Abstract
Purpose
To evaluate efficacy and vision with 2 prototype myopia control soft contact lenses with noncoaxial ring-focus designs (for enhancing efficacy [EE] and enhancing vision [EV]) compared with dual-focus (DF) and single-vision (SV) designs.
Design
Multicenter, 6-month, randomized, controlled, double-masked clinical trial.
Participants
One hundred ninety-nine myopic (−0.75 diopters [D] to −4.50 D) children aged 7 to 12 years.
Methods
Participants were randomized with stratification into myopia control (EE, EV, or DF) or SV arms at 9 clinical sites in 3 countries. Postcycloplegia axial length (AL) and spherical equivalent autorefraction (SECAR) were measured at baseline and 26 weeks. Axial length was also measured without cycloplegia at baseline, 1, 4, 13, and 26 weeks. Progression was analyzed using linear mixed models by intention-to-treat population. Visual acuity (VA) and vision quality were monitored.
Main Outcome Measures
Axial elongation, change in SECAR.
Results
A total of 185 subjects completed the study (n = 44, 49, 45, and 47 for EE, EV, DF, and SV, respectively). There were no serious/significant ocular adverse events. After 26 weeks, EE, EV, and DF all had statistically significantly less axial elongation than SV (unadjusted mean [standard deviation]: EE, 0.079 [0.125]; EV, 0.119 [0.101]; DF, 0.135 [0.117]; SV; 0.189 [0.121] mm). The estimated least-square mean (LSM) differences (adjusted 95% confidence interval) compared with SV were −0.105 (−0.149, −0.062), −0.063 (−0.106, −0.020), and −0.056 (−0.100, −0.013) mm for EE, EV, and DF, respectively. Enhancing efficacy alone had statistically significantly less progression of SECAR than SV (EE: −0.12 [0.27] D vs. SV: −0.35 [0.33] D; LSM difference: 0.22 D [0.09, 0.35]). Enhancing efficacy also had statistically significantly less axial elongation than DF (−0.049 mm [−0.093, −0.004]). Changes in AL and SECAR of EV and DF were not statistically different. All 3 myopia control lenses had mean VA close to 0.00 logarithm of the minimum angle of resolution (logMAR) with estimated 95% upper confidence limits <0.10 logMAR. Enhancing efficacy and DF produced similar reports of halos but more than EV and SV.
Conclusions
The prototype contact lenses met the design intent; EE was more efficacious in slowing axial elongation than DF with comparable vision performance, whereas EV produced comparable efficacy to DF with similar vision performance to SV.
Keywords: Axial length, Myopia control, Pediatric, Soft contact lenses, Vision
Abbreviations and Acronyms: AL, axial length; B2B, bottom-2-boxes; CI, confidence interval; D, diopter; DF, dual-focus; EE, enhanced efficacy; EV, enhanced vision; logMAR, logarithm of the minimum angle of resolution; LSM, least-square mean; MMRM, mixed models with repeated measures; SD, standard deviation; SE, standard error; SECAR, spherical equivalent cycloplegic autorefraction; SV, single-vision; VA, visual acuity
With increased prevalence of myopia worldwide, a recent focus of clinical and scientific research has been to find safe, effective methods for controlling myopia progression.1,2 Eye care practitioners have a long history of introducing relative plus power (“ADD” power) to myopia correcting lenses to slow myopia progression, albeit originally in relation to a perceived link between binocular and accommodative problems and myopia progression.3,4 Also, research in young animals shows that hyperopic defocus at the retina leads to development of myopia.5 As such, various modern optical interventions that introduce myopic defocus in the visual field have been applied for myopia control.1,6 These include a variety of “simultaneous vision” soft contact lenses, such as aspheric progressive, dual-focus (DF), traditional multifocal, and extended depth of focus designs.6,7 These optical designs inevitably introduce aberrations to the eye, affecting vision.
Despite advances in methodologies, there seem to be limitations to the extent to which myopic progression may be slowed.8 Increasing the ADD power in the treatment zone of myopia control soft lenses has been shown to result in increased treatment efficacy.9 However, increasing ADD power in simultaneous vision lenses ostensibly increases the impact on vision.10, 11, 12 Thus, it is generally held that there is a trade-off between vision quality and myopia control efficacy when manipulating the magnitude of ADD power or the size and location of the treatment zone.
In striving to limit the compromise involved in this efficacy-vision trade-off, we have developed a new concept that differs from earlier designs. Most previous soft contact lens designs generally rely on presbyopic principles to achieve myopia control effect; that is, the ADD power in the treatment zone serves to focus rays from a near point source more or less to a focal point at the retina in an unaccommodated eye. Thus, rays originating from a distant object passing through this zone will be focused in front of the retina and degrade vision. This practice sets a constraint in the ADD power, size, and location of the treatment zone before vision quality is significantly compromised and, as it turns out, is unnecessary.13 An essential design objective of the concept tested here was to break this interdependence between efficacy and vision quality.
Here, we investigated this new design concept by comparing the myopia control efficacy and vision performance of 2 prototype contact lenses (enhanced efficacy [EE] and enhanced vision [EV], designed to enhance efficacy and vision, respectively) with concentric annulus, DF and single-vision (SV) lenses. We hypothesized that both prototype lenses would show significant reduction in axial elongation compared with the SV lens. We also hypothesized that EE would outperform the DF lens in myopia control efficacy but with similar vision performance and that EV would outperform the DF lens in vision performance with similar myopia control efficacy.
Methods
The clinical trial was performed in accordance with the ethical principles of the Declaration of Helsinki and standards for Good Clinical Practice. Before participation, informed assent and informed consent were obtained from each pediatric subject and their parent(s) or legal guardian(s), respectively. The research was approved by appropriate institutional review boards or independent ethic committees and regulatory authorities. This clinical trial was registered on ClinicalTrials.gov with the identifier NCT03408444 and conformed with the 2017 Final Rule for Clinical Trials Registration and Results Information Submission in accordance with Section 801 of the United States Food and Drug Administration Amendments Act of 2007.
Study Design
This was a multisite, prospective, randomized, controlled, double-masked clinical trial of 4 study soft contact lenses with a 4-arm-parallel group design conducted between December 2017 and May 2019 at 9 international clinical sites (1 in Canada, 4 in China, and 4 in the United States). Detailed site information can be found in the acknowledgment section of this article.
Healthy male and female children between 7 and 12 years of age (inclusive) with myopia between −0.75 diopters (D) and −4.50 D (inclusive) and 1.00 D or less astigmatism were invited to participate in the study. Eligible subjects had best sphero-cylindrical corrected visual acuity (VA) of 20/25 (i.e., 0.10 logarithm of the minimum angle of resolution [logMAR]) or better in each eye and were free of ocular and systemic pathologies.
The subjects were randomly assigned in a 1:1:1:1 ratio to wear 1 of 4 lens types in a daily disposable modality for a minimum of 6 months. Randomization was first stratified by site. At each site, subjects were further stratified based on age (7–9 and 10–12 years) and baseline refraction (−0.75 to −1.75 D and −2.00 to −4.50 D). Each clinical site followed a computer-generated randomization scheme for study lens assignment. The randomization scheme was generated using randomly permuted block randomization in the SAS software (Version 9.4, SAS Institute), with each block containing 4 different lens codes that were the only identifiers of the study lenses.
After initial lens fitting and dispensing, subjects were examined at 1 week (7 ± 3 days), 4 weeks (28 ± 7 days), 13 weeks (91 ± 7 days), and 26 weeks (182 ± 14 days) for measurement of study related parameters and general contact lens wear evaluation. Contact lens power was adjusted if VA was < 20/25 during the study. The study was terminated after all subjects had completed the 26-week follow-up visit. A study duration of 6 months was selected because efficacy in a myopia control contact lens should become apparent within this timeframe. In other words, if a meaningful effect was not evident within 6 months, we considered that this would be indicative of failure of the lens design concept.
Sample size was based on treatment efficacy of > 0.08 mm (standard deviation [SD]: 0.10) in axial elongation from baseline and > 0.20 D (SD: 0.32) in change of spherical equivalent cycloplegic autorefraction (SECAR) from baseline at 26 weeks. Controlling the 2-sided type I error rate at the 0.05 level, a sample size of 40 subjects per group would yield > 80% power for detecting treatment efficacy.
Development of EE and EV Lenses
Extensive preclinical work was performed on an optical table to establish design concepts that would mitigate the compromise between efficacy and vision.13,14 Change in axial length (AL) of eyes of human subjects was measured by optical biometry to track choroidal thickness change, a biomarker for myopigenic or myopia-protective optical signals.15 A spatial light modulator was used to present various optical designs to the eye, obviating the need to manufacture prototype lenses and allowing high throughput of designs for proof-of-concept testing. A pupil-tracking device maintained the position of the design relative to the eye. Vision performance testing, including VA and contrast sensitivity, were incorporated into the apparatus. A purpose-built halometer was also added to the optical table to allow width and brightness of halos to be measured.
With the apparatus described earlier, dozens of different optical stimuli were tested to arrive at an understanding of design features that drive short-term changes of choroidal thickness and impact vision.16, 17, 18 This led to the development and optimization of 2 prototype lens designs. Like other soft lenses used for controlling myopia progression, these designs have zones with relative plus optical power compared with that required to correct myopic error. As such, the vision correction zone neutralizes refractive error to provide clear vision, whereas rays of light passing through these “plus” zones create positive retinal blur (myopic defocus) for myopia control. Unlike conventional multifocal or DF designs, the “plus” power in the prototype lenses is created without generating a coaxial point focus. Rays passing through concentric annular zones of the prototype lenses form a ring focus in front of the retina. The dispersal of these rays is such that the impact on vision can be modulated compared with existing coaxial multifocal designs while still allowing control of myopia progression.13 These 2 prototype lenses were constructed to achieve either of 2 specific objectives. Enhanced efficacy was designed to increase myopia control efficacy via introduction of a greater amount of plus power than conventional multifocal or DF lens designs while maintaining comparable visual performance. Enhanced vision was designed to optimize vision while maintaining similar myopia control efficacy to a standard DF lens. Both lenses included 2 concentric, annular zones with +7 D noncoaxial plus power for myopia control treatment, but these annular treatment zones in the EE lens were positioned closer to the center of the lens than for EV. Enhanced efficacy also included a central +10 D coaxial treatment zone that was designed to further “boost” myopia control efficacy while limiting its impact on vision. These lens prototypes were granted “Breakthrough Device” designation by the United States Food and Drug Administration.
Study Contact Lenses
Four soft contact lenses were included in the study, comprising the 2 prototype lenses with multizone, concentric annulus, noncoaxial ring-focus designs (EE and EV)13; 1 lens with standard DF design with +2.5 D coaxial plus power in the treatment zone as a positive control19; and 1 SV negative control lens.
All 4 study lenses were manufactured in silicone hydrogel material (senofilcon A) and were identical in major design aspects and manufacturing process, with the front surface optical design being the only differentiating factor. Lens dimensional parameters were determined in previous studies to provide optimal fit in pediatric patients, with a diameter of 13.8 mm and an aspheric back surface with a central curvature of 7.9 mm. There were no visible features either on visual inspection (naked eye) or under slit lamp examination to differentiate the 4 study lenses. Furthermore, all 4 lenses were manufactured with the same packaging. Preassigned lens codes were printed on the primary label of the lens blister packs and cartons of the secondary packaging as the only information identifying the 4 study lenses. Both study personnel and subjects were masked to the identity of the study lenses. There was no breaking of double-masking during the study.
Lenses were worn in daily disposable mode with a minimum compliant wear time of 8 hours per day, 5 days per week, and a recommended wear time of 10 hours or more per day, 7 days per week. Lens wear compliance was evaluated at each follow-up visit based on the subject/parent-reported typical time of lens insertion and removal (during both weekdays and weekends). From this, the average number of hours of lens wear per day and the weighted average daily wear time per week were computed for each follow-up period.
EndPoints and Procedure
The 2 coprimary efficacy endpoints were axial elongation and change in SECAR from baseline to 26 weeks. Axial length was measured with Lenstar LS 900 (Haag-Streit) at 8 clinical sites and with IOLMaster 700 (Carl Zeiss Meditec AG) at 1 clinical site in China both before and after cycloplegia at baseline and 26 weeks. Additionally, AL was measured at 1, 4, and 13 weeks without cycloplegia to provide information on the time course of axial elongation within the first 6 months of treatment. Five repeated measurements of AL were obtained at each visit. Cycloplegic autorefraction was measured at baseline and 26 weeks using an open-field autorefractor (WAM-5500, Grand Seiko, Shigiya Machinery Works Ltd). Five repeated measures of sphero-cylindrical refraction, each of which was the mean of 3 consecutive readings, were obtained at each visit. Spherical equivalent power was computed from each of the 5 repeated measures. The averages of the 5 repeated measures of AL and SECAR were used for statistical analysis. Cycloplegia was achieved by 2 drops of 1% cyclopentolate 5 minutes apart. A third drop of 1% cyclopentolate was used if residual accommodation was 2.00 D or more 30 minutes after the second drop. Postcycloplegia AL and SECAR were measured at least 30 minutes after the last drop.
Monocular and binocular visual acuities were measured with a high-contrast Landolt C logMAR VA chart (Precision Vision) under high luminance (190 cd/m2) conditions. Measurements were taken at baseline with subjects corrected with spherical lenses in a trial frame and at each subsequent visit with subjects wearing the study lenses. Patient-reported outcomes regarding vision, comfort, and handling of study lenses were collected through a pediatric Contact Lens User Experience questionnaire. This was internally developed specifically for use by pediatric contact lens wearers based on the existing CLUE questionnaire for the adult population.20 The pediatric Contact Lens User Experience questionnaire included 9 questions on vision, 7 questions on comfort, and 6 questions on handling, all of which were graded on a 5-point frequency scale (Always to Never), except selected questions on ease of handling, which were rated on a severity scale (Extremely easy to Not easy). Only results for 1 week, 4 weeks, and 26 weeks are presented in this report.
Statistical Analysis
The primary efficacy endpoint analyses were comparisons of postcycloplegic axial elongation and change in SECAR in the EE, EV, and DF groups with those of the SV group. Axial elongation and change in SECAR from baseline to 26 weeks were analyzed separately using linear mixed models with repeated measures (MMRM) on the intention-to-treat population. The MMRM model included treatment group as a fixed effect and site as a random effect (G-side). Other baseline characteristics such as age, gender, race, and the corresponding baseline measures (AL or SECAR) were included as fixed covariates. The within-subject repeated measures collected from different eyes were considered as a random effect (R-side) with the covariance of residuals modeled using the unstructured covariance structure. Adjustment for multiple comparisons to control the inflation of type I error rate was conducted using Dunnett’s method.21
The same MMRM models were used for post hoc comparisons of axial elongation and change in SECAR among EE, EV, and DF lenses. A simulation-based method was used to adjust the multiple comparisons among the 3 study lenses.22 Post hoc analysis of proportion of eyes with shrinkage effect (i.e., reduction of noncycloplegic AL from baseline) over time was conducted using a similar MMRM model by controlling for key demographic and baseline characteristics and appropriately modeling random effects. Multiplicity was adjusted using Dunnett’s method. The effect of mesopic pupil size and lens wear time in myopia progression and potential interactions with lens type was also examined in a post hoc fashion.
Contact lens VA and change of VA from baseline were analyzed separately for the 4 study groups utilizing a similar statistical approach to those described above. Safety was assessed qualitatively by summarizing the number and rate of ocular adverse events by subjects and by eyes.
Results
Subject Disposition
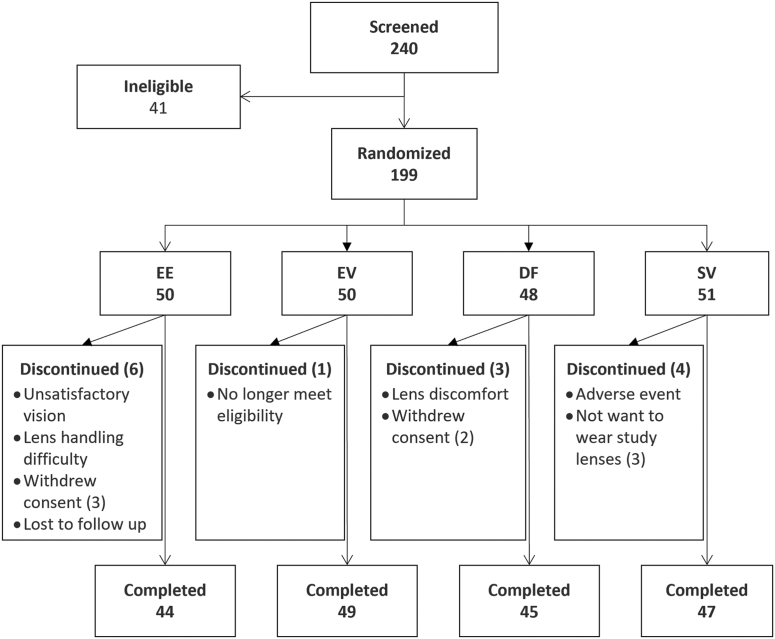
A total of 240 subjects was screened between December 2017 and October 2018, with 199 eligible subjects randomly assigned to the 4 study arms. Fourteen subjects discontinued; therefore, 185 subjects completed the study. Subject flow and reasons for discontinuation are presented in Figure 1. The study was concluded after the last subjects completed the 26-week follow-up.
Figure 1.
Flowchart illustrating the disposition of subjects from screening to completion, including reasons for subject discontinuation. DF = dual-focus lens group; EE = enhanced efficacy lens group; EV = enhanced vision lens group; SV = single-vision lens group.
Baseline Characteristics
Most of the enrolled subjects were neophytes who were either SV spectacle lens wearers (69%) or with no correction (20%) at the time of enrollment; the remaining 11% of subjects were habitual, SV, soft contact lens wearers. Demographics and baseline characteristics of the 199 randomized subjects are presented in Table 1. Of the 185 completed subjects, mean (SD) age was 10.0 (1.5) years (range: 7–12), 53% were females, 52% were Asians (50% of all completed subjects were ethnic Chinese), and 43% were White. Homogeneity testing found no statistically significant differences among the 4 groups with respect to age, gender, race, and baseline AL and SECAR in randomized (intention-to-treat) subjects.
Table 1.
Demographic and Baseline Characteristics of Enrolled Subjects
| EE | EV | DF | SV | |
|---|---|---|---|---|
| Age, yrs, mean (SD) | 10.0 (1.6) | 10.1 (1.4) | 10.2 (1.5) | 9.9 (1.6) |
| % < 10 yrs | 36 | 34 | 38 | 39 |
| % female | 48 | 56 | 60 | 49 |
| % Asian | 54 | 52 | 52 | 47 |
| Axial length, mm, mean (SD) | 24.65 (0.78) | 24.42 (0.86) | 24.31 (0.67) | 24.43 (0.79) |
| SECAR, D, mean (SD) | −2.50 (0.95) | −2.36 (0.99) | −2.34 (0.97) | −2.43 (1.00) |
| % −2.00 D or more myopia | 36 | 45 | 47 | 45 |
D = diopters; DF = dual-focus lens group; EE = enhanced efficacy lens group; EV = enhanced vision lens group; SD = standard deviation; SECAR = spherical equivalent cycloplegic autorefraction; SV = single-vision lens group.
Compliance with Wear Time
Average lens wear time (hours/day) was similar among all 4 lens groups. The mean (SD) weighted average daily wear time was 10.9 (2.4), 11.4 (1.8), 11.1 (2.1), and 11.2 (2.0) hours at 1 week and 12.6 (1.6), 12.2 (1.8), 12.7 (1.6), and 13.1 (1.4) hours at 26 weeks for EE, EV, DF, and SV groups, respectively.
Safety
There were no serious or significant ocular adverse events reported throughout the study. A total of 17 nonsignificant ocular adverse events (all reported as mild in severity) affecting 14 subjects (7% of all randomized subjects) was recorded. The most reported adverse event diagnoses were dryness (4 events in 2 subjects), followed by grade 2 or less slit lamp findings (3 events in 3 subjects) and conjunctival foreign bodies (3 events in 2 subjects). There was 1 asymptomatic, nonsignificant infiltrative event reported from 1 site in China at 26 weeks with no treatment required. Other adverse events included 1 case each of bacterial conjunctivitis, unspecified conjunctivitis, meibomian gland dysfunction, meibomianitis, stye, and chalazion. Four of the events were deemed possibly related, 6 unlikely to be related, and 7 not related to the lenses under study. None of the reported ocular adverse events led to subjects being discontinued from the study. Throughout the study, there were no observations of any grade 3 or higher slit lamp findings. There were 2 nonserious, nonocular adverse events (both “headaches”) reported from 2 subjects in the SV group that were deemed possibly related to the SV lens. One case was deemed severe in symptoms, which led to subject withdrawal from the study, the other case was deemed mild, and the subject completed the study with no action taken.
Efficacy
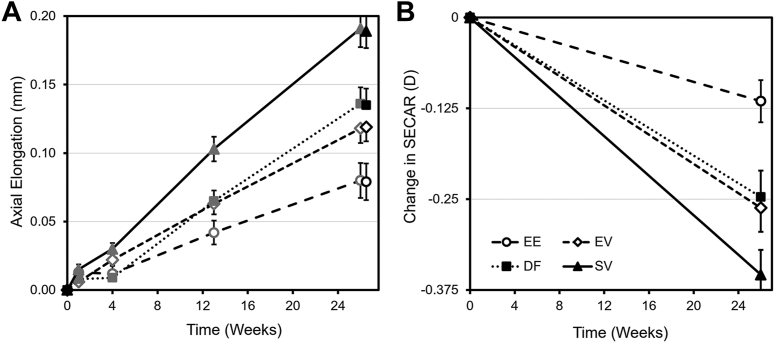
Figure 2 presents the unadjusted mean (standard error) axial elongation (Fig 2A) and change in SECAR (Fig 2B) by lens type across time. At 26 weeks, mean (SD) axial elongation from baseline was 0.079 (0.125), 0.119 (0.101), and 0.135 (0.117) mm for EE, EV, and DF, respectively, and 0.189 (0.121) mm for SV. Unadjusted mean SECAR change from baseline was EE, −0.12 (0.27); EV, −0.26 (0.32); DF, −0.25 (0.35) D; and SV, −0.35 (0.33) D. Table 2 shows differences in statistically adjusted means (least-square mean [LSM]) for the 3 myopia control lenses compared with SV with the corresponding adjusted 95% confidence intervals (95% CIs) at 26 weeks. All 3 myopia control lenses had statistically significantly less axial elongation than the SV lens, whereas EE was the only lens that also had statistically significantly less refractive progression (adjusted P < 0.05) than the SV lens. From the pairwise comparisons among the 3 myopia control lenses, EE also demonstrated statistically significantly less axial elongation than DF (LSM difference: −0.049 mm, adjusted 95% CI: −0.093 to −0.004 mm, adjusted P < 0.05).
Figure 2.
Unadjusted mean (standard error) change in (A) axial length (AL) and (B) spherical equivalent cycloplegic autorefraction (SECAR) by lens type across time. The 2 measures of AL at 26 weeks represent noncycloplegic (dark gray) and postcycloplegic measures (black). D = diopter; EE = enhanced efficacy lens group; EV = enhanced vision lens group; DF = dual-focus lens group; SV = single-vision lens group.
Table 2.
Least-Square Mean Differences with Adjusted 95% Confidence Intervals in Axial Elongation and Refractive Change with EE, EV, and DF Compared with Single-Vision Lens Group at 26 Weeks for the Intention-to-Treat Population
| Lens | EE | EV | DF |
|---|---|---|---|
| Axial elongation, mm | −0.105∗ (−0.149, −0.062) | −0.063∗ (−0.106, −0.020) | −0.056∗ (−0.100, −0.013) |
| SECAR change, D | 0.22∗ (0.09, 0.35) | 0.08 (−0.04, 0.21) | 0.12 (−0.01, 0.25) |
D = diopters; DF = dual-focus lens group; EE = enhanced efficacy lens group; EV = enhanced vision lens group; SECAR = spherical equivalent cycloplegic autorefraction.
Adjusted P < 0.05.
Reduction of AL from baseline (defined as a negative change in noncycloplegic AL) was observed at 1 week in some subjects of all 4 lens groups. The proportion of subjects showing reduced AL varied by time and lens group. The odds of showing reduced AL were significantly higher among subjects wearing EE and DF than SV at 4 weeks (47% and 34% vs. 15%; odds ratio [adjusted 95% CI] of 4.9 [1.8, 13.2] and 2.9 [1.1, 7.9], respectively; adjusted P < 0.05). At 13 and 26 weeks, EE was the only group that showed statistically significant odds of reduction of AL compared with SV (24% vs. 7% and 23% vs. 4%, respectively; odds ratio [adjusted 95% CI] of 4.0 [1.3, 12.2] and 7.4 [2.1, 26.5], respectively; adjusted P < 0.05).
At 4 weeks, significantly less mean axial elongation from baseline (LSM difference [adjusted 95% CI]) was observed for EE (−0.022 [−0.039, −0.004]) and DF (−0.022 [−0.037, −0.007]) than SV (adjusted P < 0.05). By 12 weeks, all 3 myopia control lens groups demonstrated statistically significantly less axial elongation than SV (−0.066 [−0.096, −0.035], −0.045 [−0.075, −0.015], and −0.044 [−0.074, −0.015] for EE, EV, and DF, respectively; adjusted P < 0.05), an effect that increased in magnitude by 26 weeks.
Post hoc analyses were conducted to examine the role of age, gender, baseline refraction, mesopic pupil size, and lens wear time in myopia progression (axial elongation and change in SECAR), as well as their interactions with lens type, by including these variables as covariates in statistical models. In both axial elongation and SECAR models, race was a significant factor (P < 0.0001 and P = 0.005, respectively) with Asians associated with more axial elongation and myopia progression, whereas race-by-lens type interaction was not statistically significant (P = 0.86 and P = 0.68, respectively). Age at baseline was only a significant factor for the axial elongation model (P = 0.002) with younger age associated with more axial elongation, whereas age-by-lens type interaction was not significant (P = 0.26). Baseline AL and refraction, as well as mesopic pupil size, were all found to be statistically insignificant in the axial elongation and SECAR models. Lens wear time was found to be significant (P = 0.006 and P = 0.037, respectively), and wear time-by-lens type interaction was significant in the axial elongation model at 0.15 significance level (P = 0.098), with longer wear time associated with less axial elongation.
Vision Performance
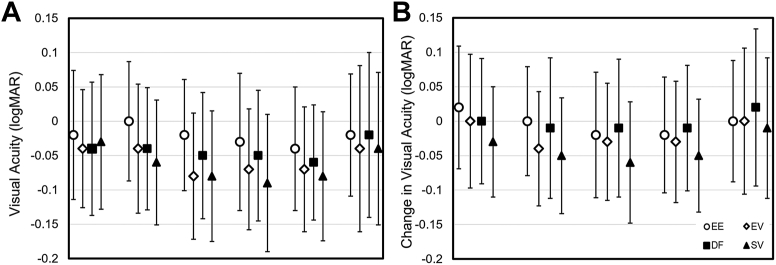
Figure 3 plots unadjusted mean (SD) monocular distance logMAR VA at baseline with best-sphere spectacle correction and with study lenses at each visit (Fig 3A), as well as changes of VA from baseline (Fig 3B). There was no significant difference in VA among the 4 lens groups at baseline. At initial lens fitting, mean (SD) best contact lens corrected VA was −0.00 (0.09), −0.04 (0.09), −0.04 (0.09), and −0.06 (0.09) logMAR for EE, EV, DF, and SV, respectively. None of the 3 groups wearing myopia control lenses had mean VA that was statistically different from 0.00 logMAR, and none were statistically worse than baseline best-sphere spectacle correction (P > 0.05). Similar results were found in follow-up visits for EE and DF, except that, at 13 weeks, EE showed statistically significant improvement in VA by 0.03 (0.00, 0.05) logMAR compared with baseline (P < 0.05). Visual acuity with both EV and SV was found at several follow-up visits to be statistically better than 0.00 logMAR (e.g., −0.06 to −0.08 logMAR) and better than baseline by 0.03 to 0.06 logMAR (P < 0.05).
Figure 3.
A, Unadjusted mean (SD) monocular distance visual acuity with study lenses. B, Change in visual acuity (SD) between baseline and different timepoints in the study. DF = dual-focus lens group; EE = enhanced efficacy lens group; EV = enhanced vision lens group; SD = standard deviation; SV = single-vision lens group.
Throughout the study, there were no clinically or statistically significant differences between EV and SV groups in vision. Visual acuity with EE and DF was statistically worse than SV at some follow-up visits. The largest difference compared with SV was 0.07 (95% CI: 0.04, 0.10) logMAR for EE at 1 week and 0.05 (95% CI: 0.02, 0.07) logMAR for DF at 4 weeks. Despite this, the statistically estimated mean VA with EE and DF was not significantly different from 0.00 logMAR at all visits.
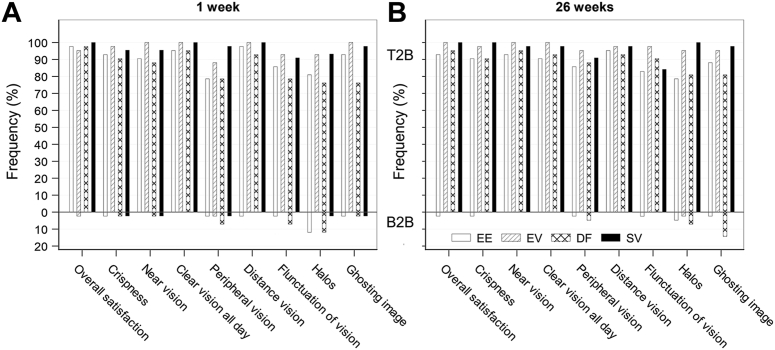
Subjective vision responses indicated that, for all 4 lens groups, > 90% of subjects reported they were “very happy” with how well they could see. Only 1 subject (in the EE group) was discontinued from the study because of unsatisfactory vision. Because of the nature of their optical designs, EE and DF were potentially expected to cause some reporting of visual symptoms, such as halos or ghost images. Figure 4 plots the rates of positive and negative responses to vision questions. Shown are the proportions of responses indicating the 2 positive (top-2-boxes) and 2 negative (bottom-2-boxes [B2B]) grades on a 5-point scale for each question in the questionnaire, respectively, for example, “always” or “usually” happy with clarity of vision throughout the day (top-2-boxes) and “rarely” or “never” happy with how well they could see (B2B).
Figure 4.
Subject reported vison outcomes at 1 week (A) and 26 weeks (B) in frequencies (%) of top-2-box (T2B, 2 best grades) and bottom-2-box (B2B, 2 worst grades) on a 5-point scale for each question in the subjective vision questionnaire. DF = dual-focus lens group; EE = enhanced efficacy lens group; EV = enhanced vision lens group; SV = single-vision lens group.
Consistent with findings of VA measures, both EE and DF groups had more subjects with negative responses than EV and SV groups. The rate of reporting seemed to decrease over time, potentially because of subjects adapting to the lenses. At 1 week, the main visual symptom reported by subjects in the EE and DF groups was halos (B2B: 12% for both). By 26 weeks, this rate decreased to 5% and 7% for subjects of the EE and DF groups, respectively, whereas there were 14% of subjects in the DF group reporting usually or always noticing ghost images at 26 weeks compared with 2% in the EE group.
Lens Fit, Handling, and Comfort Performance
No instances of unacceptable lens fit were observed in any lens group throughout the study. Because all 4 study lenses had identical lens geometry, as expected, all 4 study lenses had similar fitting characteristics. At all timepoints, all 4 groups had > 94% eyes with centered lens fit. There were no reports of substantial lens decentration. Optimal movement was reported in > 86% of eyes (4 groups combined) with no report of insufficient or excessive lens movement.
Study lenses were successfully dispensed in 96% of subjects after 1 training session for contact lens insertion and removal. The pediatric Contact Lens User Experience questionnaire indicated that, among the 191 subjects completing 4 weeks of wear, 93% and 95% agreed that it was "extremely easy," "very easy," or "easy" to insert and remove lenses, respectively.
There were no major issues identified in the pediatric Contact Lens User Experience questionnaire regarding subjective comfort. All 4 lenses performed similarly with regard to comfort. For example, rates of positive responses (top 2 grades on the 5-point scale) to the question “Were the contact lenses comfortable?” were above 85% at all follow-up visits.
Discussion
All 3 myopia control designs were effective at slowing axial elongation after 6 months of lens wear while providing good visual quality. Because EE produced greater myopia control efficacy while maintaining comparable visual performance to DF and EV provided essentially unaffected vision while maintaining similar myopia control efficacy to DF, the clinical performance of EE and EV was consistent with the design intent. The noncoaxial ring-focus technology of the prototype lenses used in this study offers potential performance advantages over traditional coaxial focus lenses and may mitigate the trade-off between efficacy and visual quality with such designs.
Axial length, as measured by optical biometry, is emerging as the preferred metric for assessing efficacy of myopia control products because it is more repeatable than refractive error measurement, may be more closely related to the risk of complications later in life, and can be measured accurately without the need for cycloplegia.8 Indeed, efficacy with respect to axial elongation was evident at 4 weeks for EE and at 13 weeks for all of the myopia control lenses, in which refractive error differences to SV remained not statistically significant for EV and DF at 26 weeks. Being able to discern a statistically significant treatment effect by optical biometry within a 4-week period may have implications for lessening the burden of screening myopia control prototype products.
Table 3 shows control of axial elongation from other studies in which contact lenses were used and 6-month myopia control data were reported. The efficacy of the EE lens in reducing axial elongation (0.105 mm compared with the SV control) observed in this study makes this lens a viable candidate for myopia control in young children.
Table 3.
Previous 6-Month Results from Studies Using Myopia Control Contact Lenses
| Study (First Author, Year)∗ | Treatment | Reduction in Axial Elongation (mm) |
|---|---|---|
| This study | SMCL | 0.11 |
| Lam et al,23 2014 | SMCL | 0.04 |
| Aller et al,24 2016 | SMCL | 0.11 |
| Cheng et al,25 2016 | SMCL | 0.11 |
| Sankaridurg et al,26 2019 | SMCL | 0.09 |
| Sankaridurg et al,26 2019 | SMCL | 0.07 |
| Sankaridurg et al,26 2019 | SMCL | 0.07 |
| Sankaridurg et al,26 2019 | SMCL | 0.08 |
| Cho et al,27 2012 | OK | 0.10 |
| Santodomingo-Rubido et al,28 2012 | OK | 0.06 |
OK = orthokeratology; SMCL = soft multifocal contact lens.
Only studies reporting 6-month efficacy using optical biometry and with data obtained over the last decade are included. Studies of spectacle lenses and orthokeratology studies with subpopulations of myopes (high myopia and significant astigmatism) are also not shown.
Our results show consistency with previous studies. Subjects wearing the SV lens showed unadjusted axial elongation and myopia progression of 0.189 mm and 0.35 D, which are not unexpected values for a group of myopic children of around 10 years of age.25, 26, 27 In addition, although myopia progression was observed to be greater in younger children and Asians,29,30 there was no evidence that myopia control efficacy varies with age or race.25,31,32 Compliance was found to impact treatment efficacy, consistent with some previous work.26,23 Some subjects showed an initial reduction in AL in response to myopia control treatment, a phenomenon that has been reported previously.8,24,33
Strengths of this study included the application of the gold standard for intervention studies, that is, a controlled, randomized, double-masked design. This study was also multisite, which is of higher value than single-center studies because the results are more generalizable.34 High completion rate, the lens wearing time considerably surpassing the target, and good safety profile demonstrate that most children can successfully wear soft contact lenses made in senofilcon A material. Furthermore, our efficacy analysis was based on intention-to-treat, so the results include those who were not compliant with minimum wearing times, reflecting what may be expected in clinical practice.
The major limitation of this study was its relatively short duration. The 6-month results presented here currently do not allow confident prediction of longer-term treatment effect. The early rate of treatment efficacy in myopia control products has been shown to reduce over time,8,35,36 so linear (or percentage) projections are inappropriate.8 Models to project longer-term treatment efficacy from short-term trials are needed because there are (i) ethical concerns around assigning children to a control group in a myopia control clinical trial for a period of, say, 3 years, when known treatments are available and (ii) logistical and resource challenges in conducting long-term studies. At the time of study, there were no myopia control soft contact lenses approved or available in the United States or China where 8 out of the 9 clinical sites were located. In light of the above considerations, we chose to proceed with a 6-month, placebo-controlled study for the purpose of validating the new design concepts. Our reasoning was that a statistically distinguishable difference in the rate of eye growth between a treatment and control group should emerge within this timeframe if the treatment is to provide a meaningful performance difference over the longer term. Because subjects were randomized and the enrollment period was extended over 10 months, seasonal effects were more likely to contribute random error than systematic bias. Nonetheless, because of the short study duration, the magnitude of the observed myopia control efficacy for the EE lens, especially in comparison to the DF lens, was limited and statistically significant for the AL end point alone. Establishment of clinically meaningful superiority requires further evaluation over a longer period of time.
In summary, the efficacy and vision performance of the EE and EV lens met the design intent, demonstrating that noncoaxial ring-focus technology offers an alternative approach with the potential to mitigate some of the limitations of conventional presbyopic coaxial principles. We consider EE a viable soft contact lens candidate for further investigation of myopia control in children. Additional international, randomized, controlled clinical trials with longer study duration are currently ongoing to gather comprehensive clinical data on the lens safety and efficacy.
Acknowledgments
The authors thank the following clinical sites and investigators:
University of Waterloo Center for Ocular Research & Education: Lyndon Jones, Jill Woods, Debbie Jones, Karen Walsh, Marc Schulze, Mike Yang, Amir M. Moezzi
Eye Hospital of Wenzhou Medical University: Jun Jiang, Feifu Wang, Wanqing Jin, Yi Shen, Ge Wu, Huiling Lin
Sun Yat-sen University Zhongshan Ophthalmic Center: Zhi Lin, Xiang Chen, Jincheng Shi, Yusu Shao, Hening Zhang
Fudan University Shanghai Eye & ENT Hospital: Xiaomen Qu, Zhi Chen, Li Zeng, Meng Li, Jiaqi Zhou, Qianni Jiang
Tianjin Medical University Eye Hospital: Ruihua Wei, Hongmei Zhang, Bei Du, Ying Zhu, Ding Han
Kannarr Eye Care: Shane R. Kannarr, Christopher J. Jacquinot, Katherine A. Painter
David W Ferris & Associates: Stephen M. Montaquila, Georgia Patsiopoulos, Alyssa M. Campagnone
Sabal Eye Care: Christopher G. Pearson
Maitland Vision Center: Bradley S. Giedd, Ryan W. Schott
Clinical Research Organization:
Graeme Young, Ruth Craven, and staff at Visioncare Research Ltd
Manuscript no. D-22-00181.
Footnotes
Disclosure(s):
All authors have completed and submitted the ICMJE disclosures form.
The author(s) have made the following disclosure(s): X.C.: Employee – Johnson & Johnson Vision; Patent – US10901237 assigned to Johnson & Johnson Vision.
J.X.: Employee – Johnson & Johnson Vision.
N.A.B.: Employee – Johnson & Johnson Vision; Patent – US10901237 assigned to Johnson & Johnson Vision.
The prototype lenses discussed in this article are subject to US Patent No 10901237, assigned to Johnson & Johnson Inc. These lenses are commercially available in some countries outside of the United States.
Funded and coordinated by Johnson & Johnson Vision.
HUMAN SUBJECTS: Human subjects were included in this study. The clinical trial was performed in accordance with the ethical principles of the Declaration of Helsinki and standards for Good Clinical Practice. Before participation, informed assent and informed consent were obtained from each pediatric subject and their parent(s) or legal guardian(s), respectively. The research was approved by appropriate Institutional Review Boards or Independent Ethic Committees and Regulatory Authorities.
No animal subjects were used in this study.
Author Contributions:
Conception and design: Cheng, Brennan
Data collection: Cheng
Analysis and interpretation: Cheng, Xu, Brennan
Obtained funding: Study was performed as part of regular employment duties at Johnson & Johnson Vision. No additional funding was provided.
Overall responsibility: Cheng, Xu, Brennan
References
- 1.Walline J.J., Lindsley K.B., Vedula S.S., et al. Interventions to slow progression of myopia in children. Cochrane Database Syst Rev. 2020;1:CD004916. doi: 10.1002/14651858.CD004916.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jonas J.B., Ang M., Cho P., et al. IMI prevention of myopia and its progression. Invest Ophthalmol Vis Sci. 2021;62:6. doi: 10.1167/iovs.62.5.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wick R.E. The use of bifocals in myopia; a case report. Am J Optom Arch Am Acad Optom. 1947;24:368–371. doi: 10.1097/00006324-194708000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Shotwell A.J. Plus lenses, prisms, and bifocal effects on myopia progression in military students. Am J Optom Physiol Opt. 1981;58:349–354. doi: 10.1097/00006324-198105000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Troilo D., Smith E.L., III, Nickla D.L., et al. IMI - report on experimental models of emmetropization and myopia. Invest Ophthalmol Vis Sci. 2019;60:M31–M88. doi: 10.1167/iovs.18-25967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang W.C., Leung M., Wong A.C., et al. In: Updates on Myopia. Ang M., Wong T.Y., editors. Springer; Singapore: 2020. Optical interventions for myopia control. [Google Scholar]
- 7.Li Q., Fang F. Advances and challenges of soft contact lens design for myopia control. Appl Opt. 2019;58:1639–1656. doi: 10.1364/AO.58.001639. [DOI] [PubMed] [Google Scholar]
- 8.Brennan N.A., Toubouti Y.M., Cheng X., Bullimore M.A. Efficacy in myopia control. Prog Retin Eye Res. 2021;83 doi: 10.1016/j.preteyeres.2020.100923. [DOI] [PubMed] [Google Scholar]
- 9.Walline J.J., Walker M.K., Mutti D.O., et al. Effect of high add power, medium add power, or single-vision contact lenses on myopia progression in children: the BLINK randomized clinical trial. JAMA. 2020;324:571–580. doi: 10.1001/jama.2020.10834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kang P., McAlinden C., Wildsoet C.F. Effects of multifocal soft contact lenses used to slow myopia progression on quality of vision in young adults. Acta Ophthalmol. 2017;95:e43. doi: 10.1111/aos.13173. –e53. [DOI] [PubMed] [Google Scholar]
- 11.Schulle K.L., Berntsen D.A., Sinnott L.T., et al. Visual acuity and over-refraction in myopic children fitted with soft multifocal contact lenses. Optom Vis Sci. 2018;95:292–298. doi: 10.1097/OPX.0000000000001207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bickle K.M., Mitchell G.L., Walline J.J. Visual performance with spherical and multifocal contact lenses in a pediatric population. Optom Vis Sci. 2021;98:483–489. doi: 10.1097/OPX.0000000000001695. [DOI] [PubMed] [Google Scholar]
- 13.Brennan N.A., Cheng X., Hernandez J.V., et al. Johnson & Johnson, assignee. Ophthalmic lens with an optically non-coaxial zone for myopia control. US Patent No 10901237. 2021 [Google Scholar]
- 14.Collins MJ, Davis BA, Yi F. Queensland University of Technology, Applicant. Apparatus, method and system for measuring the influence of ophthalmic lens design. US Patent Application US20200081269A1. 2020.
- 15.Read S.A., Collins M.J., Sander B.P. Human optical axial length and defocus. Invest Ophthalmol Vis Sci. 2010;51:6262–6269. doi: 10.1167/iovs.10-5457. [DOI] [PubMed] [Google Scholar]
- 16.Davis B., Collins M.J., Yi F., et al. The impact on eye length and vision performance for a range of positive defocus levels in two-zone bifocal contact lens adaptive optics simulations. Invest Ophthalmol Vis Sci. 2021;62:2914. [Google Scholar]
- 17.Yi F., Brennan N.A., Davis B., et al. The impact on short term axial length and vision performance of myopia control optics with coaxial and non-coaxial plus power lenslets. Invest Ophthalmol Vis Sci. 2021;62:2906. [Google Scholar]
- 18.Brennan N.A., Collins M.J., Cheng X. Design concepts for a myopia control soft contact lens. Invest Ophthalmol Vis Sci. 2022;63:1438. [Google Scholar]
- 19.Phillips J. Auckland Uniservices Ltd, assignee. Contact lens and method. US Patent No. 7832859. 2010 [Google Scholar]
- 20.Wirth R.J., Edwards M.C., Henderson M., et al. Development of the contact lens user experience: CLUE scales. Optom Vis Sci. 2016;93:801–808. doi: 10.1097/OPX.0000000000000913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dunnett C.W. A multiple comparison procedure for comparing several treatments with a control. J Am Stat Assoc. 1955;50:1096–1121. [Google Scholar]
- 22.Edwards D., Berry J.J. The efficiency of simulation-based multiple comparisons. Biometrics. 1987;43:913–928. [PubMed] [Google Scholar]
- 23.Lam C.S., Tang W.C., Tse D.Y., et al. Defocus Incorporated Soft Contact (DISC) lens slows myopia progression in Hong Kong Chinese schoolchildren: a 2-year randomised clinical trial. Br J Ophthalmol. 2014;98:40–45. doi: 10.1136/bjophthalmol-2013-303914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aller T.A., Liu M., Wildsoet C.F. Myopia control with bifocal contact lenses: a randomized clinical trial. Optom Vis Sci. 2016;93:344–352. doi: 10.1097/OPX.0000000000000808. [DOI] [PubMed] [Google Scholar]
- 25.Cheng X., Xu J., Chehab K., et al. Soft contact lenses with positive spherical aberration for myopia control. Optom Vis Sci. 2016;93:353–366. doi: 10.1097/OPX.0000000000000773. [DOI] [PubMed] [Google Scholar]
- 26.Sankaridurg P., Bakaraju R.C., Naduvilath T., et al. Myopia control with novel central and peripheral plus contact lenses and extended depth of focus contact lenses: 2 year results from a randomised clinical trial. Ophthal Physiol Opt. 2019;39:294–307. doi: 10.1111/opo.12621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cho P., Cheung S.W. Retardation of myopia in Orthokeratology (ROMIO) study: a 2-year randomized clinical trial. Invest Ophthalmol Vis Sci. 2012;53:7077–7085. doi: 10.1167/iovs.12-10565. [DOI] [PubMed] [Google Scholar]
- 28.Santodomingo-Rubido J, Villa-Collar C, Gilmartin B, Gutiérrez-Ortega R. Myopia control with orthokeratology contact lenses in Spain: refractive and biometric changes. Invest Ophthalmol Vis Sci. 2012;53(8):5060–5065. doi: 10.1167/iovs.11-8005. [DOI] [PubMed] [Google Scholar]
- 29.Donovan L., Sankaridurg P., Ho A., et al. Myopia progression rates in urban children wearing single-vision spectacles. Optom Vis Sci. 2012;89:27–32. doi: 10.1097/OPX.0b013e3182357f79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shamp W. Influence of age and race on axial elongation in myopic children. Invest Ophthalmol Vis Sci. 2022;63:257. [Google Scholar]
- 31.Berntsen D.A., Sinnott L.T., Mutti D.O., Zadnik K. A randomized trial using progressive addition lenses to evaluate theories of myopia progression in children with a high lag of accommodation. Invest Ophthalmol Vis Sci. 2012;53:640–649. doi: 10.1167/iovs.11-7769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chamberlain P., Peixoto-de-Matos S.C., Logan N.S., et al. A 3-year randomized clinical trial of misight lenses for myopia control. Optom Vis Sci. 2019;96:556–567. doi: 10.1097/OPX.0000000000001410. [DOI] [PubMed] [Google Scholar]
- 33.Kumaran A., Htoon H.M., Tan D., Chia A. Analysis of changes in refraction and biometry of atropine- and placebo-treated eyes. Invest Ophthalmol Vis Sci. 2015;56:5650–5655. doi: 10.1167/iovs.14-14716. [DOI] [PubMed] [Google Scholar]
- 34.Gold J.L., Dewa C.S. Institutional review boards and multisite studies in health services research: is there a better way? Health Serv Res. 2005;40:291–307. doi: 10.1111/j.1475-6773.2005.00354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang J., Wen D., Wang Q., et al. Efficacy comparison of 16 interventions for myopia control in children: a network meta-analysis. Ophthalmology. 2016;123:697–708. doi: 10.1016/j.ophtha.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 36.Kaphle D., Atchison D.A., Schmid K.L. Multifocal spectacles in childhood myopia: are treatment effects maintained? A systematic review and meta-analysis. Surv Ophthalmol. 2020;65:239–249. doi: 10.1016/j.survophthal.2019.10.001. [DOI] [PubMed] [Google Scholar]