Abstract
Varicella–zoster virus (VZV) is a human pathogen of the α-herpesvirus family. Some fetuses infected in utero around 8–20 weeks of pregnancy show signs of congenital varicella syndrome (CVS). Infants born to mothers who develop varicella within 5 days before and 2 days after delivery can experience severe disease with increased mortality. The best diagnostic modality is polymerase chain reaction (PCR), which can be done using vesicular swabs or scrapings, scabs from crusted lesions, tissue from biopsy samples, and cerebrospinal fluid. The prevention and management of varicella infections include vaccination, anti-VZV immunoglobulin, and specific antiviral drugs. In this article, we have reviewed the characteristics of VZV, clinical manifestations, management of perinatal infections, and short- and long-term prognosis.
Keywords: Congenital varicella syndrome, Herpes zoster, Neonatal varicella, Postexposure prophylaxis, Varicella–zoster virus, Vesicular rash, Varicella zoster immunoglobulin
Introduction
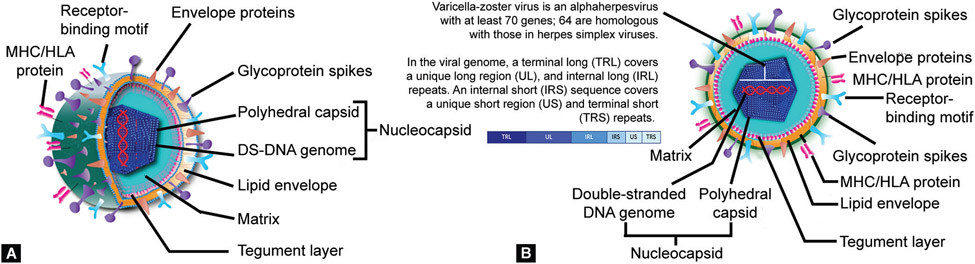
The VZV, an α-herpes virus, is an exclusively human pathogen.1 There is no animal reservoir, and so all transmission occurs from infected patients to other susceptible subjects. The primary cellular targets are epithelial cells, T-lymphocytes, and ganglion cells. In neonates, the initial infection in the upper respiratory epithelial cells is followed by viremia. These circulating viruses will disseminate to diverse locations in the skin and after an incubation period of 10–21 days, a vesicular rash develops Figure 1A shows the surface and side dissection of VZV, and its cross-section is shown in Figure 1B.2 Some of these circulating virions enter axon terminals in sensory neurons and reach the nerve cell bodies in ganglia through retrograde transport, where these establish a latent infection.3 The skin lesions contain high viral concentrations that can be transmitted to susceptible individuals.4
Figs 1A and B:
Schematic diagrams showing (A) Surface and side dissection and (B) Cross-section of the varicella–zoster virus
Epidemiology
Neonatal varicella is most often acquired from maternal infections occurring during the last 3 weeks of pregnancy.5 Nosocomial acquisition of VZV can also occur in newborn infants.
The incidence of varicella has been estimated to be 0.1–0.7/1,000 pregnancies.6 VZV infections show age-related, seasonal, and geographic variations. The seasonality is most noticeable in temperate climates, peaking in late winter or spring. Infections are seen most frequently in pre-school (1–4 years) or early elementary school-aged (5–9 years) children; the annual incidence reached 100 per 1,000 children prior to the implementation of the varicella vaccination program. In tropical climates, the virus is usually acquired at later ages.7 Skin cells shed during acute infections are probably the major source of the infectious airborne virus. Infected children without skin lesions are not contagious to others.
Viral Structure
The VZV is an α-herpesvirus with a double-stranded DNS (dsDNA) genome encased in an icosahedral capsid measuring approximately 125 nm in diameter.1 The capsid is comprised of particles in A-, B-, and C- maturational states. The A-particles are empty protein shells formed during abortive DNA packaging, B- contain a proteinaceous core of scaffold proteins, and C- are matured particles containing a DNA genome.8 The nucleocapsid is surrounded by a trilaminar lipoprotein envelope derived from the nuclear membrane of the infected host cell. The enveloped particle is spherical to pleomorphic in shape, about 180–200-nm diameter. Projecting from the lipid envelope are about 8-nm long viral glycoprotein spikes that bind specific host receptors to facilitate virus entry.9 In mature virus particles, an amorphous proteinaceous layer, the tegument, is seen covering the capsid and underneath the lipid envelope.10 The tegument consists of enzymes such as VP16 (described below) which recruits cellular proteins and enzymes into viral nucleic acid replication and the synthesis of the VHS (virion host shutoff) protein which shuts off the synthesis of host cell proteins in the cytoplasm. Detailed information is available for some of the viral components (Table 1).8-15
Table 1:
We have used a standardized table developed to describe viral pathogens. Major structural components of enteroviruses have been listed
| Structure | Available information |
|---|---|
| Lipid envelope | A trilaminar lipoprotein envelope derived from the nuclear membrane of the infected host cell covers the nucleocapsid.9 |
| Glycoproteins | About 8 nm long viral glycoprotein spikes project from the lipid envelope; bind specific host receptors to facilitate virus entry.9 |
| Receptor binding motifs | Involved in virion attachment to cell surface receptors. Motif binds integrins to promote entry into the cells.11 |
| Envelope protein | A trilaminar lipoprotein envelope containing envelope proteins (as mentioned above).9 |
| Membrane protein | Either not expressed or relevance unclear in fetal/infantile disease. |
| MHC or HLA Proteins | During primary VZV infection, both MHC I-restricted, CD8+ T lymphocytes and MHC II-restricted, CD4+ T lymphocytes are sensitized to VZV antigens.12 |
| Spike protein | About 8 nm long viral glycoprotein spikes project from the lipid envelope; bind specific host receptors to facilitate virus entry.9 |
| Surface tubules | Either not expressed or relevance unclear in fetal/infantile disease. |
| Palisade layer | Either not expressed or relevance unclear in fetal/infantile disease. |
| Viral tegument | In mature virus particles, an amorphous proteinaceous layer, the tegument, is seen covering the capsid and is underneath the lipid envelope.10 The tegument consists of enzymes such as VP16 (described below), which recruits cellular proteins and enzymes into viral nucleic acid replication and in the synthesis of the VHS (virion host shutoff) protein that shuts off the synthesis of host cell proteins in the cytoplasm. |
| Lateral bodies | Either not expressed or relevance unclear in fetal/infantile disease. |
| Capsid | The capsid is comprised of particles in A-, B-, and C- maturational states. A-particles are empty protein shells formed during abortive DNA packaging, B- contain a proteinaceous core of scaffold proteins, and the C- are matured particles containing a DNA genome.8 |
| Capsomeres | VZV open reading frame 23 (ORF23) encodes a conserved capsid protein, referred to as VP26 (UL35).8 |
| Core membrane | Either not expressed or relevance unclear in fetal/infantile disease. |
| Protein core | Details on genome-associated polyprotein described below. |
| Core fibrils | Either not expressed or relevance unclear in fetal/infantile disease. |
| Matrix | Virions penetrating the cell surface get uncoated and the viral genome functions as mRNA for the viral polyprotein.9 |
| Enzymes | Details scant. Alter the expression of host enzymes.14 |
| RNA elements | No RNA genome exists. |
| Nucleus | Either not expressed or relevance unclear in fetal/infantile disease. |
| Nucleosome | Either not expressed or relevance unclear in fetal/infantile disease. |
| DNA | Double-stranded DNA genome exists.8 |
| RNA | No RNA genome exists. |
| Genome-associated polyprotein | Either not expressed or relevance unclear in fetal/infantile disease. |
| DNA polymerase | VZV induces a DNA polymerase similar to that seen in other herpesviruses. Functions as a replicase for viral DNA synthesis in infected cells.15 |
| Reverse transcriptase | Either not expressed or relevance unclear in fetal/infantile disease. |
| Head | Either not expressed or relevance unclear in fetal/infantile disease. |
| Base plate | Either not expressed or relevance unclear in fetal/infantile disease. |
| Integrase | Either not expressed or relevance unclear in fetal/infantile disease. |
| Tail | Either not expressed or relevance unclear in fetal/infantile disease. |
| Tail fiber | Either not expressed or relevance unclear in fetal/infantile disease. |
| Neck | Either not expressed or relevance unclear in fetal/infantile disease. |
HLA, human leukocyte antigen; MHC, major histocompatibility complex
We do not have a consensus nomenclature for viral strains. Most studies use one of the four classification systems, but these seemingly similar nomenclature schemes do not correlate between methods.
Whole genome sequence/phylogenetic analyses have identified 22 strains of VZV.16 Some strains are seen more frequently, such as the European strains E1 and E2; the Japanese strain J; the Eastern Australian strains E1 and E2; and the mosaics M1 and M2.17 Single nucleotide polymorphism (SNP) analysis of whole-genome alignments shows two patterns of variation, one in the open reading frame 22 (ORF22), and the other involving ORF21 and ORF50.
Variations in the ORF22 are particularly notable in various strains of VZV. Initial attempts identified the following three major genotypes: A, B, and C; strains A in parts of Africa and Asia, genotypes B and C were found primarily in Europe, and genotype J was subsequently added to accommodate Japanese strains.18,19 Genotype B strains were presumed to have arisen from recombination between types A and C viruses. The M group were mosaics, and these strains were later subdivided into distinct M1 and M2 (and possibly M3 and M4) genotypes.
The reasons for the geographic diversity of infections among VZV strains are unclear; climatic factors and immigration patterns may be involved. A study in 18 European countries that included 342 clinical specimens showed E1 in 221 (65%); E2 in 87 (25%); M1 in 20 (6%); M2 in 3 (1%); and M4 in 11 (3%). No M3 or J strains were observed.20 The strain diversity may be broader in eastern parts of Australia than in Europe, Africa, and North America. Similarly, Japanese strains may differ considerably from those seen in the United States of America (USA), United Kingdom (UK), Europe, and eastern Australia isolates. Isolates from tropical Africa, India, Bangladesh, China, Central America, and northern Australia also seem to be distinct.21
Genome sequences of the five glycoprotein genes (gH, gI, gL, gB, and gE) and the major transactivator gene that encodes for the immediate-early protein 62 (IE62) have identified three subcategories, designated A, B, and C.22
Linear VZV genomes were differentiated based on the packing proteins that form the icosahedral nucleocapsid core, namely, that orf20, orf21, orf23, orf33, orf38, orf40, orf41 and orf54.23
Finally, in a study using complete genome sequence information, VZV was segregated into the following four genotypes: A, B, C, and D. The VZV strains circulating in Japan, Iceland, and The Netherlands have similarities, whereas genotypes circulating in different regions of the US, Thailand, Singapore, and Japan were diverse. This approach defined four distinct genotypes that were also arbitrarily designated A, B, C, and D. Viral isolates from Singapore and Japan were labeled as genotypes B and C, and those from Western Europe and the US as genotypes A and D.16
Clinical Presentations
Intrauterine VZV Infection
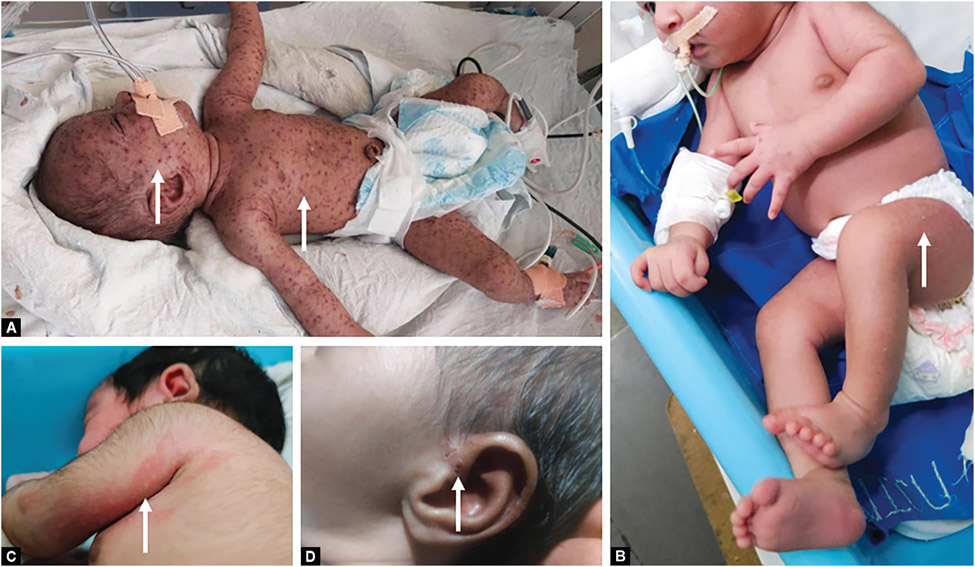
Infection of the fetus following maternal varicella during the first and the early second trimester can occasionally lead to fetal death or varicella embryopathy that can include cutaneous scarring, limb hypoplasia, eye and central nervous system (CNS) abnormalities (CVS).24,25 Severe CVS with extensive skin lesions and multisystem disease is shown in Figure 2A and perinatal CVS is shown in Figure 2B.Also shown are postnatal varicella with mild (Fig. 2C) and limited (Fig. 2D) cutaneous lesions.
Figs 2A to D:
Erythematous vesicular lesions in neonatal varicella (arrows). (A) Severe CVS with extensive skin lesions and multisystem disease. Lung involvement necessitated respiratory support; (B) Perinatal CVS. The infant had feeding difficulties; (C) and (D) Postnatal varicella with mild, limited cutaneous lesions
In 1947, CVS was first described by Laforet and Lynch.24 The frequency of CVS is very low (0.4%).26 When the maternal infection occurs between 8 and 20 weeks of gestation, CVS is seen in approximately 2% of fetuses.27 Although it is thought that maternal viremia in primary maternal varicella infections leads to placental infection and subsequent fetal infection, it is also possible that reactivation of VZV in utero can lead to CVS.28
The clinical features of CVS in affected infants include the following:
Intrauterine growth restriction;
Cicatricial (scarring) skin lesions, which may show localized depression and pigmentation in a dermatomal distribution;
Ocular defects, such as cataracts, chorioretinitis, Horner syndrome, microphthalmos, and nystagmus;
Limb abnormalities, such as localized hypoplasia of bone and muscle; and
The CNS abnormalities, such as cortical atrophy, seizures, and intellectual disability.
Acute and recurrent VZV infections are frequently accompanied by robust innate and acquired immune responses. Innate immune cells in skin and ganglion secrete type I interferon (IFN-I) and proinflammatory cytokines to control VZV. In the postneonatal period, VZV infections subvert the pattern recognition receptor sensing to modulate antigen presentation and IFN-I production. During primary infections, VZV can promote the accumulation of T-cells in skin lesions and consequent retrograde movement into the axons and ganglia. T- and B-cell memory formed within a few weeks of infection is boosted by reactivation or re-exposure.26 We could not find a strain-based propensity for varicella viruses affecting pregnant women.
Diagnosis of CVS
The clinical manifestations of CVS are enumerated in Table 2.29 The diagnostic criteria for CVS are shown in Table 3.30-32
Table 2:
Clinical manifestations of CVS
| Skin | Nervous system | Eye | Musculoskeletal | Systemic | Gastrointestinal tract |
Urinary tract |
|---|---|---|---|---|---|---|
| Cicatricial lesions | Intrauterine encephalitis | Chorioretinitis | Limb hypoplasia | Intrauterine growth retardation | Gastrointestinal reflux | Hydroureter |
| Cutaneous defects | Cortical atrophy/porencephaly | Cataracts | Muscle hypoplasia | Developmental delay | Hydronephrosis29 | |
| Hypopigmentation | Seizures | Microphthalmia | Cardiovascular defects | |||
| Mental retardation | Anisocoria | |||||
| Autonomic instability |
Table 3:
Diagnostic criteria of CVS
| S.no. | Diagnostic criteria of CVS |
|---|---|
| 1. | Appearance of maternal chickenpox during pregnancy |
| 2. | Presence of congenital skin lesions in dermatomal distribution and/or neurologic defects, eye disease, limb hypoplasia |
| 3. | Evidence of intrauterine VZV infection by detection of viral DNA in the infant |
| 4. | Presence of specific IgM |
| 5. | Persistence of IgG beyond 7 months of age |
| 6. | Appearance of zoster during early infancy |
Neonatal Varicella
The appearance of symptoms within the first 10 days after life most likely indicates a prenatal infection because of the 10–20 days incubation period.33 The highest risk for severe neonatal varicella is seen when the maternal VZV infection occurs between 5 days before and 2 days after delivery. These infants are typically exposed to high viral loads but have not had the opportunity to acquire maternal protective antibodies. Between 20 and 50% of these infants develop the life-threatening disseminated disease between postnatal days 5–10 and unless treated aggressively, can have case–fatality rates of up to 20%. Nosocomial acquisition of VZV also can occur. Newborns born to mothers who are exposed to VZV or have clinical disease manifestations within the first 2 weeks after birth are at the highest risk of developing symptomatic disease.
Symptoms appearing after postnatal day 13 are more likely to be due to postnatal acquisition of the virus. Although most infants with neonatal varicella have mild disease, some can develop a serious illness with a mortality rate of up to 30%.34,35 However, postnatally acquired varicella that occurs between 10 and 28 days after birth usually causes mild disease.36
The immunological immaturity of neonates places them at higher risk of developing the relatively severe disease compared to older infants or children.37 Premature infants are at even higher risk for nosocomial acquisition of VZV compared with infants born at term because the active transfer of maternal immunoglobulin G (IgG) antibodies occurs primarily during the third trimester of pregnancy.38 In these infants, the risk may increase with increasing postnatal age because the antibody levels decline with age.39,40
Neonatal varicella may present with fever occurring within the first 5–10 days of life, followed by a generalized vesicular eruption. The rash starts as macules and rapidly progresses to papules and then to characteristic vesicular lesions before crusting. These lesions may be noted first on the head and then on other parts of the body. The lesions may be seen in various stages of development and healing.41 The generalized distribution and appearance of lesions in different stages of development distinguish varicella from the vesicular rash seen in neonatal herpes simplex virus (HSV), which tends to occur in localized clusters. In mild cases of neonatal varicella, the lesions heal within 7–10 days. Very rarely, infants develop disseminated disease with varicella pneumonia, hepatitis, and meningoencephalitis.
Laboratory Diagnosis
The diagnosis of CVS can be accomplished by analyzing amniotic fluid or fetal blood for VZV DNA using the PCR assay together with prenatal ultrasound to detect fetal abnormalities such as limb deformity, microcephaly, hydrocephalus, polyhydramnios, soft tissue calcification and intrauterine growth restriction.31 The PCR assay for detecting VZV DNA is highly sensitive.42-44 However, amniocentesis can be usually performed after 16–18 weeks of gestation because of the risk of complications. A normal fetal ultrasound and a negative PCR performed between 17 and 21 weeks of gestation suggests low risk of CVS. The ultrasound imaging should be performed at least 5 weeks after maternal infection to detect fetal abnormalities consistent with CVS.30
Treatment and Prevention of CVS
Prevention and treatment of CVS are comprised of vaccination, antiviral agents and varicella zoster immunoglobulin (VZIG).30 A brief discussion of each strategy is provided below.
VZV vaccine is a live attenuated varicella vaccine is currently included on the World Health Organization’s list of “Essential Medicines for Children.” The VZV vaccine is routinely administered during early childhood in several countries including the USA, Canada, Japan, Australia, Brazil and few other European and Middle Eastern countries. Two doses of VZV vaccine in children has been shown to 98% effective in preventing severe disease. However, many of the developing countries and a few developed countries such as the UK have not recommended routine vaccination of all children.
Since the VZV vaccine is not secreted in breast milk, non-immune women should be vaccinated immediately after delivery.45 In most developing countries, immunity to varicella usually results from natural infection and only 3.9% of adults are non-immune.46 The VZV vaccination can prevent infection of the mother and fetus and reduce the incidence of both CVS and neonatal chickenpox.46 If a pregnant woman has had significant exposure to such as household contact with a varicella within the infectious period, sero-status of the exposed individual should be evaluated. In women who have received varicella vaccine, the risk for maternal varicella and CVS are very low.47 Nonimmune women could receive postexposure prophylaxis immediately.
It is unclear whether antiviral drugs such as acyclovir are effective in reducing the risk of varicella during pregnancy after exposure. Antiviral treatment is indicated for pregnant women with varicella infection.48-50 However, in early pregnancy, the fetal benefit is controversial.51 In women with complicated varicella infections such as varicella pneumonia, treatment with IV acyclovir is recommended because of the high mortality rate.52
Management of Pregnant Women Exposed to VZV
Significant exposure such as household contact with varicella infection during pregnancy warrants passive immunization with VZV-specific antibodies to reduce the risk of varicella infection and also to prevent severe disease. Moreover, VZIG is prepared from individuals with recent zoster or from donors screened for high VZV IgG titers. It is a purified human immune globulin preparation made from plasma containing high levels of antivaricella antibodies,53 and is recommended both for women who are exposed and for others who are susceptible to infection with the virus.54,55 Also, VZIG can prevent maternal disease and complications,56 and can reduce the risk of fetal infection.57 It should be administered within 96 hours of chickenpox exposure.56 However, Varizig is the only preparation available in the US and VZIG has been discontinued. If VZIG or Varizig is not available, intravenous immunoglobulin (IVIG) can be considered, if necessary, at a dose of 400 mg/kg. The recommended dose is 125 units/10 kg of body weight, up to a maximum of 625 units.33
The overall risk for CVS is very low and pregnant women affected by varicella during pregnancy should be reassured. This reassurance could lead to a reduced frequency of pregnancy terminations performed because of the risk of congenital anomalies.58
Herpes Zoster in Infancy
Maternal varicella during pregnancy can manifest as an infant zoster in the first or second year of life. The majority of infants who present with herpes zoster early in life do not have malformations and are asymptomatic.59 Enders et al. estimated the risk of developing herpes zoster in children with maternal varicella infection between the 13th and 36th week of gestation. They noted that 0.8–1.7% of these infants may develop herpes zoster during the first 2 years of life.60
Diagnosis
Neonatal varicella can be suspected based on the characteristic appearance of generalized vesicular skin lesions in various stages of development and healing in an infant born to a mother exposed to VZV or with clinical symptoms close to the time of delivery.
Laboratory Diagnosis
In infants with uncertain and/or severe clinical manifestations, PCR of swabs from vesicles or other samples for the detection of VZV DNA confirms the diagnosis. Using PCR, it may be possible to distinguish between wild type VZV and vaccine strains. Direct fluorescent antibody (DFA) on scrapings from active vesicular skin lesions can provide a rapid diagnosis.
The sensitivity of viral culture is significantly lower compared with PCow. Serologic testing by examining cord blood for VZV IgM antibodies can also help establish the diagnosis. However, this approach requires acute and convalescent titers and therefore it is not helpful for rapidly establishing the diagnosis. In neonatal varicella infection, acute and convalescent sera demonstrate a rise in VZV IgG titers. In uninfected neonates who received the passive transfer of maternal antibodies during pregnancy typically have low acute VZV titers and convalescent titers remain low. Moreover, VZV IgM is insensitive in neonates and false positives can occur.
The role of other diagnostic tests, including fluorescent antimembrane antibody (FAMA), latex agglutination (LA), enzyme-linked immunosorbent assay (ELISA), and complement-enhanced neutralization, is not known in neonates.
Clinical Management
Prophylaxis
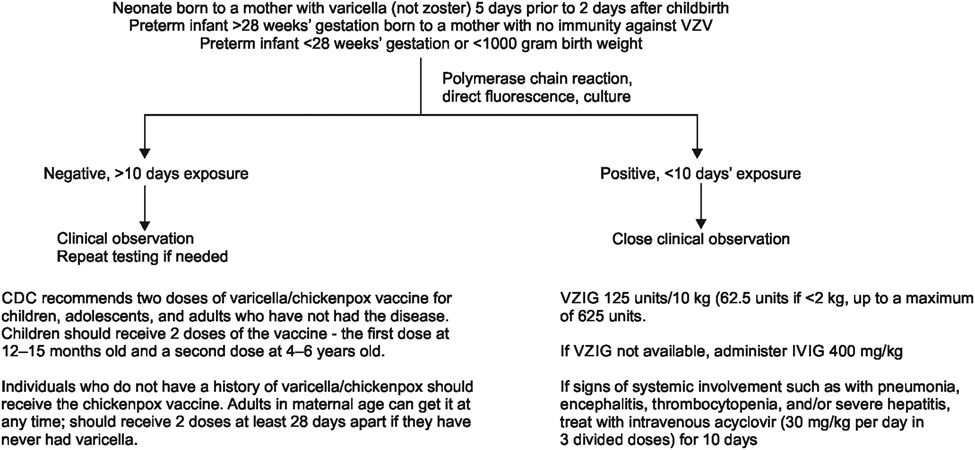
Management of newborn infants who are exposed to VZV by maternal infection or contact with affected individuals includes isolation and postexposure prophylaxis. The specific intervention depends upon the timing of exposure, the mother’s serologic status, and gestational age.
Prompt postexposure administration of VZIG or Varizig can prevent varicella in exposed neonates or ameliorate the disease course in patients in whom the infection was not fully prevented.61 The American Academy of Pediatrics (AAP), Centers for Disease Control and Prevention (CDC), and the Advisory Committee on Immunization Practices (ACIP), recommend administration of Varizig to neonates who have had a significant exposure to VZV plus one or more of the following:62
Maternal symptoms – Neonates whose mothers have signs and symptoms of varicella around the time of delivery (within 5 days before or 2 days after) should receive Variizig orVZIG.
Preterm infants above or equal to 28 weeks of gestation – Hospitalized preterm infants born at ≥28 weeks of gestation who had significant exposure to VZV and whose mothers do not have documented immunization, serologic immunity, or prior documented history of varicella infection should receive Varizig or VZIG (Flowchart 1).
Preterm infants less than 28 weeks of gestation – Hospitalized premature infants born at less than 28 weeks of gestation or who weigh less than 1,000 gm at birth who have had significant exposure to VZV should receive VZIG or Varizig regardless of maternal history of varicella or vaccination.
Flowchart 1:
Schematic flowchart showing recommendations for clinical investigation and management of infants with varicella infections
Healthy term neonates who are exposed to VZV postnatally (including infants whose mother’s rash developed 48 hours after delivery) do not require postexposure prophylaxis. This is because postnatally acquired varicella that occurs beyond the immediate newborn period in a term infant generally is mild.63 Also, VZIG is given intramuscularly at a dose is 125 units to neonates weighing above 2.1–10 kg and 62.5 units to children weighing below or equal to 2 kg.
For postexposure prophylaxis, passive immunization with VZIG or Vaizic should be offered as soon as possible and within 10 days.64 If VZIG or Varizig is unavailable, IVIG or prophylaxis with acyclovir can be considered.41
Isolation
Isolation for the mother and infant depends upon whether there is active disease and the timing of exposure. Patients who require isolation include the following:
Active disease – A mother with active VZV lesions must be isolated. The infant is isolated from the mother until she is not infectious. Any infant who develops varicella in the nursery or neonatal intensive care unit (NICU) is also isolated.
Maternal exposure during the period of 6–21 days before hospitalization – A seronegative mother exposed to VZV 6–21 days before hospital admission should be isolated from other patients and the nursery because she may develop varicella while hospitalized. This calculation takes into account the incubation period of varicella which is usually 14–16 days but sometimes ranges from 10 to 21 days after exposure.63 The incubation may be prolonged for as long as 28 days after receipt of VZIG or IVIG, and it may be shortened in immunocompromised patients. Her infant, if born at term, should be isolated from the mother. The mother and infant should be cared for only by staff with immunity to VZV. Both should be discharged as soon as possible.
Patients who generally do not require isolation include the following:
Active disease 21 days before delivery – A mother who has active varicella within 21 days of delivery that resolves before hospitalization does not need to be isolated. However, the newborn should stay in the mother’s room and be isolated from other infants.
Maternal exposure within 6 days of hospitalization – If a seronegative mother was exposed within 6 days of admission and discharged before 48 hours, isolation is not needed, because varicella would not be expected to develop during the hospital stay.
Nursery Exposure
An infant who develops varicella in the nursery or NICU should be isolated. The more common situation is nursery exposure by a visitor or hospital worker who is infectious. In the newborn nursery, exposed infants typically are discharged before they would be infectious.
Neonatal Intensive Care Unit Exposure
Exposed infants in the NICU usually are made as cohorts. They are isolated from new patients admitted between 8 and 21 days after exposure.48 Infants who received VZIG should be isolated from new patients for 28 days.
Treatment
Acyclovir
Newborns with severe disseminated VZV infection, such as with pneumonia, encephalitis, thrombocytopenia, and/or severe hepatitis are treated with intravenous acyclovir (30 mg/kg per day in 3 divided doses) for 10 days.63,65 Antiviral treatment must be started as soon as possible after the onset of symptoms because most viral replication has stopped 72 hours after the appearance of the rash.66,67 Similar to immunocompromised patients, neonates with disseminated VZV are also at increased risk of severe morbidity and higher mortality compared with older immunocompetent patients.
Breastfeeding
Whether VZV is secreted in human milk is uncertain, although VZV DNA has been detected.68 However, the transmission of VZV from breast milk is very rare, and therefore breastfeeding is encouraged in infants of mothers infected with varicella. In addition to the known benefits of breastfeeding on the overall health of the infant, breast milk contains antiviral antibodies that may provide protection.69
Outcomes
The severity of neonatal varicella that was acquired in utero is closely related to the timing of maternal infection as transplacentally-transmitted antibodies may reduce the severity of symptoms in the newborn. The risk of disseminated neonatal varicella is more likely (20%-50%) with significant mortality (~20%) if mothers develop the varicella rash between 5 days before and 2 days after delivery.6
Source of support:
U01CA260462 to SB
Footnotes
Conflict of interest: None
References
- 1.Pergam SA, Limaye AP, AST Infectious Diseases Community of Practice. Varicella–zoster virus (VZV) in solid organ transplant recipients. Am J Transplant 2009;9(Suppl. 4):S108–S115. DOI: 10.1111/j.1600-6143.2009.02901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ku CC, Zerboni L, Ito H, et al. Varicella–zoster virus transfer to skin by T cells and modulation of viral replication by epidermal cell interferon-α. J Exp Med 2004;200(7):917–925. DOI: 10.1084/jem.20040634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gilden DH, Vafai A, Shtram Y, et al. Varicella–zoster virus DNA in human sensory ganglia. Nature 1983;306(5942):478–480. DOI: 10.1038/306478a0. [DOI] [PubMed] [Google Scholar]
- 4.Zerboni L, Sen N, Oliver SL, et al. Molecular mechanisms of varicella–zoster virus pathogenesis. Nat Rev Microbiol 2014;12(3):197–210. DOI: 10.1038/nrmicro3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chapman SJ. Varicella in pregnancy. Seminars perinatol 1998;22(4):339–346. DOI: 10.1016/s0146-0005(98)80023-2. [DOI] [PubMed] [Google Scholar]
- 6.Sauerbrei A, Wutzler P. Neonatal varicella. J Perinatol 2001;21(8):545–549. DOI: 10.1038/sj.jp.7210599. [DOI] [PubMed] [Google Scholar]
- 7.Laing KJ, Ouwendijk WJD, Koelle DM, et al. Immunobiology of varicella–zoster virus infection. J Infect Dis 2018;218(Suppl. 2):S68–S74. DOI: 10.1093/infdis/jiy403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun J, Liu C, Peng R, et al. Cryo-EM structure of the varicella–zoster virus A-capsid. Nat Commun 2020;11(1):4795. DOI: 10.1038/s41467-020-18537-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edson CM, Hosler BA, Poodry CA, et al. Varicella–zoster virus envelope glycoproteins: biochemical characterization and identification in clinical material. Virology 1985;145(1):62–71. DOI: 10.1016/0042-6822(85)90201-6. [DOI] [PubMed] [Google Scholar]
- 10.Wang W, Cheng T, Zhu H, et al. Insights into the function of tegument proteins from the varicella–zoster virus. Sci China Life Sci 2015;58(8):739–749. DOI: 10.1007/s11427-015-4887-3. [DOI] [PubMed] [Google Scholar]
- 11.Li Q, Krogmann T, Ali MA, et al. The amino terminus of varicella–zoster virus (VZV) glycoprotein E is required for binding to insulin-degrading enzyme, a VZV receptor. J Virol 2007;81(16):8525–8532. DOI: 10.1128/JVI.00286-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abendroth A, Lin I, Slobedman B, et al. Varicella–zoster virus retains major histocompatibility complex class I proteins in the Golgi compartment of infected cells. J Virol 2001;75(10):4878–4888. DOI: 10.1128/JVI.75.10.4878-4888.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Visalli MA, House BL, Selariu A, et al. The varicella–zoster virus portal protein is essential for cleavage and packaging of viral DNA. J Virol 2014;88(14):7973–7986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hew K, Dahlroth SL, Veerappan S, et al. Structure of the varicella–zoster virus thymidylate synthase establishes functional and structural similarities as the human enzyme and potentiates itself as a target of brivudine. PLoS One 2015;10(12):e0143947. DOI: 10.1371/journal.pone.0143947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meier JL, Straus SE. Varicella–zoster virus DNA polymerase and major DNA-binding protein genes have overlapping divergent promoters. J Virol 1993;67(12):7573–7581. DOI: 10.1128/JVI.67.12.7573-7581.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loparev VN, Rubtcova EN, Bostik V, et al. Identification of five major and two minor genotypes of varicella–zoster virus strains: A practical two-amplicon approach used to genotype clinical isolates in Australia and New Zealand. J Virol 2007;81(23):12758–12765. DOI: 10.1128/JVI.01145-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loparev V, Martro E, Rubtcova E, et al. Toward universal varicella–zoster virus (VZV) genotyping: Diversity of VZV strains from France and Spain. J Clin Microbiol 2007;45(2):559–563. DOI: 10.1186/s12879-016-1863-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quinlivan M, Hawrami K, Barrett–Muir W, et al. The molecular epidemiology of varicella–zoster virus: Evidence for geographic segregation. J Infect Dis 2002;186(7):888–894. DOI: 10.1086/344228. [DOI] [PubMed] [Google Scholar]
- 19.Muir WB, Nichols R, Breuer J. Phylogenetic analysis of varicella–zoster virus: evidence of intercontinental spread of genotypes and recombination. J Virol 2002;76(4):1971–1979. DOI: 10.1128/jvi.76.4.1971-1979.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loparev VN, Rubtcova EN, Bostik V, et al. Distribution of varicella–zoster virus (VZV) wild-type genotypes in northern and southern Europe: Evidence for high conservation of circulating genotypes. Virology 2009;383(2):216–225. DOI: 10.1016/j.virol.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 21.Loparev VN, Gonzalez A, Deleon–Carnes M, et al. Global identification of three major genotypes of varicella–zoster virus: Longitudinal clustering and strategies for genotyping. J Virol 2004;78(15):8349–8358. DOI: 10.1128/JVI.78.15.8349-8358.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moffat J, Ku CC, Zerboni L, et al. VZV: Pathogenesis and the disease consequences of primary infection. In: Arvin A, Campadelli–Fiume G, Mocarski E, Moore PS, et al. editors. Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. Cambridge: Cambridge University Press; 2007, Chapter 37. [PubMed] [Google Scholar]
- 23.Zerboni L, Sen N, Oliver SL, et al. Molecular mechanisms of varicella–zoster virus pathogenesis. Nat Rev Microbiol 2014;12(3):197–210. DOI: 10.1038/nrmicro3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laforet EG, Lynch CL Jr. Multiple congenital defects following maternal varicella: Report of a case. N Engl Med 1947;236(15):534–537. DOI: 10.1056/NEJM194704102361504. [DOI] [PubMed] [Google Scholar]
- 25.Pastuszak AL, Levy M, Schick B, et al. Outcome after maternal varicella infection in the first 20 weeks of pregnancy. N Engl J Med 1994;330(13):901–905. DOI: 10.1056/NEJM199403313301305. [DOI] [PubMed] [Google Scholar]
- 26.Gershon AA, Breuer J, Cohen JI, et al. Varicella–zoster virus infection. Nat Rev Dis Primers 2015;1:15016. DOI: 10.1038/nrdp.2015.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kett JC. Perinatal varicella. Pediatr Rev 2013;34(1):49–51. DOI: 10.1542/pir.34-1-49. [DOI] [PubMed] [Google Scholar]
- 28.Higa K, Dan K, Manabe H. Varicella–zoster virus infections during pregnancy: Hypothesis concerning the mechanisms of congenital malformations. Obstetrics and Gynaecology 1987;69(2):214–222. [PubMed] [Google Scholar]
- 29.Dworsky M, Whitley R, Alford C. Herpes zoster in early infancy. Am J Dis Child 1980;134:618–619. DOI: 10.1001/archpedi.1980.02130180074021. [DOI] [PubMed] [Google Scholar]
- 30.Ahn KH, Park YJ, Hong SC, et al. Congenital varicella syndrome: A systematic review. J Obstet Gynaecol 2016;36(5):563–566. DOI: 10.3109/01443615.2015.1127905. [DOI] [PubMed] [Google Scholar]
- 31.Alkalay AL, Pomerance JJ, Rimoin DL. Fetal varicella syndrome. Journal of Paediatrics 1987;111(3):320–323. DOI: 10.1016/s0022-3476(87)80447-x. [DOI] [PubMed] [Google Scholar]
- 32.Paryani SG, Arvin AM. Intrauterine infection with varicella–zoster virus after maternal varicella. N EngJ Med 1986;314(24):1542–1546. DOI: 10.1056/NEJM198606123142403. [DOI] [PubMed] [Google Scholar]
- 33.Smith CK, Arvin AM. Varicella in the fetus and newborn. Semin Fetal Neonatal Med 2009;14(4):209–217. DOI: 10.1016/j.siny.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 34.Meyers JD. Congenital varicella in term infants: risk reconsidered. J Infect Dis 1974;129(2):215. DOI: 10.1093/infdis/129.2.215. [DOI] [PubMed] [Google Scholar]
- 35.American Academy of Pediatrics. Varicella–zoster virus infections. In: Red Book 2018: Report of the Committee on Infectious Diseases, 31st edition, Kim berlin DW, Brady MT, Jackson MA, editors. Itasca, Illinois, USA: American Academy of Pediatrics, 2018. pp.869–883. [Google Scholar]
- 36.Bailey JE, Toltzis P. Perinatal viral infections. In: Neonatal–Perinatal Medicine: Diseases of the Fetus and Infant, 9th edition. Martin RJ, Fanaroff AA, Walsh MC, editors. St. Louis: Elsevier Mosby, 2011. Vol. 2, p.841. [Google Scholar]
- 37.Prober CG, Gershon AA, Grose C, et al. Consensus: Varicella–zoster infections in pregnancy and the perinatal period. Pediatr Infect Dis J 1990;9(12):865–869. [PubMed] [Google Scholar]
- 38.Saji F, Samejima Y, Kamiura S, et al. Dynamics of immunoglobulins at the feto–maternal interface. Rev Reprod 1999;4(2):81–89. DOI: 10.1530/ror.0.0040081. [DOI] [PubMed] [Google Scholar]
- 39.Lipton SV, Brunell PA. Management of varicella exposure in a neonatal intensive care unit. JAMA 1989; 261:1782. [PubMed] [Google Scholar]
- 40.Ng PC, Lyon DJ, Wong MY, et al. Varicella exposure in a neonatal intensive care unit: Emergency management and control measures. J Hosp Infect 1996;32(3):229–236. DOI: 10.1016/s0195-6701(96)90149-8. [DOI] [PubMed] [Google Scholar]
- 41.Blair RJ. Varicella–zoster virus. Pediatr Rev 2019;40(7):375–377. DOI: 10.1542/pir.2017-0242. [DOI] [PubMed] [Google Scholar]
- 42.Leung J, Harpaz R, Baughman AL, et al. Evaluation of laboratory methods for diagnosis of varicella. Clin Infect Dis 2010;51(1):23–32. DOI: 10.1086/653113. [DOI] [PubMed] [Google Scholar]
- 43.Mendelson E, Aboudy Y, Smetana Z, et al. Laboratory assessment and diagnosis of congenital viral infections: Rubella, cytomegalovirus (CMV), varicella–zoster virus (VZV), herpes simplex virus (HSV), parvovirus B19 and human immunodeficiency virus (HIV). Reprod Toxicol 2006;21(4):350–382. DOI: 10.1016/j.reprotox.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 44.Mouly F, Mirlesse V, Meritet JF, et al. Prenatal diagnosis of fetal varicella–zoster virus infection with polymerase chain reaction of amniotic fluid in 107 cases. Am J Obstet Gynecol 1997;177(4):894–898. DOI: 10.1016/s0002-9378(97)70291-6. [DOI] [PubMed] [Google Scholar]
- 45.Bohlke K, Galil K, Jackson LA, et al. Postpartum varicella vaccination: Is the vaccine virus excreted in breast milk? Obstet Gynaecol 2003;102:970–977. DOI: 10.1016/s0029-7844(03)00860-3. [DOI] [PubMed] [Google Scholar]
- 46.Hanaoka M, Hisano M, Watanabe N, et al. Changes in the prevalence of the measles, rubella, varicella–zoster, and mumps virus antibody titers in Japanese pregnant women. Vaccine 2013;31(19):2343–2347. DOI: 10.1016/j.vaccine.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 47.Watson B, Civen R, Reynolds M, et al. Validity of self-reported varicella disease history in pregnant women attending prenatal clinics. Public Health Rep 2007;122(4):499–506. DOI: 10.1177/003335490712200411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Heuchan AM, Isaacs D. The management of varicella–zoster virus exposure and infection in pregnancy and the newborn period. Australasian Subgroup in Paediatric Infectious Diseases of the Australasian Society for Infectious Diseases. Med J Aust 2001;174(6):288–292. DOI: 10.5694/j.1326-5377.2001.tb143273.x. [DOI] [PubMed] [Google Scholar]
- 49.Tan MP, Koren G. Chickenpox in pregnancy: Revisited. Reprod Toxicol 2006;21(4):410–420. DOI: 10.1016/j.reprotox.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 50.Lamont RF, Sobel JD, Carrington D, et al. Varicella–zoster virus (chickenpox) infection in pregnancy. BJOG 2011;118(10):1155–1162. DOI: 10.1111/j.1471-0528.2011.02983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mandelbrot L. Fetal varicella: Diagnosis, management, and outcome. Prenat Diagn 2012;32(6):511–518. DOI: 10.1002/pd.3843. [DOI] [PubMed] [Google Scholar]
- 52.American Academy of Pediatrics Committee on Infectious Diseases. The use of oral acyclovir in otherwise healthy children with varicella. Pediatrics 1993;91(3):674–676. [PubMed] [Google Scholar]
- 53.Centers for Disease Control and Prevention (CDC). A new product (VariZIG) for postexposure prophylaxis of varicella available under an investigational new drug application expanded access protocol. MMWR Morb Mortal Wkly Rep 2006;55(8):209–210. [PubMed] [Google Scholar]
- 54.Maranich AM, Rajnik M. Varicella-specific immunoglobulin G titers in commercial intravenous immunoglobulin preparations. Pediatrics 2009;124(3):e484–e488. DOI: 10.1542/peds.2009-0047. [DOI] [PubMed] [Google Scholar]
- 55.Miller E, Cradock–Watson JE, Ridehalgh MK. Outcome in newborn babies given anti-varicella–zoster immunoglobulin after perinatal maternal infection with varicella–zoster virus. Lancet 1989;2(8659):371–373. DOI: 10.1016/s0140-6736(89)90547-3. [DOI] [PubMed] [Google Scholar]
- 56.Marin M, Guris D, Chaves SS, et al. Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2007;56(RR-4):1–40. DOI: 10.1016/s0029-7844(03)00860-3. [DOI] [PubMed] [Google Scholar]
- 57.Cohen A, Moschopoulos P, Stiehm RE, et al. Congenital varicella syndrome: the evidence for secondary prevention with varicella–zoster immune globulin. CMAJ 2011;183(2):204–208. DOI: 10.1503/cmaj.100615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Harger JH, Ernest JM, Thurnau GR, et al. Frequency of congenital varicella syndrome in a prospective cohort of 347 pregnant women. Obstet Gynecol 2002;100(2):260–265. DOI: 10.1016/s0029-7844(02)02059-8 [DOI] [PubMed] [Google Scholar]
- 59.Figueroa–Damian R, Arredondo–Garcia JL. Perinatal outcome of pregnancies complicated with varicella infection during the first 20 weeks of gestation. AmJ Perinatol 1997;14(07):411–414. DOI: 10.1055/s-2007-994170. [DOI] [PubMed] [Google Scholar]
- 60.Enders G, Miller E, Cradock–Watson J, et al. Consequences of varicella and herpes zoster in pregnancy: Prospective study of 1739 cases. Lancet 1994;343(8912):1548–1551. DOI: 10.1016/s0140-6736(94)92943-2. [DOI] [PubMed] [Google Scholar]
- 61.Tebruegge M, Pantazidou A, Curtis N. Towards evidence based medicine for paediatricians. How effective is varicella–zoster immunoglobulin (VZIG) in preventing chickenpox in neonates following perinatal exposure? Arch Dis Child 2009;94(7):559. DOI: 10.1136/adc.2008.154542. [DOI] [PubMed] [Google Scholar]
- 62.Centers for Disease Control and Prevention (CDC). Morbidity and Mortality Weekly Report. Updated recommendations for Use of VariZIG-United States. 2013. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6228a4.htm. Accessed date: 18 August, 2022 [PMC free article] [PubMed] [Google Scholar]
- 63.American Academy of Pediatrics. Varicella–zoster virus infections. In: Red Book: 2018 Report of the Committee on Infectious Diseases, 31st edition, Kim berlin DW, Brady MT, Jackson MA, et al. , editors. Itasca, IL: American Academy of Pediatrics, 2018. p. 871. [Google Scholar]
- 64.Centers for Disease Control and Prevention (CDC). FDA approval of an extended period for administering VariZIG for postexposure prophylaxis of varicella. MMWR Morb Mortal Wkly Rep 2012; 61:212. [PubMed] [Google Scholar]
- 65.Kesson AM, Grimwood K, Burgess MA, et al. Acyclovir for the prevention and treatment of varicella-zoster in children, adolescents and pregnancy. J Paediatr Child Health 1996;32(3):211. DOI: 10.1111/j.1440-1754.1996.tb01556.x. [DOI] [PubMed] [Google Scholar]
- 66.Williams H, Latif A, Morgan J, et al. Acyclovir in the treatment of neonatal varicella. J Infect 1987;15:65–67. DOI: 10.1016/s0163-4453(87)91501-5. [DOI] [PubMed] [Google Scholar]
- 67.Singalavanija S, Limpongsanurak W, Horpoapan S, Ratrisawadi V. Neonatal varicella: A report of 26 cases. J Med Assoc Thai 1999;82(10):957–962. [PubMed] [Google Scholar]
- 68.Yoshida M, Yamagami N, Tezuka T, et al. Case report: Detection of varicella–zoster virus DNA in maternal breast milk. J Med Virol 1992;38(2):108–110. DOI: 10.1002/jmv.1890380207. [DOI] [PubMed] [Google Scholar]
- 69.Grumach AS, Carmona RC, Lazarotti D, et al. Immunological factors in milk from Brazilian mothers delivering small-for-date term neonates. Acta Paediatr 1993;82(3):284–290. DOI: 10.1111/j.1651-2227.1993.tb12661.x. [DOI] [PubMed] [Google Scholar]