Abstract
Objective
This 12-week, multicenter, open-label study investigated the efficacy and tolerability of the HydraFacial Clarifying Treatment for improving skin appearance in patients who present with acne vulgaris.
Methods
Twenty eligible adult patients with mild-to-moderate acne were enrolled at one of two treatment sites in the United States and were to undergo six HydraFacial Clarifying Treatments, one every two weeks for 12 weeks. Treatment occurs in three steps: cleansing and peeling; suction to extract dead skin cells, sebum, and debris; and application of blue LED light. Acne severity was graded by investigators and by patients using the Global Acne Severity Score (GASS).
Results
The proportion of patients with no acne or almost clear skin (GASS ≤1) at baseline versus final treatment increased from 20 to 65 percent per investigator assessment (p=0.0027), and from 5 to 55 percent per patient self-report (p=0.0016). At final treatment, more than 80 to 100 percent of both investigators and patients agreed or strongly agreed there was an improvement in skin appearance across multiple assessment parameters. Treatments were generally well tolerated.
Limitations
Due to the nature of the treatment, blinding of neither investigators nor patients was feasible.
Conclusion
The results presented here suggest that a series of six HydraFacial Clarifying Treatments improves overall skin appearance in patients with active acne.
Keywords: Acne, hydradermabrasion, microdermabrasion, hydrafacial, aesthetic dermatology
Acne vulgaris is estimated to affect 45 million people in the United States, the majority of whom are young adults.1 Acne has a prevalence of over 90 percent among adolescents and persists into adulthood in approximately 12 to 14 percent of cases, often with resultant psychological and social consequences.2 Research suggests that individuals with acne are more likely to suffer from depression, anxiety, and low self-esteem.3 Thus, improving skin appearance for individuals with acne may yield benefits beyond aesthetic appearance.4
Acne pathogenesis is currently understood to be a multi-factorial inflammatory disease.5,6 Key pathogenic factors that play an important role in the development of acne include follicular hyperkeratinization, microbial colonization, sebum production, and inflammatory mechanisms.2 Contributing factors to acne development may include hormone levels, stress, family history, hair and skin products.3,7 Current treatment options range from topical agents like benzoyl peroxide, antibiotics, and retinoids; laser and light therapy; mechanical disruption of sebaceous glands; to systemic therapy including oral antibiotics, hormonal therapy, and isotretinoin.8 These therapies are often used in combination to improve patient outcomes.9
Microdermabrasion, the application of an exfoliative and/or abrasive force on the skin, is a non-pharmaceutical approach to acne management.10 The HydraFacial Clarifying Treatment (The HydraFacial Company; Long Beach, California) is a hydradermabrasion device that provides similar benefits as microdermabrasion but without the use of harsh, abrasive surfaces.11,12 Rather, it uses its proprietary vortex technology in combination with topical solutions to cleanse and exfoliate oily and congested skin.10 Exfoliation and suction remove cellular, keratinized, and sebaceous debris from follicular orifices that act as a nidus for acne lesions.6,13 This is the first clinical study to formally assess the safety and efficacy of the HydraFacial Clarifying Treatment on the appearance of patients with acne when delivered as a series of six treatments over 12 weeks.
METHODS
Study design. This was a multicenter, open-label prospective study to assess the tolerability and efficacy of the HydraFacial Clarifying Treatment in patients with mild-to-moderate acne. All patients provided written informed consent prior to undertaking any study procedures. Treatments and assessments were completed at two study sites: MBeauty Clinic in San Diego, CA, and Mount Sinai Medical Center in Miami Beach, Florida. Treatments occurred between June 20, 2018, and January 25, 2019.
Key inclusion criteria were an age of 18 years or more with mild or moderate acne, male or female gender, on a stable acne regimen (no addition of new products) for more than two weeks prior to study start, no use of oral or topical antibiotic treatment within two weeks prior to study start, and no use of other office-based or home-use acne device or facial treatments within four weeks prior to study start. Key exclusion criteria were the use of oral isotretinoin within six months prior to first study treatment, presence of cystic acne, history of frequent HSV facial outbreaks or presence of an active HSV outbreak, autoimmune disease such as HIV, lupus, hepatitis, scleroderma, or any condition that would confound the study results in the investigator’s opinion or would interfere significantly with the patient's participation in the study. Use of antibiotics and/or isotretinoin was prohibited during the study.
Each patient received the HydraFacial Clarifying Treatment via the HydraFacial Elite machine. The treatment consisted of a cleansing and peeling step using HydraFacial’s Activ-4® and GlySal® (7.5% glycolic acid and 2% salicylic acid) solutions; an exfoliation step using the Beta-HD solution; use of blue LED lights for eight minutes; and a hydration step with Anti-Ox+®. A single treatment was administered every two weeks for 12 weeks for a total of six treatments.
Assessments. Efficacy was assessed independently by the patient and investigator using the Global Acne Severity Scale (GASS) and a customized efficacy questionnaire.14 GASS grading was as follows: Grade 0 (none): clear skin with no inflammatory lesions or non-inflammatory lesions; Grade 1 (almost clear): few non-inflammatory lesions with not more than one or two small inflammatory lesions; Grade 2 (mild): some non-inflammatory lesions with no more than a few inflammatory lesions, papules/pustules only, no nodular lesions; Grade 3 (moderate): up to many non-inflammatory lesions and may have some inflammatory lesions but no more than one small nodular lesion; Grade 4 (severe): up to many non-inflammatory and inflammatory lesions but no more than one small nodular lesion. GASS was assessed at baseline prior to Treatment 1, after each treatment, and at follow-up two weeks after treatment.6 Investigator GASS was not measured after Treatment 1.
The investigator efficacy questionnaire included four skin assessments: skin looks clearer, skin has reduced erythema, skin looks less oily and congested, skin appears healthier and more radiant. The patient efficacy questionnaire included five assessments: 1) My skin looks clearer; 2) I feel more confident in my appearance; 3) My skin looks less inflamed compared to before I began treatment; 4) I see fewer acne lesions compared to before I began treatment; 5) My skin looks and feels cleaner. Both investigator and patient efficacy questionnaires used a five-point scale: strongly disagree (1), disagree (2), neither agree nor disagree (3), agree (4), or strongly agree (5). Efficacy questionnaires were performed after each treatment and at follow-up.
Tolerability was assessed after each treatment and at follow-up by investigator grading of erythema, edema, dryness, peeling on four-point scale: none (0), mild (1), moderate (2), severe (3), and patients grading of stinging, tingling, itching, burning on the same 4-point scale.
Statistical analysis. McNemar’s Chi-squared tests were used to analyze categorical improvement in GASS Grade (≤1 vs. ≥2) from baseline to final treatment. A last observation carried forward imputation method was applied for any missing data. Each customized efficacy questionnaire assessment was analyzed using Fisher’s Exact tests. All analyses were performed using R and Microsoft Excel.
RESULTS
Twenty patients enrolled in the study. Eighteen patients completed all six treatments, and two patients completed five treatments. Of the 18 patients who completed all treatments, eight returned for the two-week follow-up. Patients were predominantly female (80%) with a median age of 29.5 years (range 19-48, Table 1).
TABLE 1.
Patient Demographic Data
| CHARACTERISTIC | ALL PATIENTS (N = 20) |
|---|---|
| Age (year) | |
| Median | 29.5 |
| Range | 19–48 |
| Sex–no. (%) | |
| Male | 4 (20%) |
| Female | 16 (80%) |
| Race–no. (%) | |
| Asian | 9 (45%) |
| Black/African American | 1 (5%) |
| White | 8 (40%) |
| Other | 2 (10%) |
| Hispanic/Latino | 6 (30%) |
| Not Hispanic/Latino | 14 (70%) |
TABLE 2.
Investigator and Patient Tolerability Assessment
| TREATMENT TIME | MILD | MODERATE | SEVERE |
|---|---|---|---|
| Erythema– Investigator assessment | |||
| Any time | 9 (45%) | 9 (45%) | 0 (0%) |
| First Treatment | 12 (60%) | 3 (15%) | 0 (0%) |
| Final treatment | 8 (40%) | 1 (5%) | 0 (0%) |
| Edema– Investigator assessment | |||
| Any time | 6 (30%) | 5 (25%) | 0 (0%) |
| First Treatment | 7 (35%) | 2 (10%) | 0 (0%) |
| Final treatment | 3 (15%) | 1 (5%) | 0 (0%) |
| Dryness– Investigator assessment | |||
| Any time | 2 (10%) | 0 (0%) | 0 (0%) |
| First Treatment | 1 (5%) | 0 (0%) | 0 (0%) |
| Final treatment | 0 (0%) | 0 (0%) | 0 (0%) |
| Peeling– Investigator assessment | |||
| Any time | 1 (5%) | 0 (0%) | 0 (0%) |
| First Treatment | 1 (5%) | 0 (0%) | 0 (0%) |
| Final treatment | 0 (0%) | 0 (0%) | 0 (0%) |
| Stinging– Patient assessment | |||
| Any time | 6 (30%) | 2 (10%) | 0 (0%) |
| First Treatment | 2 (10%) | 1 (5%) | 0 (0%) |
| Final treatment | 1 (5%) | 0 (0%) | 0 (0%) |
| Tingling– Patient assessment | |||
| Any time | 6 (30%) | 1 (5%) | 1 (5%) |
| First Treatment | 4 (20%) | 0 (0%) | 0 (0%) |
| Final treatment | 2 (10%) | 0 (0%) | 0 (0%) |
| Itching– Patient assessment | |||
| Any time | 6 (30%) | 2 (10%) | 0 (0%) |
| First Treatment | 1 (5%) | 1 (5%) | 0 (0%) |
| Final treatment | 0 (0%) | 0 (0%) | 0 (0%) |
| Burning– Patient assessment | |||
| Any time | 3 (15%) | 2 (10%) | 0 (0%) |
| First Treatment | 1 (5%) | 1 (5%) | 0 (0%) |
| Final treatment | 0 (0%) | 0 (0%) | 0 (0%) |
HydraFacial Clarifying Treatment series resulted in a statistically significant improvement in both investigator and patient GASS from baseline to final treatment (Figure 1). The proportion of patients with investigator assessment of no acne to almost clear skin (GASS ≤1) increased from 20 percent to 65 percent from baseline to final treatment (p=0.0027). The proportion of patients who self-reported no acne to almost clear skin (GASS ≤1) increased from 5 to 55 percent from baseline to final treatment (p=0.0016). Mean investigator GASS decreased 37 percent from baseline to Treatment 6, from 2.10 to 1.33. Mean patient GASS decreased 34 percent from baseline to Treatment 6, from 2.45 to 1.61. Treatment effect was sustained, as GASS at the two-week follow-up were similar or improved compared to Treatment 6.
FIGURE 1.
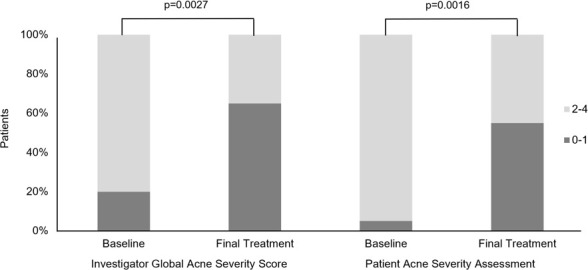
Investigator and Patient Global Acne Severity Score. GASS grading 0-4: Grade 0=none, Grade 1=almost clear, Grade 2=mild, Grade 3=moderate, Grade 4=severe.
Both investigators and patients reported an immediate improvement in skin appearance following the first treatment which increased over the course of the treatment series. Following final treatment, investigators agreed or strongly agreed that 100 percent of patients had both clearer and healthier and more radiant skin, and that more 80 percent had both reduced erythema and less oiliness and congestion (Figure 2). Following final treatment, 100 percent of patients agreed or strongly agreed that they felt more confident in their appearance and that their skin looked and felt cleaner, and more than 80 percent agreed or strongly agreed that their skin looked clearer, looked less inflamed, and had fewer acne lesions compared to baseline (Figure 3).
FIGURE 2.
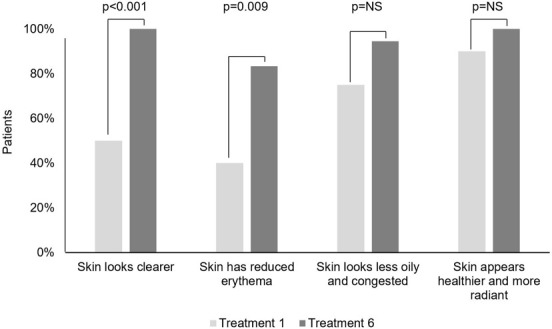
Investigator Efficacy Assessments. Percent of "Agree" and "Strongly Agree" indications after treatments 1 and 6.
NS, not significant (p>0.05).
FIGURE 3.
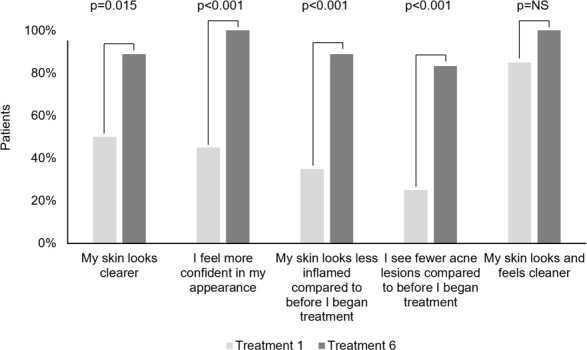
Patient Efficacy Assessments. Percent of "Agree" and "Strongly Agree" indications after treatments 1 and 6.
NS, not significant (p>0.05).
HydraFacial was well tolerated with no severe signs reported by the investigator. Across the four investigator tolerance parameters, erythema was most common, followed by edema, dryness, and peeling. Across the four patient tolerance assessment parameters, stinging and itching were the most common, followed by tingling and burning. A single event of severe tingling occurred following the first treatment which resolved by the second treatment. All signs and symptoms were most frequent following the first treatment and least frequent following final treatment.
DISCUSSION
Acne is a common condition that can be difficult to treat and prove detrimental to self-esteem and self-image.2,15 Therefore, much research has been dedicated to developing effective and efficient treatment interventions.
This is the first clinical study to formally evaluate the efficacy and tolerability of the HydraFacial Clarifying Treatment for acne. The results of this study demonstrate that a series of six HydraFacial treatments over a 12-week period resulted in a statistically significant reduction in the grade of acne severity as assessed by both investigators and patients using the well-established GASS.14 Improvement was observed after the first treatment, increased over the course of the treatment series, and was sustained through the follow-up period. In addition, the vast majority (>80-100%) of patients experienced improvement across all nine skin appearance parameters. Notably, investigators agreed or strongly agreed that 100 percent of patients had both clearer skin and healthier and more radiant skin, and 100 percent of patients agreed or strongly agreed that that their skin looked and felt cleaner. Given the relationship between acne and low self-esteem, it is meaningful that 100 percent of patients also agreed or strongly agreed that they felt more confident in their appearance following treatment.
HydraFacial treatment was generally well tolerated. The most common investigator reported sign was erythema and the most common patient reported symptoms were stinging and itching. Importantly, the incidence of signs and symptoms declined over the course of therapy, indicating improved tolerance with repeated treatments. It should be noted that as a non-pharmaceutical therapy, the HydraFacial treatment does not expose patients to potential risks associated with pharmaceutical therapies such as retinoids and antibiotics.
For both adolescents and adults overall, it is most important that acne treatments are fast-acting, non-irritating, and non-bleaching, and HydraFacial offers a treatment that aligns with all three of these attributes.16 HydraFacial is a procedure which can be administered by aestheticians, has no contra-indications, and can be used in conjunction with other treatments (although not studied here). As many offices work to attract this patient group which spends more on self-care as a proportion of disposable income than any other generation, HydraFacial’s fast-acting results at relatively low costs and with minimal side effects represents an attractive addition to aesthetic practices.17 In addition, the HydraFacial treatment series allows practitioners to assume a more active role in maintaining treatment compliance for their acne patients which is crucial for achieving successful outcomes.18
This study was not without limitations. The study was nonrandomized and unblinded; however, due to the nature of the procedure, an appropriate placebo treatment is not possible, nor would it be feasible to blind either the investigator or patient. The sample size consisted of only 20 patients; however, the results still yielded a statistically significant increase in the proportion of patients with no acne or almost clear skin following the treatment series. Given the potential to combine HydraFacial with other acne treatment options, future studies evaluating combination treatments may yield even further benefit.
CONCLUSION
This first clinical study of HydraFacial Clarifying Treatment demonstrated the therapy was well tolerated and resulted in a significant improvement in acne severity per both investigator and patient assessment following six treatments over 12 weeks. Importantly, 100 percent of patients agreed or strongly agreed that their skin looked and felt cleaner and that they felt more confident in their appearance. This data demonstrates that HydraFacial treatment is an effective and well tolerated therapy option for patients suffering from acne vulgaris.
Contributor Information
Ryan Storgard, Mr. Storgard is with Weill Cornell Medical College in New York, New York..
Tess Mauricio, Ms. Mauricio is with the Loma Linda University School of Medicine in Loma Linda, California..
Martin Zaiac, Dr. Zaiac is with Mount Sinai Medical Center in Miami Beach, Florida..
Jwala Karnik, Dr. Karnik is with The HydraFacial Company in Long Beach, California..
REFERENCES
- Lehmann H, Andrews J, Robinson K Management of Acne: Summary. 17th ed. Rockville: AHQR Evidence Report Summaries. 2001. [PMC free article] [PubMed]
- Zaenglein A, Pathy A, Schlosser B et al. Guidelines of care for the management of acne vulgaris. Journal of the American Academy of Dermatology. 2016;74(5):945–973. doi: 10.1016/j.jaad.2015.12.037. [DOI] [PubMed] [Google Scholar]
- Fried R, Wechsler A. Psychological problems in the acne patient. Dermatologic Therapy. 2006;19(4):237–240. doi: 10.1111/j.1529-8019.2006.00079.x. [DOI] [PubMed] [Google Scholar]
- https://www.aad.org/public/diseases/acne/really-acne/adult-acne American Academy of Dermatology. Adult Acne. Available at. Accessed January 22, 2018.
- Layton A. A review on the treatment of acne vulgaris. International Journal of Clinical Practice. 2006;60(1):64–72. doi: 10.1111/j.1368-5031.2005.00695.x. [DOI] [PubMed] [Google Scholar]
- Fabbrocini G, Annunziata MC, D'Arco V et al. Acne scars: pathogenesis, classification and treatment. Dermatology Research and Practice. 2010;2010(893080) doi: 10.1155/2010/893080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams H, Dellavalle R, Garner S. Acne Vulgaris. The Lancet. 2012;379(9813):361–372. doi: 10.1016/S0140-6736(11)60321-8. [DOI] [PubMed] [Google Scholar]
- Croke L. Acne Vulgaris: Treatment Guidelines from the AAD. American Family Physician. 2017;95(11):740–741. [PubMed] [Google Scholar]
- Rathi SK. Acne vulgaris treatment: the current scenario. Indian J Dermatol. 2011;56(1):7–13. doi: 10.4103/0019-5154.77543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Domyati M, Hosam W, Abdel-Azim E et al. Microdermabrasion: a clinical, histometric, and histopathologic study. Journal of Cosmetic Dermatology. 2016;15(4):503–513. doi: 10.1111/jocd.12252. [DOI] [PubMed] [Google Scholar]
- Lloyd J. The Use of Microdermabrasion for Acne: A Pilot Study. Dermatologic Surgery. 2001;27(4):329–331. doi: 10.1046/j.1524-4725.2001.00313.x. [DOI] [PubMed] [Google Scholar]
- https://www.allure.com/story/hydrafacial-treatment Allure. The HydraFacial Phenomenon. Available at. Accessed November 14, 2019.
- Kempiak S, Uebelhoer N. Superficial Chemical Peels and Microdermabrasion for Acne Vulgaris. Semin Cutan Med Surg. 2008;27(3):212–220. doi: 10.1016/j.sder.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Allen BS, Smith JG. Various parameters for grading acne vulgaris. Arch Dermatol. 1982;118(1):23–25. [PubMed] [Google Scholar]
- Tan AU, Schlosser BJ. A review of diagnosis and treatment of acne in adult female patients. Int J Womens Dermatol. 2017;4(2):56–71. doi: 10.1016/j.ijwd.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbrocini G, Cacciapuoti S, Giuseppe M. A qualitative investigation of the impact of acne on health-related quality of life (HRQL): Development of a conceptual model. Dermatol Ther (Heidelb). 2018;8(1):85–99. doi: 10.1007/s13555-018-0224-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobayed N, Nguyen J, Jagdeo J. Minimally invasive facial cosmetic procedures for the Millennial aesthetic patient. Journal of Drugs in Dermatology. 2020;19(1):100–103. doi: 10.36849/JDD.2020.4641. [DOI] [PubMed] [Google Scholar]
- Zaghloul SS, Cunliffe WJ, Goodfield MJD. Objective assessment of compliance with treatments in acne. British Journal of Dermatology. 2005;152(5):1015–1021. doi: 10.1111/j.1365-2133.2005.06357.x. [DOI] [PubMed] [Google Scholar]


