A, Le Bouguenec C. The afimbrial adhesive sheath encoded by the afa-3 gene cluster of pathogenic Escherichia coli is composed of two adhesins. Mol Microbiol. 1996;19:683–693. doi: 10.1046/j.1365-2958.1996.394935.x. [DOI] [PubMed] [Google Scholar]
- 27.Geiger B, Ayalon O. Cadherins. Annu Rev Cell Biol. 1992;8:307–332. doi: 10.1146/annurev.cb.08.110192.001515. [DOI] [PubMed] [Google Scholar]
- 28.Giron J A, Jones T, Millan Velasco F, Castro Munoz E, Zarate L, Fry J, Frankel G, Moseley S L, Baudry B, Kaper J B, Schoolnick G K, Riley L W. Diffuse-adhering Escherichia coli (DAEC) as a putative cause of diarrhea in Mayan children in Mexico. J Infect Dis. 1991;163:507–513. doi: 10.1093/infdis/163.3.507. [DOI] [PubMed] [Google Scholar]
- 29.Grasset E, Pinto M, Dussaulx E, Zweibaum A, Desjeux J F. Epithelial properties of the human colonic carcinoma cell line Caco-2: electrical parameters. Am J Physiol. 1984;247:C260–C267. doi: 10.1152/ajpcell.1984.247.3.C260. [DOI] [PubMed] [Google Scholar]
- 30.Gumbiner G M. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345–357. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- 31.Haskin J, Gu L, Wittchen E S, Hibbard J, Stevenson B R. ZO-3, a novel member of the MAGUK protein family found at the tight junction, interacts with ZO-1 and occludin. J Cell Biol. 1998;141:199–208. doi: 10.1083/jcb.141.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jallat C, Livrelli V, Darfeuille-Michaud A, Rich C, Joly B. Escherichia coli strain involved in diarrhea in France: high prevalence and heterogeneity of diffusely adhering strains. J Clin Microbiol. 1993;31:2031–2037. doi: 10.1128/jcm.31.8.2031-2037.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jepson M A, Collares-Buzato C B, Clark M A, Hirst B H, Simmons N L. Rapid disruption of epithelial barrier function by Salmonella typhimurium is associated with structural modification of intercellular junctions. Infect Immun. 1995;63:356–359. doi: 10.1128/iai.63.1.356-359.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jou T-Z, Schneeberger E E, Nelson W J. Structural and functional regulation of tight junctions by RhoA and Rac 1 small GTPases. J Cell Biol. 1998;13:101–115. doi: 10.1083/jcb.142.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Labigne-Roussel A, Schmidt M A, Waltz W, Falkow S. Genetic organization of the afimbrial adhesin operon and nucleotide sequence from a uropathogenic Escherichia coli gene encoding an afimbrial adhesin. J Bacteriol. 1985;162:1285–1292. doi: 10.1128/jb.162.3.1285-1292.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Le Bouguenec C, Garcia M I, Ouin V, Desperrier J M, Gounon P, Labigne A. Characterization of plasmid-borne afa-3 gene clusters encoding afimbrial adhesins expressed by Escherichia coli strains associated with intestinal or urinary tract infections. Infect Immun. 1993;61:5106–5114. doi: 10.1128/iai.61.12.5106-5114.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levine M M, Ferreccio C, Prado V, Cayazzo M, Abrego P, Martinez J, Maggi L, Baldini M M, Martin W, Maneval D, Kay B, Guers L, Lior H, Watermann S S, Nataro J P. Epidemiologic studies of Escherichia coli diarrheal infections in a low socioeconomic level peri-urban community in Santiago, Chile. Lancet. 1993;i:1119–1122. doi: 10.1093/oxfordjournals.aje.a116788. [DOI] [PubMed] [Google Scholar]
- 38.Li Z, Elliott E, Payne J, Isaacs J, Gunning P, O'Loughlin E V. Shiga toxin-producing Escherichia coli can impair T84 cell structure and function without inducing attaching/effacing lesions. Infect Immun. 1999;67:5938–5945. doi: 10.1128/iai.67.11.5938-5945.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Loomis W P, Moseley S L. Translational control of mRNA processing in the F1845 fimbrial operon of Escherichia coli. Mol Microbiol. 1998;30:843–853. doi: 10.1046/j.1365-2958.1998.01117.x. [DOI] [PubMed] [Google Scholar]
- 40.Louvard D, Kedinger M, Hauri H P. The differentiating intestinal epithelial cell: establishment and maintenance of functions through interactions between cellular structures. Annu Rev Cell Biol. 1992;8:157–195. doi: 10.1146/annurev.cb.08.110192.001105. [DOI] [PubMed] [Google Scholar]
- 41.Ma T Y, Hollander D, Tran L T, Nguyen D, Hoa N, Bhalla D. Cytoskeletal regulation of Caco-2 intestinal monolayer paracellular permeability. J Cell Physiol. 1995;164:533–545. doi: 10.1002/jcp.1041640311. [DOI] [PubMed] [Google Scholar]
- 42.Madara J L. Regulation of the movement of solutes across tight junctions. Annu Rev Physiol. 1998;60:143–159. doi: 10.1146/annurev.physiol.60.1.143. [DOI] [PubMed] [Google Scholar]
- 43.Madara J L, Barenberg D, Carlson S. Effects of cytochalasin D on occluding junctions of intestinal absorptive cells: further evidence that the cytoskeleton may influence paracellular permeability and junctional charge selectivity. J Cell Biol. 1986;102:2125–2136. doi: 10.1083/jcb.102.6.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marano C W, Lewis S A, Garulacan L A, Peralta Soler A, Mullin J M. Tumor necrosis factor-α increases sodium and chloride conductance across the tight junction of CACO-2 BBE, a human intestinal epithelial cell line. J Membrane Biol. 1998;161:263–274. doi: 10.1007/s002329900333. [DOI] [PubMed] [Google Scholar]
- 45.McCarthy K M, Share I B, Stankewitch M C, Furuse M, Tsukita S, Rogers R A, Lynch R D, Schneeberger E E. Occludin is a functional component of the tight junction. J Cell Sci. 1996;109:2287–2298. doi: 10.1242/jcs.109.9.2287. [DOI] [PubMed] [Google Scholar]
- 46.Menconi M J, Salzman A L, Unno N, Ezzel R M, Casey D M, Brown D A, Tsuji Y, Fink M P. Acidosis induces hyperpermeability in Caco-2BBe cultured intestinal epithelial monolayers. Am J Physiol. 1997;272:G1007–G1021. doi: 10.1152/ajpgi.1997.272.5.G1007. [DOI] [PubMed] [Google Scholar]
- 47.Nataro J P, Kaper J B. Diarrheagenic Escherichia coli. Clin Microbiol Rev. 1998;11:403–503. doi: 10.1128/cmr.11.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nowicki B, Hart A, Coyne K E, Lublin D M, Nowicki S. Short consensus repeat-3 domain of recombinant decay-accelerating factor is recognized by Escherichia coli recombinant Dr adhesin in a model of cell-cell interaction. J Exp Med. 1993;178:2115–2121. doi: 10.1084/jem.178.6.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nowicki B, Moulds J, Hull R, Hull S. A hemagglutinin of uropathogenic Escherichia coli recognizes the Dr blood group antigen. Infect Immun. 1988;56:1057–1060. doi: 10.1128/iai.56.5.1057-1060.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nusrat A, Giry M, Turner J R, Colgan S P, Parkos C A, Carnes D, Lemichez E, Boquet P, Madara J L. Rho protein regulates tight junctions and perijunctional actin organization in polarized epithelia. Proc Natl Acad Sci USA. 1995;92:10629–10633. doi: 10.1073/pnas.92.23.10629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Peiffer I, Servin A L, Bernet-Camard M-F. Piracy of decay-accelerating factor (DAF-CD55) signal transduction by the diffusely adhering strain Escherichia coli C1845 promotes cytoskeletal F-actin rearrangements in cultured human intestinal INT407 cells. Infect Immun. 1998;66:4036–4042. doi: 10.1128/iai.66.9.4036-4042.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pham T, Kaul A, Hart A, Goluszko P, Moulds J, Nowicki S, Lublin D M, Nowicki B J. Dra-related X adhesins of gestational pyelonephritis-associated Escherichia coli recognize SCR-3 and SCR-4 domains of recombinant decay-accelerating factor. Infect Immun. 1995;63:1663–1668. doi: 10.1128/iai.63.5.1663-1668.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Philpott D J, McKay D M, Mak W, Perdue M H, Sherman P M. Signal transduction pathways involved in enterohemorrhagic Escherichia coli-induced alterations in T84 epithelial permeability. Infect Immun. 1998;66:1680–1687. doi: 10.1128/iai.66.4.1680-1687.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Philpott D J, McKay D M, Sherman P M, Perdue M H. Infection of T84 intestinal epithelial cells with enteropathogenic Escherichia coli alters barrier and transport functions. Am J Physiol. 1996;270:G634–G645. doi: 10.1152/ajpgi.1996.270.4.G634. [DOI] [PubMed] [Google Scholar]
- 55.Pinto M, Robine-Leon S, Appay M D, Kedinger M, Triadou N, Dussaulx E, Lacroix B, Simon-Assmann P, Haffen K, Fogh J, Zweibaum A. Enterocyte-like differentiation and polarization of the human colon carcinoma cell line Caco-2 in culture. Biol Cell. 1983;47:323–330. [Google Scholar]
- 56.Rao R K, Baker R D, Baker S S, Gupta A, Holycross M. Oxidant-induced disruption of intestinal epithelial barrier function: role of protein tyrosine phosphorylation. Am J Physiol. 1997;273:G812–G823. doi: 10.1152/ajpgi.1997.273.4.G812. [DOI] [PubMed] [Google Scholar]
- 57.Scaletsky I C A, Silva M L, Trabulsi L R. Distinctive patterns of adherence of enteropathogenic Escherichia coli to HeLa cells. Infect Immun. 1984;43:534–536. doi: 10.1128/iai.45.2.534-536.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sears C L, Kaper J B. Enteric bacterial toxins: mechanisms of action and linkage to intestinal secretion. Microbiol Rev. 1996;60:167–215. doi: 10.1128/mr.60.1.167-215.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stevenson B R, Siliciano J D, Mooseker M S, Goodenough D A. Identification of ZO-1: a high molecular weight polypeptide associated with the tight junctions (zonula occludens) in a variety of epithelia. J Cell Biol. 1986;103:755–766. doi: 10.1083/jcb.103.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Takeichi M. Cadherins: a molecular family important in selective cell-cell adhesion. Annu Rev Biochem. 1990;59:237–252. doi: 10.1146/annurev.bi.59.070190.001321. [DOI] [PubMed] [Google Scholar]
- 61.Wachter C, Beinke C, Mattes M, Schmidt M A. Insertion of EspD into epithelial target cell membranes by infecting enteropathogenic Escherichia coli. Mol Microbiol. 1999;31:1695–1707. doi: 10.1046/j.1365-2958.1999.01303.x. [DOI] [PubMed] [Google Scholar]
- 62.Yamamoto T, Wakisaka N, Nakae T, Kamano T, Serichantalergs O, Echeverria P. Characterization of a novel hemagglutinin of diarrhea-associated Escherichia coli that has characteristics of diffusely adhering E. coli and enteraggregative E. coli. Infect Immun. 1996;64:3694–3702. doi: 10.1128/iai.64.9.3694-3702.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Youakim A, Adhieh M. Interferon-γ decreases barrier function in T84 cells by reducing ZO-1 levels and disrupting apical F-actin. Am J Physiol. 1999;276:G1279–G1288. doi: 10.1152/ajpgi.1999.276.5.G1279. [DOI] [PubMed] [Google Scholar]
- 64.Zabriskie T M, Klocke J A, Ireland C M, Marcus A H, Molinski T F, Faulkner D J, Xu C, Clardy J C. Jaspamide, a modified peptide from Jaspis sponge, with insecticidal and antifungal activity. J Am Chem Soc. 1986;108:3123–3124. [Google Scholar]
- 65.Zhang L, Foxman B, Tallman P, Cladera E, Le Bouguenec C, Marrs C F. Distribution of drb genes coding for Dr binding adhesins among uropathogenic and fecal Escherichia coli isolates and identification of new subtypes. Infect Immun. 1997;65:2011–2018. doi: 10.1128/iai.65.6.2011-2018.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zweibaum A, Laburthe M, Grasset E, Louvard D. Use of cultured cell lines in studies of intestinal cell differentiation and function. In: Schultz S J, Field M, Frizell R A, editors. Handbook of physiology. The gastrointestinal system, vol. IV. Intestinal absorption and secretion. Bethesda, Md: American Physiological Society; 1991. pp. 223–255. [Google Scholar]