Abstract
We examined the motor unit action potential amplitude versus recruitment threshold relationship (MUAPAMP-RT) as an indicator of MU-specific hypertrophy following high-intensity exercise training in females. Participants were assigned to either a high-intensity exercise (EX, n = 9) or control (CON, n = 18) condition and completed pre- (PRE) and post-testing (POST) during which maximal voluntary isometric leg extension strength (MVIT), vastus lateralis (VL) muscle cross sectional area (mCSA), whole leg skeletal muscle mass (SMMRL), and high-density surface EMG (HD-sEMG) signals were recorded from the VL during an isometric ramp contraction at 70% MVIT. The HD-sEMG signals were decomposed and yielded a MUAPAMP and an absolute (ABS; Nm) and normalized (NORM; %MVIC) RT for each MU. Individual MUAPAMP-RT slopes and intercepts were calculated for each subject. Changes in the pooled MUAPAMP-RT relationships for each group were also examined. Finally, relationships among individual changes in slopes of MUAPAMP-RT and individual changes in mCSA and SMMRL were examined. Training elicited increases in MVIT (+18%), mCSA (+12%), and mean and pooled slopes of MUAPAMP-RTNORM. The individual changes in slopes of both the MUAPAMP-RT relationships were moderately to strongly (r = 0.48-0.68) related to changes in mCSA and SMMRL. Eight-weeks of high-intensity exercise elicited increases in MUAPAMP-RT slope in females. Further, the observed change in slope was related to both VL mCSA and SMM of the tested leg. However, changes in slope for the MUAPAMP-RT relationship were more subdued when MUAPAMP was expressed relative to the absolute versus relative RT.
Keywords: resistance training, interval training, skeletal muscle hypertrophy, size principal, electromyography
INTRODUCTION
While it had long been established that motor neurons vary widely in size, the functional significance of this size variation was poorly understood until Henneman described the inverse relationship between a motor neuron’s excitability and its soma size in 19571, and subsequently that a motor unit’s (MU) contribution to graded activity is determined by its neuron size2. Based on this fundamental work, it is understood that skeletal muscle force production is largely accomplished via the progressive, orderly recruitment of MUs with increasing neuron size in response to net increases in excitatory input to the MU pool, known as the size principle.
Motor neurons with larger soma’s display greater axon diameters and innervate muscle fibers with larger diameters3,4. Therefore, larger MUs are also characterized by greater action potential amplitudes (MUAPAMP) and higher conduction velocities (MUCV)3,5 than smaller MUs. In fact, Hakansson et al.4 reported that muscle fiber action potential amplitude was greater in larger than smaller circumference fibers. Further, Tanji and Kato6 and Goldberg and Derfler7 both reported positive relationships between a MU’s recruitment threshold (RT) and MUAPAMP. Consequently, it has been proposed that the size principle can be assessed by examining the relationship between MUAPAMP and RT8.
Skeletal muscle fibers are remarkably plastic and display marked increases in both single fiber cross-sectional area9,10 and whole-muscle size11–13 in response to chronic high-intensity training. It is widely thought that fibers innervated by larger, higher-threshold motor neurons display the greatest plasticity in response to high-intensity exercise training (i.e., resistance training). This idea appears to be based on the observation of greater relative increases in type II than type I fiber size following resistance training10,14,15 and the apparent alignment of functional characteristics (i.e., size, force capacity, fatigue resistance, etc.) among fiber type and MU properties, whereby smaller, low-threshold motor neurons are thought to innervate type I fibers and larger motor neurons are thought to innervate type II fibers16,17. Furthermore, while myoblast cell lineage plays an important role in establishing muscle phenotype, evidence exists to support the contention that external stimuli such as neural impulse activity likely also strongly influence muscle phenotype16,18–21. Indeed, Dubowitz21 demonstrated in a cross-innervation study that neural activity has a profound effect on the structure and metabolic activity of skeletal muscle. Consequently, as previously posited by Pope et al.8, high-intensity exercise is likely to elicit greater adaptation in the fibers of high-threshold MUs as an artifact of the dramatically increased impulse activity in these fibers required of high effort contractions. Accordingly, recent evidence has suggested greater adaptation in the peripheral properties of higher-threshold MUs in response to high-intensity exercise interventions8,22,23.
Recent technological advancements now allow for the discrimination of MUAP waveforms and their firing trains using automated decomposition of high-density surface electromyographic (sEMG) signals recorded from multichannel sEMG electrode arrays. Importantly, Hu et al.24 illustrated that a MU’s APAMP, determined from sEMG signal decomposition, has a strong relationship with its RT in accordance with the size-principle2. Based on these premises, Pope et al.8 examined whether MUAPAMP could be used as a non-invasive indicator of MU-specific hypertrophy following resistance training. The authors8 reported that (1) resistance training elicited increases in MUAPAMP of high threshold MUs, and (2) that training-induced changes in whole muscle cross-sectional area (mCSA) were significantly related (r = 0.91) to changes in slope of each individual’s relationship between MUAPAMP and RT. Consequently, the authors concluded that the non-invasive assessment of MUAPAMP using sEMG signal decomposition is a sensitive indicator of MU-specific hypertrophy. Sterczala et al.25 recently also reported training induced changes in the MUAPAMP versus RT (MUAPAMP-RT) relationship. In contrast to Pope et al.8, however, the authors did not observe a relationship between the changes in slope and changes in mCSA. Thus, additional studies are needed to further examine the utility of this marker as an indicator of hypertrophy.
To our knowledge, these studies8,25 have also not yet been replicated in females and there are several factors that were not addressed by either study that may ultimately confound the utility of the MUAPAMP versus RT (MUAPAMP-RT) relationship as an indicator of MU specific hypertrophy. First, there was no comparative control group utilized by Pope et al. 8 and it is not clear how stable the MUAPAMP-RT relationship is across time. Moreover, in a recent study utilizing longitudinal MU tracking, it was reported that 4-weeks of resistance training decreases RT by ~15%23. Both Pope et al.8 and Sterczala et al.25 normalized RT to the MVICs at pre- and post-training when examining the MUAPAMP-RT relationship, despite the fact that strength also increased significantly (+9.6% and +17.2%, respectively). Therefore, it is plausible that the reported increase in MUAPAMP observed for MUs with recruitment thresholds >40% MVIC by Pope et al.8 and the increase in slope of the MUAPAMP-RT relationship by Sterczala et al.25 may simply be an artifact of decreasing RTs, increasing MVIC strength, or a combination of both. Finally, at present, only 39% of all participants among the published literature are female, and it has been proposed that this percentage is lower in resistance training intervention studies26. Therefore, the purpose of this study was to examine the utility of the MUAPAMP versus RT relationship as an indicator of MU-specific hypertrophy following high-intensity exercise training in females. To address the limitations of the Pope et al.8 study, we utilized a comparative control group and examined MUAPAMP relative to both normalized and absolute RT.
METHODS
Experimental Design
These data were collected as part of a larger clinical trial. Participants were assigned to either a high-intensity exercise (EX, n = 9) or one of two non-exercise control conditions (CON, n = 18) and completed pre- and post-testing during which maximal voluntary isometric leg extension strength, vastus lateralis (VL) mCSA, whole leg skeletal muscle mass of the tested leg (SMMRL), and high-density sEMG signals were recorded from the VL during an isometric ramp contraction at 70% of maximal isometric strength. The high-density sEMG signals were decomposed and used to determine the MUAPAMP and RT of accurately discriminated MUs. Participants in the EX group performed two sessions of high-intensity aerobic exercise training and two sessions of resistance training each week during the intervention period, whereas participants assigned to the CON group were asked to maintain baseline physical activity levels.
Participants
Twenty-seven untrained, young females (mean ± SD, age = 20.8 ± 3 y, height = 166.0 ± 7 cm, weight = 71.6 ± 14 kg) volunteered, completed this study, and had useable motor unit data (criteria described below). Prior to data collection, all participants were informed of the experimental procedures, signed an informed consent form, and then completed a health history questionnaire. To be eligible, participants must have been non-exercising 18–29 year old females who answered no to all questions on the PAR-Q for people aged 15–69 y and signed an informed consent form. In addition, each participant must have agreed to comply with the protocol. The study’s procedures were approved by university’s Institutional Review Board in accordance with the Declaration of Helsinki.
Isometric Strength Testing
Before and after the intervention period, participants completed isometric strength testing on a calibrated isokinetic dynamometer (Biodex System 4; Shirley, NY) with the hip and knee joints positioned at 90° angles as described in detail previously27. Following a warmup27, participants completed two maximal voluntary isometric contraction (MVIC) attempts, during which they were instructed to kick out “as hard as possible” for 5-s. Two minutes of rest was provided between attempts. If the participant’s second attempt was greater than the first, a third attempt was provided following an additional rest period. The maximal voluntary isometric torque (MVIT) achieved during a 1,000-ms epoch of the subjects MVIC attempt was recorded, used for analysis, and used to calculate the trajectory for the subsequent submaximal ramp contractions.
Following the completion of MVIC testing, participants completed submaximal isometric ramp muscle actions while tracing trapezoidal shaped torque trajectories. The torque trajectory increased linearly at 10% of MVIC·s−1, plateaued at target torque and was held for 10-s, and then decreased linearly back to baseline at −10% of MVIC·s−1. Each participant completed two submaximal ramp muscle actions at 70% MVIC with two minutes of rest provided between each attempt.
Motor Unit Recording, Processing, and Analysis
During each submaximal isometric ramp muscle action, high-density surface electromyographic (HD-sEMG) signals were recorded from the VL using a specialized 5-pin sensor and a 16-channel Bagnioli data acquisition unit (Delsys Inc., Natick, MA, USA). Before placing the sensor, the skin was carefully prepared by shaving, abrading, and cleansing with alcohol. The 5-pin sensor was then taped to the skin over the vastus lateralis with hypoallergenic tape in accordance with the recommendations of Zaheer et al.28. A reference electrode (Dermatrode, American Imex, Irvine, CA, USA) was also secured to the skin over the C7 spinous process. The four channels of raw HD-sEMG signal were stored on a personal computer and later decomposed offline using the Precision Decomposition III Algorithm described by De Luca et al.29 and improved and re-described by Nawab et al.30. Only MU’s demonstrating at least 90% accuracy, as assessed by the decompose-synthesize-decompose-compare test, were retained for analyses. For every discriminated MU, the decomposition output provided unique action potential waveforms for each sEMG channel, which represented a weighted average MUAP shape and amplitude comparable with those derived from spike-triggered averaging31. From each of these four waveforms, the peak-to-peak amplitude (MUAPAMP) was calculated, and the greatest MUAPAMP from among the four channels was used to represent that MU. In addition, each MUs recruitment threshold (RT) was defined as the relative force (%MVIC) and absolute torque (Nm) at which the MU first discharged. Based partially on the criteria described by Colquhoun et al.27, for a participant to be included in analyses, they must have had a contraction at both PRE and POST that yielded: (1) ≥6 accurately detected MUs, (2) an observed RT range ≥10% MVIC, and (3) a significant MUAPAMP vs. RT relationship (p ≤ 0.05).
Muscle Cross Sectional Area and Leg Muscle Mass
Ultrasound-based acquisition and analysis of VL mCSA has been described elsewhere in great detail32. In brief, following a five minute rest period, several panoramic ultrasound images of the VL were obtained using a clinical-grade, brightness mode (B-mode) ultrasound imaging device (General Electric LOGIQ S8, Wauwatosa, WI, USA) and a multi-frequency linear-array probe (Model ML6-15-D 4-15 MHz, 50-mm field of view) at 50% of the distance from the ASIS to the medial, superior border of the patella. The images with the highest visual contrast were used for subsequent analyses in Image-J Software (National Institutes of Health, Bethesda, MD, USA). A multifrequency, segmental medical bioimpedance analysis system (seca mBCA 515) was also used to assess skeletal muscle mass of the right leg (SMMRL), which has been shown to be highly accurate33.
Exercise Training
Participants enrolled in the exercise group completed eight weeks of supervised, progressive resistance and interval exercise training. Each participant completed two resistance sessions and two interval exercise sessions per week, and all exercise sessions were supervised by qualified personnel. During the resistance exercise sessions, participants completed nine different exercises, including the bench press, lat pulldown, lateral raise, seated row, leg press, leg extension, leg curl, biceps curl, and triceps extension. During weeks 1-4, the participants completed two sets of 15 repetitions for each exercise at ~60% of one-repetition maximum (1RM). During weeks 5-8 of training, the participants completed three sets of 12 repetitions at ~70% of 1RM. Throughout the resistance training program, the load was increased by 5-10% if the participant was able to perform the prescribed load for two repetitions more than planned during their last set on two consecutive training days. During each of the interval training sessions, participants completed a 5-min warmup consisting of low-intensity cycling before completing ten separate, 30-s intervals with a 90-s active recovery period between each interval. The intensity of the interval and active recovery periods were based on each individual’s rating of perceived exertion (RPE)34, and were set at an RPE of 7 and 3 out of 10, respectively. Following completion of the intervals, participants completed a 5-min cool-down. Both the warm-up and cool-down were completed at an RPE of 3. All interval training was completed on an air bike with a belt-driven steel fan (Rogue Echo Bike, Rogue Fitness, Columbus, OH).
Statistical Analyses
Linear regression models were fit to the MUAPAMP versus RT data, which provided slopes, y-intercepts, and correlation coefficients characterizing each participant’s MUAPAMP versus RT relationship at pre- and post-intervention. These regression analyses were performed using both normalized (MUAPAMP-RTNORM) and, to further explore whether the changes in the MUAPAMP versus normalized RT relationships were caused by changes in normalized recruitment thresholds and/or strength, absolute RT (MUAPAMP-RTABS).
Separate, two-way (Condition (EX vs. CON) × Time (PRE vs. POST) mixed-factorial repeated measures analyses of variance (ANOVAs) were then used to analyze changes in the slopes and y-intercepts of the MUAPAMP-RTNORM and MUAPAMP-RTABS relationships, mCSA, SMMRL, and MVIT from pre- to post-intervention in the exercise versus control group. Post-hoc Sidak-corrected dependent samples t-tests were used to examine the pre- to post-intervention change within each group in the event of a significant interaction. In addition, Pearson’s product-moment correlation coefficients were calculated to examine the relationships among every participant’s change (i.e., Δ = POST–PRE) in MUAPAMP-RTNORM and MUAPAMP-RTABS slope, VL mCSA, SMMRL, and MVIT. Because we hypothesized that changes in MUAPAMP-RT slope would be positively related to changes in VL mCSA, SMMRL, and MVIT, one-tailed tests were used. Further, the magnitudes of the relationships were interpreted as small (r ≤ 0.10), moderate (0.10 > r < 0.50), or large (r ≥ 0.50)35.
Regression analyses were also performed on the pooled MU data in the exercise versus control groups at pre- versus post-intervention, similar to the methods used by Pope et al. (2016). Specifically, all MU data were pooled among subjects in each group and at pre- and post-intervention before the average MUAPAMP and RT were determined in 10% normalized recruitment threshold bin widths (i.e., 0-10 %MVIC, 10-15 %MVIC, …, 60+ %MVIC) and in 13 (CON) or 14 (EX) Nm recruitment threshold bin widths, which represented 10% increments of the maximum observed RT in CON and EX, respectively. To examine the influence of the intervention on the pooled MUAPAMP versus RT relationships, the slopes and y-intercepts of the regression lines were compared using analyses of covariance as described by Zar 36 and used by Colquhoun et al.27. All statistical analyses were performed using Graphpad Prism 8 (San Diego, CA, USA) and IBM SPSS Statistics (v. 25.0, Armonk, NY, USA). The a priori, type I error rate for all analyses was set at 5%.
RESULTS
Table 1 displays the characteristics of the detected MUs in the both the EX and CON groups at PRE and POST.
TABLE 1.
The mean (± 95% confidence interval) characteristics of the discriminated MUs at pre- and post-intervention in the exercise and control groups.
| Pre-Intervention (PRE) | Post-Intervention (POST) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MU Yield | Minimum RT (%MVIC) | Maximum RT (%MVIC) | RT Range (%MVIC) | r | MU Yield | Minimum RT | Maximum RT | RT Range | r | |
| Exercise | 18.4 (±5.2) | 18.4 (±6.6) | 51.1 (±12.4) | 32.8 (±10.8) | 0.84 (±0.06) | 22.3 (±7.9) | 20.6 (±9.2) | 47.1 (±13.2) | 26.5 (±9.5) | 0.85 (±0.04) |
| Control | 22.1 (±4.6) | 27.4 (±5.1) | 60.0 (±4.3) | 32.6 (±4.0) | 0.83 (±0.05) | 23.3 (±3.3) | 26.9 (±4.0) | 61.3 (±5.1) | 34.4 (±4.3) | 0.86 (±0.04) |
MU, motor unit; RT, recruitment threshold; MVIC, maximal voluntary isometric contraction; r, correlation coefficient of the motor unit action potential amplitude versus recruitment threshold relationship
Mean Comparisons
There were significant Condition × Time interactions observed for the slopes (F1,25 = 12.1, p = 0.002, Fig. 1A) and y-intercepts (F1,25 = 5.6, p = 0.03, Fig. 1B) of the MUAPAMP-RTNORM relationship, as well as mCSA (F1,25 = 33.3, p < 0.001, Fig. 1C), and MVIT (F1,25 = 10.1, p = 0.004, Fig. 1D). The MUAPAMP-RTNORM slope increased in EX (+1.46 ± 1.42 μV·MVIC%−1, p = 0.04) and decreased in CON (−1.09 ± 1.01 μV·MVIC%−1, p = 0.03). The post-hoc comparisons revealed no changes for the MUAPAMP-RTNORM intercept in the EX (−43.49 ± 59.11 μV, p = 0.18) or CON group (28.40 ± 41.79 μV, p = 0.22), whereas VL mCSA increased in EX (+2.33 ± 1.04 cm2, p < 0.0001) and did not change in CON (+0.08 ± 0.73 cm2, p = 0.96). Similarly, MVIT increased in EX (+20.42 ± 12.1 Nm, p < 0.001) and did not change in CON (+0.62 ± 8.55 Nm, p = 0.98). There was no interaction (p = 0.14), nor were there main effects (p ≥ 0.39) observed for SMMRL.
Figure 1.
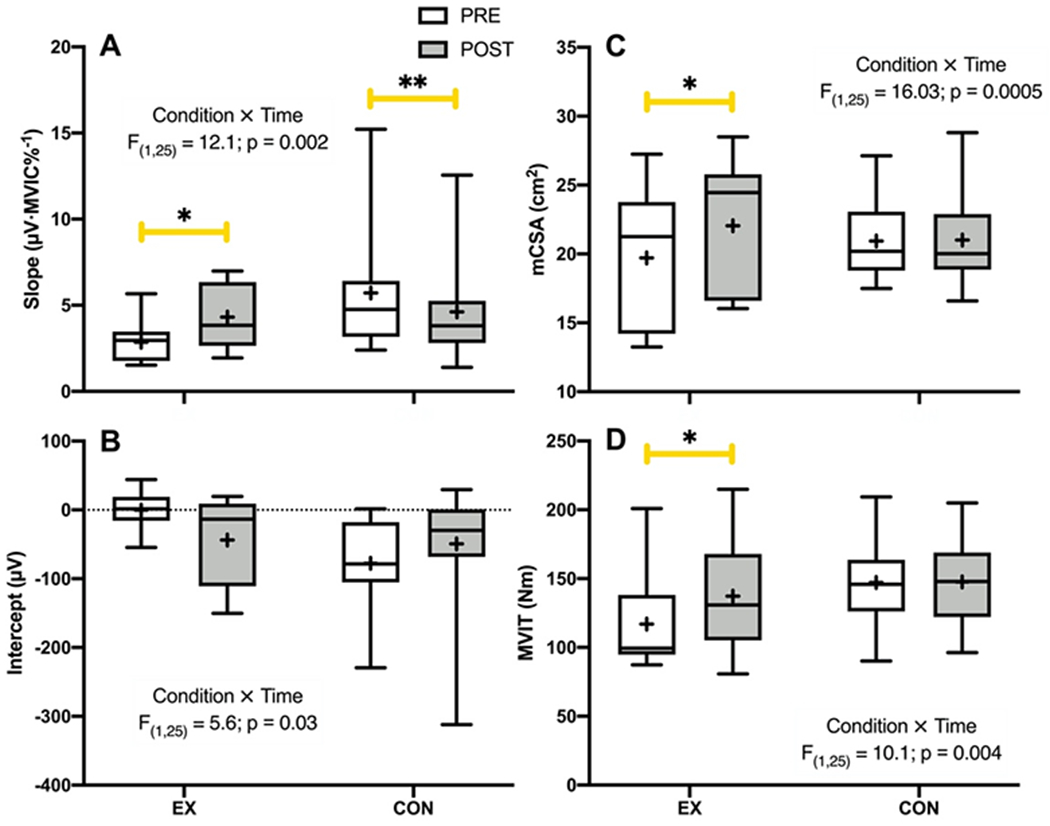
Box and whisker plots depicting the pre- (PRE) to post-intervention (POST) changes in (A) motor unit action potential amplitude versus normalized recruitment threshold (MUAPAMP-RTNORM) slope, (B) MUAPAMP-RTNORM intercept, (C) vastus lateralis muscle cross sectional area (mCSA), and (D) maximal voluntary isometric torque (MVIT) for the exercise (EX) and control (CON) groups. The middle horizontal line of the box represents the median, the lower and upper horizontal lines of the box represent the interquartile range (i.e., 1st and 3rd quartiles), the small cross represents the mean, and the whiskers depict the range. *indicates a significant increase from PRE to POST (p ≤ 0.04); **indicates a significant decrease from PRE to POST (p = 0.03).
While there were also significant Condition × Time interactions for the slope (F1,25 = 4.7, p = 0.04) and y-intercept (F1,25 = 5.4, p = 0.03) of the MUAPAMP-RTABS relationship, the post-hoc comparisons revealed no significant changes for either in the EX (both p ≥ 0.18) or CON (both p ≥ 0.16) group.
Pooled Analyses
The slope of the pooled regression line for the MUAPAMP-RTNORM relationship increased from PRE to POST (1.58 vs. 3.36 μV·MVIC%−1; F1,10 = 10.13, p = 0.0098) in the EX group (Figure 2A). There were no changes in either the slope (F1,8 = 3.16, p = 0.11) or the y-intercept (F1,8 = 0.79, p = 0.40) in the CON group (Figure 2B).
Figure 2.
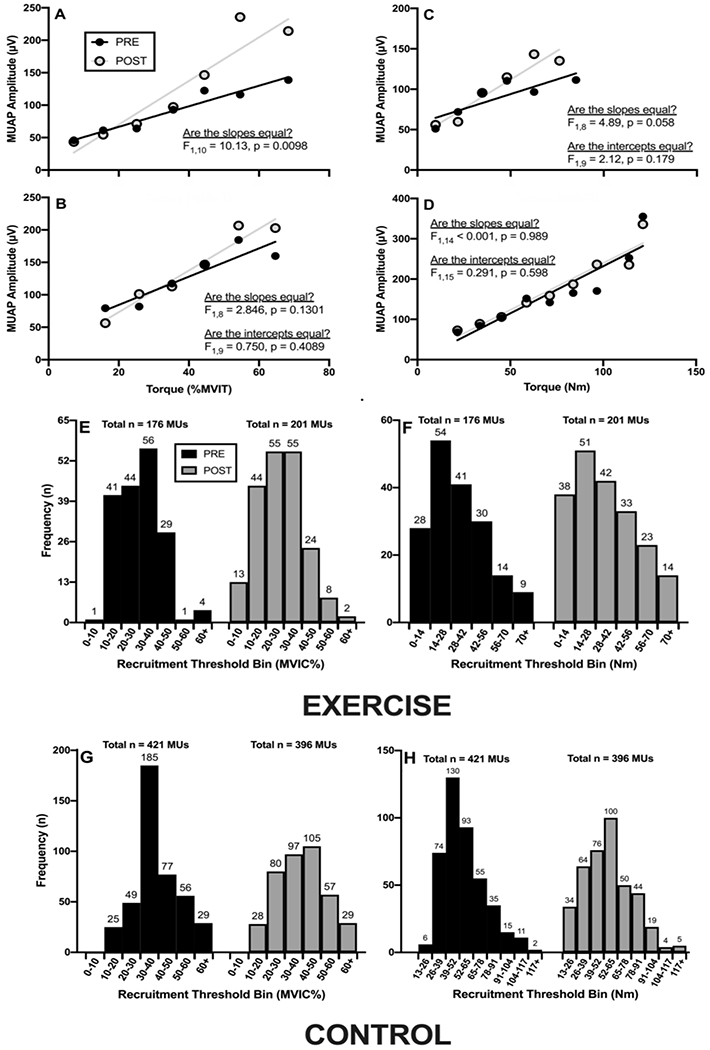
The pooled motor unit action potential (MUAP) amplitude versus normalized and absolute recruitment threshold (RT) relationships before (PRE) and after (POST) the intervention period in the exercise (panels A and C, respectively) and control (panels B and D, respectively) groups. Panels E-H depict the number and distribution of the MUs utilized in the pooled analyses.
However, neither the slope (0.73 vs. 1.42 μV·MVIC%−1; F1,8 = 4.89, p = 0.058) or intercept (F1,9 = 2.12, p = 0.18) of the pooled regression line for the MUAPAMP-RTABS relationship changed significantly from PRE to POST in the EX group (Figure 2C). There were also no changes in the slope (F1,14 = 0.001, p = 0.97) or y-intercept (F1,15 = 0.28, p = 0.61) in the CON group (Figure 2D).
Relationships
A significant, large correlation (r = 0.68, p = 0.023) was observed between Δslope of the MUAPAMP-RTNORM relationship and ΔmCSA in the EX group (Figure 3A), but not in the CON group (Figure 3C). Similarly, there was a large relationship (r = 0.65, p = 0.028) between Δslope of the MUAPAMP-RTABS relationship and ΔmCSA in the EX (Figure 3B), but not CON group (Figure 3D). Finally, Δslope of the normalized and absolute MUAPAMP-RT relationship were moderately and strongly (r = 0.48 and 0.56, respectively) related to ΔSMMRL in the EX group (Figures 3E and 3F, respectively), although they were not statistically significant (p = 0.098 and 0.06, respectively). There were no significant relationships between Δslope and ΔSMMRL in CON (Figures 3G and 3H).
Figure 3.
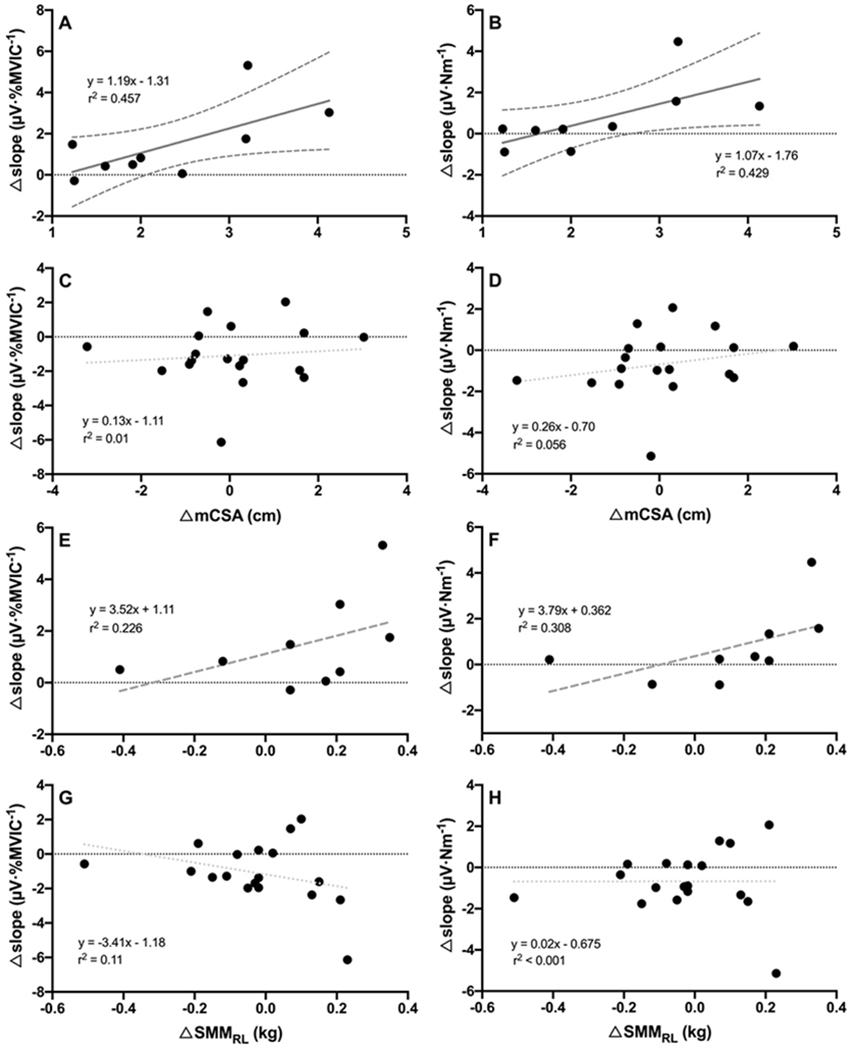
Panels A-D depict the relationships among each subject’s change in MUAPAMP-RTNORM slope and change in MUAPAMP-RTABS slope versus change in mCSA in the (A and B) EX group and (C and D) CON group. Panels E-H depict the relationships among each subject’s change in MUAPAMP-RTNORM slope and change in MUAPAMP-RTABS slope versus change in whole leg skeletal muscle mass (SMMRL) in the (E and F) EX group and (G and H) CON group. Solid dark grey regression lines (with 95% confidence intervals) indicate a strong, significant relationship. Dashed dark grey regression lines indicate a moderate to strong, but non-significant relationship (0.05 > p ≤ 0.10). Dotted light grey lines indicate small, non-significant relationships.
DISCUSSION
This investigation was the first to examine the utility of the MUAPAMP-RT relationship as an indicator of MU-specific hypertrophy following high-intensity exercise training in females. Our study also expanded on and addressed several limitations in the original study that proposed the MUAPAMP-RT relationship as a non-invasive indicator of hypertrophy8, including the inclusion of a comparative non-exercise control group and examination of MUAPAMP relative to both normalized and absolute RTs. We observed significant increases in mCSA, the slope of the MUAPAMP-RTNORM relationship, and MVIT in the EX, but not CON group. The changes in slope of both the normalized and absolute MUAPAMP-RT relationships were in fact strongly related (r = 0.68 and 0.65, respectively) to the change in mCSA, and moderately to strongly related (r = 0.48 and 0.56, respectively) to the change in SMMRL in the EX group. Further, when we examined changes in the pooled regression lines characterizing the normalized and absolute MUAPAMP-RT relationships, we observed a significant increase in slopes in the EX, but not CON group. Therefore, overall, our data demonstrate that 8 weeks of high-intensity exercise training elicits an increase in the rate of change in MUAPAMP with increments in RT (i.e., muscle force) in females. Furthermore, the magnitude of this change was strongly related to the magnitude of change in whole muscle CSA and moderately to strongly related to the magnitude of change in skeletal muscle mass of the tested leg. However, our data also reveal some important considerations for investigators interested in utilizing the MUAPAMP-RT relationship as a longitudinal assessment of MU-specific hypertrophy, which we discuss below.
The high-intensity exercise training program elicited significant improvements in both knee extensor strength and VL mCSA in the EX, but not CON group (Figure 1C & D). The observed 11.8% increase in mCSA and 17.5% increase in MVIT in the EX group after 8-weeks are in agreement with the significant 7-10% increases in VL muscle size37–40 and 10-20% increases in isometric knee extensor muscle strength40 reported previously following 8-10 week resistance training programs in untrained females. One significant implication of the increase in strength in the EX group is that the torque requirement of the 70% tracing increased (p = 0.003) by an average of 14.3 ± 10.1 Nm. As mentioned in the introduction, this increase could confound the interpretation of changes in the MUAPAMP-RTNORM relationship and, consequently, we also examined changes in the MUAPAMP-RTABS relationship following training.
In accordance with several previous studies using the same technology8,16,41,42, we observed strong, positive relationships between MUAPAMP and RT for each subject at both PRE and POST (PRE, r2 = 0.70 ± 0.15; POST, r2 = 0.74 ± 0.13). Thus, in accordance with the size principle, as a MU’s RT increased, so too did its MUAPAMP, and this relationship was observed both before and after the intervention period. Furthermore, both the mean changes and changes in the pooled MUAPAMP-RTNORM and MUAPAMP-RTABS relationships support the conclusion that the 8-week high intensity exercise program resulted in preferential adaptation to high-threshold MUs. Specifically, while the slope of MUAPAMP-RTNORM relationship decreased for CON, high-intensity exercise training elicited an increase in slope for EX. Similarly, while post-hoc analyses did not reveal significant changes, there was an interaction present for the slope of the MUAPAMP-RTABS relationship that was caused by an increase (+27%) in slope for EX, and a decrease (−18%) for CON. Furthermore, the slope of the pooled MUAPAMP-RTNORM increased in response to training in EX (Figure 2A). In contrast, there was no change in either the slope or y-intercept of the pooled MUAPAMP-RTNORM relationship for CON (Figure 2B). Thus, together, these data provide evidence that combined resistance and interval exercise training elicited adaptations in the peripheral properties of MUs which were greater for MUs with greater RTs, and this is likely because the high-intensity exercise employed herein predominantly stressed this population of MUs22.
Pope et al.8 previously reported a very strong relationship (r = 0.91) between the changes in slope of the MUAPAMP-RTNORM relationship and mCSA of the VL. In the present study, we also observed strong relationships between the change in slopes of both the normalized and absolute MUAPAMP-RT relationships and the change in mCSA (r = 0.68 and 0.65, respectively). Moreover, we utilized segmental BIA analyses to examine the change in SMMRL, which was also moderately and strongly related to the change in slopes of both the normalized (r = 0.48) and absolute (r = 0.56) MUAPAMP-RT relationships. Thus, while our data agree with those of Pope et al.8 in that the changes observed in the slope coefficients of the MUAPAMP-RT relationships were related to the changes in VL mCSA, our correlations were not as strong and changes in slope were only able to explain 46% of the variance in the change in muscle size, at most.
It is also worth noting that the changes in the MUAPAMP-RTABS relationships were more subdued than those of the MUAPAMP-RTNORM relationship. This was evidenced by both the lower relative increases in the mean and pooled slopes in the EX group for the MUAPAMP-RTABS than for the MUAPAMP-RTNORM relationship, as well as the fact that the changes in slope for the MUAPAMP-RTABS relationship were not statistically significant. This may suggest that training-induced changes in the MUAPAMP-RTNORM are not entirely a result of MU-specific fiber hypertrophy, but could also be influenced by changes in normalized RTs and/or muscle strength. Indeed, Casolo et al.23 reported “substantial and consistent” decreases (−15%) of normalized RTs following resistance training, but did not observe significant changes in absolute RTs. Together, these results suggest that participants in the EX group were able to achieve the prescribed torques by recruiting MUs at lower intensities relative to their maximum torque capability (e.g., MVIT) following training, and likely explains the greater observed changes in the MUAPAMP-RTNORM versus MUAPAMP-RTABS relationships.
Overall, our data suggest that an 8-week high-intensity exercise program elicited increases in the slope of the MUAPAMP-RT relationship in females. The observed change in slope of this relationship was significantly related to both VL mCSA and SMMRL. However, the observed changes in slope for the MUAPAMP-RT relationship were more subdued when MUAPAMP was expressed as a function of absolute (Nm) versus relative (%MVIC) RT, suggesting that changes in normalized RTs may play a role in the training-induced increase in MUAPAMP-RT slope. Therefore, investigators should carefully consider the role that training-induced changes in normalized RTs may have when utilizing outcomes reliant on normalized RT (e.g,, MUAPAMP-RTNORM, MUCV-RTNORM, etc) before attributing these changes solely to the dependent variable of these relationships (e.g., MUAPAMP and MUCV).
ACKNOWLEDGMENTS:
The authors would like to acknowledge PT for his contribution to data collection.
FUNDING: This study was supported by the Center for Integrative Research on Childhood Adversity under Award Number P20GM109097.
References
- 1.Henneman E Relation between size of neurons and their susceptibility to discharge. Science. 1957;126(3287):1345–1347. [DOI] [PubMed] [Google Scholar]
- 2.Henneman E, Somjen G, Carpenter DO. Functional Significance of Cell Size in Spinal Motoneurons. J Neurophysiol. 1965;28:560–580. [DOI] [PubMed] [Google Scholar]
- 3.Del Vecchio A, Negro F, Felici F, Farina D. Distribution of muscle fibre conduction velocity for representative samples of motor units in the full recruitment range of the tibialis anterior muscle. Acta Physiol (Oxf). 2018;222(2). [DOI] [PubMed] [Google Scholar]
- 4.Hakansson CH. Conduction velocity and amplitude of the action potential as related to circumference in the isolated fibre of frog muscle. Acta Physiol Scand. 1956;37(1):14–34. [DOI] [PubMed] [Google Scholar]
- 5.McPhedran AM, Wuerker RB, Henneman E. Properties of Motor Units in a Homogeneous Red Muscle (Soleus) of the Cat. J Neurophysiol. 1965;28:71–84. [DOI] [PubMed] [Google Scholar]
- 6.Tanji J, Kato M. Recruitment of motor units in voluntary contraction of a finger muscle in man. Exp Neurol. 1973;40(3):759–770. [DOI] [PubMed] [Google Scholar]
- 7.Goldberg LJ, Derfler B. Relationship among recruitment order, spike amplitude, and twitch tension of single motor units in human masseter muscle. J Neurophysiol. 1977;40(4):879–890. [DOI] [PubMed] [Google Scholar]
- 8.Pope ZK, Hester GM, Benik FM, DeFreitas JM. Action potential amplitude as a noninvasive indicator of motor unit-specific hypertrophy. J Neurophysiol. 2016;115(5):2608–2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitchell CJ, Churchward-Venne TA, West DW, et al. Resistance exercise load does not determine training-mediated hypertrophic gains in young men. J Appl Physiol (1985). 2012;113(1):71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campos GE, Luecke TJ, Wendeln HK, et al. Muscular adaptations in response to three different resistance-training regimens: specificity of repetition maximum training zones. Eur J Appl Physiol. 2002;88(1-2):50–60. [DOI] [PubMed] [Google Scholar]
- 11.Jenkins NDM, Miramonti AA, Hill EC, et al. Greater Neural Adaptations following High- vs. Low-Load Resistance Training. Front Physiol. 2017;8:331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moritani T, deVries HA. Neural factors versus hypertrophy in the time course of muscle strength gain. Am J Phys Med. 1979;58(3):115–130. [PubMed] [Google Scholar]
- 13.Seynnes OR, de Boer M, Narici MV. Early skeletal muscle hypertrophy and architectural changes in response to high-intensity resistance training. J Appl Physiol (1985). 2007;102(1):368–373. [DOI] [PubMed] [Google Scholar]
- 14.Roberts MD, Mobley CB, Vann CG, et al. Synergist ablation-induced hypertrophy occurs more rapidly in the plantaris than soleus muscle in rats due to different molecular mechanisms. Am J Physiol Regul Integr Comp Physiol. 2020;318(2):R360–R368. [DOI] [PubMed] [Google Scholar]
- 15.Staron RS, Malicky ES, Leonardi MJ, Falkel JE, Hagerman FC, Dudley GA. Muscle hypertrophy and fast fiber type conversions in heavy resistance-trained women. Eur J Appl Physiol Occup Physiol. 1990;60(1):71–79. [DOI] [PubMed] [Google Scholar]
- 16.Colquhoun RJ, Magrini MA, Haun CT, et al. Muscle phenotype is related to motor unit behavior of the vastus lateralis during maximal isometric contractions. Physiol Rep. 2018;6(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trevino MA, Sterczala AJ, Miller JD, et al. Sex-related differences in muscle size explained by amplitudes of higher-threshold motor unit action potentials and muscle fibre typing. Acta Physiol (Oxf). 2019;225(4):e13151. [DOI] [PubMed] [Google Scholar]
- 18.Gundersen K Determination of muscle contractile properties: the importance of the nerve. Acta Physiol Scand. 1998;162(3):333–341. [DOI] [PubMed] [Google Scholar]
- 19.Buller AJ, Eccles JC, Eccles RM. Interactions between motoneurones and muscles in respect of the characteristic speeds of their responses. J Physiol. 1960;150:417–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lomo T, Westgaard RH, Dahl HA. Contractile properties of muscle: control by pattern of muscle activity in the rat. Proc R Soc Lond B Biol Sci. 1974;187(1086):99–103. [DOI] [PubMed] [Google Scholar]
- 21.Dubowitz V, Newman DL. Change in enzyme pattern after cross-innervation of fast and slow skeletal muscle. Nature. 1967;214(5090):840–841. [DOI] [PubMed] [Google Scholar]
- 22.Martinez-Valdes E, Farina D, Negro F, Del Vecchio A, Falla D. Early Motor Unit Conduction Velocity Changes to High-Intensity Interval Training versus Continuous Training. Med Sci Sports Exerc. 2018;50(11):2339–2350. [DOI] [PubMed] [Google Scholar]
- 23.Casolo A, Farina D, Falla D, Bazzucchi I, Felici F, Del Vecchio A. Strength Training Increases Conduction Velocity of High-Threshold Motor Units. Med Sci Sports Exerc. 2019. [DOI] [PubMed] [Google Scholar]
- 24.Hu X, Rymer WZ, Suresh NL. Motor unit pool organization examined via spike-triggered averaging of the surface electromyogram. J Neurophysiol. 2013;110(5):1205–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sterczala AJ, Miller JD, Dimmick HL, Wray ME, Trevino MA, Herda TJ. Eight weeks of resistance training increases strength, muscle cross-sectional area and motor unit size, but does not alter firing rates in the vastus lateralis. Eur J Appl Physiol. 2020;120(1):281–294. [DOI] [PubMed] [Google Scholar]
- 26.Hagstrom AD, Marshall PW, Halaki M, Hackett DA. The Effect of Resistance Training in Women on Dynamic Strength and Muscular Hypertrophy: A Systematic Review with Meta-analysis. Sports Med. 2019. [DOI] [PubMed] [Google Scholar]
- 27.Colquhoun RJ, Tomko PM, Magrini MA, Muddle TWD, Jenkins NDM. The influence of input excitation on the inter- and intra-day reliability of the motor unit firing rate versus recruitment threshold relationship. J Neurophysiol. 2018;120(6):3131–3139. [DOI] [PubMed] [Google Scholar]
- 28.Zaheer F, Roy SH, De Luca CJ. Preferred sensor sites for surface EMG signal decomposition. Physiol Meas. 2012;33(2):195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Luca CJ, Adam A, Wotiz R, Gilmore LD, Nawab SH. Decomposition of surface EMG signals. J Neurophysiol. 2006;96(3):1646–1657. [DOI] [PubMed] [Google Scholar]
- 30.Nawab SH, Chang SS, De Luca CJ. High-yield decomposition of surface EMG signals. Clin Neurophysiol. 2010;121(10):1602–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herda TJ, Parra ME, Miller JD, Sterczala AJ, Kelly MR. Measuring the accuracies of motor unit firing times and action potential waveforms derived from surface electromyographic decomposition. J Electromyogr Kinesiol. 2020;52:102421. [DOI] [PubMed] [Google Scholar]
- 32.Jenkins ND, Housh TJ, Bergstrom HC, et al. Muscle activation during three sets to failure at 80 vs. 30% 1RM resistance exercise. Eur J Appl Physiol. 2015;115(11):2335–2347. [DOI] [PubMed] [Google Scholar]
- 33.Bosy-Westphal A, Jensen B, Braun W, Pourhassan M, Gallagher D, Muller MJ. Quantification of whole-body and segmental skeletal muscle mass using phase-sensitive 8-electrode medical bioelectrical impedance devices. Eur J Clin Nutr. 2017;71(9):1061–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ciolac EG, Mantuani SS, Neiva CM, Verardi C, Pessoa-Filho DM, Pimenta L. Rating of perceived exertion as a tool for prescribing and self regulating interval training: a pilot study. Biol Sport. 2015;32(2):103–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cohen J. A power primer. Psychol Bull. 1992;112(1):155–159. [DOI] [PubMed] [Google Scholar]
- 36.Zar JH. Biostatistical Analysis. Englewood Cliffs, NJ: Prentice-Hall; 1984. [Google Scholar]
- 37.Tinsley GM, Moore ML, Graybeal AJ, et al. Time-restricted feeding plus resistance training in active females: a randomized trial. Am J Clin Nutr. 2019;110(3):628–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stefanaki DGA, Dzulkarnain A, Gray SR. Comparing the effects of low and high load resistance exercise to failure on adaptive responses to resistance exercise in young women. J Sports Sci. 2019;37(12):1375–1380. [DOI] [PubMed] [Google Scholar]
- 39.Loenneke JP, Rossow LM, Fahs CA, Thiebaud RS, Grant Mouser J, Bemben MG. Time-course of muscle growth, and its relationship with muscle strength in both young and older women. Geriatr Gerontol Int. 2017;17(11):2000–2007. [DOI] [PubMed] [Google Scholar]
- 40.Botton CE, Radaelli R, Wilhelm EN, Rech A, Brown LE, Pinto RS. Neuromuscular Adaptations to Unilateral vs. Bilateral Strength Training in Women. J Strength Cond Res. 2016;30(7):1924–1932. [DOI] [PubMed] [Google Scholar]
- 41.Muddle TWD, Colquhoun RJ, Magrini MA, Luera MJ, DeFreitas JM, Jenkins NDM. Effects of fatiguing, submaximal high- versus low-torque isometric exercise on motor unit recruitment and firing behavior. Physiol Rep. 2018;6(8):e13675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sterczala AJ, Herda TJ, Miller JD, Ciccone AB, Trevino MA. Age-related differences in the motor unit action potential size in relation to recruitment threshold. Clin Physiol Funct Imaging. 2018;38(4):610–616. [DOI] [PubMed] [Google Scholar]


