Objective:
To determine the morbidity, mortality, and pathologic outcomes of transanal total mesorectal resection (taTME) versus laparoscopic total mesorectal excision (laTME) among patients with rectal cancer with clinical stage I to III rectal cancer below the peritoneal reflection.
Background:
Studies with sufficient numbers of patients allowing clinical acceptance of taTME for rectal cancer are lacking. Thus, we launched a randomized clinical trial to compare the safety and efficacy of taTME versus laTME.
Methods:
A randomized, open-label, phase 3, noninferiority trial was performed at 16 different hospitals in 10 Chinese provinces. The primary endpoints were 3-year disease-free survival and 5-year overall survival. The morbidity and mortality within 30 days after surgery, and pathologic outcomes were compared based on a modified intentiontotreat principle; this analysis was preplanned.
Results:
Between April 13, 2016, and June 1, 2021, 1115 patients were randomized 1:1 to receive taTME or laTME. After exclusion of 26 cases, modified intentiontotreat set of taTME versus laTME groups included 544 versus 545 patients. There were no significant differences between taTME and laTME groups in intraoperative complications [26 (4.8%) vs 33 (6.1%); difference, −1.3%; 95% confidence interval (CI), −4.2% to 1.7%; P=0.42], postoperative morbidity [73 (13.4%) vs 66 (12.1%); difference, 1.2%; 95% CI, −2.8% to 5.2%; P=0.53), or mortality [1 (0.2%) vs 1 (0.2%)]. Successful resection occurred in 538 (98.9%) versus 538 (98.7%) patients in taTME versus laTME groups (difference, 0.2%; 95% CI, −1.9% to 2.2%; P>0.99).
Conclusions:
Experienced surgeons can safely perform taTME in selected patients with rectal cancer.
Keywords: rectal cancer, taTME, morbidity, mortality
Since the advent of total mesorectal excision (TME) in 1982,1 the surgical outcomes for rectal cancer have improved significantly. Currently, transabdominal TME with laparoscopic technology (laTME) has become one of the general modalities to manage resectable rectal cancer.2 However, laTME operating in the confined space of the pelvis is often technically challenging due to several tumor-related and patient-related factors, especially for mid-low rectal cancer. Worse yet, due to the “up-to-bottom” technical hurdles, radical resection following laTME is not always guaranteed; difficult patients are significantly predisposed to suffer suboptimal TME or resection margin involvement. These pitfalls highlight an unmet clinical need to develop an optimal approach for rectal cancer surgery.
In 2010, Sylla et al3 reported the first instance of transanal total mesorectal excision (taTME) assisted by laparoscopy for a patient with a T2N2M0 rectal cancer. As a “bottom-to-up” and “inside-to-outside” approach, taTME addresses some of the challenges inherent to laTME. The novel transanal vantage point could facilitate access to the mid and distal rectum, enhance visualization of the dissection plane, and improve resection margins. The ability to refine the surgical quality of TME for mid-low rectal cancer has the potential to significantly improve the surgical outcomes of a large portion of patients with rectal cancer.
Currently, taTME as an alternative to laTME in selected patients with rectal cancer has become popular in skilled surgeons. A recent meta-analysis demonstrated similar technical success with acceptable oncologic and perioperative outcomes in rectal cancer patients treated by taTME versus laTME,4 and other systematic reviews verified these trends.5,6 By contrast, an international taTME registry found a relatively high percentage of perioperative complications including an anastomotic failure rate of 15.7%,7 and a Norwegian case review of 110 taTME procedures indicated increased early multifocal local recurrences, resulting in a moratorium on taTME.8 Therefore, the role of taTME is still a matter of debate pending the outcomes from the multicenter ongoing randomized controlled trials (RCTs) COLOR III and ETAP-GRECCAR 11.9,10 Against this background, the Chinese Transanal Endoscopic Surgery Collaborative (CTESC) group launched a phase 3, open-labeled, multicenter, noninferiority RCT to assess the surgical safety and oncologic outcomes of taTME versus laTME in patients with rectal cancer. Herein, we report the initial results on the morbidity and mortality as well as pathologic outcomes; this early analysis was preplanned.
METHODS
Study Design
This trial of transanal versus conventional laparoscopic TME for rectal cancer (TaLaR) is a phase 3, open-labeled, multicenter, randomized, controlled, noninferiority trial conducted at 16 centers in 10 Chinese provinces from April 2016 to June 2021. The primary endpoints of this trial were 3-year disease-free survival (DFS) and 5-year overall survival (OS) after surgery. Approval was obtained from the institutional review board of each participating hospital, and all patients provided written informed consent. This trial is registered with ClinicalTrials.gov, NCT 02966483. The approved study protocol and statistical analysis plan are available in Supplemental Digital Content (SDC) (http://links.lww.com/SLA/D870).
Inclusion and Exclusion Criteria
Eligible patients were aged 18 to 75 years; had an American Society of Anesthesiologists class I to III; had clinical stage I to III rectal adenocarcinoma below peritoneal reflection based on preoperative imaging; and were expected to undergo a sphincter-sparing procedure via TME principles for curative intent. Patients were excluded if they had T1 cancers that can be locally resected; had involvement of the circumferential resection margin (CRM) as indicated by preoperative magnetic resonance imaging; had tumors with ingrowth in the internal sphincter or levator ani; and had contraindications for surgery. Detailed eligibility criteria are shown in the study protocol in SDC (http://links.lww.com/SLA/D870).
Randomization
Eligible patients were randomly assigned in a 1:1 ratio to undergo either taTME or laTME using a Web site–based randomization system using the central dynamic and with stratification by the center. Participating centers submitted the patient information to the data center at the Sixth Affiliated Hospital of Sun Yat-sen University, Guangzhou, China, where central randomization was done. Information on treatment assignment was subsequently sent to each participating center. The surgeons and patients were not masked to treatment allocation.
Eligibility of Surgeons
Surgeons were selected from the members of the CTESC group who met the following criteria: (1) have a minimum of 100 laTME and 50 taTME procedures performed by each surgical team; (2) were confirmed to be qualified surgeons by the CTESC Research Committee based on the evaluation of the unedited videos of 2 laTME and 2 taTME procedures in obese male patients [body mass index (BMI) ≥28 kg/m2] with rectal cancer below the peritoneal reflection. After approval by the committee, 17 surgical teams at 16 centers (1 surgical team per center except in 1 center) participated in the trial.
Surgical Quality Control
Surgical quality control was maintained by using unedited videos of all the surgeries. These videos were reviewed by the CTESC Research Committee, and feedback on the assessment was regularly provided to the investigators. The taTME and laTME procedures are performed according to the TME principles. The key distinction between the taTME and laTME procedures was that TME was performed from a transanal bottom-up approach for patients receiving taTME. In both treatment arms, the anastomosis methods were not uniform, as either a handsewn or stapled anastomosis was allowed.
Outcome Measurements for Surgical Safety Analysis
Morbidity and mortality were monitored within 30 days after surgery. Anastomotic leakage was defined as clinical evidence of a defect of the integrity of the intestinal wall at the anastomotic site or the presence of a pelvic abscess adjacent to the anastomosis. A specific complication was diagnosed according to either an image-based physical evaluation or obvious clinical evidence and then was stratified by the Clavien-Dindo classification system. Intraoperative complications were defined as unexpected surgical adverse events occurring during surgery [eg, iatrogenic injury of the blood vessels, bowel, or other organs; hemorrhage; carbon dioxide (CO2) embolism]. Vascular injury was defined as a laceration or break of the presacral vessels. Intraoperative hemorrhage was defined as blood loss of >200 mL in the absence of vascular injury. Any diagnosis on perioperative complications was made by the surgeons and was confirmed by the CTESC Research Committee every 3 months. Detailed measurements are shown in the study protocol in SDC (http://links.lww.com/SLA/D870).
Pathologic Assessment
Pathologic outcomes included the TME quality, CRM, distal resection margin (DRM), number of harvested lymph nodes and number positive, length from the inferior of tumor to DRM, length of the resected sample, lymphovascular and nerve invasion. Measurements for surgical resection included a composite of CRM (>1 mm from the tumor to the mesorectal fascia), DRM (>1 mm between the closest tumor to the cut edge of the tissue), and TME quality (complete or nearly complete as previously described).11 All 3 of the parameters (CRM, DRM, and TME quality) must have been achieved for the surgery to be considered a “success.” The pathologic outcomes were evaluated by 2 specialized pathologists in each participating institution in a blinded manner and were reviewed by the CTESC Research Committee every 3 months.
Statistical Analysis
Sample size calculation of this trial was on the basis of 3-year DFS and 5-year OS; but the sample size according to 5-year OS was larger than that based on 3-year DFS. The 3-year DFS and 5-year OS among patients with clinical stage I to III rectal cancer treated with laTME were assumed to be 74.6% and 77.4%, respectively. According to a log-rank test with an α error of 2.5% (in a 2-sided test), power of 80%, and a noninferiority margin of 10%, at least 610 and 910 patients would be required to sufficiently declare taTME noninferior to laTME in 3-year DFS and 5-year OS, respectively. Assuming a dropout rate of 20%, a total of 1114 patients were planned to enroll for this trial.
A modified intentiontotreat method (excluding patients who no longer met inclusion criteria after randomization) was adopted here. Continuous variables were shown as the median and interquartile range (IQR) and were analyzed using a Mann-Whitney U test if not normally distributed, or were presented as the mean and SD and analyzed using t test if normally distributed. Categorical variables were expressed as number (%) and were analyzed by Fisher exact test or χ2 test as appropriate. The Newcombe method with an adjustment for randomization strata was used to calculate the 95% confidence intervals (CIs) for between-group differences in intraoperative and postoperative complication rates, as well as successful resection rates. SAS 9.3 software (SAS Institute, Cary, NC) was used for all statistical analyses and a 2-sided P value <0.05 indicated significance.
RESULTS
Between April 13, 2016, and June 1, 2021, 1115 enrolled patients from 16 centers in China were randomized 1:1 to receive taTME (n=558) or laTME (n=557). After the exclusion of 26 cases, 544 patients in the taTME group and 545 patients in the laTME group were included in the final modified intentiontotreat analysis of the morbidity, mortality, and pathologic outcomes (Fig. 1). The baseline characteristics of the patients are provided in Table 1, which were well balanced between the 2 groups. The mean distance from the inferior margin of the tumor to the anal verge was 5.2 cm (SD: 1.5 cm). As indicated by preoperative imaging, the 2 groups had no difference in clinical staging at baseline.
FIGURE 1.
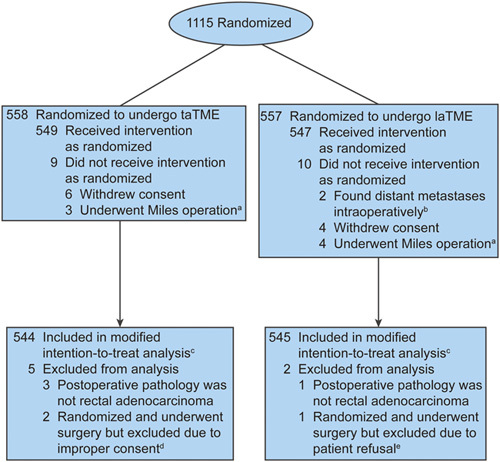
CONSORT flow diagram of patient enrollment and randomization. aSurgeons decided to perform the Miles operation for these patients according to the specific intraoperative circumstances. bPatients were found to have peritoneal metastasis (n=1) or liver metastasis (n=1) intraoperatively. cThe modified intent-to-treat set excluded patients who no longer met inclusion criteria after randomization. dThe 2 patients allocated to taTME did not receive proper consent; hence, no data could be used in any analysis. eAfter surgery, the patient refused to participate and have any data used in any analysis. CONSORT indicates Consolidated Standards of Reporting Trials.
TABLE 1.
Demographic and Clinical Characteristics
| n (%) | ||
|---|---|---|
| taTME (N=544) | laTME (N=545) | |
| Male sex | 359 (66.0) | 333 (61.1) |
| Age [median (IQR)] (y) | 58 (50–67) | 60 (52–67) |
| BMI [median (IQR)] (kg/m2) | 22.9 (20.7–24.9) | 22.8 (20.9–24.8) |
| ASA class | ||
| I | 228 (41.9) | 219 (40.2) |
| II | 279 (51.3) | 270 (49.5) |
| III | 37 (6.8) | 56 (10.3) |
| Tumor distance from anal verge [median (IQR)] (cm) | 5.0 (3.9–6.0) | 5.5 (4.4–6.6) |
| Preoperative therapy | 211 (38.8) | 179 (32.8) |
| Chemotherapy plus radiation | 93 (17.1) | 59 (10.8) |
| Chemotherapy alone | 116 (21.3) | 120 (22.0) |
| Radiation alone | 2 (0.4) | 0 |
| Preoperative clinical stage | ||
| I | 105 (19.3) | 89 (16.3) |
| II | 220 (40.4) | 243 (44.6) |
| III | 219 (40.3) | 213 (39.1) |
ASA indicates American Society of Anesthesiologists.
Surgical Outcomes
The surgical outcomes appeared in Table 2. Among the 544 patients receiving taTME, 358 (65.8%) underwent a 2-team taTME procedure. The taTME versus laTME groups had similar surgical time [mean: 209.5 minutes (SD: 71.0) vs 214.7 minutes (84.4); P=0.28], but a post hoc test showed, compared with laTME, the 1-team taTME [mean: 238.6 minutes (SD: 67.5)] took longer, whereas the 2-team taTME [mean: 194.5 minutes (SD: 68.2)] took shorter. The intraoperative estimated blood loss was comparable [median: 50 mL (IQR, 40–100) vs 50 mL (IQR, 40–100); P=0.29]. Six (1.1%) of 545 patients in the laTME arm were converted to the transanal surgery due to the tumor-related technical difficulty, but no patients in the taTME arm required a conversion. Despite no significant differences in postoperative hospital stay [median: 8.0 days (IQR, 7.0–10.0) vs 9.0 days (IQR, 7.0–10.0); P=0.22], patients undergoing taTME versus laTME displayed faster postoperative recovery (Supplemental Figs. 1A–C, http://links.lww.com/SLA/D870), as indicated by less time to first flatus [median: 2.0 days (IQR, 1.0–3.0) vs 2.0 days (IQR, 2.0–3.0); P<0.001], to first intake [median: 2.0 days (IQR, 2.0–3.0) vs 3.0 days (IQR, 2.0–4.0); P<0.001], and to ambulation [median: 2.0 days (IQR, 1.0–3.0) vs 2.0 days (IQR, 1.0–3.0); P=0.001].
TABLE 2.
Surgical Outcomes for taTME and laTME Groups
| taTME (N=544) | laTME (N=545) | P | |
|---|---|---|---|
| Operative time [mean (SD)] (min) | |||
| Total group | 209.5 (71.0) | 214.7 (84.4) | 0.28 |
| 1-team | 238.6 (67.5) | <0.001* | |
| 2-team | 194.5 (68.2) | <0.001* | |
| Surgical approach [n (%)] | |||
| ISR | 81 (14.9) | 48 (8.8) | 0.002 |
| LAR | 463 (85.1) | 497 (91.2) | |
| Estimated blood loss [median (IQR)] (mL) | 50 (40–100) | 50 (40–100) | 0.29 |
| Intraoperative blood transfusion [n (%)] | 8 (1.5) | 13 (2.4) | 0.38 |
| Anastomotic technique [n (%)] | |||
| Handsewn | 154 (28.3) | 48 (8.8) | <0.001 |
| Stapled | 390 (71.7) | 497 (91.2) | |
| Enterostomy [n (%)] | 303 (55.7) | 292 (53.6) | 0.50 |
| Conversion [n (%)]† | 0 | 6 (1.1) | 0.03 |
| Postoperative recovery [median (IQR)] (d) | |||
| Time to first flatus | 2.0 (1.0–3.0) | 2.0 (2.0–3.0) | <0.001 |
| Time to first intake | 2.0 (2.0–3.0) | 3.0 (2.0–4.0) | <0.001 |
| Time to ambulation | 2.0 (1.0–3.0) | 2.0 (1.0–3.0) | 0.001 |
| Postoperative hospital stays | 8.0 (7.0–10.0) | 9.0 (7.0–10.0) | 0.22 |
Compared with the laTME group.
Six cases in the laTME group were converted to transanal surgery.
ISR indicates intersphincteric resection; LAR, low anterior resection.
Surgical Complications
Morbidities and mortalities are described in Table 3. Intraoperative complications occurred in 26 (4.8%) of 544 patients in the taTME arm (hemorrhage in 10 patients, vascular injury in 7, intestinal injury in 4, urethral injury in 2, ureter injury in 1, subcutaneous emphysema in 1, and CO2 embolism in 2) and 33 (6.1%) of 545 in the laTME arm (hemorrhage in 12 patients, vascular injury in 5, intestinal injury in 5, ureter injury in 4, subcutaneous emphysema in 3, spleen injury in 3, and pancreatic injury in 1). These rates had no significant difference (difference, −1.3%; 95% CI, −4.2% to 1.7%; P=0.42). Moreover, there was no significant difference in the overall postoperative complication rate of 13.4% in the taTME and 12.1% in the laTME groups (difference, 1.2%; 95% CI, −2.8% to 5.2%; P=0.53). In the first 30 days after surgery, 1 patient in the taTME group died of septic shock as a result of abdominal infection, and 1 patient in the laTME group died of a cerebrovascular accident. Based on the Clavien-Dindo classification of surgical complications, the distribution of severity between the 2 groups did not differ. Specifically, the overall rate of anastomotic leakage was 6.2% [39 (7.2%) of 544 vs 29 (5.3%) of 545; difference, 1.9%; 95% CI, −1.1% to 5.0%; P=0.21]. In addition, 24 (4.4%) and 19 (3.5%) in the taTME and laTME groups required secondary surgery within 30 days after surgery as a result of anastomotic leakage (21 vs 12), anastomotic bleeding (1 vs 4), intestinal obstruction (0 vs 2), abdominal/pelvic infection (1 vs 1), and intraperitoneal bleeding (1 vs 0).
TABLE 3.
Morbidity and Mortality for taTME and laTME Groups
| n (%) | ||||
|---|---|---|---|---|
| taTME (N=544) | laTME (N=545) | Difference [% (95% CI)]* | P † | |
| Intraoperative complications‡ | 26 (4.8) | 33 (6.1) | −1.3 (−4.2 to 1.7) | 0.42 |
| Hemorrhage | 10 (1.8) | 12 (2.2) | −0.4 (−2.4 to 1.9) | 0.83 |
| Vascular injury | 7 (1.3) | 5 (0.9) | 0.4 (−1.8 to 2.7) | 0.58 |
| Intestinal injury | 4 (0.7) | 5 (0.9) | −0.2 (−2.8 to 1.9) | >0.99 |
| Urologic injury | 3 (0.6)‡ | 4 (0.7)§ | −0.2 (−2.7 to 1.9) | >0.99 |
| Subcutaneous emphysema | 1 (0.2) | 3 (0.6) | −0.4 (−2.9 to 2.1) | 0.62 |
| CO2 embolism | 2 (0.4) | 0 | 0.4 (−0.1 to 0.9) | 0.25 |
| Spleen injury | 0 | 3 (0.6) | −0.6 (−1.2 to 0.1) | 0.25 |
| Pancreatic injury | 0 | 1 (0.2) | −0.2 (−0.5 to 0.2) | >0.99 |
| Postoperative complications‖ | 73 (13.4) | 66 (12.1) | 1.2 (−2.8 to 5.2) | 0.53 |
| Anastomotic leakage | 39 (7.2) | 29 (5.3) | 1.9 (−1.1 to 5.0) | 0.21 |
| Anastomotic bleeding | 4 (0.7) | 7 (1.3) | −0.5 (−2.2 to 1.6) | 0.55 |
| Intestinal obstruction | 10 (1.8) | 22 (4.0) | −2.2 (−4.5 to 0.3) | 0.05 |
| Uroschesis | 7 (1.3) | 4 (0.7) | 0.5 (−1.3 to 2.2) | 0.39 |
| Incisional infection | 1 (0.2) | 1 (0.2) | 0.0 (−2.4 to 2.4) | >0.99 |
| Abdominal/pelvic infection | 8 (1.5) | 6 (1.1) | 0.3 (−1.5 to 2.1) | 0.61 |
| Deep vein thrombosis | 1 (0.2) | 1 (0.2) | 0.0 (−2.4 to 2.4) | >0.99 |
| Pulmonary embolism | 1 (0.2) | 0 | 0.2 (−0.2 to 0.6) | 0.50 |
| Cerebral infarction | 0 | 1 (0.2) | −0.2 (−0.5 to 0.2) | >0.99 |
| Others | 7 (1.3) | 3 (0.6) | 0.7 (−1.1 to 2.4) | 0.22 |
| Mortality | 1 (0.2) | 1 (0.2) | 0.0 (−2.4 to 2.4) | >0.99 |
| Clavien-Dindo classification | ||||
| I–II | 47 (8.6) | 45 (8.3) | 0.86¶ | |
| III–IV | 25 (4.6) | 20 (3.7) | ||
| V | 1 (0.2) | 1 (0.2) | ||
Calculated by Newcombe method with adjustment for center effect.
Calculated by Fisher exact method unless otherwise indicated.
Urethral injury occurred in 2 patients, and ureter injury occurred in 1 patient.
Ureter injury occurred in 4 patients.
More than 1 complication could have occurred per patient.
Calculated by χ2 test.
Pathologic Outcomes
Successful resection, measured by a negative CRM and DRM and complete or nearly complete TME, occurred in 538 (98.9%) patients in the taTME group and 538 (98.7%) patients in the laTME group (difference, 0.2%; 95% CI, −1.9% to 2.2%; P>0.99) (Table 4). In considering components of surgical resection quality, DRM result was negative in 99.6% and 99.3% of patients receiving taTME and laTME, respectively. Clear CRM was obtained in 99.1% of patients irrespective of the type of surgery. The quality of the TME specimen was complete (87.8%) and nearly complete (12.2%) in all of the 1089 cases, whereas none of the patients in had an incomplete TME.
TABLE 4.
Surgical Success Outcomes
| n (%) | |||
|---|---|---|---|
| taTME (N=544) | laTME (N=545) | P | |
| Successful resection* | 538 (98.9) | 538 (98.7) | >0.99 |
| TME | |||
| Complete | 487 (89.5) | 469 (86.1) | 0.10 |
| Nearly complete | 57 (10.5) | 76 (13.9) | |
| Incomplete | 0 | 0 | |
| CRM | |||
| Positive (≤1 mm) | 5 (0.9) | 5 (0.9) | >0.99 |
| Negative (>1 mm) | 539 (99.1) | 540 (99.1) | |
| DRM | |||
| Positive (≤1 mm) | 2 (0.4) | 4 (0.7) | 0.69 |
| Negative (>1 mm) | 542 (99.6) | 541 (99.3) | |
| No. harvested lymph nodes [median (IQR)] | 14 (10–19) | 15 (11–20) | 0.15 |
| Length of resected sample [mean (SD)] (cm) | 12.3 (5.4) | 12.9 (5.2) | 0.06 |
| Lymphovascular invasion | 71 (13.1) | 84 (15.4) | 0.30 |
| Nerve invasion | 63 (11.6) | 56 (10.3) | 0.50 |
| Pathology stage | |||
| 0† | 27 (5.0) | 24 (4.4) | 0.87‡ |
| I | 174 (32.0) | 165 (30.3) | |
| II | 179 (32.9) | 183 (33.6) | |
| III | 164 (30.1) | 173 (31.7) | |
| Tumor differentiation | |||
| Well | 45 (8.3) | 34 (6.2) | 0.43‡ |
| Moderate | 430 (79.0) | 443 (81.3) | |
| Poor | 42 (7.7) | 44 (8.1) | |
Difference, 0.2%; 95% CI, −1.9% to 2.2%, by Newcombe method with adjustment for center effect.
These patients had received preoperative therapy, and had pathologic complete response.
Calculated by χ2 test.
DISCUSSION
The key findings of our TaLaR trial demonstrated taTME could yield similar surgical safety and pathologic outcomes as laTME, and thus it generally seems to be safe and feasible for skilled surgeons. To our knowledge, this study is the first reported multicenter RCT with sufficient power to directly compare taTME with laTME. The primary outcomes of 3-year DFS and 5-year OS are expected on a future date.
Given its technical merits, taTME has the potential to improve the quality of resected specimens with superior radicality, particularly for patients with distal rectal cancer, visceral obesity, bulky tumors, or a narrow pelvis.12,13 However, controversy with respect to the surgical safety and efficacy of taTME persists.14 An analysis of 17 reports comparing 600 patients receiving taTME versus 639 cases undergoing laparoscopic/robotic TME showed taTME is associated with a lower risk of positive CRM.5 By contrast, a recent Norwegian moratorium challenged the status quo of taTME by showing a relatively high local recurrence rate of 9.5% after a median postoperative interval of 11 months.8 In such a contradictory context, our results are of great significance. The initial results from this trial established the surgical safety and feasibility of taTME performed by skilled surgeons in selected patients with rectal cancer. More importantly, pathologic outcomes indicated had taTME could yield adequate surgical resection, similar to laTME.
Due to the very distinct anatomical landmarks as relative to laTME, taTME has shown to be technically challenging and associated with substantial perioperative complications which might impede its feasibility in widespread use in the clinic.15 Treated by skilled surgeons, patients in the taTME group had an acceptable intraoperative complication rate of 4.8%.7 Specifically, the CO2 embolism occurred in 2 of 544 patients in the taTME group, similar to previous report.16 With regard to the postoperative morbidity, the 2 groups had comparable rates of anastomotic leakage; these rates are acceptable and seems to be lower than previous studies.7,17 These data indicate safe implementation of taTME in terms of morbidity in this trial. Moreover, the surgical specimens were extracted through the anus in 512 (94.1%) of 544 patients in the taTME group. This benefit of taTME could reduce surgical incision requirement and provide earlier postoperative recovery, with less time to first flatus, first intake, and ambulation in the taTME group than that in laTME group. Six patients underwent conversion from laTME to transanal surgery due to the difficulties in treating the distal tumors, while conversion occurred in zero case in the taTME group; this could truly reflect the technical advantages of the “bottom-to-up” approach of taTME.
In the current literature, the surgical resection quality for patients receiving taTME varies greatly.18,19 A meta-analysis assessed the pathologic outcomes of taTME versus laTME for middle and low rectal cancer, and results indicated that the positive CRM ranged from 0/46 to 4/34 (11.8%) and that the DRM ranged from 0/41 to 3/41 (7.3%).20 Recently, an observational study using data from an online registry system showed that incomplete TME, positive CRM, and positive DRM were noted in 0.1% (1/849), 2.8% (22/849), and 0.7% (7/849) of patients, respectively.21 Although the findings from different reports could not be directly compared, this trial showed a relatively high rate of successful resection. This phenomenon could be ascribed to the expertise of the surgical teams and the exclusion of patients with the involvement of the CRM as indicated by preoperative magnetic resonance imaging. In addition, all patients who participated in this study were Chinese. Taking the characteristics of Asian populations into consideration, especially the average BMI, which is lower than that observed for non-Asian populations,22 the results might also contribute to the good pathologic outcomes of this trial.
Despite similar surgical safety and pathologic outcomes of taTME versus laTME in this trial, several potential disadvantages of taTME technique should be put forward. First, all the surgeons participating in this trial had technical expertise in taTME. The steep learning curve resulting from the complexity of taTME inevitably impedes the generalizability of our findings. Thus, the establishment of an effective learning system is necessary to foster optimal taTME training. Second, a higher rate of intersphincteric resection was found in patients receiving taTME in this trial, and the sacrifice of the internal sphincter could potentially impair functional outcomes. Third, due to the use of a wide anal platform, patients undergoing taTME suffered from the prolonged anal dilatation, which was likely to interfere with functional results as well. Last, this trial demonstrated the 1-team taTME procedure took longer than laTME, and this might represent a potential disadvantage of taTME. Given these potential limitations of taTME, the clinical acceptance of taTME requires further efforts.
There were several limitations in our study. First, the TME grading was assessed by pathologists in each participating institution. Although an independent review by the CTESC Research Committee was routinely performed, an overestimation bias for successful resection still remains a certain possibility. Second, all study patients enrolled into this trial were subjected to sphincter-sparing surgery. As such, this study cannot present the effects of taTME on sphincter preservation. Third, all participating centers in this trial were in China. Given the unique demographic characteristics of Asian populations, our findings warrant careful consideration when applied to patients from other countries or races.
CONCLUSIONS
In summary, taTME in selected patients with rectal cancer can be safely performed by experienced surgeons and provide satisfactory oncologic radicality. However, the applicability of taTME to rectal cancer patients should continue to be considered until the long-term oncologic outcomes from this well-established RCT are analyzed.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the patients who participated in this study and their families, as well as the nursing and research staff at the study centers.
Footnotes
H.L., Z.Z., H.Z., and M.W. are joint first authors.
P.L., W.T., M.R., J.W., and L.K. are equally contributing senior authors.
Supported by the Sun Yat-sen University Clinical Research 5010 Program (2016005), Science and Technology Projects in Guangzhou (202206010062), China Postdoctoral Science Foundation (2021M703723), and Guangdong Basic and Applied Basic Research Foundation (2021A1515111011).
The authors report no conflicts of interest.
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website, www.annalsofsurgery.com.
This is an open access article distributed under the terms of the Creative Commons Attribution-Non Commercial-No Derivatives License 4.0 (CCBY-NC-ND), where it is permissible to download and share the work provided it is properly cited. The work cannot be changed in any way or used commercially without permission from the journal.
Contributor Information
Huashan Liu, Email: liuhshan@mail2.sysu.edu.cn.
Ziwei Zeng, Email: zengzw@mail2.sysu.edu.cn.
Hong Zhang, Email: haojiubujian1203@sina.cn.
Miao Wu, Email: 13990905852@163.com.
Dan Ma, Email: 1054727918@qq.com.
Quan Wang, Email: wangquanjdyy@163.com.
Ming Xie, Email: 2581303091@qq.com.
Qing Xu, Email: renjixuqing@163.com.
Jun Ouyang, Email: 1847039906@qq.com.
Yi Xiao, Email: xiaoy@pumch.cn.
Yongchun Song, Email: dr.songyongchun@qq.com.
Bo Feng, Email: fengbo2022@163.com.
Qingwen Xu, Email: xuqwen@21cn.com.
Yanan Wang, Email: wyn8116@163.com.
Yi Zhang, Email: yzhangxy3@126.com.
Yuantao Hao, Email: haoyt@mail.sysu.edu.cn.
Shuangling Luo, Email: luoshl3@mail.sysu.edu.cn.
Xingwei Zhang, Email: zhangxw9@mail.sysu.edu.cn.
Zuli Yang, Email: yangzuli@mail.sysu.edu.cn.
Junsheng Peng, Email: pengjsh@mail.sysu.edu.cn.
Xiaojian Wu, Email: wuxjian@mail.sysu.edu.cn.
Donglin Ren, Email: rendl@mail.sysu.edu.cn.
Meijin Huang, Email: hmjin@mail.sysu.edu.cn.
Ping Lan, Email: lanping@mail.sysu.edu.cn.
Weidong Tong, Email: vdtong@163.com.
Mingyang Ren, Email: 2861746489@qq.com.
Jianping Wang, Email: wjp@mail.sysu.edu.cn.
Liang Kang, Email: kangl@mail.sysu.edu.cn.
REFERENCES
- 1. Heald RJ, Husband EM, Ryall RD. The mesorectum in rectal cancer surgery—the clue to pelvic recurrence? Br J Surg. 1982;69:613–616. [DOI] [PubMed] [Google Scholar]
- 2. You YN, Hardiman KM, Bafford A, et al. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the management of rectal cancer. Dis Colon Rectum. 2020;63:1191–1222. [DOI] [PubMed] [Google Scholar]
- 3. Sylla P, Rattner DW, Delgado S, et al. NOTES transanal rectal cancer resection using transanal endoscopic microsurgery and laparoscopic assistance. Surg Endosc. 2010;24:1205–1210. [DOI] [PubMed] [Google Scholar]
- 4. Zhang X, Gao Y, Dai X, et al. Short- and long-term outcomes of transanal versus laparoscopic total mesorectal excision for mid-to-low rectal cancer: a meta-analysis. Surg Endosc. 2019;33:972–985. [DOI] [PubMed] [Google Scholar]
- 5. Lei P, Ruan Y, Yang X, et al. Trans-anal or trans-abdominal total mesorectal excision? A systematic review and meta-analysis of recent comparative studies on perioperative outcomes and pathological result. Int J Surg. 2018;60:113–119. [DOI] [PubMed] [Google Scholar]
- 6. Wasmuth HH, Gachabayov M, Bokey L, et al. Statistical, clinical, methodological evaluation of local recurrence following transanal total mesorectal excision for rectal cancer: a systematic review. Dis Colon Rectum. 2021;64:899–914. [DOI] [PubMed] [Google Scholar]
- 7. Penna M, Hompes R, Arnold S, et al. Incidence and risk factors for anastomotic failure in 1594 patients treated by transanal total mesorectal excision: results from the International TaTME Registry. Ann Surg. 2019;269:700–711. [DOI] [PubMed] [Google Scholar]
- 8. Larsen SG, Pfeffer F, Kørner H. Norwegian Colorectal Cancer Group. Norwegian moratorium on transanal total mesorectal excision. Br J Surg. 2019;106:1120–1121. [DOI] [PubMed] [Google Scholar]
- 9. Deijen CL, Velthuis S, Tsai A, et al. COLOR III: a multicentre randomised clinical trial comparing transanal TME versus laparoscopic TME for mid and low rectal cancer. Surg Endosc. 2016;30:3210–3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lelong B, de Chaisemartin C, Meillat H, et al. A multicentre randomised controlled trial to evaluate the efficacy, morbidity and functional outcome of endoscopic transanal proctectomy versus laparoscopic proctectomy for low-lying rectal cancer (ETAP-GRECCAR 11 TRIAL): rationale and design. BMC Cancer. 2017;17:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fleshman J, Branda M, Sargent DJ, et al. Effect of laparoscopic-assisted resection vs open resection of stage II or III rectal cancer on pathologic outcomes: the ACOSOG Z6051 Randomized Clinical Trial. JAMA. 2015;314:1346–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fernández-Hevia M, Delgado S, Castells A, et al. Transanal total mesorectal excision in rectal cancer: short-term outcomes in comparison with laparoscopic surgery. Ann Surg. 2015;261:221–227. [DOI] [PubMed] [Google Scholar]
- 13. Rouanet P, Mourregot A, Azar CC, et al. Transanal endoscopic proctectomy: an innovative procedure for difficult resection of rectal tumors in men with narrow pelvis. Dis Colon Rectum. 2013;56:408–415. [DOI] [PubMed] [Google Scholar]
- 14. Caycedo-Marulanda A, Brown CJ, Chadi SA, et al. Canadian taTME expert collaboration (CaTaCO) position statement. Surg Endosc. 2020;34:3748–3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Veltcamp Helbach M, van Oostendorp SE, Koedam TWA, et al. Structured training pathway and proctoring; multicenter results of the implementation of transanal total mesorectal excision (TaTME) in The Netherlands. Surg Endosc. 2020;34:192–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dickson EA, Penna M, Cunningham C, et al. Carbon dioxide embolism associated with transanal total mesorectal excision surgery: a report from the International Registries. Dis Colon Rectum. 2019;62:794–801. [DOI] [PubMed] [Google Scholar]
- 17. Perdawood SK, Kroeigaard J, Eriksen M, et al. Transanal total mesorectal excision: the Slagelse experience 2013-2019. Surg Endosc. 2021;35:826–836. [DOI] [PubMed] [Google Scholar]
- 18. Zeng Z, Liu Z, Huang L, et al. transanal total mesorectal excision in mid-low rectal cancer: evaluation of the learning curve and comparison of short-term results with standard laparoscopic total mesorectal excision. Dis Colon Rectum. 2021;64:380–388. [DOI] [PubMed] [Google Scholar]
- 19. Ong GK, Tsai B, Patron RL, et al. Transanal total mesorectal excision achieves equivalent oncologic resection compared to laparoscopic approach, but with functional consequences. Am J Surg. 2021;221:566–569. [DOI] [PubMed] [Google Scholar]
- 20. Lin D, Yu Z, Chen W, et al. Transanal versus laparoscopic total mesorectal excision for mid and low rectal cancer: a meta-analysis of short-term outcomes. Wideochir Inne Tech Maloinwazyjne. 2019;14:353–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yao H, An Y, Zhang H, et al. Transanal total mesorectal excision: short-term outcomes of 1283 cases from a Nationwide Registry in China. Dis Colon Rectum. 2021;64:190–199. [DOI] [PubMed] [Google Scholar]
- 22. WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. [DOI] [PubMed] [Google Scholar]


