Objective:
The aim of this study was to demonstrate the ability of the Versius Surgical System to successfully and safely complete cholecystectomy.
Background:
The system has been developed in-line with surgeon feedback to overcome limitations of conventional laparoscopy to enhance surgeon experience and patient outcomes. Here we present results from the cholecystectomy cohort from a completed early clinical trial, which was designed to broadly align with Stage 2b of the Idea, Development, Exploration, Assessment, Long-term follow-up framework for surgical innovation.
Methods:
Procedures were performed between March 2019 and September 2020 by surgical teams consisting of a lead surgeon and operating room (OR) assistants. Male or female patients aged 18 years and over and requiring cholecystectomy were enrolled. The primary endpoint was the rate of unplanned conversion from robot-assisted surgery to conventional laparoscopic or open surgery. Adverse events (AEs) and serious AEs were adjudicated by video review of the surgery and patient study reports by an independent Clinical Expert Committee.
Results:
Overall, 134/143 (93.7%) cholecystectomies were successfully completed using the device. Of the 9 (6.3%) conversions to another surgical modality, 7 were deemed to be related to the device. A total of 6 serious AEs and 3 AEs occurred in 8 patients (5.6%), resulting in 4 (2.8%) readmissions to hospital within 30 days of surgery and 1 death.
Conclusions:
This study demonstrates cholecystectomy performed using the device is as safe and effective as conventional laparoscopy and supports the implementation of the device on a wider scale, pending instrument modifications, in alignment with Idea, Development, Exploration, Assessment, Long-term follow-up Stage 3 (Assessment).
Keywords: cholecystectomy, clinical trial, general surgery, minimal access surgery, robotic surgical system
Research has demonstrated multiple advantages of minimal access surgery (MAS) over open surgery. These include lower blood loss, reduced incidence of postoperative adhesions, fewer wound complications, reduced postoperative pain, earlier recovery, shortened hospital stays, and improved cosmesis. 1–3 Furthermore, MAS has the potential to reduce surgery’s economic burden on healthcare systems by reducing complication rates and improving recovery times. 4 However, anatomical challenges, two-dimensional vision and restricted instrument range of movement during conventional MAS makes accurate dissection and suturing difficult. 5,6 Thus, many surgeons face a long learning-curve when achieving competency in MAS. 6 Additionally, performing procedures can come at a considerable physical cost to surgeons, which can result in work absence and early retirement. 7–10 More ergonomically designed devices are needed to overcome this challenge. 7,9–11
Compared with conventional laparoscopy, robot-assisted surgery improves surgeon visualization, dexterity and precision, making challenging maneuvers easier to perform. 6,12–14 As such, robotic assistance may allow more surgeons to perform MAS by overcoming technical difficulties with a shallower associated learning-curve. 15 Therefore, robot-assisted surgery may allow a greater range of patients to benefit from MAS, such as those with high body mass indices (BMI). 16,17
The Versius Surgical System (CMR Surgical Ltd, Cambridge, UK) is a new tele-operated robotic surgical system designed to enhance surgeon experience and patient outcomes in MAS. The device was designed to mimic the articulation of the human arm and developed using surgeon feedback. 18,19 The device was designed to provide maximum flexibility within operating rooms (ORs). 18,19 The portable bedside units allow a flexible choice of port placement, enabling surgeons to use their preferences from conventional MAS. 18,19 The combination of the V-wrist and articulated instruments allow 7 degrees of motion within the body to provide greater surgical access and reach compared with standard MAS. 18,19 The console was designed to provide variation in surgeon working position, allowing either sitting or standing. 18,19 The open console design aids communication within ORs. 18,19
The Idea, Development, Exploration, Assessment, Long-term follow-up (IDEAL-D) framework was developed to provide guidance for the safe introduction of complex medical technology and generating a thorough evidence base throughout the process. 20,21 Previous studies on device development, preclinical and clinical testing have aimed to broadly align with different stages of IDEAL-D. Initial studies were designed to align with Stages 0 and 1 (Idea). 8,19,22,23 The usability of the device by surgeons and surgical teams was successfully assessed and a 3.5-day device-specific surgeon training program was validated. 18,24 Preclinical cadaver and live porcine studies demonstrated the device can be used to complete a range of surgeries. 8,22,23 An interim study from this clinical trial broadly aligned with Stage 2a (Development), detailing the device’s use to assist a small number of minor and intermediate surgeries. 25 These surgeries included 9 cholecystectomies, which were all completed successfully without conversion to conventional MAS or open surgery. 25
This study reports a full safety analysis and the ability of the device to successfully complete surgery in a larger cohort of patients requiring cholecystectomy, in addition to the 9 patients from the interim study. 25 This aims to satisfy many of the criteria of Stage 2b (Exploration). 20,21 The focus of this study was patient safety, with device usability assessed in previous studies. 18 The study’s goal is to support the device’s future implementation on a larger scale, to align with Stage 3 (Assessment).
Methods
Ethical Board Review Statement
This study was reviewed and approved by the study hospitals’ Institutional Ethics Committees: Deenanath Mangeshkar Hospital & Research Center, Erandwane, Pune, Maharashtra, India, on February 23, 2019, and the Healthcare Global Manavata Cancer Centre, Mumbai Naka, Nashik, Maharashtra, on October 11, 2019. The study is registered on the Indian Clinical Trials Register (CTRI/ 2019/02/017872). All study activities were performed in compliance with Drugs and Cosmetic Rules 1945-Schedule Y, Indian Council of Medical Research and ISO14155 standards.
Surgeons
Procedures were completed by 5 surgeons, all of whom are accredited, practicing, high-volume general consultant surgeons with extensive experience in MAS. All study surgeons had limited or no experience of using the device in humans before the clinical trial (≤2 cases/surgeon).
One of the operating surgeons is not an author as they joined at the end of the study and did not fulfill all criteria for authorship. Immediately before study commencement, all surgical team members completed and passed a validated 3.5 day training program. 24 This was in addition to completing a didactic online program and simulated practice using the device.
Patients
Patients were recruited as inpatients or outpatients from study hospital surgical lists, if they experienced symptomatic gallbladder disease. Patients were approached directly by their surgeon and provided with relevant study information. Upon entering the study, patients provided both written and audio-visual consent. To be eligible, patients must have been over 18 years and not have been pregnant. Patients were excluded if they suffered from a clinically significant unstable medical disorder or life-threatening disease or, in the investigator’s opinion, there was any reason they were contraindicated to undergo surgery. Patients were excluded if their physical status was American Society of Anesthesiologists (ASA) Class III or higher. 26 In June 2020, this exclusion criterion was changed to ASA Class IV, to extend patient eligibility once several procedures had been safely performed.
Study Design
Patients underwent robot-assisted cholecystectomy at the study hospitals between March 11, 2019 and July 25, 2020. As part of the continual process of performance improvement, modifications were made to the device subsequent to March 2019. After a regulatory request for “Final Finished Device’’ data, an additional 25 surgeries using an updated model were conducted as part of a bridging study between August 22 and September 12, 2020. These procedures took place at a third center due to the COVID-19 pandemic limiting access to the initiating hospitals: Healing Hands Clinic, Fourth Floor, Dhole Patil Rd, Pune, Maharashtra 411001, India.
Patients who passed preoperative screening underwent robotassisted cholecystectomy on Day 1 and received the same pre- and peri-operative standard of care as patients undergoing conventional laparoscopic cholecystectomy. After discharge, patients received follow-up telephone or in-clinic consultations on Day 30 (±2 days) days) and Day 90 (±7 days) (Fig. 1). Patients in the bridging study were followed up to at least Day 30 (±2 days) and all other study procedures were consistent.
Figure 1.
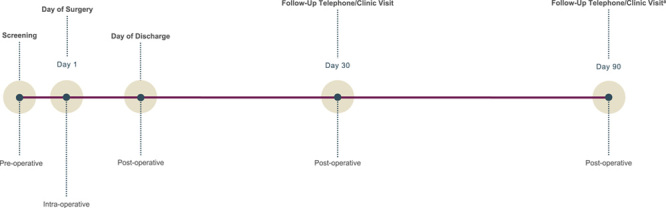
Study design. aPatients in the bridging study were only followed up to at least Day 30.
OR Layout
The system consisted of a surgeon console, 3 instrument bedside units and 1 visualization bedside unit (see Supplemental Digital Content Figures 1 and 2, http://links.lww.com/SLA/D678 which shows an overview of the system). The most common port placements and bedside unit positions are detailed in Fig. 2.
Figure 2.
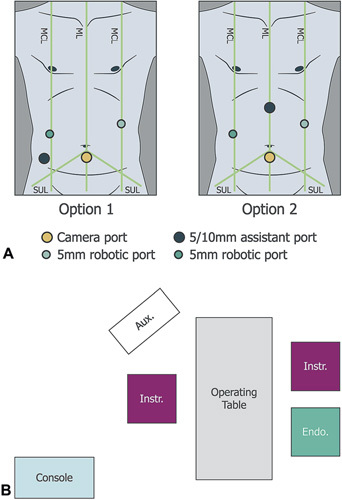
Port positioning and OR layout. Adapted from Kelkar et al. 25 Examples of common port positioning options for cholecystectomy (A) with corresponding bedside unit positions (B). The assistant port was for non-robotic laparoscopic instruments. Umbilicus is where the ML crosses the SUL. Five millimiters robotic ports were positioned on the left and right midclavicular lines, with a third 5 or 10 mm assistant port positioned either superior to the iliac crest (Fig. 2A, option 1) or in the epigastrium (Fig. 2A, option 2). The camera port was usually positioned up to 2 cm below the umbilicus on the midline. However, in high BMI patients, the camera port was positioned above the umbilicus. Aux., auxiliary monitor; Console, surgeon console; Endo., endoscope; Instr., instrument; MCL, midclavicular line; ML, midline; OR, operating room; SUL, supine-umbilical line.
Study Procedures and Evaluations
The primary endpoint was the rate of unplanned conversion of robot-assisted surgery to conventional MAS or open surgery. Secondary endpoints were total operating time (from incision to skin closure); intraoperative complications; estimated intraoperative blood loss; intraoperative blood transfusions; postoperative complications classified by Clavien-Dindo score up to 90 days post-surgery; returns to surgery within 24 hours; and readmissions to hospital within 30 days of cholecystectomy. Ninety-day mortality was also recorded.
In addition, telemetry data were collected for each procedure. These data were not formally assessed except in cases of reported instrument or system malfunction, when they were used to inform future device improvements. They are not reported in this study.
Adverse Events
A Clinical Events Committee (CEC), consisting of experienced surgeons, was established to independently assess adverse events (AEs) and serious adverse events (SAEs) across multiple clinical trials assessing the device. Initially, the CEC comprised of four surgeons (details listed in the Acknowledgements). Due to limited CEC member availability to attend all ongoing adjudication meetings, the CEC was expanded to incorporate 2 further surgeons in 2021.
All possible AEs and SAEs were identified by study surgeons, who recorded detailed information in the study database. The CEC were provided this information along with surgery video recordings and further details regarding SAEs (including SAE ethics committee notifications, SAE follow-up reports, summary operative notes and recovery notes). After reviewing this material individually, CEC members convened at a series of meetings to reach consensus on event classification.
AEs were any untoward medical occurrence, unintended disease or injury, or untoward clinical signs. SAEs included all medical occurrences that were life-threatening or led to death, required hospital admission or prolonged hospitalization, or resulted in persistent disability or permanent damage; 27 and any other event the CEC judged to be “medically significant.” The CEC determined expectedness (expected/unexpected) based on whether the complication was typically listed on a cholecystectomy consent form, and device-relatedness and/or relatedness to “user error’’ (related/probably related/possibly related/not related) based on CEC member consensus. AEs and SAEs were also graded by severity on the Clavien-Dindo Classification system. 28 With this addition of 2 new CEC members in 2021, AEs deemed related to the device were readjudicated.
Statistical Analysis
A sample size of 120 patients was determined to be sufficient to estimate conversion rates with satisfactory accuracy with 95% confidence intervals of an appropriate size, using an alpha of 0.05 and a conversion rate of 6.2% (based upon a literature search). Unless stated otherwise, data summaries were used to present the number of observations, the median and the range. Data were collected in Statistical Analysis System format and all analyses were performed using Statistical Analysis System version 9.4.
Results
Patient Disposition and Baseline Characteristics
A total of 161 patients consented to be included in the study (Fig. 3). Of these, 15 patients were excluded during preoperative screening at the surgeons’ discretion: 13 based upon endoscopic examination (11 due to adhesions; 1 due to adhesions, wall thickening and the presence of gallstones; 1 due to abnormal anatomy), 1 due to preoperative shortness of breath and 1 due to failure to attend their screening appointment. Three patients passed preoperative screening but underwent more complex robot-assisted procedures due to disease state and were excluded from the final analyses (1 total hysterectomy and cholecystectomy; 1 colectomy and cholecystectomy; 1 unilateral inguinal hernia repair and cholecystectomy). †
Figure 3.
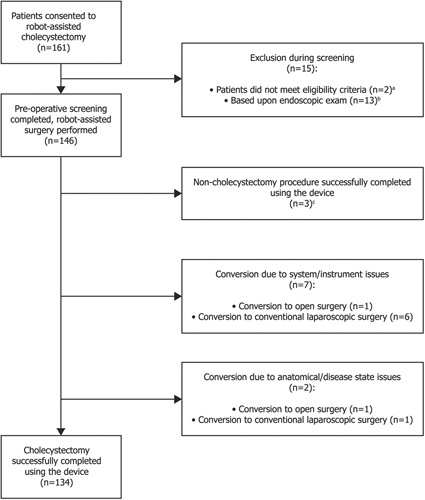
Study CONSORT diagram. aOne patient did not return to the site for screening; bPreoperative laparoscopic endoscopic examination of surgical site revealed anatomical challenges, for example, dense adhesions, resulting in surgeons electing to proceed with conventional laparoscopic or open surgery; cThese procedures included 1 total hysterectomy and cholecystectomy; 1 colectomy and cholecystectomy; 1 unilateral inguinal hernia repair and cholecystectomy.
Of the patients analyzed, 92/143 (64.3%) were female and the median age was 41.0 years (range: 18–72 years; Table 1). The median patient BMI was 26.1 kg/m2 (range 17–40 kg/m2). Of these patients, 27/143 (18.9%) had previously experienced abdominal or pelvic surgery, but none within the year before cholecystectomy (Table 1). The majority (107/143; 74.8%) of patients underwent cholecystectomy to treat cholelithiasis (Table 1).
Table 1.
Patient Characteristics and Surgical History
| Cholecystectomy (n = 143)a | |
|---|---|
| Characteristic | |
| Sex, female | 92 (64.3) |
| Age (yr), median (range) | 41.0 (18–72) |
| Height (cm), median (range) | 158.0 (142–183) |
| Weight (kg), median (range) | 65.0 (41–109) |
| BMI (kg/m2), median (range) | 26.1 (17–40) |
| <18.5 | 4 (2.8) |
| 18.5–<25 | 59 (41.3) |
| 25–<30 | 49 (34.3) |
| 30–<40 | 31 (21.7) |
| ≥40 | 0 (0.0) |
| ASA Status | |
| Class I | 115 (80.4) |
| Class II | 25 (17.5) |
| Class III | 3 (2.1) |
| Diagnoses | |
| Cholecystitis | 26 (18.2) |
| Symptomatic cholelithiasis | 107 (74.8) |
| Uncomplicated cholelithiasis | 104 (72.7) |
| Complicated cholelithiasis | 7 (4.9) |
| Choledocholithiasis | 3 (2.1) |
| Calculous cholecystitis | 2 (1.4) |
| Biliary colic | 1 (0.7) |
| Biliary colic with cholecystitis | 1 (0.7) |
| Benign gallbladder tumor | 3 (2.1) |
| Other | 1 (0.7) |
| Surgical history | |
| Previous abdominal/pelvic surgeries | |
| Yes | 27 (18.9) |
| No | 116 (81.1) |
Data are expressed as n (%) unless specified otherwise.
ASA, American Society of Anesthesiologists; BMI, body mass index.
Intraoperative Endpoints
Median operative time (from incision to skin closure) was 92 minutes (range: 25–304 minutes; Fig. 4A). In the first 50 patients, median operative time was 102 minutes, decreasing to 88.5 minutes in the second 50 patients and 74 minutes in the final 43 patients.
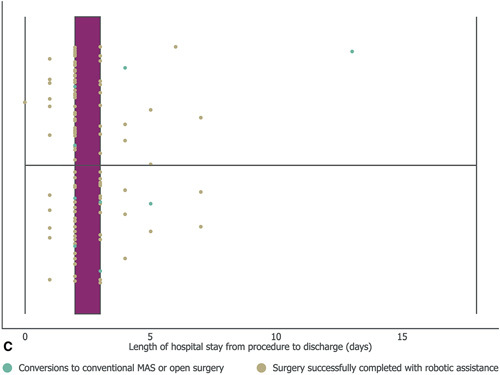
Figure 4.
Outcomes of robot-assisted cholecystectomy. Operative time from first incision to skin closure (A);estimated intraoperative blood loss (B); and number of days from operation to dischargec (C). For A and C middle vertical lines represent the medians, box edges represent the first and third quartiles, and lower and upper whiskers extend to the respective lowest and highest values. aIncludes patients recorded as <100 ml. bIncludes patients recorded as <500 ml;cBoth first quartile and the median were 2 days.
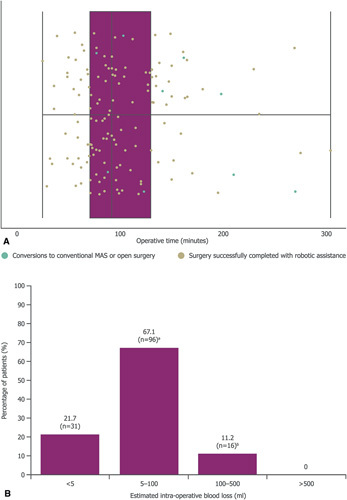
Estimated intraoperative blood loss was low, with 31/143 (21.7%) patients reporting less than 5 ml, and no patient losing more than 500 ml or requiring a blood transfusion (Fig. 4B).
Postoperative Endpoints
The median time from procedure to discharge was 2 days (range: 0–18 days; Fig. 4C). Two patients were hospitalized for over 10 days postoperatively. One patient was preemptively hospitalized for 18 days, as they lived a long distance from the study site and faced transportation issues if needing to reattend hospital. This patient experienced no postoperative complications. Another patient remained hospitalized for 13 days postoperatively while experiencing autoimmune hemolytic anemia and breathlessness after common bile duct stent removal.
Conversions and Adverse Events
Of the robot-assisted cholecystectomies, 134/143 (93.7%) were completed successfully (Fig. 3), 7/143 (4.8%) required conversion to laparoscopic surgery and 2/143 (1.4%) required conversion to open surgery (Table 2). On 7 occasions conversion was device related. Five of these conversions were due to robotic grasper failure to grip the gallbladder; 1 was due to robotic arm instability; and 1 was due to device inability to retrieve spilled stones.
Table 2.
Conversions
| Surgery Number | Conversion Technique | Previous Abdominal Surgery? | Reason for Conversion |
|---|---|---|---|
| 37 | Laparoscopic | No | Failure of fenestrated graspers |
| 43 | Laparoscopic | No | Spilled stones could not be retrieved robotically |
| 47 | Laparoscopic | Yes | Obscure anatomy and densely frozen Calot’s Triangle |
| 60 | Laparoscopic | No | Failure of graspers to hold thickened gallbladder wall |
| 62 | Laparoscopic | Yes | Failure of graspers to hold gallbladder wall |
| 75 | Laparoscopic | Yes | Robotic arm unstable |
| 110 | Open | No | Failure of graspers to hold thickened gallbladder wall |
| 112 | Laparoscopic | No | Failure of graspers to hold thickened gallbladder wall |
| 114 | Open | No | Dense pericholecystic adhesions |
After evaluation by the CEC, 8/143 (5.6%) patients were classified as experiencing a total of 3 AEs and 6 SAEs (Table 3). All of these occurred postoperatively. No patients returned to surgery within 24hours and 4 (2.8%) patients were readmitted to hospital within 30 days.
Table 3.
Adverse Events
| Surgery Number | Adverse Event | Days After Procedure | Seriousness (AE/SAE)a | CDC Grade | Reltedness to Device | Reltedness to “User Error”a | Expectednessa | Outcome |
|---|---|---|---|---|---|---|---|---|
| 5 | Acute gastroenteritis | 7 | AE | I | Not related | Not related | Unexpected | Re-hospitalization. Medical treatment. Recovered |
| 6 | Abdominal pain and gastroenteritis | 33 | SAE | I | Not related | Not related | Expected | Re-hospitalization. Medical treatment. Recovered |
| 23 | Leak in cystic duct stump | 3 | SAE | IIIb | Not relatedb | Related | Expected | Re-hospitalization. ERCP, sphincterotomy and CBD stenting performed. Recovered |
| 41 | Gastritis | 2 | SAE | I | Not related | Not related | Expected | Re-hospitalization. Medical treatment. Recovered |
| 65 | Abdominal pain | 25 | AE | II | Not related | Not related | Expected | Medical treatment. Recovered |
| 98 | Abdominal pain | 16 | AE | II | Not related | Not related | Expected | Medical treatment. Recovered |
| 110c | Fever | 4 | SAE | II | Not related | Not related | Unexpected | Patient was diagnosed with autoimmune hemolytic anemia |
| Breathlessness after common bile duct stent removal | 7 | SAE | IVa | Related | Not related | Unexpected | Patient not discharged. Patient moved to ICU for close monitoring and medical treatment. Recovered | |
| 141 | Abdominal pain and breathlessness after suspect cystic duct leak | 4 | SAE | V | Possibly relatedd | Related | Unexpected | Re-hospitalization. Patient received ventilation support. Patient went into septic shock. Fatal |
AEs adjudicated by the CEC.
Initial adjudication from the CEC ruled this event to be related to the device.
Surgery converted to open surgery after robotic grasper failure.
Initial adjudication from the CEC ruled this event to be probably related to the device.
AE, adverse event; CBD, common bile duct; CDC, Clavien-Dindo Classification; CEC, Clinical Events Committee; ERCP, endoscopic retrograde cholangiopancreatography; ICU, intensive care unit; SAE, serious adverse event.
Three patients who successfully underwent robot-assisted cholecystectomy experienced 1 AE each. These AEs included acute gastroenteritis and abdominal pain. None of these AEs were judged to be device related or user error. One of these patients was electively readmitted to hospital due to acute gastroenteritis 7 days postsurgery, where they made a full recovery. Of these 3 AEs, only this acute gastroenteritis was deemed to be unexpected.
Four patients who successfully underwent robot-assisted cholecystectomy experienced 1 SAE each, and 1 patient experienced 2 separate SAEs. Two patients experienced gastrointestinal symptoms, 2 and 33 days after their surgery, respectively, and were readmitted to hospital as emergencies and made full recoveries. Both cases were judged to be expected and unrelated to the device or user error.
One patient was re-hospitalized as an emergency 3 days postsurgery after a cystic duct stump leak. Initially, the CEC deemed this SAE to be expected and device related, due to intraoperative surgeon difficulty with instrumentation. However, with the addition of 2 new CEC members, 2 surgeons (1 new member and 1 existing member) reassessed this event to not be related to the device, but related to user error. This patient recovered after endoscopic retrograde cholangiopancreatography, sphincterotomy and common bile duct stenting.
One patient experienced 2 SAEs before discharge. This patient’s cholecystectomy was converted to open surgery, due to grasper failure to grip a grossly thickened gallbladder. This patient additionally underwent successful endoscopic retrograde cholangiopancreatography, sphincterotomy and common bile duct stenting 2 days post-cholecystectomy. They experienced 2 SAEs: fever 4 days after cholecystectomy, which was diagnosed as autoimmune hemolytic anemia and deemed unrelated to the device or user error; and breathlessness after removal of their common bile duct stent 7 days post-cholecystectomy. This resulted in the patient being moved to an intensive care unit for close monitoring and medical treatment. The patient recovered and was discharged from hospital 13 days postoperatively. As the surgery was converted due to instrument performance, the CEC felt it was reasonable to assume this was the root cause of this second SAE and this was, therefore, device related. When 2 further members were added to the CEC, this event was readjudicated, but the previous decision of device relatedness was upheld. Both SAEs were classified as unexpected.
One death occurred in the study. The patient was a participant in the bridging study and was the 141st of the 143 patients to undergo robot-assisted cholecystectomy. Before surgery, the patient was diagnosed with cholecystitis and categorized as ASA Class III, meaning they either had severe systemic disease that was not incapacitating; or moderate or moderate-to-severe systemic disease with operative and anesthetic risk. 26 Their procedure was completed without conversion and no AEs were noted before discharge the following day. The patient was readmitted to hospital 4 days postoperatively, with complaints of abdominal pain and breathlessness. COVID-19 was ruled out by polymerase chain reaction testing. The patient was returned to theatre for laparoscopic examination and signs of suspected cystic duct leak were observed. In total, 1 liter of abdominal fluid was drained from the abdomen. After a 5-liter saline lavage, a drain was inserted. The following morning, after further reports of breathlessness and a diagnosis of septic shock, the patient was placed on a ventilator. The patient’s death was reported that afternoon, 5 days post-surgery with “septic shock with acute cardiorespiratory arrest’’ listed as the cause of death. On video review, the CEC noted that there was a gross distortion of the patient anatomy due to adhesions and at no point was the surgeon able to get a clear surgical view. However, the surgeon also experienced difficulty using the available instruments to grasp sufficiently and commented that “there was a combination of surgeon and device error.’’ Considering this was a new device and, given the difficulties with the distorted anatomy, the surgeon could have considered converting to a more familiar approach. Therefore, the CEC judged this death to be probably related to the device. At re-adjudication after the addition of 2 new CEC members, 2 surgeons (1 new member and 1 existing member) re-assessed this event to be possibly related to the device and related to user error.
Discussion
This prospective clinical study demonstrates surgeons were able to successfully complete multiple robot-assisted cholecystectomies using the device, experiencing few surgical difficulties and without raising safety concerns.
Of the 143 robot-assisted cholecystectomies performed, 134 were successfully completed without conversion to another surgical modality. In all 9 cases of conversion, cholecystectomy was successfully completed using the alternative modality. Of the 9 converted surgeries, 7 were device-related conversions, most commonly due to failure of the graspers. However, no intraoperative device-related AEs were recorded and device failure is a known risk with robotic surgical systems. 29 Nevertheless, surgeon feedback and surgical reports from this early study are being used to inform ongoing instrument improvement programs, including refinement of the graspers.
Median operative time was approximately 1.5 hours and estimated intraoperative blood loss was minimal. Median operative times decreased throughout the study, suggesting they may decrease as surgeons gain experience with the device. Additionally, operative times in this study were comparable to those seen in early use of other robotic devices for cholecystectomy. 12 However, the focus of this study and the participating surgeons was on patient safety and not operative speed. Time taken to set up the device and operate will be investigated in later studies, to assess cost-effectiveness in line with IDEAL-D Stage 3 (Assessment). 20,21 These studies will also assess deviations from average procedure time and report learning curves for surgeons new to the device (eg, see Supplemental Digital Contents 3 and 4, http://links.lww.com/SLA/D679 which show an individual control chart and a normalized cumulative sum learning curve for procedure duration for 1 of the surgeons from this study).
Postoperatively, no patients were re-admitted to the OR within 24 hours and most patients were discharged from hospital within 3 days and recovered without incident. Lengths of hospital stay were comparable to patients undergoing laparoscopic cholecystectomy. 30 Generally, patients did not exceed the 4-day postoperative stay protocol often enacted at the study hospital, due to patient difficulties in re-attending because of travel limitations.
AEs and SAEs were adjudicated by the CEC, comprised of experienced surgeons reviewing video recordings of the procedures and patients’ hospital records. The CEC ensured independent and consensus-based decisions were reached with regards to the seriousness, expectedness and device-relatedness of all reported AEs and SAEs. The level of scrutiny and transparency offered by independent video review is important in accountably verifying device safety. Although this practice has been used in a small number of studies, it is still relatively rare in the robotic surgery field. 31–33 In total, 3 AEs and 6 SAEs were recorded, all of which occurred postoperatively. Two SAES were deemed to be device related and 1 SAE deemed to be related to user error.
One of the SAEs was the death (due to septic shock) of a patient 5 days after a successful robot-assisted cholecystectomy, which the CEC judged to be related to user error. The patient was categorized as ASA Class III, a known risk factor for mortality in cholecystectomy. 34 Nevertheless, the mortality rate in this study (1/ 143, 0.7%) is greater compared with previous clinical trials investigating the early use of innovation in cholecystectomy. 35 This demonstrates that persisting in unfavorable anatomy in early clinical trials can be challenging, when individual adverse events can potentially skew data against new devices due to low patient numbers. Therefore, surgeons must be carefully selected in the early stages of device development and should make conservative decisions when facing surgical difficulty. Furthermore, continual efforts are required to ensure any trends in patient safety data are quickly highlighted and promptly addressed. As such, data collected in all clinical studies using the device are entered into the Versius registry, an ongoing collection of real-world data to evaluate ongoing patient safety. This registry has been designed to broadly align with IDEAL-D Stage 4 (Long-Term Study), by enabling surveillance of rare events, long-term clinical outcomes and quality assurance. 20,21
This cholecystectomy cohort was studied in tandem with a cohort requiring gynecological surgery, which found the device to be safe and effective when performing hysterectomies. 36
Conclusions
This study demonstrates cholecystectomy can be performed with the assistance of the device with a similar efficacy and safety profile to conventional laparoscopy but may not be suitable for cases of severe inflammation or thickening of the gallbladder wall pending further investigations and device modification. This study broadly aligns with IDEAL-D Stage 2b (Exploration), and supports the implementation of the robot on a wider scale in alignment with Stage 3 (Assessment), to provide evidence of middle- and long-term clinical outcomes. 20,21
Supplementary Material
Acknowledgments
The authors thank the patients, the investigators and their teams who took part in this study.
Footnotes
Dr Lewis Stevens is a paid consultant for CMR Surgical and Dr Mark Slack is Chief Medical Officer and founder of CMR Surgical.
This study was sponsored by CMR Surgical. Support for third-party writing assistance for this article was funded by CMR Surgical in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
Clinical Events Committee: The authors thank the members of the Clinical Events Committee for their independent adjudication of all adverse events: Miss Elly Brockbank, Barts Health NHS Trust, London, UK; Prof. James Fleshman, Baylor University Medical Center, Dallas, TX, USA; Mr Richard Hardwick, Cambridge University Hospitals NHS Foundation Trust, Cambridge, UK; Prof. Catherine Matthews, Wake Forest Baptist Health, Winston-Salem, NC, USA; Dr Adrian Park, Anne Arundel Medical Center, Johns Hopkins University, Annapolis, MD, USA; and Professor Steven Schwaitzberg, Jacobs School of Medicine and Biomedical Sciences, University of Buffalo, Buffalo, NY, USA.
Medical Writing Support: The authors also acknowledge Timothy Jones, VetMB, Marc Lynch, PhD, and Emma Phillips, PhD from Costello Medical, UK, for medical writing and editorial assistance based on the authors’ input and direction.
Authors’ contributions: Substantial contributions to study conception and design: DK, UK, LS, GW, MS; substantial contributions to analysis and interpretation of the data: DK, UK, LS, GW, MS; drafting the article or revising it critically for important intellectual content: DK, UK, LS, GW, MS; final approval of the version of the article to be published DK, UK, LS, GW, MS.
The authors report no conflicts of interest.
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website, www.annalsofsurgery.com.
One study surgeon performed 2 surgeries in humans using the device before study commencement (1 appendectomy and 1 bilateral oophorectomy), 2 study surgeons performed 1 surgery each (1 appendectomy and 1 left hemicolectomy) and the remaining 2 study surgeons had not used the device in humans before.
All of these procedures were completed successfully with Versius assistance and without device-related AEs.
References
- 1. Keus F, de Jong JA, Gooszen HG, et al. Laparoscopic versus open cholecystectomy for patients with symptomatic cholecystolithiasis. Cochrane Database Syst Rev. 2006;18:Cd006231. [DOI] [PubMed] [Google Scholar]
- 2. Abu Gazala M, Wexner SD. Re-appraisal and consideration of minimally invasive surgery in colorectal cancer. Gastroenterol Rep (Oxf). 2017;5:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Weaver A, Steele S. Robotics in colorectal surgery. F1000Res. 2016;5. [DOI] [PMC free article] [PubMed]
- 4. Xu T, Hutfless SM, Cooper MA, et al. Hospital cost implications of increased use of minimally invasive surgery. JAMA Surg. 2015;150:489–490. [DOI] [PubMed] [Google Scholar]
- 5. Giulianotti PC, Coratti A, Angelini M, et al. Robotics in general surgery: personal experience in a large community hospital. Arch Surg. 2003;138:777–784. [DOI] [PubMed] [Google Scholar]
- 6. Morris B. Robotic surgery: applications, limitations, and impact on surgical education. MedGenMed. 2005;7:72. [PMC free article] [PubMed] [Google Scholar]
- 7. Janki S, Mulder E, Ijzermans J, et al. Ergonomics in the operating room. Surg Endosc. 2017;31:2457–2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Morton J, Hardwick RH, Tilney HS, et al. Preclinical evaluation of the versius surgical system, a new robot-assisted surgical device for use in minimal access general and colorectal procedures. Surg Endosc. 2021;35:2169–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Reyes DA, Tang B, Cuschieri A. Minimal access surgery (MAS)-related surgeon morbidity syndromes. Surg Endosc. 2006;20:1–13. [DOI] [PubMed] [Google Scholar]
- 10. Stucky CH, Cromwell KD, Voss RK, et al. Surgeon symptoms, strain, and selections: systematic review and meta-analysis of surgical ergonomics. Ann Med Surg (Lond). 2018;27:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Morton J, Stewart G. The burden of performing minimal access surgery: ergonomics survey results from 462 surgeons across Germany, the UK and the USA. J Robot Surg. 2022;16:1347–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jayaraman S, Davies W, Schlachta CM. Getting started with robotics in general surgery with cholecystectomy: the Canadian experience. Can J Surg. 2009;52:374–378. [PMC free article] [PubMed] [Google Scholar]
- 13. Moorthy K, Munz Y, Dosis A, et al. Dexterity enhancement with robotic surgery. Surg Endosc. 2004;18:790–795. [DOI] [PubMed] [Google Scholar]
- 14. Prasad SM, Prasad SM, Maniar HS, et al. Surgical robotics: impact of motion scaling on task performance. J Am Coll Surg. 2004;199:863–868. [DOI] [PubMed] [Google Scholar]
- 15. Ahlering TE, Skarecky D, Lee D, et al. Successful transfer of open surgical skills to a laparoscopic environment using a robotic interface: initial experience with laparoscopic radical prostatectomy. J Urol. 2003;170:1738–1741. [DOI] [PubMed] [Google Scholar]
- 16. Gehrig PA, Cantrell LA, Shafer A, et al. What is the optimal minimally invasive surgical procedure for endometrial cancer staging in the obese and morbidly obese woman? Gynecol Oncol. 2008;111:41–45. [DOI] [PubMed] [Google Scholar]
- 17. Seamon LG, Cohn DE, Henretta MS, et al. Minimally invasive comprehensive surgical staging for endometrial cancer: Robotics or laparoscopy? Gynecol Oncol. 2009;113:36–41. [DOI] [PubMed] [Google Scholar]
- 18. Haig F, Medeiros ACB, Chitty K, et al. Usability assessment of Versius, a new robot-assisted surgical device for use in minimal access surgery. BMJ Surg Interv Health Technol. 2020;2:e000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hares L, Roberts P, Marshall K, et al. Using end-user feedback to optimize the design of the Versius Surgical System, a new robot-assisted device for use in minimal access surgery. BMJ Surg Interv Health Technol. 2019;1:e000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McCulloch P, Altman DG, Campbell WB, et al. No surgical innovation without evaluation: the IDEAL recommendations. Lancet. 2009;374:1105–1112. [DOI] [PubMed] [Google Scholar]
- 21. Sedrakyan A, Campbell B, Merino JG, et al. IDEAL-D: a rational framework for evaluating and regulating the use ofmedical devices. BMJ. 2016;353:i2372. [DOI] [PubMed] [Google Scholar]
- 22. Carey M, Bali A, Pandeva I, et al. Preclinical evaluation of a new robotassisted surgical system for use in gynecology minimal access surgery. Gynecol Surg. 2020;17:2. [Google Scholar]
- 23. Thomas. Preclinical evaluation of the Versius Surgical System, a new robotassisted surgical device for use in minimal access renal and prostate surgery. Eur Urol Focus. 2020;7:444–452. [DOI] [PubMed] [Google Scholar]
- 24. Butterworth J, Sadry M, Julian D. Assessment of the training program for Versius, a new innovative robotic system for use in minimal access surgery. BMJ Surg Interv Health Technol. 2021;3:e000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kelkar D, Borse MA, Godbole GP, et al. Interim safety analysis of the first-inhuman clinical trial of the Versius surgical system, a new robot-assisted device for use in minimal access surgery. Surg Endosc. 2020;35:5193–5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Daabiss M. American Society of Anaesthesiologists physical status classification. Indian J Anaesth. 2011;55:111–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. FDA. Reporting serious problems to the FDA: what is a serious adverse event? 2016. Available online at: https://www.fda.gov/safety/medwatch/howtoreport/ucm053087.htm. Accessed February 18, 2022.
- 28. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Alemzadeh H, Raman J, Leveson N, et al. Adverse events in robotic surgery: a retrospective study of 14 years of FDA data. PLoS One. 2016;11:e0151470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rosenmüller M, Haapamaki MM, Nordin P, et al. Cholecystectomy in Sweden 2000–2003: a nationwide study on procedures, patient characteristics, and mortality. BMC Gastroenterol. 2007;7:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bonrath EM, Gordon LE, Grantcharov TP. Characterising ’near miss’ events in complex laparoscopic surgery through video analysis. BMJ Qual Saf. 2015;24:516–521. [DOI] [PubMed] [Google Scholar]
- 32. Jung JJ, Jüni P, Lebovic G, et al. First-year analysis of the operating room black box study. Ann Surg. 2020;271:122–127. [DOI] [PubMed] [Google Scholar]
- 33. Moulton D. Surgical black box may sew up malpractice cases. CMAJ. 2015;187:794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sandblom G, Videhult P, Crona Guterstam Y, et al. Mortality after a cholecystectomy: a population-based study. HPB (Oxford). 2015;17:239–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Noguera JF, Cuadrado A, Dolz C, et al. Prospective randomized clinical trial comparing laparoscopic cholecystectomy and hybrid natural orifice transluminal endoscopic surgery (NOTES) (NCT00835250). Surg Endosc. 2012;26:3435–3441. [DOI] [PubMed] [Google Scholar]
- 36. Borse M, Godbole G, Kelkar D, et al. Early evaluation of a next-generation surgical system in robot-assisted total laparoscopic hysterectomy: A prospective clinical cohort study. Acta Obstet Gynecol Scand. 2022;101:978–986. [DOI] [PMC free article] [PubMed] [Google Scholar]


