Abstract
Intravital microscopy of the pulmonary microcirculation in research animals is of great scientific interest for its utility in identifying regional changes in pulmonary microcirculatory blood flow. Although feasibility studies have been reported, the pulmonary window can be further refined into a practical tool for pharmaceutical research and drug development. We have established a method to visualize and quantify dynamic changes in three key features of lung function: microvascular red blood cell velocity, flow direction, and hemoglobin saturation. These physiological parameters were measured in an acute closed-chest pulmonary window, which allows real-time images to be captured by fluorescence and multispectral absorption microscopy; images were subsequently quantified using computerized analysis. We validated the model by quantifying changes in microcirculatory blood flow and hemoglobin saturation in two ways: 1) after changes in inspired oxygen content and 2) after pharmacological reduction of pulmonary blood flow via treatment with the β1 adrenergic receptor blocker metoprolol. This robust and relatively simple system facilitates pulmonary intravital microscopy in laboratory rats for pharmacological and physiological research.
Keywords: thoracic window in rats, pulmonary intravital microscopy in rats, red blood cell flux, hemoglobin saturation
the lung is of major interest for pathophysiological research and drug development, and, therefore, methods to accurately analyze its physiology in preclinical models are in high demand. Intravital microscopy holds promise for measuring and subsequently understanding dynamic changes in the pulmonary microcirculation (2). However, pulmonary microcirculation is difficult to image without compromising its overall function. The importance of maintaining intrapleural negative pressure is paramount. Furthermore, the lung surface is fragile and easily damaged in the process of creating a window. In addition, functional microscopy requires a lengthy period of immobilization, which can interfere with the continuous movement during breathing of a healthy lung. Two main strategies have been employed to overcome these technical hurdles: 1) a window has been inserted into, and anchored to, the chest wall via surgical suture or glue to permit visualization of the thoracic cavity; 2) more recently, a position-stabilized window has been inserted where local negative pressure was used to eliminate motion of the lung surface in relation to the window (5, 6, 12). Although this advanced technique allows for cell tracking and quantification of particle movement, it is difficult to determine how the application of a local vacuum will alter natural capillary blood flow, and how it may stimulate arteriovenous shunting. In addition, manual cell tracking, which is the principal method to analyze blood cell movement, limits assessment of changes in network hemodynamics. Thus, although progress has been made, functional intravital microscopy in the rodent lung is not yet at a development level that allows its widespread use in preclinical research.
Here, we aimed to develop a simplified surgical approach to facilitate measurement of rat pulmonary microcirculation, and to test its reproducibility under control and manipulated conditions. We have independently established an approach to measure red blood cell flow and capillary blood oxygenation in the rat lung, using automated blood cell tracking, and multispectral absorption imaging. We have investigated the utility of this method to serially measure physiological changes in the pulmonary microcirculation in response to two conditions: 1) inspired hypoxia, which alters hemoglobin saturation, and 2) treatment with cardiovascular drugs that decrease pulmonary perfusion.
MATERIALS AND METHODS
Surgery and pulmonary window implantation.
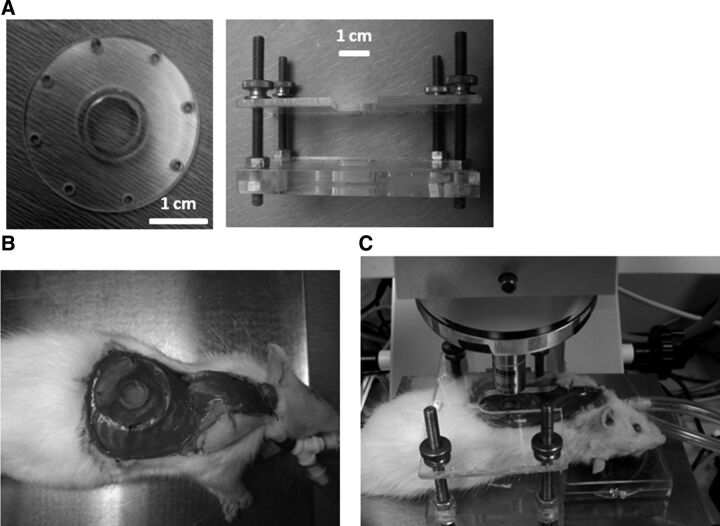
All surgical procedures were performed in accordance with Duke University Medical Center Institutional Animal Care and Use Committee (IACUC) protocols. Rats were anesthetized using an intraperitoneal injection of 50 mg/kg pentobarbital to achieve surgical-level anesthesia. Anesthetized animals were shaved on the right chest and neck areas and fixed in the supine position on a metal plate placed on a 37°C heating pad. Vital signs were monitored using pulse oximetry (MouseOx, Starr Life Sciences, Oakmont, PA), with blood oxygenation and heart rate recorded. A cervical skin incision was made horizontally above the jugular notch of the manubrium, and the cervical trachea was exposed by vertical separation of the sternohyoid and sternocleidomastoid muscles. The trachea was dissected and separated from surrounding muscle and connective tissue; a transverse incision was made on the anterior tracheal wall between the second and third tracheal rings. A 2.5- to 3.0-mm “Y” tracheal cannula (Kent Scientific, Torrington, CT) was inserted into the trachea and secured with a suture. The animal was then repositioned in left lateral recumbancy. The tracheal cannula was connected to a pressure-cycled ventilator (Kent Scientific) to maintain positive pulmonary pressure. The ventilator was connected in line with an OxyDial oxygen blender (Starr Life Sciences). O2 (100%) was delivered during the surgical procedure. Once connected to the ventilator, the superficial and serratus anterior muscles were dissected from the right lateral chest wall. The intercostal muscles were left intact until the next step, which involved removal of a portion of the sixth and seventh ribs. Both ribs were cut dorsally and ventrally to isolate a length of 1.2 cm. These ribs were removed as follows. To minimize damage to the lung surface by the edge of the ribs and minimize the bleeding of the intercostal vessels, the middle of the rib was held tightly with forceps. The medial side of the rib was cut first, with the rib lifted gently with the forceps. The lateral side of the same rib was then separated. After removal of the rib segments and intercostal muscles, the parietal pleura was excised to create a 1.5-cm-diameter chest wall perforation. This procedure exposed the lung surface. Positive pressure in the lung was maintained by the ventilator with expiration tube connected with a water bottle (the tube merged in water ∼6 cm). A custom-fabricated lung window, consisting of a 1-cm-diameter cover glass glued to a Plexiglas socket (Fig. 1A) was placed in the resulting perforation in the chest wall to maintain direct contact with the visceral pleura of the lung. The window frame was then sutured via perforations at its edge to intercostal muscle using 4.0 monofilament synthetic thread. As it was sutured, residual air was allowed to escape until a seal was created (Fig. 1B). Following surgery and stabilization of vital signs, the animal was moved to a microscope stage covered with a thermostatic heating blanket set at 37°C. The animal was positioned in a custom-designed frame to reduce z-directional movement (Fig. 1, B and C).
Fig. 1.
Surgical procedures for pulmonary window fenestration. A: Plexiglas window holder with socket and window glued to it, and rat restrainer to eliminate z-directional movement. B: anesthetized intubated rat with implanted pulmonary window. C: rat with window in a restrainer, placed on a heating pad under a fluorescence upright microscope.
Preparation of fluorescently labeled red blood cells.
Donor rats (female Sprague-Dawley) were deeply anesthetized with pentobarbital (50 mg/kg ip). Rats were exsanguinated using cardiac puncture, and 10–14 ml of blood was collected in heparin. The blood was then centrifuged at 3,000 g for 3 min at 4°C. Plasma was removed and replaced with sterile saline, and the blood cell pellet was centrifuged and washed three times. Packed red blood cells (100 μl) were mixed with 100 μl of 0.5 mg/ml DiI (1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate) solution (Sigma Aldrich, St. Louis, MO), 2 ml of 5% dextrose solution in water, and brought up to a 10-ml final volume with saline. DiI labels cells by integrating into the plasma membrane and moving laterally until the whole cell is labeled (10). The solution was incubated at room temperature in the dark for 30 min and then centrifuged and washed three times with saline. Before injection, the red blood cells were suspended in saline to achieve a hematocrit of ∼30%. Then 500 μl of this red cell suspension was infused into the jugular vein of the anesthetized rat over ∼30 s.
Blood flow measurements.
Animals were anesthetized with 60 mg/kg of pentobarbital. Redosing with 10 mg/kg pentobarbital was done at least 15 min before starting the experiment for measurements of red blood cell velocity. Animals were injected with the labeled red blood cells after the surgery was complete and at least 15 min before imaging began. Depth of anesthesia was such that redosing of anesthetics was not required during the imaging cycle. There were nine image sequences (65–100 images/sequence, frame rate of 16.4–23.6 Hz, frame rate and stack size kept constant throughout each experiment) taken, with 5-min intervals between each consecutive image set. After three imaging cycles, 10 mg/kg metoprolol was injected intravenously, and three cycles later the injection was repeated. For both flow and HbO2 measurements, mechanical ventilation was deliberately paused for a maximum of 15 s. The animals were ventilated with 100% O2, and arterial hemoglobin oxygen saturation was maintained above 99%.
Measurement of hemoglobin saturation using hyperspectral microscopy.
Anesthesia procedures, surgery, intubation, and ventilation were as described in the previous section. The level of inspired oxygen was maintained at 100% for at least three images, 12% or 16% for three consecutive images, and then 100% for the remaining three images. The 100% inspired oxygen typically yields 99% arterial hemoglobin oxygen saturation. Images were acquired during a short period of apnea, as described above. Optical microscopy was performed using an upright Zeiss fluorescence microscope (Axioscop, Carl Zeiss, Thornwood, NY). The imaging techniques and analyses have been described in detail previously (14) but are briefly outlined here. The rat, on the ventilator, was placed under the microscope objective. To enable bright field imaging, a separate optical fiber lamp was used to illuminate the window from a position adjacent to the objective (Techniquip, Roseville, CA). The illuminated lung surface was imaged at a series of discrete wavelengths using a liquid crystal tunable filter that spectrally filtered the light before incidence upon the camera (VariSpec, CRi). Reflectance images were acquired at wavelengths from 500 to 590 nm, in 10-nm increments. Before processing, the spectral profile of the imaging system was acquired using a 99% reflectance standard (Labsphere, SRS-99-010). The ratio of the reflectance standard to the tissue intensity was taken as a function of wavelength and the logarithm of this ratio describes the spectral absorption of the tissue according to the equation:
where Ical is the calibrated reflectance standard intensity and Itissue is the tissue reflected intensity at the same wavelength (after the dark signal offset is subtracted from each term). These values were normalized to exposure time. The total absorption term (A) can be broken down into constitutive components: b0 is a constant offset term, and μeff is the effective attenuation coefficient calculated for representative skin optical properties at each wavelength (http://omlc.ogi.edu/news/jan98/skinoptics.html) and accounting for attenuation due to non-hemoglobin absorption and scattering. b1 is a free parameter modulating the magnitude of this term. εi(λ) gives the extinction coefficient of the ith absorber of interest and is a fixed parameter. In this case, oxygenated and deoxygenated hemoglobin are the absorbers of interest, and their extinction coefficients are known (http://omlc.ogi.edu/spectra/hemoglobin/). A linear, nonnegative least squares-fitting algorithm is used to extract the terms b0, b1, and Ci on a pixel-by-pixel basis. Ci is a composite term that represents the product of the concentration and path length of light attenuated by each absorber. This model, within which only a single composite path length is used to represent the distribution of path lengths present within each pixel region, is simplified to facilitate processing of the large number of pixels on a given image. Under this assumption, the hemoglobin oxygen saturation can be calculated as CoxyHb/(CdeoxyHb + CoxyHb) × 100%.
Analysis of red blood cell velocity and automated red blood cell tracking.
Flow speed and direction maps were calculated using an algorithm developed by our laboratory, which will be published as part of a separate paper but is explained in the following. Briefly, video sequences of 65–100 images were collected using a charge-coupled device microscope camera (Andor Technology, Belfast, Northern Ireland) and converted into three-dimensional matrices for processing in MATLAB. Movement of the background (e.g., due to heartbeat or respiration) was corrected for, using a custom algorithm that repositioned adjacent images based on maximizing structural similarity.
To enable the reconstruction of red blood cell moving patterns, the stack was filtered to consist only of grayscale patterns that reflect moving blood cells. This was done by excluding all grayscale information that did not fluctuate throughout the z direction within a set degree of synchronization with its neighboring pixels.
Blood cell movement was tracked by performing cross-correlation analysis of the z-directional temporal grayscale profiles of every pixel with that of every other pixel in its set local area. In this analysis, the z-directional, linear profiles of the two compared pixels are aligned with each other in multiple positions, and grayscale values of corresponding pixels are multiplied with each other. Corresponding Z-profiles result in the highest sums of products, whereas non-corresponding profiles yield low product sums. The relative distance of the matched profiles on the XY map are used to calculate the flow velocity at any given point, or pixel, whereas the relative position of pixels with matching Z-profiles to each other enables determination of flow direction. This strategy of analysis is based, in principle, on the dual-slit technique, introduced by Baker and Wayland in 1974 (1), and has here been applied to analyze flow velocity in two-dimensional arrays rather than up- and downstream of a single vessel.
To reduce computational expense, cross-correlation operations were performed in the Fourier domain by employing the convolution theorem, which states that the Fourier transform of two convolved signals is equivalent to the product of their individual transforms. Since it was our goal to calculate the cross-correlation of the signals rather than their convolution, one signal was reversed at a time. This time reversal was performed in the Fourier domain by taking the complex conjugate of one of the transformed signals. Thus, by finding the transform of the zero-mean matrix wherein the one-dimensional Fourier transform along the temporal dimension is calculated at every spatial coordinate, the computationally intensive cross-correlation operation was simplified to an inner product operation. The Fourier transform matrix was calculated in the initial stage of the algorithm, and as two temporal signals were compared, their corresponding Fourier signals were extracted from the matrix and multiplied (after taking the complex conjugate of one signal), and the inverse Fourier transform of this product yielded the cross-correlated signal. From this vector, the maximum value was found, with its index representing the best correlated temporal offset. This process was performed for every coordinate in the local region, generating a two-dimensional temporal-offset matrix for the particular pixel of interest.
Image analysis of the velocity maps was done on 16-bit grayscale maps in Image J by evaluation of regions of interest and cancellation of areas with no flow, using appropriate thresholding.
Blood gas analysis from pulmonary arterial and carotid arterial blood.
Rats were anesthetized with ketamine/xylazine mixture at 80/8 mg/kg and placed on their back onto a water-circulated heating pad. Indwelling catheters filled with heparinized saline were placed in the carotid (PE-50) and pulmonary (PE-10) artery as previously described (8). A pulse oximetry foot clip was attached to the left hindlimb of the animal (MouseOx, Starr Life Sciences) for computerized recording of arterial hemoglobin oxygen saturation (HbO2) and heart rate. The oxygen content of the breathing air was controlled with a gas blender (Oxydial, StarrLife Sciences), which allowed for adjustment of different gas mixtures at constant flow rates. After 5 min of 100% oxygen breathing, a concentration of 12% oxygen was gradually dialed in and was maintained through the time of blood sampling. A sample of 1 ml was then collected through each catheter into a gas-tight syringe (GE Healthcare, Pittsburgh, PA), sealed, placed on ice, and then analyzed for hemoglobin oxygen content using a clinical blood gas analyzer (Instrumentation Laboratory, Bedford, MA).
RESULTS
Hemoglobin saturation and blood gas analyses.
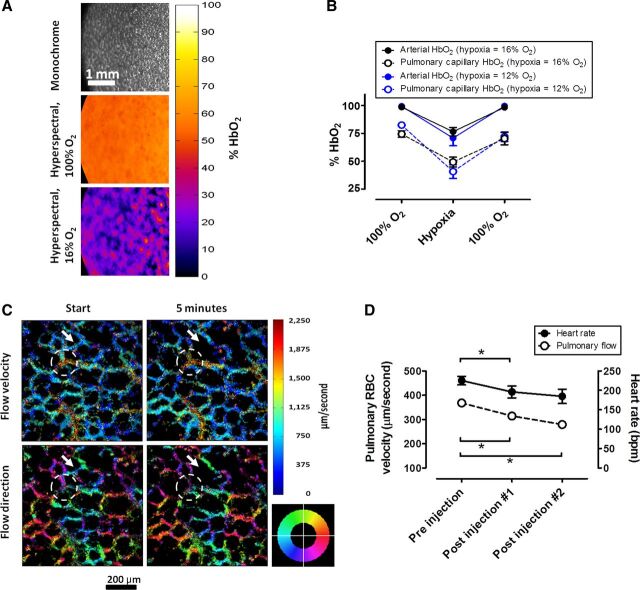
A study was conducted to investigate changes in pulmonary capillary and arterial HbO2 under hypoxia using 12% and 16% of inspired oxygen. Under baseline conditions [100% inspired oxygen fraction (FiO2)], average arterial HbO2 was 98.9 ± 0.9% (SD). Under 16% FiO2, the average arterial saturation was 76.5 ± 7.5%, and under 12% it was 70.8 ± 12.0%. Average pulmonary capillary oxygen saturation under baseline conditions was 74.8 ± 3.6%. Under 16% FiO2, average pulmonary capillary saturation was 49.2 ± 8.8%, and under 12% it was 40.6 ± 10.6% (Fig. 1, A and B). To evaluate the stability of the method over time, we have calculated the coefficient of variation of the respective baseline measurements that were acquired before the onset of hypoxia. During oxygen breathing in (four experiments over three measurement time points), the coefficients of variation across subsequent imaging time points were 3.3%, 14.2%, 14.0%, and 3.3%.
To validate the HbO2 data calculated from the intravital microscopy experiments, blood gas analyses were done on blood samples from (nonsurgically fenestrated) anesthetized rats breathing 12% hypoxic air. Blood was sampled from the pulmonary artery (deoxygenated) and from the carotid artery (fully oxygenated) in four animals. The mean HbO2 in pulmonary arterial blood was 25.4 ± 10.7%, and the mean HbO2 in carotid arterial blood was 63.9 ± 3.3%.
Red blood cell blood flow.
On a microscopic level, patterns of precapillary, capillary, and postcapillary blood flow were distinguishable from each other by a combination of structural and functional criteria. Precapillary blood flow manifested as appearance of cells approaching from the depth, or z-direction, of the image in a “source” or “fountain-type” fashion, and then diverging into several different directions. As blood cells diverged into multiple vessel branches, their flow velocity markedly decreased. Capillary blood flow could be distinguished as cells flowing at low velocity in a curved path, accurately outlining the alveolar walls, which could be identified from the nonspecific background fluorescence. Postcapillary blood flow was characterized by noticeable acceleration of blood cell movement, convergent blood flow into collecting vessels, and followed by traveling of blood cells at high velocity for some distance in a direction parallel to the image plane, before they would turn toward the depth of the image and disappear. These flow patterns were reflected by the blood flow/direction mapping algorithm, with low-speed capillary flow tracks outlining alveolar walls and collecting venules manifesting as areas of high blood flow velocity (Fig. 2C; see supplemental video available at the Journal website). Differential recruitment of pulmonary capillaries, which has been described in the past (11), could be observed as spontaneous cessation of blood flow through particular capillaries after subsequent imaging events (Fig. 2C).
Fig. 2.
Hemodynamic and microcirculatory measurements using pulmonary intravital microscopy. A: brightfield reflectance image at 590 nm (top), color-encoded hemoglobin oxygen saturation maps under 100% O2 (middle), and 16% O2 (botom). B: time course of concomitant measurements of HbO2 in the lung using pulmonary intravital microscopy and in the arteries of the hindlimb using pulse oximetry after 15 min at 100% O2, 15 min of hypoxia, and 15 min at 100% O2. Hypoxia was 12% and 16% O2. Each data point represents an average from three sequential measurements per animal, and an average between three (12% O2) and four (16% O2) animals. C: color encoded maps of red blood cell velocity (top) and directionality (bottom) in the pulmonary microcirculation. Image pairs were acquired in 5-min intervals. The arrow marks what is likely a single vessel where blood flow has stalled in between imaging events. The dashed line marks an area of venous blood flow, as visible by converging movement of blood cells. The colored compass wheel is the legend for the directionality map, with colors indicating the direction in which the blood flows. D: changes in heart rate and red blood cell velocity in the pulmonary microcirculation after repeated injection of metoprolol (10 mg/kg). For each individual rat, three measurements were made, before/after injections, separated by 5-min intervals. Sequential measurements from each pre-/posttreatment period were averaged per animal, and each data point on the graph represents an average from all three rats for the respective period. Significant changes in heart rates and flow are indicated with a star (paired t-test, P < 0.05, n = 3).
We then tested whether our model would be sensitive to agents that are capable of modifying pulmonary blood flow. Metoprolol is a selective beta-1 adrenergic receptor agonist and a negative chronotrope (4, 7). In our experiments, the average blood cell velocity before treatment was 368.1 ± 14.1 μm/s at an average heart rate of 225.1 ± 18.9 beats/min. Intravenous injection of 10 mg/kg metoprolol significantly reduced the heart rate of the animals to 195.8 ± 26.7 beats/min (first injection) and 185.5 ± 31.9 beats/min (second injection). Concomitantly, pulmonary blood flow velocity was decreased to 314.3 ± 6.9 μm/s (first injection) and 279.1 ± 17.3 μm/s (second injection) (paired t-test, P < 0.05; n = 3; Fig. 2D).
The coefficient of variation between the average velocity of single image sequences was determined by performing triplicate measurements in three rats. The coefficients of variation between the average of three sequential baseline measurements of red blood cell velocity, measured in three animals, were 2.6%, 2.4%, and 6.6% of the mean.
DISCUSSION
Several approaches have been made in the past to image the pulmonary microcirculation of laboratory animals, including the manual assessment of blood flow velocity (3, 5, 16) and hemoglobin saturation (9). However, to improve the ability of this method to measure kinetic changes of the pulmonary physiology and advance its availability to a broader range of laboratories, it is necessary to automate and facilitate data acquisition and analysis procedures. In this publication, we demonstrate, for the first time, an intravital pulmonary microscopy system that permits an automated evaluation of blood flow and oxygen saturation in the pulmonary microcirculation.
The most important obstacle in establishing intravital microscopy of the lung is the movement of the lung due to respiratory and cardiac activity. One possible solution to this problem has been the application of negative pressure where vertical movement of the lung is eliminated by completely opening the rib cage, and the field of view is maintained stationary by applying a gentle vacuum between the cover glass and the lung surface (9). Although this method has shown great promise for imaging immune cell movement in mouse lungs, several aspects of it, such as the local vacuum, the continuous contact with the open air, the removal of the mechanical influence of the heart, and the lack of direct contact of the lung with the pleural wall, may change the characteristics of the pulmonary microcirculation. We have chosen a model based on an earlier study by Fingar et al. (5) using a socketed window that seals the thoracic wall. This maintains contact of the lung surface with the pleura, which in principle would better preserve the characteristics of an unperturbed microcirculation. Such a model has the additional advantage to be potentially further developed for long-term implantation as part of a survival surgery procedure.
Hemoglobin saturation.
Using previously published computational tools (14) to image vascular hemoglobin saturation, we investigated the utility of our surgical model to enable the measurement of microvascular hemoglobin saturation and blood oxygen loading under maximum oxygen loading, i.e., 100% FiO2, and under two levels of hypoxia, i.e., 12% and 16%. Expectedly, the oxygen saturation in the pulmonary vascular network was consistently lower than the arterial saturation because pulmonary capillary blood consists of mixed venous (partially deoxygenated) blood and blood that is freshly loaded with oxygen. It is known, for instance, that under normoxia in humans, mixed venous blood has a Po2 of ∼40 Torr, equivalent to 75% HbO2 (13, 18). The O2 saturation values we measured in the rat's pulmonary microvasculature are therefore in the range of what would be predicted. To validate the oxygen saturation we measured in the pulmonary capillaries, we performed blood gas analyses in mixed venous and in arterial blood of rats breathing hypoxic air. We found that the HbO2 saturation in mixed venous blood at 12% FiO2 was appreciably lower than the values measured in the pulmonary capillaries, and the difference between the mixed venous and the arterial hemoglobin saturation values bracketed our intravital measurements, therefore serving as confirmation of the validity of our measurements in the pulmonary microvasculature. The finding that pulmonary microvascular hemoglobin oxygen saturation was higher under 16% than under 12% oxygen further supports the accuracy of our model to measure microcirculatory HbO2 at different percentages of inspired oxygen.
Blood flow velocity.
We provided an automated method to quantify blood flow in the pulmonary microcirculation using a newly created computer algorithm that converted the tracks of fluorescently labeled red blood cells into velocity and directionality maps. We selected DiI as a labeling dye for the blood cells because the extraordinary stability of the labeled blood cells reported in previous studies indicates that they do not appreciably alter the rheology, i.e., membrane stiffness and flow behavior, of the blood cells (17). The resulting color maps showed the expected patterns of blood flow, mostly outlining alveolar walls, as shown in Fig. 2C and in the supplemental video. Most of the blood flow tracks were between ∼5 and 30 μm thick. Although it is difficult to reconstruct precisely from the thickness of the flow tracks on the diameter and hierarchical position of vessels, this range is indicative for capillaries (≤20 μm) and small venules and arterioles (20–30 μm) (16). As has been described in previous work, red blood cells accelerated in collecting venules (9). The average velocity values in untreated animals of ∼370 μm/s are in excellent agreement with previous work in other mammalian lungs, acquired by offline manual tracking, reporting velocity values of ∼400 μm/s in rabbits (6), and 160–1,420 μm/s in canine pulmonary capillaries (12, 15). To test the ability of our method to detect changes in pulmonary blood flow, we have investigated whether pharmacological reduction of the heart rate would lead to a detectable decrease in pulmonary blood flow rate. Indeed, after injection of the negative chronotrope metoprolol, a beta-1-specific adrenergic receptor blocker (4), at an appropriate dose (7), we found a significant reduction of pulmonary blood flow in our animals, arguing that our model is indeed capable of measuring pharmacologically induced changes in blood flow velocity.
Potential limitations to our method.
Some limitations apply to our model: the field of view is restricted to the peripheral microvasculature of the caudal areas of the lung, limiting measurements to local rather than global changes. Also, functional imaging on a closed window requires a short period of apnea, which by itself might change the microcirculation. This could be improved by abbreviating imaging periods or by gating the image acquisition. Third, inflation of the lung in our model is maintained by positive pressure via a ventilator instead of the naturally occurring negative pressure in the chest. This would also impact on the physiology of the pulmonary circulation.
Although our method allows for a considerably easier acquisition of microvascular flow data than manual tracking, it also permits acquisition of much more data in a shorter time. Importantly, our method enables for the analysis of whole vascular networks instead of single, individual vessels. The relatively low coefficients of variation of both hemoglobin oxygen saturation and blood flow measurements argue that, given natural variation between time points, our method is robust and precise.
In summary, we present a refined method to precisely quantify changes in hemoglobin saturation, blood flow velocity, and directionality in the pulmonary microcirculation in the rat lung. Using this integrated technology, we have demonstrated the feasibility of measuring changes in capillary hemoglobin oxygen saturation over time. Due to its technological robustness, ease of application, and relatively low costs, this model has the potential to turn intravital microscopy of the rodent lung, facilitated through the thoracic window model presented here, into a workhorse application for pharmaceutical and physiological research in the pulmonology field.
GRANTS
This work was funded by a grant from the Defense Advanced Research Projects Agency (DARPA), Prime Award No. N66001-10-C-2134, and by Department of Defense Fellowship Grant No. BC083195.
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: G.H., G.P., S.S., D.R.R., D.C.I., A.B., C.A.P., G.B., M.W.D., T.J.M., and T.S. conception and design of research; G.H., S.S., Y.Z., A.B., G.B., and T.S. performed experiments; G.H., A.F., G.P., G.B., and T.S. analyzed data; G.H., G.P., S.S., C.A.P., G.B., and T.S. interpreted results of experiments; G.H. and T.S. prepared figures; G.H. and T.S. drafted manuscript; G.H., A.F., G.P., S.S., D.R.R., D.C.I., K.L.H., A.B., C.A.P., G.B., M.W.D., T.J.M., and T.S. edited and revised manuscript; G.H., A.F., G.P., S.S., D.R.R., Y.Z., D.C.I., K.L.H., A.B., C.A.P., G.B., M.W.D., T.J.M., and T.S. approved final version of manuscript.
Supplemental Video
Video S1 - .avi (4.5 MB) - The video demonstrates an example of a microscope image sequence taken from an image sequence acquired from a pulmonary window experiment, after injection of fluorescently labeled red blood cells. Al three video panels are derived from the same image sequence. Center panel: raw grayscale fluorescence image sequence, visualizing labeled red blood cell movement through the microvasculature around the alveolae. The light blue dashed lines outline venous vasculature, with convergent blood flow (light blue arrows). Note that the flow velocity in these areas is considerably higher than the other parts of the microvasculature. Areas marked with pink dashed lines represent arterial vasculature, defined by diverging flow (pink arrows). Selected blood cells leaving the arterial area on the right side were marked with colors, to facilitate visualization of divergent blood flow. Cells marked green, orange, and yellow, are fully transgressing from the arterial region to the venous area on the upper right, whereas the pink, red, and blue cells find a different destination. Left side panel: color encoded velocity map, co-registered with the image sequence of origin. Blue color indicates low velocity, whereas red colors mark high velocity. Right panel: color encoded directionality map, co-registered with the image sequence of origin. The circular color compass legend indicates the direction in which cells are moving.
ACKNOWLEDGMENTS
We thank Kenneth Young for excellent technical support, Dr. W. Michael Foster (Duke Department of Medicine) for reviewing the manuscript, and Dr. Jahar Bhattacharya (Department of Physiology and Cellular Biophysics, Columbia University Medical Center) for advice in developing the model.
REFERENCES
- 1. Baker M , Wayland H. On-line volume flow rate and velocity profile measurement for blood in microvessels. Microvasc Res 7: 131–143, 1974. [DOI] [PubMed] [Google Scholar]
- 2. Bhattacharya J. Seeing is believing. Nat Methods 8: 57–58, 2011. [DOI] [PubMed] [Google Scholar]
- 3. Bickenbach J , Dembinski R , Czaplik M , Meissner S , Tabuchi A , Mertens M , Knels L , Schroeder W , Pelosi P , Koch E , Kuebler WM , Rossaint R , Kuhlen R. Comparison of two in vivo microscopy techniques to visualize alveolar mechanics. J Clin Monit Comput 23: 323–332, 2009. [DOI] [PubMed] [Google Scholar]
- 4. Cucherat M , Borer JS. Reduction of resting heart rate with antianginal drugs: review and meta-analysis. Am J Ther 19: 269–280, 2012. [DOI] [PubMed] [Google Scholar]
- 5. Fingar VH , Taber SW , Wieman TJ. A new model for the study of pulmonary microcirculation: determination of pulmonary edema in rats. J Surg Res 57: 385–393, 1994. [DOI] [PubMed] [Google Scholar]
- 6. Groh J , Kuhnle GE , Sckell A , Ney L , Goetz AE. Isoflurane inhibits hypoxic pulmonary vasoconstriction. An in vivo fluorescence microscopic study in rabbits. Anesthesiology 81: 1436–1444, 1994. [DOI] [PubMed] [Google Scholar]
- 7. Hocht C , Di Verniero C , Opezzo JA , Taira CA. Pharmacokinetic-pharmacodynamic properties of metoprolol in chronic aortic coarctated rats. Naunyn Schmiedebergs Arch Pharmacol 370: 1–8, 2004. [DOI] [PubMed] [Google Scholar]
- 8. Irwin DC , Foreman B , Morris K , White M , Sullivan T , Jacobs R , Monnet E , Hackett T , TissotvanPatot MC , Hamilton KL , Gotshall RW. Polymerized bovine hemoglobin decreases oxygen delivery during normoxia and acute hypoxia in the rat. Am J Physiol Heart Circ Physiol 295: H1090–H1099, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kuebler WM. Real-time imaging assessment of pulmonary vascular responses. Proc Am Thorac Soc 8: 458–465, 2011. [DOI] [PubMed] [Google Scholar]
- 10. Lassailly F , Griessinger E , Bonnet D. “Microenvironmental contaminations” induced by fluorescent lipophilic dyes used for noninvasive in vitro and in vivo cell tracking. Blood 115: 5347–5354, 2010. [DOI] [PubMed] [Google Scholar]
- 11. Okada O , Presson RG , Kirk KR , Godbey PS , Capen RL , Wagner WW. Capillary perfusion patterns in single alveolar walls. J Appl Physiol 72: 1838–1844, 1992. [DOI] [PubMed] [Google Scholar]
- 12. Presson RG , Graham JA , Hanger CC , Godbey PS , Gebb SA , Sidner RA , Glenny RW , Wagner WW. Distribution of pulmonary capillary red blood cell transit times. J Appl Physiol 79: 382–388, 1995. [DOI] [PubMed] [Google Scholar]
- 13. Severinghaus JW. Simple, accurate equations for human blood O2 dissociation computations. J Appl Physiol 46: 599–602, 1979. [DOI] [PubMed] [Google Scholar]
- 14. Sorg BS , Moeller BJ , Donovan O , Cao Y , Dewhirst MW. Hyperspectral imaging of hemoglobin saturation in tumor microvasculature and tumor hypoxia development. J Biomed Opt 10: 44004, 2005. [DOI] [PubMed] [Google Scholar]
- 15. Staub NC , Schultz EL. Pulmonary capillary length in dogs, cat and rabbit. Respir Physiol 5: 371–378, 1968. [DOI] [PubMed] [Google Scholar]
- 16. Tabuchi A , Mertens M , Kuppe H , Pries AR , Kuebler WM. Intravital microscopy of the murine pulmonary microcirculation. J Appl Physiol 104: 338–346, 2008. [DOI] [PubMed] [Google Scholar]
- 17. Unthank JL , Lash JM , Nixon JC , Sidner RA , Bohlen HG. Evaluation of carbocyanine-labeled erythrocytes for microvascular measurements. Microvasc Res 45: 193–210, 1993. [DOI] [PubMed] [Google Scholar]
- 18. Wagner PD , West JB. Effects of diffusion impairment on O2 and CO2 time courses in pulmonary capillaries. J Appl Physiol 33: 62–71, 1972. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1 - .avi (4.5 MB) - The video demonstrates an example of a microscope image sequence taken from an image sequence acquired from a pulmonary window experiment, after injection of fluorescently labeled red blood cells. Al three video panels are derived from the same image sequence. Center panel: raw grayscale fluorescence image sequence, visualizing labeled red blood cell movement through the microvasculature around the alveolae. The light blue dashed lines outline venous vasculature, with convergent blood flow (light blue arrows). Note that the flow velocity in these areas is considerably higher than the other parts of the microvasculature. Areas marked with pink dashed lines represent arterial vasculature, defined by diverging flow (pink arrows). Selected blood cells leaving the arterial area on the right side were marked with colors, to facilitate visualization of divergent blood flow. Cells marked green, orange, and yellow, are fully transgressing from the arterial region to the venous area on the upper right, whereas the pink, red, and blue cells find a different destination. Left side panel: color encoded velocity map, co-registered with the image sequence of origin. Blue color indicates low velocity, whereas red colors mark high velocity. Right panel: color encoded directionality map, co-registered with the image sequence of origin. The circular color compass legend indicates the direction in which cells are moving.