Abstract
Women are more vulnerable to stress-induced craving, which may be associated with increased vulnerability to relapse. Susceptibility to stress-induced craving also appears to be modulated by the menstrual cycle and is negatively correlated with circulating progesterone levels in women. However, the factors that contribute to relapse vulnerability are poorly characterized in female animals. In this study, we assessed whether chronic ethanol exposure, estrous cycle, or exogenous progesterone administration modulated vulnerability to stress-induced reinstatement. To model ethanol dependence, adult female C57Bl/6J mice underwent chronic intermittent ethanol (CIE) exposure via vapor inhalation. Seventy-two hours after the final ethanol exposure, food-restricted mice began training in a conditioned place preference paradigm (CPP) for a food reward, followed by extinction training. Mice were then subjected to forced swim stress and assessed for reinstatement of their preference for the reward-paired chamber. CIE did not affect stress-induced reinstatement. However, stress-induced reinstatement was attenuated during the diestrus phase, when endogenous levels of progesterone peak in female mice. Further, administration of exogenous progesterone mimicked the attenuated reinstatement observed in diestrus. These findings indicate that circulating hormone levels modulate susceptibility to relapse-like behaviors and implicate progesterone as a potential target for treating stress-induced relapse in women.
Keywords: Progesterone, Estrous cycle, Reinstatement, Reward seeking, Stress, Female
Introduction
Alcohol use disorder (AUD) is a devastating neuropsychiatric illness, characterized by a high propensity to relapse even after extended abstinence. It has become increasingly evident that existing pharmacotherapies are not equally effective in men and women [1–3]. Among individuals that engage in heavy drinking, women exhibit greater alcohol craving in response to stress than men [4] which places them at elevated risk of stress-induced relapse. Intensity of drug craving is modulated across the menstrual cycle [5] and correlates with circulating hormone levels [6]. Increased progesterone levels are associated with reduced stress and drug cue-induced craving in women [6], which may reduce the likelihood of relapse [7]. Craving induced by administration of the pharmacological stressor yohimbine was attenuated in women with high levels of progesterone compared to women with low levels of progesterone [8]. This has been recapitulated in a preclinical model of stress-induced relapse-like behavior where administration of the progesterone metabolite allopregnanolone reduced yohimbine-induced reinstatement of cocaine seeking in female, but not male, rats [9]. These findings are broadly consistent across preclinical models and human findings and across substance classes [10–12], with progesterone and its metabolite, allopregnanolone, showing potential promise for cocaine and nicotine, though data on the therapeutic potential for AUD are limited [13].
A history of chronic ethanol exposure may elevate risk for inflexible reward seeking and relapse-related behaviors e.g., [14,15]. Importantly, a history of chronic ethanol exposure may also impact response to pharmacological approaches to reduce reinstatement, a model of relapse-related behavior [16–18]. Both the factors regulating reinstatement and outcomes of chronic ethanol exposure are poorly characterized in females, but though evidence for sex differences in these systems is growing [19–22]. To determine what factors regulate stress-induced reinstatement in female mice, the effects of chronic intermittent ethanol (CIE) exposure, estrous cycle, and progesterone administration on stress-induced reinstatement were investigated. Findings indicated that while CIE did not promote stress-induced reinstatement, stress-induced reinstatement was attenuated during the diestrus phase of the estrous cycle, at a time when endogenous progesterone levels are elevated in mice [23–27]. Further, stress-induced reinstatement was attenuated following exogenous progesterone administration.
Together, our findings join a growing literature implicating progesterone as a therapeutic target in the regulation of stress-induced reinstatement with efficacy in chronic ethanol-exposed females.
Materials and methods
Subjects
Female C57Bl/6J (9 weeks) mice were obtained from Jackson Laboratories and group housed in same sex cages at Drexel University College of Medicine under standard 12h:12h light conditions. Mice had ad libitum access to food (LabDiet 5053; PicoLab Rodent Diet) and water until the commencement of behavioral experiments, when they were food restricted to 90% of their free feeding body weight. All behavioral experiments took place during the light cycle. All procedures were approved by the Institutional Animal Use and Care Committee at Drexel University.
Vaginal cytology
Mice were monitored for phase of the estrous cycle using vaginal cytology. Vaginal smears were performed immediately following assessment of stress-induced reinstatement (Group 1). In order to assess whether endogenous progesterone impacted reward seeking, proestrus, estrus, and metestrus were combined into one group (nondiestrus) and compared to diestrus, the phase where progesterone peaks in mice [28].
Chronic Intermittent Ethanol (CIE) exposure
One group of female mice underwent two cycles of chronic intermittent ethanol (CIE) (n = 22) or air exposure (n = 24) by vapor inhalation (Group 1). One cycle consisted of 16 hours/day of vapor inhalation for 4 days and each cycle was separated by 72 hours of abstinence. CIE-exposed mice received an intraperitoneal injection of 1.6g/kg ethanol and 1mmol pyrazole to maintain stable blood ethanol concentrations prior to being placed in the ethanol vapor chamber. Air controls received 1mmol pyrazole in saline prior to being placed in the air chamber. To measure blood ethanol concentrations, submandibular bleeds were collected immediately following removal from the chamber once/ week. All behavioral training began 72 hrs following the final CIE exposure.
Food conditioned place preference training and extinction
Behavioral testing took place in a standard three-chamber conditioned place preference (CPP) apparatus with retractable doors (Med Associates). The CPP box consisted of a black chamber with grid floors and white chamber with wire mesh floors, separated by a neutral gray chamber with a solid floor. Time spent, latency to enter, entries, and locomotor behavior in each chamber were detected using photocell beam breaks by Med-PC V software. During pre-conditioning, mice were placed in the neutral chamber and allowed to freely explore all three chambers for 20 min. The black or white chamber was paired with food, counterbalancing for initial preference. During six 30-minute conditioning sessions (3 paired, 3 unpaired), mice were confined to either the black or white chamber on alternating days. For each mouse, the paired chamber contained a clear dish with 30 grain pellets (Bio-Serv Dustless Precision Pellets®) and the unpaired chamber contained an empty dish. To assess whether mice expressed a CPP, following conditioning, mice were placed in the neutral chamber and allowed to freely explore the 3 chambers for 20 minutes. Mice then underwent 8 extinction sessions which were identical to CPP test sessions. Mice were subjected to 10 minutes of forced swim stress 24 hours following the last extinction assessment. Mice were returned to the CPP apparatus 24 hours following FSS to determine if they reinstate their preference for the food-paired chamber. The CPP difference score was calculated by subtracting the time spent (sec) in the reward-paired chamber during the CPP test from the time spent in the reward-paired chamber during pre-conditioning. The reinstatement score was calculated by subtracting the time spent in the reward-paired chamber during the last day of extinction from the time spent in the reward-paired chamber during reinstatement assessment.
Forced Swim Stress (FSS)
Mice were subjected to 10 min of forced swim test prior to reinstatement. The apparatus consisted of a 2L glass beaker containing 1.8L of water at 24–26°C. Following FSS, mice were placed in a clean cage to dry under a heat lamp before being returned to their home cage.
Progesterone administration
A separate group of ethanol-naïve female mice (n = 14 vehicle, 14 progesterone) was assessed for the effect of progesterone treatment on stress-induced reinstatement (Group 2). Mice underwent behavioral training, extinction, and reinstatement as described above. Mice were randomly assigned to receive a subcutaneous injection of either sesame oil or progesterone (5mg/kg, Sigma-Aldrich, St. Louis MO) 30 minutes prior to assessment of reinstatement. Mice in all phases received progesterone administration.
Experimental design and statistical analysis
Data were analyzed in GraphPad using 2-way ANOVA for comparing CIE vs estrous phase, followed by Sidak’s corrected posthoc comparisons when significant interactions were observed. All other data were analyzed using unpaired two-tailed t-tests (comparison between groups) or one sample t-tests (comparison to 0). Alpha was set at 0.05.
Results
Estrous phase, but not chronic ethanol exposure, impacted stress-induced reinstatement of reward seeking
Chronic ethanol exposure resulted in a significant decrease in pellet consumption during conditioning. A rm ANOVA revealed a significant effect of CIE exposure on pellet consumption [F(1,45) = 4.303, p = 0.044] and pairing session [F(1, 83) = 11.13, p < 0.001], but no interaction [F(2,90) = 2.492, p = 0.089] (Fig. 1A). Post hoc analyses indicated that food pellets consumed in pairing 2 (p < 0.0001) and pairing 3 (p = 0.008) were significantly greater than pairing 1. However, CIE did not affect the acquisition or expression of a CPP in females as both CIE- and air-exposed females exhibited a difference score during CPP that was significantly greater than 0 [air, t (23) = 2.565, p = 0.022, CIE, t (22) = 2.965, p = 0.007, one sample t-test]. The magnitude of the CPP score did not differ between air- and CIE-exposed mice [t (45) = 0.002, p = 0.99] (Fig. 1b). CIE did not affect latency to enter the reward-paired chamber [t (45) = 0.232, p = 0.817] (Fig. 1c), activity in the reward-paired chamber [t (45) = 1.183, p = 0.243] (Fig. 1d), or entries into the reward-paired chamber [t (45) = 0.542, p = 0.591] (Fig. 1e). Blood ethanol concentrations did not differ between cycle 1 and cycle 2 of CIE exposure [t(34) = 0.155, p = 0.88, unpaired t-test] (Fig. 1e).
Fig. 1. Effects of ethanol dependence on conditioned place preference.
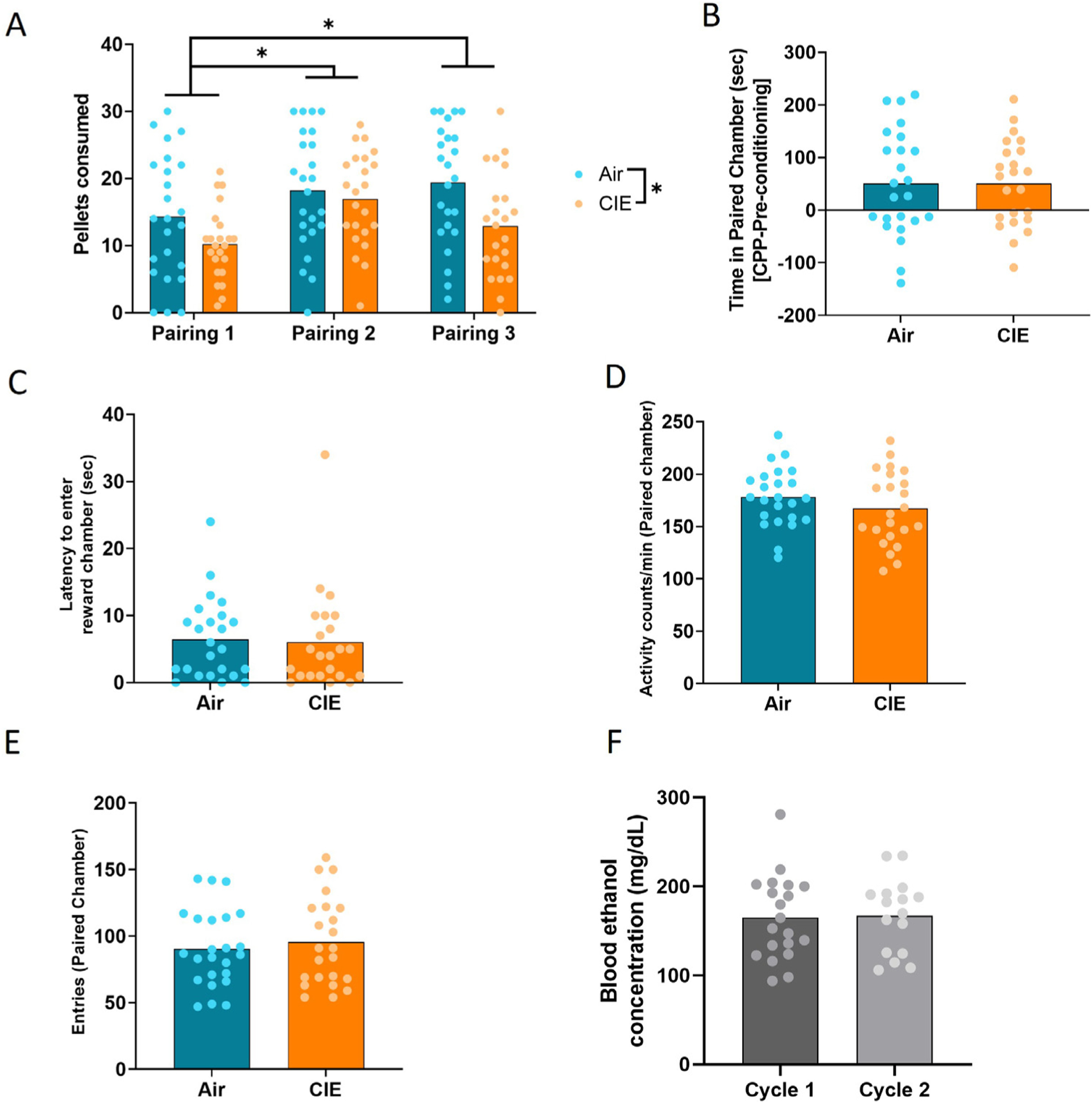
(a) Pellets consumed during each of the three food-paired conditioning sessions. (b) CPP difference scores [time in reward-paired chamber (sec), CPP – pre-conditioning] for nondependent and dependent female mice during CPP. (c) Latency to enter the reward-paired chamber during CPP test. (d) Activity levels of nondependent and dependent females during CPP test. (e) Entries into the reward-paired chamber during CPP test. (f) Blood ethanol concentrations during CIE cycles 1 and 2. Bars represent means. *p < 0.05.
In order to determine if CIE exposure or estrous phase impacted stress-induced reinstatement of reward seeking, mice underwent subsequent extinction sessions, stress exposure, and were assessed for reinstatement of their preference (Fig. 2a). Following assessment of reinstatement, estrous phase was determined using vaginal cytology. Proestrus was characterized by predominantly nucleated epithelial cells, estrus by cornified epithelial cells, diestrus by predominantly leukocytes, and metestrus by a mix of all three cell types (Supplemental Fig. 1). Based on the hypothesis that stress-induced reinstatement was attenuated during the diestrus phase, a two-way ANOVA revealed a significant effect of estrous phase on reinstatement score [F(1,43) = 4.19, p = 0.047], but no effect of CIE [F(1,43) = 0.267, p = 0.61] and no interaction [F(1,43) = 0.532, p = 0.47] (Fig. 2b). Neither CIE nor estrous phase impacted the latency to enter the reward-paired chamber, locomotor activity in the reward-paired chamber, or entries into the reward-paired chamber. A two-way ANOVA of latency to enter the reward-paired chamber revealed no effect of estrous phase [F(1,43) = 0.995, p = 0.324] or CIE [F(1,43) = 0.194, p = 0.66] and no interaction [F(1,43) = 0.0005, p = 0.984] (Fig. 2c). A two-way ANOVA of activity in the reward-paired chamber revealed no effect of estrous phase [F(1,43) = 1.85, p = 0.18] or CIE [F(1,43) = 3.21, p = 0.08] and no interaction [F(1,43) = 1.26, p = 0.27] (Fig. 2d). A two-way ANOVA for entries into the reward-paired chamber revealed no effect of estrous phase [F(1,43) = 1.65, p = 0.21] or CIE [F(1,43) = 0.050, p = 0.82] and no interaction [F(1,43) = 1.65, p = 0.21] (Fig. 2e).
Fig. 2. Estrous cycle-dependent modulation of stress-induced reinstatement of reward seeking.
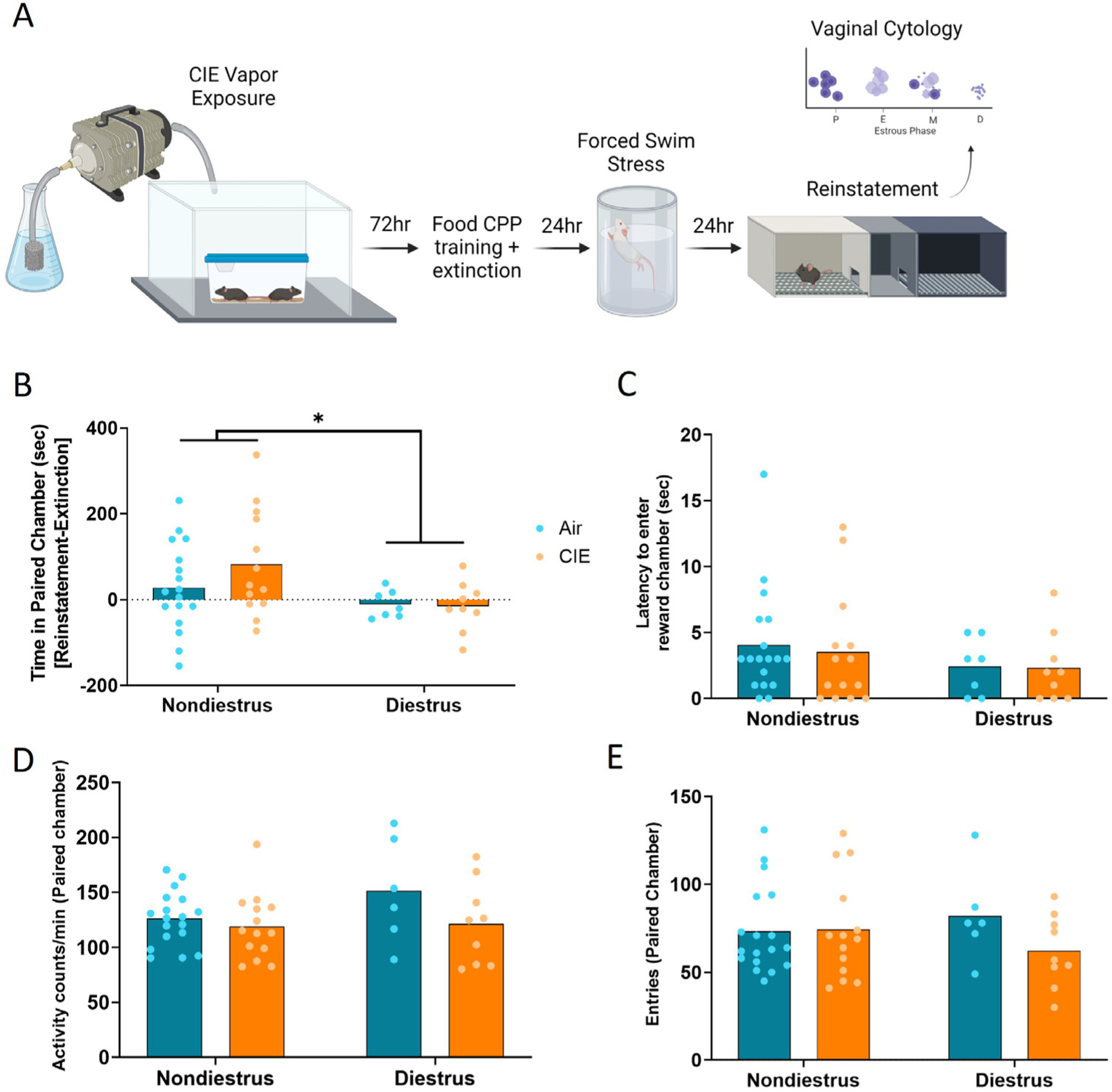
(a) Timeline of CIE exposure and behavioral testing. (b) Reinstatement scores [time in reward-paired chamber (sec), reinstatement-extinction] for nondependent and dependent female mice in nondiestrus and diestrus phases. (c) Latency to enter the reward-paired chamber during reinstatement. (d) Activity levels of nondependent and dependent females in nondiestrus and diestrus phases during reinstatement. (e) Entries into the reward-paired chamber during reinstatement. Bars represent means. *p < 0.05.
Progesterone administration attenuates stress-induced reinstatement of reward seeking
As reinstatement was attenuated during the diestrus phase of the estrous cycle, when progesterone levels peak in mice, we assessed whether progesterone administration would affect stress-induced reinstatement of reward seeking. Progesterone administration resulted in a significantly lower reinstatement score than sesame oil treatment [t(25) = 2.407, p = 0.039] (Fig. 3a). Progesterone administration did not impact latency to enter the paired chamber [t(25) = 1.61, p = 0.12] (Fig. 3c), locomotor activity during reinstatement [t(25) = 0.39, p = 0.70] (Fig. 3c). or entries into the paired chamber [t(25) = 0.31, p = 0.76] (Fig. 3d).
Fig. 3. Progesterone administration attenuates stress-induced reinstatement.
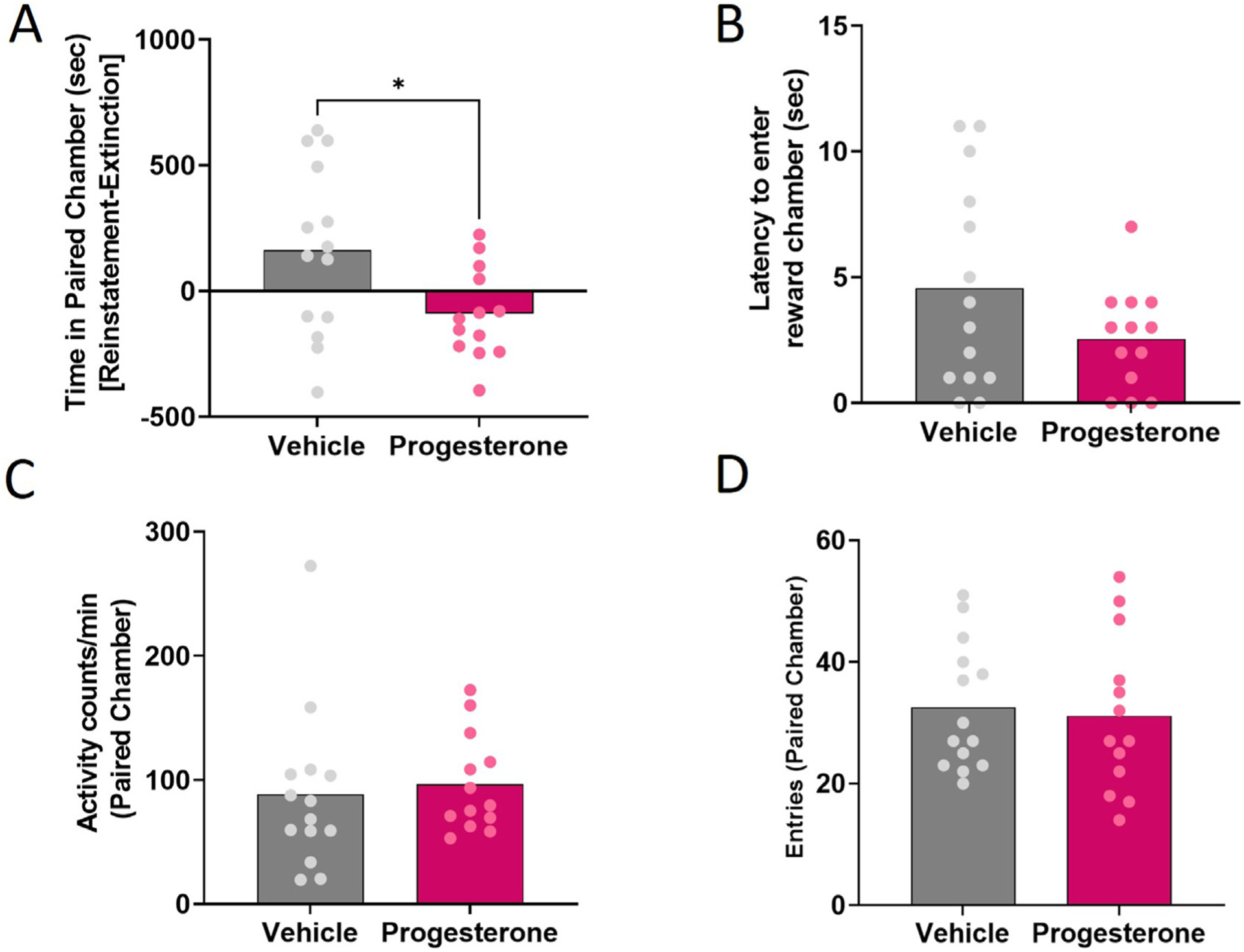
(a) Reinstatement scores for vehicle- and progesterone-treated female mice. (b) Latency of vehicle- and progesterone-treated female mice to enter the reward-paired chamber during reinstatement. (c) Activity levels in the reward-paired chamber for vehicle- and progesterone-treated female mice during reinstatement. (d) Entries into the reward-paired chamber for vehicle- and progesterone-treated female mice during reinstatement. Bars represent means. *p < 0.05.
Discussion
Our findings demonstrated that stress-induced reinstatement of reward seeking was modulated by the estrous cycle, such that reinstatement was suppressed during the diestrus phase, but was not affected by chronic ethanol exposure. Further, attenuated reinstatement observed during the diestrus was mimicked through exogenous administration of progesterone. Together these findings implicate progesterone as a regulator of stress-induced reward seeking.
Stress-induced reinstatement was selectively attenuated during the diestrus phase, when circulating progesterone levels peak in mice. Further, progesterone administration also attenuated stress-induced reinstatement. These results support a substantial preclinical literature suggesting that progesterone or its metabolites may be protective for drug seeking behavior, especially cocaine seeking. In addition to attenuated stress-induced reinstatement of cocaine seeking by progesterone in female rats [9], higher plasma progesterone levels are also associated with blunted cocaine-primed reinstatement, suggesting a generally protective role for progesterone in cocaine seeking [29]. These results are also recapitulated in clinical data from women which indicate that stress-induced craving for alcohol is attenuated during the midluteal phase, when progesterone peaks in women [5] and with findings that stress-induced craving for cocaine is negatively correlated with progesterone levels [6,8]. As craving appears to be reduced when progesterone levels are high, this may represent an optimal time for women to achieve abstinence. It is unclear from the current data set whether administration of exogenous progesterone will be similarly effective across cycle as the ability of progesterone administration to reduce stress-induced reinstatement was assessed across all phases. Others have found that the effects of progesterone administration on cocaine-seeking behavior were phase-dependent in rats [30]. Indeed, findings on ethanol intake across the menstrual cycle in women suggest interactions between estradiol and progesterone levels contribute to drinking [31]. It will be important in future studies to determine whether approaches to reducing stress-induced reinstatement are similarly efficacious under all hormonal conditions, especially as estradiol may confer risk for drug seeing behavior c.f., [32].
While the current study did not measure progesterone levels, the finding that progesterone levels are elevated during diestrus in mice has been frequently reported [23–27]. This was found in both C57 mice [24,26,27] – the strain used in the current study - and in CD1 mice [23,25], but see [33] which identified no significant differences in progesterone levels across the estrous cycle in C57Bl6 mice. Overall, hormone cycling in the C57 mouse diverges from patterns observed in rats [34,35], and may differ from less common mouse strains including the IVCS [36] and CHI mouse strains [37], highlighting the importance of considering both rodent species and strains in designing and in interpreting studies.
In contrast to our hypothesis, chronic ethanol exposure did not impact stress-induced reinstatement of food seeking. CIE exposure has previously been shown to promote baseline ethanol self-administration in male rats and mice [38–40], though this was not observed in female mice [19]. In rats, this was shown to be specific to ethanol seeking as saccharin self-administration was unaffected by CIE exposure [38]. Thus, it is possible that CIE differentially effects different components of reward seeking, with disparate outcomes for self-administration versus reinstatement following extinction, and that this may further depend on reward type. Alternatively, CIE enhancement of stress-induced reinstatement may be specific to male rodents. However, it has also been shown that CIE exposure did not affect stress-induced reinstatement of either ethanol or sweetened condensed milk in male or female rats [41]. Another possibility for the lack of effect of CIE exposure on reinstatement is that the abbreviated 2-week length of CIE used in the current study was not sufficient to induce changes in behavior. However, the previous study utilized a 6-week CIE exposure paradigm [41]. A separate study found that 12 days of continuous ethanol vapor exposure promoted cue-, stress-, and stress + cue-induced reinstatement of ethanol seeking in male rats [42]. Further, we have previously demonstrated that 2 weeks of CIE exposure is sufficient to induce impairments in extinction learning in female mice [43]. The effects of CIE exposure on reinstatement may be specific to the form of reinstatement as CIE exposure promoted reinstatement via the kappa opioid receptor agonist, U50,488, in male rats [44]. However, forced swim stress is sufficient to promote escalated home cage ethanol drinking in ethanol-dependent male mice [45].
One potential caveat of our study is that vaginal smears were only performed on the day of testing. This enabled avoidance of potential behavioral confounds as vaginal lavage prior to testing can induce a conditioned place preference in female rats [46]. Thus, it is possible that abnormally cycling mice were included. However, CIE-exposed mice were captured in each phase of the estrous cycle, suggesting that mice were cycling. The current study did not assess the effects of ethanol on estrous cycle. While alcohol intake does not appear to be correlated with disruptions in menstrual cycling in women [47,48], there is some evidence that ethanol can impact cycling in female rats and a mouse line selectively bred for seizure propensity during withdrawal [49–51]. For example, rats fed an ethanol liquid diet for 5 weeks exhibited disruptions in estrous cycling, with higher frequency of days in metestrus and diestrus than controls [49]. Similarly, administration of ethanol through intragastric intubations yielded an increase in the incidence of the diestrus phase and reduction in proestrus and estrous following 12 days of 4 or 8 g/kg ethanol exposure, but not lower doses [51]. Notably, normal cycling returned within 20 days after cessation of ethanol exposure.
Progesterone regulates neuronal function through interactions with multiple neurotransmitter systems, including glutamate and γ-Aminobutyric acid (GABA). Effects on both systems are largely mediated through the progesterone metabolite, allopregnanolone. To impact glutamatergic signaling, allopregnanolone regulates behavior through its effects on expression of GLT1 and glutamine synthetase in the NAc [52]. Both chronic intermittent ethanol (CIE) exposure [53] and acute stress [54] increase glutamate release in the NAc, which subsequently can promote heightened reward seeking. Modulation of glutamate levels in the NAc by targeting the astrocytic transporter GLT1 or antiporter, system xc-, also regulates reinstatement-like behaviors [55]. Pharmacological upregulation of system xc- attenuates reinstatement of ethanol seeking by increasing glutamatergic tone on mGluR2/3 receptors to reduce glutamate release [56]. In contrast, pharmacological GLT1 upregulation by N-acetylcysteine administration promotes glutamate clearance [56] and attenuates stress- and cue-induced reinstatement of ethanol and cocaine seeking [57]. System xc- is also reduced in mice that exhibit enhanced susceptibility to stress-induced reinstatement [58].
Allopregnanolone impacts GABAergic signaling in part through its actions on the GABAA receptor, located on neuronal and astrocyte populations [59,60]. In studies in both rat and mouse, GABAA receptor expression is altered following chronic ethanol exposure in a structure- and withdrawal time point-dependent manner, though the majority of this work was conducted in males [61–63]. Tonic inhibition through GABAA receptors is modulated across the estrous cycle through convergent mechanisms – allopregnanolone binding and signaling through the receptor [60] and alterations in receptor subunit composition across the cycle [64]. These alterations in receptor composition can be mimicked through exogenous progesterone administration, upregulating GABAA δ subunits in males and females, as is observed in the high progesterone diestrus phase of cycling females [64,64]. Global [65] and brain region specific [66] loss of δ subunit-containing GABAA receptors drove reductions in ethanol intake, though this may be specific to discrete anatomical substrates [66]. In addition to direct effects on reward seeking of progesterone as reported here and elsewhere [29,32,60,67], progesterone administration can also modulate anxiety-like behavior [68], which may be mediated through its effects on the GABAA receptor [64,69]. This suggests that there may be dual mechanisms by which progesterone may act to blunt stress-induced reinstatement, potentially through actions on the GABAergic system – general suppression of response to acute stressors or suppression of reward-seeking behavior.
As astrocytes play a critical role in maintaining basal neurotransmitter levels and signaling, one potential mechanism by which progesterone modulates reward seeking behavior is by acting on astrocytes to regulate neurotransmission. Astrocytes in the NAc have been implicated in self-administration, extinction, and reinstatement of reward seeking [58,70–73]. Chemogenetic activation of NAc astrocytic Gq signaling in male rats has been shown to decrease ethanol motivation to self-administer ethanol following abstinence [74] and attenuate cue-induced reinstatement of cocaine seeking [72]. The role of astrocytes in the regulation of reinstatement has not been characterized in females. However, we have also previously demonstrated that activation of astrocytic Gg signaling attenuates ethanol dependence-induced impairments in extinction of reward seeking in female mice [43]. Future studies should address the consequences of estrous cycle and progesterone administration of astrocytic protein expression and function in the context of stress-induced reinstatement.
Together, these findings are consistent with progesterone as a promising therapeutic target for stress-induced relapse as both endogenous levels of progesterone and exogenous progesterone administration attenuated stress-induced reinstatement.
Supplementary Material
Funding
This research was supported by NIH grants AA024499 (JMB) and AA027629 (JMB), Mary DeWitt Pettit Fellowship (JMB), and the Hartwell Foundation Postdoctoral Fellowship (LLG).
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi: 10.1016/j.addicn.2022.100035.
References
- [1].Martin EL, EM Doncheck, Reichel CM, McRae-Clark AL, Consideration of sex as a biological variable in the translation of pharmacotherapy for stress-associated drug seeking, Neurobiol. Stress 15 (2021) 100364 [accessed 2022 Jul 11], doi: 10.1016/J.YNSTR.2021.100364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].McKee SA, Smith PH, Kaufman M, Mazure CM, Weinberger AH, Sex differences in varenicline efficacy for smoking cessation: a meta-analysis, Nicotin Tob. Res 18 (5) (2016) 1002–1011 [accessed 2022 Jul 11] https://academic.oup.com/ntr/article/18/5/1002/2511548, doi: 10.1093/NTR/NTV207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].McKee SA, McRae-Clark AL, Consideration of sex and gender differences in addiction medication response, Biol Sex Differ 13 (1) (2022) 1–18 13:1. 2022 [accessed 2022 Jul 11] https://bsd.biomedcentral.com/articles/10.1186/s13293-022-00441-3, doi: 10.1186/s13293-022-00441-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hartwell EE, Ray LA, Sex moderates stress reactivity in heavy drinkers, Addict. Behav 38 (11) (2013) 2643–2646, doi: 10.1016/j.addbeh.2013.06.016. [DOI] [PubMed] [Google Scholar]
- [5].Warren JG, Goodwin L, Gage SH, Rose AK, The effects of menstrual cycle stage and hormonal contraception on alcohol consumption and craving: a pilot investigation, Comprehens. Psychoneuroendocrinol 5 (2021) 100022, doi: 10.1016/j.cpnec.2020.100022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Sofuoglu M, Sinha R, Hong K-I, Morgan PT, Bergquist KT, Fox H, Sex steroid hormones, stress response, and drug craving in cocaine-dependent women: implications for relapse susceptibility, Exp. Clin. Psychopharmacol 15 (5) (2007) 445–452, doi: 10.1037/1064-1297.15.5.445. [DOI] [PubMed] [Google Scholar]
- [7].Higley AE, Crane NA, Spadoni AD, Quello SB, Goodell V, Mason BJ, Craving in response to stress induction in a human laboratory paradigm predicts treatment outcome in alcohol-dependent individuals, Psychopharmacology (Berl.) 218 (1) (2011) 121–129, doi: 10.1007/s00213-011-2355-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Moran-Santa Maria MM, McRae-Clark A, Baker NL, Ramakrishnan V, Brady KT, Yohimbine administration and cue-reactivity in cocaine-dependent individuals, Psychopharmacology (Berl.) 231 (21) (2014) 4157–4165, doi: 10.1007/s00213-014-3555-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Anker JJ, Carroll ME, Sex differences in the effects of allopregnanolone on yohimbine-induced reinstatement of cocaine seeking in rats, Drug Alcohol Depend 107 (2) (2010) 264–267, doi: 10.1016/j.drugalcdep.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Carroll ME, Smethells JR, Sex differences in behavioral dyscontrol: role in drug addiction and novel treatments, Front Psychiatry 6 (FEB) (2015) 175 [accessed 2022 Jul 12], doi: 10.3389/FPSYT.2015.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Cooper ZD, Foltin RW, Evans SM, Effects of menstrual cycle phase on cocaine self-administration in rhesus macaques, Horm. Behav 63 (1) (2013) 105 [accessed 2022 Jul 12], doi: 10.1016/J.YHBEH.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Evans SM, Foltin RW, Does the response to cocaine differ as a function of sex or hormonal status in human and non-human primates? Horm. Behav 58 (1) (2010) 13 [accessed 2022 Jul 12], doi: 10.1016/J.YHBEH.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Peltier MKR, Sofuoglu M, Role of exogenous progesterone in the treatment of men and women with substance use disorders: a narrative review, CNS Drugs 32 (5) (2018) 421–435 [accessed 2022 Jul 11] https://pubmed.ncbi.nlm.nih.gov/29761343/, doi: 10.1007/S40263-018-0525-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Gass JT, McGonigal JT, Chandler LJ, Deficits in the extinction of ethanol-seeking behavior following chronic intermittent ethanol exposure are attenuated with positive allosteric modulation of mGlu5, Neuropharmacology 113 (Pt A) (2017) 198–205 [accessed 2019 Oct 1] http://www.ncbi.nlm.nih.gov/pubmed/27725153, doi: 10.1016/j.neuropharm.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Renteria R, Baltz ET, Gremel CM, Chronic alcohol exposure disrupts top-down control over basal ganglia action selection to produce habits, Nat. Commun 9 (1) (2018) 211 [accessed 2019 Jan 22] http://www.nature.com/articles/s41467-017-02615-9, doi: 10.1038/s41467-017-02615-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ciccocioppo R, Lin D, Martin-Fardon R, Weiss F, Reinstatement of ethanol-seeking behavior by drug cues following single versus multiple ethanol intoxication in the rat: effects of naltrexone, Psychopharmacology (Berl.) 168 (1–2) (2003) 208–215 [accessed 2022 Apr 8] https://link.springer.com/article/10.1007/s00213-002-1380-z, doi: 10.1007/s00213-002-1380-z/FIGURES/5. [DOI] [PubMed] [Google Scholar]
- [17].Kufahl PR, Martin-Fardon R, Weiss F, Enhanced sensitivity to attenuation of conditioned reinstatement by the mGluR2/3 agonist LY379268 and increased functional activity of mGluR2/3 in rats with a history of ethanol dependence, Neuropsychopharmacology 36 (13) (2011) 2762–2773 36:13. 2011 [accessed 2022 Apr 8] https://www.nature.com/articles/npp2011174, doi: 10.1038/npp.2011.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Sidhpura N, Weiss F, Martin-Fardon R, Effects of the mGlu2/3 agonist LY379268 and the mGlu5 antagonist MTEP on ethanol seeking and reinforcement are differentially altered in rats with a history of ethanol dependence, Biol. Psychiatry 67 (9) (2010) 804–811 [accessed 2013 Jun 16], doi: 10.1016/j.biopsych.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Jury NJ, DiBerto JF, Kash TL, Holmes A, Sex differences in the behavioral sequelae of chronic ethanol exposure, Alcohol 58 (2017) 53–60, doi: 10.1016/j.alcohol.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Feltenstein MW, Henderson AR, See RE, Enhancement of cue-induced reinstatement of cocaine-seeking in rats by yohimbine: Sex differences and the role of the estrous cycle, Psychopharmacology (Berl.) 216 (1) (2011) 53–62, doi: 10.1007/s00213-011-2187-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bertholomey ML, Nagarajan V, Torregrossa MM, Sex differences in reinstatement of alcohol seeking in response to cues and yohimbine in rats with and without a history of adolescent corticosterone exposure, Psychopharmacology (Berl.) 233 (12) (2016) 2277–2287 [accessed 2018 Jun 13] http://link.springer.com/10.1007/s00213-016-4278-x, doi: 10.1007/s00213-016-4278-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Sneddon EA, Rasizer LN, Cavalco NG, Jaymes AH, Ostlie NJ, Minshall BL, Masters BM, Hrncir H, Arnold AP, Radke AK, Gonadal hormones and sex chromosome complement differentially contribute to ethanol intake, preference, and relapse-like behavior in Four Core Genotypes mice, bioRxiv (2021. Sep 15) [accessed 2022 Apr 8]:2021.04.28.441845 https://www.biorxiv.org/content/10.1101/2021.04.28.441845v2, doi: 10.1101/2021.04.28.441845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Walmer DK, Wrona MA, Hughes CL, Nelson KG, Lactoferrin expression in the mouse reproductive tract during the natural estrous cycle: correlation with circulating estradiol and progesterone, Endocrinology 131 (3) (1992) 1458–1466 [accessed 2022 Jul 11] https://academic.oup.com/endo/article/131/3/1458/3034824, doi: 10.1210/ENDO.131.3.1505477. [DOI] [PubMed] [Google Scholar]
- [24].Fata JE, Chaudhary V, Khokha R, Cellular turnover in the mammary gland is correlated with systemic levels of progesterone and not 17beta-estradiol during the estrous cycle, Biol. Reprod 65 (3) (2001) 680–688 [accessed 2022 Jul 11] https://pubmed.ncbi.nlm.nih.gov/11514328/, doi: 10.1095/BIOLREPROD65.3.680. [DOI] [PubMed] [Google Scholar]
- [25].Bastida CM, Cremades A, Castells MT, López-Contreras AJ, López-García C, Tejada F, Peñafiel R, Influence of ovarian ornithine decarboxylase in folliculogenesis and luteinization, Endocrinology 146 (2) (2005) 666–674 [accessed 2022 Jul 11] https://academic.oup.com/endo/article/146/2/666/2878203, doi: 10.1210/EN.2004-1004. [DOI] [PubMed] [Google Scholar]
- [26].Jaric I, Rocks D, Greally JM, Suzuki M, Kundakovic M, Chromatin organization in the female mouse brain fluctuates across the oestrous cycle, Nat. Commun 10 (1) (2019) [accessed 2022 Jul 11] https://pubmed.ncbi.nlm.nih.gov/31253786/, doi: 10.1038/S41467-019-10704-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Maguire JL, Stell BM, Rafizadeh M, Mody I, Ovarian cycle–linked changes in GABAA receptors mediating tonic inhibition alter seizure susceptibility and anxiety, Nat. Neurosci 8 (6) (2005) 797–804 8:6. 2005 [accessed 2022 Jul 11] https://www.nature.com/articles/nn1469, doi: 10.1038/nn1469. [DOI] [PubMed] [Google Scholar]
- [28].McLean AC, Valenzuela N, Fai S, Bennett SAL, Performing vaginal lavage, crystal violet staining, and vaginal cytological evaluation for mouse estrous cycle staging identification, J. Vis. Exp (67) (2012), doi: 10.3791/4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Feltenstein MW, See RE, Plasma progesterone levels and cocaine-seeking in freely cycling female rats across the estrous cycle, Drug Alcohol Depend 89 (2–3) (2007) 183–189 [accessed 2022 Jul 11] https://pubmed.ncbi.nlm.nih.gov/17240083/, doi: 10.1016/J.DRUGALCDEP.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Feltenstein MW, Byrd EA, Henderson AR, See RE, Attenuation of cocaine-seeking by progesterone treatment in female rats, Psychoneuroendocrinology 34 (3) (2009) 343–352 [accessed 2022 Jul 11], doi: 10.1016/j.psyneuen.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Martel MM, Eisenlohr-Moul T, Roberts B, Interactive effects of ovarian steroid hormones on alcohol use and binge drinking across the menstrual cycle, J. Abnorm. Psychol 126 (8) (2017) 1104–1113 [accessed 2022 Jul 12] https://pubmed.ncbi.nlm.nih.gov/29154570/, doi: 10.1037/ABN0000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Peart DR, Andrade AK, Logan CN, Knackstedt LA, Murray JE, Regulation of cocaine-related behaviours by estrogen and progesterone, Neurosci. Biobehav. Rev 135 (2022) [accessed 2022 Jul 11] https://pubmed.ncbi.nlm.nih.gov/35189163/, doi: 10.1016/J.NEUBIOREV.2022.104584. [DOI] [PubMed] [Google Scholar]
- [33].ME Nilsson, Vandenput L, Tivesten Å, Norlén AK, Lagerquist MK, Windahl SH, Börjesson AE, Farman HH, Poutanen M, Benrick A, et al. , Measurement of a comprehensive sex steroid profile in rodent serum by high-sensitive gas chromatography-tandem mass spectrometry, Endocrinology 156 (7) (2015) 2492–2502 [accessed 2022 Jul 11] https://academic.oup.com/endo/article/156/7/2492/2422943, doi: 10.1210/EN.2014-1890. [DOI] [PubMed] [Google Scholar]
- [34].Inoue T, Tsuchiya K, Koyama T, Regional changes in dopamine and serotonin activation with various intensity of physical and psychological stress in the rat brain, Pharmacol. Biochem. Behav 49 (4) (1994) 911–920 [accessed 2013 Jul 18] http://www.ncbi.nlm.nih.gov/pubmed/7886107. [DOI] [PubMed] [Google Scholar]
- [35].Haim S, Shakhar G, Rossene E, Taylor AN, Ben-Eliyahu S, Serum levels of sex hormones and corticosterone throughout 4- and 5-day estrous cycles in Fischer 344 rats and their simulation in ovariectomized females, J. Endocrinol. Invest 26 (10) (2003) 1013–1022 [accessed 2022 Jul 11] https://pubmed.ncbi.nlm.nih.gov/14759076/, doi: 10.1007/BF03348201. [DOI] [PubMed] [Google Scholar]
- [36].Kosaka T, Saito TR, Takahashi KW, Changes in plasma progesterone levels during the estrous cycle and pregnancy in 4-day cyclic Mice, Exp. Anim 37 (3) (1988) 351–353 [accessed 2022 Jul 11], doi: 10.1538/EXPANIM1978.37.3_351. [DOI] [PubMed] [Google Scholar]
- [37].Guttenberg I, Plasma levels of “Free” progestin during the estrous cycle in the mouse, Endocrinology 68 (6) (1961) 1006–1009 [accessed 2022 Jul 11] https://academic.oup.com/endo/article/68/6/1006/2702540, doi: 10.1210/ENDO-68-6-1006. [DOI] [PubMed] [Google Scholar]
- [38].O’Dell LE, Roberts AJ, Smith RT, Koob GF, Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure, Alcohol.: Clin. Exp. Res 28 (11) (2004) 1676–1682, doi: 10.1097/01.ALC.0000145781.11923.4E. [DOI] [PubMed] [Google Scholar]
- [39].McCool BA, Chappell AM, Chronic intermittent ethanol inhalation increases ethanol self-administration in both C57BL/6J and DBA/2J mice, Alcohol 49 (2) (2015) 111–120, doi: 10.1016/j.alcohol.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Barker JM, Bryant KG, Montiel-Ramos A, Goldwasser B, Chandler LJ, Selective deficits in contingency-driven ethanol seeking following chronic ethanol exposure in male mice, Alcohol.: Clin. Exp. Res 44 (9) (2020) 1896–1905, doi: 10.1111/acer.14418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].A Matzeu, Martin-Fardon R, Blockade of orexin receptors in the posterior paraventricular nucleus of the thalamus prevents stress-induced reinstatement of reward-seeking behavior in rats with a history of ethanol dependence, Front. Integr. Neurosci 14 (2020) 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Liu X, Weiss F, Additive effect of stress and drug cues on reinstatement of ethanol seeking: exacerbation by history of dependence and role of concurrent activation of corticotropin-releasing factor and opioid mechanisms, J. Neurosci 22 (18) (2002) 7856LP–787861, doi: 10.1523/JNEUROSCI.22-18-07856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Giacometti LL, Chandran K, Figueroa LA, Barker JM, Astrocyte modulation of extinction impairments in ethanol-dependent female mice, Neuropharmacology 179 (2020) 108272 http://www.sciencedirect.com/science/article/pii/S0028390820303403, doi: 10.1016/j.neuropharm.2020.108272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Funk D, Coen K, Tamadon S, Lê AD, Effect of chronic alcohol vapor exposure on reinstatement of alcohol seeking induced by U50,488, Neuropharmacology 148 (2019) 210–219, doi: 10.1016/j.neuropharm.2019.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Lopez MF, Anderson RI, Becker HC, Effect of different stressors on voluntary ethanol intake in ethanol-dependent and nondependent C57BL/6J mice, Alcohol (Fayetteville, N.Y.) 51 (2016) 17–23, doi: 10.1016/j.alcohol.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Walker Q, Nelson CJ, Smith D, Kuhn CM, Vaginal lavage attenuates cocaine-stimulated activity and establishes place preference in rats, Pharmacol. Biochem. Behav 73 (4) (2002) 743–752 [accessed 2022 Aug 9] https://pubmed.ncbi.nlm.nih.gov/12213518/, doi: 10.1016/S0091-3057(02)00883-3. [DOI] [PubMed] [Google Scholar]
- [47].Schliep KC, Zarek SM, Schisterman EF, Wactawski-Wende J, Trevisan M, Sjaarda LA, Perkins NJ, Mumford SL, Alcohol intake, reproductive hormones, and menstrual cycle function: a prospective cohort study, Am. J. Clin. Nutr 102 (4) (2015) 933–942 [accessed 2022 Aug 9] https://pubmed.ncbi.nlm.nih.gov/26289438/, doi: 10.3945/AJCN.114.102160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Lyngsø J, Toft G, Høyer BB, Guldbrandsen K, Olsen J, Ramlau-Hansen CH, Moderate alcohol intake and menstrual cycle characteristics, Hum. Reprod 29 (2) (2014) 351–358 [accessed 2022 Aug 9] https://pubmed.ncbi.nlm.nih.gov/24287817/, doi: 10.1093/HUMREP/DET417. [DOI] [PubMed] [Google Scholar]
- [49].Sanchis R, Esquifino A, Guerri C, Chronic ethanol intake modifies estrous cyclicity and alters prolactin and LH levels, Pharmacol. Biochem. Behav 23 (2) (1985) 221–224 [accessed 2022 Aug 9] https://pubmed.ncbi.nlm.nih.gov/4059308/, doi: 10.1016/0091-3057(85)90560-X. [DOI] [PubMed] [Google Scholar]
- [50].Forquer MR, Hashimoto JG, Roberts ML, Wiren KM, Elevated testosterone in females reveals a robust sex difference in altered androgen levels during chronic alcohol withdrawal, Alcohol 45 (2) (2011) 161–171 [accessed 2022 Aug 9], doi: 10.1016/J.ALCOHOL.2010.08.013. [DOI] [PubMed] [Google Scholar]
- [51].Eskay RL, Ryback RS, Goldman M, Majchrowicz E, Effect of chronic ethanol administration on plasma levels of LH and the estrous cycle in the female rat, Alcohol Clin. Exp. Res 5 (2) (1981) 204–206 [accessed 2022 Aug 9] https://pubmed.ncbi.nlm.nih.gov/7018298/, doi: 10.1111/J.1530-0277.1981.TB04889.X. [DOI] [PubMed] [Google Scholar]
- [52].Nangaku M, Yoshino K, Oda Y, Kimura M, Kimura H, Hirose Y, Shirayama, Iyo M, Astroglial glutamate transporter 1 and glutamine synthetase of the nucleus accumbens are involved in the antidepressant-like effects of allopregnanolone in learned helplessness rats, Behav. Brain Res 401 (2021), doi: 10.1016/j.bbr.2020.113092. [DOI] [PubMed] [Google Scholar]
- [53].Griffin WC, Haun HL, Hazelbaker CL, Ramachandra VS, Becker HC, Increased extracellular glutamate in the nucleus accumbens promotes excessive ethanol drinking in ethanol dependent mice, Neuropsychopharmacology 39 (3) (2014) 707–717, doi: 10.1038/npp.2013.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].P Rada, Moreno SA, Tucci S, Gonzalez LE, Harrison T, Chau DT, Hoebel BG, Hernandez L, Glutamate release in the nucleus accumbens is involved in behavioral depression during the Porsolt swim test, Neuroscience 119 (2) (2003) 557–565, doi: 10.1016/S0306-4522(03)00162-3. [DOI] [PubMed] [Google Scholar]
- [55].Giacometti LL, Barker JM, Sex differences in the glutamate system: implications for addiction, Neurosci. Biobehav. Rev 113 (2020) 157 [accessed 2022 Apr 7], doi: 10.1016/J.NEUBIOREV.2020.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Qrunfleh AM, Alazizi A, Sari Y, Ceftriaxone, a beta-lactam antibiotic, attenuates relapse-like ethanol-drinking behavior in alcohol-preferring rats, J. Psychopharmacol 27 (6) (2013) 541–549, doi: 10.1177/0269881113482529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Garcia-Keller C, Smiley C, Monforton C, Melton S, Kalivas PW, Gass J, N-Acetylcysteine treatment during acute stress prevents stress-induced augmentation of addictive drug use and relapse, Addict. Biol 25 (5) (2020) [accessed 2022 Apr 6] https://pubmed.ncbi.nlm.nih.gov/31282090/, doi: 10.1111/ADB.12798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Amaral VCS, Morais-Silva G, Laverde CF, Marin MT, Susceptibility to extinction and reinstatement of ethanol-induced conditioned place preference is related to differences in astrocyte cystine-glutamate antiporter content, Neurosci. Res (2020), doi: 10.1016/j.neures.2020.07.002. [DOI] [PubMed] [Google Scholar]
- [59].Liu J, Feng X, Wang Y, Xia X, Zheng JC, Astrocytes: GABAceptive and GABAergic cells in the brain, Front. Cell Neurosci 16 (2022) [accessed 2022 Jul 11] https://pubmed.ncbi.nlm.nih.gov/35755777/, doi: 10.3389/FNCEL.2022.892497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Frye CA, Paris JJ, Walf AA, Rusconi JC, Effects and mechanisms of 3 α,5 α,-THP on emotion, motivation, and reward functions involving pregnane xenobiotic receptor, Front. Neurosci 0 (JAN) (2012) 136 [accessed 2022 Jul 11], doi: 10.3389/FNINS.2011.00136/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Sheela Rani CS, Ticku MK, Comparison of chronic ethanol and chronic intermittent ethanol treatments on the expression of GABA(A) and NMDA receptor subunits, Alcohol (Fayetteville, N.Y.) 38 (2) (2006) 89–97 [accessed 2022 Jul 11] https://pubmed.ncbi.nlm.nih.gov/16839855/, doi: 10.1016/J.ALCOHOL.2006.05.002. [DOI] [PubMed] [Google Scholar]
- [62].Follesa P, Floris G, Asuni GP, Ibba A, Tocco MG, Zicca L, Mercante B, Deriu F, Gorini G, Chronic intermittent ethanol regulates hippocampal GABA(A) receptor delta subunit gene expression, Front Cell Neurosci 9 (NOVEMBER) (2015) [accessed 2022 Jul 11] https://pubmed.ncbi.nlm.nih.gov/26617492/, doi: 10.3389/FNCEL.2015.00445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].BA Harlan, Becker HC, Woodward JJ, Riegel AC, Opposing actions of CRF-R1 and CB1 receptors on VTA-GABAergic plasticity following chronic exposure to ethanol, Neuropsychopharmacology 43 (10) (2018) 2064–2074 [accessed 2022 Jul 11] https://pubmed.ncbi.nlm.nih.gov/29946104/, doi: 10.1038/S41386-018-0106-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Maguire J, Mody I, Neurosteroid synthesis-mediated regulation of GABAA receptors: relevance to the ovarian cycle and stress, J. Neurosci 27 (9) (2007) 2155–2162 [accessed 2022 Jul 11] https://www.jneurosci.org/content/27/9/2155, doi: 10.1523/JNEUROSCI.4945-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Mihalek RM, Bowers BJ, Wehner JM, Kralic JE, VanDoren MJ, Morrow AL, Homanics GE, GABA(A)-receptor delta subunit knockout mice have multiple defects in behavioral responses to ethanol, Alcohol. Clin. Exp. Res 25 (12) (2001) 1708–1718 [accessed 2022 Jul 12] https://pubmed.ncbi.nlm.nih.gov/11781502/, doi: 10.1097/00000374-200112000-00003. [DOI] [PubMed] [Google Scholar]
- [66].Nie H, Rewal M, Gill TM, Ron D, Janak PH, Extrasynaptic delta-containing GABAA receptors in the nucleus accumbens dorsomedial shell contribute to alcohol intake, Proc. Nat. Acad. Sci. U.S.A 108 (11) (2011) 4459–4464 [accessed 2022 Jul 12] https://pubmed.ncbi.nlm.nih.gov/21368141/, doi: 10.1073/PNAS.1016156108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Quinones-Jenab V, Jenab S, Progesterone attenuates cocaine-induced responses, Horm. Behav 58 (1) (2010) 22–32 [accessed 2022 Jul 11], doi: 10.1016/j.yhbeh.2009.10.002. [DOI] [PubMed] [Google Scholar]
- [68].Paris JJ, Fenwick J, McLaughlin JP, Progesterone protects normative anxiety-like responding among ovariectomized female mice that conditionally express the HIV-1 regulatory protein, Tat, in the CNS, Horm. Behav 65 (5) (2014) 445–453 [accessed 2022 Jul 11] https://pubmed.ncbi.nlm.nih.gov/24726788/, doi: 10.1016/J.YHBEH.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Antonoudiou P, Colmers PLW, Walton NL, Weiss GL, Smith AC, Nguyen DP, Lewis M, Quirk MC, Barros L, Melon LC, et al. , Allopregnanolone Mediates Affective Switching Through Modulation of Oscillatory States in the Basolateral Amygdala, Biol. Psychiatry 91 (3) (2022) 283–293 [accessed 2022 Jul 11] https://pubmed.ncbi.nlm.nih.gov/34561029/, doi: 10.1016/J.BIOPSYCH.2021.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Kofuji P, Araque A, Astrocytes and Behavior 44 (2022. Apr 8) 49–67 [accessed] https://www.annualreviews.org/doi/abs/10.1146/annurev-neuro-101920-112225, doi: 10.1146/annurev-neuro-101920-112225.2021. doi:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Park K, Lee SJ, Deciphering the star codings: astrocyte manipulation alters mouse behavior, Exp. Mol. Med 52 (7) (2020) 1028–1038 52:7. 2020 [accessed 2022 Apr 8] https://www.nature.com/articles/s12276-020-0468-z, doi: 10.1038/s12276-020-0468-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Scofield MD, Boger HA, Smith RJ, Li H, Haydon PG, Kalivas PW, Gq-DREADD selectively initiates glial glutamate release and inhibits cue-induced cocaine seeking, Biol. Psychiatry (2015), doi: 10.1016/j.biopsych.2015.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Reissner KJ, Gipson CD, Tran PK, Knackstedt LA, Scofield MD, Kalivas PW, Glutamate transporter GLT-1 mediates N-acetylcysteine inhibition of cocaine reinstatement, Addict. Biol 20 (2) (2015) 316–323, doi: 10.1111/adb.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Bull C, Syed WA, Minter SC, Bowers MS, Differential response of glial fibrillary acidic protein-positive astrocytes in the rat prefrontal cortex following ethanol self-administration, Alcohol.: Clin. Exp. Res 39 (4) (2015) 650–658, doi: 10.1111/acer.12683. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


