Summary
Background
In the phase 3 LACC trial and a hsubsequent population-level review, minimally invasive radical hysterectomy was shown to be associated with worse disease-free survival and higher recurrence rates than was open radical hysterectomy in patients with early stage cervical cancer. Here, we report the results of a secondary endpoint, quality of life, of the LACC trial.
Methods
The LACC trial was a randomised, open-label, phase 3, non-inferiority trial done in 33 centres worldwide. Eligible participants were women aged 18 years or older with International Federation of Gynaecology and Obstetrics (FIGO) stage IA1 with lymphovascular space invasion, IA2, or IB1 adenocarcinoma, squamous cell carcinoma, or adenosquamous carcinoma of the cervix, with an Eastern Cooperative Oncology Group performance status of 0 or 1, who were scheduled to have a type 2 or 3 radical hysterectomy. Participants were randomly assigned (1:1) to receive open or minimally invasive radical hysterectomy. Randomisation was done centrally using a computerised minimisation program, stratified by centre, disease stage according to FIGO guidelines, and age. Neither participants nor investigators were masked to treatment allocation. The primary endpoint of the LACC trial was disease-free survival at 4·5 years, and quality of life was a secondary endpoint. Eligible patients completed validated quality-of-life and symptom assessments (12-item Short Form Health Survey [SF-12], Functional Assessment of Cancer Therapy–Cervical [FACT-Cx], EuroQoL-5D [EQ-5D], and MD Anderson Symptom Inventory [MDASI]) before surgery and at 1 and 6 weeks and 3 and 6 months after surgery (FACT-Cx was also completed at additional timepoints up to 54 months after surgery). Differences in quality of life over time between treatment groups were assessed in the modified intention-to-treat population, which included all patients who had surgery and completed at least one baseline (pretreatment) and one follow-up (at any timepoint after surgery) questionnaire, using generalised estimating equations. The LACC trial is registered with ClinicalTrials.gov, NCT00614211.
Findings
Between Jan 31, 2008, and June 22, 2017, 631 patients were enrolled; 312 assigned to the open surgery group and 319 assigned to the minimally invasive surgery group. 496 (79%) of 631 patients had surgery completed at least one baseline and one follow-up quality-of-life survey and were included in the modified intention-to-treat analysis (244 [78%] of 312 patients in the open surgery group and 252 [79%] of 319 participants in the minimally invasive surgery group). Median follow-up was 3·0 years (IQR 1·7–4·5). At baseline, no differences in the mean FACT-Cx total score were identified between the open surgery (129·3 [SD 18·8]) and minimally invasive surgery groups (129·8 [19·8]). No differences in mean FACT-Cx total scores were identified between the groups 6 weeks after surgery (128·7 [SD 19·9] in the open surgery group vs 130·0 [19·8] in the minimally invasive surgery group) or 3 months after surgery (132·0 [21·7] vs 133·0 [22·1]).
Interpretation
Since recurrence rates are higher and disease-free survival is lower for minimally invasive radical hysterectomy than for open surgery, and postoperative quality of life is similar between the treatment groups, gynaecological oncologists should recommend open radical hysterectomy for patients with early stage cervical cancer.
Funding:
MD Anderson Cancer Center and Medtronic.
Keywords: cervical cancer, quality of life, radical hysterectomy, robotic surgery, laparoscopic surgery, minimally invasive surgery
Introduction
New technologies are frequently adopted into surgical oncology subspecialties before prospective, randomized studies confirm their safety and efficacy. One prime example is the widespread acceptance of minimally invasive radical hysterectomy for the treatment of early-stage cervical cancer, based first on individual experiences and opinions and later on institutional retrospective studies.1-3 The recently completed Laparoscopic Approach to Cervical Cancer (LACC) Trial showed that in patients with early-stage cervical cancer, the risk of death after the minimally invasive procedure is six times the risk of death when compared to a radical hysterectomy performed through a traditional open incision (laparotomy). The minimally invasive procedure is also associated with higher recurrence rates.4 A review of the minimally invasive approach to radical hysterectomy based on population-level data from the National Cancer Database confirmed that women with cervical cancer who undergo this surgery have worse outcomes than women with cervical cancer who have the procedure performed via laparotomy.5 Since publication of those two studies, multiple retrospective studies have been published confirming these findings.6-9
Although studies demonstrate that survival is worse with minimally invasive radical hysterectomy, some surgeons might consider continued utilization of this approach because of the possibility that the procedure offers decreased operative morbidity/mortality. However, data on adverse events from the LACC Trial showed that although blood loss was higher for women who underwent open surgery, there was no difference between the open and minimally invasive surgery groups in terms of intraoperative complications, early or delayed postoperative adverse events, or major adverse events.10
Surgeons might also argue that vastly improved quality of life after minimally invasive surgery justifies continuing to offer patients minimally invasive radical hysterectomy.11 However, prospective studies are far from conclusive regarding the quality of life benefits of the minimally invasive approach. For women with endometrial cancer undergoing simple hysterectomy, two prospective, randomized trials, the LAP2 and LACE studies, reported different findings for quality of life in the postoperative period.12,13 The LAP2 study showed a modest advantage for minimally invasive surgery in terms of body image and return to work 6 weeks after surgery, but scores from the Functional Assessment of Cancer Therapy—General (FACT-G) questionnaire from the two groups (open and minimally invasive surgery) did not meet the pre-defined minimally important difference at this early milestone. At 6 months after surgery, there was no difference between the open and minimally invasive surgery groups in any of the quality of life measurements ascertained.12 In contrast, the LACE study showed improved FACT-G scores favoring the minimally invasive approach over open surgery for endometrial cancer at 6 weeks after surgery, and this difference persisted even at 6 months after surgery.13
For women who undergo radical hysterectomy for the treatment of cervical cancer, no prospective, randomized study comparing quality of life after the open and minimally invasive approaches has been done. Observational studies show no difference in long-term quality of life between the two approaches in cervical cancer survivors.14 In fact, long-term quality of life after open radical hysterectomy does not seem to differ even between patients who have undergone open radical hysterectomy and matched controls who have not undergone any type of hysterectomy (simple or radical).15
The recently completed LACC Trial was designed with the primary endpoint of comparing progression-free survival between the open and minimally invasive approaches. Secondary objectives included comparisons of overall survival, patterns of recurrence, treatment-related morbidities, and quality of life in the acute (≤ 6 weeks after surgery) and late (≥ 3 months after surgery) phases of recovery utilizing multiple instruments. In this manuscript, we present the quality of life results from the LACC Trial.
Methods
Study design and participants
The LACC Trial was a phase III non-inferiority study comparing disease-free survival after open versus minimally invasive radical hysterectomy. The trial was approved by the institutional review boards at each of the 33 participating centers, and patients gave written informed consent. The selection criteria, randomisation and masking, and treatment approach for the LACC Trial were previously reported.4 Patients at least 18 years of age with adenocarcinoma, squamous cell carcinoma, or adenosquamous carcinoma of the cervix, ECOG performance status of 0-1, and FIGO (2008) clinical stage IA1 disease with lymphovascular space invasion, IA2 disease, or IB1 disease (≤ 4 cm and limited to the cervix) were randomized 1:1 to open or minimally invasive radical hysterectomy. Patients could undergo either a type II or a type III radical hysterectomy (Piver classification) and pelvic lymphadenectomy. Postoperative adjuvant radiation therapy was recommended according to the Sedlis criteria, which are widely accepted.16
Patients were included in the quality of life analyses if they completed at least one baseline (pretreatment) questionnaire and one follow-up (at any time point) questionnaire. Patients were excluded if surgery was abandoned or if they withdrew from the study prior to surgery; however, patients who were randomized to the minimally invasive arm but had a conversion to open surgery were included in the minimally invasive arm. Finally, as none of the questionnaires were available in Korean, none of the patients recruited at Korean sites were included.
Randomization and masking
Randomization was performed through minimization with an equal allocation between the two treatment groups and stratified according to center, disease stage as determined clinically according to FIGO guidelines, and patient’s age (≤ 70 and > 70 years). Randomization was performed via a web-based computer randomization procedure coordinated centrally in the Biostatistical Department of the School of Population Health, University of Queensland, Australia using a computerized minimization program. As the primary outcome compared open to minimally invasive surgery, blinding of participants or investigators was not possible.
Procedures
Four self-administered quality of life questionnaires were administered to patients by study coordinators in person at baseline (before surgery) and at each follow-up appointment after surgery: the Functional Assessment of Cancer Therapy—Cervical (FACT-Cx), the MD Anderson Symptom Inventory (MDASI), the Short Form-12 (SF-12), and the EuroQoL-5D (EQ-5D). These instruments were chosen based on prior publications in gynecologic oncology as well as in consultation with experts from the MD Anderson Assessment, Intervention, and Measurement (AIM) Core Resource Center.12,13 The FACT-Cx was administered before surgery and at 1 and 6 weeks and at 3, 6, 18, 30, 42, and 54 months after surgery. The MDASI, SF-12, and EQ-5D were administered before surgery and at 6 weeks and at 3 and 6 months after surgery. In an effort to limit survey burden for patients, we collected quality of life data for all four instruments for 6 months after surgery and then the FACT-Cx only for an additional 4 years (up to 54 months) since the FACT-Cx is the most commonly utilized quality of life instrument for assessing cervical cancer patients across all types of studies (surgical, medical, radiation, supportive care). As there is no standard for collection of patient reported outcomes after surgery, time points for quality of life assessments were chosen based on patient follow-up schedule for other endpoints in the LACC study including recurrence rates, progression free survival, and postoperative complications. The instruments comprised 78 items total, and the estimated time to complete all four instruments was 20-25 minutes.
Typically, higher scores correlate with better functioning on the SF-12 and FACT-Cx instruments, while lower scores correlate with better functioning on the MDASI and EQ-5D instruments. However, in order to make interpretation easier, all survey scales were transformed to a scale of 0–100 with higher scores correlating with better quality of life outcome.
The FACT-Cx is a 42-item survey that has been widely used in oncology because it is a multidimensional instrument that is easy to administer. The series of FACT questionnaires are also particularly well regarded because they contain several disease-specific subscales, including the FACT-Cx cervical cancer subscale. This subscale was developed to incorporate several issues specific to cervical cancer treatment, both physical and emotional, including sexual function and fertility.17
The MDASI is a 19-item questionnaire. The first 13 items assess patient symptoms during the prior 24 hours. Symptoms assessed include pain, fatigue, nausea/vomiting, anorexia, sleep symptoms, and distress. The last six items assess how those symptoms have interfered with the patient’s general well-being, including their general activity, mood, ability to walk and perform normal work, and relationships with others and enjoyment of life.18
The SF-12 measures generic health concepts relevant regardless of a patient’s age, disease, or treatment. This instrument is designed to assess health from the patient's point of view and covers eight areas: physical functioning, role functioning—physical, bodily pain, general health, vitality, social functioning, role functioning—emotional, and mental health. Results are expressed in terms of two meta-scores: a physical component summary and a mental component summary.19
The EQ-5D is a standardized instrument for measuring health outcome. It provides a descriptive profile and a single index value for health status. The EQ-5D was originally designed to complement the SF-12. Serial administrations of the EQ-5D can be used to measure changes in health status and quality of life and may be used to calculate the quality-adjusted life years gained with an intervention.20
From these four questionnaires, six quality of life outcomes were analyzed: FACT-Cx total score, SF-12 physical and mental components, MDASI scores for symptoms (“symptom score”) and interference of symptoms with daily life (“interference score”), and EQ-5D total score (“body state score”).
Outcomes
The LACC Trial was a phase III non-inferiority study comparing open versus minimally invasive radical hysterectomy with a primary endpoint of disease-free survival. Secondary endpoints included comparisons between the two arms with regards to 1) overall survival, 2) patterns of recurrence, 3) quality of life, 4) treatment associated morbidity, 5) pelvic floor dysfunction, 6) feasibility of sentinel nodes, and 7) cost-effectiveness. Quality of life was measured using four validated instruments: the Functional Assessment of Cancer Therapy—Cervical (FACT-Cx), the MD Anderson Symptom Inventory (MDASI), the Short Form-12 (SF-12), and the EuroQoL-5D (EQ-5D).
Statistical methods
The primary endpoint in this non-inferiority trial was progression free survival. We hypothesized that disease free survival at 4.5 years would be similar for women with early stage cervical cancer undergoing open versus minimally invasive radical hysterectomy. Based on these numbers, for a 4.5-year accrual and 4.5-year follow-up, a total of 740 patients (370 per arm) would be sufficient to declare equivalence with an equivalence margin of 7.2% or less at 4.5 years.
All statistical analyses were conducted based on the modified intention-to-treat principle. As only one patient in each arm did not receive the randomized treatment, analysis by actual treatment was not performed. All analyses were performed at the 5% level of significance (two-sided) and conducted in SAS version 9·3 (SAS Institute, Inc, Cary, NC) and STATA version 14·1 (StataCorp, Texas). No statistical adjustments to the analysis were made for multiple testing or to account for missing data.
The number of completed questionnaires at each time point of interest was summarised. Patients were excluded from analysis if they did not complete at least one baseline (pretreatment) questionnaire, did not complete at least one follow-up (at any time point) questionnaire, or did not undergo surgery.
Patient demographic and clinical characteristics were presented as frequencies for categorical variables and mean (standard deviation [SD]) or median (interquartile range) for continuous variables. Quality of life scores were summarised as mean (SD) at each time point by treatment group. In an effort to standardize multiple quality of life instruments for ease of interpretation, survey scores have been transformed into 0–100 scale with higher scores correlating with better quality of life outcome. Original, untransformed data are also presented in the appendix (pages 2–5).13,21 Plots were constructed of mean scores with corresponding 95% confidence intervals (CIs) over time for each quality of life outcome.
Change in quality of life was calculated from baseline to an early (6 weeks) and a late (3 months) postoperative time point for each variable. Change scores were summarised by treatment group for each time period. Differences in change scores between open and minimally invasive surgery were compared at each time period using generalised estimating equations with a time-by-treatment interaction term included alongside the main effects of time and treatment. This method allows the inclusion of all participants, regardless of whether they have missing data at the early or late time point, and was adopted to take into account the within-patient correlation. As no other covariates were included in the model, the assumption is that treatment and time explain any missing data. Results are presented in a forest plot with positive differences representing an absolute advantage for minimally invasive surgery.
Change scores were used to assess change in quality of life over time for all four instruments. Change in quality of life between baseline and the early and late time points was dichotomised to show first a 5% and then a 10% improvement from baseline. The change scores were set at 5% and 10% differences in an effort to quantify which patient’s quality of life improved over time as the overall averages in the quality of life instruments does not provide information on which individual patients actually show and improvement or decrease in quality of life. The assessment of these change scores were predefined based on previous studies as clinically significant.13,22-24 The difference in proportions between the open surgery and minimally invasive surgery groups was calculated and is presented alongside the corresponding 95% CI and p-value.
Finally, we performed a subset analysis for FACT-Cx composite scores at early and late time points for age (<60 vs. ≥60 years), country of residence (developed vs. developing according to the United Nations Department of Economic and Social Affairs), ECOG performance status (0 vs. 1), incision type for laparotomy (vertical vs. transverse), and adverse events (< grade 0-1 vs. grade 2+ adverse events per CTCAE criteria). As grade 2+ adverse events was the only predictor of decreased quality of life scores on the FACT-Cx composite scores, we also evaluated that endpoint for the five composite scores from the other three instruments at the early and late time points.
This study is registered with ClinicalTrials.gov, number NCT00614211.
Role of the funding source
This study was funded by a departmental research fund of the Department of Gynecologic Oncology and Reproductive Medicine, The University of Texas MD Anderson Cancer Center, and by an unrestricted research grant from Medtronic. Medtronic had no role in the study design or implementation, nor did they participate in the collection, analysis, or interpretation of the data or in the writing of this report. MF, KPR, VG, RA, AO, and PTR were the only authors with access to all of the raw data. The corresponding author had full access to all of the data and the final responsibility to submit for publication. MF, RLC, and PTR are also supported by the NIH/NCI under award number P30CA016672.
Results
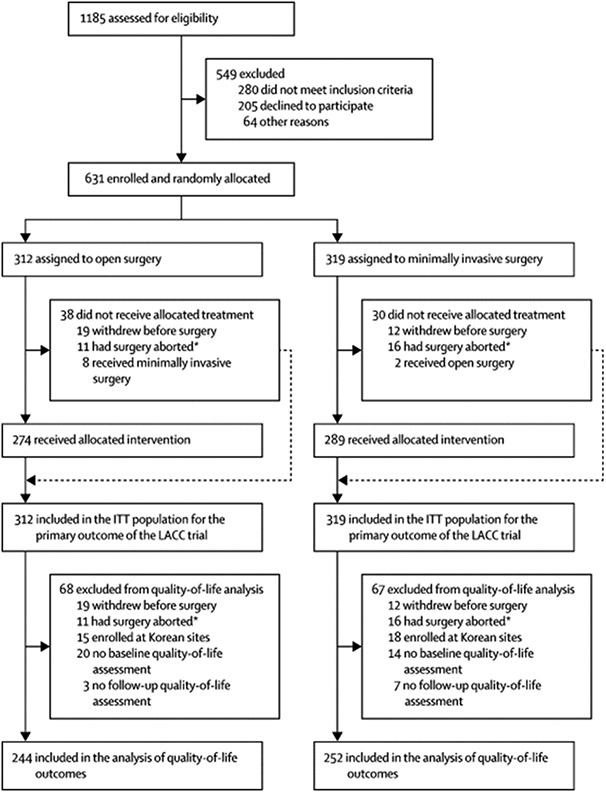
Between January 31, 2008 and June 22, 2017, 631 patients were enrolled in the LACC Trial at 33 centers worldwide. Of these patients, 312 were randomized to open (abdominal) radical hysterectomy, and of these, 244 (78·2%) completed at least one baseline quality of life survey. Completion rates for each instrument at each time point are shown in the Appendix (pages 2-5). The other 319 patients were randomized to minimally invasive radical hysterectomy, and of these, 252 (79·0%) completed at least one baseline quality of life survey (Figure 1). Patient characteristics for each group are summarised in Table 1. As 20% of baseline forms were not completed, the analysis is according to the ITT principle (patients analyzed in the groups to which they were originally assigned regardless of whether they actually received the allocated treatment) however this may be considered by some to not be strictly ITT (eg modified ITT).25 Additionally, no baseline variables were identified as being associated with missing quality of life forms suggesting that non-completion of forms was random.
Figure 1. Trial profile.
ITT=intention to treat. *Surgery was started, but not completed due metastatic disease.
Table 1.
Baseline characteristics in the modified intention-to-treat population
| Empty Cell |
Empty Cell | Open surgery (n=244) |
Minimally invasive surgery (n=252) |
|---|---|---|---|
| Age, years | |||
| Mean (SD) | 45·6 (10·4) | 45·4 (1·4) | |
| Median (IQR) | 45·0 (22·0–73·1) | 44·1 (22·4–71·3) | |
| Body-mass index, kg/m2 | 26·5 (5·5) | 27·3 (5·7) | |
| ECOG performance status | |||
| 0 | 223 (91%) | 229 (91%) | |
| 1 | 21 (9%) | 23 (9%) | |
| Geographical region | |||
| Asia | 37 (15%) | 40 (16%) | |
| Australia or New Zealand | 42 (17%) | 41 (16%) | |
| Europe | 20 (8%) | 27 (11%) | |
| North America | 27 (11%) | 29 (12%) | |
| South America | 118 (48%) | 115 (46%) | |
| Histological subtype | |||
| Squamous cell carcinoma | 174 (71%) | 175 (69%) | |
| Adenocarcinoma | 64 (26%) | 71 (28%) | |
| Adenosquamous | 6 (2%) | 6 (2%) | |
| FIGO clinical disease stage | |||
| IA1 (with lymphovascular space invasion) | 4 (2%) | 4 (2%) | |
| IA2 | 15 (6%) | 17 (7%) | |
| IB1 | 225 (92%) | 231 (92%) | |
| Treatment received | |||
| Open | 243 (100%) | 0 | |
| Minimally invasive surgery | 1 (<1%) | 244 (97%) | |
| Surgery converted to TARH | NA | 8 (3%) | |
| Adjuvant therapy | |||
| Chemotherapy or radiotherapy | 66 (27%) | 66 (26%) | |
| ≥1 cycle of chemotherapy | 48 (20%) | 47 (19%) | |
| ≥1 dose of radiotherapy | 55 (23%) | 57 (23%) | |
| Incision type | |||
| Vertical midline | 141 (58%) | 7 (3%) | |
| Low transverse | 103 (42%) | 1 (<1%) | |
| Did not have open surgery | 0 | 244 (97%) | |
Data are mean (SD), or n (%), unless stated otherwise. ECOG=Eastern Cooperative Oncology Group. FIGO=International Federation of Gynaecology and Obstetrics. TARH=total abdominal radical hysterectomy. NA=not applicable.
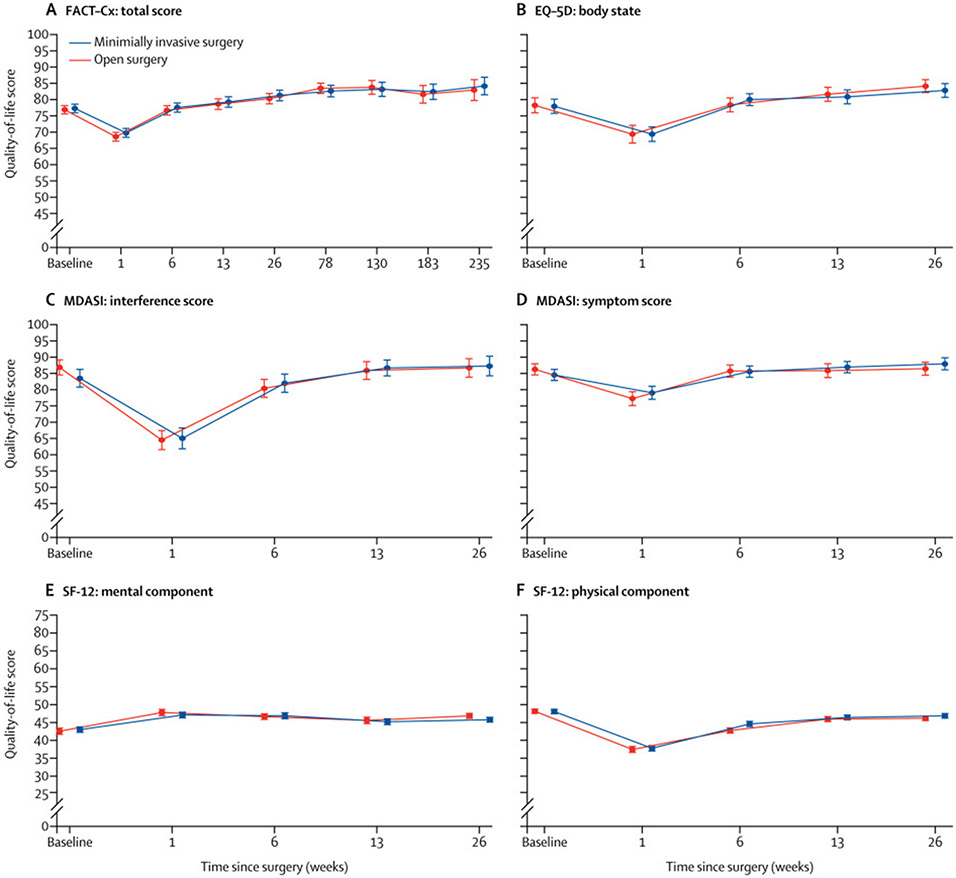
Baseline surveys prior to surgery showed no difference between the open and minimally invasive surgery groups in any of the six composite scores: FACT-Cx total score, SF-12 physical and mental components, MDASI symptom and interference scores, and EQ-5D body state score. Postoperatively, there was no difference in any of the six composite scores between the open and minimally invasive surgery groups at any time point. The transformed survey scales (0–100 with higher scores correlating with better quality of life outcome) are shown in Figure 2. Appendix pages 2-3 shows the transformed scores for each instrument at each time point. Appendix pages 4-5 shows the untransformed scores.
Figure 2. Change in quality-of-life scores over time.
Datapoints represent means and error bars denote 95% CIs. Higher quality-of-life scores represents better quality of life. Timepoints for all surveys were the same for both treatment groups. Figure timepoints have been offset slightly to better show data without overlap. FACT-Cx=Functional Assessment of Cancer Therapy–Cervical. EQ-5D=EuroQoL-5D. MDASI=MD Anderson Symptom Inventory. SF-12=12-item Short Form Health Survey.
Postoperative recovery was evaluated at early (6 weeks after surgery) and late (3 months after surgery) time points. For those randomized to open surgery, the median time from surgery to response for the early time point was 6 weeks (IQR 6·0 - 6·4 weeks) and for the late time point 3 months (IQ 3·0 - 3·3 months). For those randomized to minimally invasive surgery, the median time from surgery to response for the early time point was 6 weeks (IQR 6·0 - 6·6 weeks) and for the late time point 3 months (IQ 3·0 - 3·2 months). At the early time point (6 weeks after surgery), patients in both the open surgery group and the minimally invasive surgery group had a significant reduction in the physical component score of the SF-12, with patients in the open surgery group reporting a greater reduction (Appendix page 6). Although the decrease in the SF-12 physical component score was greater for the open group as compared to the minimally invasive group, there was no difference in the composite scores between the two groups at the 6-week time point. The decrease from baseline to 6 weeks in the SF-12 physical component score was 3·3 (95% CI 2·3 to 4·3) for the minimally invasive surgery group and 5·3 (95% CI 4·2 to 6·5) for the open surgery group (difference: 1·9 [95% CI: 0·7 to 3·2], p=0·003). Both groups also had a significant increase from baseline to 6 weeks in the mental component score of the SF-12, but there was no difference between groups. At 6 weeks after surgery, there was no difference between the open surgery and minimally invasive surgery groups in the change scores for any of the other quality of life measures (Figure 3). By the 3-month postsurgery (late) time point, there was no difference between the two groups in the change scores for any of the quality of life measures analyzed, including the SF-12 physical component score, although both groups reported a reduction from baseline (Figure 3 and Appendix page 7). Finally, there was no difference between the open and minimally invasive surgery groups in change scores when changes were dichotomised to show either a 5% or 10% improvement at the early or late time point (Table 2).
Table 2.
Proportion of patients whose quality of life had improved by at least 5% or 10% from baseline at 6 weeks and 3 months after surgery by treatment group
| Empty Cell | Open surgery |
Minimally invasive surgery |
Difference (95% CI)* |
p value |
|---|---|---|---|---|
| FACT-Cx total score | ||||
| 5% improvement at 6 weeks | 75/210 (36%) | 69/214 (32%) | −3·5% (−18·9 to 12·0) | 0·66 |
| 5% improvement at 3 months | 78/198 (39%) | 91/211 (43%) | 3·7% (−11·1 to 18·6) | 0·62 |
| 10% improvement at 6 weeks | 38/210 (18%) | 33/214 (15%) | −2·7% (−20·0 to 14·7) | 0·76 |
| 10% improvement at 3 months | 46/198 (23%) | 45/211 (21%) | −1·9% (−19·0 to 15·2) | 0·83 |
| MDASI: interference score | ||||
| 5% improvement at 6 weeks | 50/211 (24%) | 57/213 (27%) | 3·1% (−13·4 to 19·5) | 0·72 |
| 5% improvement at 3 months | 53/199 (27%) | 69/210 (33%) | 6·2% (−10·0 to 22·5) | 0·45 |
| 10% improvement at 6 weeks | 34/211 (16%) | 43/213 (20%) | 4·1% (−13·2 to 21·3) | 0·64 |
| 10% improvement at 3 months | 38/199 (19%) | 54/210 (26%) | 6·6% (−10·5 to 23·7) | 0·45 |
| MDASI: symptom score | ||||
| 5% improvement at 6 weeks | 73/210 (35%) | 70/215 (33%) | −2·2% (−17·7 to 13·3) | 0·78 |
| 5% improvement at 3 months | 73/202 (36%) | 79/211 (37%) | 1·3% (−14·0 to 16·6) | 0·87 |
| 10% improvement at 6 weeks | 43/210 (20%) | 42/215 (20%) | −0·9% (−17·9 to 16·1) | 0·91 |
| 10% improvement at 3 months | 47/202 (23%) | 52/211 (25%) | 1·4% (−15·4 to 18·2) | 0·87 |
| SF-12: mental component | ||||
| 5% improvement at 6 weeks | 84/195 (43%) | 87/204 (42%) | −0·9% (−15·7 to 14·0) | 0·91 |
| 5% improvement at 3 months | 78/185 (42%) | 72/202 (35%) | −6·9% (−22·5 to 8·6) | 0·38 |
| 10% improvement at 6 weeks | 48/195 (25%) | 46/204 (22%) | −2·3% (−19·5 to 14·9) | 0·79 |
| 10% improvement at 3 months | 41/185 (22%) | 39/202 (19%) | −3·1% (−20·8 to 14·7) | 0·73 |
| SF-12: physical component | ||||
| 5% improvement at 6 weeks | 18/195 (9%) | 26/204 (13%) | 3·4% (−15·1 to 21·9) | 0·72 |
| 5% improvement at 3 months | 26/185 (14%) | 24/202 (12%) | −2·3% (−20·9 to 16·3) | 0·81 |
| 10% improvement at 6 weeks | 9/195 (5%) | 7/204 (3%) | −1·2% (−20·5 to 18·0) | 0·90 |
| 10% improvement at 3 months | 9/185 (5%) | 9/202 (4%) | −0·5% (−19·9 to 19·0) | 0·96 |
| EQ-5D: body state | ||||
| 5% improvement at 6 weeks | 69/213 (32%) | 78/215 (36%) | 3·9% (−11·5 to 19·2) | 0·62 |
| 5% improvement at 3 months | 79/202 (39%) | 84/211 (40%) | 0·7% (−14·3 to 15·7) | 0·93 |
| 10% improvement at 6 weeks | 44/213 (21%) | 54/215 (25%) | 4·5% (−12·2 to 21·1) | 0·60 |
| 10% improvement at 3 months | 47/202 (23%) | 59/211 (28%) | 4·7% (−12·0 to 21·3) | 0·58 |
Data are n/N (%). FACT-Cx=Functional Assessment of Cancer Therapy–Cervical. MDASI=MD Anderson Symptom Inventory. SF-12=12-item Short Form Health Survey. EQ-5D=EuroQoL-5D.
Change in score from baseline (minimally invasive surgery group minus open surgery group).
In exploratory analyses, we evaluated whether certain subgroups noted significant changes from baseline FACT-Cx scores at the early (6 weeks) or late (3 months) time point. At 6 weeks after surgery, patients with body mass index (BMI) <30 mg/m2 had an increase of 1·87 (95% CI: −0·33 to 4·08) in their total FACT-Cx score, and patients with BMI ≥30 mg/m2 had a decrease of 0·49 (95% CI: −1·77 to 0·79) in their total FACT-Cx score (difference: −2·35 [95% CI: −4·54 to −0·16], p=0·04). This difference between these two groups had resolved at the late time point.
On further exploratory analyses, patients who experienced a grade 2+ adverse event had a worse Total Score on the FACT-Cx at 6 weeks after surgery than those women who did not experience a grade 2 or worse adverse event. We then expanded this comparison to all instruments and found worse quality of life on all of six composite scores for the four instruments at the early (6 week) time point (Table 3). At the late (3 month) time point, the cohort with grade 2+ adverse events continued to experience worse quality of life as measured by the FACT-Cx Global Score, MDASI Symptom and Interference Scores, and SF-12 Physical Component Score but not on the SF-12 Mental Component or EQ-5D Body State scores.
Table 3.
Subgroup analysis of quality-of-life scores at 6 weeks and 3 months after surgery, stratified by adverse event severity
| Empty Cell | Grade 0–1 adverse events | Grade ≥2 adverse events | p value* | ||
|---|---|---|---|---|---|
| Empty Cell | Patients, n | Mean score† (SD) | Patients, n | Mean score† (SD) | Empty Cell |
| FACT-Cx total score | |||||
| 6 weeks | 357 | 78·0 (11·6) | 78 | 73·2 (12·8) | 0·0058 |
| 3 months | 360 | 79·8 (12·9) | 59 | 73·8 (13·3) | 0·0023 |
| SF-12: physical component | |||||
| 6 weeks | 343 | 44·0 (6·4) | 77 | 42·1 (7·3) | 0·025 |
| 3 months | 349 | 46·6 (5·8) | 56 | 43·6 (7·3) | 0·0051 |
| SF-12: mental component | |||||
| 6 weeks | 343 | 47·2 (6·9) | 77 | 44·6 (7·8) | 0·0016 |
| 3 months | 349 | 45·5 (7·5) | 56 | 44·5 (6·1) | 0·38 |
| MDASI: symptom score | |||||
| 6 weeks | 357 | 86·8 (13·9) | 78 | 80·5 (17·7) | 0·0014 |
| 3 months | 362 | 87·4 (14·8) | 58 | 80·4 (18·2) | 0·0092 |
| MDASI: interference score | |||||
| 6 weeks | 357 | 83·3 (20·6) | 77 | 71·7 (26·2) | 0·0002 |
| 3 months | 360 | 87·6 (19·4) | 57 | 78·3 (23·5) | 0·026 |
| EQ-5D body state score | |||||
| 6 weeks | 354 | 80·6 (15·5) | 80 | 73·2 (17·2) | 0·0033 |
| 3 months | 361 | 82·2 (16·1) | 58 | 75·2 (21·5) | 0·066 |
FACT-Cx=Functional Assessment of Cancer Therapy–Cervical. SF-12=12-item Short Form Health Survey. MDASI=MD Anderson Symptom Inventory. EQ-5D=EuroQoL-5D.
Two-sided.
All quality-of-life scores were transformed to a 0–100 scale, with higher scores indicating better quality of life.
There was no difference in change in FACT-Cx score between baseline and the early or late time point for any of the other subgroups analyzed, including subgroups defined by age (<60 vs. ≥60 years), country of residence (developed vs. developing according to the United Nations Department of Economic and Social Affairs), ECOG performance status (0 vs. 1), or incision type for laparotomy (vertical vs. transverse). (Appendix page 8). Finally, we explored whether change in scores between baseline and either the early or the late time point predicted future recurrence or death from disease and found no correlation.
Discussion
In this study, we found no difference in quality of life between women undergoing open radical hysterectomy and those undergoing minimally invasive radical hysterectomy in the acute (≤6 weeks) or late (≥3 months) phase of recovery on any of the four quality of life instruments utilized. These four validated instruments assess the effects on quality of life of an array of acute and chronic health conditions. In addition to performing cross-cohort comparisons at multiple time points, we evaluated within-patient changes in quality of life scores from baseline (pre-surgery) to 6 weeks after surgery (early time point) and 3 months after surgery (late time point) for the two groups. In these 12 comparisons, the only statistically significant difference was a difference in the physical component score of the SF-12 at 6 weeks after surgery favoring (i.e., indicating a less severe decrease in quality of life) the minimally invasive surgery group. Although statistically significant, the absolute difference was only 2% between the two groups, which calls into question the clinical significance of this finding. By 3 months after surgery, this difference had resolved. Finally, in a hypothesis generating exploratory analysis, we found that adverse events (CTCAE grade 2 or worse) correlated with worse quality of life on all scales 6 weeks after surgery and this association persisted on multiple instruments 3 months postoperatively.
These findings may be surprising to some surgeons who anecdotally may feel certain that minimally invasive surgery correlates with better quality of life than laparotomy. The literature, however, does not consistently support that sentiment. There are few randomized, prospective surgical studies comparing open and minimally invasive approaches for cancer treatment and even fewer that incorporate quality of life endpoints in their outcomes. The few studies to date show generally short-lived or minimal quality of life advantages for minimally invasive surgery. For example, in a large prospective, randomized study comparing open and minimally invasive colectomy for colon cancer, Weeks et al.26 found a difference in the single-item global rating scale at 2 weeks after surgery favoring minimally invasive surgery but no difference at that time point on the Symptom Distress Scale pain intensity score, the Symptom Distress Scale summary score, or the Quality of Life Index. And at 2 months after surgery, there was no difference in any of the scales measured. Other prospective, randomized studies on the surgical treatment of colon cancer have shown a difference in quality of life favoring a minimally invasive approach lasting up to 1 year after surgery.27,28 In the treatment of esophageal cancer, quality of life at 6 weeks after surgery also was better for patients who underwent esophagectomy via a minimally invasive approach than for those who underwent open esophagectomy.29 In that study, quality of life was not measured beyond the 6 week time point.
In the field of surgery for gynecologic cancers, as mentioned in the introduction, the LAP2 and LACE studies reported conflicting results for quality of life measured with the FACT-G survey after open and minimally invasive simple hysterectomy for endometrial cancer.12,13 In the LAP2 study, there was a slight difference favoring minimally invasive surgery at 6 weeks, but this difference did not meet the predetermined “minimally important difference,” and by 6 months, this difference had disappeared.12 In the LACE study, there was a difference in all subscales of the FACT-G at 6 weeks favoring the minimally invasive surgery group, and this difference did persist at 6 months.13
There are multiple possible explanations for the lack of difference in quality of life between the two cohorts in the LACC Trial that we report herein. Among these potential explanations is that early postoperative quality of life scores may correlate more closely with surgical morbidity than with surgical approach.30 In explaining their results, authors of the LAP2 study hypothesized that one reason clinically significant quality of life differences were not observed between the open and laparoscopic surgery groups was that clinical outcomes (including intraoperative complications) were similar in the two cohorts.12 Similarly, in the LACC Trial, there were no differences in intraoperative complications or postoperative morbidities between the minimally invasive and open surgery groups.10 However, when we evaluated quality of life in those patients who had a grade 2+ adverse event related to the surgical procedure, we found worse quality of life across all scales regardless of surgical approach. We hypothesize surgical approach (open vs. minimally invasive) may be irrelevant for quality of life if patients have an uncomplicated postoperative course.
The lack of difference in intraoperative and postoperative complications may be due to the relatively low rates of obesity in the patient population studied. Obese and morbidly obese patients have more postoperative complications and longer hospitalizations than women with BMI <30 kg/m2 (the threshold between overweight and obesity).31 In the LACC Trial, the average BMI in both the open and minimally invasive surgery groups was approximately 27 kg/m2. In the LAP2 study, in which there was a lack of clinically meaningful differences in postoperative quality of life scores, the median BMI was also lower than the obesity threshold, at 28-29 kg/m2.12 In contrast, in the LACE study, in which postoperative quality of life scores did differ among the open and minimally invasive surgery groups, the median BMI in the overall patient population was 33 kg/m2.13 In the exploratory analyses in the study we report herein, change scores at the early time point differed between obese and morbidly obese patients (BMI ≥30 kg/m2) and normal-weight and overweight patients (BMI <30 kg/m2).
Adding to the multifactorial influences that may affect postoperative quality of life is the adoption of “enhanced recovery after surgery” programs in many institutions worldwide. These programs apply standardized, evidence-based treatment algorithms or “bundles” to the patient’s surgical journey starting days before the surgery and lasting through discharge and beyond.32 This multidisciplinary approach, which involves surgeons, anesthesia teams, and nursing support, has led to significant decreases in postoperative pain and hospital length of stay, and faster return to baseline functioning after laparotomy in a variety of surgical fields.33 Implementation of an enhanced recovery program in patients undergoing laparotomy for ovarian cancer resection showed improvement in MDASI scores in the cohort of women who had their postoperative care managed under enhanced recovery compared to those who had conventional perioperative management.34 Although we do not have any data about the number of centers in the LACC Trial that had adopted enhanced recovery pathways for their patients undergoing laparotomy, this approach has become ubiquitous in our field and likely played some role in improving postoperative quality of life in patients who underwent laparotomy.
Our study has limitations inherent to international surgical trials. First, although patients were randomized to open or minimally invasive surgery, blinding of researchers and patients was impossible. Furthermore, patients were randomized well ahead of their surgery, and patients’ knowledge of their group assignment may have biased the results. Also, the study was powered to show non-inferiority in disease free survival between the two arms so the sample size may not be adequate to detect a difference in the quality of life outcomes. The exclusion of Korean patients due to the lack of validated instruments in that language may also have introduced bias. In addition, although we did perform some exploratory analyses on other confounders that may effect quality of life (e.g. age, country of residence, ECOG performance status, incision type, BMI, postoperative morbidity), we are unable to evaluate other possible factors such as length of hospital stay. As the study was performed at 33 sites across multiple countries, there was a wide range of postoperative lengths of stay. For example, in the United States, the median length of stay after minimally invasive surgery was 2 days compared to 14 days for patients in China. However, it would be difficult to determine the association of quality of life with length of stay as the latter is driven by a wide variety of factors such as varying clinical practice, surgeon preference, patient/cultural expectations, and institutional norms and not necessarily on surgical approach or adverse events/complications alone. Finally, the majority of patients were recruited from academic centers around the world, so the applicability of our results to specific patient populations may be limited.
In conclusion, our analysis of quality of life outcomes in the LACC Trial shows that women with early-stage cervical cancer can expect similar postoperative quality of life at 6 weeks after surgery and beyond regardless of whether they undergo open or minimally invasive radical hysterectomy. In light of these results and the previously reported findings of worse progression-free and overall survival after minimally invasive radical hysterectomy than after open surgery and no difference in early or late postoperative morbidity between these two surgical approaches, surgeons must seriously re-evaluate the role of minimally invasive surgery in the treatment of early-stage cervical cancer. We would strongly argue that minimally invasive radical hysterectomy should no longer be offered to women with early-stage cervical cancer.
Supplementary Material
Research in Context.
Evidence before this study
Before developing the concept for the LACC Trial and producing the subsequent protocol, we performed a comprehensive review of the medical literature to evaluate the use of minimally invasive radical hysterectomy for the treatment of early-stage cervical cancer. We searched PubMed and clinicaltrials.gov with no date or language restrictions. Search terms included “radical hysterectomy”, “cervical cancer”, “minimally invasive”, “laparoscopic”, “robotic”, “robotic-assisted”, and “quality of life”. We identified multiple small, single-institution retrospective reports detailing the use of minimally invasive radical hysterectomy for early-stage cervical cancer. We identified no prospective, randomized studies comparing open and minimally invasive radical hysterectomy. We also identified no studies, prospective or retrospective, comparing quality of life after open or minimally invasive radical hysterectomy. In addition, we identified very few prospective, randomized studies comparing quality of life after open and minimally invasive surgeries across all surgical subspecialties and procedures.
Added value of this study
Contrary to the belief of most surgeons that minimally invasive surgery is superior to open surgery with respect to quality of life after surgery, the LACC Trial showed no difference in quality of life between patients who underwent open and minimally invasive radical hysterectomy for early-stage cervical cancer.
Implications of all the available evidence
Given the results of our current study and previously reported findings of worse progression-free and overall survival after minimally invasive radical hysterectomy than after open surgery and no difference in early or late postoperative morbidity between these procedures, surgeons must seriously re-evaluate the role of minimally invasive surgery in the treatment of early-stage cervical cancer. We would strongly argue that minimally invasive radical hysterectomy should no longer be offered to women with early-stage cervical cancer.
Acknowledgements
The LACC trial was funded by MD Anderson Cancer Center and Medtronic
We would like to thank Stephanie Deming for providing medical editing services for the manuscript.
Footnotes
Declaration of Interests
MF reports grants and personal fees from Novadaq/Stryker, grants from Navidea, personal fees from Johnson and Johnson, personal fees from Genetech, grants from Astra Zeneca, outside the submitted workRLC reports grants from NIH, grants from Gateway Foundation, grants from V Founation, during the conduct of the study; grants and personal fees from AstraZeneca, grants from Merck, personal fees from Tesaro, personal fees from Medivation, grants and personal fees from Clovis, personal fees from Gamamab, grants and personal fees from Genmab, grants and personal fees from Roche/Genentech, grants and personal fees from Janssen, personal fees from Agenus, personal fees from Regeneron, personal fees from OncoQuest, outside the submitted work.
AO reports other from CEO of SurgicalPerformance, a surgical audit software, grants from AO received travel grants from the O.R. Company, non-financial support from AO is a consultant for Covidien, NSW, Australia, outside the submitted work; .
The other authors declare no conflict of interest.
Contributor Information
Prof. Michael Frumovitz, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Prof. Andreas Obermair, Queensland Centre for Gynaecological Cancer, Australia, and School of Medicine, The University of Queensland, Australia.
Prof. Robert L. Coleman, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Prof. Rene Pareja, Instituto Nacional de Cancerología, , Bogotá and Clínica de Oncología Astorga, Medellín, Colombia..
Aldo Lopez, Instituto Nacional de Enfermedades Neoplásicas, Lima, Av. Angamos 2520, Surquillo 15038, Peru.
Reitan Ribero, Erasto Gaertner Hospital, , Curitiba - PR, 81520-060, Brazil.
Prof. David Isla, Instituto Nacional de Cancerología, Mexico.
Prof. Gabriel Rendon, Instituto de Cancerologia - Las Americas, Medellín, Colombia.
Marcus Q. Bernadini, Princess Margaret Cancer Center, Toronto, Ontario, Canada.
Alessandro Buda, San Gerardo Hospital, Monza MB, Italy.
Renato Moretti-Marquez, Hospital Israelita Albert Einstein, Centro de Oncologia e Hematologia, São Paulo - SP, Brazil.
Albert Zevallos, Instituto Nacional de Enfermedades Neoplasicas, Lima Peru.
Marcelo A. Vieira, Barretos Cancer Hospital, Barretos/SP, Brazil.
Tao Zhu, Institute of Cancer Research and Basic Medical Sciences of Chinese Academy of Sciences, Cancer Hospital of University of Chinese Academy of Sciences, , Hangzhou, Zhejiang Province, China 310022.
Russell P. Land, Queensland Centre for Gynaecological Cancer, Australia, and School of Medicine, The University of Queensland, Australia.
James Nicklin, Queensland Centre for Gynaecological Cancer, Australia, and School of Medicine, The University of Queensland, Australia.
Rebecca Asher, National Health and Medical Research Council Clinical Trials Centre, University of Sydney, Camperdown, Sydney, NSW Australia.
Kristy P. Robledo, National Health and Medical Research Council Clinical Trials Centre, University of Sydney, Camperdown, Sydney, NSW Australia.
Prof. Val Gebski, National Health and Medical Research Council Clinical Trials Centre, University of Sydney, Camperdown, Sydney, NSW Australia.
Prof. Pedro T. Ramirez, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Data Sharing Statement
Individual participant data that underlie the results reported in this article, after de-identification (text, tables, figures, and appendices) will be shared. In addition, study protocol will be shared. Data will be available beginning 9 months and ending 36 months after publication. Data may be shared with investigators whose proposed use of the data has been approved by an independent review committee (“learned intermediary”) identified for this purpose. Data may be utilized for indiviudal participant data meta-analysis. Proposals may be submitted up to 36 months following article publication. After 36 months the data will be available in our university’s data warehouse but without investigator support other than deposited metadata.
References
- 1.Sert BM, Boggess JF, Ahmad S, et al. Robot-assisted versus open radical hysterectomy: A multi-institutional experience for early-stage cervical cancer. Eur J Surg Oncol 2016; 42: 513–22. [DOI] [PubMed] [Google Scholar]
- 2.Nam JH, Park JY, Kim DY, Kim JH, Kim YM, Kim YT. Laparoscopic versus open radical hysterectomy in early-stage cervical cancer: long-term survival outcomes in a matched cohort study. Ann Oncol 2012; 23: 903–11. [DOI] [PubMed] [Google Scholar]
- 3.Corrado G, Vizza E, Legge F, et al. Comparison of Different Surgical Approaches for Stage IB1 Cervical Cancer Patients: A Multi-institution Study and a Review of the Literature. Int J Gynecol Cancer 2018; 28: 1020–8. [DOI] [PubMed] [Google Scholar]
- 4.Ramirez PT, Frumovitz M, Pareja R, et al. Minimally Invasive versus Abdominal Radical Hysterectomy for Cervical Cancer. N Engl J Med 2018; 379: 1895–904. [DOI] [PubMed] [Google Scholar]
- 5.Melamed A, Margul DJ, Chen L, et al. Survival after Minimally Invasive Radical Hysterectomy for Early-Stage Cervical Cancer. N Engl J Med 2018; 379: 1905–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cusimano MC, Baxter NN, Gien LT, et al. Impact of surgical approach on oncologic outcomes in women undergoing radical hysterectomy for cervical cancer. Am J Obstet Gynecol 2019. [DOI] [PubMed] [Google Scholar]
- 7.Doo DW, Kirkland CT, Griswold LH, et al. Comparative outcomes between robotic and abdominal radical hysterectomy for IB1 cervical cancer: Results from a single high volume institution. Gynecol Oncol 2019; 153: 242–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim SI, Cho JH, Seol A, et al. Comparison of survival outcomes between minimally invasive surgery and conventional open surgery for radical hysterectomy as primary treatment in patients with stage IB1-IIA2 cervical cancer. Gynecol Oncol 2019; 153: 3–12. [DOI] [PubMed] [Google Scholar]
- 9.Paik ES, Lim MC, Kim MH, et al. Comparison of laparoscopic and abdominal radical hysterectomy in early stage cervical cancer patients without adjuvant treatment: Ancillary analysis of a Korean Gynecologic Oncology Group Study (KGOG 1028). Gynecol Oncol 2019; 154: 547–53. [DOI] [PubMed] [Google Scholar]
- 10.Obermair A, Asher R, Pareja R, et al. Incidence of adverse events in minimally invasive versus open radical hysterectomy in early cervical cancer: Results of a randomized controlled trial. Am J Obstet Gynecol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kimmig R, Ind T. Minimally invasive surgery for cervical cancer: consequences for treatment after LACC Study. J Gynecol Oncol 2018; 29: e75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kornblith AB, Huang HQ, Walker JL, Spirtos NM, Rotmensch J, Cella D. Quality of life of patients with endometrial cancer undergoing laparoscopic international federation of gynecology and obstetrics staging compared with laparotomy: a Gynecologic Oncology Group study. J Clin Oncol 2009; 27: 5337–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Janda M, Gebski V, Brand A, et al. Quality of life after total laparoscopic hysterectomy versus total abdominal hysterectomy for stage I endometrial cancer (LACE): a randomised trial. Lancet Oncol 2010; 11: 772–80. [DOI] [PubMed] [Google Scholar]
- 14.Xiao M, Gao H, Bai H, Zhang Z. Quality of life and sexuality in disease-free survivors of cervical cancer after radical hysterectomy alone: A comparison between total laparoscopy and laparotomy. Medicine (Baltimore) 2016; 95: e4787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frumovitz M, Sun CC, Schover LR, et al. Quality of life and sexual functioning in cervical cancer survivors. J Clin Oncol 2005; 23: 7428–36. [DOI] [PubMed] [Google Scholar]
- 16.Sedlis A, Bundy BN, Rotman MZ, Lentz SS, Muderspach LI, Zaino RJ. A randomized trial of pelvic radiation therapy versus no further therapy in selected patients with stage IB carcinoma of the cervix after radical hysterectomy and pelvic lymphadenectomy: A Gynecologic Oncology Group Study. Gynecol Oncol 1999; 73: 177–83. [DOI] [PubMed] [Google Scholar]
- 17.Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol 1993; 11: 570–9. [DOI] [PubMed] [Google Scholar]
- 18.Cleeland CS, Mendoza TR, Wang XS, et al. Assessing symptom distress in cancer patients: the M.D. Anderson Symptom Inventory. Cancer 2000; 89: 1634–46. [DOI] [PubMed] [Google Scholar]
- 19.Ware J Jr., Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996; 34: 220–33. [DOI] [PubMed] [Google Scholar]
- 20.The EuroQol Group. EuroQol--a new facility for the measurement of health-related quality of life. Health Policy 1990; 16: 199–208. [DOI] [PubMed] [Google Scholar]
- 21.Smith MJ, Gill PG, Wetzig N, et al. Comparing patients' and clinicians' assessment of outcomes in a randomised trial of sentinel node biopsy for breast cancer (the RACS SNAC trial). Breast Cancer Res Treat 2009; 117: 99–109. [DOI] [PubMed] [Google Scholar]
- 22.Cella D. Quality of life outcomes: measurement and intervention. J Support Oncol 2005; 3: 133–4. [PubMed] [Google Scholar]
- 23.Sloan JA. Assessing the minimally clinically significant difference: scientific considerations, challenges and solutions. COPD 2005; 2: 57–62. [DOI] [PubMed] [Google Scholar]
- 24.Cella D, Zagari MJ, Vandoros C, Gagnon DD, Hurtz HJ, Nortier JW. Epoetin alfa treatment results in clinically significant improvements in quality of life in anemic cancer patients when referenced to the general population. J Clin Oncol 2003; 21: 366–73. [DOI] [PubMed] [Google Scholar]
- 25.White IR, Horton NJ, Carpenter J, Pocock SJ. Strategy for intention to treat analysis in randomised trials with missing outcome data. BMJ 2011; 342: d40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weeks JC, Nelson H, Gelber S, Sargent D, Schroeder G, for the Clinical Outcomes of Surgical Therapy Study Group. Short-term quality-of-life outcomes following laparoscopic-assisted colectomy vs open colectomy for colon cancer: a randomized trial. JAMA 2002; 287: 321–8. [DOI] [PubMed] [Google Scholar]
- 27.Toritani K, Watanabe J, Nakagawa K, et al. Randomized controlled trial to evaluate laparoscopic versus open surgery in transverse and descending colon cancer patients. Int J Colorectal Dis 2019; 34: 1211–20. [DOI] [PubMed] [Google Scholar]
- 28.Braga M, Frasson M, Vignali A, Zuliani W, Civelli V, Di Carlo V. Laparoscopic vs. open colectomy in cancer patients: long-term complications, quality of life, and survival. Dis Colon Rectum 2005; 48: 2217–23. [DOI] [PubMed] [Google Scholar]
- 29.Biere SS, van Berge Henegouwen MI, Maas KW, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet 2012; 379: 1887–92. [DOI] [PubMed] [Google Scholar]
- 30.Torphy RJ, Chapman BC, Friedman C, et al. Quality of Life Following Major Laparoscopic or Open Pancreatic Resection. Ann Surg Oncol 2019. [DOI] [PubMed] [Google Scholar]
- 31.Gunderson CC, Java J, Moore KN, Walker JL. The impact of obesity on surgical staging, complications, and survival with uterine cancer: a Gynecologic Oncology Group LAP2 ancillary data study. Gynecol Oncol 2014; 133: 23–7. [DOI] [PubMed] [Google Scholar]
- 32.Nelson G, Bakkum-Gamez J, Kalogera E, et al. Guidelines for perioperative care in gynecologic/oncology: Enhanced Recovery After Surgery (ERAS) Society recommendations-2019 update. Int J Gynecol Cancer 2019; 29: 651–68. [DOI] [PubMed] [Google Scholar]
- 33.Ljungqvist O, Scott M, Fearon KC. Enhanced Recovery After Surgery: A Review. JAMA Surg 2017; 152: 292–8. [DOI] [PubMed] [Google Scholar]
- 34.Meyer LA, Lasala J, Iniesta MD, et al. Effect of an Enhanced Recovery After Surgery Program on Opioid Use and Patient-Reported Outcomes. Obstet Gynecol 2018; 132: 281–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Individual participant data that underlie the results reported in this article, after de-identification (text, tables, figures, and appendices) will be shared. In addition, study protocol will be shared. Data will be available beginning 9 months and ending 36 months after publication. Data may be shared with investigators whose proposed use of the data has been approved by an independent review committee (“learned intermediary”) identified for this purpose. Data may be utilized for indiviudal participant data meta-analysis. Proposals may be submitted up to 36 months following article publication. After 36 months the data will be available in our university’s data warehouse but without investigator support other than deposited metadata.