Mini-abstract
Cellular senescence and smooth muscle cells are key features of the atherosclerotic plaque; however, how senescent cells regulate smooth muscle cells is largely unknown. Herein, a new study in Nature Aging illuminates this interplay, providing insights into plaque dynamics and stability with potentially profound implications for heart attack and stroke.
Atherosclerosis is characterized by plaque build-up in the arterial wall, and rupture of these lesions are the leading cause of myocardial infarction and stroke. Older adults are particularly prone to atherosclerosis and the devastating cardiovascular pathologies associated with it. Despite dietary and potent pharmacological strategies to control elevated blood lipids, an important risk factor for the disease, atherosclerosis continues to wreak havoc on the health of patients. Hence, therapies beyond lipid lowering are desperately needed to reduce the burden of this disease on human health. In this issue of Nature Aging, Child et al. report the important and therapeutically relevant finding that senescent cells (SNCs) inhibit the recruitment of smooth muscle cells (SMCs) to the atherosclerotic lesion and in particular to its fibrous cap, which protects against plaque rupture and thus, cardiovascular insults1. These results shed new light on how the elimination of SNCs by senolytics may potentially help improve clinical outcomes in atherosclerosis.
Cellular senescence was initially described sixty years ago as cessation of proliferation of cultured cells2. It was subsequently associated with wound healing and aging, and its underlying mechanisms were revealed as involving the convergence of diverse stimuli and pathways on activating cell cycle inhibitors p16, p21 and p53. Senescence is a pro-inflammatory process that is observed throughout atherogenesis and augments disease burden in mice and humans3-5. The selective killing of senescent cells (SNCs), known as senolysis, is a provocative therapeutic strategy for atherosclerosis. Indeed, in hypercholesterolemic mouse models, senolysis has been shown through a pharmacological approach to improve vasomotor function6; however, whether or not genetic approaches to induce apoptosis of p16+ cells attenuate atherosclerotic burden appears to be context dependent4,7. In the atherosclerotic plaque, SNCs include modified versions of endothelial cells (ECs), smooth muscle cells (SMCs), macrophages and T-cells3,4,8. SMCs play key roles in atherogenesis with rare SMC progenitors from the vascular wall giving rise to cells in the nascent plaque in response to sub-EC lipid accumulation and inflammation. These rare SMC progenitors clonally expand contributing ~30-70% of the total cellularity of an advanced plaque, and cells of the lineage assume plaque stabilizing fates, such as forming the protective fibrous cap, or transition into destabilizing fates9-11.
Child et al. further evaluated the efficacy of senolysis in atherosclerosis and explored some of the underlying mechanisms (Fig. 1). This study utilized atherosclerosis-prone LDL-receptor knock out mice [Ldlr(−/−)] fed a high-fat diet (HFD) to induce profound hypercholesterolemia and plaque formation, and in many experiments, these mice were subsequently switched to a chow diet to reduce cholesterol levels. The authors used this approach in an attempt to mimic the medical management of elevated lipids following diagnosis of atherosclerosis in humans; however, it’s important to recognize that hyperlipidemia is, in general, very effectively treated with medications, such as statins, whereas even on a chow diet, Ldlr(−/−) mice have an elevated cholesterol (~230-240 mg/dl) compared to that of wild type mice (~100-120 mg/dl)12. To evaluate the effects of senolysis during various stages of atherogenesis or in the established plaque, mice were predominantly treated with the senolytic compound Navitoclax (ABT263), which does not alter circulating lipids. The differing effect of ABT263 on peripheral leukocyte count in this study (no significant change) and a recently published paper (reduced)7 again speaks to the importance of the context of these investigations. As an alternative to ABT263, in a subset of experiments, the authors utilize genetic strategies to eliminate SNCs (INK-ATTAC or p16-3MR transgenic models4). Importantly, senolysis enhanced fibrous cap thickness and/or lesional SMC content in early, moderate and advanced staged plaques. As noted by the authors, this beneficial senolytic effect at diverse plaque stages is critical for potential translation to humans as the atherosclerotic arterial tree in humans includes plaques at differing stages.
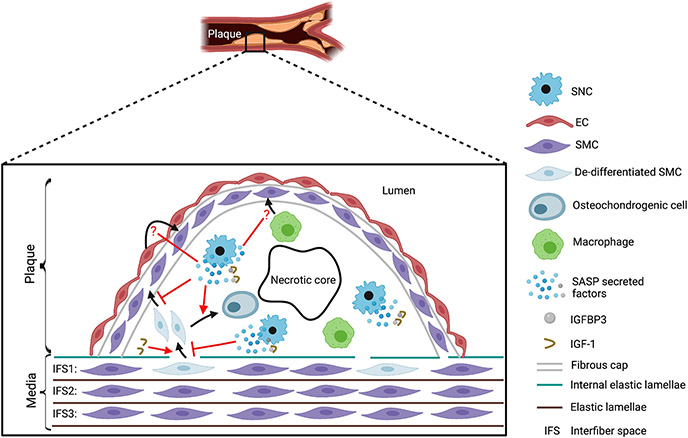
Figure 1. Senescent cells (SNCs) regulate the smooth muscle cell (SMC) lineage in the atherosclerotic plaque.
De-differentiated SMCs are recruited into the atherosclerotic plaque and assume diverse fates, including osteochondrogenic cells of the core and SMCs stabilizing the fibrous cap as well as other fates not depicted (e.g., macrophage-like cells, foam cells). Using the ABT263 senolytic agent, Childs et al. suggest that SNCs decrease the number of cells located in internal elastic lamellae breaks at the tunica media-plaque juncture and the incorporation of SMC marker+ SMC-derived cells in the cap. Conversely, SNCs are implicated in increasing the SMC-derived osteochondrogenic cells in the core. SNCs release the insulin-like growth factor binding protein 3 (IGFBP3), a component of the senescence-associated secretory phenotype (SASP), which sequesters insulin-like growth factor 1 (IGF-1). As exogenously administered IGF-1 increases both cells at the media-plaque juncture and SMA+ lesional cells, the SASP is implicated in reducing SMCs in the fibrous cap and thus, destabilizing the plaque. In addition to medial SMCs, endothelial cells (ECs) and macrophages have previously been implicated as a source of fibrous cap SMCs. The effect of SNCs on the recruitment of these cell types to the cap is not delineated and warrants further investigation (see question marks).
In the normal arterial wall, the internal elastic lamellae separates the tunica intima from the underlying tunica media. The tunica intima is a single cell-thick EC layer. The tunica media consists of multiple alternating circumferential layers of SMCs and elastic fibers, and the authors refer to the position where SMCs in a layer are located as an interfiber space (IFS). In atherosclerosis, the plaque is sandwiched between the EC layer and the media, greatly expanding this space. A particularly intriguing and novel aspect of this paper is the analysis of cells at the juncture between the first IFS (IFS1) of the tunica media and the plaque in breaks of the internal elastic lamellae. Most of these “crossing” cells express the intermediate filament protein vimentin (Vim) but not the SMC marker alpha-smooth muscle actin (SMA). Interestingly, SMA− Vim+ cells are present in substantial numbers in the IFS1 underneath the plaque, but neither in IFS2 or 3 underneath the plaque nor in the IFS1 adjacent to the plaque. Fate mapping studies with HFD-fed Ldlr(−/−), Myh11-CreERT2, CAG-LSL-tdTomato-WPRE mice indicate that the overwhelming majority of SMA−Vim+ cells in IFS1 are tdTomato+ and thus, derive from SMCs. Moreover, ABT263 treatment increases the total number of crossing cells as well as the percentage of IFS1 cells that have the SMA−Vim+ molecular signature. Given that the architecture of SMC-derived cells in the plaque has been shown to consist of rare but large clonally-related patches9,11, these findings suggest that the IFS1 may be a fertile niche for rare progenitors to clonally expand and give rise to daughter cells that migrate into the plaque.
During atherogenesis, recent studies indicate that most SMC-derived plaque cells transition through a “pioneer” or “intermediate” cell phenotype and subsequently differentiate into diverse other phenotypes, including osteochondrocyte-like, inflammatory (e.g., macrophagelike) or extracellular matrix-rich, or they return to a SMC molecular signature13,14. In the current study, ABT263 treatment reduced plaque calcification as assessed by transcriptomic analysis and by histological staining with Alizarin red. Furthermore, fate mapping studies of Ldlr(−/−) mice fed 14 weeks of HFD followed by 9 weeks of chow diet indicate that the large majority of SMA+ cells in the lesion, particularly in the fibrous cap, are of SMC origin. In contrast, a prior study using diverse models of atherosclerosis-prone mice, including Ldlr nulls, fed a HFD for 18 weeks, reported a substantially more limited contribution of the SMC lineage to fibrous cap SMA+ cells and implicated ECs and macrophages as additional important sources15. SMC-derived cells have previously been shown to initially coat the cap of the nascent plaque and subsequently transdifferentiate into other non-SMC fates as they populate the core of the more advanced lesion11,13. Cells of the osteochondrogenic lineage express Runx1, and herein, the authors report that fate mapping studies indicate that in Ldlr(−/−) mice fed 14 weeks of HFD and then 9 weeks of chow diet, ~85% of SMC-derived core cells are Runx1+. This very large percentage is reduced to ~70% with ABT263 treatment. Conversely, ABT263 treatment enhances the percentage of fibrous cap SMA+ cells that derive from SMCs. Thus, SNCs are implicated in driving SMCs away from the protective SMC marker+ fate in the fibrous cap. Taken in the context of the prior literature, these studies raise a number of further important and unanswered questions. First, what underlies the dramatic difference in SMC contribution to the fibrous cap SMA+ cells in atherosclerosis-prone mice fed a HFD for 18 weeks in comparison to mice exposed to 14 weeks of HFD and followed by 9 weeks of chow diet? Second, what is the effect of SNCs on the fate of ECs and macrophages and their contribution to the cap in the plaque? Third, what are the cell autonomous effects of SMC senescence on SMC fate in the atherosclerotic plaque?
Finally, the authors turn their attention to the critical issue of how SNCs regulate SMCs in the plaque. Senescent cells exhibit the pro-inflammatory senescence-associated secretory phenotype (SASP). Cellular secretions of the SASP include cytokines, chemokines, proteases, growth factors and growth factor modulators. One such modulator is insulin-like growth factor binding protein (IGFBP)-3, a potent inhibitor of insulin-like growth factor (IGF)-1, and IGF-1 regulates SMC migration and differentiation state. The current study demonstrates that a high percentage of SNCs in advanced plaques express IGFBP3 and ABT263 treatment substantially reduces the percent of lesional cells that are IGFBP3+. In addition, administration of a IGF-1 variant that is resistant to IGFBP3 binding during a 33 day HFD exposure results in an increase of crossing cells and of SMA+ lesional cells. Finally, the effect of anti-IGFBP3 treatment during ex vivo culture of plaques isolated from mice or humans was examined. In murine plaques, IGFBP3 inhibition alters the composition of the IFS1 cellular pool: decreasing SMA+Vim− cells and increasing SMA−Vim+ cells. In human plaques, this treatment leads to an increase of Vim expression in SMA+ cells. Follow-up studies are needed to further delineate the role of the IGFBP3 - IGF-1 axis in SNC-mediated regulation of the SMC lineage. Moreover, beyond IGFBP3, other factors secreted by SASP+ cells undoubtedly also modulate SMCs in atherogenesis. In the future, “-omic” studies to evaluate the specific gene expression signatures of plaque SMC-derived cells and SNCs in the absence and presence of senolysis will add substantially to the field. And spatial transcriptomics will further integrate the key element of location of the SNC-SMC interaction within the plaque and underlying media.
This stimulating study emphasizes the importance of the interplay between SNCs and SMCs during atherogenesis. As is typical with provocative investigations, many important unresolved issues are raised. For instance, an in depth understanding of the identity of SNCs that regulate SMCs in the plaque and the molecular underpinnings of this complex and dynamic interaction will markedly advance the field. In sum, this study is an important step in the road ahead towards devising further effective therapies beyond lipid lowering strategies - perhaps, such as senolysis - for atherosclerosis.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
References
- 1.Childs BG, et al. Senescent cells suppress innate smooth muscle cell repair functions in atherosclerosis. Natue Aging 8:698–714 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hayflick L & Moorhead PS The serial cultivation of human diploid cell strains. Exp Cell Res 25, 585–621 (1961). [DOI] [PubMed] [Google Scholar]
- 3.Wang J, et al. Vascular Smooth Muscle Cell Senescence Promotes Atherosclerosis and Features of Plaque Vulnerability. Circulation 132, 1909–1919 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Childs BG, et al. Senescent intimal foam cells are deleterious at all stages of atherosclerosis. Science 354, 472–477 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holdt LM, et al. Expression of Chr9p21 genes CDKN2B (p15(INK4b)), CDKN2A (p16(INK4a), p14(ARF)) and MTAP in human atherosclerotic plaque. Atherosclerosis 214, 264–270 (2011). [DOI] [PubMed] [Google Scholar]
- 6.Roos CM, et al. Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging Cell 15, 973–977 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garrido AM, et al. Efficacy and limitations of senolysis in atherosclerosis. Cardiovasc Res (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stojanovic SD, Fiedler J, Bauersachs J, Thum T & Sedding DG Senescence-induced inflammation: an important player and key therapeutic target in atherosclerosis. Eur Heart J 41, 2983–2996 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Basatemur GL, Jorgensen HF, Clarke MCH, Bennett MR & Mallat Z Vascular smooth muscle cells in atherosclerosis. Nat Rev Cardiol 16, 727–744 (2019). [DOI] [PubMed] [Google Scholar]
- 10.Shankman LS, et al. KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis. Nat Med 21, 628–637 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Misra A, et al. Integrin beta3 regulates clonality and fate of smooth muscle-derived atherosclerotic plaque cells. Nat Commun 9, 2073 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishibashi S, et al. Hypercholesterolemia in low density lipoprotein receptor knockout mice and its reversal by adenovirus-mediated gene delivery. J Clin Invest 92, 883–893 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alencar GF, et al. Stem Cell Pluripotency Genes Klf4 and Oct4 Regulate Complex SMC Phenotypic Changes Critical in Late-Stage Atherosclerotic Lesion Pathogenesis. Circulation 142, 2045–2059 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pan H, et al. Single-Cell Genomics Reveals a Novel Cell State During Smooth Muscle Cell Phenotypic Switching and Potential Therapeutic Targets for Atherosclerosis in Mouse and Human. Circulation 142, 2060–2075 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Newman AAC, et al. Multiple cell types contribute to the atherosclerotic lesion fibrous cap by PDGFRbeta and bioenergetic mechanisms. Nat Metab 3, 166–181 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]