Abstract
Background
Screening and diagnostic assessments tools for autism spectrum disorder (ASD) are important to administer during childhood to facilitate timely entry into intervention services that can promote developmental outcomes across the lifespan. However, assessment services are not always readily available to families, as they require significant time and resources. Currently, in-person screening and diagnostic assessments for ASD are limited due to the COVID-19 pandemic and will continue to be a concern for situations that limit in-person contact. Thus, it is important to expand the modalities in which child assessments are provided, including the use of technology.
Aims
This systematic review aims to identify technologies that screen or assess for ASD in 0–12 year-old children, summarizing the current state of the field and suggesting future directions.
Methods
An electronic database search was conducted to gather relevant articles to synthesize for this review.
Outcomes and results
16 studies reported use of novel technology to assess children suspected of ASD.
Conclusions and implications
Results strongly supported live-video evaluations, video observations, and online or phone methods, but there is a need for research targeting the feasibility of these methods as it applies to the stay-at-home orders required by the pandemic, and other situations that limit clients from seeing providers in-person.
Keywords: Autism, Technology, Telehealth, Assessment, Diagnosis
What this paper adds?
This systematic review aims to understand the current technology-based screenings and assessments for children who are suspected to have ASD. Given the limitations of the COVID-19 pandemic and future situations with similar restrictions, there is strong need to incorporate telehealth into assessment administration. Additionally, these methods have important potential beyond the pandemic for improving access to underserved communities. The 16 studies discuss promising results that support the effectiveness of using technology-based tools as it applies to clinical and remote settings, but more research is necessary to further examine these tools.
1. Introduction
The COVID-19 pandemic has exposed the urgent need for telehealth technologies that can aid in the identification of mental and behavioral health concerns. Due to public health restrictions, it has become difficult, and in many cases impossible, to see clients in person and complete behavioral observations that are often crucial to the diagnostic process. This presents a particular challenge for the screening and assessment of autism spectrum disorder (ASD; Maenner, Shaw, & Baio, 2020). Currently, a gold standard autism evaluation includes parent interview, such as the Autism Diagnostic Interview-Revised, (ADI-R; Lord, Rutter, & Le Couteur, 1994) and an in-person behavioral observation, such as the Autism Diagnostic Observation Schedule, 2nd edition (ADOS-2; Lord et al., 2012). Because of difficulties accessing in-person observations, children are likely to experience delays in diagnosis, which is especially exacerbated in underserved communities, who are at a higher risk for experiencing health disparities during the pandemic beyond the current barriers of accessibility (e.g., financial challenges, geographic isolation, lack of resources) (Janvier et al., 2016). Furthermore, clinics will likely develop backlogs that delay ASD assessment and treatment.
Delayed ASD diagnosis is a public health concern, given the preponderance of evidence that early ASD identification and intervention are important for achieving positive outcomes (Mandell, Novak, & Zubritsky, 2005). As a result, it is critical that ASD assessment adapt to this changing landscape through innovation in screening and assessment strategies. A previous systematic review by our research team identified technologies that have been developed for remote diagnostic screening and assessment of early signs of ASD from ages 0–3 years, to identify tools conducted across distances and outside of a clinic setting (Dahiya, McDonnell, DeLucia, & Scarpa, 2020). In the current review, we aim to expand on these findings, in order to provide a comprehensive understanding of autism screening and assessment (hereby referred to as assessments) strategies using communication technology.
1.1. Current review
The current review aims to synthesize all papers using communication and information technology (e.g., videos, online tools, mobile applications, phones, tablets) to identify ASD signs or symptoms. This will extend our previous review by (1) including a broader age range of children 0–12 years, and (2) focusing on a wider range of technologies not captured in our prior review. The papers from the prior review were included in if they met inclusion criteria to provide a comprehensive synthesis of these assessments. The goals of the current review were to (1) examine information and communication technology as a method for delivering ASD assessments to children from ages 0–12 years, including diagnostic and screening accuracy and user satisfaction, (2) examine the representativeness of research in this area by reporting socio-demographic factors of participants, and (3) discuss implications for future development of technology-based ASD assessment methods and implementation during the pandemic. By identifying technologies that screen for or diagnose ASD, this paper intends to summarize the current state of the field and suggest future directions.
2. Method
2.1. Search strategy
A literature search was conducted using two electronic databases (EBSCOhost/PsychINFO and PubMed) during June and July 2020. Specific terms (see supplemental materials for the list of search terms) involved a combination of autism diagnostic labels, assessment or screening terms (e.g., assessment, tool, screening, evaluation, etc.), ASD signs (e.g., social orienting, imitation, repetitive behaviors, etc.), and technology terms (e.g., technology, phone, video, mobile, online, etc.). The search was filtered by age, with a focus on participants aged 0–12 years. The search yielded a total of 3360 articles, which were screened to eliminate duplicates, review papers, posters, presentations, study protocols, dissertation, or theses.
2.2. Selection criteria
Abstracts and full-text articles were screened independently by two investigators (AD & ED) to determine if they met inclusion criteria, including: (1) published in English in a peer reviewed journal, (2) included a population of children between ages 0–12 years, (3) included participants who were suspected to have ASD, (4) used some form of information and communication technology as a diagnostic assessment or screening tool (e.g., videos, online tools, mobile applications, phones, tablets, etc.), and (5) determined diagnostic criteria based on the Diagnostic Statistical Manual (DSM) IV or above (American Psychiatric Association, 2000, 2013). Any disagreements among investigators were resolved by consensus.
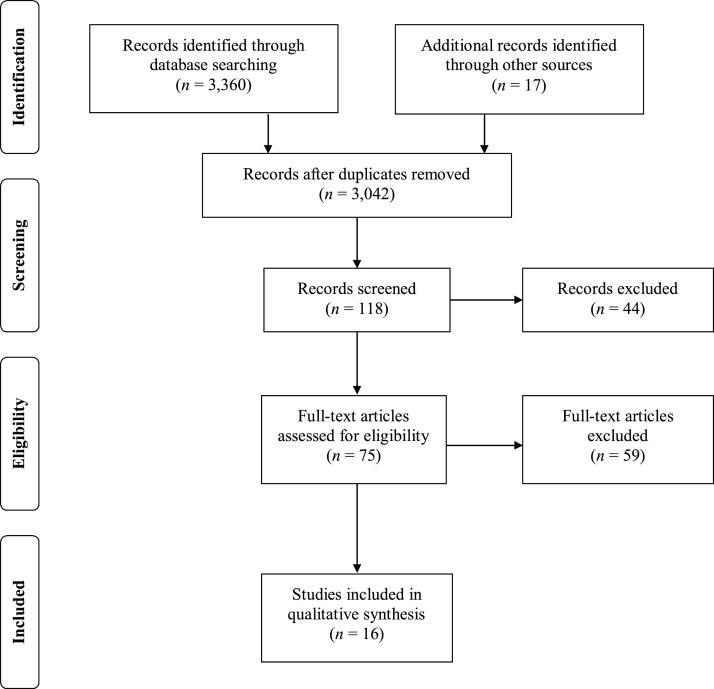
Studies were excluded if they met any of the following criteria: (1) single-subject designs/case studies, (2) treatment studies, (3) lab-based studies (e.g., eye tracking), or (4) retrospective video analysis studies. This comprehensive review resulted in 16 articles. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA; Moher, Liberati, Tetzlaff, Altman, & The PRISMA Group, 2009) guidelines were used to determine study inclusion (see Fig. 1 ).
Fig. 1.
PRISMA flow diagram for determining study inclusion.
3. Results
3.1. Demographic information
Across the 16 studies examined, 10 conducted research on technology-based screening tools for ASD, whereas six utilized technology for ASD diagnostic assessments. Overall, a total of 1584 children participated in some form of technology-based ASD assessment, with participant ages ranging from 18 months to 12 years across samples. Race and ethnic background varied across studies, with three studies not reporting a breakdown of ethnicities. Of the other 13 studies, nine noted participants that were primarily White, as many assessments took place in Southern or Midwestern United States (e.g., Kansas, Tennessee, Ohio). Two studies included a majority of African or African American participants (Maleka, Van Der Linde, Page Glascoe, & Swanepoel, 2016; Obeid, Beekman, Roizen, Ciccia, & Short, 2019). Smith et al. (2017) reported that 41 % of their participants were White and 43 % were Hispanic. Another study consisted of predominantly Hispanic participants (97 %), which took place in a clinic that caters to underserved neighborhoods of Los Angeles, California (Nelson et al., 2019). See Table 1 for a breakdown of demographic data.
Table 1.
Demographic Variables of Child Participants.
| Article | N | Mean Age in Years; yrs. or Months; mo. (SD) | Gender (% male) | Location | Race/Ethnicity (%) |
|---|---|---|---|---|---|
| Ben-Sasson et al. (2018) | 115 | 25.06 mo. (4.46) | 58.3 | Israel | Not Reported |
| Bishop et al. (2017) | ASD: 159 Non-ASD: 130 |
ASD: 8.15 yrs. Non-ASD: 8.4 yrs. |
ASD: 76.1 Non-ASD: 66.2 |
Michigan & Ohio, U.S.A. | W: 71 (ASD); 57.7 (Non-ASD) B: 8.8 (ASD); 29.2 (Non-ASD) Other: 15.1 (ASD); 13.1 (Non-ASD) |
| Chambers et al. (2017) | Total: 26 ASD: 10 Non-ASD: 16 |
ASD: 38.03 mo. (5.28) Non-ASD: 22.79 mo. (4.82) |
ASD: 70 Non-ASD: 81 |
South Africa | KwaZulu-Natal province in South Africa |
| Corona, Weitlauf et al. (2020) | Total: 51 ASD: 35 DD: 10 TD: 6 |
Total: 2.49 yrs. (0.35) ASD: 2.51 yrs. (0.32) DD: 2.43 yrs. (0.46) TD: 2.45 yrs. (0.37) |
Total: 70.6 ASD: 74 DD: 70 TD: 50 |
Tennessee, U.S.A. | W: 63 (ASD); 60 (DD); 67 (TD) B: 17 (ASD); 30 (DD); 17 (TD)H: 9 (ASD) Other: 20 (ASD); 10 (DD); 17 (TD) |
| Duda et al. (2016) | Total: 222 ASD: 60 Non-ASD: 109 |
5.8 yrs. (4.6) | 76.15 | Massachusetts, U.S.A. | Not reported |
| Gabrielsen et al. (2015) | Total: 42 ASD: 14 LD: 14 TD: 14 |
Total: 23.4 mo. (5.2) ASD: 22.7 mo. (4.8) LD: 23.0 mo. (5.5) TD: 24.5 mo. (5.5) |
Overall: 71 ASD: 86 Language:64 TD: 64 |
Suburban pediatric practice in Utah, U.S.A. | W: 57 B: 2 NA: 2 H: 29 PI: 7 A: 2 |
| Juárez et al. (2018) | Study 1: 20 Study 2: 45 |
Study 1: 26.7 mo. (4.49) Study 2: 26.8 mo. (3.12) |
Study 1: 80 Study 2:77.78 |
Tennessee, U.S.A. Study 1: University- Medical Center Study 2: RuralRegional Health Center |
Study 1: not reported Study 2: W: 66.67 B: 20 H: 6.67 Other: 6.67 |
| Maleka et al., 2016) | 207 | 1.94 yrs. (1.35) | Not reported | South Africa | B: 99.9 Other: 0.5 |
| Morgan et al. (2008) | ASD: 50 DD: 25 TD: 50 |
ASD: 44.18 mo. (14.09) DD: 47.33 mo. (14.51) |
ASD: 86 DD: 76 TD: 86 |
Not Reported | W: 72 (ASD); 68 (DD); 84 (TD) B: 16 (ASD); 20 (DD); 14 (TD) H: 8 (ASD); 8 (DD); 2 (TD) A: 4 (ASD); 4 (DD) |
| Nelson et al. (2019) | 152 | 24.5 mo. (8.8.) | 76 | Underserved areas in Los Angeles, CA, U.S.A. | H: 97 W: 1 B: 2 Other: 1 |
| Obeid et al. (2019) | 49 | 39 mo. | 49 | Cleveland, Ohio, U.S.A. | B: 93 |
| Other: 7 | |||||
| Reese et al. (2013) | Total: 21 ASD: 11 DD: 10 |
Not reported: Ages ranged from 3−5 yrs. | 85.7 | Kansas, U.S.A. | W: 90.4 B: 4.8 Other: 4.8 |
| Reese et al. (2015) | 17 | 4.47 yrs. | 70.6 | Kansas, U.S.A. | W: 88.2 B: 5.9 H: 5.9 |
| Smith et al. (2017) | Total: 51 ASD: 41 TD: 11 |
50.6 mo. (16.84) | 70.6 | Southwestern area of U.S.A. | W: 41.2 B: 7.8 H: 43.1 Other: 7.8 |
| Sturner et al. (2016) | 98 | 22.9 mo. (6.1) | 74.5 | Baltimore, MD, U.S.A. (Kennedy Krieger Institute) | W: 65.3 B: 11.2 A: 5.1 NA: 2 Other: 16.3 % |
| Thomas et al. (2016) | 54 | 18 mo. | 48 | Family medicine clinic in Calgary, Alberta, Canada | Not Reported |
Note. A: Asian; ASD: Autism Spectrum Disorder; B: Black/African American; DD: Developmental Delay; F: Female; H: Hispanic; LD: Language Delay; M: Male; NA: Native American; PI: Pacific Islander; TD: Typically Developing; W: White.
3.2. Technology types
The 16 articles were grouped by type of technology used to assess or screen for ASD. Two studies utilized phone interviews (Bishop et al., 2017; Nelson et al., 2019), six studies promoted use of web-based tools (Ben-Sasson, Robins, & Yom-Tov, 2018; Duda, Daniels, & Wall, 2016; Maleka et al., 2016; Obeid et al., 2019; Sturner et al., 2016; Thomas, Spragins, Mazloum, Cronkhite, & Maru, 2016), and four studies implemented live video evaluations to administer ASD screening or assessments (Corona, Weitlauf et al., 2020; Juárez et al., 2018; Reese et al., 2013, 2015), while four studies completed video observations that did not contain live diagnostic measurements (Chambers et al., 2017; Morgan, Wetherby, & Barber, 2008; Smith et al., 2017). See Table 2 for details.
Table 2.
Description of technology and sample type, research design, signs assessed, assessment tools, and outcomes.
| Article | Screening | Diagnostic | Sample Type | ResearchDesign | Signs of ASD Assessed | Tele-Assessment Tools | Outcomes |
| Ben-Sasson et al. (2018) | Online/Web-Based Source | Parents who endorsed concerns about child's social & communication development | Parents reported via interview and completed an online screening form. Machine learning was used to predict ASD risk based on parent narrative alone or parent narrative plus one additional screening question. |
Social-communication development |
M-CHAT-R/F M-CHAT-R ASQ |
Moderate correlations between expert & MCHAT-R/F (r= .36) & MCHAT-R (r=.43). The additional screening question improved accuracy vs. the parent narrative alone. |
|
| Bishop et al. (2017) | Phone Interview | Clinical sample (validation phase) | Measure development was based on an adaptation of the ADI-R. Parents completed the interview over the phone prior to IPA. |
Language delays ASD characteristics: direct gaze, nodding, gesture, response to name, group play, circumscribed interests. |
ASI ADI-R items |
Verbal algorithm: Sensitivity: .87, Specificity: .62. Positive predictive value: .72, Negative predictive value: .81. | |
| Chambers et al. (2017) | Video Observations | Parents with no developmental concerns (recruited by community) Parents of children at risk/suspected to have ASD (recruited through autism society & health center) |
Families were seen in the home for the ESAC, CSBS, and home evaluation, and in the clinic for the ADOS-2 South African & U.S. based study teams watched videos of CSBS, home observation, and ADOS to made a clinical diagnosis. |
Eye gaze Communication Gestures Sounds Words Understanding use of objects |
ESAC CSBS SORF ADOS-2 Naturalistic home observation |
The U.S. & South African teams had 100% diagnostic agreement. There was a significant group difference on the ESAC, CSBS, and SORF on the CSBS and the home observation. The ASD group showed significantly more red flags in CSBS vs. home observation. |
|
| Corona, Weitlauf et al. (2020) | Live Video Evaluations | Clinical sample | Participants were randomized into one of two tele-screening protocols (TELE-STAT or TELE-ASD-PEDS). | Directed speech/sounds Eye contact Odd vocalizations RRBs Gestures Speech |
TELE-STAT TELE-ASD-PEDS |
Remote clinicians had 86% diagnostic agreement. Parents reported feeling comfortable during the tele-screening and stated that the instructions for working with the remote assessor were easy to follow. No significant differences between both. |
|
| Duda et al. (2016) | Online/Web Based Source | Clinical sample | Parents were asked to answer a 7-item measure of ASD symptoms. Answers were run through a machine learning program to identify typical vs atypical development. | Communication Social Skills Play |
MARA |
N=25 received an ASD diagnosis. Sensitivity: 89.9%, Specificity: 79.7% |
|
| Gabrielsen et al. (2015) | Video Observations | Community sample (middle to lower SES families from diverse racial and ethnic backgrounds) | Children were grouped into three categories: suspected ASD, suspected LD without ASD, and TD. |
Social responding Vocalization Play Social initiation Response to name |
ADOS-2 (from two 10-minute segments at minute 0 to 10 and minute 30 to 40) | Differences in social responding quality, repetitive sounds, & response to name. Rater judgment on referral was most inaccurate for ASD group. Sensitivity of ASD: 61%; Specificity: 82%; Positive predictive value: 63%; Negative predictive value: 81% |
|
| Juárez et al. (2018) | Live Video Evaluations | Clinically referred sample | Study 1: Compared IPA (including MSEL, VABS-II, and ADOS-2) vs. remote assessment conducted by a trained research assistant while a remote psychologist observed. Study 2: Compared IPA vs. remote assessment conducted by early intervention provider while a remote psychologist observed. |
Play Imitation Directing attention Requests |
Medical/psychosocial interview STAT DSM-5 ASD diagnostic interview for toddlers |
Study 1: Telemedicine assessment sensitivity: 78.95%. Psychologists were "certain" or "very certain" of their diagnosis in 75% of telemedicine cases. Study 2: Psychologists provided diagnoses for 64.44% of children, ruled out ASD in 22% and deferred 13.33% for a full evaluation. Psychologists reported satisfaction with telemedicine 80% of the time. 91% of families reported being very satisfied with the remote assessment. |
|
| Maleka et al. (2016) | Online/Web Based Source | Community-based primary healthcare clinic | The PEDS was administered by speech language pathologists, (SLPs) via pen-and-paper and community health workers (CHWs) via smartphone. | Language Motor Skills Self-Help Academics Social-Emotional health |
PEDS PEDS-DM |
99% correspondence between paper (SLP) and smartphone (CHW) | |
| Morgan et al. (2008) | Video Observation | Clinically referred sample | Children with a communication delay were assigned to the ASD or DD group. | Social communication Repetitive, stereotyped movements |
CSBS (at age 2 years) RSMS ADOS |
The RSMS significantly correlated with the ADOS SA domain in the ASD (.32) & DD (.26) groups. The RSMS body movements significantly predicted the ADOS SA & RRB domains. |
|
| Nelson et al. (2019) | Phone Interview | Clinical sample (recruited through primary care clinics & were not enrolled in intervention for DD) | Families were randomized to an intervention or control group. This was followed by a phone screen interview conducted by a trained RA (control) or by a 211LA care coordinator (intervention). Children with moderate or high developmental risk were referred for an evaluation. |
Developmental or behavioral concerns | Structured interview PHDS PEDS PEDS Developmental Milestones (PEDS-DM) M-CHAT-R |
More children in the control group met high-risk criteria on the PEDS but there was no statistically significant difference compared to the intervention group. There was a statistically significant difference between groups for evaluation referrals, completed evaluations, eligibility for services, and receipt of services, with more care in the intervention group. |
|
| Obeid et al. (2019) | Online/Web Based Source | Community-based urban health clinic for minority, low-income families | Social skills Speech Symbolic play Play performance |
INvesT application | N= 6 with developmental concerns; n=26 with high risk for developmental delay; n=18 with high risk for specific developmental delays | ||
| Reese et al. (2013) | Live Video Evaluation | Clinical sample | Participants were randomized to either IPA or IVC. Parents were instructed on how to administer ADOS presses/social bids either via IPA or IVC. | Items from the ADOS & the ADI-R were used to assess ASD symptom domains. | ADOS ADI-R |
No significant differences were reported between IPA & IVC on both the ADOS and the ADI-R. Clinicians had nearly 100% diagnostic agreement (on 20 of 21 participants). Parents reported high levels of satisfaction on both IPA & IVC. |
|
| Reese et al. (2015) | Live Video Evaluation | Clinical sample | Participants were randomized to either IPA or IVC, and were screened with a BASC-2 and ASQ before their study visit. | Items from the ADOS & the ADI-R were used to assess ASD symptom domains and elicit behaviors related to ASD. | Unstructured 20-minute play observation Modified ADOS-2 activities Modified ADI-R interview Medical & family history |
IPA diagnostic accuracy: 82% Specificity: 78% Sensitivity: 88% IVC diagnostic accuracy: 86% Specificity: 88% Sensitivity: 83% |
|
| Smith et al., (2017) | Video Observations | Clinical sample | Participants received both IPA and NODA. Agreement between the IPA and NODA, as well as sensitivity and specificity of NODA, were assessed. | Social impairment Verbal/nonverbal impairment RRBs |
IPA: ADI-R ADOS-2 VABS-III NODA: Developmental history 4 pre-recorded 10-min videos of child |
88.2% agreement between IPA and NODA (kappa =.75) Sensitivity of NODA 84.9%; specificity of NODA 94.4% |
|
| Sturner et al. (2016) | Online/Web-Based Source | Community sample | PCPs and trained RAs completed a follow up interview with online platform for all children who screened positive on the MCHAT. | Scores from the ADOS-2 and MSEL were compared with the MCHAT/F scores | M-CHAT/F (with online follow up) | 86% agreement on the M-CHAT/F between PCPs & RAs No differences on accuracy, sensitivity, specificity, or positive predicted values between PCPs and trained RAs. |
|
| Thomas et al. (2016) | Online/Web-Based Source | Community sample | Children were randomized to "usual care" or evidence-based screening via computer questionnaires. In the evidence-based group, parents completed screening questionnaires on a computer during an 18-month PCP visit. |
Gross motor skills Expressive/receptive language Fine motor skills Social emotional skills Other developmental domains |
M-CHAT PEDS PEDS-DM |
No referrals were made in the evidence-based group for high risk for developmental disabilities. No differences were noted in the referral rates between the “usual care” and evidence-based groups. However, three children from each group required a 3 mo. follow-up. |
Note. 211LA: 2-1-1- Los Angeles County; ADI-R: Autism Diagnostic Interview-Revised; ADOS-2: Autism Diagnostic Observation Schedule, 2nd edition; ASI: Autism Symptom Interview; ASQ: Ages and Stages Questionnaire; BASC-2: Behavior Assessment System for Children, 2nd Edition; CSBS: Communication and Symbolic Behavior Scales; ESAC: Early Screening for Autism and Communication Disorders; IPA: In-Person Assessment; IVC: Interactive Video Conferencing; LD: Language delay; MARA: Mobile Autism Risk Assessment; M-CHAT: Modified Checklist for Autism in Toddlers; M-CHAT/F: Modified Checklist for Autism in Toddlers with Follow-Up Interview; M-CHAT-R/F: Modified Checklist for Autism in Toddlers-Revised, with Follow Up; MSEL: Mullen Scales of Early Learning; NODA: Naturalistic Observation Diagnostic Assessment; NV: Non-verbal; PCP: Primary care physician; PEDS: Parents Evaluation of Developmental Status; PEDS-DM: PEDS-Developmental Milestone; PHDS: Promoting Healthy Development Survey; RA: Research Assistant; RRB: Restricted & Repetitive Behaviors; RSMS: Repetitive and Stereotyped Movement Scales: Companion to the CSBS; SA: Social Affect; SES: Socioeconomic status; SORF: Systematic Observation of Red Flags; TELE-ASD-PEDS: A tool for telemedicine-based assessments for ASD in children under 36 months of age; TELE-STAT: Telehealth Screening Tool for Autism in Toddlers and Young Children; TD: Typically developing; VABS-II: Vineland Adaptive Behavior Scale, 2nd Edition; VABS-III: Vineland Adaptive Behavior Scale, 3rd Edition.
3.3. Signs of ASD assessed
The reviewed articles primarily examined symptoms or characteristics pulled from standardized assessment protocols (i.e., ADOS-2, ADI-R) or screening tools (i.e., PEDS, M-CHAT; Robins et al., 2014). Specifically, some studies directly applied items from the ADOS and ADI-R in a live video evaluation method (coached by a clinician) compared to in-person administration. The video observation studies targeted a range of features including emotions, eye gaze, communication, gestures, and repetitive, stereotyped movements (see Table 2).
The literature that examined online tools screened several signs of social and communication development, including eye contact, directed speech, gestures, as well as repetitive play or movements (Ben-Sasson et al., 2018) while other sources that were implemented in a community sample focused on online administration of well-established ASD screeners (Sturner et al., 2016; Thomas et al., 2016). The two phone-screen studies (Bishop et al., 2017; Nelson et al., 2019), evaluated broader domains of ASD symptoms, such as developmental or behavioral difficulties.
3.4. Study designs and outcomes
3.4.1. Live video evaluations
Evidence of the use of live video conferencing for assessments seems to be growing in light of the limitations of the COVID-19 pandemic, especially for families in remote areas or for those who otherwise cannot go to a hospital or clinic (e.g., being immune-compromised, disabled, or have no transportation). Reese et al. (2013, 2015) examined interactive videoconferencing (IVC) as a possible method for diagnostic assessments for children suspected to have ASD. The 2013 study evaluated toddlers from 3−5 years old using the ADOS and the ADI-R through IVC compared to the standard in-person assessment (IPA). During the IVC condition, parents were given instructions on how to provide ADOS presses for social behaviors via videoconference, and were also administered the ADI-R through this modality. In the IPA condition, parents were instructed on how to provide the ADOS presses in person, and they were also administered the ADI-R in person. No differences were noted between the IVC and IPA conditions on the ADOS observations, the ADI-R scores, and the nearly 100 % diagnostic accuracy. Additionally, parents reported high satisfaction ratings in both conditions.
Corona, Weitlauf et al. (2020), which is the most recent study examined in this review, compared two different tele-screening protocols, comparing the TELE-STAT (the remote version of the Screening Tool for Autism in Toddlers & Young Children;Stone & Ousley, 2008) and the TELE-ASD-PEDS (Corona, Hine et al., 2020), which have both been widely disseminated since the start of the pandemic. It is important to note that the TELE-ASD-PEDS tool is not a telehealth adaptation of the PEDS developed by Brothers, Glascoe, and Robertshaw (2008), which is a tool that is discussed later in this review. Participants were randomized to complete one of these protocols. The remote clinicians who administered these screeners reported 86 % diagnostic agreement, with no significant difference between the screeners. Additionally, parents reported satisfaction and comfort with the remote screening tool.
In the 2015 study by Reese and colleagues, the same aforementioned conditions were examined in children between 2.5–6 years old. However, the evaluation procedures were slightly more comprehensive compared to the 2013 study. The protocol included a 20-minute unstructured play observation, facilitated ADOS-2 activities, a structured interview consisting of the algorithm items from the ADI-R, and collection of medical and family history. Parents were provided with details on each aspect of the assessment and were also instructed to watch a sixteen-minute video modeling the instructions for each item of the play observation. The IVC and IPA conditions differed based on who was present in the assessment room, such that the IVC participants were directed through the evaluation by means of the video-conferencing technology and the IPA participants were directed through an in-person evaluation. When compared to a full interdisciplinary diagnostic evaluation, the IVC condition reported an 86 % diagnostic accuracy with high specificity (88 %) and sensitivity (83 %) and the IPA condition reported an 82 % diagnostic accuracy with high specificity (78 %) and sensitivity (88 %).
Similar to Juárez et al. (2018), Reese et al. (2013, 2015) compared IPA to remote assessments to gather data on whether remote assessments can report high diagnostic accuracy when compared to the gold standard ASD assessment protocol. This study examined children between ages 20–34 months by setting up tele-evaluation rooms that permitted the remote assessor to observe and communicate through a video camera mounted in the assessment room. The procedure included a brief observation, the STAT, and a diagnostic interview. A licensed psychologist conducted interviews through the tele-evaluation rooms and observed the STAT through the same technology, while a trained research assistant or early intervention provider conducted the observation. Following this procedure, licensed psychologists reported their confidence in their diagnostic decisions as “certain” or “very certain” for 75 % of the participants. Additionally, the sensitivity of the telemedicine assessment was promising (78.9 %), indicating that this method can accurately diagnose significant proportion of children with ASD. Psychologists (80 %) and families (91 %) reported high levels of satisfaction with the remote assessment. These outcomes are similar to Reese et al. (2013, 2015), suggesting that video conferencing is likely comparable to standardized in-person evaluations for ASD.
3.4.2. Video observations
Although in-vivo conferencing is a promising method to observe an individual while guiding them through an evaluation, live observation is not always feasible. Chambers et al. (2017) examined previously recorded videos (as opposed to live video sessions) to achieve diagnostic accuracy for children in South Africa, specifically for those who did not speak English, and instead conducted the assessment in their native language of isiZulu. Parents of children from ages 12–48 months at risk of ASD were recruited for an evaluation conducted in their home, which included the Early Screening for Autism and Communication Disorder (ESAC; Wetherby et al., 2009), the Communication and Symbolic Behavior Scales-Developmental Profile Behavior Sample (CSBS; Wetherby & Prizant, 2002), the Systematic Observation of Red Flags of ASD (Wetherby et al., 2016), and a naturalistic home observation, all of which were conducted in the home by a trained speech-language pathologist and recorded to review and clarify a diagnostic decision. Both study teams in the United States and South Africa had 100 % diagnostic agreement among participants.
Gabrielsen et al. (2015) applied the video observation method to a community sample consisting of middle to lower class SES families of diverse backgrounds, in which children were grouped into categories of suspected ASD, suspected language delay (LD) without ASD, or typically developing (TD). Two 10-minute video samples from the ADOS-2 (administered by trained clinicians) were observed by licensed psychologists to assess for risk of ASD and rate five behavior categories: social responding, vocalization, play, social initiation, and response to name. Following each video clip, the raters were asked if they would refer the child for a full ASD assessment. Although there appeared to be some difficulty in detecting atypical behaviors across groups and a somewhat low sensitivity rate (61 %) was reported for ASD, there was a higher rate of specificity (82 %) in ruling-out an ASD diagnoses.
Although video observations can be used diagnostically to assess for ASD, it is also feasible to observe videos as a screening tool for specific signs of ASD and predict future diagnosis. Morgan et al. (2008) conducted a study to detect the presence of repetitive and stereotyped movements (RSM) in children with ASD or a developmental delay (DD) using videotaped behavior samples. When administering the CSBS behavior sample (administered by trained clinicians) to collect data on social communication in social, speech, and symbolic composites, a video was recorded in order to score RSM with body and RSM with objects using the Repetitive and Stereotyped Movements Scales: Companion to the CSBS (RSMS; Wetherby & Morgan, 2007). Both the rate of RSM occurrence and the variety of different RSM were coded from the video. The RSMS body movements significantly predicted scores on the SA and Restricted, Repetitive Behavior (RRB) domains of the ADOS. This supports the predictive value of this measure such that the RSMS can predict specific autism symptoms, suggesting that RSM can be present in children with ASD at less than 24 months of age. As such, utilizing video-based behavioral samples can assist with early detection of RRBs, which can be conducive to situations where there are restrictions to visiting a clinic.
A study from our previous review also examined the utility of video analysis for identifying signs of ASD. Smith et al. (2017) investigated a method called the Naturalistic Observation Diagnostic Assessment (NODA). The researchers asked parents to perform a series of “presses” similar in nature to what clinicians present in diagnostic observations, such as saying the child’s name to get their attention or interacting with them playfully. The parent recorded four 10-minute videos, which were then evaluated by trained clinicians.
3.4.3. Online or web-based tools
Parents often report concerns in their child’s development as early as 5–6 months, but they are not able to obtain an ASD diagnosis for their child until closer to 4 years old (Guinchat et al., 2016). That gap between age of concern and age of evaluation is critical in child development, and highlights the importance of screening, particularly for families who are unable to readily access diagnostic services. Ben-Sasson et al. (2018) examined the use of online screening measures through the M-CHAT-R/F (Robins et al., 2014) and the Ages and Stages Questionnaire (ASQ; Squires, Twombly, Bricker, & Potter, 2009). Participants included 115 children between 16–30 months, whose parents were asked to report on their child’s social-emotional development concerns and family history of ASD, followed by the online screeners. Clinicians provided a risk rating based on just the parent narrative, and a machine-learning approach was used to predict risk of ASD based on the parent narrative alone or the parent narrative plus an additional random screening question taken from the M-CHAT-R, using an algorithm. When comparing these approaches, findings revealed that the additional screening question improved diagnostic accuracy.
As opposed to the clinical sample in the previous study, Sturner et al. (2016) and Thomas et al. (2016) applied their online screeners to a community sample in a primary care setting. Thomas et al. (2016) utilized the Parents Evaluation of Developmental Status (PEDS) and the PEDS: DM (Development Milestones) in addition to the M-CHAT, to conduct a validated screening as part of an 18-month primary care visit for children. Participants were randomized to a “usual care” group or evidence-based screening group, in which the latter prompted parents to complete the above-mentioned screening questionnaires on a computer. In the evidence-based group, parents were able to complete the questionnaires within 10 min before their child’s appointment and could then identify more concerns when given the opportunity to do so. On the other hand, parents in the “usual care” group reported significantly more concerns later on, suggesting the importance of early screening for developmental problems to increase rates of detection as early as 18-months of age.
Sturner et al. (2016) also emphasized the importance of early screening via the use of the M-CHAT-Follow Up Interview (Robins, Fein, & Barton, 1999) through a web-based platform by conducting administrations at 18- and 24- month primary care visits and then comparing results to the ADOS-2 and Mullen Scales for Early Learning (MSEL; Mullen, 1995). For all children who screened positively on the M-CHAT, a primary care physician (PCP) and a trained RA conducted a follow-up interview through the online platform. Results revealed an 86 % agreement on the M-CHAT/F across both assessors, and no differences were noted in terms of diagnostic accuracy, sensitivity, or specificity. This suggests that it is possible to administer these developmental screeners through different methods, and it is essential to implement them as a routine part of general PCP visits, either through telemedicine or in person.
Three studies from our previous review also evaluated mobile and web applications. Duda et al. (2016) evaluated a mobile tool that screens for ASD using a series of multiple-choice questions. The study found that this screening method had a sensitivity of 89.9 % and specificity of 79.7 % for diagnosis of ASD. Similarly, Maleka et al. (2016) examined a mobile screening tool in South Africa, finding that a mobile version of the PEDS had high agreement with a pen-and-paper version when completed by community health workers. Finally, Obeid et al. (2019) created a web version of a model that categorized ASD risk in 12–36 month old children based on parent report of developmental concerns. However, it is unclear whether such technologies have successfully been applied to older children, and if they can be tested in a clinic setting to provide implications for use in remote areas.
3.4.4. Phone interviews
Because the ADI-R is a widely used, gold-standard autism assessment tool that gathers valuable information on an individual’s developmental history, Bishop et al. (2017) developed the Autism Symptom Interview (ASI) based on questions from the ADI-R, in order to provide a shorter measurement that lasts only 15−20 min and requires limited training. The ASI provides a “Yes/No” answer at the completion of the interview, indicating whether there is an indication of an ASD presentation. The validation of the ASI resulted in strong internal consistency of the algorithm items (.92). For the verbal algorithm, sensitivity was reported at .97, and specificity was .62. Additionally, the sample size for the nonverbal algorithm was too small to report these statistics, but 13 items were identified as being the best differentiating items. Overall, it appears that ASI can be a useful tool to screen children suspected to have ASD, but a standardized observation would likely still be required to make an accurate diagnostic decision, as this simplified screener is different from a diagnostic confirmation, since a standardized observation also assesses for current behaviors.
Nelson et al. (2019) utilized a somewhat different approach to phone screen interviews that categorized children as low, moderate, or high risk of developmental concerns as opposed to the ASI’s “Yes/No” approach. Additionally, this study utilized several screeners administered over the phone, including a structured interview asking about developmental concerns as well as socio-demographic and health information, the Promoting Healthy Development Survey (PHDS; Bethell, Peck, & Schor, 2001), the PEDS, the PEDS: DM, and the M-CHAT-R (Robins, Fein, & Barton, 2009). Those involved in coordinated care received more referrals, evaluations, eligibility appointments, and treatment services compared to the control group.
4. Discussion
The goal of this review was to understand the state of the current literature in regard to the use of information and communication technology to facilitate screening and diagnostic assessment for ASD in children without the use of in-person contact. The studies identified offer several promising methods for using technology assessments for ASD, including live video observations, delayed video observations, web and mobile tools, and phone screening interviews. The results of our review suggest that while technology-based ASD screening and assessment is in its infancy, these methods hold promise for improving access to diagnostic services. The changing landscape of clinical services in the context of the COVID-19 pandemic serves to highlight the urgency of future research in this field.
Several studies investigated in-vivo video observation as a strategy for assessing for ASD. This method is consistent with psychology’s current shift toward teletherapy (Wright & Caudill, 2020) and teleassessment (e.g. Farmer et al., 2020) during COVID-19. These studies largely utilized existing ASD screening and assessment tools, such as the ADOS, ADI-R, and STAT, modified for use via telehealth. On the whole, these studies provided very promising results for the in-vivo administration of assessment protocols via telehealth, had good diagnostic accuracy and sensitivity, and reported high levels of satisfaction with the in vivo procedure. These positive results suggest that in-vivo tele-assessments may provide a feasible and accurate alternative to in-person contact, which can be useful in the context of the pandemic. It is important to note that the studies that utilized in-vivo observation took place in a clinical setting with standard materials. As such, this may not be feasible during a pandemic lockdown, as many standardized materials often need to be sent to parents. Several of the studies had parents conduct the assessment procedures, and therefore face-to-face contact with providers can be minimized if more caregiver-facilitated interactions that do not require standardized materials are developed. In the context of COVID-19, more research in this area is necessary to understand whether similarly high rates of diagnostic accuracy can be found with at-home administrations.
Many studies also utilized video recordings that were observed at a later date, rather than live observed. This also proved to be a promising strategy. Relatively high levels of specificity are observable in several of the studies that used video recording (Chambers et al., 2017; Gabrielsen et al., 2015; Smith et al., 2017). However, Gabrielsen et al. reported lower sensitivity of identifying ASD from 20-minute video observations, suggesting that video review may be more effective as a screening method rather than as a diagnostic assessment. Furthermore, some early evidence suggests that video recording can also be used prospectively to screen for children with higher likelihood of receiving a later ASD diagnosis (Morgan et al., 2008). This finding is consistent with earlier research, which shows that it is possible to differentiate children who will be diagnosed with ASD through retrospective video analysis (Baranek, 1991; Osterling, Dawson, & Munson, 2002). Taken together, this evidence on video observation suggests that this technique may be a useful screening tool.
The third category of studies examined online and web applications for ASD screening. These studies utilized online administrations of screening questionnaires both at home and in primary care offices. The studies using these methods suggested that online screening tools for ASD are feasible in multiple settings. Ben-Sasson et al. (2018) found that administering a single question from the MCHAT-R in addition to asking parents to explain their concerns in narrative improved the accuracy of a machine-learning approach to assessing ASD likelihood, similar to the MARA tool (Duda et al., 2016). The INvesT model also utilized similar methods to gather information on developmental concerns (Obied et al., 2019). Lastly, two studies in this area reviewed in this area took place in primary care settings (Sturner et al., 2016; Thomas et al., 2016), whereas Maleka et al. (2016) was implemented by community health workers, supporting the feasibility of evidence-based screening in diverse health care settings. Finally, our review also summarized studies on telephone screening methods for children with developmental concerns (Bishop et al., 2017; Nelson et al., 2019), suggesting that phone screening has the potential to facilitate entry into the developmental service system, which families regularly describe as confusing and difficult to navigate (Lappé et al., 2018).
The studies reviewed provide a number of options for technology-assisted ASD assessment. As innovation in this area continues, a clearer picture is likely to emerge regarding how these tools can be used in conjunction to allow for a smooth screening and diagnosis assessment process utilizing technology. Larger studies utilizing technological methods are already underway; Barbaro and Yaari (2020) are examining a mobile app called ASDetect in a sample of 1000 children, and will investigate whether ASDetect can accurately detect ASD. Similar large-scale studies have the potential to build on the findings of smaller studies on assessment and screening technologies, such as those discussed in this review.
4.1. Limitations
This review summarized a number of different types of assessment tools that utilized technology as a novel method for ASD identification in children from 0–12 years. However, several limitations were noted. Many of the studies had small sample sizes, which limits the generalizability of their results to the broader population. Furthermore, the majority of the studies focused on using technology in younger age groups. Therefore, more work is needed to understand how these technologies may be applied to screening and diagnosis for older children. In addition, several of the papers reviewed (Corona, Weitlauf et al., 2020; Juárez et al., 2018; Reese et al., 2013, 2015) tested tele-assessment in clinical settings, with implications for remote use in the future. Standardized assessments, such as the ADOS-2, cannot be administered remotely; thus, there is a need for flexible measures that do not depend on costly standardized materials and can be disseminated to more providers. As such, more research is needed to understand whether at-home remote technologies are feasible and accurate for ASD assessments.
5. Conclusion
The use of communication and information technology in childhood assessments for ASD appears to be a novel approach for conducting assessments. This systematic review builds upon our prior review on detecting early signs for ASD, providing promising results related to several types of teleassessment and informing the future development of technology-based assessments. These strategies will be particularly important as the COVID-19 pandemic progresses in the United States, with families often being required to stay at home and clinics and hospitals operating at limited capacities. In vivo tele-assessment strategies, in particular, hold promise for diagnosis and are consistent with current telepsychology practices during COVID-19. These methods have important potential beyond the pandemic to improve access for underserved communities. Additionally, while this current review included technologies that could be used remotely in the future, there is still much work to be done to develop and identify valid evidence-based tools that can be used remotely. Future research has the opportunity to investigate these assessment and screening protocols in large, community samples, providing additional evidence to support their use.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Angela V. Dahiya: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Resources, Writing - original draft, Writing - review & editing, Visualization, Data curation, Supervision, Project administration. Elizabeth DeLucia: Conceptualization, Methodology, Investigation, Resources, Visualization, Data curation, Writing - original draft, Writing - review & editing. Christina G. McDonnell: Conceptualization, Writing - review & editing, Supervision. Angela Scarpa: Conceptualization, Writing - review & editing, Supervision.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.ridd.2021.103852.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- American Psychiatric Association . 4th ed. Author; Washington, DC: 2000. Diagnostic and statistical manual of mental disorders. text rev. [Google Scholar]
- American Psychiatric Association . American Psychiatric Pub.; 2013. Diagnostic and statistical manual of mental disorders (DSM-5®) [Google Scholar]
- Baranek G.T. Autism during infancy: A retrospective video analysis of sensory-motor and social behaviors at 9–12 months of age. Journal of Autism and Developmental Disorders. 1991;29(3):213–224. doi: 10.1023/a:1023080005650. [DOI] [PubMed] [Google Scholar]
- Barbaro J., Yaari M. Study protocol for an evaluation of ASDetect-a Mobile application for the early detection of autism. BMC Pediatrics. 2020;20(1):21. doi: 10.1186/s12887-019-1888-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Sasson A., Robins D.L., Yom-Tov E. Risk assessment for parents who suspect their child has autism spectrum disorder: Machine learning approach. Journal of Medical Internet Research. 2018;20(4):e134. doi: 10.2196/jmir.9496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethell C., Peck C., Schor E. Promoting healthy development survey. PsycTESTS Dataset. 2001 doi: 10.1037/t28447-000. [DOI] [PubMed] [Google Scholar]
- Bishop S.L., Farmer C., Bal V., Robinson E.B., Willsey A.J., Werling D.M.…Thurm A. Identification of developmental and behavioral markers associated with genetic abnormalities in autism spectrum disorder. American Journal of Psychiatry. 2017;174(6):576–585. doi: 10.1176/appi.ajp.2017.16101115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brothers K.B., Glascoe F.P., Robertshaw N.S. PEDS: Developmental milestones—An accurate brief tool for surveillance and screening. Clinical Pediatrics. 2008;47(3):271–279. doi: 10.1177/0009922807309419. [DOI] [PubMed] [Google Scholar]
- Chambers N.J., Wetherby A.M., Stronach S.T., Njongwe N., Kauchali S., Grinker R.R. Early detection of autism spectrum disorder in young isiZulu-speaking children in South Africa. Autism. 2017;21(5):518–526. doi: 10.1177/1362361316651196. [DOI] [PubMed] [Google Scholar]
- Corona L., Hine J., Nicholson A., Stone C., Swanson A., Wade J.…Warren Z. Vanderbilt University Medical Center; 2020. TELE-ASD-PEDS: A telemedicine-based ASD evaluation tool for toddlers and young children. [Google Scholar]
- Corona L.L., Weitlauf A.S., Hine J., Berman A., Miceli A., Nicholson A.…Vehorn A. Parent perceptions of caregiver-mediated telemedicine tools for assessing autism risk in toddlers. Journal of Autism and Developmental Disorders. 2020:1–11. doi: 10.1007/s10803-020-04554-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahiya A.V., McDonnell C., DeLucia E., Scarpa A. A systematic review of remote telehealth assessments for early signs of autism spectrum disorder: Video and mobile applications. Practice Innovations. 2020 doi: 10.1037/pri0000121. [DOI] [Google Scholar]
- Duda M., Daniels J., Wall D.P. Clinical evaluation of a novel and mobile autism risk assessment. Journal of Autism and Developmental Disorders. 2016;46:1953–1961. doi: 10.1007/s10803-016-2718-4. https://doi-org.ezproxy.lib.vt.edu/10.1007/s10803-016-2718-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer R.L., McGill R.J., Dombrowski S.C., McClain M.B., Harris B., Lockwood A.…Benson N.F. Teleassessment with children and adolescents during the coronavirus (COVID-19) pandemic and beyond: Practice and policy implications. PsyArXiv. 2020 doi: 10.1037/pro0000349. July 22. [DOI] [Google Scholar]
- Gabrielsen T.P., Farley M., Speer L., Villalobos M., Baker C.N., Miller J. Identifying autism in a brief observation. Pediatrics. 2015;135(2):e330–e338. doi: 10.1542/peds.2014-1428. [DOI] [PubMed] [Google Scholar]
- Guinchat V., Chamak B., Bonniau B., Bodeau N., Perisse D., Cohen D.…Danion A. Very early signs of autism reported by parents include many concerns not specific to autism criteria. Res Autism Spectrum Disorder. 2016;6(2):589–601. doi: 10.1016/j.rasd.2011.10.005. [DOI] [Google Scholar]
- Janvier Y.M., Harris J.F., Coffield C.N., Louis B., Xie M., Cidav Z.…Mandell D.S. Screening for autism spectrum disorder in underserved communities: Early childcare providers as reporters. Autism. 2016;20(3):364–373. doi: 10.1177/1362361315585055. [DOI] [PubMed] [Google Scholar]
- Juárez A.P., Weitlauf A.S., Nicholson A., Pasternak A., Broderick N., Hine J.…Warren Z. Early identification of ASD through telemedicine: Potential value for underserved populations. Journal of Autism and Developmental Disorders. 2018;48(8) doi: 10.1007/s10803-018-3524-y. 2601-2610. 18) 48:2601–2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappé M., Lau L., Dudovitz R.N., Nelson B.B., Karp E.A., Kuo A.A. The diagnostic Odyssey of autism spectrum disorder. Pediatrics. 2018;141(Supplement 4):S272–S279. doi: 10.1542/peds.2016-4300C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C., Rutter M., Le Couteur A. Autism diagnostic interview-revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Lord C., Rutter M., DiLavore P., Risi S., Gotham K., Bishop S. Western Psychological Corporation; Los Angeles, CA: 2012. Autism diagnostic observation schedule–2nd edition (ADOS-2) [Google Scholar]
- Maenner M.J., Shaw K.A., Baio J. Prevalence of autism spectrum disorder among children aged 8 years—Autism and developmental disabilities monitoring network, 11 sites, United States, 2016. MMWR Surveillance Summaries. 2020;69(4):1. doi: 10.15585/mmwr.ss6904a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maleka B.K., Van Der Linde J., Page Glascoe F., Swanepoel D.W. Developmental screening—Evaluation of an m-Health version of the parents evaluation developmental status tools. Telemedicine and e-Health. 2016;22:1013–1018. doi: 10.1089/tmj.2016.0007. https://doi-org.ezproxy.lib.vt.edu/10.1089/tmj.2016.0007 [DOI] [PubMed] [Google Scholar]
- Mandell D.S., Novak M.M., Zubritsky C.D. Factors associated with age of diagnosis among children with autism spectrum disorders. Pediatrics. 2005;116(6):1480–1486. doi: 10.1542/peds.2005-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D.G., The PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Medicine. 2009;6(7) doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan L., Wetherby A.M., Barber A. Repetitive and stereotyped movements in children with autism spectrum disorders late in the second year of life. Journal of Child Psychology and Psychiatry. 2008;49(8):826–837. doi: 10.1111/j.1469-7610.2008.01904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen E.M. AGS; Circle Pines, MN: 1995. Mullen scales of early learning; pp. 58–64. [Google Scholar]
- Nelson B.B., Thompson L.R., Herrera P., Biely C., Zarate D.A., Aceves I.…Chung P.J. Telephone-based developmental screening and care coordination through 2-1-1: A randomized trial. Pediatrics. 2019;143(4) doi: 10.1542/peds.2018-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeid R., Beekman L., Roizen N., Ciccia A., Short E.J. Using Telehealth to address disparities in cognitive, language, and emotion regulation problems in young children: A case illustration using the INvesT model. Birth Defects Research. 2019;111:1154–1164. doi: 10.1002/bdr2.1537. https://doi-org.ezproxy.lib.vt.edu/10.1002/bdr2.1537 [DOI] [PubMed] [Google Scholar]
- Osterling J., Dawson G., Munson J. Early recognition of 1-year-old infants with autism spectrum disorder versus mental retardation. Development and Psychopathology. 2002;14(2):239–251. doi: 10.1017/s0954579402002031. [DOI] [PubMed] [Google Scholar]
- Reese R.M., Jamison T.R., Braun M., Wendland M., Black W., Hadorn M.…Prather C. Brief report: Use of interactive television in identifying autism in young children: Methodology and preliminary data. Journal of Autism and Developmental Disorders. 2015;45(5):1474–1482. doi: 10.1007/s10803-014-2269-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese R.M., Jamison R., Wendland M., Fleming K., Braun M.J., Schuttler J.O.…Turek J. Evaluating interactive videoconferencing for assessing symptoms of autism. Telemedicine and e-Health. 2013;19(9):671–677. doi: 10.1089/tmj.2012.0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins D.L., Casagrande K., Barton M., Chen C.M.A., Dumont-Mathieu T., Fein D. Validation of the modified checklist for autism in toddlers, revised with follow-up (M-CHAT-R/F) Pediatrics. 2014;133(1):37–45. doi: 10.1542/peds.2013-1813. https://doi-org.ezproxy.lib.vt.edu/10.1542/peds.2013-1813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins D.L., Fein D., Barton M.L. 1999. Follow-up interview for the modified checklist for autism in toddlers (M-CHAT FUI) Self-published. [Google Scholar]
- Robins D.L., Fein D., Barton M. 2009. The modified checklist for autism in toddlers, revised with follow-up (M-CHAT-R/F) Self-published. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C.J., Rozga A., Matthews N., Oberleitner R., Nazneen N., Abowd G. Investigating the accuracy of a novel telehealth diagnostic approach for autism spectrum disorder. Psychological Assessment. 2017;29(3):245. doi: 10.1037/pas0000317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squires J., Twombly E., Bricker D., Potter L. 3rd ed. Paul H. Brookes; Baltimore, MD: 2009. Ages and stages questionnaires user’s guide. [Google Scholar]
- Stone W., Ousley O.Y. Vanderbilt University; 2008. Screening tool for autism in toddlers and young children (STAT) [Google Scholar]
- Sturner R., Howard B., Bergmann P., Morrel T., Andon L., Marks D.…Landa R. Autism screening with online decision support by primary care pediatricians aided by M-CHAT/F. Pediatrics. 2016;138(3) doi: 10.1542/peds.2015-3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R.E., Spragins W., Mazloum G., Cronkhite M., Maru G. Rates of detection of developmental problems at the 18‐month well‐baby visit by family physicians’ using four evidence‐based screening tools compared to usual care: A randomized controlled trial. Child: Care, Health and Development. 2016;42(3):382–393. doi: 10.1111/cch.12333. [DOI] [PubMed] [Google Scholar]
- Wetherby A.M., Prizant B.M. Paul H Brookes Publishing Co.; 2002. Communication and symbolic behavior scales: Developmental profile. [Google Scholar]
- Wetherby A.M., Woods J., Nottke C., Stronach S., Dow D., McCoy D. Florida State University; Tallahassee, FL: 2016. Systematic observation of red flags of ASD (SORF) manual. [Google Scholar]
- Wright J.H., Caudill R. Remote treatment delivery in response to the COVID-19 pandemic. Psychotherapy and Psychosomatics. 2020;89(3):1. doi: 10.1159/000507376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherby A.M., Morgan L. Florida State University; Tallahassee, FL: 2007. Repetitive and stereotyped movement scales: Companion to the CSBS. [Google Scholar]
- Wetherby A., Lord C., Woods J., Guthrie W., Pierce K., Shumway S., Ozonoff S. The Early Screening for Autism and Communication Disorders (ESAC): Preliminary field-testing of an autism-specific screening tool for children 12 to 36 months of age. Paper presented at the International Meeting for Autism Research (IMFAR); Chicago, IL; 2009. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.