Abstract
A concentrated bacterial culture supernatant from the hemolytic Moraxella bovis strain UQV 148NF was used to immunize mice and generate monoclonal antibodies (MAbs). One, MAb G3/D7, neutralized the hemolytic activity of M. bovis and recognized a 94-kDa protein by Western blot analysis in hemolytic M. bovis strains representing each of the different fimbrial serogroups. Exposure of corneal epithelial cells to M. bovis concentrated culture supernatants demonstrated a role for an exotoxin in the pathogenesis of infectious bovine keratoconjunctivitis, while neutralization of hemolytic and cytotoxic activities by MAb G3/D7 implies that these activities are related or have common epitopes. The action of M. bovis hemolysin was further characterized in sheep erythrocyte preparations with a binding step and Ca2+ required for lysis to proceed, similar to the RTX family of bacterial exotoxins. Neutralization of lytic activity in vitro is evidence for the presence of M. bovis antigens, which may be capable of protecting cattle from the development of infectious bovine keratoconjunctivitis.
Moraxella bovis is a gram-negative bacterium implicated in the pathogenesis of infectious bovine keratoconjunctivitis (IBK), a disease causing significant economic losses in cattle industries worldwide (10, 35, 37). Strains of M. bovis must be fimbriated and hemolytic in cell culture to cause clinical disease, suggesting that these characteristics are important virulence traits. Fimbriae mediate attachment to corneal epithelium (28), while toxin production may cause corneal epithelial cell damage (16, 34).
The role of these virulence traits in disease implies the potential of these proteins as vaccine candidates. To date, most investigations have used fimbrial proteins in vaccine preparations, and both native and recombinant M. bovis fimbriae induce serogroup-specific protection (22, 23). However, there is increasing evidence that inclusion of fimbriae from all seven identified serogroups is necessary for protection, which may lead to antigenic competition (33). Therefore, the aim of this investigation was to characterize the hemolysin of M. bovis and assess its potential as an alternative vaccine candidate, as described for other bacterial hemolysins (30).
There has been limited characterization of the hemolysin of M. bovis with previous investigations associating hemolytic activity in cell-free preparations to fractions with molecular mass greater than 300 kDa (6). This has been attributed to its release in membrane-bound vesicles and association with cell-wall proteins and has foiled efforts to obtain purified toxin (3). However, several similarities have been observed between the activity of M. bovis hemolysin and the activity of recently documented family of pore-forming bacterial toxins, the RTX exotoxins (1, 4, 11, 15). These activities include pore formation in target-cell membranes and a role for Ca2+ in the lytic activity of M. bovis hemolysin (11). Members of the RTX toxin family include the secreted toxins of Escherichia coli, Pasteurella hemolytica, Actinobacillus pleuropneumoniae, and Actinobacillus actinomycetemcomitans (36, 39). Speculation that the hemolysin of M. bovis may be related to this toxin family has been supported by preliminary findings that a monoclonal antibody (MAb) recognizing E. coli hemolysin also recognizes a 100-kDa protein in hemolytic M. bovis cultures (16). However, the inclusion of the hemolysin of M. bovis in this toxin family has not been confirmed (21).
In addition to lytic action on erythrocytes, in vitro exposure of bovine corneal epithelial cells to culture supernatants of hemolytic M. bovis strains results in cell lysis (16, 19, 20). Although nonhemolytic M. bovis strains do not cause epithelial cell lysis, it is not known whether the loss of hemolytic phenotype alone is responsible for the observed loss of cytotoxicity. Consequently, a possible role for separate hemolytic and cytotoxic proteins in the pathogenesis of IBK has been debated (16, 19, 20).
Here, we report the generation of a MAb which neutralizes both the hemolytic and epithelial cytotoxic potential of M. bovis culture supernatants. This MAb was used to identify the putative hemolysin in cell-free extracts of hemolytic M. bovis strains by Western blot analysis and to investigate a role for Ca2+ in lytic activity of the hemolysin of M. bovis following exposure to erythrocytes.
MATERIALS AND METHODS
Bacterial strains and toxin preparation.
Seven field strains of hemolytic M. bovis representing the different fimbrial serogroups were used (serogroup A, strain 276; serogroup B, serogroup 3WO7; serogroup C, Dal2d; serogroup D, 593L; serogroup E, TAT 849; serogroup F, 218R; and serogroup G, Fleur 462 [27]). Two nonfimbriated M. bovis variants were also used, one hemolytic, UQV 148NF, and one nonhemolytic, Gordon 26L3 (3). To maximize hemolysin production, M. bovis cultures were grown in Mueller-Hinton broth (Oxoid, Basingstoke, Hertshire, United Kingdom) and were harvested in late log phase (6) with whole cells pelleted by centrifugation at 10,000 × g for 30 min at 4°C. Culture supernatants were volume concentrated by tangential ultrafiltration across a Minitan-S low-protein-binding cellulose membrane (Millipore, Sydney, Australia) with a 300-kDa cutoff as previously described (6), and the resulting concentrated culture supernatants (CCS) and filtrates (<300 kDa) were used in investigations. In some experiments, further concentration of CCS was achieved by subjecting samples to ultracentrifugation at 100,000 × g for 6 h at 4°C. Following ultracentrifugation, the resulting pellet contained >90% of hemolytic activity and was used in investigations, while the supernatant was discarded.
MAb production.
Three female BALB/c mice were immunized intraperitoneally on days 1, 28, and 42 with 9 μg of CCS from hemolytic M. bovis strain UQV 148NF. Splenocytes were harvested 3 days after the final immunization and were fused with P3-X63-Ag8-653 (X653) myeloma cells and selected in hypoxanthine-aminopterine-thymidine medium (Flow Laboratories, Sydney, Australia). Single-cell clones were obtained by limiting dilution, and antibodies were isotyped by using IsoStrip (Boehringer GmbH, Mannheim, Germany).
Enzyme-linked immunosorbent assay (ELISA) to determine MAb specificity.
Flat-bottom 96-well microtiter plates (Maxi-Sorp; Nunc A/S, Roskilde, Denmark) were coated with CCS (2.5 μg/ml in 50 mM carbonate buffer [pH 9.6]) from hemolytic M. bovis UQV 148NF and were incubated at 4°C overnight. Following blocking in 5% sodium caseinate in phosphate-buffered saline (PBS), hybridoma culture supernatants were added, and plates were incubated for 1 h at 37°C. Rabbit anti-murine immunoglobulin G (IgG)-alkaline phosphatase (Sigma Chemical Co., St. Louis, Mo.) was added, and plates were incubated for 1 h before the addition of substrate (p-nitrophenyl phosphate disodium [Sigma] dissolved in 1 M diethanolamine-HCl buffer [pH 9.8]) and measurement of absorbance at 405 nm. Plates were washed three times between each step by using PBS with 0.05% Tween 20. Controls included serum from CCS-immunized mice and culture supernatant from unfused X653 myeloma cell cultures.
Determination of hemolytic activity.
The hemolytic activity of CCS was assessed by using a hemolytic assay as previously described (3). Briefly, twofold dilutions of CCS were made in 96-well microtiter plates, and an equal volume of a 1% solution of fresh, defibrinated sheep erythrocytes in PBS (sRBC) was added. Plates were incubated for 3 h at 37°C, and hemolysis was assessed visually. For detecting neutralization of hemolytic activity, CCS was incubated with hybridoma culture supernatant for 1 h at 37°C prior to the addition of sRBC as previously described (6). Controls included sera from nonimmunized mice, myeloma culture supernatant from cell line X653, and PBS.
The hemolytic activity of CCS over time was also investigated, and the role of Ca2+ was examined. CCS (1.2 μg) from hemolytic M. bovis strain UQV 148NF was incubated in the presence of PBS, EGTA (10 mM), or MAb G3/D7 for 1 h at 37°C prior to centrifugation at 12,000 × g for 5 min and addition to sRBC. Incubation was continued for a further 1 h at 37°C with aliquots collected at 0, 5, 10, 20, 23, and 60 min and subjected to centrifugation at 12,000 × g for 5 min. The extent of sRBC lysis was determined by hemoglobin release into supernatants, with absorbance measured at 545 nm. sRBC-membrane pellets were collected after centrifugation and were washed twice at 12,000 × g for 5 min in PBS or EGTA (500 μl) before being subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot analysis. CCS (1.2 μg) from nonhemolytic M. bovis strain Gordon 26L3 was used as a control.
Corneal epithelial cell cytotoxicity assay.
Primary cultures of bovine corneal epithelial cells were derived from trephined explants of bovine corneas and were rinsed for 20 min in sterile PBS (CSL Ltd., Melbourne, Australia) containing 1% penicillin (5,000 IU)-streptomycin (5,000 μg/ml) before washing in dispase II (Boehringer) overnight at 4°C. Following washing and removal of corneal stroma, explants were placed into 24-well flat-bottomed tissue culture plates (Costar, Cambridge, Mass.) with 1 ml of Dulbecco modified Eagle medium–Ham's F-12 Medium (CSL) supplemented with 20% fetal calf serum, 20 mM l-glutamine, and 1% penicillin-streptomycin. Cell cultures were passaged less than three times prior to use in assays.
Cytotoxicity assays were similar to those previously described (16). Briefly, corneal epithelial cells were transferred to 96-well tissue culture plates, and 150 μl of fresh media was added prior to use in assays. CCS (1.2 μg) from M. bovis UQV 148NF was added to each well, and plates were incubated for 3 h at 37°C. Nonhemolytic M. bovis strain Gordon 26L3 and Mueller-Hinton broth preparations were included as controls. For neutralization assays, CCS was incubated with 100 μl of culture supernatant from MAb G3/D7 for 1 h at 37°C prior to addition to corneal epithelial cell cultures. Culture supernatant from MAb 45/5, recognizing the protease of Dichelobacter nodosus, was used as a control. Cytotoxicity and neutralization of cytotoxicity were evaluated by light microscopy for dehiscence of confluent corneal epithelial cell cultures.
SDS-PAGE and Western blot analysis.
SDS-PAGE and Western blot analysis were performed by using CCS (0.9 μg of protein) or sRBC membrane preparations. Samples were separated by electrophoresis in SDS–10% polyacrylamide gels and were stained with Coomassie blue-picric acid. For Western blot analysis, proteins were transferred via a wet transfer system (Bio-Rad, Richmond, Calif.) following SDS-PAGE, and nitrocellulose membranes were blocked with 5% skim milk powder in Tris-buffered saline containing 0.5% Tween 20. Following washing in Tris-buffered saline–0.5% Tween 20, blots were incubated with a 1:5 dilution of tissue culture supernatant of MAb G3/D7 before further washing and addition of horseradish peroxidase-conjugated rabbit anti-mouse IgG (Sigma). Bound immunoglobulin was detected by using ECL substrate (Amersham International, Little Chalfont, United Kingdom).
RESULTS
Characterization of MAbs.
Sera of mice immunized three times with M. bovis CCS demonstrated hemolysis-neutralizing antibody titers between 1:64 and 1:128. Therefore, hybridomas were generated and screened by ELISA against CCS from hemolytic M. bovis strain UQV 148NF. Hybridoma lines with ELISA absorbances greater than 0.8 were expanded, resulting in six different clones. All hybridoma cell lines produced antibodies belonging to the IgG2a subclass, except for one, MAb 1/H8, which produced antibodies of the IgG1 subclass (Table 1).
TABLE 1.
Summary of MAb clones generated against CCS from hemolytic M. bovis UQV 148NF
| MAb | Results of:
|
||
|---|---|---|---|
| ELISA | Hemolysis neutralization | Isotype | |
| 2/H5 | + | − | IgG2a |
| 1/G4 | + | − | IgG2a |
| E6/E8 | + | − | IgG2a |
| G3/D7 | + | + | IgG2a |
| G3/C5 | + | − | IgG2a |
| 1/H8 | + | ± | IgG1 |
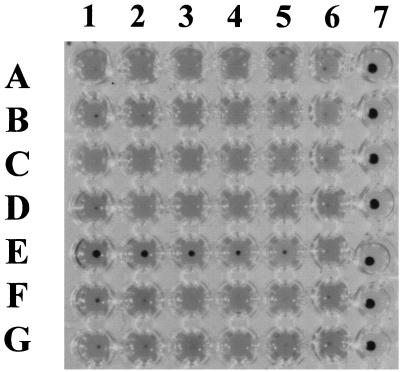
Hybridoma culture supernatants positive by ELISA were also screened by hemolysis inhibition assay, and single cell clones obtained by limiting dilution were tested for antigen specificity and hemolysis neutralization. This resulted in the isolation of one clone, MAb G3/D7, which produced antibodies with hemolysis-neutralizing titers of 1:16 (Fig. 1). In addition, weak neutralization of hemolytic activity was observed with MAb 1/H8. Culture supernatants from myeloma cell line X653 and other generated clones did not demonstrate hemolysis-neutralizing activity (Fig. 1).
FIG. 1.
Screening for hemolysis neutralization of CCS incubated with sRBC. Lanes 1 to 5, serial twofold dilutions of supernatant from MAb clones. Row A, 1/E6; B, 1/H8; C, 3/F2; D, 4/G11; E, G3/D7; F, 2/H5; G, X653. Lane 6, no added MAb; lane 7, sRBC only.
Detection of hemolysin by SDS-PAGE and Western blot analysis.
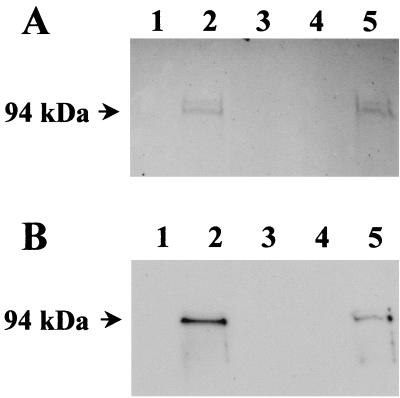
CCS from hemolytic and nonhemolytic M. bovis strains was subjected to SDS-PAGE and Western blot analysis. Two predominant protein bands with apparent molecular masses of 94 and 97 kDa, respectively, were observed in CCS from hemolytic M. bovis strain UQV 148NF following staining with Coomassie blue-picric acid (Fig. 2A, lanes 2 and 5). Western blot analysis was performed by using hemolysis-neutralizing MAb G3/D7 and identified the same 94-kDa protein (Fig. 2B, lanes 2 and 5). Bound immunoglobulin was not observed in filtrates (<300 kDa) from hemolytic M. bovis strain UQV 148NF (Fig. 2, lane 1) nor in CCS preparations from nonhemolytic M. bovis strain Gordon 26L3 (Fig. 2, lanes 3 and 4).
FIG. 2.
M. bovis preparations (0.9 μg) following SDS-PAGE and (A) stained with Coomassie blue-picric acid or (B) subjected to Western blot analysis using MAb G3/D7 tissue culture supernatant diluted 1:5. Lane 1, low-molecular-weight filtrate from UQV 148NF; lane 2, CCS from UQV 148NF following ultracentrifugation; lane 3, CCS from Gordon 26L3; lane 4, CCS from Gordon 26L3 following ultracentrifugation; lane 5, CCS from UQV 148NF. Protein molecular mass markers are indicated to the left of the figure.
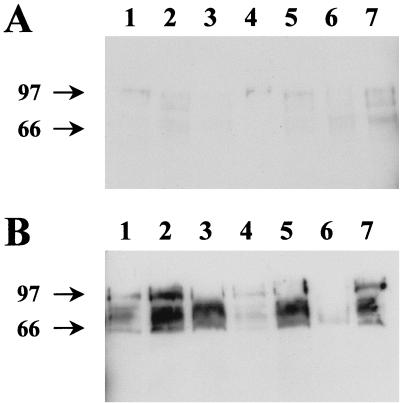
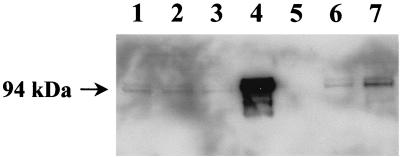
The presence of cross-reactive antigens was demonstrated by Western blot analysis of culture supernatants (without concentration) and cell pellets from late-log-phase cultures of hemolytic M. bovis strains representing the seven known M. bovis fimbriae serogroups (Fig. 3). MAb G3/D7 identified proteins with apparent molecular mass ranging from 60 to 105 kDa in all hemolytic M. bovis strains used (Fig. 3). Antibody binding was observed in both cell pellet (Fig. 3A) and culture supernatant preparations (Fig. 3B), although increased binding was apparent in culture supernatants.
FIG. 3.
Western blot analysis using MAb G3/D7 of preparations from M. bovis strains representing different fimbrial serogroups. (A) Cell pellets and (B) culture supernatants harvested in log phase. Lane 1, 276R (serogroup A); lane 2, 3WO7 (serogroup B); lane 3, Dal2d (serogroup C); lane 4, 593L (serogroup D); lane 5, TAT 849 (serogroup E); lane 6, 218R (serogroup F); lane 7, Fleur 462 (serogroup G). Protein molecular mass markers are indicated to the left of the figure.
Corneal epithelial cell cytotoxicity assays.
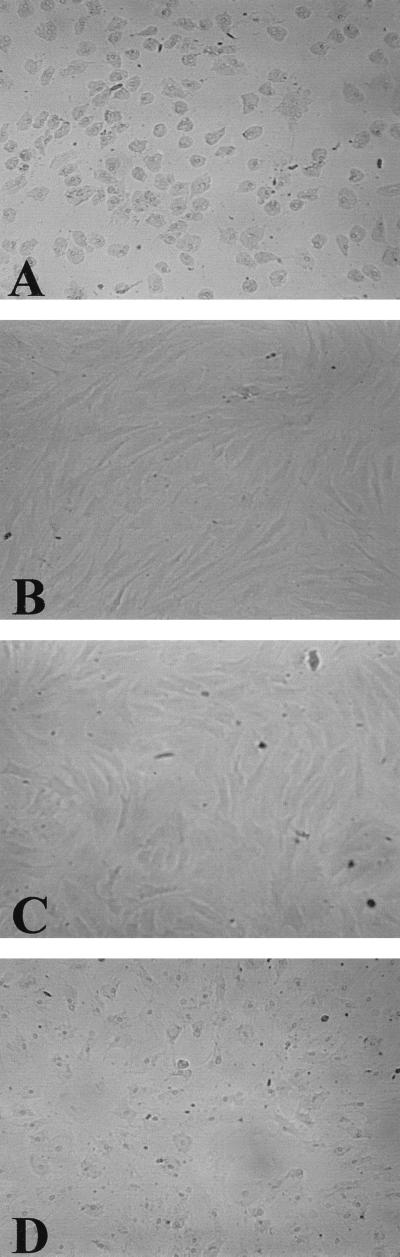
The cytotoxic potential of hemolytic M. bovis strain UQV 148NF was assessed qualitatively after incubation with bovine corneal epithelial cells for 3 h at 37°C. Epithelial cell monolayers were disrupted with many cells detached following exposure to CCS from hemolytic M. bovis (Fig. 4A). Trypan blue dye exclusion confirmed that the few remaining cells were dead. In contrast, bovine corneal epithelial cells incubated with CCS from nonhemolytic M. bovis strain Gordon 26L3 showed no monolayer disruption (Fig. 4B).
FIG. 4.
Neutralization of corneal epithelial cytotoxicity using MAb G3/D7. Incubation of bovine corneal epithelial cells with CCS from (A) hemolytic M. bovis UQV 148NF and (B) nonhemolytic M. bovis Gordon 26L3. Preincubation of CCS from M. bovis UQV 148NF prior to addition to corneal epithelial cells with a 1:5 dilution of MAb tissue culture supernatant from (C) MAb G3/D7 and (D) MAb against D. nodosus.
Neutralization of cytotoxic activity was investigated by incubating CCS from M. bovis strain UQV 148NF with MAb G3/D7 prior to addition to bovine corneal epithelial cells. No disruption of corneal epithelial cell monolayers was observed following preincubation with MAb G3/D7 (Fig. 4C). In contrast, monolayer disruption was evident following preincubation of CCS with a control MAb recognizing an unrelated D. nodosus antigen (Fig. 4D).
Activity of M. bovis hemolysin.
The hemolytic activity of CCS from M. bovis strain UQV 148NF over time was assessed by measuring released hemoglobin from lysed sRBC. No lysis was observed after 0, 1, or 5 min of incubation before a gradual increase in released hemoglobin was observed with total hemolysis occurring after incubation for 60 min (Table 2). No hemoglobin liberation was observed following incubation of sRBC with CCS from nonhemolytic M. bovis strain Gordon 26L3.
TABLE 2.
Hemolytic activity of CCS from M. bovis over time
| M. bovis CCS sample incubated with sRBC | Time (min) | A545 |
|---|---|---|
| Hemolytic CCS | 0 | 0.004 |
| Hemolytic CCS | 5 | 0.006 |
| Hemolytic CCS | 10 | 0.159 |
| Hemolytic CCS | 20 | 0.160 |
| Hemolytic CCS | 23 | 0.162 |
| Hemolytic CCS | 60 | 0.180 |
| Hemolytic CCS plus EGTA | 60 | 0.004 |
| Hemolytic CCS plus MAb G3/D7 | 60 | 0.007 |
| Nonhemolytic CCS | 60 | 0.001 |
| Distilled water | 60 | 0.178 |
Resulting sRBC membrane pellets were also collected over time and were subjected to Western blot analysis by using hemolysis-neutralizing MAb G3/D7. Two predominant proteins were detected in all samples with apparent molecular masses in SDS-PAGE of 94 and 97 kDa (Fig. 5), although bound immunoglobulin was increased in samples incubated for longer periods (Fig. 5, lanes 1 to 3). Similar-sized antigens were noted in CCS controls not exposed to sRBC (Fig. 5, lane 4). However, bound MAb was not identified in sRBC membrane preparations incubated with CCS from nonhemolytic M. bovis strain Gordon 26L3 (Fig. 5, lane 5).
FIG. 5.
Western blot detection of hemolysin bound to sRBC over time using a 1:5 dilution of MAb G3/D7 tissue culture supernatant. Lanes 1 to 3, different incubation periods of sRBC with hemolytic CCS from UQV 148NF. Lane 1, 60 min; lane 2, 10 min; lane 3, 0 min; lane 4, CCS from M. bovis UQV 148NF without sRBC; lane 5, nonhemolytic CCS incubated with sRBC for 60 min; lane 6, hemolytic CCS incubated with MAb G3/D7 prior to addition to sRBC; lane 7, hemolytic CCS incubated with sRBC in the presence of 10 mM EGTA. Protein molecular mass markers are indicated to the left of the figure.
Hemoglobin was not released from sRBC following exposure to CCS from hemolytic M. bovis UQV 148NF in the presence of 10 mM EGTA nor following preincubation of CCS with MAb G3/D7 (Table 2). Although hemolysis was not detected, 94- and 97-kDa antigens were identified in these sRBC preparations by Western blot analysis (Fig. 5, lane 6 and 7).
DISCUSSION
M. bovis hemolysin is a bacterial exotoxin implicated in the pathogenicity of M. bovis, as nonhemolytic strains do not initiate disease (3). We have generated a hemolysis-neutralizing MAb (G3/D7) that identifies an antigen in CCS of hemolytic M. bovis strain UQV 148NF by Western blot analysis, enabling characterization of the hemolysin of M. bovis without further purification. The antigen identified by MAb G3/D7 had an apparent molecular mass of 94 kDa and corresponded to a protein detected by Coomassie blue-picric acid after SDS-PAGE of similar preparations from hemolytic M. bovis strain UQV 148NF. A protein was not identified in equivalent preparations from nonhemolytic M. bovis strain Gordon 26L3, suggesting that nonhemolytic strains do not express this protein. Alternatively, MAb G3/D7 may recognize an activated form of the toxin not present in nonhemolytic strains. Identification of a similar size protein in M. bovis cultures using a MAb to the E. coli alpha-hemolysin provides further evidence that MAb G3/D7 specifically recognizes the hemolysin (16).
MAb G3/D7 detected more than one protein band in CCS, which has been reported in the characterization of other microbial exotoxins by using neutralizing MAbs (18). Association of the hemolysin of E. coli with lipopolysaccharide has been proposed to account for a size and charge heterogeneity in SDS-PAGE analysis of this toxin (9), although it is not known whether M. bovis hemolysin associates with lipopolysaccharide. Proteins of different size may also result from posttranslational modification, as demonstrated for M. bovis fimbriae (25, 32), or from proteolytic cleavage. Proteolytic activity has been reported in M. bovis cell cultures (26) and could account for the rapid loss of activity occurring in hemolysin preparations (3). A similar phenomenon has been reported for the exotoxins of E. coli and A. pleuropneumoniae (4, 14, 31).
Previous investigations have demonstrated lysis of corneal epithelial cells by cell-free M. bovis extracts in vitro in addition to hemolytic activity, and it has been speculated that a separate cytotoxin exists (2, 3, 16, 17; L. W. George and G. M. Kagonyera, 15 January 1990, international patent application no. PCT/US90/00106). Therefore, MAb G3/D7 was used to investigate whether more than one exotoxin is secreted by hemolytic M. bovis strains. Our study demonstrated corneal epithelial cell toxicity could be neutralized following preincubation of hemolytic M. bovis toxin preparations with a single (epitope-specific) hemolysis-neutralizing MAb. The most plausible explanation for this observation is that hemolytic and cytotoxic activities are due to the action of a single toxin. However, the existence of a common epitope on different toxins cannot be excluded. This would require control by a common regulatory gene to enable simultaneous expression, as reported in the regulation of virulence factors of Bordetella pertussis (38). Alternatively, MAb G3/D7 may have recognized an accessory protein required for lysis to proceed as described for the RTX toxins (24).
The unique neutralization properties of MAb G3/D7 presented the opportunity to investigate binding of the hemolysin to cell membranes. Previously, investigations using pore-forming bacterial exotoxin preparations, including an M. bovis hemolysin preparation, have suggested that osmotic lysis occurs following a rapid influx of Ca2+ (7, 8, 11, 12, 36). This effect was concentration dependent, suggesting time of exposure and toxin load were influencing factors (36). In agreement with previous reports, our investigations also demonstrated increased lysis of sRBC over time after exposure to M. bovis hemolysin. In addition, Western blot analysis using MAb G3/D7 detected a rapid initial binding step of toxin to sRBC, and bound protein identified before hemoglobin release was detected spectrophotometrically. A role for extracellular Ca2+ in the lytic process was supported by the absence of sRBC lysis in the presence of 10 mM EGTA. However, MAb G3/D7 recognized membrane-bound protein in these sRBC preparations, indicating binding of toxin to target cells was able to proceed unhindered. This does not reject a requirement of Ca2+ in the binding of M. bovis hemolysin to sRBC membranes, as Ca2+ was not excluded from bacterial growth medium in the preparation of CCS. Toxin-incorporated Ca2+ may be inaccessible to EGTA and, therefore, may still be able to contribute to the binding of hemolysin to sRBC membranes.
Further evidence for a two-step mechanism for hemolysis was obtained by investigating neutralization of activity following preincubation of CCS with MAb G3/D7. Preincubation with MAb G3/D7 neutralized hemolytic activity of M. bovis CCS, although Western blot analysis detected membrane-bound protein in sRBC preparations, albeit in decreased quantities. Therefore, binding of the M. bovis hemolysin to sRBC membranes can occur without lysis, which is evidence that these two events are temporally separable. This supports similar findings for the hemolysin of E. coli and the leukotoxins of A. actinomycetemcomitans and P. hemolytica (1, 13, 15, 36). The results of the present study support previous investigations suggesting that the hemolysin of M. bovis is a member of the RTX toxin family, although cloning and sequencing of the hemolysin are still required for confirmation of a relationship.
The hemolysin of M. bovis has been proposed as a vaccine candidate (3, 6). Vaccines based on fimbrial antigens require all known serogroups to be included for protection in the field, leading to potential problems with antigenic competition (33). Therefore, we assessed whether MAb G3/D7 could recognize the hemolysin from M. bovis strains representative of the seven identified fimbrial serogroups. Common epitopes were demonstrated in all strains. The increased binding observed in supernatant preparations suggested recognition of a protein predominantly secreted rather than cell associated, although some antibody was associated with the cell, with MAb G3/D7 identifying toxin leaving the cell-surface. This supports the proposed mechanism for toxin secretion in membrane vesicles (3). The apparent molecular mass range where antibody binding was observed is comparable to a previous report using immune sera from cattle vaccinated with CCS, which also identified antigens with apparent molecular masses of 70 to 110 kDa by Western blot analysis (6). Therefore, a vaccine preparation containing the antigen identified by MAb G3/D7 may protect cattle from developing IBK.
In summary, MAb G3/D7 is a hemolysis-neutralizing antibody specific for M. bovis hemolysin. This MAb is currently being used to clone the hemolysin gene of M. bovis, which will allow further characterization of this toxin as a virulence factor in IBK and contribute to an understanding of its relationship with other recognized bacterial toxins.
ACKNOWLEDGMENTS
J. Woolcock (University of Queensland, Australia) generously donated M. bovis UQV 148NF. We thank Stuart Davies for advice and assistance in corneal epithelial cell culture (CRC for Lens Research and Development, New South Wales, Australia). F.M.B. thanks Philippa O'Brien for reviewing the manuscript and offering comment.
F.M.B. was the recipient of a Junior Research Fellowship from the Meat Research Corporation of Australia.
REFERENCES
- 1.Bauer M E, Welch R A. Association of RTX toxins with erythrocytes. Infect Immun. 1996;64:4665–4672. doi: 10.1128/iai.64.11.4665-4672.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beard M K M. Ph.D. thesis. Sydney, Australia: University of Sydney; 1991. [Google Scholar]
- 3.Beard M K, Moore L J. Reproduction of bovine keratoconjunctivitis with a purified hemolytic and cytotoxic fraction of Moraxella bovis. Vet Microbiol. 1994;42:15–33. doi: 10.1016/0378-1135(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 4.Bhakdi S, Tranum-Jensen J. Damage to cell membranes by pore-forming bacterial cytolysins. Prog Allergy. 1988;40:1–43. [PubMed] [Google Scholar]
- 5.Bhakdi S, Mackman N, Nicaud J M, Holland I B. Escherichia coli hemolysin may damage target cell membranes by generating transmembrane pores. Infect Immun. 1986;52:63–69. doi: 10.1128/iai.52.1.63-69.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Billson F M, Hodgson J L, Egerton J R, Lepper A W, Michalski W P, Schwartzkoff C L, Lehrbach P R, Tennent J M. A hemolytic cell-free preparation of Moraxella bovis confers protection against infectious bovine keratoconjunctivitis. FEMS Microbiol Lett. 1994;124:69–73. doi: 10.1111/j.1574-6968.1994.tb07263.x. [DOI] [PubMed] [Google Scholar]
- 7.Boehm D F, Welch R A, Snyder I S. Calcium is required for binding of Escherichia coli hemolysin to erythrocyte membranes. Infect Immun. 1990;58:1951–1958. doi: 10.1128/iai.58.6.1951-1958.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boehm D F, Welch R A, Snyder I S. Domains of Escherichia coli hemolysin (HlyA) involved in binding of calcium and erythrocyte membranes. Infect Immun. 1990;58:1959–1964. doi: 10.1128/iai.58.6.1959-1964.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bohach G A, Snyder I S. Composition of affinity-purified α-hemolysin of Escherichia coli. Infect Immun. 1986;53:435–437. doi: 10.1128/iai.53.2.435-437.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown M H, Brightman A H, Fenwick B W, Rider M A. Infectious bovine keratoconjunctivitis: a review. J Am Vet Med Assoc. 1998;12:259–266. doi: 10.1111/j.1939-1676.1998.tb02120.x. [DOI] [PubMed] [Google Scholar]
- 11.Clinkenbeard K D, Thiessen A E. Mechanism of action of M. bovis hemolysin. Infect Immun. 1991;59:1148–1152. doi: 10.1128/iai.59.3.1148-1152.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clinkenbeard K D, Mosier D A, Confer A W. Transmembrane pore size and role of cell swelling in cytotoxicity caused by Pasteurella hemolytica leukotoxin. Infect Immun. 1989;57:420–425. doi: 10.1128/iai.57.2.420-425.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cruz W T, Young R, Chang Y F, Struck D K. Deletion analysis resolves cell-binding and lytic domains of the Pasteurella leukotoxin. Mol Microbiol. 1990;4:1933–1939. doi: 10.1111/j.1365-2958.1990.tb02042.x. [DOI] [PubMed] [Google Scholar]
- 14.Devenish J, Rosendal S. Immunological characterization of breakdown peptides of the 104 kilodalton hemolysin of Actinobacillus pleuropneumoniae serotype 1. Vet Microbiol. 1991;29:85–93. doi: 10.1016/0378-1135(91)90112-s. [DOI] [PubMed] [Google Scholar]
- 15.Eberspacher B, Hugo F, Bhakdi S. Quantitative study of the binding and hemolytic efficiency of Escherichia coli hemolysin. Infect Immun. 1989;57:983–988. doi: 10.1128/iai.57.3.983-988.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gray J T, Fedorka-Cray P J, Rogers D G. Partial characterization of a Moraxella bovis cytolysin. Vet Microbiol. 1995;43:183–196. doi: 10.1016/0378-1135(94)00084-a. [DOI] [PubMed] [Google Scholar]
- 17.Hoien-Dalen P S, Rosenbusch R F, Roth J A. Comparative characterization of the leukocidic and hemolytic activity of Moraxella bovis. Am J Vet Res. 1990;51:191–196. [PubMed] [Google Scholar]
- 18.Ji G E, O'Hanley P. Epitopes of Escherichia coli hemolysin: identification of monoclonal antibodies that prevent hemolysis. Infect Immun. 1990;58:3029–3035. doi: 10.1128/iai.58.9.3029-3035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kagonyera G M, George L W, Miller M. Effects of Moraxella bovis and culture filtrates on 51Cr-labeled bovine neutrophils. Am J Vet Res. 1989;50:18–21. [PubMed] [Google Scholar]
- 20.Kagonyera G M, George L W, Munn R. Cytopathic effects of Moraxella bovis on cultured bovine neutrophils and corneal cells. Am J Vet Res. 1989;50:10–17. [PubMed] [Google Scholar]
- 21.Kuhnert P, Heyberger-Meyer B, Burnens A P, Nicolet J, Frey J. Detection of RTX toxin genes in gram-negative bacteria with a set of specific probes. Infect Immun. 1997;63:2258–2265. doi: 10.1128/aem.63.6.2258-2265.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lepper A W. Vaccination against infectious bovine keratoconjunctivitis: protective efficacy and antibody response induced by pili of homologous and heterologous strains of Moraxella bovis. Aust Vet J. 1988;65:310–316. doi: 10.1111/j.1751-0813.1988.tb14513.x. [DOI] [PubMed] [Google Scholar]
- 23.Lepper A W D, Atwell J L, Lehrbach P R, Schwartzkoff C L, Egerton J R, Tennent J M. The protective efficacy of cloned Moraxella bovis pili in monovalent and multivalent vaccine formulations against experimentally induced infectious bovine keratoconjunctivitis. Vet Microbiol. 1995;45:129–138. doi: 10.1016/0378-1135(94)00123-e. [DOI] [PubMed] [Google Scholar]
- 24.Ludwig A, Goebel W. The family of the multigenic encoded RTX toxins. In: Alouf J E, Freer H, editors. The comprehensive sourcebook of bacterial protein toxins. II: membrane damaging toxins. 2nd ed. London, England: Academic Press; 1999. pp. 330–348. [Google Scholar]
- 25.Michalski W P, Tennent J M. Glycosylation status of type IV pilin proteins from Gram-negative bacteria. Prot Sci. 1995;4(Suppl. 1):128. [Google Scholar]
- 26.Michalski W P, Crooks J K, Prowse S J, Tennent J M, Lepper A W. Purification of proteases by immobilized-bacitracin A chromatography. Protein purification and biochemical engineering, Keystone Symposia on Molecular and Cellular Biology, Santa Fe, New Mexico. J Cell Biochem. 1993;53(Suppl. 17A):50. [Google Scholar]
- 27.Moore L J, Lepper A W. A unified serotyping scheme for Moraxella bovis. Vet Microbiol. 1991;29:75–83. doi: 10.1016/0378-1135(91)90111-r. [DOI] [PubMed] [Google Scholar]
- 28.Moore L J, Rutter J M. Antigenic analysis of fimbrial proteins from Moraxella bovis. J Clin Microbiol. 1987;25:2063–2070. doi: 10.1128/jcm.25.11.2063-2070.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.New J C. Costs of veterinary services and vaccines/drugs used for prevention and treatment of diseases in 60 Tennessee cow-calf operations (1987–1988) J Am Vet Med Assoc. 1991;198:1334–1340. [PubMed] [Google Scholar]
- 30.O'Hanley P, Marcus R, Baek K H, Denich K, Ji G E. Genetic conservation of hlyA determinants and serological conservation of HlyA: basis for developing a broadly cross-reactive subunit Escherichia coli alpha-hemolysin vaccine. Infect Immun. 1993;61:1091–1097. doi: 10.1128/iai.61.3.1091-1097.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oropeza-Wekerle R L, Muller E, Kern P, Meyermann R, Goebel W. Synthesis, inactivation, and localization of extracellular and intracellular Escherichia coli hemolysins. J Bacteriol. 1989;171:2783–2788. doi: 10.1128/jb.171.5.2783-2788.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parge H E, Forest K T, Hickey M J, Christensen D A, Getzoff E D, Tainer J A. Structure of the fibre-forming protein pilin a 2.6A resolution. Nature. 1995;378:32–38. doi: 10.1038/378032a0. [DOI] [PubMed] [Google Scholar]
- 33.Raadsma H W, O'Meara T J, Egerton J R, Lehrbach P R, Schwartzkoff C L. Protective antibody titres and antigenic competition in multivalent Dichelobacter nodosus fimbrial vacines using characterized rDNA antigens. Vet Immunol Immunopathol. 1994;40:253–274. doi: 10.1016/0165-2427(94)90024-8. [DOI] [PubMed] [Google Scholar]
- 34.Rogers D G, Cheville N F, Pugh G W. Pathogenesis of corneal lesions caused by Moraxella bovis in gnotobiotic calves. Vet Pathol. 1987;24:287–295. doi: 10.1177/030098588702400401. [DOI] [PubMed] [Google Scholar]
- 35.Slatter D H, Edwards M E, Hawkins C D, Wilcox G E. A national survey of the clinical features, treatment and importance of infectious bovine keratoconjunctivitis. Aust Vet J. 1982;59:69–72. doi: 10.1111/j.1751-0813.1982.tb02729.x. [DOI] [PubMed] [Google Scholar]
- 36.Taichman N S, Iwase M, Lally E T, Shattil S J, Cunningham M E, Korchak H M. Early changes in cytosolic calcium and membrane potential induced by Actinobacillus actinomycetemcomitans leukotoxin in susceptible and resistant target cells. J Immunol. 1991;147:3587–3594. [PubMed] [Google Scholar]
- 37.Thrift F A, Overfield J R. Impact of pinkeye (infectious bovine keratoconjunctivitis) on weaning and postweaning performance of hereford calves. J Anim Sci. 1974;38:1179–1184. doi: 10.2527/jas1974.3861179x. [DOI] [PubMed] [Google Scholar]
- 38.Weiss A A, Hewlett E L, Meyers G A, Falkow S. Pertussis toxin and extracytoplasmic adenylate cyclase as virulence factors of Bordetella pertussis. J Infect Dis. 1984;150:219–222. doi: 10.1093/infdis/150.2.219. [DOI] [PubMed] [Google Scholar]
- 39.Welch R A, Forestier C, Lobo A, Pellett S, Thomas W, Jr, Rowe G. The synthesis and function of the Escherichia coli hemolysin and related RTX exotoxins. FEMS Microbiol Immunol. 1992;5:29–36. doi: 10.1111/j.1574-6968.1992.tb05883.x. [DOI] [PubMed] [Google Scholar]