SUMMARY
Liver cancer epidemiology is changing due to increasing alcohol consumption, rising prevalence of obesity, and advances in hepatitis B virus (HBV) and hepatitis C virus (HCV) treatment. However, the impact of these changes on global liver cancer burden remains unclear. We estimated global and regional temporal trends in the burden of liver cancer and the contributions of various liver disease etiologies using the methodology framework of the Global Burden of Disease study. Between 2010 and 2019, there was a 25% increase in liver cancer deaths. Age-standardized death rates (ASDRs) increased only in the Americas and remained stable or fell in all other regions. Between 2010 and 2019, non-alcoholic steatohepatitis (NASH) and alcohol had the fastest growing ASDRs, while HCV and HBV declined. Urgent measures are required at a global level to tackle underlying metabolic risk factors and slow the growing burden of NASH-associated liver cancer, especially in the Americas.
In brief
Huang et al. estimate global and regional trends in the burden of liver cancer and the contributions of various liver disease etiologies from 2010 to 2019. They determined that NASH was the fastest growing cause of age-adjusted liver cancer deaths globally, while age-adjusted deaths from hepatitis B and C declined.
Graphical Abstract
INTRODUCTION
Liver cancer is the third leading cause of cancer death worldwide (Sung et al., 2021). The major etiologies for liver cancer are hepatitis B virus (HBV), hepatitis C virus (HCV), alcohol, and non-alcoholic steatohepatitis (NASH) (Global Burden of Disease Liver Cancer Collaboration et al., 2017). Over the past decade, there have been changes in the burden and etiology of liver diseases (Asrani et al., 2019).
Increasing vaccination coverage for HBV, coupled with the widespread availability of antiviral therapy, has reduced HBV-associated liver cancer burden worldwide (Huang et al., 2021b; Huang and Lim, 2018, 2020; Lok et al., 2016; Thomas, 2019). Safe and effective oral antivirals have been available for HCV since 2014, which can similarly significantly reduce hepatocellular carcinoma (HCC) risk, although the impact of HCV treatment on global liver cancer burden remains unclear (Singal and El-Serag, 2015). Increasing economic wealth has fueled an increase in global alcohol-per-capita consumption, which may have increased the burden of alcohol-associated liver cancer (El-Serag et al., 2020; Seitz et al., 2018). Finally, the prevalence of NASH has also risen in parallel with obesity and diabetes, leading to demonstrated rises in NASH-associated liver cancer in the United States, Europe, and Asia; however, recent global data are still lacking (Dyson et al., 2014; El-Serag et al., 2020; Huang et al., 2021a; Le et al., 2021; Loomba et al., 2021; Loomba and Sanyal, 2013).
Previous studies on liver cancer epidemiology have largely focused on country-, region-, or etiology-specific data. Updated data from a global perspective are limited. Herein, we report temporal trends in liver cancer incidence, mortality, and disability-adjusted life-years (DALYs) and the contributions of various liver disease etiologies across 204 countries and territories from 2010 to 2019.
RESULTS
Global burden of liver cancer in 2019
Globally, in 2019, there were 534,364 incident cases (95% uncertainty interval [UI] 486,550–588,639), 484,577 deaths (95% UI 444,091–525,798), and 12.5 million (95% UI 11.4–13.7 million) DALYs due to liver cancer (Tables 1, 2, and S4; Figure 1A). In 2019, the estimated age-standardized incident rate (ASIR), age-standardized death rate (ASDR), and age-standardized DALYs (ASDALYs) of liver cancer were 6.51 per 100,000 (95% UI 5.95–7.16), 5.95 per 100,000 (95% UI 5.44–6.44), and 151.08 per 100,000 (95% UI 137.53–167.82), respectively. Between 2010 and 2019, there was a 27% increase in the frequency of incident liver cancer cases, a 25% increase in liver cancer deaths, and 21% increase in DALYs. Over this period, the estimated annual percentage changes (APCs) of the ASIR and ASDR were stable, with APCs of 0.03% (95% CI −0.01 to 0.05) and −0.10% (95% CI −0.25 to 0.06), respectively, whereas ASDALYs decreased (APC −0.27%, 95% CI −0.34 to −0.21).
Table 1.
Incident cases and age-standardized incidence rates of liver cancer in 2010 and 2019 and the temporal trend of age-standardized incident rates from 2010 to 2019
| 2010 | 2019 | ||||
|---|---|---|---|---|---|
|
|
|
|
|
||
| No. incident cases (95% UI) | ASIR per 100,000 (95% UI) | No. incident cases (95% UI) | ASIR per 100,000 (95% UI) | Annual percentage change of ASIR (95% CI) | |
|
| |||||
| Global | 420,196 (398,639–440,763) | 6.50 (6.15–6.81) | 534,364 (486,550–588,639) | 6.51 (5.95–7.16) | 0.03 (−0.01 to 0.05) |
|
| |||||
| Sex | |||||
|
| |||||
| Male | 292,049 (276,249–310,447) | 9.54 (9.03–10.13) | 376,483 (421,982–335,003) | 9.71 (8.69–10.84) | 0.21 (0.20–0.23) |
| Female | 128,147 (119,003–134,959) | 3.76 (3.49–3.96) | 157,881 (140,436–176,052) | 3.63 (3.23–4.05) | −0.39 (−0.41 to −0.37) |
|
| |||||
| Socio-demographic index | |||||
|
| |||||
| Low SDI | 16,006 (14,421–17,603) | 3.90 (3.53–4.28) | 19,279 (16,951–21,648) | 3.69 (3.27–4.11) | −0.62 (−0.64 to −0.59) |
| Low-middle SDI | 41,378 (38,129–44,607) | 3.89 (3.55–4.20) | 55,345 (50,136–61,558) | 4.05 (3.67–4.51) | 0.49 (0.42–0.55) |
| Middle SDI | 152,103 (141,917–165,051) | 7.85 (7.33–8.49) | 185,567 (162,261–210,710) | 8.28 (7.24–9.47) | 0.68 (0.52–0.84) |
| High-middle SDI | 89,077 (83,223–95,370) | 5.46 (5.10–5.84) | 106,792 (94,151–120,908) | 5.34 (4.70–6.05) | −0.25 (−0.40 to −0.10) |
| High SDI | 121,477 (112,975–126,370) | 8.01 (7.54–8.29) | 140,145 (125,500–154,013) | 7.61 (6.88–8.36) | −0.58 (−0.74 to −0.42) |
|
| |||||
| Region | |||||
|
| |||||
| Africa | 17,073 (15,331–18,873) | 4.43 (3.99–4.86) | 21,024 (18,482–23,886) | 4.08 (3.63–4.57) | −0.89 (−0.91 to −0.87) |
| Eastern Mediterranean | 21,819 (20,220–23,921) | 6.76 (6.28–7.38) | 28,024 (22,850–34,563) | 6.45 (5.32–7.90) | −0.50 (−0.53 to −0.47) |
| Europe | 60,706 (58,037–62,220) | 4.42 (4.25–4.53) | 68,698 (61,389–76,767) | 4.39 (3.94–4.90) | −0.15 (−0.38 to 0.09) |
| Americas | 35,370 (33,973–36,349) | 3.56 (3.42–3.66) | 49,708 (43,650–56,326) | 3.92 (3.45–4.44) | 1.11 (1.09–1.15) |
| Southeast Asia | 53,133 (48,832–62,220) | 4.16 (3.81–4.46) | 69,181 (60,277–79,273) | 4.04 (3.52–4.63) | −0.34 (−0.39 to −0.28) |
| Western Pacific | 230,265 (212,955–249,156) | 11.02 (10.19–11.89) | 295,484 (257,479–339,134) | 11.02 (9.62–12.61) | 0.02 (−0.05 to 0.09) |
|
| |||||
| Etiology | |||||
|
| |||||
| Alcohol | 74,377 (61,771–88,219) | 1.16 (0.96–1.37) | 98,463 (79,034–120,127) | 1.19 (0.96–1.45) | 0.34 (0.33–0.36) |
| Hepatitis B | 172,897 (154,745–192,114) | 2.57 (2.30–2.86) | 218,855 (186,488–254,887) | 2.62 (2.24–3.05) | 0.23 (0.17–0.29) |
| Hepatitis C | 123,598 (108,700–128,172) | 2.00 (1.75–2.24) | 152,225 (131,581–174,627) | 1.90 (1.64–2.17) | −0.60 (−0.67 to −0.54) |
| NASH | 26,220 (21,628–31,705) | 0.41 (0.34–0.50) | 36,339 (29,494–44,855) | 0.45 (0.37–0.55) | 0.88 (0.79–0.98) |
| Other causes | 23,104 (19,666–26,849) | 0.35 (0.30–0.41) | 28,482 (23,574–34,082) | 0.35 (0.29–0.42) | 0.12 (0.01–0.24) |
ASIR, age-standardized incidence rate; SDI, socio-demographic index; NASH, nonalcoholic steatohepatitis.
Table 2.
Deaths and age-standardized death rates of liver cancer in 2010 and 2019 and the temporal trend of age-standardized death rates from 2010 to 2019
| 2010 | 2019 | ||||
|---|---|---|---|---|---|
|
|
|
|
|
||
| No. deaths (95% UI) | ASDR per 100,000 (95% UI) | No. deaths (95% UI) | ASDR per 100,000 (95% UI) | Annual percentage change of ASDR (95% CI) | |
|
| |||||
| Global | 386,342 (366,367–404,997) | 6.05 (5.72–6.33) | 484,577 (444,091–525,798) | 5.95 (5.44–6.44) | −0.10 (−0.25 to 0.06) |
|
| |||||
| Sex | |||||
|
| |||||
| Male | 263,323 (249,598–278,647) | 8.78 (8.31–9.27) | 333,673 (299,581–368,334) | 8.73 (7.88–9.60) | −0.06 (−0.17 to 0.05) |
| Female | 123,018 (113,977–129,867) | 3.63 (3.36–3.83) | 150,904 (134,123–167,013) | 3.46 (3.08–3.83) | −0.50 (−0.65 to −0.35) |
|
| |||||
| Socio-demographic index | |||||
|
| |||||
| Low SDI | 16,435 (14,822–18,089) | 4.17 (3.77–4.57) | 20,756 (18,217–23,330) | 3.93 (3.49–4.38) | −0.66 (−0.72 to −0.61) |
| Low-middle SDI | 42,132 (38,776–45,219) | 4.09 (3.73–4.39) | 57,241 (52,130–63,452) | 4.23 (3.86–4.68) | 0.40 (0.08–0.71) |
| Middle SDI | 147,231 (137,694–158,985) | 7.83 (7.32–8.41) | 196,959 (172,833–223,210) | 7.92 (6.97–8.93) | 0.39 (0.11–0.67) |
| High-middle SDI | 83,440 (78,200–88,646) | 5.15 (4.83–5.46) | 97,189 (87,228–108,111) | 4.83 (4.34–5.38) | −0.69 (−0.91 to −0.46) |
| High SDI | 96,952 (89,685–100,974) | 6.24 (5.83–6.48) | 112,240 (102,489–118,738) | 5.89 (5.44–6.21) | −0.65 (−0.74 to −0.55) |
|
| |||||
| Region | |||||
|
| |||||
| Africa | 17,599 (15,794–19,425) | 4.76 (3.84–4.13) | 21,537 (18,873–24,287) | 4.37 (3.90–4.87) | −0.92 (−0.97 to −0.88) |
| Eastern Mediterranean | 21,615 (20,010–23,703) | 6.92 (6.40–7.55) | 27,219 (22,054–33,580) | 6.49 (5.33–7.91) | −0.72 (−0.83 to −0.61) |
| Europe | 56,407 (53,637–57,839) | 4.03 (3.84–4.13) | 63,501 (58,916–67,531) | 3.95 (3.67–4.19) | 0.31 (−0.47 to −0.15) |
| Americas | 32,449 (30,926–33,426) | 3.26 (3.11–3.36) | 46,132 (42,506–49,469) | 3.61 (3.33–3.88) | 1.09 (0.97–1.22) |
| Southeast Asia | 53,647 (49,252–57,466) | 4.37 (4.01–4.68) | 70,029 (60,641–80,604) | 4.22 (3.67–4.84) | −0.53 (−0.73 to −0.33) |
| Western Pacific | 202,914 (187,913–219,829) | 9.84 (9.11–10.66) | 254,054 (221,667–289,533) | 9.50 (8.31–10.78) | −0.29 (−0.64 to 0.06) |
|
| |||||
| Etiology | |||||
|
| |||||
| Alcohol | 68,980 (57,146–82,279) | 1.08 (0.90–1.29) | 90,740 (73,349–109,402) | 1.10 (0.89–1.33) | 0.23 (0.09–0.37) |
| Hepatitis B | 156,307 (139,827–175,398) | 2.35 (2.10–2.64) | 191,736 (161,861–223,727) | 2.31 (1.95–2.69) | −0.20 (−0.31 to −0.09) |
| Hepatitis C | 114,657 (100,138–128,567) | 1.89 (1.64–2.11) | 141,810 (121,787–161,828) | 1.78 (1.53–2.04) | −0.62 (−0.79 to −0.46) |
| NASH | 25,249 (20,882–30,666) | 0.40 (0.33–0.49) | 34,729 (28,395–43,182) | 0.43 (0.35–0.53) | 0.70 (0.43–0.97) |
| Other causes | 21,147 (18,055–24,682) | 0.32 (0.28–0.38) | 25,560 (21,229–30,491) | 0.32 (0.27–0.38) | −0.02 (−0.17 to 0.12) |
ASDR, age-standardized death rate; SDI, sociodemographic index; NASH, nonalcoholic steatohepatitis.
Figure 1. Estimated global death cases and age-standardized death rates of liver cancer from 2010 to 2019.

(A) Frequency of liver cancer deaths in 2010 versus 2019, global and by World Health Organization region.
(B) Frequency of liver cancer deaths by World Health Organization region from 2010 to 2019.
(C) Contribution to global liver cancer deaths in 2019 by World Health Organization region.
(D) Age-standardized death rates of liver cancer by World Health Organization region in 2010 versus 2019.
(E) Age-standardized death rates of liver cancer by World Health Organization region from 2010 to 2019.
(F) Frequency of liver cancer deaths in 2010 versus 2019 by sociodemographic index.
(G) Age-standardized death rates of liver cancer from 2010 to 2019 by sociodemographic index ASDR, age-standardized death rate; SDI, sociodemographic index.
Burden of liver cancer by World Health Organization region
The estimated frequencies of incident liver cancer cases, deaths, DALYs, and rates (ASIRs, ASDRs, and ASDALYs) in different World Health Organization (WHO) regions are summarized in Tables 1, 2, and S4. In 2019, the Western Pacific region had the highest frequency of incident liver cancer cases (n = 295,484), deaths (n = 254,054), and DALYs (6.7 million) (Figures 1A and 1B). However, the Americas had the greatest increase between 2010 and 2019 in liver cancer incident cases (+41%), deaths (+42%), and DALYs (+36%). The proportions of global liver cancer deaths in 2019 contributed by each WHO region are shown in Figure 1C.
During the study period, ASIRs increased in the Americas (APC: 1.11%, 95% CI 1.09–1.15), remained stable in the Western Pacific and Europe, and decreased in all other WHO regions, with the greatest decrease in Africa (APC: −0.89%, 95% CI −0.91 to −0.87) (Table 1). Similarly, ASDRs increased in the Americas between 2012 and 2019 (APC 1.09%, 95% CI 0.97–1.22), remained stable in the Western Pacific, but decreased in Europe and all other WHO regions, with the greatest decrease in Africa (APC: −0.92%, 95% CI −0.97 to −0.88) (Table 2; Figures 1D and 1E). Finally, ASDALYs increased in the Americas (APC: 0.86%, 95% CI 0.65–1.07), remained stable in the Western Pacific, and decreased in all other regions, with the greatest decrease in the Eastern Mediterranean (APC: −0.99%, 95% CI −1.09 to −0.89) (Table S4).
Age-standardized death rates by country
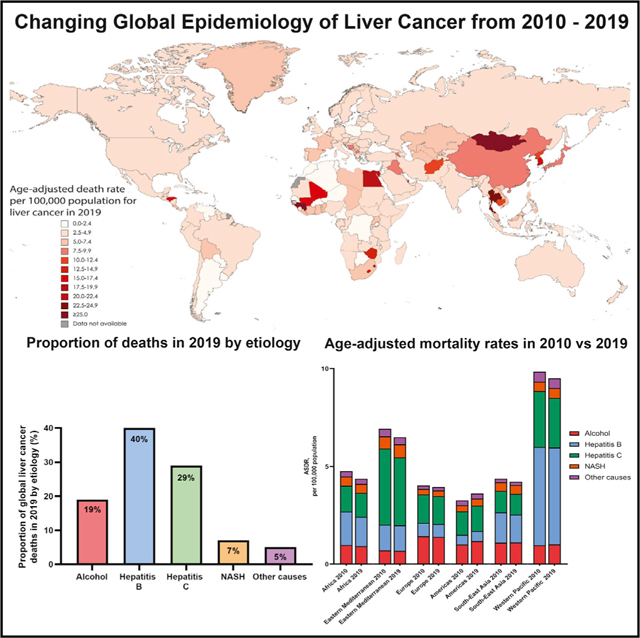
The estimated ASDRs of liver cancer in 2019 by country are shown in Figure 2. The estimated ASDRs ranged from 0.65 deaths per 100,000 (95% UI 0.49–0.84) in Niger to 115.23 deaths per 100,000 (95% UI 91.48–142.48) in Mongolia.
Figure 2. Estimated age-standardized death rates from liver cancer in 2019 by country.

Burden of liver cancer by sociodemographic index
The numbers of incident liver cancer cases, deaths, and DALYs, as well as ASIR and ASDR by country’s sociodemographic index (SDI), are summarized in Tables 1, 2, and S4. The highest frequency of incident cases (n = 185,567), deaths (n = 196,959), and DALYs (5.5 million) in 2019 took place in middle SDI countries (Figure 1F). Between 2010 and 2019, the greatest increase in the frequency of incident cases (+39% ) and ASIR (APC: 0.68%, 95% CI 0.52–0.84) occurred in middle SDI countries, whereas the greatest increase in the frequency of deaths (+36%), ASDR (APC: 0.40%, 95% CI 0.08–0.71), and ASDALYs (APC: 0.33%, 95% CI 0.17–0.49) occurred in low-middle SDI countries (Tables 1, 2, and S4; Figures 1F and 1G). By contrast, the greatest reduction in ASIRs, ASDRs, and ASDALYs occurred in low SDI countries, high-middle SDI countries, and high SDI countries, respectively.
Trends in etiology of liver cancer
The frequency of incident cases, deaths, ASIRs, ASDRs, and DALYs by etiology of liver disease is summarized in Tables 1, 2, and S4 and Figure 3A. In 2019, HBV accounted for 40% of the frequency of global liver cancer deaths, followed by HCV (29%), alcohol (19%), NASH (7%), and other causes (5%) (Figure 3B). Between 2010 and 2019, NASH was the fastest growing etiology of incident liver cancer cases (+39%) worldwide, while HCV had the lowest increase (+23%). Similarly, NASH was the fasting growing etiology of liver cancer deaths (+38%), while other causes had the lowest increase (+21%) (Figure 3A). The frequency of liver cancer deaths by etiology and region is summarized in Figure 3C.
Figure 3. Temporal trends in the etiology of liver cancer from 2010 to 2019.
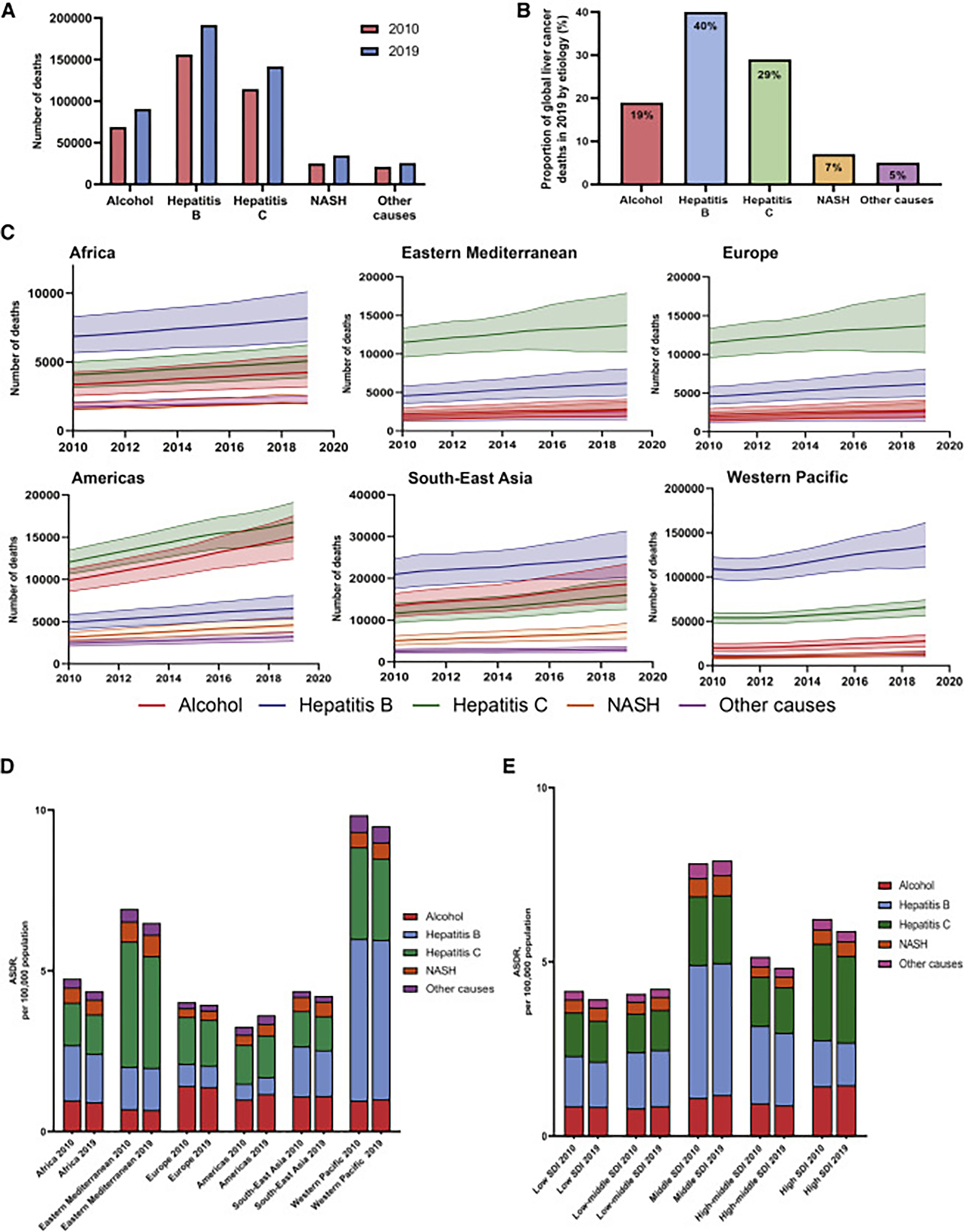
(A) Frequency of liver cancer deaths in 2010 versus 2019 by etiology.
(B) Contribution to frequency of liver cancer deaths by etiology in 2019.
(C) Frequency of deaths from liver cancer by region and etiology from 2010 to 2019.
(D) Estimated age-standardized death rates of liver cancer in 2010 versus 2019 by World Health Organization region and etiology.
(E) Estimated age-standardized death rates of liver cancer in 2010 versus 2019 by sociodemographic index and etiology.
NASH; nonalcoholic steatohepatitis; ASDR, age-standardized death rate; SDI, sociodemographic index.
The ASIRs, ASDRs, ASDALYs, and the APCs in these rates between 2010 and 2019 stratified by etiology and region are summarized in Tables S5–S7 and Figure 3D. The global ASIRs for HCV-related HCC decreased (APC: −0.60%, 95% CI −0.67 to −0.54), while the ASIRs for the other four etiologies increased (Table 1). NASH was the etiology with the largest increment in liver cancer ASIRs (APC: 0.88%, 95% CI 0.79–0.98). The ASIRs for NASH-associated liver cancer increased in five of six WHO regions, with the greatest increase in the Americas (APC: 1.33%, 95% CI 1.25–1.41), while the only region with a decrease in ASIR was Africa (APC: −0.19%, 95% CI −0.23 to −0.14) (Table S5). The ASIRs for alcohol-associated liver cancer increased in the Americas, Southeast Asia, and the Western Pacific but decreased in Africa, the Eastern Mediterranean, and Europe, with the greatest increase in the Americas (APC 1.81%, 95% CI 1.76–1.86). ASIRs for HCV-associated liver cancer increased only in the Americas, remained stable in Europe, and decreased in the other four regions. ASIRs for HBV-associated liver cancer increased in the Americas, the Western Pacific, and the Eastern Mediterranean, while the ASIRs decreased in the other three regions.
Between 2010 and 2019, the global ASDRs of liver cancer due to NASH (APC: 0.70%, 95% CI 0.43–0.97) and alcohol (APC: 0.23%, 95% CI 0.09–0.37) increased, while the ASDRs of liver cancer due to HCV (APC: −0.62%, 95% CI −0.79 to −0.46) and HBV (APC: −0.20%, 95% CI −0.31 to −0.09) declined, and other etiologies remained stable (Table 2). The ASDRs for NASH-associated liver cancer increased in the Americas, Eastern Mediterranean, Western Pacific, and Europe (Table S6; Figure 3D), with the greatest rise in the Americas (APC: 1.33%, 95% CI 1.25–1.42). The ASDRs for NASH-associated liver cancer remained stable in Africa and Southeast Asia. The ASDRs for alcohol-associated liver cancer increased in the Americas (APC: 1.78%, 95% CI 1.64–1.93) and the Western Pacific (APC: 0.48%, 95% CI 0.22–0.73); decreased in Africa, the Eastern Mediterranean, and Europe; but remained stable in Southeast Asia. The ASDRs for HBV-associated liver cancer increased only in the Americas (APC: 0.66%, 95% CI 0.56–0.75) but decreased in Africa, Europe, and Southeast Asia and remained stable in the Western Pacific. The ASDRs for HCV-associated liver cancer increased only in the Americas (APC: 0.70%, 95% CI 0.42–0.99) and decreased in all other WHO regions, with the greatest decrease in the Western Pacific (APC: −1.36%, 95% CI −1.51 to −1.21).
The increase in the liver cancer ASDRs between 2010 and 2019 among middle SDI countries was largely driven by an increase in NASH (APC: 1.15%, 95% CI 0.82–1.48) and alcohol (APC 0.87%, 95% CI 0.51–1.24). (Table S8; Figure 3E). Similarly, the increase in ASDRs among low-middle SDI countries was driven by NASH (APC: 1.08%, 95% CI 0.87–1.27), alcohol (APC: 1.04%, 95% CI 0.83–1.26), and HCV (APC: 0.48%, 95% CI 0.25–0.70). By contrast, low, high-middle, and high SDI countries experienced reductions in the ASDRs from HCV, HBV, and other causes.
DISCUSSION
Main findings
Utilizing data from the 2019 GBD study, we determined that there were an estimated 534,000 incident cases, 485,000 deaths, and 12.5 million DALYs due to liver cancer. Between 2010 and 2019, there was a 27% increase in the frequency of incident liver cancer cases and a 25% increase in the frequency of liver cancer deaths, although there were no significant changes in age-standardized incidence and death rates. The growth as well as aging of the world population may contribute to the observed disconnect in the temporal trends of frequency and age-standardized incidence and death rates. Although the Western Pacific contributed more than half of the global liver cancer deaths in 2019, ASDRs (APC: −0.29%) remained stable between 2010 and 2019. By contrast, ASDRs (APC: 1.09%) rose sharply in the Americas but declined in all other WHO regions.
The global ASDR due to HBV-associated liver cancer decreased (APC: −0.20%) between 2010 and 2019, likely due to the impact of successful vaccination programs, aflatoxin reduction programs, and antiviral therapy (Cox et al., 2020). However, the ASDR of HBV-associated liver cancer in the Americas increased, in contrast to all other regions, possibly due to underdiagnosis and a lack of disease awareness (Le et al., 2020). There was a reduction in the global ASDR of HCV-associated liver cancer (APC: −0.62%), due to decreasing death rates in all WHO regions except for the Americas, contributed by the availability of highly effective oral antiviral therapy (Dang et al., 2020). However, there remain major gaps in diagnosis and linkage to care (Desai et al., 2021; Huang et al., 2021c; Thomas, 2019; Ye et al., 2022), and substantial improvements are required in infection control, screening, and treatment rates to achieve the elimination targets set by the WHO (Heffernan et al., 2019).
NASH was the fastest growing cause of liver cancer deaths globally, especially in the Americas, driven by rapidly rising obesity rates (Ward et al., 2019). The incidence of liver cancer due to NASH is projected to increase further in the next decade in the United States, Europe, and Asia (Estes et al., 2018a, 2018b, 2020). Urgent measures are required at a global level to tackle the underlying metabolic risk factors and slow the growing burden of NASH-related liver cancer (Estes et al., 2018a; Huang et al., 2022; Lazarus et al., 2022; Loomba et al., 2010; Singal and El-Serag, 2022; Tan et al., 2022). Alcohol had the second fastest rising cause of liver cancer ASDRs globally (APC: 0.23%), with the highest increase in the Americas (APC: 1.78%). The global alcohol-per-capita consumption is projected to rise further, especially in the Western Pacific and Southeast Asia (Vos et al., 2020). Implementing policies such as an increased price and taxation for alcohol may be considered at a national level to reduce the burden of alcohol-associated liver cancer in countries with a high alcohol-per-capita consumption (Sheron, 2016). It is notable that the greatest increase in liver cancer ASDRs between 2010 and 2019 took place in middle and low-middle SDI countries, driven by a combination of NASH and alcohol.
In context with published literature
Our study builds on two previous studies of liver cancer using GBD 2015 and GBD 2017 (Global Burden of Disease Liver Cancer Collaboration et al., 2017; Paik et al., 2020) and provides an in-depth analysis of the temporal trends of liver cancer and the contributions of various etiologies using updated data from GBD 2019. The GBD 2017 study included deaths secondary to both primary and secondary cancers under liver cancers (GBD 2017 Disease and Injury Incidence and Prevalence Collaborators, 2018). GBD 2019 proportionally redistributed cases of metastases to the liver, resulting in more accurate estimates of liver cancer mortality in our study. Our findings validate several country-specific studies that have reported the growing impact of NASH and alcohol on the burden of liver cancer and provide important data for care providers and policy makers (Dyson et al., 2014; Ganne-Carrié and Nahon, 2019; Julien et al., 2020; Tohra et al., 2021; Younossi et al., 2019, 2015). The current study provides a global overview of the temporal trends in liver cancer burden and the contributions of various liver disease etiologies from 2010 to 2019, unlike most of the other studies that focused on a specific country/region or etiology of liver disease. These data may help promote future investment into liver cancer diagnosis management and research. However, it remains important to continue to track the epidemiology of liver cancer over time, particularly given early signals of potential changes in trends in some countries (Petrick et al., 2020).
Conclusions
The frequency of new cases, deaths, and DALYs from liver cancer increased substantially between 2010 and 2019. Age-standardized incidence and death rates remained stable at the global level but increased substantially in the Americas. NASH and alcohol are the fastest growing causes of age-standardized liver cancer mortality, highlighting the need for urgent measures to tackle these growing issues.
Limitations of study
Our study shares the limitations of the GBD 2019, namely the availability of primary data that is dependent on the quality of each country’s registry (Vos et al., 2020). In countries or regions with missing data, the estimates rely on modeling studies and past trends, potentially resulting in heterogeneity and discrepancies in the accuracy of the data. In addition, there was likely to be under-reporting of data in certain regions such as Africa, due to a lack of disease awareness and access to care. The estimates for the burden of liver cancer are susceptible to some degree of reporter or ascertainment bias, which are inherent issues with utilizing data from the GBD study. Therefore, cautious interpretation is required. The GBD 2019 study classified individuals with cryptogenic liver disease under other causes of liver disease. It is possible that some of these individuals may have had NASH, resulting in an underestimate of the burden of NASH-associated liver cancer, especially in the United States, given the lack of ICD codes and adjudication for NASH. However, we determined that NASH was the fastest rising cause of liver cancer despite the possible underestimate of NASH-associated liver cancer burden. Although NASH and non-alcoholic fatty liver disease (NAFLD) were not specifically listed in the search terms used by the GBD 2019 study, studies were only included if they were population based and representative of the proportions of the etiologies of liver cancer for the respective location. Liver cancer cases were then attributed to NASH when the study explicitly listed the etiology to be NASH or NAFLD. It is less likely to have resulted in missed data for NASH, given the requirement for the included studies to be population based. Finally, data regarding the histological subgroups of liver cancer such as HCC or cholangiocarcinoma are lacking in the GBD 2019 study; therefore, further studies are required to determine the global burden of each histological subgroup.
STAR★METHODS
RESOURCE AVAILABILITY
Lead contact
Further information and requests for resources should be directed to and will be fulfilled by the lead contact, Rohit Loomba (roloomba@ucsd.edu).
Materials availability
No new reagents or materials were generated in this study.
Data and code availability
This paper utilizes publicly available data. Access to the data is available from http://ghdx.healthdata.org/gbdresults-tool.
This study did not generate any unique datasets or code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
METHOD DETAILS
Data source
This study was performed using data from the GBD 2019, a systematic effort to estimate the burden caused by 369 diseases and 87 risk factors in 204 countries/territories (Vos et al., 2020). Annual frequencies and age-standardized rates (ASRs) of liver cancer-related incidence, mortality and DALYs, from 2010 to 2019 by sex, age, region, and country were obtained from an online data source, the GlobalHealth Data Exchange (GHDx) query tool (http://ghdx.healthdata.org/gbd-results-tool), which is maintained by an ongoing multinational collaboration and coordinated by the Institute for Health Metrics and Evaluation.
Estimation methods
The general estimation methods for the GBD 2019 and the methods for liver cancer disease burden estimation were previously described (Global Burden of Disease Liver Cancer Collaboration et al., 2017; GBD 2019 Diseases and Injuries Collaborators, 2020; Paik et al., 2020). Briefly, data were obtained from population-based cancer registries, vital registration systems or verbal autopsy studies (GBD 2019 Diseases and Injuries Collaborators, 2020). The ICD-10 codes used by the GBD 2019 study for liver cancer deaths were C22.0-C22.8, D13.4, and the ICD-9 codes used were 155–155.1, 155.3–155.9 and 211.5. The GBD study provides a simple quality assessment from 0 to 5 to assess the quality of the data provided by each country/territory. Data quality ratings for the causes of death data from each country are available in Table S1.
Previous estimates of liver cancer deaths in the GBD 2017 included deaths secondary to both primary and secondary cancers (Global Burden of Disease Liver Cancer Collaboration et al., 2017; Paik et al., 2020). In GBD 2019, these deaths were proportionally distributed to both primary liver cancer and extrahepatic primary sites that metastasized to the liver, resulting in fewer deaths attributed to liver cancer in GBD 2019 compared to the GBD 2017 (GBD 2019 Diseases and Injuries Collaborators, 2020). Multiple statistical methods were utilized to minimize data heterogeneity, including misclassification correction, garbage code redistribution and noise reduction algorithms (GBD 2019 Diseases and Injuries Collaborators, 2020). A Cause of Death Ensemble model (CODEm), a Bayesian geospatial regression analysis, was utilized to estimate mortality by age, sex, location, and year. Incidence was estimated by dividing mortality estimates by mortality-to-incidence ratios. DALYs were calculated as the sum of years of life lost and years lived with disability.
To derive the proportions of liver cancer cases due to the five etiology groups included in GBD (hepatitis B; hepatitis C; alcohol; NASH; and other causes), the GBD collaborators performed a systematic literature search of Pubmed (Table S2) and included only population-based studies that provided data for the contribution of liver cancer etiologies. Cases where the etiology was described as “cryptogenic”, “idiopathic”, or “unknown” were included within the “other causes” category. Other etiologies of liver disease, such as haemochromatosis, autoimmune hepatitis, Wilson’s disease, were also included in the “other causes” category. The proportions of liver cancer due to the five specific risk factors were calculated for each study, and the pooled proportions were then used in five separate DisMod-MR 2.1 models (a Bayesian meta-regression-type model) to determine the proportion of liver cancers due to the five liver disease etiologies, stratified by country/territory, gender, and year. When multiple risk factors were reported for individual patients, these were assigned proportionally to the individual risk factors. These estimated proportions were used to split the overall liver cancer estimates into estimates for their respective etiologies of liver disease. As the proportion models were run independently of each other, the final proportion models were scaled to sum to 100% within each age, sex, year, and location, by dividing each proportion by the sum of the five models.
To group countries with similar development status, a Sociodemographic Index (SDI) was used, which combines total fertility rate, average educational attainment in the population over age 15, and measures of income per capita (Table S3). The STROBE statement is enclosed in Table S9.
QUANTIFICATION AND STATISTICAL ANALYSIS
Estimates for the frequency of incident cases and deaths were reported with 95% uncertainty intervals (UIs) as 2.5th and 97.5th ranked values across all 1,000 draws from a posterior distribution. Age-standardized rates were derived using the direct method to the GBD 2019 population estimate (GBD 2019 Diseases and Injuries Collaborators, 2020). The change in any category between 2010 and 2019 was calculated as follows: (value in 2019 - value in 2010)/ value in 2010). For changes of age-standardized rates over time, we calculated annual percent change (APC) and 95% confidence interval (CI) in age-standardized rates using the Joinpoint regression program, version 4.6.0.0 (Statistical Research and Applications Branch, National Cancer Institute, Bethesda). When the annualized rate of change and the lower boundary of its 95% CI were both positive, this was considered as an increasing trend. By contrast, when the annualized rate of change and the upper boundary were negative, this was considered as a decreasing trend. Statistical analyses were conducted using RStudio (Version 4.1.1) and GraphPad Prism.
Supplementary Material
KEY RESOURCES TABLE.
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
|
| ||
| Software and algorithms | ||
|
| ||
| RStudio (Version 4.1.1) | R Foundation for Statistical Computing | https://www.r-project.org/ |
| GraphPad Prism | GraphPad Software company | https://www.graphpad.com/ |
| Mapchart | Mapchart | https://www.mapchart.net/ |
| Joinpoint regression program (version 4.6.0.0) | Statistical Research and Applications Branch, National Cancer Institute, Bethesda | https://surveillance.cancer.gov/joinpoint/download |
Highlights.
In 2019, there were an estimated 485,000 deaths globally due to liver cancer
Between 2010 and 2019, death rates from liver cancer increased only in the Americas
NASH is the fastest growing cause of age-adjusted liver cancer deaths globally
Age-adjusted liver cancer deaths from hepatitis B and C declined
ACKNOWLEDGMENTS
The world map was created using https://www.mapchart.net/. R.L. receives funding support from NIAAA (U01AA029019), NIEHS (5P42ES010337), NCATS (5UL1TR001442), NIDDK (U01DK130190, U01DK061734, R01DK106419, P30DK120515, R01DK121378, and R01DK124318), NHLBI (P01HL147835), and DOD PRCRP (W81XWH-18-2-0026). R.L.’s institutions received research grants from Arrowhead Pharmaceuticals, AstraZeneca, Boehringer-Ingelheim, Bristol-Myers Squibb, Eli Lilly, Galectin Therapeutics, Galmed Pharmaceuticals, Gilead, Intercept, Hanmi, Intercept, Inventiva, Ionis, Janssen, Madrigal Pharmaceuticals, Merck, NGM Biopharmaceuticals, Novo Nordisk, Merck, Pfizer, Sonic Incytes, and Terns Pharmaceuticals. D.Q.H. receives funding support from Singapore Ministry of Health’s National Medical Research Council under its NMRC Research Training Fellowship (MOH-000595-01). A.G.S. receives funding support from National Cancer Institute R01 MD012565, U01 CA230694, R01 212008, R01 222900, and R01 256977. Y.K. receives funding support from NIH 1R01CA194307 and 1R01CA215520-01 and research support from GE Healthcare, Canon Medical Systems Co., Lantheus Medical Imaging, and Bracco Diagnostics Inc. H.B.E.-S. receives funding support from NIH P30 DK 56338, Cancer Prevention Research Institute of Texas CAP-CAC RP190641, and VA CSR&D Merit Award IIR 16I31.HB.
Footnotes
DECLARATION OF INTERESTS
R.L. serves as a consultant to Aardvark Therapeutics, Altimmune, Anylam/Regeneron, Amgen, Arrowhead Pharmaceuticals, AstraZeneca, Bristol-Myer Squibb, CohBar, Eli Lilly, Galmed, Gilead, Glympse Bio, Hightide, Inipharma, Intercept, Inventiva, Ionis, Janssen Inc., Madrigal, Metacrine, Inc., NGM Biopharmaceuticals, Novartis, Novo Nordisk, Merck, Pfizer, Sagimet, Theratechnologies, 89 Bio, Terns Pharmaceuticals, and Viking Therapeutics and is cofounder of LipoNexus Inc. D.Q.H. has served as an advisory board member for Eisai. A.G.S. has served as a consultant or on advisory boards for Genentech, AstraZeneca, Bayer, Eisai, Exelixis, Wako Diagnostics, Exact Sciences, Roche, Glycotest, and GRAIL.
SUPPLEMENTAL INFORMATION
Supplemental information can be found online at https://doi.org/10.1016/j.cmet.2022.05.003.
REFERENCES
- Asrani SK, Devarbhavi H, Eaton J, and Kamath PS (2019). Burden of liver diseases in the world. J. Hepatol. 70, 151–171. [DOI] [PubMed] [Google Scholar]
- Cox AL, El-Sayed MH, Kao J-H, Lazarus JV, Lemoine M, Lok AS, and Zoulim F (2020). Progress towards elimination goals for viral hepatitis. Nat. Rev. Gastroenterol. Hepatol. 17, 533–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang H, Yeo YH, Yasuda S, Huang CF, Iio E, Landis C, Jun DW, Enomoto M, Ogawa E, Tsai PC, et al. (2020). Cure with interferon-free direct-acting antiviral is associated with increased survival in patients with hepatitis C virus-related hepatocellular carcinoma from both east and west. Hepatology 71, 1910–1922. [DOI] [PubMed] [Google Scholar]
- Desai N, Rich NE, Jain MK, Blackwell JM, Murphy CC, Perryman P, McBryde J, Quirk L, Clark C, Villarreal D, et al. (2021). Randomized clinical trial of inreach with or without mailed outreach to promote hepatitis C screening in a difficult-to-reach patient population. Am. J. Gastroenterol. 116, 976–983. [DOI] [PubMed] [Google Scholar]
- Dyson J, Jaques B, Chattopadyhay D, Lochan R, Graham J, Das D, Aslam T, Patanwala I, Gaggar S, Cole M, et al. (2014). Hepatocellular cancer: the impact of obesity, type 2 diabetes and a multidisciplinary team. J. Hepatol. 60, 110–117. [DOI] [PubMed] [Google Scholar]
- El-Serag HB, Kanwal F, Feng Z, Marrero JA, Khaderi S, and Singal AG; Texas Hepatocellular Carcinoma Consortium (2020). Risk factors for cirrhosis in contemporary hepatology practices-findings from the Texas hepatocellular carcinoma consortium cohort. Gastroenterology 159, 376–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes C, Anstee QM, Arias-Loste MT, Bantel H, Bellentani S, Caballeria J, Colombo M, Craxi A, Crespo J, Day CP, et al. (2018a). Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016–2030. J. Hepatol. 69, 896–904. [DOI] [PubMed] [Google Scholar]
- Estes C, Razavi H, Loomba R, Younossi Z, and Sanyal AJ (2018b). Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 67, 123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes C, Chan HLY, Chien RN, Chuang WL, Fung J, Goh GBB, Hu TH, Huang JF, Jang BK, Jun DW, et al. (2020). Modelling NAFLD disease burden in four Asian regions-2019–2030. Aliment. Pharmacol. Ther. 51, 801–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganne-Carrié N, and Nahon P (2019). Hepatocellular carcinoma in the setting of alcohol-related liver disease. J. Hepatol. 70, 284–293. [DOI] [PubMed] [Google Scholar]
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators (2018). Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392, 1789–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GBD 2019 Diseases and Injuries Collaborators (2020). Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 396, 1204–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Global Burden of Disease Liver Cancer Collaboration, Akinyemiju T, Abera S, Ahmed M, Alam N, Alemayohu MA, Allen C, Al-Raddadi R, Alvis-Guzman N, Amoako Y, et al. (2017). The burden of primary liver cancer and underlying etiologies From 1990 to 2015 at the global, regional, and national level: results from the Global Burden of Disease Study 2015. JAMA Oncol. 3, 1683–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffernan A, Cooke GS, Nayagam S, Thursz M, and Hallett TB (2019). Scaling up prevention and treatment towards the elimination of hepatitis C: a global mathematical model. Lancet 393, 1319–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang QD, and Lim S-G (2018). New advances in hepatitis B vaccination for adults. Curr. Hepatol. Rep. 17, 466–474. [Google Scholar]
- Huang DQ, and Lim SG (2020). Hepatitis B: who to treat? A critical review of international guidelines. Liver Int. 40 (Suppl 1), 5–14. [DOI] [PubMed] [Google Scholar]
- Huang DQ, El-Serag HB, and Loomba R (2021a). Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 18, 223–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang DQ, Hoang JK, Leong J, Riveiro-Barciela M, Maeda M, Yang JD, Accarino EV, Thin K, Trinh L, Cheung RC, et al. (2021b). Differential characteristics and outcomes of Asian and non-Asian patients with HBV-related hepatocellular carcinoma. Liver Int. 41, 1922–1932. [DOI] [PubMed] [Google Scholar]
- Huang DQ, Li X, Le MH, Le AK, Yeo YH, Trinh HN, Zhang J, Li J, Wong C, Wong C, et al. (2021c). Natural history and hepatocellular carcinoma risk in untreated chronic hepatitis B patients with indeterminate phase. Clin. Gastroenterol. Hepatol. 10.1016/j.cgh.2021.01.019. [DOI] [PubMed] [Google Scholar]
- Huang DQ, Fowler KJ, Liau J, Cunha GM, Louie AL, An JY, Bettencourt R, Jung J, Gitto Z, Hernandez C, et al. (2022). Comparative efficacy of an optimal exam between ultrasound versus abbreviated MRI for HCC screening in NAFLD cirrhosis: a prospective study. Aliment. Pharmacol. Ther. 55, 820–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julien J, Ayer T, Bethea ED, Tapper EB, and Chhatwal J (2020). Projected prevalence and mortality associated with alcohol-related liver disease in the USA, 2019–40: a modelling study. Lancet Public Health 5, e316–e323. [DOI] [PubMed] [Google Scholar]
- Lazarus JV, Mark HE, Anstee QM, Arab JP, Batterham RL, Castera L, Cortez-Pinto H, Crespo J, Cusi K, Dirac MA, et al. (2022). Advancing the global public health agenda for NAFLD: a consensus statement. Nat. Rev. Gastroenterol. Hepatol. 19, 60–78. [DOI] [PubMed] [Google Scholar]
- Le MH, Yeo YH, Cheung R, Henry L, Lok AS, and Nguyen MH (2020). Chronic hepatitis B prevalence among foreign-born and U.S.-born adults in the United States, 1999–2016. Hepatology 71, 431–443. [DOI] [PubMed] [Google Scholar]
- Le MH, Yeo YH, Li X, Li J, Zou B, Wu Y, Ye Q, Huang DQ, Zhao C, Zhang J, et al. (2021). 2019 global NAFLD prevalence: a systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 10.1016/j.cgh.2021.12.002. [DOI] [PubMed] [Google Scholar]
- Lok AS, McMahon BJ, Brown RS Jr., Wong JB, Ahmed AT, Farah W, Almasri J, Alahdab F, Benkhadra K, Mouchli MA, et al. (2016). Antiviral therapy for chronic hepatitis B viral infection in adults: a systematic review and meta-analysis. Hepatology 63, 284–306. [DOI] [PubMed] [Google Scholar]
- Loomba R, and Sanyal AJ (2013). The global NAFLD epidemic. Nat. Rev. Gastroenterol. Hepatol. 10, 686–690. [DOI] [PubMed] [Google Scholar]
- Loomba R, Yang HI, Su J, Brenner D, Iloeje U, and Chen CJ (2010). Obesity and alcohol synergize to increase the risk of incident hepatocellular carcinoma in men. Clin. Gastroenterol. Hepatol. 8, 891–898. [DOI] [PubMed] [Google Scholar]
- Loomba R, Friedman SL, and Shulman GI (2021). Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell 184, 2537–2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik JM, Golabi P, Younossi Y, Mishra A, and Younossi ZM (2020). Changes in the global burden of chronic liver diseases from 2012 to 2017: the growing impact of NAFLD. Hepatology 72, 1605–1616. [DOI] [PubMed] [Google Scholar]
- Petrick JL, Florio AA, Loomba R, and McGlynn KA (2020). Have incidence rates of liver cancer peaked in the United States? Cancer 126, 3151–3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitz HK, Bataller R, Cortez-Pinto H, Gao B, Gual A, Lackner C, Mathurin P, Mueller S, Szabo G, and Tsukamoto H (2018). Alcoholic liver disease. Nat. Rev. Dis. Primers 4, 16. [DOI] [PubMed] [Google Scholar]
- Sheron N (2016). Alcohol and liver disease in Europe – simple measures have the potential to prevent tens of thousands of premature deaths. J. Hepatol. 64, 957–967. [DOI] [PubMed] [Google Scholar]
- Singal AG, and El-Serag HB (2015). Hepatocellular carcinoma from epidemiology to prevention: translating knowledge into practice. Clin. Gastroenterol. Hepatol. 13, 2140–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singal AG, and El-Serag HB (2022). Rational HCC screening approaches for patients with NAFLD. J. Hepatol. 76, 195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, and Bray F (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249. [DOI] [PubMed] [Google Scholar]
- Tan DJH, Ng CH, Lin SY, Pan XH, Tay P, Lim WH, Teng M, Syn N, Lim G, Yong JN, et al. (2022). Clinical characteristics, surveillance, treatment allocation, and outcomes of non-alcoholic fatty liver disease-related hepatocellular carcinoma: a systematic review and meta-analysis. Lancet Oncol. 23, 521–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DL (2019). Global elimination of chronic hepatitis. N. Engl. J. Med. 380, 2041–2050. [DOI] [PubMed] [Google Scholar]
- Tohra S, Duseja A, Taneja S, Kalra N, Gorsi U, Behera A, Kaman L, Dahiya D, Sahu S, Sharma B, et al. (2021). Experience with changing etiology and nontransplant curative treatment modalities for hepatocellular carcinoma in a real-life setting—a retrospective descriptive analysis. J. Clin. Exp. Hepatol. 11, 682–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos T, Lim SS, Abbafati C, Abbas KM, Abbasi M, Abbasifard M, Abbasi-Kangevari M, Abbastabar H, Abd-Allah F, Abdelalim A, et al. (2020). Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 396, 1204–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward ZJ, Bleich SN, Cradock AL, Barrett JL, Giles CM, Flax C, Long MW, and Gortmaker SL (2019). Projected U.S. state-level prevalence of adult obesity and severe obesity. N. Engl. J. Med. 381, 2440–2450. [DOI] [PubMed] [Google Scholar]
- Ye Q, Kam LY, Yeo YH, Dang N, Huang DQ, Cheung R, and Nguyen MH (2022). Substantial gaps in evaluation and treatment of patients with hepatitis B in the US. J. Hepatol. 76, 63–74. [DOI] [PubMed] [Google Scholar]
- Younossi ZM, Otgonsuren M, Henry L, Venkatesan C, Mishra A, Erario M, and Hunt S (2015). Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004 to 2009. Hepatology 62, 1723–1730. [DOI] [PubMed] [Google Scholar]
- Younossi Z, Stepanova M, Ong JP, Jacobson IM, Bugianesi E, Duseja A, Eguchi Y, Wong VW, Negro F, Yilmaz Y, et al. (2019). Nonalcoholic steatohepatitis is the fastest growing cause of hepatocellular carcinoma in liver transplant candidates. Clin. Gastroenterol. Hepatol. 17, 748–755.e3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This paper utilizes publicly available data. Access to the data is available from http://ghdx.healthdata.org/gbdresults-tool.
This study did not generate any unique datasets or code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.


