Abstract
Previous studies have shown that Gardnerella vaginalis can utilize iron-loaded human lactoferrin as a sole source of iron. In this study, G. vaginalis cells were shown to bind digoxigenin (DIG)-labeled human lactoferrin in a dot blot assay. Using the DIG-labeled human lactoferrin, a 120-kDa human lactoferrin-binding protein was detected by Western blot analysis of G. vaginalis proteins. The lactoferrin-binding activity of this protein was found to be heat stable. Competition studies indicated that this binding activity was specific for human lactoferrin. Treatment of G. vaginalis cells with proteases suggested that this protein was surface exposed. An increase in lactoferrin binding by the 120-kDa protein was observed in G. vaginalis cells grown under iron-restrictive conditions, suggesting that this activity may be iron regulated.
Gardnerella vaginalis is the predominant microorganism associated with bacterial vaginosis (BV), a common disorder of women of reproductive age. BV is characterized by a shift in the microbiological flora of the lower vagina in which the Lactobacillus-predominant flora is replaced by a number of different microorganisms, including G. vaginalis, Mobiluncus spp., Peptostreptococcus spp., Prevotella spp., Bacteroides spp., and Mycoplasma hominis (18, 19, 46). Other characteristics include (i) the presence of a homogenous discharge, (ii) an amine (fishy) odor, (iii) the presence of vaginal epithelial “clue cells,” and (iv) an increase in the pH of the vagina to >4.5 (11, 46). Although found at low concentrations in healthy subjects, G. vaginalis is found in higher concentrations in BV patients. BV is a significant risk factor for upper genital tract infections (12, 34) in pregnant women, which can result in adverse outcomes of pregnancy, including preterm delivery and low birth weight of infants (21), premature rupture of membranes (29), premature labor (22), and impaired fetal development (13). More recent studies (44) indicate that BV increases the risk of human immunodeficiency virus (HIV) infection. Furthermore, it has also been demonstrated that the microflora associated with BV could activate HIV type 1 (HIV-1) expression in a promonocytic cell line chronically infected with HIV-1 (1, 32). It is postulated that a BV microflora-associated HIV-inducing factor may contribute to HIV transmission.
G. vaginalis is a fastidious, nonmotile, beta-hemolytic, unencapsulated, rod-shaped bacterium (6). Although G. vaginalis cells stain gram variable, this organism possesses a gram-positive cell wall (37). In addition to being associated with BV, G. vaginalis has been isolated from or detected in a number of infections, including intra-amniotic and chorioamniotic infections (14, 15, 20), intrauterine infections (26), and urinary tract and bladder infections (27, 45), as well as pelvic inflammatory disease (12). However, little is known about the mechanism of G. vaginalis pathogenesis. One potential virulence factor is a 60-kDa hemolysin that lyses human red blood cells, neutrophils, and endothelial cells (7). G. vaginalis also possesses pili and an exopolysaccharide coat that are involved in the adherence of G. vaginalis to vaginal epithelial cells (“clue cells”) and red blood cells (4, 43). However, their specific roles in the establishment of G. vaginalis infection remain to be determined. Of great importance, recent work by Hashemi et al. demonstrated that G. vaginalis cell lysates could stimulate HIV-1 gene expression in human cell cultures, suggesting that G. vaginalis may play a role in the increased rate of HIV transmission in BV patients (17).
Virtually all microorganisms require iron for their survival. For many bacterial pathogens, the ability to acquire iron is related to their virulence potential (16, 28, 47). However, in the human host, free iron is found in limited amounts as a result of being sequestered in compounds such as heme, ferritin, and hemoglobin or bound by high-affinity iron-binding proteins such as transferrin or lactoferrin (33, 47). To overcome this iron-withholding capacity of the host, bacteria have developed several high-affinity mechanisms to obtain this essential nutrient. One mechanism is the utilization of siderophores (8, 28). Siderophores are low-molecular-weight, high-affinity iron chelators which remove iron from carrier molecules. After binding iron, siderophores are bound by outer surface receptors for import of the iron or iron-siderophore complex into the bacterial cell (8, 28). A second mechanism is the direct binding of iron-containing compounds (such as heme, hemoglobin, heme-hemopexin, lactoferrin, and transferrin) by specific cell surface receptors (28, 33, 47). Other mechanisms include the production of hemolysins or cytolysins which lyse host cells, presumably resulting in the release of iron-containing compounds (28), and the utilization of iron reductases (25).
Little is known about iron acquisition by G. vaginalis. Previous studies demonstrated that G. vaginalis could utilize several iron-containing compounds, including iron salts, heme, hemoglobin, and lactoferrin, as a sole source of iron (24). G. vaginalis was also shown to produce siderophores and express iron-regulated proteins (24). Preliminary work in our lab (C. B. Land and G. P. Jarosik, Abstr. 99th Gen. Meet. Am. Soc. Microbiol., abstr. B/D-156, 1999; C. B. Land, M. S. Smith, and G. P. Jarosik, Abstr. 98th Gen. Meet. Am. Soc. Microbiol., abstr. D-102, p. 230, 1998) suggested that G. vaginalis could directly bind several iron-containing compounds, including human lactoferrin (hLf). In this study, the interaction between G. vaginalis strains and hLf was examined. G. vaginalis cells were shown to directly bind hLf. Additionally, we demonstrate the detection of a G. vaginalis hLf-binding protein.
MATERIALS AND METHODS
Bacterial strains, reagents, media, and growth conditions.
The G. vaginalis type strain, 594 (ATCC 14018), and G. vaginalis 317 (ATCC 14019), a clinical isolate, were obtained from the American Type Culture Collection (Manassas, Va.). G. vaginalis strains were routinely grown on human blood bilayer Tween agar plates obtained from Remel (Lenexa, Kans.) or on basal medium (36) supplemented with 0.3% soluble starch (BMS) but lacking heme. The G. vaginalis strains were colistin and nalidixic acid resistant and beta-hemolytic when cultured on human blood bilayer Tween plates, hydrogen peroxide sensitive, and catalase negative. G. vaginalis cultures were incubated at 37°C in an atmosphere of 5% CO2. Culture stocks were stored at −75°C in Proteose Peptone 3 (Difco, Detroit, Mich.) broth with 50% glycerol. Escherichia coli strain DH5αMCR, which does not bind lactoferrin (35), was routinely cultured on Luria-Bertani medium (38) at 37°C. All iron-containing compounds, the iron chelator 2,2′-dipyridyl, trypsin, proteinase K, and the protease inhibitor phenylmethylsulfonyl fluoride were purchased from Sigma Chemical Company (St. Louis, Mo.). All iron compounds were freshly prepared by dissolving the compounds in distilled water followed by filter sterilization, with the exception of heme, which was dissolved in 0.02 N sodium hydroxide prior to filter sterilization.
DIG labeling of hLf.
Labeling of hLf with digoxigenin (DIG) was performed using the DIG Protein Labeling Kit (Boehringer Mannheim, Indianapolis, Ind.) according to the manufacturer's instructions. Briefly, 1 mg of hLf dissolved in phosphate-buffered saline (pH 7.4) was incubated with 100 μg of DIG (digoxigenin-3-O-succinyl-ɛ-aminocaproic acid-N-hydroxy-succinimide ester) for 2 h at room temperature with gentle mixing. Unbound DIG was removed by dialysis in phosphate-buffered saline buffer followed by ultrafiltration using a Centricon-30 microconcentrator (Millipore, Bedford, Mass.). The concentration of DIG-labeled hLf (DIG-hLf) was adjusted to 1 mg/ml, and the solution was stored at −20°C.
Solid-phase dot blot binding assay.
Cultures of G. vaginalis strains grown on BMS were resuspended in BMS broth to a concentration of 109 cells/ml, and 10 μl of the cell suspensions were vacuum blotted onto nitrocellulose filters (Immobilon-NC; Millipore) using the Bio-Rad (Hercules, Calif.) Bio-Dot Blot apparatus. After drying for 1 h at 37°C, the filters were incubated for 1 h with a blocking solution consisting of 3% (wt/vol) skim milk dissolved in Tris-buffered saline (TBS) (pH 7.4). DIG-hLf was then added to a final concentration of 1 to 2 μg/ml. After incubation with DIG-hLf for 1 h at room temperature, the filters were washed three times with TBS (pH 7.4), followed by a 1-h incubation with the 3% skim milk blocking solution. Anti-DIG antibody coupled to horseradish peroxidase (anti-DIG-POD; Boehringer Mannheim) (150 U/ml) was added to a 1:25,000 final dilution and incubated for 1 h at room temperature. After three TBS buffer washes, the filters were subjected to chemiluminescence analysis using the ECL chemiluminescence detection agents (Amersham Pharmacia Biotech, Piscataway, N.J.) according to the manufacturer's instructions.
Protein analysis.
Except where noted, G. vaginalis cells (109/ml) were routinely lysed by incubation in lysis buffer (2% sodium dodecyl sulfate [SDS], 50 mM Tris, pH 6.8) overnight at 4°C. Protein concentrations were determined using the dotMETRIC protein assay (Gene Technology, St. Louis, Mo.) or the Bio-Rad DC protein assay kits. Separation of proteins was routinely performed by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) using 10% polyacrylamide gels and the Tris-glycine buffer system (38). For Western blot analysis, the separated proteins were then electroblotted onto nitrocellulose filter paper. After blocking for 1 h with 3% skim milk, the filters were probed with DIG-hLf (1- to 2-μg/ml final concentration), washed with TBS, and then incubated with anti-DIG-POD antibody (1:25,000 final dilution). After the unbound antibody was removed by washing, the blots were developed utilizing the chemiluminescence detection kit as described above.
Competitive binding assays.
The specificity of the binding activity of the 120-kDa protein was examined in a competition assay using Western blot analysis. Briefly, Western blot analysis of G. vaginalis proteins was performed as described above with the exception that the nitrocellulose filters were preincubated with the following unlabeled iron-containing compounds for 1 h prior to the addition of the DIG-hLf: hLf, human transferrin, bovine lactoferrin, catalase, hemin, or hemoglobin. The final concentration of the competitor compounds was 100 μg/ml.
Proteolytic treatment of G. vaginalis cells.
One milliliter of G. vaginalis cells (108/ml) was incubated with proteinase K (50 μg/ml) or trypsin (50 μg/ml) for 30 min at 37°C. After being washed with BMS broth to remove the proteases, the cells were resuspended (108/ml) in BMS broth containing phenylmethylsulfonyl fluoride at a final concentration of 1 mM and lysed with Laemmli SDS-PAGE sample buffer. The cell proteins were then analyzed by SDS-PAGE and Western blot analysis as described above.
RESULTS
Binding of hLf by G. vaginalis cells.
We have previously reported that G. vaginalis could utilize iron-loaded hLf as a sole source of iron (24). Previous work has demonstrated that some bacteria which utilize lactoferrin as an iron source can directly bind this iron-containing compound (10, 23, 41, 42). To determine if G. vaginalis cells could bind hLf, a solid-phase dot blot assay was performed utilizing DIG-hLf as a probe. As shown in Fig. 1, chemiluminescence was detected from G. vaginalis strains 594 and 317 (Fig. 1, lanes a and b), indicating the binding of DIG-hLf by these cells. As a negative control, a dot blot assay was performed using E. coli DH5αMCR, and no lactoferrin binding was observed (Fig. 1, lane c). In control experiments assaying for nonspecific binding of the reagents, no chemiluminescence was detected from G. vaginalis cells incubated with only DIG-hLf or the anti-DIG-POD antibody (data not shown).
FIG. 1.
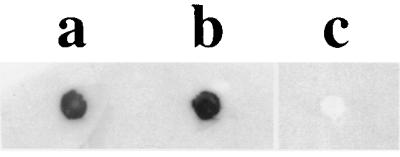
Binding of DIG-hLf by G. vaginalis cells. G. vaginalis cells were blotted onto nitrocellulose filters and probed with DIG-hLf as described in the text. Lanes: a, G. vaginalis 594; b, G. vaginalis 317; c, E. coli DH5αMCR.
Detection of a 120-kDa Lbp.
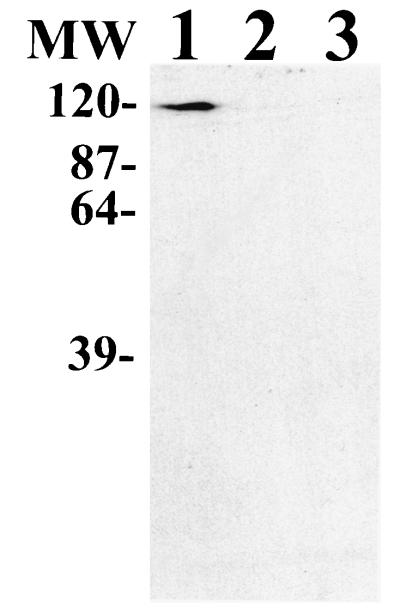
Many bacteria which utilize lactoferrin as an iron source express one or more specific receptors which bind this glycoprotein (41). Since G. vaginalis cells could utilize and bind lactoferrin, we wanted to identify a potential G. vaginalis protein(s) involved in the binding of DIG-hLf by utilizing Western blot analysis. Proteins from G. vaginalis whole-cell lysates separated via SDS-PAGE were electroblotted onto nitrocellulose filters and probed with DIG-hLf. Figure 2 shows that an hLf-binding protein with an estimated molecular mass of 120 kDa was detected from G. vaginalis strains 594 and 317. In control experiments assaying for nonspecific binding of DIG-hLf, the 120-kDa lactoferrin-binding protein (Lbp) was not detected from blots incubated with only the DIG-hLf or only the anti-DIG-POD antibody (data not shown). The 120-kDa hLf-binding protein was also observed from cell lysates heated to 100°C prior to SDS-PAGE, indicating that the binding activity was heat stable and that the 120-kDa protein was not comprised of smaller protein subunits (Fig. 3).
FIG. 2.
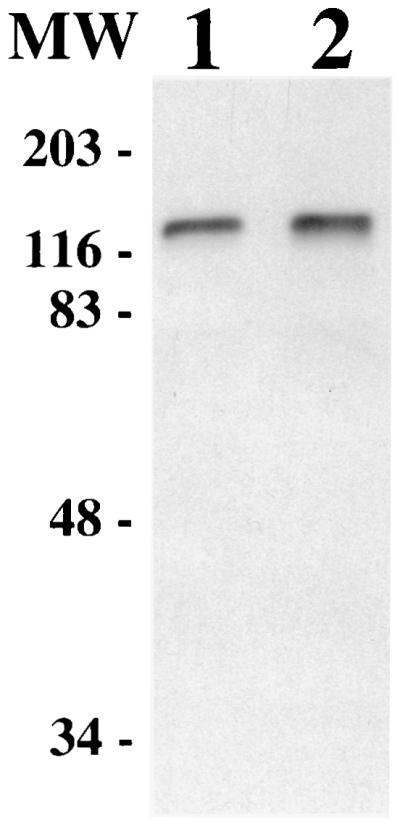
Detection of a 120-kDa Lbp via Western blot analysis. G. vaginalis proteins from whole-cell lysates were electroblotted onto nitrocellulose and probed with DIG-hLf. Lane 1, G. vaginalis 594; lane 2, G. vaginalis 317. MW, molecular mass standards in kilodaltons.
FIG. 3.
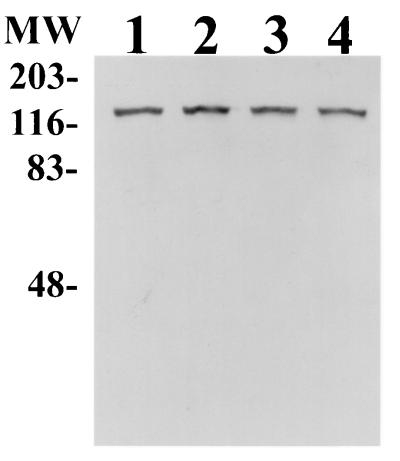
Heat stability of the 120-kDa protein lactoferrin-binding activity. Proteins from whole-cell fractions of G. vaginalis 594 (lanes 1 and 2) and G. vaginalis 317 (lanes 3 and 4) were not boiled (lanes 1 and 3) or boiled (lanes 2 and 4) prior to SDS-PAGE and Western blot analysis. MW, molecular mass standards in kilodaltons.
To determine if the 120-kDa Lbp binding activity could be detected from G. vaginalis grown under different iron conditions, Western blot analysis of G. vaginalis proteins was performed utilizing whole-cell lysates from G. vaginalis 317 cells grown under iron-replete conditions (BMS), iron-supplemented conditions (BMS supplemented with 100 μM ferric chloride), or iron-deficient conditions (BMS supplemented with 100 μM 2,2′-dipyridyl). The results are shown in Fig. 4. An increase in lactoferrin-binding activity was detected from G. vaginalis 317 cells grown under iron-restrictive conditions (Fig. 4, lane 3) compared to cells grown under iron-replete or iron-supplemented conditions (Fig. 4, lanes 1 and 2), suggesting that this activity may be iron regulated.
FIG. 4.
Western blot analysis of proteins from G. vaginalis cells grown under different iron conditions. Equal amounts of protein (35 μg) from whole-cell lysates of G. vaginalis 317 grown under iron-replete (lane 1), iron-supplemented (lane 2), or iron-restrictive (lane 3) conditions (see text) were probed with DIG-hLf. The arrow indicates the 120-kDa G. vaginalis Lbp.
Specificity of the 120-kDa Lbp binding activity.
Previous work in our laboratory (24; Land and Jarosik, Abstr. 99th Gen. Meet. Am. Soc. Microbiol.; Land et al., Abstr. 98th Gen. Meet. Am. Soc. Microbiol.) indicated that G. vaginalis could utilize and directly bind a number of host iron-containing compounds, including catalase, hemoglobin, and hemin. In order to examine the specificity of the 120-kDa Lbp, a competitive binding assay utilizing various iron-containing proteins was performed. Briefly, Western blot analysis of G. vaginalis proteins was performed as described above with the exception that the nitrocellulose blot paper was preincubated with unlabeled iron-containing compounds for 1 h prior to the addition of the DIG-hLf. The unlabeled iron compounds included human transferrin and bovine lactoferrin, which are structurally and functionally similar to hLf, as well as catalase, hemin, hemoglobin, and hLf. The results are shown in Fig. 5. Preincubation with unlabeled hemin, hemoglobin, catalase, bovine lactoferrin, or human transferrin did not affect the ability of the 120-kDa protein to bind DIG-hLf (Fig. 5C, D, E, F, and G), whereas preincubation with unlabeled hLf inhibited the binding of DIG-hLf by the 120-kDa protein (Fig. 5B). These results indicated that the binding activity of the 120-kDa Lbp was specific for hLf.
FIG. 5.
Specificity of the G. vaginalis 120-kDa Lbp. Equal amounts of protein from whole-cell lysates of G. vaginalis 594 (lanes 1) or G. vaginalis 317 (lanes 2) were electroblotted onto nitrocellulose filters. Prior to exposure to DIG-hLf, the filters were preincubated with various unlabeled iron-containing compounds. (A) no pretreatment; (B) hLf; (C) hemin; (D) hemoglobin; (E) catalase; (F) bovine lactoferrin; (G) human transferrin. The arrow indicates the 120-kDa G. vaginalis Lbp.
Proteolytic treatment of G. vaginalis cells.
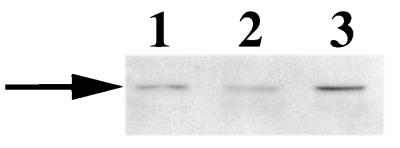
To examine if the 120-kDa Lbp may be surface exposed, G. vaginalis 317 cells were exposed to either proteinase K or trypsin prior to Western blot analysis. The results are shown in Fig. 6. Binding of DIG-hLf by the 120-kDa protein was detected from cells not treated with the proteases (Fig. 6, lane 1). However, the 120-kDa Lbp activity was not detected from cells treated with either proteinase K or trypsin prior to Western blot analysis (Fig. 6, lanes 2 and 3), suggesting that this protein may be surface exposed. It is also possible that a second protein sensitive to the proteolytic treatment is required for the hLf-binding activity of the 120-kDa Lbp.
FIG. 6.
Sensitivity of the 120-kDa protein lactoferrin-binding activity to protease treatment. Western blot analysis was performed to examine the 120-kDa protein hLf-binding activity from G. vaginalis 594 cells treated with proteases prior to cell lysis. Lane 1, no protease treatment; lane 2, proteinase K treatment; lane 3, trypsin treatment. MW, molecular mass standards in kilodaltons.
DISCUSSION
hLf is an extracellular iron-binding glycoprotein which can be found in mucosal secretions, including those found in the respiratory tract, gastrointestinal tract, and urogenital tract. It is also released by neutrophils at sites of infection. During infection, the binding of iron by lactoferrin is proposed to reduce the amount of free extracellular iron. This process, known as the hypoferremia of infection, is thought to further limit the free iron available to invading microorganisms. However, a number of bacterial pathogens, including Helicobacter pylori (23), Neisseria meningitidis (41), Neisseria gonorrhoeae (41), Moraxella catarrhalis (5), and Staphylococcus aureus (31), possess the ability to utilize lactoferrin as an iron source. Some bacteria, for example, Bordetella pertussis and Bordetella bronchiseptica (30), utilize siderophores to capture iron from lactoferrin. However, a number of bacteria are able to sequester iron from lactoferrin through the use of specific surface receptors. The lactoferrin receptors found in Neisseria and Moraxella species have been the best studied (41, 42). For example, N. gonorrhoeae expresses an Lbp designated LbpA, whereas two Lbps, LbpA and LbpB, have been identified in N. meningitidis (41, 42). M. catarrhalis possesses three Lbps, namely, LbpA, LbpB, and CopB (41). However, mutational analysis indicates that LbpA, but not LbpB, is essential for iron acquisition from lactoferrin in these two pathogens (3, 41, 42). Lbps have been identified in other bacteria, including a 70-kDa hLf receptor in H. pylori (10), a 40-kDa hLf receptor in the oral pathogen Prevotella nigrescens (9), and a 450-kDa Lbp complex in S. aureus (31). However, whether the Lbps from these organisms play a direct role in iron acquisition from lactoferrin remains to be determined.
Previous studies demonstrated that G. vaginalis could utilize hLf as a sole source of iron in vitro (24). In this study, we began work examining the possible interaction between G. vaginalis cells and hLf. Using a solid-phase dot blot assay, it was shown that G. vaginalis cells could directly bind DIG-hLf (Fig. 1). To determine if this binding could be mediated by a receptor, proteins from G. vaginalis whole cells were analyzed using Western blots probed with DIG-hLf. This resulted in the detection of an hLf-binding protein with an apparent molecular mass of about 120 kDa (Fig. 2). This activity was also detected from protein samples heated to 100°C under reducing conditions prior to SDS-PAGE (Fig. 3), suggesting the hLf-binding activity is heat stable and that the 120-kDa protein is a monomer. The heat-stable activity of this protein is different from the activity of other Lbps. For example, the lactoferrin-binding activities of N. meningitidis LbpA and LbpB and the 40-kDa P. nigrescens Lbp are heat labile (2, 9). The 450-kDa S. aureus hLf-binding protein complex was found to consist of two protein subunit components of 62 and 67 kDa upon exposure to reducing conditions (31).
In this study, the binding activity of the G. vaginalis Lbp was shown to be specific for hLf. Competition binding assays utilizing unlabeled iron compounds which G. vaginalis can utilize as iron sources did not inhibit the hLf-binding activity of the 120-kDa protein, whereas preincubation with unlabeled hLf did inhibit this activity (Fig. 5). Interestingly, bovine lactoferrin or human transferrin, two compounds which are structurally and functionally very similar to human lactoferrin, also did not inhibit the hLf-binding activity of the 120-kDa protein. These observations correlate with studies demonstrating that G. vaginalis cannot utilize human transferrin (24) or bovine lactoferrin (G. P. Jarosik, unpublished data) as an iron source. This suggests that the 120-kDa Lbp may interact with amino acid residues or structural motifs unique to the hLf molecule. Other bacteria express Lbps possessing similar binding specificities. For example, the Lbps of N. meningitidis, N. gonorrhoeae, M. catarrhalis, and H. pylori specifically bind human lactoferrin (10, 41, 42).
In addition to the evidence suggesting their direct involvement in iron acquisition, additional studies have suggested that the LbpA and LbpB proteins of N. meningitidis and M. catarrhalis are surface exposed and that the expression of these proteins is iron regulated (41, 42). Whether the G. vaginalis 120-kDa Lbp is directly involved in the acquisition of iron from hLf remains to be determined. Furthermore, it is not known if G. vaginalis siderophores may be involved in the acquisition of iron from hLf. It is possible that G. vaginalis may utilize two mechanisms, siderophores and the direct binding of lactoferrin, to obtain iron from lactoferrin. However, results from this study indicate that the 120-kDa Lbp is surface exposed (Fig. 6) and that the binding activity of this protein is iron regulated (Fig. 4), suggesting that this protein may play a role in iron acquisition. In the gram-positive bacterium Corynebacterium diphtheriae, the regulation of several proteins is mediated by the DtxR protein and iron (39, 40). It is possible that the increase in the hLf-binding activity by the G. vaginalis 120-kDa Lbp under iron-restrictive conditions, and presumably its expression, is mediated by a DtxR homolog. The binding activity of the 120-kDa Lbp was also detected from cells grown under iron-supplemented conditions (Fig. 4). Thus, the binding activity of the 120-kDa Lbp under iron-replete conditions as well as an increase in lactoferrin-binding activity under iron-restrictive conditions could potentially allow G. vaginalis to more effectively compete with other vaginal microorganisms for lactoferrin-bound iron. Whether this contributes to the changes in the vaginal microflora, such as those associated with BV, remains to be determined. Further studies will be required to determine what role the G. vaginalis Lbp plays in iron acquisition and to determine the mechanism by which its activity is iron regulated.
ACKNOWLEDGMENTS
We thank Alan Biel for critical reading of the manuscript.
This study was supported in part by a grant from the Joe W. and Dorothy Dorsett Brown Foundation and in part by grant no. LEQSF(1999-02)-RD-A-05 from the State of Louisiana Board of Regents.
REFERENCES
- 1.Al-Harthi L, Roebuck K A, Olinger G G, Landay A, Sha B E, Hashemi F B, Spear G T. Bacterial vaginosis-associated microflora isolated from the female genital tract activates HIV-1 expression. J Acquir Immune Defic Syndr. 1999;21:194–202. doi: 10.1097/00126334-199907010-00003. [DOI] [PubMed] [Google Scholar]
- 2.Bonnah R A, Schryvers A B. Preparation and characterization of Neisseria meningitidis mutants deficient in production of the human lactoferrin-binding proteins LbpA and LbpB. J Bacteriol. 1998;180:3080–3090. doi: 10.1128/jb.180.12.3080-3090.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonnah R A, Wong H, Loosmore S M, Schryvers A B. Characterization of Moraxella (Branhamella) catarrhalis lbpB, lbpA, and lactoferrin receptor orf3 isogenic mutants. Infect Immun. 1999;67:1517–1520. doi: 10.1128/iai.67.3.1517-1520.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boustouller Y L, Johnson A P, Taylor-Robinson D. Pili on Gardnerella vaginalis studied by electron microscopy. J Med Microbiol. 1987;23:327–329. doi: 10.1099/00222615-23-4-327. [DOI] [PubMed] [Google Scholar]
- 5.Campagnari A A, Shanks K L, Dyer D W. Growth of Moraxella catarrhalis with human transferrin and lactoferrin: expression of iron-repressible proteins without siderophore production. Infect Immun. 1994;62:4909–4914. doi: 10.1128/iai.62.11.4909-4914.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Catlin B W. Gardnerella vaginalis: characteristics, clinical considerations, and controversies. Clin Microbiol Rev. 1992;5:213–237. doi: 10.1128/cmr.5.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cauci S, Monte R, Ropele M, Missero C, Not T, Quadrifoglio F, Menestrina G. Pore-forming and haemolytic properties of the Gardnerella vaginalis cytolysin. Mol Microbiol. 1993;9:1143–1155. doi: 10.1111/j.1365-2958.1993.tb01244.x. [DOI] [PubMed] [Google Scholar]
- 8.Crosa J H. Genetics and molecular biology of siderophore-mediated iron transport in bacteria. Microbiol Rev. 1989;53:517–530. doi: 10.1128/mr.53.4.517-530.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Lillo A, Fierro J F. Identification of a lactoferrin-binding protein in Prevotella nigrescens. FEMS Microbiol Lett. 1997;150:61–64. doi: 10.1111/j.1574-6968.1997.tb10350.x. [DOI] [PubMed] [Google Scholar]
- 10.Dhaenens L, Szczebara F, Husson M O. Identification, characterization, and immunogenicity of the lactoferrin-binding protein from Helicobacter pylori. Infect Immun. 1997;65:514–518. doi: 10.1128/iai.65.2.514-518.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eschenbach D A. History and review of bacterial vaginosis. Am J Obstet Gynecol. 1993;169:441–445. doi: 10.1016/0002-9378(93)90337-i. [DOI] [PubMed] [Google Scholar]
- 12.Faro S, Martens M, Maccato M, Hammill H, Pearlman M. Vaginal flora and pelvic inflammatory disease. Am J Obstet Gynecol. 1993;169:470–473. doi: 10.1016/0002-9378(93)90344-i. [DOI] [PubMed] [Google Scholar]
- 13.Germain M, Krohn M A, Hillier S L, Eschenbach D A. Genital flora in pregnancy and its association with intrauterine growth and retardation. J Clin Microbiol. 1994;32:2162–2168. doi: 10.1128/jcm.32.9.2162-2168.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gibbs R S. Chorioamnionitis and bacterial vaginosis. Am J Obstet Gynecol. 1993;169:460–462. doi: 10.1016/0002-9378(93)90341-f. [DOI] [PubMed] [Google Scholar]
- 15.Gibbs R S, Weiner M H, Walmer K, St. Clair P J. Microbiologic and serologic studies of Gardnerella vaginalis in intra-amniotic infection. Obstet Gynecol. 1987;70:187–190. [PubMed] [Google Scholar]
- 16.Griffiths E. Iron and bacterial virulence—a brief overview. Biol Metals. 1991;4:7–13. doi: 10.1007/BF01135551. [DOI] [PubMed] [Google Scholar]
- 17.Hashemi F B, Ghassemi M, Roebuck K A, Spear G T. Activation of human immunodeficiency virus type-1 expression by Gardnerella vaginalis. J Infect Dis. 1999;179:924–929. doi: 10.1086/314674. [DOI] [PubMed] [Google Scholar]
- 18.Hill G B. The microbiology of bacterial vaginosis. Am J Obstet Gynecol. 1993;169:450–454. doi: 10.1016/0002-9378(93)90339-k. [DOI] [PubMed] [Google Scholar]
- 19.Hillier S L. Diagnostic microbiology of bacterial vaginosis. Am J Obstet Gynecol. 1993;169:455–459. doi: 10.1016/0002-9378(93)90340-o. [DOI] [PubMed] [Google Scholar]
- 20.Hillier S L, Martius J, Krohn M, Kiviat N, Holmes K K, Eschenbach D A. A case-control study of chorioamnionic infection and histologic chorioamnionitis in prematurity. N Engl J Med. 1988;319:972–978. doi: 10.1056/NEJM198810133191503. [DOI] [PubMed] [Google Scholar]
- 21.Hillier S L, Nugent R P, Eschenbach D A, Krohn M A, Gibbs R S, Martin D H, Cotch M F, Edelman R, Pastorek II J G, Rao A V, McNellis D, Regan J A, Carey J C, Klebanoff M A. Association between bacterial vaginosis and preterm delivery of a low-birth-weight infant. N Engl J Med. 1995;333:1737–1742. doi: 10.1056/NEJM199512283332604. [DOI] [PubMed] [Google Scholar]
- 22.Holst E, Goffeng A R, Andersch B. Bacterial vaginosis and vaginal microorganisms in idiopathic premature labor and association with pregnancy outcome. J Clin Microbiol. 1994;32:176–186. doi: 10.1128/jcm.32.1.176-186.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Husson M-O, Legrand D, Spik G, Leclerc H. Iron acquisition by Helicobacter pylori: importance of human lactoferrin. Infect Immun. 1993;61:2694–2697. doi: 10.1128/iai.61.6.2694-2697.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jarosik G P, Land C B, Duhon P, Chandler R, Jr, Mercer T. Acquisition of iron by Gardnerella vaginalis. Infect Immun. 1998;66:5041–5047. doi: 10.1128/iai.66.10.5041-5047.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson W, Varner L, Poch M. Acquisition of iron by Legionella pneumophila: role of iron reductase. Infect Immun. 1991;59:2376–2381. doi: 10.1128/iai.59.7.2376-2381.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kristiansen F V, Øster S, Frost L, Boustouller Y, Korsager B, Møller B R. Isolation of Gardnerella vaginalis in pure culture from the uterine cavity of patients with irregular bleedings. Br J Obstet Gynaecol. 1987;94:979–984. doi: 10.1111/j.1471-0528.1987.tb02273.x. [DOI] [PubMed] [Google Scholar]
- 27.Lam M H, Birch D F, Fairley K F. Prevalence of Gardnerella vaginalis in the urinary tract. J Clin Microbiol. 1988;26:1130–1133. doi: 10.1128/jcm.26.6.1130-1133.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Litwin C M, Calderwood S B. Role of iron in the regulation of virulence genes. Clin Microbiol Rev. 1993;6:137–149. doi: 10.1128/cmr.6.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McGregor J A, French J I, Seo K. Premature rupture of membranes and bacterial vaginosis. Am J Obstet Gynecol. 1993;169:463–466. doi: 10.1016/0002-9378(93)90342-g. [DOI] [PubMed] [Google Scholar]
- 30.Moore C H, Foster L-A, Gerbig D G, Jr, Dyer D W, Gibson B W. Identification of alcaligin as the siderophore produced by Bordetella pertussis and B. bronchiseptica. J Bacteriol. 1995;177:1116–1118. doi: 10.1128/jb.177.4.1116-1118.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naidu A S, Andersson M, Forsgren A. Identification of a human lactoferrin-binding protein in Staphylococcus aureus. J Med Microbiol. 1992;36:177–183. doi: 10.1099/00222615-36-3-177. [DOI] [PubMed] [Google Scholar]
- 32.Olinger G G, Hashemi F B, Sha B E, Spear G T. Association of indicators of bacterial vaginosis with a female genital tract factor that induces expression of HIV-1. AIDS. 1999;13:1905–1912. doi: 10.1097/00002030-199910010-00013. [DOI] [PubMed] [Google Scholar]
- 33.Otto B R, Verweij-van Vught A M J J, MacLaren D M. Transferrins and heme-compounds as iron sources for pathogenic bacteria. Crit Rev Microbiol. 1992;18:217–233. doi: 10.3109/10408419209114559. [DOI] [PubMed] [Google Scholar]
- 34.Peipert J F, Montagno A B, Cooper A S, Sung C J. Bacterial vaginosis as a risk factor for upper genital tract infection. Am J Obstet Gynecol. 1997;177:1184–1187. doi: 10.1016/s0002-9378(97)70038-3. [DOI] [PubMed] [Google Scholar]
- 35.Pettersson A, Maas A, Tommassen J. Identification of the iroA gene product of Neisseria meningitidis as a lactoferrin receptor. J Bacteriol. 1994;176:1764–1766. doi: 10.1128/jb.176.6.1764-1766.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Piot P, Van Dyck E, Goodfellow M, Falkow S. A taxonomic study of Gardnerella vaginalis (Haemophilus vaginalis) Gardner and Dukes 1955. J Gen Microbiol. 1980;119:373–396. doi: 10.1099/00221287-119-2-373. [DOI] [PubMed] [Google Scholar]
- 37.Sadhu K, Domingue P A G, Chow A W, Nelligan J, Cheng N, Costerton J W. Gardnerella vaginalis has a gram-positive cell-wall ultrastructure and lacks classical cell-wall lipopolysaccharide. J Med Microbiol. 1989;29:229–235. doi: 10.1099/00222615-29-3-229. [DOI] [PubMed] [Google Scholar]
- 38.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 39.Schmitt M P, Holmes R K. Iron-dependent regulation of diphtheria toxin and siderophore expression by the cloned Corynebacterium diphtheriae repressor gene dtxR in C. diphtheriae C7 strains. Infect Immun. 1991;59:1899–1904. doi: 10.1128/iai.59.6.1899-1904.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmitt M P, Talley B G, Holmes R K. Characterization of lipoprotein IRP1 from Corynebacterium diphtheriae, which is regulated by the diphtheria toxin repressor (DtxR) and iron. Infect Immun. 1997;65:5364–5367. doi: 10.1128/iai.65.12.5364-5367.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schryvers A B, Bonnah R, Yu R, Wong H, Retzer M. Bacterial lactoferrin receptors. Adv Exp Med Biol. 1998;443:123–133. doi: 10.1007/978-1-4757-9068-9_15. [DOI] [PubMed] [Google Scholar]
- 42.Schryvers A B, Stojiljkovic I. Iron acquisition systems in the pathogenic Neisseria. Mol Microbiol. 1999;32:1117–1123. doi: 10.1046/j.1365-2958.1999.01411.x. [DOI] [PubMed] [Google Scholar]
- 43.Scott T G, Curran B, Smyth C J. Electron microscopy of adhesive interactions between Gardnerella vaginalis and vaginal epithelial cells, McCoy cells, and human red blood cells. J Gen Microbiol. 1989;135:475–480. doi: 10.1099/00221287-135-3-475. [DOI] [PubMed] [Google Scholar]
- 44.Sewankambo N, Gray R H, Wawer M J, Paxton L, McNaim D, Wabwire-Mangen F, Serwadda D, Li C, Kiwanuka N, Hillier S L, Rabe L, Gaydos C A, Quinn T C, Konde-Lule J. HIV-1 infection associated with abnormal vaginal flora morphology and bacterial vaginosis. Lancet. 1997;350:546–550. doi: 10.1016/s0140-6736(97)01063-5. [DOI] [PubMed] [Google Scholar]
- 45.Smith S M, Ogbara T, Eng R H K. Involvement of Gardnerella vaginalis in urinary tract infections in men. J Clin Microbiol. 1992;30:1575–1577. doi: 10.1128/jcm.30.6.1575-1577.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spiegel C A. Bacterial vaginosis. Clin Microbiol Rev. 1991;4:484–502. doi: 10.1128/cmr.4.4.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wooldridge K G, Williams P H. Iron uptake mechanisms of pathogenic bacteria. FEMS Microbiol Rev. 1993;12:325–348. doi: 10.1111/j.1574-6976.1993.tb00026.x. [DOI] [PubMed] [Google Scholar]