Abstract
Background
Seasonal epidemics of respiratory syncytial virus (RSV) cause a clinically significant burden of disease among young children. Non-pharmaceutical interventions targeted at SARS-CoV-2 have affected the activity of other respiratory pathogens. We describe changes in the epidemiology of RSV among children younger than 5 years in England since 2020.
Methods
Surveillance data on RSV infections, comprising laboratory-confirmed cases, proportion of positive tests, hospital admissions for RSV-attributable illness, and syndromic indicators for RSV-associated disease (emergency department attendances for acute bronchitis or bronchiolitis, non-emergency health advice telephone service [NHS 111] calls for cough, general practitioner [GP] in-hours consultations for respiratory tract infections, and GP out-of-hours contacts for acute bronchitis or bronchiolitis) were analysed from Dec 29, 2014 to March 13, 2022, for children younger than 5 years. Data were extracted from national laboratory, clinical, and syndromic surveillance systems. Time-series analyses using generalised linear models were used to estimate the effect of non-pharmaceutical interventions targeting SARS-CoV-2 on RSV indicators, with absolute and relative changes calculated by comparing observed and predicted values.
Findings
RSV-associated activity was reduced for all RSV indicators during winter 2020–21 in England, with 10 280 (relative change –99·5% [95% prediction interval –100·0 to –99·1]) fewer laboratory-confirmed cases, 22·2 (–99·6%) percentage points lower test positivity, 92 530 (–80·8% [–80·9 to –80·8]) fewer hospital admissions, 96 672 (–73·7% [–73·7 to –73·7]) fewer NHS 111 calls, 2924 (–88·8% [–90·4 to –87·2]) fewer out-of-hours GP contacts, 91 304 (–89·9% [–90·0 to –89·9]) in-hours GP consultations, and 27 486 (–85·3% [–85·4 to –85·2]) fewer emergency department attendances for children younger than 5 years compared with predicted values based on winter seasons before the COVID-19 pandemic. An unprecedented summer surge of RSV activity occurred in 2021, including 11 255 (1258·3% [1178·3 to 1345·8]) extra laboratory-confirmed cases, 11·6 percentage points (527·3%) higher test positivity, 7604 (10·7% [10·7 to 10·8]) additional hospital admissions, 84 425 (124·8% [124·7 to 124·9]) more calls to NHS 111, 409 (39·0% [36·6 to 41·8]) more out-of-hours GP contacts, and 9789 (84·9% [84·5 to 85·4]) more emergency department attendances compared with the predicted values, although there were 21 805 (–34·1% [–34·1 to –34·0]) fewer in-hours GP consultations than expected. Most indicators were also lower than expected in winter 2021–22, although to a lesser extent than in winter 2020–21.
Interpretation
The extraordinary absence of RSV during winter 2020–21 probably resulted in a cohort of young children without natural immunity to RSV, thereby raising the potential for increased RSV incidence, out-of-season activity, and health-service pressures when measures to restrict SARS-CoV-2 transmission were relaxed.
Funding
None.
Introduction
Respiratory syncytial virus (RSV) is a common respiratory pathogen and a leading cause of bronchitis and bronchiolitis among young children.1 Most children have been infected with RSV by 2 years of age, and, although most have mild respiratory symptoms, RSV infection can cause severe disease in some groups, including infants, premature infants, and immunocompromised individuals.2 RSV infection has a substantial global impact, representing the second most frequent cause of death in infants (second only to malaria) and imparting annual global inpatient and outpatient costs of approximately €5 billion.3, 4 Transmission of RSV is seasonal, with annual epidemics occurring during the winter months in the northern hemisphere.5 In England, annual RSV activity consistently peaks in young children during December and is associated with an estimated 20 000 hospital admissions annually in children younger than 1 year.5, 6
Research in context.
Evidence before this study
Respiratory syncytial virus (RSV) is a major cause of respiratory illness in young children and has predictable seasonality with northern hemisphere epidemics occurring in mid-winter. The burden of disease is significant, with a systematic review published in 2022 estimating that more than 30 million cases and over 100 000 deaths occur annually in children younger than 5 years. During the COVID-19 pandemic, widespread non-pharmaceutical interventions, such as population lockdowns, travel restrictions, and physical distancing have been introduced to reduce the spread of the SARS-CoV-2 virus; these non-pharmaceutical interventions also have the potential to affect the circulation of RSV. We searched PubMed to identify relevant literature until March 10, 2022, using the key words “respiratory syncytial virus”, “RSV”, “epidemiology”, “SARS-CoV-2”, “COVID-19” and “pandemic”. Southern hemisphere countries initially reported the altered epidemiology of RSV in the first winter since SARS-CoV-2 non-pharmaceutical interventions were introduced. At the commencement of our study, no reports from the northern hemisphere were identified in the literature search.
Added value of this study
We assessed the change in epidemiology of RSV among children in England during the COVID-19 pandemic compared with previous years. A key strength of this study was the use of multiple national surveillance systems (laboratory, clinical, and syndromic) in the analyses to provide a comprehensive understanding of the reduction in RSV activity across a range of of health-care systems. The RSV-associated indicators studied were cases, test positivity, hospital admissions, emergency department attendances, calls to a non-emergency medical helpline, and primary care consultations. We estimated the effect of non-pharmaceutical interventions by comparing RSV indicator activity in the 2020–21 winter season, 2021 summer season, and 2021–22 winter season with data from 2015–16 onwards, using interrupted time-series analyses. Our models accounted for seasonality, trend, and changes to indicator denominator data over time.
Implications of all the available evidence
The results of our study add to the evidence of the broader impact of non-pharmaceutical interventions on the circulation of other respiratory pathogens besides SARS-CoV-2. For RSV, there are concerns around the potential for more severe outcomes (such as a higher proportion of children developing clinically severe disease requiring hospital admission) in forthcoming seasons because of an immunity debt—the concept that a greater proportion of the population is susceptible to disease after a long period of reduced exposure. Our findings will be of interest to health policy makers and planners in the context of ongoing SARS-CoV-2 transmission.
COVID-19 has had a major impact on individuals, societies, and health-care systems. In response to the pandemic, unprecedented interventions have been adopted around the globe, including lengthy whole-country lockdowns and travel restrictions, aimed at preventing SARS-CoV-2 transmission. In England, the government announced physical distancing requirements from March 12, 2020, and implemented the first national lockdown from March 26, 2020. The public were ordered to stay at home, work from home, and only leave home for essential purposes.7 From June, 2020, restrictions were eased but localised lockdowns or enhanced non-pharmaceutical interventions were used until a second 4 week national lockdown started on Nov 5, 2020, which was again followed by localised lockdowns or enhanced non-pharmaceutical interventions. The third national lockdown commenced on Jan 6, 2021, and lasted until March, 2021, when a phased easing occurred until many restrictions were lifted on July 19, 2021. This series of non-pharmaceutical interventions occurred before, during, and after the typical RSV epidemic season during the winter of 2020–21. Minimal non-pharmaceutical interventions were in place after July 19, 2021.
Like SARS-CoV-2, RSV is primarily transmitted through respiratory droplets (ie, coughs and sneezes), including via indirect contact through contaminated surfaces. Widely implemented non-pharmaceutical interventions against SARS-CoV-2—eg, stay-at-home orders, wearing of face coverings, physical distancing, and promotion of improved hygiene, such as handwashing—all have the potential to prevent transmission of other communicable (particularly respiratory) diseases.8 Enforcement of protracted public health interventions has created quasi or natural experimental conditions in which the effect of non-pharmaceutical interventions on other pathogens can be observed at a population level.
During the 2 years since COVID-19 was declared a pandemic, research regarding the impact of the pandemic on the circulation of other pathogens in the community has grown.8 We studied data from laboratory, clinical, and syndromic surveillance to describe the epidemiology of RSV during the pandemic and quantified the change in RSV-attributable disease among children in England since the introduction of public health measures to reduce transmission of SARS-CoV-2. We aimed to investigate the effect of non-pharmaceutical interventions on community transmission of respiratory infections, considering the potential for increased effects of RSV in future seasons.
Methods
Study design and population
We conducted a retrospective observational study to estimate the effect of COVID-19 public health measures on RSV activity using an interrupted time-series approach and generalised linear modelling. The period for which data relating to each indicator were extracted was from Dec 29, 2014 (International Organization for Standardization [ISO] week 1, 2015), to March 13, 2022 (ISO week 10, 2022), inclusive, unless otherwise stated below.
The study population consisted of children younger than 5 years who were identified through public health surveillance systems (operated within the UK Health Security Agency [UKHSA] and National Health Service [NHS]) as having confirmed RSV infection, an RSV test, or RSV-associated respiratory disease.
Analyses were limited to secondary use of datasets routinely collected as part of the public health function of the UKHSA. This study is not considered research as defined by the UK Policy Framework for Health and Social Care Research, determined using the Health Research Authority decision tool; therefore, review by a research ethics committee was not required.9, 10
Data sources
RSV surveillance in England is coordinated through three types of surveillance: laboratory data (cases and tests); clinical data on hospital admissions, categorised under confirmed and non-specific diagnoses (such as pneumonia or acute bronchiolitis or bronchitis); and a variety of syndromic surveillance systems, including calls to the remote health advice telephone line managed by the NHS (NHS 111), primary care (ie, general practitioner [GP]) consultations, and hospital emergency department attendances. This study reviewed RSV indicators across all three surveillance types. Data specifications are summarised below and in the appendix (p 1).
Laboratory data
The UKHSA Second Generation Surveillance System (SGSS) database holds records of all pathogenic isolates from samples submitted from UKHSA, NHS, and private microbiology laboratories in England.11 Record-level data for RSV isolates from children younger than 5 years (at the time of testing) were extracted from SGSS. Records for the same individual within 6 weeks were deduplicated, with the earliest record retained.
The Respiratory DataMart System (RDS) is a UKHSA surveillance dataset recording information on laboratory testing, including confirmed RSV infections. It differs from SGSS in that it contains both positive and negative test results, includes the results of multiplex PCR testing only, and has sentinel coverage.12 RDS data were deduplicated on the basis of a 6 week episode period. The number of cases (numerator) and total tests (denominator) for RSV were extracted for children younger than 5 years (at time of testing) diagnosed in England.
Clinical data
The Hospital Episode Statistics (HES) database contains routinely collected data on all admissions to NHS hospitals in England.13 HES records diagnoses using International Classification of Diseases 10th Revision (ICD-10) codes, with each admission allocated a primary diagnosis code describing the main reason for the hospitalisation. For the purpose of our study, an admission refers to a single HES episode, encompassing admission through to discharge at a single hospital.
The clinical case definition of a hospital admission for RSV-attributable respiratory disease was a HES episode with a primary diagnosis of bronchiolitis (ICD-10 code J21), pneumonia (J12–18), unspecified lower respiratory tract infection (J22), bronchitis (J20), or upper respiratory tract infection (J00–06). These diagnoses have been shown to be sensitive indicators for RSV.6 Only the primary diagnosis was included in order to avoid double counting admissions that contained two or more of these diagnoses.
Total admissions (denominator) and admissions for RSV-attributable respiratory disease (numerator) were extracted for children younger than 5 years (at admission). At the time of extraction, HES data were available only until Feb 28, 2022 (start of ISO week 9, 2022)
Syndromic data
The UKHSA Real-time Syndromic Surveillance Team coordinates the national syndromic surveillance systems operated within the UKHSA.14 Syndromic surveillance is the process of collecting, analysing, and interpreting health-related data to provide an early warning of health threats requiring public health action.14 Based on symptoms and clinical diagnosis of a disease, the anonymised data are aggregated by a number of syndromic indicators.14 Data from four UKHSA syndromic surveillance systems were included: calls to NHS 111; GP out-of-hours contacts; GP in-hours consultations; and emergency department attendances.
A range of syndromic indicators have been shown to be sensitive to RSV activity, including NHS 111 calls for cough, GP in-hours consultations for upper and lower respiratory tract infections, GP out-of-hours contacts for acute bronchitis or bronchiolitis, and emergency department attendances for acute bronchitis or bronchiolitis.6, 15, 16, 17, 18 Counts of calls, consultations, contacts, and attendances for these RSV-type indicators among children younger than 5 years were extracted. Emergency Department data were available only from ISO week 14, 2018, when the national system was established.
Statistical analysis
Indicator data were aggregated into weekly counts and data were presented graphically by ISO week. Total indicator activity in the 2020–21 winter season, 2021 summer season, and 2021–22 winter season were each calculated and compared with the mean in the equivalent season in previous years. The winter season for RSV activity was defined as ISO week 40 to week 10 of the following year, and the summer season as ISO week 11 to week 39, based on previous literature and visual inspection of the data.2 Where available, descriptive calculations were stratified by age group (children aged <1 year and aged 1–4 years).
Interrupted time-series analyses using generalised linear models were used to estimate the effect of COVID-19 non-pharmaceutical interventions on RSV-attributable illness measured by each surveillance indicator. The intervention period started from ISO week 11, 2020 (including March 12, 2020, when physical distancing was implemented in England). Negative binomial regression models were fitted for each indicator to account for over-dispersion. Where available, denominator data were included as an offset variable to allow for changes in denominator activity over time (appendix p 6). A binary variable was included to account for weeks that included a bank holiday, which could introduce systematic differences in weekly data. Harmonic (sine and cosine) terms were included to model annual seasonality using Fourier transformations. Where deemed appropriate by Akaike Information Criterion scores, time was included in the models to account for longer-term trends.
Each model estimated the epidemiological trend of each surveillance indicator trained on historical data before ISO week 11, 2020. These models were used as comparisons with the actual observed indicator activity to give a counterfactual estimation of expected activity if the COVID-19 non-pharmaceutical interventions had not been implemented. Absolute and relative changes in activity during the winter 2020–21, summer 2021, and winter 2021–22 compared with counterfactual models were calculated for each indicator, with prediction intervals (PIs) at the 95% level presented for percentage change.
All analyses were done with R software (version 4.0.2) using the MASS, tidyverse, and gpplot2 packages.
Role of the funding source
There was no funding source for this study.
Results
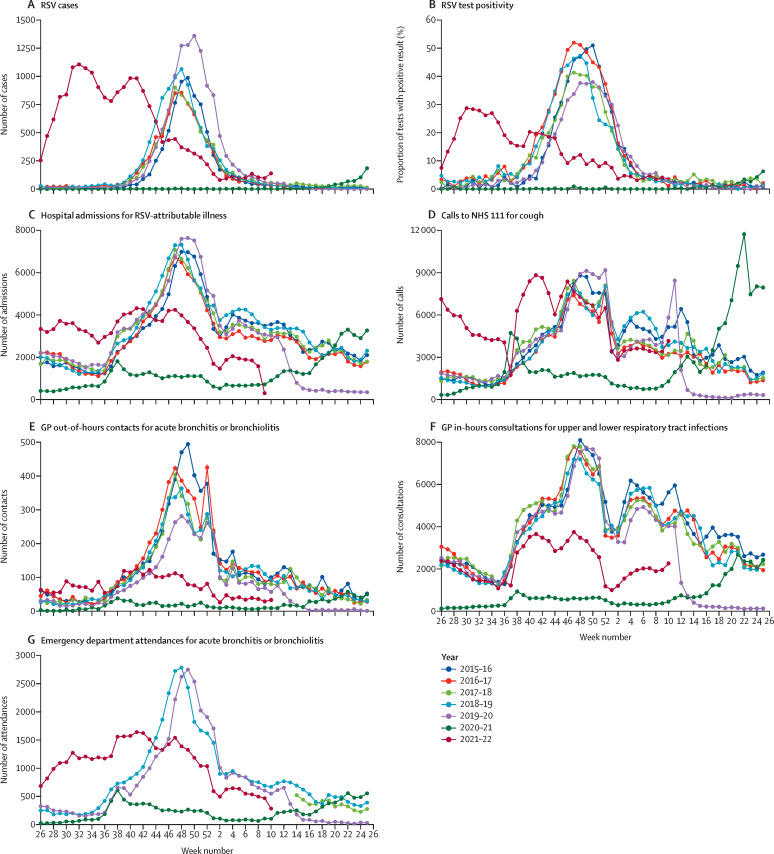
Distinct seasonal trends were observed for laboratory-confirmed cases of RSV in children younger than 5 years during the winter seasons from 2015–16 to 2019–20 (figure 1A ). A peak in cases was typically observed between weeks 47 and 50, with a mean of 8697 cases (SD 1554; range 7453–11 571) reported in each winter season (appendix p 2). Most cases were reported in children younger than 1 year (appendix pp 2–4).
Figure 1.
RSV-associated indicator activity among children younger than 5 years, 2015–22
GP=general practitioner. RSV=respiratory syncytial virus.
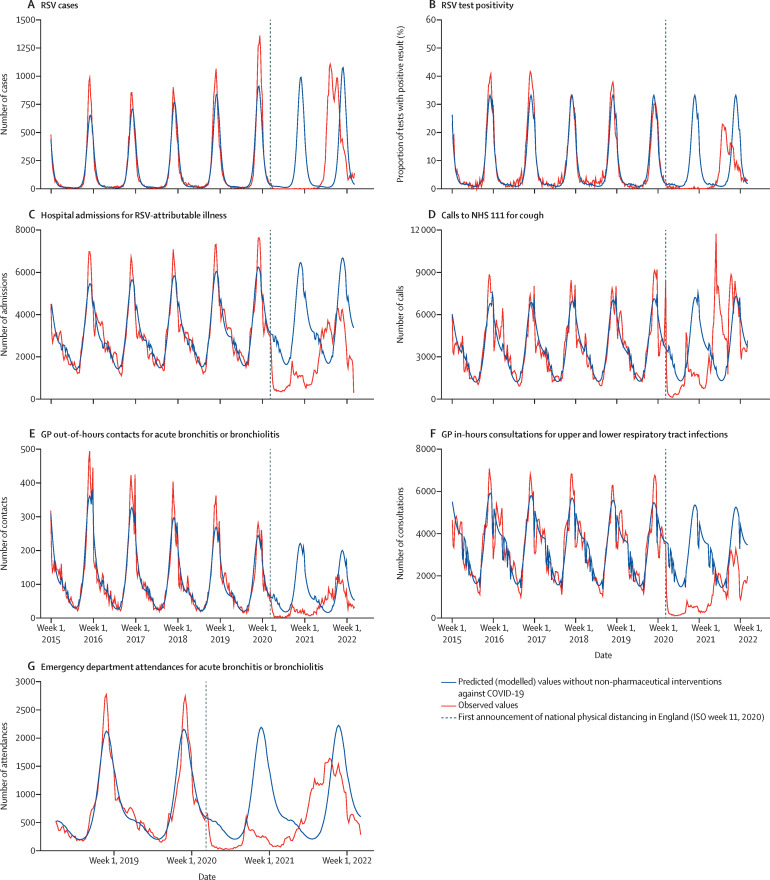
No winter peak in RSV cases was detected in the 2020–21 winter season, with a total of 47 cases reported (figure 1A; appendix p 3). Regression modelling suggested that 10 280 (–99·5% [95% PI –99·1 to –100·0]) fewer cases were reported to SGSS in the 2020–21 winter season, compared with what would have been expected had COVID-19 public health interventions not affected RSV activity (table 1 ; figure 2A ). An atypical peak in cases occurred during summer, 2021 (figure 1A), with 12 150 cases reported during weeks 11–39, in contrast to the 895 predicted cases, representing a 1258·3% increase (1345·8 to 1178·3; table 2 ; figure 2A). Following this summer activity, cases decreased, with no peak during winter 2021–22 (relative change –26·9% [–27·0 to –26·8] compared with predicted value; table 3 ; Figure 1, Figure 2).
Table 1.
Interrupted time-series analyses of predicted versus observed RSV-associated indicator activity during winter 2020–21 (week 40, 2020, to week 10, 2021)
|
Indicator activity, winter 2020–21 |
Difference in indicator activity (observed minus predicted) |
|||
|---|---|---|---|---|
| Predicted (95% PI) | Observed | Absolute change | Percentage change (95% PI) | |
| Laboratory data | ||||
| Total number of cases, SGSS data | 10 327 (10 276 to 10 378) | 47 | −10 280 | −99·5% (−100·0 to −99·1) |
| Mean percentage test positivity, RDS data | 22·3%* | 0·1% | −22·2 | −99·6%* |
| Clinical data | ||||
| Total number of admissions for RSV-attributable respiratory disease, HES data | 114 454 (114 394 to 114 514) | 21 924 | −92 530 | −80·8% (−80·9 to −80·8) |
| Syndromic data | ||||
| Total number of NHS 111 calls for cough | 131 155 (131 095 to 131 214) | 34 483 | −96 672 | −73·7% (−73·7 to −73·7) |
| Total number of GP out-of-hours contacts for acute bronchitis or bronchiolitis | 3293 (3234 to 3352) | 369 | −2924 | −88·8% (−90·4 to −87·2) |
| Total number of GP in-hours consultations for upper and lower respiratory tract infections | 101 552 (101 490 to 101 614) | 10 248 | −91 304 | −89·9% (−90·0 to −89·9) |
| Total number of emergency department attendances for acute bronchitis or bronchiolitis | 32 226 (32 175 to 32 277) | 4740 | −27 486 | −85·3% (−85·4 to −85·2) |
Note that RSV status is confirmed in laboratory data and presumed in clinical and syndromic data. RSV=respiratory syncytial virus. PI=prediction interval. SGSS=Second Generation Surveillance System. RDS=Respiratory DataMart system. HES=Hospital Episode Statistics. GP=general practitioner.
95% PIs were not estimated because of repeated zero counts in weekly reports.
Figure 2.
Time-series analyses of predicted and actual RSV-associated indicator activity among children younger than 5 years, 2015–22
GP=general practitioner. RSV=respiratory syncytial virus.
Table 2.
Interrupted time-series analyses of predicted versus observed RSV-associated indicator activity during summer 2021 (weeks 11–39, 2021)
|
Indicator activity, summer 2021 |
Difference in indicator activity (observed minus predicted) |
|||
|---|---|---|---|---|
| Predicted (95% PI) | Observed | Absolute change | Percentage change (95% PI) | |
| Laboratory data | ||||
| Total number of cases, SGSS data | 895 (834 to 955) | 12 150 | 11 255 | 1258·3% (1178·3 to 1345·8) |
| Mean percentage test positivity, RDS data | 2·2%* | 13·8% | 11·6 | 527·3%* |
| Clinical data | ||||
| Total number of admissions for RSV-attributable respiratory disease, HES data | 70 766 (70 697 to 70 835) | 78 370 | 7604 | 10·7% (10·7 to 10·8) |
| Syndromic data | ||||
| Total number of NHS 111 calls for cough | 67 645 (67 575 to 67 715) | 152 070 | 84 425 | 124·8% (124·7 to 124·9) |
| Total number of GP out-of-hours contacts for acute bronchitis or bronchiolitis | 1049 (979 to 1118) | 1458 | 409 | 39·0% (36·6 to 41·8) |
| Total number of GP in-hours consultations for upper and lower respiratory tract infections | 63 990 (63 918 to 64 062) | 42 185 | −21 805 | −34·1% (−34·1 to −34·0) |
| Total number of emergency department attendances for acute bronchitis or bronchiolitis | 11 530 (11 468 to 11 591) | 21 319 | 9789 | 84·9% (84·5 to 85·4) |
Note that RSV status is confirmed in laboratory data and presumed in clinical and syndromic data. RSV=respiratory syncytial virus. PI=prediction interval. SGSS=Second Generation Surveillance System. RDS=Respiratory DataMart system. HES=Hospital Episode Statistics. GP=general practitioner.
95% PIs were not estimated because of repeated zero counts in weekly reports.
Table 3.
Interrupted time-series analysis of predicted versus observed RSV-associated indicator activity during winter 2021–22 (week 40, 2021, to week 10, 2022)
|
Indicator activity, winter 2021–22 |
Difference in indicator activity (observed minus predicted) |
|||
|---|---|---|---|---|
| Predicted (95% PI) | Observed | Absolute change | Percentage change (95% PI) | |
| Laboratory data | ||||
| Total number of cases, SGSS data | 11 078 (11 029 to 11 127) | 8097 | −2981 | −26·9% (−27·0 to −26·8) |
| Mean percentage test positivity, RDS data | 22·7%* | 10·1% | −12·6 | −55·5%* |
| Clinical data | ||||
| Total number of admissions for RSV-attributable respiratory disease, HES data† | 111 553 (111 498 to 111 609) | 63 478 | −48 075 | −43·1% (−43·1 to −43·1) |
| Syndromic data | ||||
| Total number of NHS 111 calls for cough | 128 142 (128 084 to 128 199) | 130 905 | 2763 | 2·2% (2·2 to 2·2) |
| Total number of GP out-of-hours contacts for acute bronchitis or bronchiolitis | 2881 (2824 to 2938) | 1664 | −1217 | −42·2% (−43·1 to −41·4) |
| Total number of GP in-hours consultations for upper and lower respiratory tract infections | 97 819 (97 759 to 97 878) | 50 714 | −47 106 | −48·2% (−48·2 to −48·1) |
| Total number of emergency department attendances for acute bronchitis or bronchiolitis | 31 965 (31 916 to 32 014) | 23 283 | −8683 | −27·2% (−27·2 to −27·1) |
Note that RSV status is confirmed in laboratory data and presumed in clinical and syndromic data. RSV=respiratory syncytial virus. PI=prediction interval. SGSS=Second Generation Surveillance System. RDS=Respiratory DataMart system. HES=Hospital Episode Statistics. GP=general practitioner.
95% PIs were not estimated because of repeated zero counts in weekly reports.
HES data up to Feb 28, 2022 (latest available at time of extraction).
The trend in the proportion of tests that were positive for RSV was similar to the trend in number of cases. Before COVID-19, a peak in test positivity was typically observed during weeks 48–50 each year, with a low proportion of positive tests in the summer (figure 1B). There was no seasonal increase in test positivity in winter 2020–21, with seven (0·1%) of 7094 tests being positive (table 1; appendix p 2), with an estimated relative reduction of 99·6% in test positivity (table 1; figure 2B). Test positivity increased from week 22, 2021 (summer), and reached a peak of 28·7% in week 30 (figure 2B). Across weeks 11–39, 2021, test positivity was 527·3% higher than expected (table 2). Test positivity declined throughout the rest of the year, and no typical epidemic activity was observed in winter 2021–22, with an estimated 55·5% reduction in test positivity compared with the predicted value (table 3).
Hospital admissions showed clear seasonal trends from 2015–16 to 2019–20 (figure 1C), typically peaking in weeks 47–48. RSV-attributable admissions fell rapidly after the first announcement of physical distancing rules in week 11, 2020. Admissions remained low until week 36, 2020, when they began to increase (around the same time as in previous years), although they did not reach the same levels seen in previous years. Time-series modelling indicated that more than 92 530 hospital admissions for RSV-attributable disease among children younger than 5 years were averted in winter 2020–21 compared with the expected number (relative change –80·8% [–80·9 to –80·8]; table 1; figure 2C). During summer 2021, observed admissions were 10·7% (10·8 to 10·7) higher than predicted, an absolute difference of 7604 admissions (table 2; figure 1C). From week 47, 2021, admissions decreased as in previous years, but to lower levels, with modelling indicating that 48 075 (–43·1% [–43·1 to –43·1]) fewer admissions for RSV-attributable disease occurred during winter 2021–22 relative to the predicted number (table 3; figure 2C).
Before COVID-19, all syndromic RSV-type indicators showed clear seasonal activity during the winter each year, increasing from approximately week 36 with a peak in weeks 48–50. Following week 11, 2020, all indicators dropped immediately, and no seasonal increases were observed in winter 2020–21 (figure 1D–G). An estimated 96 672 (–73·7% [–73·7 to –73·7]) fewer NHS 111 calls for cough were made in winter 2020–21 compared with the predicted number (table 1; figure 2D). The number of GP out-of-hours contacts for acute bronchitis or bronchiolitis was 2924 (–88·8% [–90·4 to –87·2]) lower and GP in-hours consultations for lower and upper respiratory tract infections 91 304 (–89·9% [–90·0 to –89·9]) lower in 2020–21 compared with the modelled values (table 1; figure 2E–F). The absence of the 2020–21 winter RSV epidemic resulted 27 486 (–85·3% [–85·4 to –85·2]) fewer emergency department attendances for acute bronchitis or bronchiolitis being reported through the emergency department syndromic surveillance system network (table 1; figure 2G).
Peaks outside of the typical winter season following winter 2020–21 were also observed in the syndromic data, but the extent to which these peaks occurred varied between systems. All except GP in-hours consultations for upper and lower respiratory tract infections showed increased activity in summer 2021 compared with the predicted values, most notably NHS 111 calls for cough and emergency department attendances for acute bronchitis or bronchiolitis (table 2). 84 425 (124·8% [124·7 to 124·9]) additional calls to NHS 111 for cough were made compared with the predicted number (table 2; figure 2D), largely driven by a peak in calls at week 22, 2021, at a frequency that exceeded any previous winter season (figure 1D).
For GP out-of-hours contacts for acute bronchitis or bronchiolitis, an increase of 39·0% (36·6 to 41·8) above predicted levels was observed in summer 2021 (table 2; figure 1F), but no typical increase was observed the following winter (–42·2% [–43·1 to –41·4] compared with predicted; table 3). For GP in-hours consultations for upper and lower respiratory tract infections, after the absence of a winter epidemic in 2020–21, the summer 2021 period showed trends similar to those in previous years. The number of consultations was 21 805 (–34·1% [–34·1 to –34·0]) lower than predicted, but this change was attributable to fewer consultations for lower and upper respiratory tract infections at the start of 2021 compared with the same time during previous years (table 2; figure 1F). The magnitude of the winter peak in 2021–22 was reduced, with 47 106 (–48·2% [–48·2 to –48·1]) fewer consultations than expected (table 3).
The trend for emergency department attendances for acute bronchitis or bronchiolitis diverged from the seasonal norm from week 22, 2021, onwards (figure 1G). In summer 2021, 9789 (84·9% [84·5 to 85·4]) more attendances than predicted were observed (table 2). The period of increased attendances was sustained through the summer and started to decline from week 47, 2021 (figure 1G). The number of attendances was 8683 (–27·2% [–27·2 to –27·1]) lower than predicted for the winter of 2021–22 (table 3).
Most surveillance indicators showed reduced activity in winter 2020–21, but increased activity in summer 2021, and therefore the net indicator activity (compared with modelled) was calculated during the 2 full years following the introduction of COVID-19 non-pharmaceutical interventions (week 11, 2020, to week 10, 2022; appendix p 5). The net differences included 2592 fewer cases (–11·3% [–11·2 to –11·4]); 178 575 fewer hospital admissions for RSV-attributable disease (–48·9% [–48·9 to –49·0); 214 634 fewer GP in-hours consultations (–65·2% [–65·2 to –65·3]) and 33 016 fewer emergency department attendances for acute bronchitis or bronchiolitis (–38·0% [–37·9 to –38·1]).
Discussion
These results show an unprecedented reduction in RSV activity during the 2020–21 winter season, followed by an atypical surge of activity during the following summer, which coincides with the implementation and subsequent relaxation of national public health interventions aimed at limiting SARS-CoV-2 spread in the community. Reduced RSV activity was observed in the subsequent winter season of 2021–22 compared with winters before the COVID-19 pandemic.
Although some reduction in RSV would have been expected given that the transmission routes of RSV and SARS-CoV-2 are the same, the estimated effect size for winter 2020–21 is substantial. Laboratory-confirmed cases of RSV in children were 99% lower in winter 2020–21 than would be expected if non-pharmaceutical interventions against COVID-19 had not been implemented. This reduction cannot be explained by any reduction in laboratory testing, as test-positivity was reduced by a similar order of magnitude. Our findings align with reports from other countries that have reported significantly reduced RSV transmission in the first winter following the introduction of COVID-19 public health measures; for example, Western Australia and New Zealand found over 99% reductions in RSV detections compared with previous years.19, 20, 21, 22, 23, 24, 25, 26 It should be noted the UKHSA emergency department and GP syndromic surveillance systems do not have full population coverage, so our estimated reductions are an underestimation of the reduction across England.
Although the result of smaller winter epidemics might appear beneficial, there is growing evidence of the potential of medium-term negative effects through immunity debt, in which a greater proportion of the population is susceptible to a disease after a long period of reduced exposure.27 This poses a threat with regard to the size of the epidemic and subsequent health-care pressures, as well as the timing of RSV epidemics.27, 28 The unprecedented suppression of RSV activity during winter 2020–21 is likely to have resulted in a large cohort of immunologically naive children younger than 5 years who have not been exposed to natural RSV infection but who became exposed upon the lifting of restrictions. A summer epidemic of RSV had never been observed in UK surveillance data, but our analyses showed an increase of more than 1000% in laboratory-confirmed cases in summer 2021 compared with predicted (where laboratory-confirmed cases reached numbers expected of a pre-pandemic winter), and an increase of more than 80% in emergency department attendances. The disproportionate difference observed between laboratory-based and clinical or syndromic indicators of RSV activity might be explained by an increased propensity to test for respiratory pathogens during the COVID-19 pandemic, and the potential effect of summer meteorological conditions lessening the severity of symptoms and thereby the likelihood of admissions or emergency department visits. Before the COVID-19 pandemic, the greatest burden of laboratory-confirmed RSV was observed in the predominantly RSV-naive population of children younger than 1 year. During the summer RSV epidemic, however, the relative burden of laboratory-confirmed RSV in children aged 1–4 years was greater than in pre-COVID-19 winters, and activity peaked at levels higher than in pre-COVID-19 winters. This finding is possibly a result of the increased population cohort of RSV-naive children in the 1–4 years age group following the period of population lockdown during the pandemic, when exposure to RSV was minimal.
Modelling from the USA projects that large future outbreaks of respiratory viruses might occur after a period of extended non-pharmaceutical interventions.28 Indeed, inter-seasonal RSV resurgence following an RSV-free winter, associated with the relaxation of COVID-19 measures, has also been observed in the USA, Australia, and South Africa, among other countries.29, 30, 31 In England, this resurgence meant unprecedented measures were taken to mitigate the risks of RSV-associated severe disease and hospitalisation, including the approval of prescribing of palivizumab (a passive immunoprophylaxis for RSV) earlier than the usual October start date and to an expanded group of eligible patients. Inter-seasonal surges of other respiratory infections, including parainfluenza and rhinovirus, were also reported across the country, and the NHS brought forward its usual winter planning, escalation, and emergency processes.11, 32 The impact of out-of-season respiratory pathogen activity on hospitals was, however, still considerable.33
Ongoing monitoring of non-COVID-19 respiratory disease indicators will be required to inform future health-care system planning in the context of ongoing COVID-19 transmission. Widespread simultaneous community transmission of RSV and SARS-CoV-2 could exacerbate physical pressures on the primary and secondary NHS care systems, which are already working with reduced capacity because of physical distancing among other infection control precautions. Moreover, COVID-19 vaccines are not currently licensed for children younger than 5 years in England, and the effect of potential interactions between SARS-CoV-2 and other respiratory viruses in terms of disease severity is a potential concern.34
A major strength of our study is the breadth of surveillance data incorporated into the analysis. Using data from laboratory testing, hospital admissions, and syndromic surveillance we triangulated signals from across the spectrum of health care, from confirmed and hospitalised cases to health care-seeking (or advice-seeking) behaviour in the community. The systems presented here are commonplace in public health systems internationally, and our methods and findings are therefore applicable across other settings and respiratory pathogens.
However, the study had some limitations. We applied a binary threshold for the COVID-19 intervention period in time-series analyses, starting in ISO week 11, 2020, when physical distancing was first implemented in England by the UK Government. It was not possible for us to disentangle the relative effects of different interventions (eg, full lockdown, physical distancing, school or workplace closures, reduced travel, or hygiene behaviours) that occurred at different stages of the pandemic response. As a result, we can only present a crude estimate of the impact of non-pharmaceutical interventions as a whole. We also present data at the national population level, which might mask subnational differences in RSV activity.
While being sensitive to RSV activity, the indicators from syndromic and hospital surveillance systems are not specific to RSV, which could lead to an overestimation of RSV-associated disease. This lack of specificity is especially pertinent during times of enhanced COVID-19 transmission, given that some respiratory symptoms are common to COVID-19 and RSV infection. However, the magnitude of reduction across all our RSV indicators was so large that we would not expect this potential overestimation to substantially change our findings. In addition, some of the syndromic surveillance systems included were sentinel and might not be fully representative of England nationally. Furthermore, during pre-pandemic summer periods, these non-specific RSV metrics might overestimate RSV activity because they are likely to be less specific for RSV during a period when, typically, there is lower RSV activity. These syndromic indicators should, therefore, be interpreted alongside other more specific RSV indicators such as laboratory tests.
The study population did not include those people with RSV who did not use any health services, which could lead to an underestimation of the true burden of RSV occurring in the community. This limitation was particularly relevant during the initial phases of COVID-19, when the population was encouraged to avoid social contact and reduce the burden on the NHS, which resulted in large decreases in health care-seeking behaviour.35 However, parents with infants with RSV-associated clinical symptoms are more likely to present to health-care services than are other patients with acute, mild, or self-limiting presentations. Therefore, we believe that the decreases were more likely to reflect decreases in the burden of disease rather than changes in community health care-seeking behaviours.
Finally, there were some methodological limitations. Although the interrupted time series-based methodology accounted for important confounders such as changes in surveillance denominator, seasonality, and trend, there were other confounders that were not accounted for in the analyses (eg, demographic characteristics). Given the estimated effect size across the breadth of surveillance systems studied, however, we do not believe this will have substantially affected our overall findings.
In conclusion, the absence of RSV activity in England during the winter of 2020–21 and then atypical activity in summer 2021 was unprecedented in the modern epidemiological era, and was most likely due to the introduction and subsequent relaxation of public health non-pharmaceutical interventions to mitigate the spread of COVID-19. This finding has been shown across a range of surveillance systems monitoring RSV in England. These systems will be invaluable for informing seasonal RSV planning in the context of ongoing COVID-19 transmission, as the magnitude and timing of future RSV epidemics remains to be seen.
Data sharing
Applications for requests to access relevant anonymised data included in this study should be submitted to the UKHSA Office for Data Release (https://www.gov.uk/government/publications/accessing-ukhsa-protected-data/accessing-ukhsa-protected-data).
Declaration of interests
We declare no competing interests.
Acknowledgments
Acknowledgments
We thank Edward Harding for extraction of SGSS data, Mark Dancox for assistance with extraction of HES data, and Alison Waldram for supervision and support. We also acknowledge the UKHSA Real-time Syndromic Surveillance Team for technical expertise in delivering the daily syndromic service. We thank syndromic data providers NHS 111 and NHS Digital; out-of-hours providers submitting data to the GP out-of-hours system; emergency department clinicians and NHS Trusts and NHS Digital supporting Emergency Department Syndromic Surveillance System; and participating The Phoenix Partnership practices supporting GP in-hours, ambulance trusts, and the Association of the Ambulance Chief Executives. RM, CRB, GES, and AJE are affiliated with the National Institute for Health Research Health Protection Research Unit (NIHR HPRU) in Emergency Preparedness and Response at King's College London. CRB is affiliated with the NIHR HPRU in Behavioural Science and Evaluation at the University of Bristol. GES, HEH and AJE are affiliated to the NIHR HPRU in Gastrointestinal Infections at University of Liverpool. The views expressed in this Article are those of the authors and not necessarily those of the UKHSA or the Department of Health and Social Care.
Contributors
AJE, GES, RAM, and HEH conceived the study. AJE, GES, RAM, HEH, CRB, and MB designed the study. MB led on data processing and data management. MB and RAM planned the statistical analysis, analysed the data, generated the tables and figures, and accessed and verified all the data. CHW and JE provided topic-area expertise. HZ curated the RDS data. MB drafted the manuscript, and all authors reviewed and revised drafts and approved the final version. All authors had access to the data and accept responsibility for the decision to submit for publication.
Supplementary Material
References
- 1.Li Y, Wang X, Blau DM, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis. Lancet. 2022;399:2047–2064. doi: 10.1016/S0140-6736(22)00478-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Obando-Pacheco P, Justicia-Grande AJ, Rivero-Calle I, et al. Respiratory syncytial virus seasonality: a global overview. J Infect Dis. 2018;217:1356–1364. doi: 10.1093/infdis/jiy056. [DOI] [PubMed] [Google Scholar]
- 3.Zhang S, Akmar LZ, Bailey F, et al. Cost of respiratory syncytial virus-associated acute lower respiratory infection management in young children at the regional and global level: a systematic review and meta-analysis. J Infect Dis. 2020;222(suppl 7):S680–S687. doi: 10.1093/infdis/jiz683. [DOI] [PubMed] [Google Scholar]
- 4.University of Oxford Vaccine Knowledge Project What is respiratory syncytial virus (RSV)? Nov 22, 2019. https://vk.ovg.ox.ac.uk/vk/rsv
- 5.Goddard NL, Cooke MC, Gupta RK, Nguyen-Van-Tam JS. Timing of monoclonal antibody for seasonal RSV prophylaxis in the United Kingdom. Epidemiol Infect. 2007;135:159–162. doi: 10.1017/S0950268806006601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reeves RM, Hardelid P, Gilbert R, Warburton F, Ellis J, Pebody RG. Estimating the burden of respiratory syncytial virus (RSV) on respiratory hospital admissions in children less than five years of age in England, 2007-2012. Influenza Other Respir Viruses. 2017;11:122–129. doi: 10.1111/irv.12443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.UK Parliament Coronavirus: a history of English lockdown laws. Dec 22, 2021. https://commonslibrary.parliament.uk/research-briefings/cbp-9068/
- 8.Baker RE, Park SW, Yang W, Vecchi GA, Metcalf CJE, Grenfell BT. The impact of COVID-19 nonpharmaceutical interventions on the future dynamics of endemic infections. Proc Natl Acad Sci USA. 2020;117:30547–30553. doi: 10.1073/pnas.2013182117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.NHS Health Research Authority Is my study research? http://www.hra-decisiontools.org.uk/research/
- 10.Health Research Authority Defining research. October, 2017. http://www.hra-decisiontools.org.uk/research/docs/DefiningResearchTable_Oct2017-1.pdf
- 11.Public Health England National flu and COVID-19 surveillance reports. Oct 8, 2020. https://www.gov.uk/government/statistics/national-flu-and-covid-19-surveillance-reports
- 12.Public Health England Sources of UK flu data: influenza surveillance in the UK. Jan 7, 2014. https://www.gov.uk/guidance/sources-of-uk-flu-data-influenza-surveillance-in-the-uk
- 13.NHS Digital Hospital Episode Statistics (HES) 2021. https://digital.nhs.uk/data-and-information/data-tools-and-services/data-services/hospital-episode-statistics
- 14.Public Health England Syndromic surveillance: systems and analyses. 2021. https://www.gov.uk/government/collections/syndromic-surveillance-systems-and-analyses
- 15.Harcourt SE, Morbey RA, Smith GE, et al. Developing influenza and respiratory syncytial virus activity thresholds for syndromic surveillance in England. Epidemiol Infect. 2019;147:e163. doi: 10.1017/S0950268819000542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hughes HE, Morbey R, Hughes TC, et al. Emergency department syndromic surveillance providing early warning of seasonal respiratory activity in England. Epidemiol Infect. 2016;144:1052–1064. doi: 10.1017/S0950268815002125. [DOI] [PubMed] [Google Scholar]
- 17.Morbey RA, Elliot AJ, Harcourt S, et al. Estimating the burden on general practitioner services in England from increases in respiratory disease associated with seasonal respiratory pathogen activity. Epidemiol Infect. 2018;146:1389–1396. doi: 10.1017/S0950268818000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morbey RA, Harcourt S, Pebody R, et al. The burden of seasonal respiratory infections on a national telehealth service in England. Epidemiol Infect. 2017;145:1922–1932. doi: 10.1017/S095026881700070X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Britton PN, Hu N, Saravanos G, et al. COVID-19 public health measures and respiratory syncytial virus. Lancet Child Adolesc Health. 2020;4:e42–e43. doi: 10.1016/S2352-4642(20)30307-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang QS, Wood T, Jelley L, et al. Impact of the COVID-19 nonpharmaceutical interventions on influenza and other respiratory viral infections in New Zealand. Nat Commun. 2021;12 doi: 10.1038/s41467-021-21157-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yeoh DK, Foley DA, Minney-Smith CA, et al. Impact of coronavirus disease 2019 public health measures on detections of influenza and respiratory syncytial virus in children during the 2020 Australian winter. Clin Infect Dis. 2021;72:2199–2202. doi: 10.1093/cid/ciaa1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Friedrich F, Ongaratto R, Scotta MC, et al. Early impact of social distancing in response to coronavirus disease 2019 on hospitalizations for acute bronchiolitis in infants in Brazil. Clin Infect Dis. 2021;72:2071–2075. doi: 10.1093/cid/ciaa1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sullivan SG, Carlson S, Cheng AC, et al. Where has all the influenza gone? The impact of COVID-19 on the circulation of influenza and other respiratory viruses, Australia, March to September 2020. Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.47.2001847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olsen SJ, Azziz-Baumgartner E, Budd AP, et al. Decreased influenza activity during the COVID-19 pandemic-United States, Australia, Chile, and South Africa, 2020. Am J Transplant. 2020;20:3681–3685. doi: 10.1111/ajt.16381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tempia S, Walaza S, Bhiman JN, et al. Decline of influenza and respiratory syncytial virus detection in facility-based surveillance during the COVID-19 pandemic, South Africa, January to October 2020. Euro Surveill. 2021;26 doi: 10.2807/1560-7917.ES.2021.26.29.2001600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pellegrinelli L, Galli C, Bubba L, et al. Respiratory syncytial virus in pediatric influenza-like illness cases in Lombardy, Northern Italy, during seven consecutive winter seasons (from 2014–2015 to 2020–2021) Influenza Other Respir Viruses. 2022;16:481–491. doi: 10.1111/irv.12940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hatter L, Eathorne A, Hills T, Bruce P, Beasley R. Respiratory syncytial virus: paying the immunity debt with interest. Lancet Child Adolesc Health. 2021;5:e44–e45. doi: 10.1016/S2352-4642(21)00333-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng Z, Pitzer VE, Shapiro ED, Bont LJ, Weinberger DM. Estimation of the timing and intensity of reemergence of respiratory syncytial virus following the COVID-19 pandemic in the US. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.41779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Foley DA-O, Yeoh DK, Minney-Smith CA, et al. The interseasonal resurgence of respiratory syncytial virus in Australian children following the reduction of coronavirus disease 2019-related public health measures. Clin Infect Dis. 2021;73:e2829–e2830. doi: 10.1093/cid/ciaa1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.National Institute for Communicable Diseases Increase in respiratory syncytial virus (RSV) cases 2020. Oct 13, 2020. https://www.nicd.ac.za/increase-in-respiratory-syncytial-virus-rsv-cases-2020/
- 31.US Centers for Disease Control and Prevention Increased interseasonal respiratory syncytial virus (RSV) activity in parts of the southern United States. June 10, 2021. https://emergency.cdc.gov/han/2021/han00443.asp
- 32.Public Health England Health chiefs issue warning as childhood respiratory infections rise ahead of winter. July 23, 2021. https://www.gov.uk/government/news/health-chiefs-issue-warning-as-childhood-respiratory-infections-rise-ahead-of-winter
- 33.Limb M. RSV: the year the respiratory infection “took its gloves off”. BMJ. 2021;374 doi: 10.1136/bmj.n2078. [DOI] [PubMed] [Google Scholar]
- 34.Evans C, Lim WS, Semple C, et al. NERVTAG: respiratory viral infections, their interactions with SARS-CoV-2 and implications for a winter resurgence of COVID-19. July 31, 2020. https://www.gov.uk/government/publications/nervtag-respiratory-viral-infections-their-interactions-with-sars-cov-2-and-implications-for-a-winter-resurgence-of-covid-19-16-july-2020
- 35.Hughes HE, Hughes TC, Morbey R, et al. Emergency department use during COVID-19 as described by syndromic surveillance. Emerg Med J. 2020;37:600–604. doi: 10.1136/emermed-2020-209980. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Applications for requests to access relevant anonymised data included in this study should be submitted to the UKHSA Office for Data Release (https://www.gov.uk/government/publications/accessing-ukhsa-protected-data/accessing-ukhsa-protected-data).