Abstract
Objectives
To explore the anti-inflammatory effect and the potential mechanism of dexmedetomidine in ARDS/ALI.
Materials and Methods
C57BL/6 mice and EL-4 cells were used in this research. The ALI model was established by CLP. The level of inflammatory cytokines in the lung and blood, the severity of lung injury, the expression of Foxp3, and the proportion of Tregs were detected before and after dexmedetomidine treatment. The expression of the AMPK/SIRT1 after dexmedetomidine treatment was detected in vivo and in vitro. After blocking the AMPK/SIRT1 pathway or depleting Tregs in vivo, the level of the inflammatory response, tissue injury, and Tregs differentiation were detected again to clarify the effect of dexmedetomidine.
Results
Dexmedetomidine significantly reduced systemic inflammation and lung injury in CLP mice. Dexmedetomidine enhanced the Foxp3 expression in the lungs and the frequency of Tregs in the spleen. Dexmedetomidine up-regulated the protein expression of p-AMPK and SIRT1 in lungs and EL-4 cells and facilitated the differentiation of naïve CD4+ T cells into Tregs in vitro. Meanwhile, DEX also increased the expression of Helios in Treg cells.
Conclusions
DEX could improve ARDS/ALI by facilitating the differentiation of Tregs from naïve CD4+ T cells via activating the AMPK/SIRT1 pathway.
Keywords: Dexmedetomidine, Acute respiratory distress syndrome, Acute lung injury, Regulatory T cells, AMP-activated protein kinase, Sirtuin1
Introduction
The definition of acute respiratory distress syndrome (ARDS) was first established by Ashbaugh in 1967. This clinical syndrome is usually caused by pulmonary infection, sepsis, aspiration of gastric contents, and severe trauma (Matthay et al. 2019). Acute lung injury (ALI), the early and continuous pathophysiological process of ARDS, will lead to acute respiratory failure and ARDS when it develops to a serious stage (Butt et al. 2016). 10% of the inpatients in Intensive Care Units (ICUs) would develop into ARDS every year eventually (Fan et al. 2018; Matthay et al. 2019; Meyer et al. 2021). Moreover, the mortality varies from 35 to 46%, according to the degree of hypoxemia and the severity of this syndrome (Fan et al. 2018; Reilly et al. 2019). Therefore, intervening in the early stage of ARDS actively could prevent its occurrence (Butt et al. 2016; Yadav Thompson and Gajic 2017). Especially the Corona Virus Disease 2019 (COVID-19) pandemic has led to a sharp increase in ARDS and highlighted challenges associated with this syndrome, such as the lack of effective pharmacotherapy (Meyer et al. 2021). Unlike ventilator and fluid management of ARDS, pharmacotherapies for ARDS have failed to identify any consistently effective drugs (Meyer et al. 2021). Corticosteroid usage, surfactant replacement (Hentschel et al. 2020), and drugs targeting biological pathways (Meyer et al. 2021), which involves numerous factors such as dosage, mode of action, biological metabolic effect, etc., have not proven consistently effective. ARDS is an over-inflammatory disease of the lung (Mahida et al. 2020), and inflammatory cytokines storm is a critical part of the syndrome. Therefore, inflammation regulation may become an important means for the treatment of ARDS (Chai et al. 2020; Thompson et al. 2017; Yadav et al. 2017). Currently, programmed death (PD) -1/PD-L1 and cytotoxic T-lymphocyte associated Protein 4 (CTLA4) are focused as immune targets to discuss the precise treatment of tumors (Kalbasi and Ribas 2020). ARDS patients will benefit from immunotherapy, especially precise immunomodulation (Y. Zhang et al. 2020).
Dexmedetomidine (DEX) is a selective α 2-adrenergic receptor agonist, which is widely used in analgesia and sedation of patients undergoing surgery and ICU patients (Turan et al. 2020; Weerink et al. 2017; Yuan et al. 2020). DEX may be the preferred sedative in patients with or at risk of ARDS (Hu, Zhong, Li, Zhang, & Li, 2021; Rosenberg and Traube 2019). At present, the potential anti-inflammatory mechanism of DEX is also valued. Studies mainly focus on the inflammatory pathways, including PYD domains-containing protein 3 (NLRP3) (Y. Zhang et al. 2018), nuclear factor kappa-B (NF-κB) pathway (Zhao et al., 2018), and α-2R/PI3K/Akt pathway (Li et al. 2018). Animal experiments have confirmed the anti-inflammatory protective effect of DEX in the treatment of ALI (Z. Zhang 2020). DEX may be a promising therapy for treating ARDS as well as chronic diseases by directly targeting epithelial cells (Sun et al. 2019) and reducing lung cell apoptosis through α-2R/PI3K/Akt pathway (Shi et al. 2021). Moreover, DEX can ameliorate ALI by preserving mitochondrial dynamic equilibrium through the HIF-1a/HO-1 signaling pathway (Shi et al. 2021). However, individualized precise immune modulation depends on the disease course and immune status. In addition, some studies showed that DEX exerts beneficial effects to ameliorate renal ischemia–reperfusion (rI/R) -induced ALI in alveolar macrophages (Y. Chen et al. 2020b,a). Few existing studies are exploring the therapeutic effects of DEX on ARDS from the aspects of immune cells, the core of inflammation. Therefore, research on immune cells as targets can further explore the therapeutic effects of DEX on ARDS.
Regulatory T cells (Tregs) are a distinctive subset of CD4+ T cells, which occupy an extraordinary position in regulating autoimmune responses, inhibiting excessive inflammatory reactions, and maintaining tissue homeostasis (K. Wang and Fu 2020). There is an activation of Tregs difference in ARDS patients (Halter et al. 2020). The proportion of Tregs in CD4+ T cells in the peripheral venous blood collected within the first week is an important predictor of clinical prognosis in patients with ARDS between survivors and non-survivors (Halter et al. 2020). The immunomodulatory effect of Treg has been testified to inhibit the inflammatory response during ALI (Nadeem et al. 2020). Tregs can improve ALI by regulating immune response, promoting alveolar epithelial cell proliferation, and tissue repair (Mock et al. 2020, 2019). A study has shown that Treg is one of the critical effectors to protect against transfusion-related ALI (Kapur et al. 2017). It could be recognized as a key molecular target for immunotherapy in ARDS. Recent research found that DEX increases the differentiation of Th1 and Tregs and reduces the PD-1 expression in CD4+ T cells, which assists in ameliorating postoperative pain and attenuating proinflammatory response (Y. Wang et al. 2021a, b). DEX might have the potential of regulating Tregs in ARDS. More importantly, Tregs come from naïve CD4+ T cells. The differentiation of Treg is influenced by various factors, such as the immune microenvironment, and this process is accompanied by metabolic changes (Kurniawan et al. 2020). We found that the inflammatory microenvironment of ARDS occupies an important position in the differentiation of Tregs and affects the progression of this disease (Chai et al. 2020; Xie et al. 2021). Therefore, the regulatory mechanism of DEX on Tregs may involve the development of Tregs from naïve CD4+ T cells.
To test the above hypothesis, we established an ALI mouse model induced by CLP to reveal the role of DEX in lung injury, Treg polarization, and inflammation response in ALI. In vitro experiments, murine naïve CD4+ T cells and EL-4 cells were treated with or without DEX or Compound C (a specific inhibitor of AMPK) to verify the role of DEX on the differentiation of Tregs and the related signal transduction pathway.
Materials and Methods
Reagents and antibodies
Dexmedetomidine (Sigma-Aldrich, SML0956); Dorsomorphin (Compound C) (Selleck, S7306); Cell Counting Kit8 (MCE, HY-K0301); Human lymphocyte separation solution (GE Healthcare, FICOLL-PAQUE PLUS, 17,144,003); EasySep™ Mouse Naive CD4+ T Cell Isolation Kit (Stem cell, 19,765); IL-2 (Peprotech, 212–12); TGF-β1 (Peprotech, 100–21); Anti-Mouse CD3 antibody (eBioscience, 16–0032-86), Anti-Mouse CD28 antibody (eBioscience, 16–0281-85); Anti-Mouse CD25 antibody (eBioscience, 16–0251-38); CD4 Monoclonal antibody (GK1.5), FITC (eBioscience, 11–0041-82); Rat IgG2b kappa Isotype Control (eB149/10H5), FITC (eBioscience, 11–4031-82); CD25 Monoclonal Antibody (PC61.5), PE-Cyanine7 (eBioscience, 25–0251-82); Rat IgG1 kappa Isotype Control (eBRG1), PE-Cyanine7 (eBioscience, 25–4301-82); HELIOS Monoclonal Antibody (22F6), PE (eBioscience, 12–9883-42); Armenian Hamster IgG Isotype Control (eBio299Arm), PE (eBioscience, 12–4888-81); FOXP3 Monoclonal Antibody (FJK-16 s), APC (eBioscience, 17–5773-82); Rat IgG2a kappa Isotype Control (eBR2a), APC (eBioscience, 17–4321-81). Since the antibodies used in immunohistochemistry, immunofluorescence, and western blotting experiments are not identical, information on these antibodies will be described in the corresponding methods section.
Animals
Male, 8-to-10-week, 18-22 g C57BL/6 mice were obtained from the Experimental Animal Center of Chongqing Medical University. Before the operation, mice were reared in specific pathogen-free (SPF) level animal barrier facilities, at 22 ℃ constant temperature, in a 12 h light/12 h dark cycle, free to eat and drink. All mice were raised under the above conditions for seven days to adapt to the new environment. The protocol was reviewed and approved by the Animal Welfare and Use Committee of Chongqing Medical University and the Ethics Committee of the First Affiliated Hospital of Chongqing Medical University.
Medium and cell culture
EL-4 cells were kindly provided by Stem Cell Bank, Chinese Academy of Sciences. All cells were cultured with complete RPMI-1640 medium (RPMI-1640 + 10% Fetal Bovine Serum + 1% Penicillin/Streptomycin, RPMI-1640, Fetal Bovine Serum, Penicillin/Streptomycin were purchased from Gibco, USA) in an incubator with 5% CO2 at 37 ℃.
Cecal ligation and puncture model
Isoflurane was used to induce and maintain anesthesia in mice with the aid of an animal anesthesia machine throughout the operation. An incision of approximately 1 cm was cut along the middle line of the abdomen to find the cecum carefully. The cecum was ligated 1 cm away from the blind end and punctured with a No. 22 needle to squeeze out a few feces and put the cecum back into the abdominal cavity, suture the incision carefully. Mice in the sham group were not subjected to cecal ligation and puncture.
Isolation and purification of naive CD4+T cells
Naive CD4+ T cells were isolated and purified from the spleen with the use of a naive CD4+ T cell isolation kit. Naive CD4+ T cells were treated with DEX (1 μg/mL), Compound C (100 nM), and their combination under TCR activation (Anti-Mouse CD3 and CD28 antibody) and Treg polarization condition (IL-2 and TGF-β).
Isolation of human PBMCs
Peripheral blood mononuclear cells (PBMCs) were isolated from the venous blood of ARDS patients by human lymphocyte separation solution combined with density gradient centrifugation. The selection of patients with ARDS follows the 2012 Berlin definition (Meyer et al. 2021; Ranieri et al. 2012). The expression of AMPK and SIRT1 in PBMCs was detected by RT-qPCR after treatment by DEX (1 μg/mL) for 24 h.
Drug intervention
DEX was injected intraperitoneally at a dose of 50 μg/kg 30 min after CLP operation, while Dorsomorphin (Compound C) was injected intraperitoneally 30 min before the operation at a dose of 5 mg/kg, anti-mouse CD25 antibody (eBioscience, 16–0251-38) was given at a dose of 10 mg/kg 1 h before CLP operation. EL-4 cells and PBMCs were treated with DEX (1 μg/mL) and/or Compound C (1 μM), mouse naïve CD4+ T cells were treated with DEX (1 μg/mL) and/or Compound C (100 nM) under Treg polarization condition.
Histological analysis
The right upper lobe of the lung was fixed in 4% paraformaldehyde solution for 24 h. The thickness of the sections was 4 μm. The paraffin sections were stained with hematoxylin and eosin (HE). The Mikawa method was used to evaluate the degree of lung injury after the pathological images were collected under the optical microscope (Mikawa et al. 2003). We assessed the degree of histopathological damage to mouse lungs based on alveolar congestion, hemorrhage, neutrophil infiltration, alveolar wall thickening, or hyaline membrane. Each item was scored on a 5-point scale as follows: 1 = minimal damage, 2 = mild damage, 3 = moderate damage, 4 = severe damage, and 5 = maximal damage. The total score is ARDS pathological score. The higher the score, the more serious the injury.
TUNEL staining
TUNEL assay was detected by In-situ Apoptosis Detection Kit (Nanjing Jiancheng Bioengineering Institute, G002-2–2). In short, paraffin sections were soaked in xylene for five minutes and repeated twice, then soaked in gradient ethanol (100, 95, 90, 80, 70%) for three minutes, respectively. After blocking and protease K treatment, prepare the TUNEL reaction mixture and react at room temperature for 60 min. Add 50 μL TUNEL reaction mixture to the dried slices, covered with slides or sealing film, incubate in dark for 60 min at 37 ℃, and rinsed with PBS three times. After the slides were dried, 50 μL converter pod was added to the specimens, dark incubated at 37 ℃ for 30 min, and rinsed with PBS three times. Add 100 μL of DAB substrate to the tissue and incubate for ten minutes at room temperature, using PBS to wash three times. After taking photos, hematoxylin or methyl green was used to dye the cell nucleus and rinsed with PBS three times. Finally, observe and take photos under the microscope.
Immunohistochemistry
Paraffin-embedded sections were conventional dewaxing and rehydration. Blocked sections in goat serum for 30 min at room temperature after antigen retrieval. Anti-mouse myeloperoxidase (MPO) (Abcam, ab208670, 1:200), IL-10 (Servicebio, GB12108, 1:200), IL-17 (Abcam, ab79056, 1:200), FOXP3 (CST, 72,338, 1:200) antibody were added to the slices and stored in a humidified chamber at 4 ℃ overnight. The biotin-labeled secondary antibody was incubated at 37 ℃ for 3 min. The images were obtained under the microscope.
Immunofluorescence
Foxp3 and IL-10 expression in EL-4 cells was detected by immunofluorescence. Anti-FOXP3 antibody was purchased from CST (72,338), and Anti-IL-10 antibody was purchased from Servicebio (GB12108). Goat Anti-Mouse IgG(H + L) FITC-conjugated (S0007) and Goat Anti-Rabbit IgG (H + L) Fluor647-conjugated (S0013) were purchased from Affinity Bioscience. Images were taken under a laser confocal microscope after the completion of staining according to the usual method of immunofluorescence.
Determination of cytokines in the serum of mice by ELISA
Sera from mice were stored at—80 ℃ until analysis. The concentration of TNF-α and IL-6 was detected by ELISA kits (JINGMEI BIOTECHNOLOGY). After the main steps of antibody incubation, washing, and enzyme labeling, read the absorbance at 450 nm. Obtained the corresponding concentration of samples according to the standard curve.
Flow cytometry
The mononuclear cells of the mouse spleen were collected and washed with phosphate buffer solution (PBS). We first labeled CD4 and CD25 because they are located on the cell membrane. The cells should be fixed and permeated by Foxp3/Transcription Factor Staining Buffer Kit (eBioscience, 00–5523-00) before labeling Foxp3 and Helios. The antibody should be incubated for 30 min in dark. Rat IgG2b kappa Isotype Control (eB149/10H5), FITC; Rat IgG1 kappa Isotype Control (eBRG1), PE-Cyanine7; Armenian Hamster IgG Isotype Control (eBio299Arm), PE; Rat IgG2a kappa Isotype Control (eBR2a), APC were used as the isotype control, respectively. The samples separately marked by FITC, PE and APC were used to adjust the voltage compensation of the fluorescent channel. Negative control was used to gate CD4+ T cells, then gated CD25+Foxp3+ and Helios+Foxp3+Tregs in CD4 + T cells.
Total RNA isolation, reverse transcription, and qPCR
Total RNA was isolated from lung tissue and cultured cells by RNAiso plus (Takara, 9109). The relative expression of mRNAs was detected by RT-qPCR with specific primers, and β-Actin was used as the internal reference. The primer sequence was as follows, Mouse Foxp3: forward 5’- TGGATGAGAAAGGCAAGGC-3’ reverse 5’- CTGAGTACTGGTGGCTACGATG-3’; Mouse β-Actin: forward 5’-CCACCATGTACCCAGGCATT-3’ reverse 5’-CAGCTCAGTAACAGTCCGCC-3’; Human SIRT1: forward 5’-GCAGATTAGTAGGCGGCTTG-3’ reverse 5’-TCTGGCATGTCCCACTATCA-3’(Ma et al. 2020); Human AMPK: forward 5’-AAAGTCGGCGTCTGTTCCAA-3’ reverse 5’-CATGTGTGCATCAAGCAGGAC-3’; Human β-Actin: forward 5’- CCTTCCTGGGCATGGAGTC -3’ reverse 5’-TGATCTTCATTGTGCTGGGTG -3’.
RT-qPCR was executed by using TB Green™ Premix Ex Taq™ II (Tli RNaseH Plus) (Takara, RR820A) on the ABI PRISM 7000 PCR system (Bio-Rad, USA). Finally, data were analyzed via the method.
Western blotting
In the presence of PMSF and phosphatase inhibitors, proteins in lung tissue and cultured cells were harvested by RIPA lysis buffer (Beyotime, P0013B). Protein concentration was measured by the BCA protein assay kit (Beyotime, P0011). Then protein was separated by the SDS-PAGE method and transferred into the PVDF membrane subsequently. The primary antibody of AMPK (1:1000, CST 2532), p-AMPK (1:1000, CST 2537), SIRT1(1:1000, CST 8469), FOXP3(1:2000, Biolegend, 126,402), meanwhile GAPDH (1:1000, Abcam, ab8245) or β-Actin (1:10,000, ABclonal, AC026) was used loading control. The blots were incubated with the HRP-linked secondary antibody (1:3000, Affinity, S0001, S0009) at room temperature for 60 min. Finally, the gel imaging system (Fusion, Vilber, French) was used to detect and analyze protein expression by chemiluminescence.
Statistical analysis
The data analysis was completed by using GraphPad Prism 8 (GraphPad Software, USA) and SPSS 22.0 (IBM, USA). All data were expressed as mean ± standard deviation (SD). Student t-test (two groups) or one-way ANOVA (multiple groups) was used to determine the statistical significance of differences. Tukey’s HSD for post hoc test. P < 0.05 indicates the results are statistically significant. N = 5 in each group. All tests were repeated three times. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001.
Results
DEX alleviates acute lung injury and systemic inflammation in CLP mice
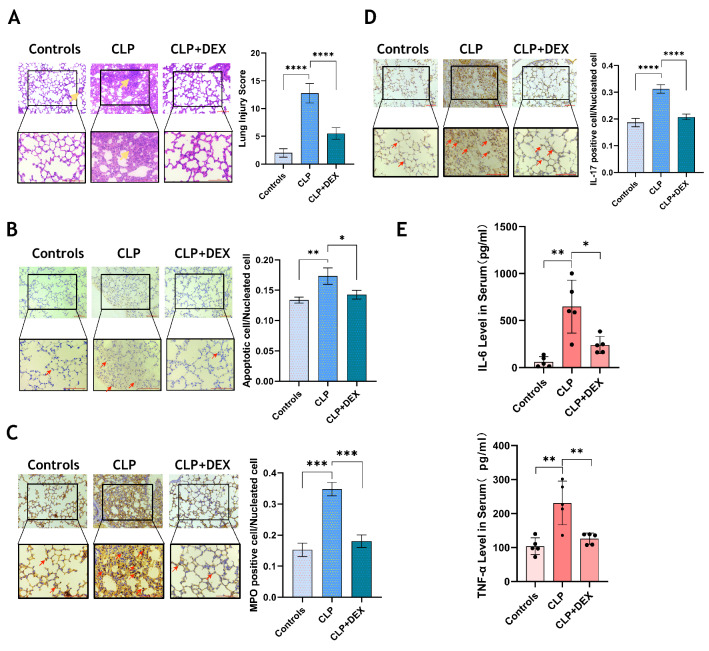
To evaluate the effect of DEX on CLP mice, the mice were sacrificed 24 h after the operation to obtain lung tissue and serum for detection. The degree of pulmonary injury was evaluated through the histopathological method. The pathological score of lung injury in the CLP group was significantly higher than controls, and DEX intervention significantly improved the pathological damage in the lungs of CLP mice (p < 0.0001, Fig. 1A). DEX also reduced the apoptosis of lung tissue cells (p < 0.05, Fig. 1B). Immunohistochemical staining images showed that the level of MPO and IL-17 in lung tissue decreased to a great extent after the intervention of DEX (p < 0.001, Fig. 1C, D). In addition, inflammatory cytokines such as IL-6 and TNF-α in serum were significantly reduced after DEX treatment (p < 0.05, Fig. 1E).
Fig. 1.
DEX alleviates acute lung injury and systemic inflammation in CLP mice. A HE staining was used to detect the pathological injury of mouse lung, and the lung injury score was obtained by the Mikawa method. The total histopathological scores were: Control (2 ± 0.63); CLP (12.8 ± 1.6); CLP + DEX (5.4 ± 0.8); B TUNEL staining was used to detect lung apoptosis. (C-D) The expression of MPO and IL-17 in lung tissue was detected by immunohistochemistry. All images were randomly obtained at 200 and 400 visual fields. E The concentration of inflammatory cytokines (TNF-α, IL-6) in mouse serum was measured by ELISA. N = 5 in each group. All tests were repeated three times. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001
DEX promotes Foxp3 expression and Treg differentiation
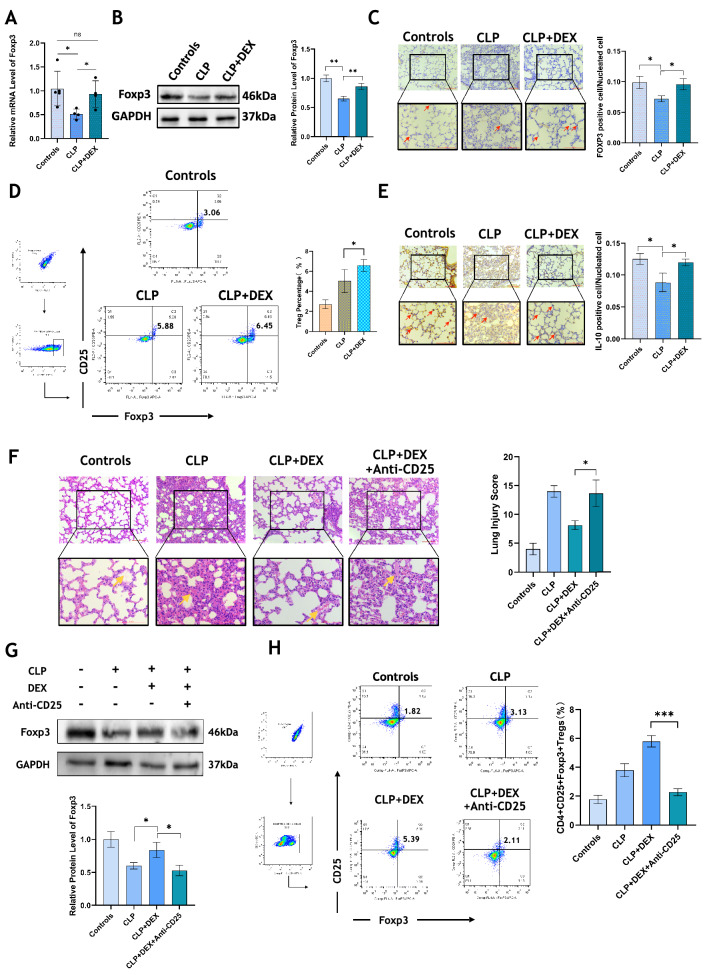
Lung tissues of mice were collected for qPCR and western blotting, and we found an obvious decline in Foxp3 expression in the CLP group than that in controls, and the mRNA and protein expression of Foxp3 in the lungs of mice underwent CLP surgery could be up-regulated by DEX apparently (p < 0.05, Fig. 2A, B). Immunohistochemistry also indicated that DEX promoted the expression of FOXP3 and IL-10 in the lung of CLP mice (p < 0.05, Fig. 2C, E). Mononuclear cells were extracted from the mouse spleen and the frequency of CD4+ CD25+ Foxp3+ Tregs was detected by flow cytometry. After DEX treatment, a significant increase in the proportion of Tregs was observed in CLP mice (p < 0.05, Fig. 2D). After administration of the anti-CD25 antibody, CD4+ CD25+ Foxp3+ Tregs in the spleen of mice in the DEX treatment group were partially depleted (p < 0.001, Fig. 2H), meanwhile, the expression of FOXP3 protein in lung tissue was also inhibited (p < 0.05, Fig. 2G). As shown in Fig. 2 F significant aggravation of lung tissue damage could be observed after Tregs depletion although DEX was used (p < 0.05).
Fig. 2.
DEX promotes Foxp3 expression and Treg differentiation. A The mRNA level of Foxp3 in lungs was detected by qRT-PCR. B Western blotting was used to measure the expression of FOXP3 protein in the lung tissue. C The expression of FOXP3 in lungs was determined by immunohistochemistry. D The proportion of CD4+ CD25+ Foxp3+ Treg cells in the spleen was detected by flow cytometry. E The IL-10 expression in lung tissue was detected by immunohistochemistry. All images were randomly acquired at 200 and 400 visual fields. F HE staining showed the lung injury of mice. The lung injury score was obtained according to the Mikawa method. All images were randomly obtained at 200 and 400 visual fields. The total histopathological scores were: Control (4 ± 0.89); CLP (14 ± 0.89); CLP + DEX (8 ± 0.63); CLP + DEX + Anti-CD25 (13.6 ± 1.74); G The FOXP3 protein expression level in lungs of controls, CLP, CLP + DEX, CLP + DEX + anti-CD25 Ab groups was detected by western blotting. H After anti-mouse CD25 antibody injection, the percentage of CD4+ CD25+ Foxp3+ Tregs in the spleen of mice in controls, CLP, CLP + DEX, CLP + DEX + anti-CD25 antibody groups was detected by flow cytometry. N = 5 in each group. All tests were repeated three times. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001
DEX up-regulates AMPK/SIRT1 signaling pathway in vivo, and blocking AMPK leads to increased inflammation and injury
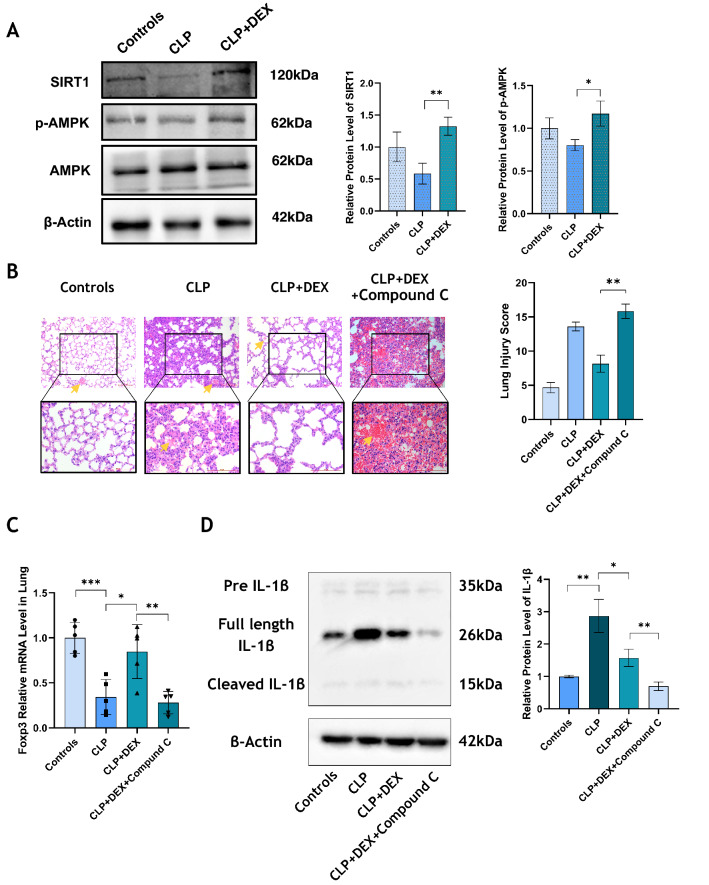
The protein expression level of SIRT1 and phosphorylation degree of AMPK in lung tissue of mice in the CLP group was detected using western blotting, we found that DEX treatment could restore the expression of p-AMPK and SIRT1, which was inhibited by CLP operation (p < 0.05, Fig. 3A). Compound C is a specific inhibitor of AMPK. We injected Compound C into DEX-treated CLP mice, resulting in significantly aggravated lung injury and increased lung histopathological score (p < 0.01, Fig. 3B). When the AMPK pathway was inhibited by Compound C, the mRNA expression of Foxp3 in the lungs was down-regulated markedly (p < 0.01, Fig. 3C). We also discovered that the relative protein level of IL-1β in the lung tissue of CLP mice was higher than in controls, while DEX reduced the expression of IL-1β. When Compound C was used to block the AMPK pathway, the expression level of IL-1 β was even lower (p < 0.05, Fig. 3D).
Fig. 3.
DEX up-regulates AMPK / SIRT1 signaling pathway in vivo, and blocking AMPK leads to increased inflammation and injury. A The level of SIRT1 and p-AMPK protein in lungs of mice in each group was detected by western blotting, and GAPDH was used as internal reference. B After Compound C (a specific inhibitor of AMPK) was used to intervene in mice, the degree of lung injury of mice in Controls, CLP, CLP + DEX and CLP + DEX + Compound C groups was assessed by using HE staining, and the lung pathological injury score was calculated according to the Mikawa method. All images were randomly obtained at 200 and 400 visual fields. The total histopathological scores were: Control (4.7 ± 0.6); CLP (13.6 ± 0.49); CLP + DEX (8.2 ± 1.03); CLP + DEX + Compound C (15.8 ± 0.81); C The mRNA level of Foxp3 in lungs was detected by qPCR. D IL-1β was detected by Western blotting. N = 5 in each group. All tests were repeated three times. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001
DEX facilitates the differentiation from naive CD4+T cells to Tregs in vitro.
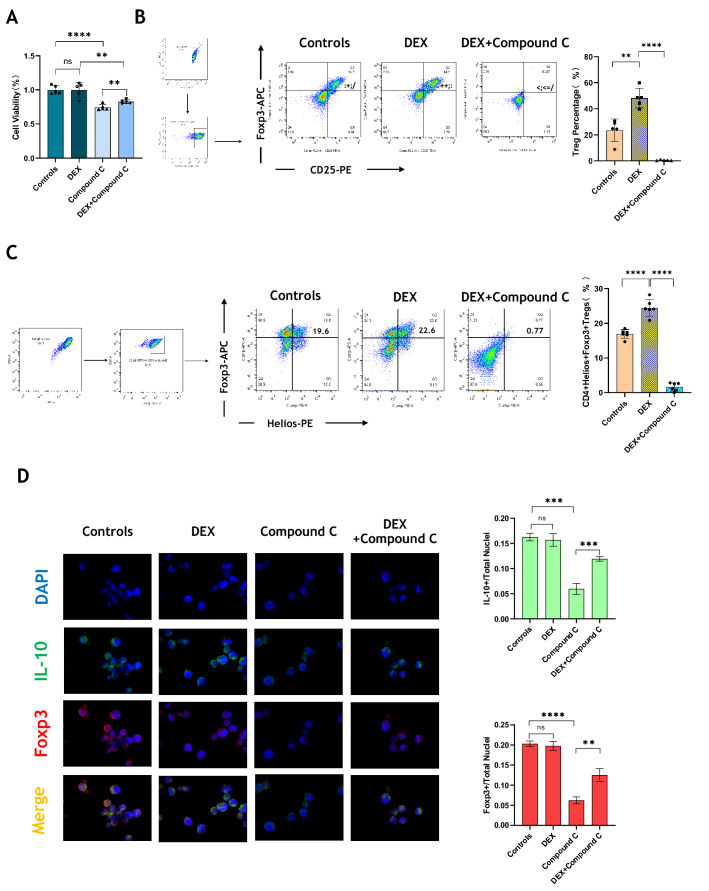
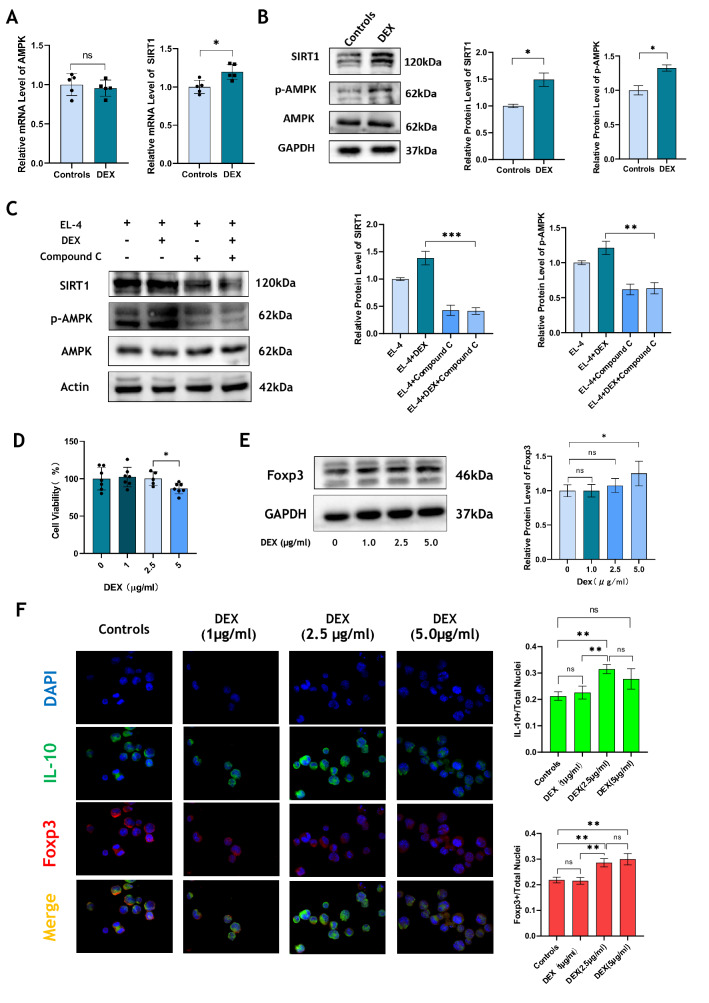
We first verified the effects of DEX and Compound C on the proliferation of EL-4 cells. We found that using Compound C (1 μM) to block AMPK could inhibit cell viability prominently. Although DEX had no effect by a single use, while it could offset against the inhibitory effect of Compound C partly (p < 0.01, Fig. 4A). Naïve CD4+ T cells were extracted and purified from mouse spleen. DEX (1 μg/mL) and Compound C (100 nM) were given under Treg polarization conditions. DEX increased the frequency of CD4+ CD25+ Foxp3+ Tregs, while Compound C almost inhabited it completely (p < 0.05, Fig. 4B). We also paid attention to the expression of Helios. DEX up-regulated the proportion of CD4+ Helios+ Foxp3+ Tregs, which decreased apparently in the Compound C treatment group (p < 0.0001, Fig. 4C). Immunofluorescence detection showed that DEX at a concentration of 1 μg/mL did not seem to affect the proportion of FOXP3+ or IL-10+ EL-4 cells, but when Compound C inhibited their expression, DEX intervention recovered the production of FOXP3 and IL-10 (p < 0.01, Fig. 4D).
Fig. 4.
DEX facilitates the differentiation from naive CD4 + T cells to Tregs in vitro. A Cell Counting kit-8 (CCK8) was used to detect the effect of DEX and Compound C on the proliferation of EL-4 cells. B Naive CD4+ T cells were extracted and purified from spleen. After cultured under Treg polarization for 72 h, the frequency of CD4+ CD25+ Foxp3+ Tregs in Controls, DEX, Compound C and DEX + Compound C groups was detected by flow cytometry. C The frequency of CD4+ Helios+ Foxp3+ Tregs in each group was detected by flow cytometry. D The expression of FOXP3 and IL-10 in EL-4 cells was detected by immunofluorescence. N = 5 in each group. All tests were repeated three times. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001
DEX up-regulates AMPK/SIRT1 signaling pathway in vitro, also enhances Foxp3 expression in T cells in a concentration-dependent manner.
We extracted PBMCs from sepsis patients and intervened PBMCs with DEX (1 μg/mL) for 24 h. It was found that DEX could apparently increase the mRNA expression of SIRT1 (p < 0.05), while the mRNA level of AMPK had no significant differences (Fig. 5A). Different from the control group, a notable increase of SIRT1 and p-AMPK protein levels in the DEX treatment group was observed (p < 0.05, Fig. 5B). However, Compound C could inhibit the production of SIRT1 and p-AMPK, and even the addition of DEX in the Compound C treatment group could not restore the expression level of SIRT1 and p-AMPK (p < 0.01, Fig. 5C). To explore whether the effects of DEX on the expression of FOXP3 are dose-dependent, we used DEX to treat EL-4 cells at the concentrations of 0, 1.0, 2.5, and 5.0 (μg/mL), respectively. Although a high concentration of DEX could inhibit the proliferation of EL-4 cells (Fig. 5D), however, a clear direct concentration-dependent relationship between DEX and FOXP3 expression in EL-4 cells was observed (Fig. 5E, F).
Fig. 5.
DEX up-regulates AMPK/SIRT1 signaling pathway in vitro, also enhanced Foxp3 expression in T cells in a concentration-dependent manner. A PBMCs from patients with sepsis were extracted and treated with DEX for 24 h. The mRNA level of AMPK and SIRT1 was detected by qRT-PCR. B The level of AMPK, p-AMPK and SIRT1 protein in EL-4 cells before and after DEX treatment was detected by Western blotting. C After Compound C was given to intervene EL-4 cells under DEX treatment condition, the protein level of AMPK, p-AMPK and SIRT1 was detected by Western blotting. D Cell Counting kit-8 (CCK8) was used to measure the cell viability under different concentration of DEX. E Western blotting was used to detect the expression of FOXP3 protein under different concentration of DEX. F The level of FOXP3 and IL-10 was detected by immunofluorescence under different concentration of DEX. N = 5 in each group. All tests were repeated three times. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001
There is a stable molecular docking between DEX and Foxp3
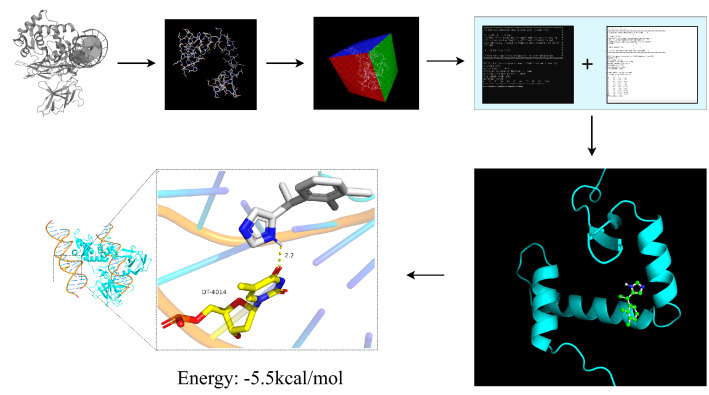
Download the pdb file of FOXP3 from the PDB database (https://www.rcsb.org/) and the SDF file of DEX was got from the PubChem database (https://pubchem.ncbi.nlm.nih.gov/). First, use PyMOL to convert the format of SDF file, then use autodocktools 1.5.6 software for molecular docking, and finally use PyMOL to beautify the results. From the result, the binding site of DEX and Foxp3 is dt-4014. Through hydrogen bond interaction, the free binding energy is—5.5 kcal / mol (Fig. 6), and the free binding energy is less than—6 (kcal/mol), which is relatively stable.
Fig. 6.
Molecular docking between DEX and Foxp3, the docking free energy is − 5.5 kcal/mol
Discussion
ARDS is a clinically heterogeneous syndrome, which can cause serious pulmonary diffuse inflammatory changes. It is a major cause of death in the ICUs (Matthay et al. 2019). Despite the continuous improvement of treatment, the mortality of ARDS remains high (Matthay et al. 2020; Ramanathan et al. 2020; Yadav et al. 2017). At present, we have recognized that the nature of ARDS is excessive, uncontrolled immune inflammatory response. The immune regulation imbalance and cytokine storm are the key links to this syndrome. Therefore, screening for inflammatory markers such as cytokines (like IL-33, IL-6, TNF-α), sRAGE in plasma, and Ang-2, endothelial injury factors (vWF) may help us to identify ARDS phenotypes early (Jabaudon et al. 2021). In addition, it provides assistance in anti-inflammatory interventions as soon as possible, which may improve the prognosis of ARDS (Fajgenbaum and June 2020; Henderson et al. 2020).
ARDS impairs the ability of the lungs to oxygenate. Reducing oxygen consumption is key to a successful strategy for ARDS (Marini 2015). The management of sedation and analgesia in patients with ARDS is extremely important, especially in patients needed mechanical ventilation (Chanques et al. 2020). It could reduce the oxygen consumption of ARDS. A cohort study systematically showed that the risk of death was reduced in patients with ARDS treated with DEX, and it may be the preferred sedative (Hu et al. 2021). Furthermore, the progression of hypoxemia is associated with pulmonary as well as systemic inflammatory responses. DEX could reduce inflammation and the mortality of sepsis animal models, possibly because it enhanced the activity of the immune system, but the reduced systemic response and the concentration of proinflammatory cytokines (TNF-α, IL-1β, IL-6)(Dardalas et al. 2019). Previous research pointed out that DEX may exert an anti-inflammatory function in ALI through multiple complex molecular mechanisms, including improving epithelial sodium channel (ENaC) (Jiang et al. 2021), upregulating tumor necrosis factor-α-induced protein-8-like 2 (TIPE2)(Kong et al. 2020), and activating the NLRP3 inflammasome in alveolar macrophages (Y. Chen et al. 2020b, a). We established an ALI model induced by CLP, and then DEX was used for treatment. We found that DEX can indeed alleviate lung injury and reduce lung and systemic inflammatory response in CLP mice. In addition, it can reduce the expression of MPO and IL-17 in lung tissue and down-regulates the level of inflammatory cytokines like TNF-α, IL-6 in the serum (Fig. 1). It suggests that DEX has great anti-inflammatory and immunomodulatory effects in the occurrence of ALI. The release of inflammatory factors is related to immune cell activation. Then does the anti-inflammatory molecular mechanism of DEX in ARDS associated with some special immune cell.
CD4+ CD25+ Foxp3+ Tregs is a special subset of CD4+ T cells with an immunosuppressive function that directly or indirectly limits ALI-inflicted tissue damage through a variety of mechanisms. Tregs play a pivotal role in ALI development (Lin et al. 2018). Tregs could exert their immunosuppressive function to prevent from activated immune response via three main mechanisms: direct contact and killing cytotoxic lymphocytes, inhibition of cytokine secretion by cytotoxic cells, and direct generation of immunoregulatory cytokines (Askenasy et al. 2008; Deng et al. 2019; Mikami et al. 2020). Foxp3+ Tregs promote lung epithelial proliferation (Mock et al. 2014), and inhibiting its DNA methyltransferase can accelerate the resolution of lung inflammation (Singer et al. 2015). In pulmonary inflammation, Treg depletion might impair the reciprocal conversion between Treg, Th1, and Th17 immune cells and result in decreased Th1 and Th17 immune responses related to tissue repair (Liu et al. 2015; Tan et al. 2019). When ARDS was in its early stages, the ratio of Th17 to Treg cells had been regarded as an important risk indicator (Yu et al. 2015). One study showed that DEX increases the differentiation of Tregs, which is related to attenuating proinflammatory response (Y. Wang et al. 2021a, b). In our research, we found that DEX could increase the proportion of Tregs in the spleen of CLP mice (Fig. 2D). On the other hand, Foxp3 is a pivotal transcription factor specifically expressed by Tregs, which maintains the phenotypic stability and immune regulation function of Tregs, especially its suppressive activity (Barbi et al. 2014; Ohkura and Sakaguchi, 2020). In our experiment, the expression of Foxp3 in the lung was up-regulated after DEX treatment (Fig. 2A, B, C, and supplementary figure). Simultaneously, we detected the level of cytokines IL‐10 in the lung, its expression was up-regulated after DEX treatment (Fig. 2E, and supplementary figure). The anti-inflammatory cytokine IL-10 plays an important role in ARDS (Kapur et al. 2017). In a pro-inflammatory cytokine-rich microenvironment, this cytokine is secreted by Tregs and plays a critical role in promoting the differentiation of Th17 cells (Rubtsov et al. 2008; Tan et al. 2019). Therefore, DEX has excellent anti-inflammatory activity in improving ALI, and it might promote Tregs, which is a potential molecular therapeutic target for ARDS. CD25 on the cell surface is indispensable for Tregs to develop immune tolerance (Ohkura and Sakaguchi 2020). To verify the above conjecture, we injected anti-mouse CD25 antibody into mice intraperitoneally, then detected the spleen and lung. Depleting Tregs (by injecting anti-mouse CD25 antibody) interfered with the remission of inflammation and the repair of the impaired pulmonary epithelium (Fig. 2F). Although DEX was given at the same time, the damage caused by Treg depletion could not be reversed (Fig. 2F). Moreover, the frequency of Treg cells in the spleen of CLP mice decreased almost by half after injection of anti-CD25 antibody. Even if DEX was used again, it was difficult to restore the proportion of Tregs, which were depleted by the anti-CD25 antibody (Fig. 2H). Similar results were obtained when we use western blotting to detect the FOXP3 protein expression level in lung tissue (Fig. 2G). These results show that the protective effect of DEX on ARDS was exerted by promoting Tregs, and the anti-inflammatory effect of DEX was almost lost when Treg was inhibited.
Mature CD4+ T cells could preserve and show their differentiation plasticity under specific conditions (Liu et al. 2015). Our previous research found that promoting the differentiation of naïve CD4+ T cells into Tregs can reduce the severity of ALI and uncontrolled inflammation (Chai et al. 2020; Xie et al. 2021). To further clarify the effect of DEX on Tregs, naïve CD4+ T cells were extracted from mouse spleen and treated with DEX in vitro. Flow cytometry showed that DEX could significantly facilitate the differentiation of naive CD4+ T cells into Tregs (Fig. 4B). Helios is regarded as another functional marker of Tregs, which is expressed in 60–70% of Tregs in mice and humans (Thornton and Shevach 2019). Helios+ and Helios− Tregs subpopulations are different in phenotypic stability and function. Helios also seems to play a positive role in the expression of Foxp3; moreover, the coordination of Helios and Foxp3 can enhance the immunosuppressive activity of Treg (Seng et al. 2020; Thornton et al. 2019). After treating naïve CD4+ T cells with DEX and culturing them for 72 h, we detected the expression of Helios in each group by flow cytometry. It showed that DEX intervention could markedly up-regulate the percentage of Helios+ Foxp3+ Tregs compared with controls (Fig. 4C). In summary, the therapeutic mechanism of DEX in ARDS through Tregs includes three main aspects: promote the differentiation of Tregs, increase the number of Tregs, and enhance the function of Tregs.
Tregs development depends on the key activities of Foxp3, the master-switch transcription factor (Piccirillo 2020). Altering the metabolic pathways and generation of metabolites can regulate Tregs (Lu et al. 2021; W. Wang et al. 2021a, b). Based on the observations in colonic tissues, it was found that butyrate derived from commensal microbes induces Treg differentiation (Braun 2021; Furusawa et al. 2013). Tregs can transform through different main energy metabolism pathways, including glycolysis, FAO, or OXPHOS according to their state changes (Furusawa et al. 2013). With activation, Drastic biosynthesis increases in T lymphocytes must be slowed down depending on the activity of AMP-activated protein kinase (AMPK), to avoid excessive consumption of cellular ATP (Andris and Leo 2015; Braun, 2021). SIRT1 is an NAD+-dependent deacetylase, whose activity is closely related to cell aging (C. Chen et al. 2020b, a). Furthermore, SIRT1 can also regulate many cellular processes such as inflammation, oxidative stress, and apoptosis (Hwang et al. 2013; Ong and Ramasamy 2018; Singh and Ubaid 2020). It is found that targeting SIRT1 can promote Treg differentiation and maintain its stability, regulate the balance between Th17 and Tregs, and play a protective role in a variety of autoimmune diseases (Daenthanasanmak et al. 2019; Gao et al. 2021; Lv et al. 2018). SIRT1 and AMPK are not independent of each other. AMPK enhances SIRT1 activity by increasing intracellular NAD+ levels, resulting in deacetylation and regulation of the activity of downstream SIRT1 targets (Cantó et al. 2009). AMPK/SIRT1 signaling pathway is very important for Treg differentiation. Studies have found that melatonin prevents Th17/Treg imbalance by activating the AMPK/SIRT1 pathway, thereby improving necrotizing enterocolitis (Ma et al. 2020), and methylene blue could improve autoimmune encephalomyelitis by regulating the AMPK/SIRT1 signaling pathway and Th17/Treg immune response in animal experiment (J. Wang et al. 2016). Obviously, DEX can enhance the phosphorylation of AMPK and the expression of SIRT1 in the lung tissue of CLP mice and PBMCs (Figs. 3A, 5A, B). To explore whether DEX mediates Treg differentiation through the AMPK/SIRT1 signaling pathway, we treated EL-4 cells with Compound C (a specific inhibitor of AMPK) in vitro to block the AMPK signal pathway. The results showed blocking the AMPK signal pathway by Compound C can significantly reduce cell proliferation activity in vitro (Fig. 4A). Then, Compound C could decrease the expression level of SIRT1 and p-AMPK protein (Fig. 5C). The promoting effect of DEX on Treg differentiation and function maintenance was significantly inhibited by Compound C (Fig. 4B, C). Meanwhile, in vivo experiments confirmed that the positive effect of DEX on acute lung injury in CLP mice was eliminated when Compound C was used (Fig. 3 B). It suggests that DEX plays its protective role by promoting the differentiation of Tregs via AMPK/SIRT1 pathway. AMPK is indispensable for energy stress and metabolic regulation in cells (Herzig and Shaw 2018). Notable, unusual IL-1β decreased expression was observed in the Compound C treated group, which may be related to abnormal intracellular metabolism (Fig. 3D). The potential mechanism is worthy of further exploration. On the other hand, low-concentration DEX seems not to improve FOXP3 expression in EL-4 cells, there is a concentration-dependent effect of DEX on promoting FOXP3 expression (Fig. 5 E, F). Network pharmacology approaches as well as bioinformatics revealed a possible stable docking between DEX and Foxp3 (Fig. 6). It may help explain the concentration-dependent effect of DEX on Foxp3 expression, while the exact mechanism needs further exploration.
In summary, DEX can alleviate lung injury, and reduce systemic inflammatory response by facilitating the differentiation from naïve CD4+ T cells into CD4+ CD25+ Foxp3+ Tregs. The mechanism may be related to DEX activating downstream SIRT1 by activating AMPK phosphorylation, promoting the development and differentiation of Tregs, and maintaining their functional phenotype.
Conclusion
DEX could improve ARDS/ALI by facilitating the differentiation from naïve CD4+ T cells into Tregs via activating the AMPK/SIRT1 pathway.
Acknowledgements
This research was supported by the project of Chongqing Postgraduate Research and Innovation (CYS21215, to ZT Zhang and F Xu), National Natural Science Foundation grants of China (8217216, to F Xu), the Joint project of Chongqing Municipal Science and Technology Bureau and Chongqing Health Commission (2020GDRC001 to F Xu), and Clinical Medicine Postgraduate Joint Training Base of Chongqing Medical University-the First Affiliated Hospital of Chongqing Medical University (lpjd202001), and the project of Chongqing talents(cstc2022ycjh-bgzxm0131 to F Xu).
Author contribution
The experimental design was completed by Z-tZ, S-hL, and FX. Z-tZ, KX, R-jL, D-yZ, Z-wH and K-fL are responsible for all experimental operations and data analysis. The manuscript was jointly finished by Z-tZ and FX then finally revised by FX.
Funding
The Project of Chongqing Postgraduate Research and Innovation, (CYS21215): Zheng-tao Zhang; National Natural Science Foundation grants of China, (8217216): Fang Xu; The Joint project of Chongqing Municipal Science and Technology Bureau and Chongqing Health Commission, (2020GDRC001): Fang Xu; Clinical Medicine Postgraduate Joint Training Base of Chongqing Medical University-the First Affiliated Hospital of Chongqing Medical University, (lpjd202001): Not applicable; The project of Chongqing talents, (cstc2022ycjh-bgzxm0131): Fang Xu
Data availability
There is no associated data in this manuscript.
Declarations
Conflict of interest
All authors declare that there is no conflict of interest.
Ethical approval
The protocol was approved by the Ethics Committee of the First Affiliated Hospital of Chongqing Medical University. The number is Lot 2020–850.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Andris F, Leo O. AMPK in lymphocyte metabolism and function. Int Rev Immunol. 2015;34(1):67–81. doi: 10.3109/08830185.2014.969422. [DOI] [PubMed] [Google Scholar]
- Askenasy N, Kaminitz A, Yarkoni S. Mechanisms of T regulatory cell function. Autoimmun Rev. 2008;7(5):370–375. doi: 10.1016/j.autrev.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Barbi J, Pardoll D, Pan F. Treg functional stability and its responsiveness to the microenvironment. Immunol Rev. 2014;259(1):115–139. doi: 10.1111/imr.12172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun MY. The natural history of T cell metabolism. Int J Mol Sci. 2021 doi: 10.3390/ijms22136779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt Y, Kurdowska A, Allen TC. Acute lung injury: a clinical and molecular review. Arch Pathol Lab Med. 2016;140(4):345–350. doi: 10.5858/arpa.2015-0519-RA. [DOI] [PubMed] [Google Scholar]
- Cantó C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Auwerx J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458(7241):1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai YS, Chen YQ, Lin SH, Xie K, Wang CJ, Yang YZ, Xu F. Curcumin regulates the differentiation of naïve CD4+ T cells and activates IL-10 immune modulation against acute lung injury in mice. Biomed Pharmacother. 2020;125:109946. doi: 10.1016/j.biopha.2020.109946. [DOI] [PubMed] [Google Scholar]
- Chanques G, Constantin JM, Devlin JW, Ely EW, Fraser GL, Gélinas C, Kress JP. Analgesia and sedation in patients with ARDS. Intensive Care Med. 2020;46(12):2342–2356. doi: 10.1007/s00134-020-06307-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Zhou M, Ge Y, Wang X. SIRT1 and aging related signaling pathways. Mech Ageing Dev. 2020;187:111215. doi: 10.1016/j.mad.2020.111215. [DOI] [PubMed] [Google Scholar]
- Chen Y, Huang Y, Xiong B, Luo H, Song X. Dexmedetomidine ameliorates renal ischemia reperfusion-mediated activation of the NLRP3 inflammasome in alveolar macrophages. Gene. 2020;758:144973. doi: 10.1016/j.gene.2020.144973. [DOI] [PubMed] [Google Scholar]
- Daenthanasanmak A, Iamsawat S, Chakraborty P, Nguyen HD, Bastian D, Liu C, Yu XZ. Targeting Sirt-1 controls GVHD by inhibiting T-cell allo-response and promoting Treg stability in mice. Blood. 2019;133(3):266–279. doi: 10.1182/blood-2018-07-863233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dardalas I, Stamoula E, Rigopoulos P, Malliou F, Tsaousi G, Aidoni Z, Pourzitaki C. Dexmedetomidine effects in different experimental sepsis in vivo models. Eur J Pharmacol. 2019;856:172401. doi: 10.1016/j.ejphar.2019.05.030. [DOI] [PubMed] [Google Scholar]
- Deng G, Song X, Fujimoto S, Piccirillo CA, Nagai Y, Greene MI. Foxp3 post-translational modifications and treg suppressive activity. Front Immunol. 2019;10:2486. doi: 10.3389/fimmu.2019.02486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fajgenbaum DC, June CH. Cytokine Storm. N Engl J Med. 2020;383(23):2255–2273. doi: 10.1056/NEJMra2026131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan E, Brodie D, Slutsky AS. Acute respiratory distress syndrome: advances in diagnosis and treatment. JAMA. 2018;319(7):698–710. doi: 10.1001/jama.2017.21907. [DOI] [PubMed] [Google Scholar]
- Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Ohno H. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504(7480):446–450. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- Gao X, Liu W, Gao P, Li S, Chen Z, Ma F. Melatonin-induced lncRNA LINC01512 prevents Treg/Th17 imbalance by promoting SIRT1 expression in necrotizing enterocolitis. Int Immunopharmacol. 2021;96:107787. doi: 10.1016/j.intimp.2021.107787. [DOI] [PubMed] [Google Scholar]
- Halter S, Aimade L, Barbié M, Brisson H, Rouby JJ, Langeron O, Monsel A. T regulatory cells activation and distribution are modified in critically ill patients with acute respiratory distress syndrome: a prospective single-centre observational study. Anaesth Crit Care Pain Med. 2020;39(1):35–44. doi: 10.1016/j.accpm.2019.07.014. [DOI] [PubMed] [Google Scholar]
- Henderson LA, Canna SW, Schulert GS, Volpi S, Lee PY, Kernan KF, Nigrovic PA. On the alert for cytokine storm: immunopathology in COVID-19. Arthritis Rheumatol. 2020;72(7):1059–1063. doi: 10.1002/art.41285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentschel R, Bohlin K, van Kaam A, Fuchs H, Danhaive O. Surfactant replacement therapy: from biological basis to current clinical practice. Pediatr Res. 2020;88(2):176–183. doi: 10.1038/s41390-020-0750-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzig S, Shaw RJ. AMPK: guardian of metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol. 2018;19(2):121–135. doi: 10.1038/nrm.2017.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu AM, Zhong XX, Li Z, Zhang ZJ, Li HP. Comparative effectiveness of midazolam, propofol, and Dexmedetomidine in patients With or at risk for acute respiratory distress syndrome: a propensity score-matched cohort study. Front Pharmacol. 2021;12:614465. doi: 10.3389/fphar.2021.614465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang JW, Yao H, Caito S, Sundar IK, Rahman I. Redox regulation of SIRT1 in inflammation and cellular senescence. Free Radic Biol Med. 2013;61:95–110. doi: 10.1016/j.freeradbiomed.2013.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabaudon M, Blondonnet R, Ware LB. Biomarkers in acute respiratory distress syndrome. Curr Opin Crit Care. 2021;27(1):46–54. doi: 10.1097/mcc.0000000000000786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Xia M, Xu J, Huang Q, Dai Z, Zhang X. Dexmedetomidine alleviates pulmonary edema through the epithelial sodium channel (ENaC) via the PI3K/Akt/Nedd4-2 pathway in LPS-induced acute lung injury. Immunol Res. 2021;69(2):162–175. doi: 10.1007/s12026-021-09176-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalbasi A, Ribas A. Tumour-intrinsic resistance to immune checkpoint blockade. Nat Rev Immunol. 2020;20(1):25–39. doi: 10.1038/s41577-019-0218-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur R, Kim M, Aslam R, McVey MJ, Tabuchi A, Luo A, Semple JW. T regulatory cells and dendritic cells protect against transfusion-related acute lung injury via IL-10. Blood. 2017;129(18):2557–2569. doi: 10.1182/blood-2016-12-758185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong Q, Wu X, Qiu Z, Huang Q, Xia Z, Song X. Protective Effect of Dexmedetomidine on Acute lung injury via the upregulation of tumour necrosis factor-α-induced protein-8-like 2 in septic mice. Inflammation. 2020;43(3):833–846. doi: 10.1007/s10753-019-01169-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurniawan H, Soriano-Baguet L, Brenner D. Regulatory T cell metabolism at the intersection between autoimmune diseases and cancer. Eur J Immunol. 2020;50(11):1626–1642. doi: 10.1002/eji.201948470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Chen Q, He X, Alam A, Ning J, Yi B, Gu J. Dexmedetomidine attenuates lung apoptosis induced by renal ischemia-reperfusion injury through α(2)AR/PI3K/Akt pathway. J Transl Med. 2018;16(1):78. doi: 10.1186/s12967-018-1455-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Wu H, Wang C, Xiao Z, Xu F. Regulatory T cells and acute lung injury: cytokines, uncontrolled inflammation, and therapeutic implications. Front Immunol. 2018;9:1545. doi: 10.3389/fimmu.2018.01545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HP, Cao AT, Feng T, Li Q, Zhang W, Yao S, Cong Y. TGF-β converts Th1 cells into Th17 cells through stimulation of Runx1 expression. Eur J Immunol. 2015;45(4):1010–1018. doi: 10.1002/eji.201444726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Liang Y, Meng H, Zhang A, Zhao J, Zhang C. Metabolic controls on epigenetic reprogramming in regulatory T Cells. Front Immunol. 2021;12:728783. doi: 10.3389/fimmu.2021.728783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv Q, Wang K, Qiao S, Yang L, Xin Y, Dai Y, Wei Z. Norisoboldine, a natural AhR agonist, promotes Treg differentiation and attenuates colitis via targeting glycolysis and subsequent NAD(+)/SIRT1/SUV39H1/H3K9me3 signaling pathway. Cell Death Dis. 2018;9(3):258. doi: 10.1038/s41419-018-0297-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma F, Hao H, Gao X, Cai Y, Zhou J, Liang P, Li S. Melatonin ameliorates necrotizing enterocolitis by preventing Th17/Treg imbalance through activation of the AMPK/SIRT1 pathway. Theranostics. 2020;10(17):7730–7746. doi: 10.7150/thno.45862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahida RY, Matsumoto S, Matthay MA. Extracellular vesicles: a new frontier for research in acute respiratory distress syndrome. Am J Respir Cell Mol Biol. 2020;63(1):15–24. doi: 10.1165/rcmb.2019-0447TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marini, J. J. (2015). Advances in the support of respiratory failure: putting all the evidence together. Crit Care, 19 Suppl 3(Suppl 3), S4. 10.1186/cc14722 [DOI] [PMC free article] [PubMed]
- Matthay MA, Zemans RL, Zimmerman GA, Arabi YM, Beitler JR, Mercat A, Calfee CS. Acute respiratory distress syndrome. Nat Rev Dis Primers. 2019;5(1):18. doi: 10.1038/s41572-019-0069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthay MA, Aldrich JM, Gotts JE. Treatment for severe acute respiratory distress syndrome from COVID-19. Lancet Respir Med. 2020;8(5):433–434. doi: 10.1016/s2213-2600(20)30127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer NJ, Gattinoni L, Calfee CS. Acute respiratory distress syndrome. Lancet. 2021;398(10300):622–637. doi: 10.1016/s0140-6736(21)00439-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikami N, Kawakami R, Sakaguchi S. New Treg cell-based therapies of autoimmune diseases: towards antigen-specific immune suppression. Curr Opin Immunol. 2020;67:36–41. doi: 10.1016/j.coi.2020.07.004. [DOI] [PubMed] [Google Scholar]
- Mikawa K, Nishina K, Takao Y, Obara H. ONO-1714, a nitric oxide synthase inhibitor, attenuates endotoxin-induced acute lung injury in rabbits. Anesth Analg. 2003;97(6):1751–1755. doi: 10.1213/01.Ane.0000086896.90343.13. [DOI] [PubMed] [Google Scholar]
- Mock JR, Garibaldi BT, Aggarwal NR, Jenkins J, Limjunyawong N, Singer BD, D'Alessio FR. Foxp3+ regulatory T cells promote lung epithelial proliferation. Mucosal Immunol. 2014;7(6):1440–1451. doi: 10.1038/mi.2014.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mock JR, Dial CF, Tune MK, Gilmore RC, O'Neal WK, Dang H, Doerschuk CM. Impact of regulatory T cells on Type 2 alveolar epithelial cell transcriptomes during resolution of acute lung injury and contributions of IFN-γ. Am J Respir Cell Mol Biol. 2020;63(4):464–477. doi: 10.1165/rcmb.2019-0399OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mock, J. R., Dial, C. F., Tune, M. K., Norton, D. L., Martin, J. R., Gomez, J. C., Doerschuk, C. M. (2019). transcriptional analysis of Foxp3+ Tregs and functions of two identified molecules during resolution of ALI. JCI Insight, 4(6). 10.1172/jci.insight.124958 [DOI] [PMC free article] [PubMed]
- Nadeem A, Al-Harbi NO, Ahmad SF, Al-Harbi MM, Alhamed AS, Alfardan AS, Albassam H. Blockade of interleukin-2-inducible T-cell kinase signaling attenuates acute lung injury in mice through adjustment of pulmonary Th17/Treg immune responses and reduction of oxidative stress. Int Immunopharmacol. 2020;83:106369. doi: 10.1016/j.intimp.2020.106369. [DOI] [PubMed] [Google Scholar]
- Ohkura N, Sakaguchi S. Transcriptional and epigenetic basis of Treg cell development and function: its genetic anomalies or variations in autoimmune diseases. Cell Res. 2020;30(6):465–474. doi: 10.1038/s41422-020-0324-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong ALC, Ramasamy TS. Role of Sirtuin1-p53 regulatory axis in aging, cancer and cellular reprogramming. Ageing Res Rev. 2018;43:64–80. doi: 10.1016/j.arr.2018.02.004. [DOI] [PubMed] [Google Scholar]
- Piccirillo CA. Transcriptional and translational control of Foxp3(+) regulatory T cell functional adaptation to inflammation. Curr Opin Immunol. 2020;67:27–35. doi: 10.1016/j.coi.2020.07.006. [DOI] [PubMed] [Google Scholar]
- Ramanathan K, Antognini D, Combes A, Paden M, Zakhary B, Ogino M, Shekar K. Planning and provision of ECMO services for severe ARDS during the COVID-19 pandemic and other outbreaks of emerging infectious diseases. Lancet Respir Med. 2020;8(5):518–526. doi: 10.1016/s2213-2600(20)30121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Slutsky AS. Acute respiratory distress syndrome: the berlin definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- Reilly JP, Calfee CS, Christie JD. Acute respiratory distress syndrome phenotypes. Semin Respir Crit Care Med. 2019;40(1):19–30. doi: 10.1055/s-0039-1684049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg L, Traube C. Sedation strategies in children with pediatric acute respiratory distress syndrome (PARDS) Ann Transl Med. 2019;7(19):509. doi: 10.21037/atm.2019.09.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J, Castelli L, Ye X, Rudensky AY. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28(4):546–558. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- Seng A, Krausz KL, Pei D, Koestler DC, Fischer RT, Yankee TM, Markiewicz MA. Coexpression of FOXP3 and a Helios isoform enhances the effectiveness of human engineered regulatory T cells. Blood Adv. 2020;4(7):1325–1339. doi: 10.1182/bloodadvances.2019000965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Yu T, Song K, Du S, He S, Hu X, Yu J. Dexmedetomidine ameliorates endotoxin-induced acute lung injury in vivo and in vitro by preserving mitochondrial dynamic equilibrium through the HIF-1a/HO-1 signaling pathway. Redox Biol. 2021;41:101954. doi: 10.1016/j.redox.2021.101954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer BD, Mock JR, Aggarwal NR, Garibaldi BT, Sidhaye VK, Florez MA, D’Alessio FR. Regulatory T cell DNA methyltransferase inhibition accelerates resolution of lung inflammation. Am J Respir Cell Mol Biol. 2015;52(5):641–652. doi: 10.1165/rcmb.2014-0327OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh V, Ubaid S. Role of silent information regulator 1 (SIRT1) in regulating oxidative stress and inflammation. Inflammation. 2020;43(5):1589–1598. doi: 10.1007/s10753-020-01242-9. [DOI] [PubMed] [Google Scholar]
- Sun J, Zheng S, Yang N, Chen B, He G, Zhu T. Dexmedetomidine inhibits apoptosis and expression of COX-2 induced by lipopolysaccharide in primary human alveolar epithelial type 2 cells. Biochem Biophys Res Commun. 2019;517(1):89–95. doi: 10.1016/j.bbrc.2019.07.023. [DOI] [PubMed] [Google Scholar]
- Tan W, Zhang C, Liu J, Miao Q. Regulatory T cells promote pulmonary repair by modulating T helper cell immune responses in lipopolysaccharide-induced acute respiratory distress syndrome. Immunology. 2019;157(2):151–162. doi: 10.1111/imm.13060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson BT, Chambers RC, Liu KD. Acute respiratory distress syndrome. N Engl J Med. 2017;377(6):562–572. doi: 10.1056/NEJMra1608077. [DOI] [PubMed] [Google Scholar]
- Thornton AM, Shevach EM. Helios: still behind the clouds. Immunology. 2019;158(3):161–170. doi: 10.1111/imm.13115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton AM, Lu J, Korty PE, Kim YC, Martens C, Sun PD, Shevach EM. Helios(+) and Helios(-) Treg subpopulations are phenotypically and functionally distinct and express dissimilar TCR repertoires. Eur J Immunol. 2019;49(3):398–412. doi: 10.1002/eji.201847935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turan A, Duncan A, Leung S, Karimi N, Fang J, Mao G, Sessler DI. Dexmedetomidine for reduction of atrial fibrillation and delirium after cardiac surgery (DECADE): a randomised placebo-controlled trial. Lancet. 2020;396(10245):177–185. doi: 10.1016/s0140-6736(20)30631-0. [DOI] [PubMed] [Google Scholar]
- Wang K, Fu W. Transcriptional regulation of treg homeostasis and functional specification. Cell Mol Life Sci. 2020;77(21):4269–4287. doi: 10.1007/s00018-020-03534-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Zhao C, Kong P, Bian G, Sun Z, Sun Y, Li B. Methylene blue alleviates experimental autoimmune encephalomyelitis by modulating AMPK/SIRT1 signaling pathway and Th17/Treg immune response. J Neuroimmunol. 2016;299:45–52. doi: 10.1016/j.jneuroim.2016.08.014. [DOI] [PubMed] [Google Scholar]
- Wang W, Wang X, Lu S, Lv H, Zhao T, Xie G, Xu L. Metabolic disturbance and Th17/Treg Imbalance are associated with progression of gingivitis. Front Immunol. 2021;12:670178. doi: 10.3389/fimmu.2021.670178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Lei Y, Gu Y, Kong X, Bian Z, Ji F. Effect of dexmedetomidine on CD4+ T cells and programmed cell death protein-1 in postoperative analgesia: a prospective, randomized, controlled study. Minerva Anestesiol. 2021;87(4):423–431. doi: 10.23736/s0375-9393.20.14581-4. [DOI] [PubMed] [Google Scholar]
- Weerink MAS, Struys M, Hannivoort LN, Barends CRM, Absalom AR, Colin P. Clinical pharmacokinetics and pharmacodynamics of dexmedetomidine. Clin Pharmacokinet. 2017;56(8):893–913. doi: 10.1007/s40262-017-0507-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie K, Chai YS, Lin SH, Xu F, Wang CJ. Luteolin regulates the differentiation of regulatory T Cells and activates IL-10-dependent macrophage polarization against acute lung injury. J Immunol Res. 2021;2021:8883962. doi: 10.1155/2021/8883962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav H, Thompson BT, Gajic O. Fifty years of research in ARDS Is acute respiratory distress syndrome a preventable disease? Am J Respir Crit Care Med. 2017;195(6):725–736. doi: 10.1164/rccm.201609-1767CI. [DOI] [PubMed] [Google Scholar]
- Yu ZX, Ji MS, Yan J, Cai Y, Liu J, Yang HF, Zheng JX. The ratio of Th17/Treg cells as a risk indicator in early acute respiratory distress syndrome. Crit Care. 2015;19(1):82. doi: 10.1186/s13054-015-0811-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan D, Liu Z, Kaindl J, Maeda S, Zhao J, Sun X, Kobilka BK. Activation of the α(2B) adrenoceptor by the sedative sympatholytic dexmedetomidine. Nat Chem Biol. 2020;16(5):507–512. doi: 10.1038/s41589-020-0492-2. [DOI] [PubMed] [Google Scholar]
- Zhang Z. Dexmedetomidine for the treatment of acute lung injury: a fact or fiction? J Invest Surg. 2020;33(6):584–586. doi: 10.1080/08941939.2018.1542471. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wang X, Liu Z, Yu L. Dexmedetomidine attenuates lipopolysaccharide induced acute lung injury by targeting NLRP3 via miR-381. J Biochem Mol Toxicol. 2018;32(11):e22211. doi: 10.1002/jbt.22211. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Chen Y, Meng Z. Immunomodulation for Severe COVID-19 pneumonia: the state of the Art. Front Immunol. 2020;11:577442. doi: 10.3389/fimmu.2020.577442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Jia L, Yang HJ, Xue X, Xu WX, Cai JQ, Cao CC. Taurine enhances the protective effect of Dexmedetomidine on sepsis-induced acute lung injury via balancing the immunological system. Biomed Pharmacother. 2018;103:1362–1368. doi: 10.1016/j.biopha.2018.04.150. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
There is no associated data in this manuscript.