ABSTRACT
We evaluated safety, reactogenicity, and immunogenicity when the WHO-prequalified single-dose Typhoid Vi-polysaccharide conjugate vaccine, Typbar-TCV®, was administered concomitantly with measles (MV) or measles-mumps-rubella (MMR) vaccines in 8- or 9-month-old children. We enrolled 493 children who were randomized 2:1:1:1 to four groups to receive either TCV (0.5 mL intramuscularly) and MV (0.5 ml subcutaneously) concomitantly at 9 months of age (Group 1) with two subgroups given TCV booster 28 days (Group 1A) or 180 days (Group 1B) later, or MV on Day 0 and TCV on Day 28 (Group 2); or TCV at 8 months of age and MV 28 days later (Group 3), or MV only at 9 months of age (Group 4). All children received MMR at 15 months of age. We observed no statistically significant differences between group rates of solicited or unsolicited adverse events assessed throughout the study. Seroconversion rates for measles, mumps, and rubella antibodies were unaffected by concomitant administration with TCV, being similar in Groups 1, 2, and 3 and comparable to Group 4 (Control). IgG anti-Vi antibody titers were similar in all groups after primary Typbar-TCV® vaccination and were not increased by a second dose 28 days later. A small response to a booster dose of Typbar-TCV® given at 180 days did not achieve the high titers observed after the first dose, suggesting that booster vaccination may be more effective after a longer interval than 6 months. Typbar-TCV® can be safely co-administered with measles and MMR vaccines in children aged ≥9 months.
Clinical trial registration number: CTRI/2014/04/004532.
KEYWORDS: Typhoid, Typbar-TCV, TCV, measles, mumps, rubella, vaccine
Introduction
Typhoid fever is an acute infection caused by a gram-negative bacterium Salmonella enterica serovar Typhi (Salmonella Typhi) transmitted through ingestion of food or water contaminated with feces of infected persons. The most common symptoms are abdominal pain, pyrexia, chills, headache, and weakness.1 Global Burden Disease (GBD) survey data from 2017 show that there were 10.9 million cases and more than 116,000 deaths due to typhoid fever globally which declined to 9.24 million cases and 110,000 deaths in 2019, with highest incidence is among individuals living in emergent nations in Asia and Sub-Saharan Africa.2,3 Typhoid disease incidence is low in the neonatal period and increasingly evident in children with increasing age.4 US Centers for Disease Control and Prevention (CDC) surveillance recorded 3–30 cases per 100,000 travelers with the highest incidence rate (59%) in travelers who visited India.5 Emergence of new typhoid fever antibiotic-resistant strains is making treatment for enteric fever more complicated and more expensive to treat, making effective vaccination programs a priority.1
Until 2017, only two typhoid fever vaccines were available, the parenteral S. Typhi capsular Vi polysaccharide vaccine was licensed for use in individuals aged ≥2 years of age and a live oral Ty21a vaccine was licensed in the USA and Canada for use in individuals aged ≥5 years.6 In 2017, the WHO prequalified the Bharat Biotech typhoid conjugate vaccine, Typbar-TCV® which consists of the S. Typhi Vi capsular polysaccharide conjugated to tetanus toxoid carrier protein for administration as a single intramuscular dose in individuals aged >6 months to 45 years.7
Following demonstration of its safety and robust immunogenicity in a phase 3 study,8 in October 2017 the Strategic Advisory Group of Experts (SAGE) on immunization advised the WHO to recommend Typbar-TCV® for routine use in children over six months of age in typhoid endemic countries and also called for its introduction to be prioritized for countries with the highest burden of typhoid disease or of antibiotic resistance to Salmonella Typhi.6 Consequently, the Typhoid Vaccine Acceleration Consortium has introduced Typbar-TCV® to emergent nations including Bangladesh, Burkina Faso, Malawi and Nepal. In 2019 Pakistan and in 2021 Liberia, Samoa and Zimbabwe have introduced Typbar-TCV® into routine childhood immunization programs.9
The introduction of TCV into the already clustered routine EPI Immunization schedule visits will require concomitant administration with other vaccines. The most appropriate health visit for TCV administration, before most natural infections occur (see Figure S1) is at 9 months of age when measles vaccine (MV) is routinely administered so potential immunological interference is a concern.10 Further, in different countries it is likely that during introductory or catch-up campaigns TCV may be administered concomitantly with measles-mumps rubella vaccine (MMR) at an older age. Therefore we performed the current study to assess the safety and immunogenicity of co-administrating Typbar-TCV® with measles vaccine (MV) or MMR in Indian children aged 9 to 15 months.
Methods
Study design
This was a phase IV, randomized, factorial assigned, open-label study, performed in healthy children at 8 or 9 months of age at six sites in India: KLE-Belgaum, Khalatkar Hospital and Mogre Children’s Hospital in Nagpur, Cheluvamba Hospital, Mysore, King George Hospital, Vizag and Institute of Child Health in Kolkata. The trial was approved by the Central Drugs Standard Control Organization (CDSCO) and the respective ethical committee of each site (see Table S1) and was conducted in compliance with local (Drugs and Cosmetics Act, 2005, Indian Council of Medical Research, 2006) and international Good Clinical Practices and ethical guidelines for biomedical research on human participants.
Participants
Healthy 8- or 9-month-old children were recruited at the health care facilities of the clinical trial sites during routine immunization visits and screened for the eligibility criteria. Informed consent as signature or a thumbprint on the consent form was obtained from parents and caregivers, who were given general information orally in the local language. Both audio and video recordings of the entire consent process were made for documentation purposes.
Children were eligible for enrollment if they were healthy, ≥8 – ≤9 months of age inclusive, were available for the next 2 years for follow-up and had not previously received any typhoid or measles vaccine. Children were excluded if they had any of the following: presence of any illness requiring hospital referral on the day of vaccine administration, known immunodeficiency, receipt of blood products in the last 6 months, known case of HIV or other major immunological abnormalities, received systemic immunosuppressant or systemic corticosteroids or had any household contact on immunosuppressant, known allergy to any component of the vaccine or any other condition identified by the investigators to interfere with the evaluation of the vaccine or to represent health risk concerning study participation.
At enrollment all children were randomly assigned into three groups (Groups 1, 2 and 4) in a 2:1:1 ratio using a computer-generated randomization code, and were assigned a unique treatment allocation code generated by a third-party agency (Sensaas India Solutions Pvt. Ltd) with a randomization sheet provided to the investigators for the administration of vaccines. Randomization included further stratification of participants in Group 1 were into two sub-groups (Groups 1 A & B). Participants in Group 3 were enrolled at 8 months of age and were not included in the randomization. They received their first TCV vaccination at 8 months to allow the measles vaccine to be administered alone according to the Indian immunization schedule at 9 months and so act as controls for concomitant vaccination.
Vaccines
Each 0.5 mL dose of Typbar-TCV® (Bharat Biotech, Hyderabad, India) vaccine containing 25 µg of S. Typhi Vi capsular polysaccharide conjugated to tetanus toxoid is administered by intramuscular injection. The commercially available freeze-dried measles vaccine (M-Vac™, Serum Institute of India, Pune) I.P. contained live attenuated Measles virus (Edmonston Zagreb Strain). The freeze-dried measles, mumps and rubella vaccine (TRESIVAC®: Serum Institute of India, Pune) I.P. is prepared from live attenuated strains of Edmonston-Zagreb Measles virus, L-Zagreb Mumps virus and Wistar RA 27/3 Rubella virus. Before use the MV or MMR vaccines were reconstituted in the supplied diluent and each 0.5 mL dose was administered by deep subcutaneous injection.
Procedures
In Group 1, children aged 9 months received TCV and MV concomitantly on Day 0. To study the effect of a booster dose of TCV, this group was further stratified into two subgroups, Groups 1A and 1B to receive a booster dose of TCV 28 (±2) and 180 (±7) days after the first dose, respectively. In Group 2, children aged 9 months received MV on Day 0 and TCV on Day 28; in Group 3 children received TCV on Day 0 when they were 8 months of age so they could receive MV on schedule at 9 months of age. In Group 4 children received MV vaccine at 9 months of age on Day 0 and no TCV. All children in all four groups received MMR at 15 months of age.
Participants were observed on-site for immediate post-vaccination reactions for 30 minutes. Study visits took place on Days 0 (baseline), 28 (±2), 56 (±7), 180 (±15), 360 (±30) and 720 (±30) when 2-mL blood samples were collected for immunogenicity testing. An additional blood sample was collected on Day 84 (±7) from Groups 2 and 3, and from Group 1B on Day 210 to evaluate the boosting effect.
Safety and reactogenicity were assessed as solicited adverse events (AEs) recorded by parents or caregivers for the 7 days after each vaccination using paper-based diary cards. Diaries were collected at the subsequent study visit (coinciding with the blood draws). Study team members contacted the parents or caregivers by telephone to ensure they completed the diaries punctually. Any unsolicited adverse events or serious AEs (SAE) occurring throughout the study were to be reported to the study team immediately for documentation by a study physician, and parents or caregivers were asked at each study visit to ensure no events had not been reported. An SAE was defined as any adverse event that was life-threatening or life-changing event, resulted in hospitalization or death.
Immunogenicity
Antibody responses against Typhoid Vi, measles, mumps and rubella were measured using validated commercially available Enzyme-Linked Immunosorbent Assay (ELISA) kits (see Supplementary Methods for details) by laboratory teams blinded to group and vaccine allocation. Seroconversion was defined as a four-fold rise in post-vaccination titer compared with the pre-vaccination titer, and seroconversion rates are the group percentages achieving seroconversion.
Outcomes
Primary study outcomes included noninterference of immune responses against measles, mumps, and rubella when MV and MMR were co-administered with Typbar-TCV® at 9 and 15 months of age. Secondary outcomes included the effect of a booster dose of TCV in Groups 1A and 1B on Days 28 and 180, respectively, compared with the standard one-dose regimens in Groups 2 and 3. Other secondary outcomes included the proportions of children who experienced solicited AEs, unsolicited AEs and SAEs.
Statistical analysis
Sample size was calculated based on the primary objective of noninterference with measles vaccine using PASS software. Sample size assumptions included an alpha of 0.05 (one-sided), power of 80% allowing for 20% loss due to dropouts or failure to collect sufficient serum. At 9 months comparing a single antigen using an 89.6% seroconversion rate for measles, the sample size needed was 98 per group. To study interference with measles at 9 months, the sample size needed was 82 in each group with and without concomitant TCV administration. The power to detect a difference in seroconversion for TCV immune responses was 100%. A total of 100 children per group was considered adequate to generate exploratory and descriptive data on TCV, so 500 children were recruited into four groups with a 2:1:1:1 ratio to allow 200 children recruited in Group 1 to be divided into two subgroups with 100 children each to study the booster dose effect. The chi-square test with Yates’ correction was used to compare the proportions of participants who seroconverted in the different groups. Differences in rates of adverse reactions between groups were analyzed by Chi-square or Fisher exact test. A statistical value of p < .05 denotes there is statistically significant difference between the proportions of subjects seroconverting, or having any particular adverse event between groups.
Results
The date of first enrollment was 3 April 2014 and the last participant completed on 26 September 2015. Of 500 children screened 493 (255 male and 238 female) were enrolled and vaccinated, consent was not given for the other 7 children. In total 434 (87%) children completed the study up to day 720 with completion in the different groups is illustrated in Figure 1. Most cases of loss to follow-up were due to blood draw failures and many such participants were re-included at later stages. Baseline demographic characteristics were comparable with no differences between study groups (Table 1).
Figure 1.
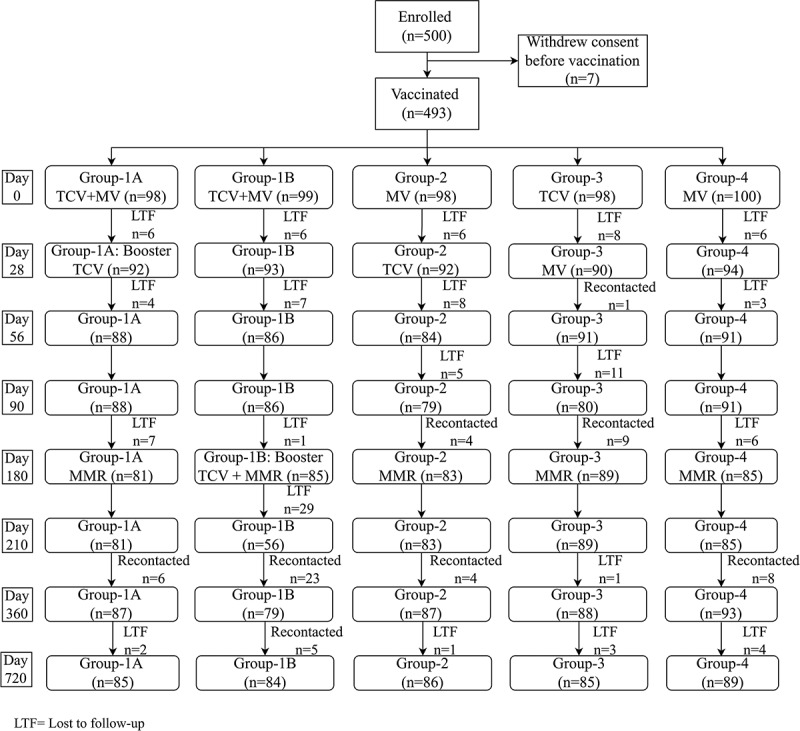
Study flow chart of the vaccination and follow-up of the study population.
Table 1.
Demography and other baseline characteristics of all enrolled participants.
| Parameter | Statistic | Group 1A (N = 98) |
Group 1B (N = 99) |
Group 2 (N = 98) |
Group 3 (N = 98) |
Group 4 (N = 100) |
|---|---|---|---|---|---|---|
| Gender, n (%) | Male | 46 (47%) | 50 (51%) | 51 (52%) | 54 (55%) | 52 (52%) |
| Female | 51 (52%) | 49 (49%) | 47 (48%) | 44 (45%) | 48 (48%) | |
| Age (months) | n | 97 | 99 | 98 | 98 | 100 |
| Mean (SD) | 8.77 (0.46) | 8.72 (0.51) | 8.74 (0.53) | 8.11 (0.39) | 8.73 (0.53) | |
| Median | 9.0 | 9.0 | 9.0 | 8.1 | 9.0 | |
| Range | 8.00–9.29 | 8.00–10.08 | 7.24*–10.13 | 7.07*–9.08 | 8.00–10.26 | |
| Weight (kg) | n | 97 | 99 | 98 | 98 | 100 |
| Mean (SD) | 7.70 (1.03) | 7.79 (0.87) | 7.71 (0.88) | 7.50 (0.85) | 7.79 (0.85) | |
| Median | 7.8 | 8.0 | 7.9 | 7.6 | 7.8 | |
| Range | 4.50–10.20 | 5.50–10.00 | 5.40–9.50 | 5.40–9.91 | 5.50–10.20 | |
| Height (cm) | n | 97 | 99 | 98 | 98 | 100 |
| Mean (SD) | 69.26 (3.99) | 70.01 (4.14) | 69.72 (4.25) | 68.95 (4.55) | 70.13 (4.53) | |
| Median | 70 | 70 | 70 | 69 | 70 | |
| Range | 59–76 | 62–80 | 60–80 | 55–82 | 61–88 |
Safety
The safety profiles for each of the vaccination regimens were comparable and clinically acceptable. Vaccinations were well-tolerated with no immediate reactions observed within the first 30 minutes. Solicited local adverse events were relatively infrequent, occurring in 3–11% of children in each TCV group, and each event occurring in fewer than 3% of any group (Table 2). In contrast, systemic AEs were recorded for 50–59% of each group. The most frequently observed solicited systemic AE was fever, reported in 20–26% of the TCV groups and 15% of the MV only group (Group 4). There were no statistical differences in incidence rates of solicited AEs among all the groups.
Table 2.
Solicited local and systemic adverse events (AEs) reported in the 7 days after vaccine administration.
| Group 1A | Group 1B | Group 2 | Group 3 | Group 4 | |
|---|---|---|---|---|---|
| Vaccines received | TCV + Measles | TCV + Measles | TCV & Measles | TCV & Measles) | Measles |
| AE Term | (N = 98) | (N = 99) | (N = 98) | (N = 98) | (N = 100) |
| Local AE | |||||
| Any | 10 (10) | 7 (7) | 3 (3) | 3 (3) | 0 |
| Pain | 1 (1) | 3 (3) | 2 (2) | 1 (1) | 0 |
| Redness | 3 (3) | 0 | 1 (1) | 1 (1) | 0 |
| Swelling | 2 (2) | 1 (1) | 0 (0) | 1 (1) | 0 |
| Induration | 2 (2) | 1 (1) | 0 | 0 | 0 |
| Itching | 0 | 1 (1) | 0 | 0 | 0 |
| Tenderness | 2 (2) | 1 (1) | 0 | 0 | 0 |
| Systemic AE | |||||
| Any | 49 (50) | 56 (57) | 53 (54) | 58 (59) | 53 (53) |
| Fever | 20 (20) | 25(25) | 22 (22) | 25 (26) | 15 (15) |
| Headache | 0 | 0 | 0 | 0 | 1 (1) |
| Nausea | 0 | 1 (1) | 1 (1) | 0 | 0 |
| Vomiting | 1 (1) | 2 (2) | 0 | 1 (1) | 2 (2) |
| Other | |||||
| Anaemia | 0 | 0 | 0 | 0 | 2 (2) |
| Cold | 12 (12) | 12 (12) | 13 (13) | 13 (13) | 21 (21) |
| Constipation | 1 (1) | 0 | 3 (3) | 1 (1) | 3 (3) |
| Cough | 7 (7) | 7 (7) | 8 (8) | 11 (11) | 1 (1) |
| Crying | 2 (2) | 1 (1) | 1 (1) | 1 (1) | 2 (2) |
| Dehydration | 1 (1) | 0 | 0 | 0 | 0 |
| Diarrhoea | 4 (4) | 5 (5) | 2 (2) | 5 (5) | 5 (5) |
| Ecchymosis | 0 | 0 | 1 (1) | 0 | 0 |
| Itching | 1 (1) | 0 | 0 | 0 | 0 |
| Rash over the body | 0 | 3 (3) | 0 | 1 (1) | 1 (1) |
| Redness | 0 | 0 | 1 (1) | 0 | 0 |
| Running Nose | 0 | 0 | 1 (1) | 0 | 0 |
Unsolicited AEs occurred in 8 children in Group 1, 4 children in Group 2, and 7 children in Groups 3 and 4. The most common unsolicited AEs were cough in Groups 3 (4 cases) and 4 (2 cases) and diarrhea in Group 1A (2 cases), Group 1B (2 cases), and one case in Group 2.
There were no deaths, but 8 SAEs were reported (3 each in Group 1 and 3, and 1 each in Group 2 and 4). All SAEs were graded as moderate and resolved without any sequelae and were considered to be unrelated to the vaccine following assessment by the site investigators (see Table S2 for details).
Immunogenicity
The control references for anti-Vi antibodies are the seroconversion rate (SCR) and GMT in Group 3 when Typbar-TCV® was administered alone without concomitant measles vaccine. On Day 28 post-vaccination the SCR was 93%, 93% and 88.9% in Groups 1A, 1B, and 3, respectively. There was only a small further increase by Day 56 to 96.6% in Group 1A after a second TCV dose on Day 28, which was still similar to rates of 97.7% and 91.2%, in Groups 1B and 3, respectively. A similar increase to 89.3% was observed in Group 2 at Day 56 following TCV vaccination on Day 28. Geometric mean titers (GMTs) of antibodies in each of the groups were comparable (Figure 2). There was a trend for SCR and GMTs to decline in Group 2 and Group 3 by Day 90 and this was more evident by Day 180 in all four groups. A small increase in GMT observed in Group 1B at Day 210 following a booster dose on Day 180, did not achieve the same level seen after the first dose, but did increase the SCR to 100%. Titers then declined further at Days 360 and 720 but levels remained above baseline (see Table S3). The SCR remained at 77.1–81.0% at Day 360 across groups with a further decline to 31.8–40.5% at Day 720 with no apparent interference by concomitant administration of MV or MMR (Table 3).
Figure 2.
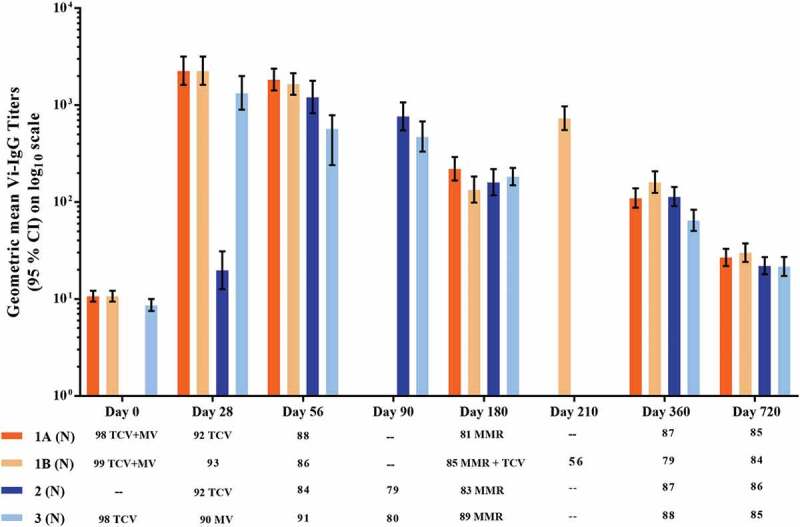
Geometric mean anti-Vi IgG antibody titers measured by ELISA (Group 1: Typbar-TCV® and MV co-administered at 9 months; Group 2: MV administered at 9 months and Typbar-TCV® at 10 months; Group 3: Typbar-TCV® administered at 8 months and MV at 9 months; and Group 4: only MV administered at 9 months. To assess TCV booster doses Group 1 was stratified into Group 1A, given a TCV booster at 10 months of age (28 ± 2 days after the first dose) and Group 1B, given a TCV booster at 15 months of age (180 ± 15 days post the first dose).
Table 3.
Seroconversion rates for the anti-Vi IgG ELISA responses.
| Seroconversion rate, % (95% CI) |
||||
|---|---|---|---|---|
| Day | Group 1A | Group 1B | Group 2 | Group 3 |
| 28 | 93.0% (87.9, 98.1) |
93.0% (87.9, 98.1) |
– | 88 9% (80.5, 94.5) |
| 56 | 96.6% (90.4, 99.3) |
97.7% (91.9, 99.7) |
89.3% (80.6, 95.0) |
91.2% (83.4, 96.1) |
| 90 | – | – | 94.1% (86.7, 98.0) |
88.8% (79.7, 94.7) |
| 180 | 87.7% (78.5, 93.9) |
85.9% (76.6, 92.5) |
84.3% (76.1, 92.3) |
94.4% (97.4, 98.2) |
| 210 | – | 100% (93.6, 100) |
– | – |
| 360 | 77.1% (66.8, 85.4) |
81.0% (70.6, 89.0) |
97.9% (91.9, 99.7) |
77.2% (67.1, 85.5) |
| 720 | 38.8% (28.4, 50.0) |
40.5% (29.9, 51.8) |
33.7% (23.9, 44.7) |
31.8% (22.1, 42.8) |
Group 1A: TCV and measles co-administered at day 0 (9 months of age) and booster TCV vaccine at day 28.
Group 1B: TCV and measles co-administered at day 0 (9 months of age) and booster TCV vaccine at day 180.
Group 2: Measles administered at day 0 (9 months of age) and TCV at day 28.
Group 3: TCV at day −28 (8 months of age) and measles administered at day 0 (9 months of age).
The control references for the anti-measles response are the GMT and the SCR for anti-measles IgG at Day 28 in Groups 2 and 4, i.e., following measles vaccine administration with no concomitant TCV. At Day 28 the SCR for anti-measles IgG in Groups 2 and 4 were not significantly different from Groups 1A and 1B, in which MV was administered concomitantly with Typbar-TCV®. Post-vaccination the SCR for measles was increased in Groups 1A, 1B, 2 and 4 on Days 28, 56, 180, 360, and 720, respectively. The anti-measles IgG GMT and SCR in all groups were similar at all time points and persisted at high levels to Day 180 when MMR vaccination was performed, and the GMT then persisted at high levels in all groups through Days 360 and 720 (Table 4 and Figure 3).
Table 4.
Seroconversion rates of MMR vaccine antigens as measured by anti-measles, mumps and rubella IgG ELISA responses.
| Seroconversion rate, % (95% CI) |
|||||
|---|---|---|---|---|---|
| Day | Group 1A | Group 1B | Group 2 | Group 3 | Group 4 |
| Measles | |||||
| 28 | 82.7% (75.0, 90.4) |
82.7% (75.0, 90.4) |
78.3% (68.4, 86.2) |
– | 87.2% (78.86, 93.2) |
| 56 | 83.0% (73. 5, 90.1) |
83.7% (74.2, 90.8) |
84.5% (75.0, 91.5) |
79.1% (69.3, 86.9) |
78.0% (68.1, 86.0) |
| 90 | – | – | 79.8% (69.2, 88.0) |
76.3% (65.4, 85.1) |
– |
| 180 | 82.7% (72.7, 90.2) |
87.1% (78.0, 93.4) |
83.1% (73.3, 90.5) |
85.39% (76.3, 92.0) |
82.4% (72.6, 90.0) |
| 210 | – | 98.2% (90. 5, 100) |
– | – | – |
| 360 | 93.1% (82.6, 97.4) |
91.1% (82.6, 96.4) |
97.7% (92.0, 99.7) |
95.5% (88.8, 98.8) |
90.3% (82.4, 95.5) |
| 720 | 90.5% (82.1, 95.8) |
89.4% (80.9, 95.0) |
97.7% (91.8, 99.7) |
85.9% (76.6, 92.5) |
80.9% (71.4, 88.2) |
| Mumps | |||||
| 210 | – | 36.8% (24.4, 50.7) |
– | – | – |
| 360 | 65.4% (54.0, 75.7) |
63.8% (51.3, 75.0) |
59.8% (48.3, 70.4) |
65.5% (54.3, 75.5) |
52.9% (41.9, 63.7) |
| 720 | 62.2% (50.8, 72.7) |
61.7% (50.3, 72.3) |
64.3% (53.1, 74.5) |
63.4% (51.1, 74.5) |
59.3% (48.2, 69.9) |
| Rubella | |||||
| 210 | – | 24.6% (14.1, 37.8) |
– | – | – |
| 360 | 64.2% (52.8, 74.6) |
71.4% (59.4, 81.6) |
67.1% (55.8, 77.1) |
76.2% (65.7, 84.8) |
60.9% (49.9, 71.2) |
| 720 | 73.2% (62.2, 82.4) |
65.4% (54.0, 75.7) |
69.1% (58.0, 78.7) |
76.1% (64.5, 85.4) |
68.6% (57.7, 78.2) |
Figure 3.
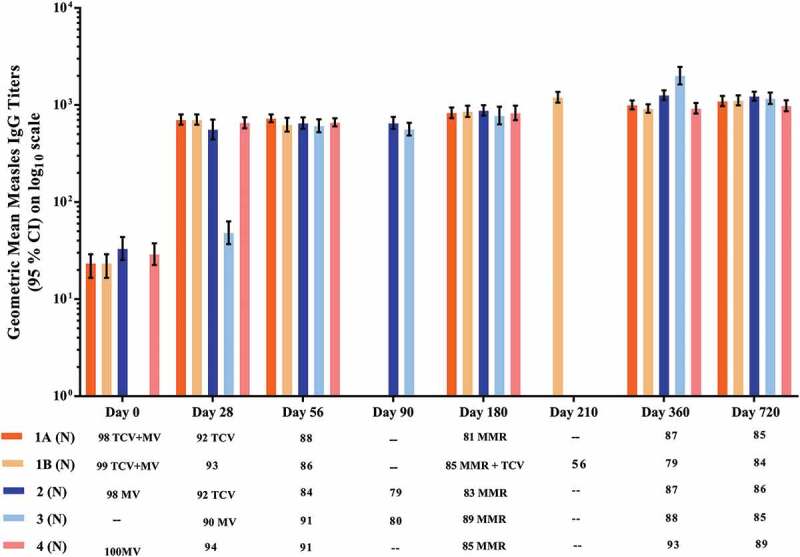
Geometric mean anti-measles IgG antibody titers measured by ELISA. All groups received MV at 9 months of age: concomitantly with Typbar-TCV® in Group 1; separately from Typbar-TCV® which was given at 10 months in Group 2; separately from Typbar-TCV® which was given at 8 months in Group 3; and with no Typbar-TCV® dose in Group 4. All groups received MMR at 15 months, which was administered concomitantly with Typbar-TCV® in Group 1B.
Similar patterns of response to the mumps and rubella components of the MMR vaccine were observed in all five study groups expressed as SCR (Table 4) or IgG antibody GMTs (Figure 4), including in the children in Group 1B who received Typbar-TCV® concomitantly with MMR.
Figure 4.
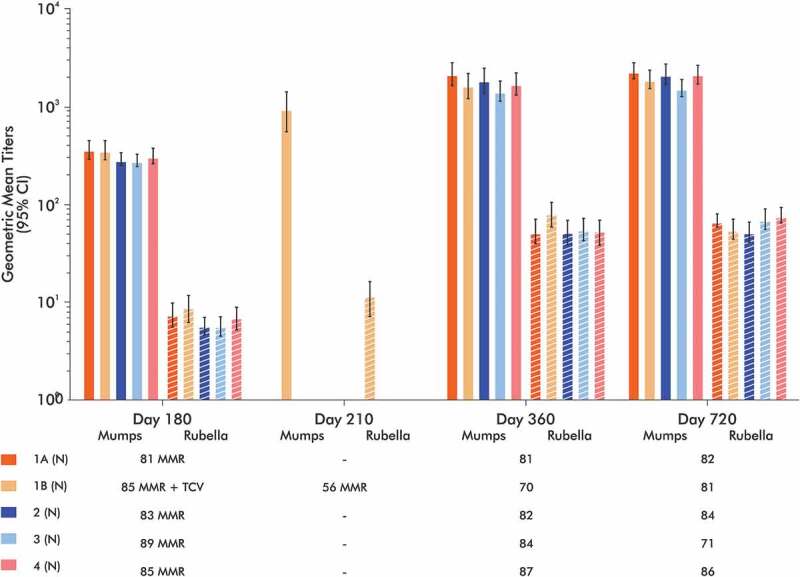
Geometric mean anti-mumps and anti-rubella IgG titers measured by ELISA. All groups received MV vaccination at 9 months of age and then MMR vaccination at 15 months, which was administered concomitantly with Typbar-TCV® in Group 1B.
Discussion
The current study was performed as a pre-requisite for WHO pre-qualification, with concomitant vaccination of Typbar-TCV® with measles or MMR vaccines to show that Typbar-TCV® is safe and there is no interference with the immune response. Reactogenicity and tolerance of the vaccinations, and the magnitude of the immune responses to typhoid, measles, mumps, and rubella were not impacted by concomitant administration of TCV in age-eligible children. Immune responses against all vaccine antigens were comparable across groups throughout the study duration. The IgG antibody responses against Vi and measles persisted until day 720, two years after the primary vaccination series. Immune responses to either Vi or measles antigens were non-inferior in terms of IgG GMT or seroconversion rate when Typbar-TCV® and MV were administered concomitantly at 9 months of age compared with separate administration of the vaccines alone.
The efficacy of TCV vaccination against S. Typhi has been demonstrated in several studies. In a population-based immunization campaign in Pakistan in 2018 involving 23,407 children from 6 months to 10 years of age living in high-risk areas TCV demonstrated 95% effectiveness against culture-confirmed S. Typhi and 97% effectiveness against extremely drug resistant (XDR) S. Typhi.11 In Malawi effectiveness in vaccinees from 9 months to 12 years of age was 80.7%,12,13 and an efficacy of 81.6% was reported among Nepalese children aged 9 months to 16 years.14,15 Subsequently, a two cohort study conducted in Burkina Faso showed Typbar-TCV® can be safely co-administered with measles-rubella (MR) and yellow fever (YF) vaccines at 9 months or with meningococcal A vaccine at 15 months with no immune interference, supporting large-scale uptake in sub-Saharan Africa.16,17 In addition, a single-dose of TCV significantly decreased the incidence of typhoid in Zimbabwe after a mass vaccination campaign in response to an outbreak conducted in 2019 among children aged 6 months to 15 years, with an effectiveness of 84%.18,19 Total vaccine protection was evidenced as 85% in children aged 9 months to 16 years of Bangladesh.20 Our study provides further corroborative data showing Typbar-TCV® can be safely co-administered with measles or MMR vaccines without any interference.
In 2018, the Global Advisory Committee on Vaccine Safety (GACVS) concluded that as the safety profile of Typbar-TCV® vaccine is reassuring as no signals of serious adverse events had presented. The GACVS recommended that countries introducing TCV into their routine immunization schedule or campaigns make every effort to ensure robust monitoring of safety.21 Also in 2018, based on the data obtained in the present study the WHO recommended that Typbar-TCV® can be co-administered with MV or MMR vaccine.22
This study demonstrated that a single dose of Typbar-TCV® at 9 months increased anti-Vi IgG antibodies titers and the seroconversion rate 28 days after vaccination, but a second dose at days 28 or 180 did not produce any significant further increase in titer. This may be due the gradual maturation of the immune system during infancy.23 The immature immune system in infants lacks certain key immunological features necessary to provide long-lasting immunity and the magnitude of infant antibody response to dosing schedules reflects the interval between two doses, i.e, a longer interval elicits a higher response.24 From our observation of the lack of response up to 180 days after the primary dose it seems likely that a booster schedule with a longer interval may be required. It may be appropriate to administer a booster dose approximately 5 years after the primary vaccination for better longevity of protection as suggested in our previous publications.8,25 This would allow the booster to be co-administered with the routine DTP/DP/Td booster dose given at school-going age in India, but this requires further investigation.
The current study has some limitations. Firstly, as it is open-label certain biases may have been introduced. Although all immunogenicity assessments were performed in a blinded manner the safety comparisons must be interpreted cautiously as the study was not powered to evaluate comparisons, but it was notable that parentally reported events were consistent across all groups. We could not measure the lack of interference with mumps and rubella antigens at 9 months of age as MMR vaccine was not licensed for use at this age in India when the study was performed. Further, there were differences in GMTs and SCR in this study from those in our previously conducted phase 3 study which is attributed the differences in the median age of the children compared with adults and infants.8,25
Conclusions
This study has shown that concomitant use of Typbar-TCV® with measles or measles-mumps-rubella vaccines does not affect the immune response to any vaccine component in Indian children. Further, we found that administering a second dose of Typbar-TCV less than 180 days after the first dose is ineffective in boosting antibody titers.
Supplementary Material
Acknowledgments
The authors are grateful to all the principal investigators and their staff who oversaw the conduct of the study at their respective sites and to all the children and their parents/guardians who participated in this study. The authors thank Mr. Siddharth Reddy, Ms. Sandhya Nandala, and Ms. Aparna Bathula for conduct of the trial, Dr. Raches Ella, Dr. Vinay Kumar Aileni, and Ms K. Akhila for the preparation of the draft manuscript. We specifically thank Dr. Keith Veitch for critical review of the manuscript.
Funding Statement
This study was funded by Bharat Biotech International Ltd.
Abbreviations
- AE
Adverse Events
- MV
Measles Vaccine
- MMR
Measles Mumps Rubella Vaccine
- SCR
Seroconversion rate
- SAE
Serious Adverse Event
Author contributions
KM contributed to the conduct of the trial and study’s data analysis, RD carried out all the serological assays (while being blinded), NSM, VK, KM, SM, and MM enrolled the participants. All authors reviewed and approved the different drafts of the manuscript and agreed to the submission of the final version for publication.
Disclosure statement
KMV and RD are full-time employees of the study sponsor. No potential conflict of interest was reported by the other authors.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2022.2150030
References
- 1.Mukhopadhyay B, Sur D, Gupta SS, Ganguly NK.. Typhoid fever: control & challenges in India. Indian J Med Res. 2019;150(5):437–9. doi: 10.4103/ijmr.IJMR_411_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GBD 2017 Typhoid and Paratyphoid Collaborators . The global burden of typhoid and paratyphoid fevers: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Infect Dis. 2019;19(4):369–81. doi: 10.1016/S1473-3099(18)30685-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Institute for Health Metrics and Evaluation . Typhoid fever – level 4 cause. [accessed 2022 Oct 30]. https://www.healthdata.org/results/gbd_summaries/2019/typhoid-fever-level-4-cause.
- 4.Crump JA. Progress in typhoid fever epidemiology. Clin Infect Dis. 2019;68(Suppl 1):S4–9. doi: 10.1093/cid/ciy846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention (CDC) . National typhoid and paratyphoid fever surveillance annual summary, 2015. Atlanta (GA): US Department of Health and Human Services, CDC; 2018. [accessed 2022 Oct 30]. https://www.cdc.gov/typhoid-fever/reports/annual-report-2015.html. [Google Scholar]
- 6.WHO . Typhoid vaccines: WHO position paper – March 2018. Weekly Epidemiological Record. 2018;93:153–72. [Google Scholar]
- 7.WHO . Prequalification of medical products (IVDs, medicines, vaccines and immunization devices, vector control) – Typbar-TCV. [accessed 2022 Oct 30]. https://extranet.who.int/pqweb/content/typbar-tcv.
- 8.Vadrevu Krishna Mohan, Varanasi V, Singh A, Pasetti MF, Levine MM, Venkatesan R, Ella KM . Safety and immunogenicity of a Vi polysaccharide-tetanus toxoid conjugate vaccine (Typbar-TCV) in healthy infants, children, and adults in typhoid endemic areas: a multicenter, 2-cohort, open-label, double-blind, randomized controlled phase 3 study. Clin. Infect. Dis. 2015;61(3):393–402. doi: 10.1093/cid/civ295. [DOI] [PubMed] [Google Scholar]
- 9.TyVAC . Take on typhoid – typhoid vaccines. [accessed 2022 Oct 30]. https://www.coalitionagainsttyphoid.org/the-issues/typhoid-vaccines/.
- 10.Singh K, Mehta S. The clinical development process for a novel preventive vaccine: an overview. J Postgrad Med. 2016;62(1):4–11. doi: 10.4103/0022-3859.173187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yousafzai MT, Karim S, Qureshi S, Kazi M, Memon H, Junejo A, Khawaja Z, Rehman NU, Ansari MS, Ali R, et al. Effectiveness of typhoid conjugate vaccine against culture-confirmed Salmonella enterica serotype Typhi in an extensively drug-resistant outbreak setting of Hyderabad, Pakistan: a cohort study. Lancet Glob Health. 2021;9(8):e1154–62. doi: 10.1016/S2214-109X(21)00255-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel PD, Patel P, Liang Y, Meiring JE, Misiri T, Mwakiseghile F, Tracy JK, Masesa C, Msuku H, Banda D, et al. Safety and efficacy of a typhoid conjugate vaccine in Malawian children. N Engl J Med. 2021;385(12):1104–15. doi: 10.1056/NEJMoa2035916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nampota-Nkomba N, Nyirenda OM, Khonde L, Mapemba V, Mbewe M, Ndaferankhande JM, Msuku H, Masesa C, Misiri T, Mwakiseghile F, et al. Safety and immunogenicity of a typhoid conjugate vaccine among children aged 9 months to 12 years in Malawi: a nested substudy of a double-blind, randomised controlled trial. Lancet Glob Health. 2022;10(9):e1326–35. doi: 10.1016/S2214-109X(22)00275-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shakya M, Colin-Jones R, Theiss-Nyland K, Voysey M, Pant D, Smith N, Liu X, Tonks S, Mazur O, Farooq YG, et al. Phase 3 efficacy analysis of a typhoid conjugate vaccine trial in Nepal. N Engl J Med. 2019;381(23):2209–18. doi: 10.1056/NEJMoa1905047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shakya M, Voysey M, Theiss-Nyland K, Colin-Jones R, Pant D, Adhikari A, Tonks S, Mujadidi YF, O’Reilly P, Mazur O, et al. Efficacy of typhoid conjugate vaccine in Nepal: final results of a phase 3, randomised, controlled trial. Lancet Glob Health. 2021;9(11):e1561–68. doi: 10.1016/S2214-109X(21)00346-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sirima SB, Ouedraogo A, Barry N, Siribie M, Tiono AB, Nébié I, Konaté AT, Berges GD, Diarra A, Ouedraogo M, et al. Safety and immunogenicity of Vi-typhoid conjugate vaccine co-administration with routine 9-month vaccination in Burkina Faso: a randomized controlled phase 2 trial. Int J Infect Dis. 2021;108:465–72. doi: 10.1016/j.ijid.2021.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sirima SB, Ouedraogo A, Barry N, Siribie M, Tiono AB, Nébié I, Konaté AT, Berges GD, Diarra A, Ouedraogo M, et al. Safety and immunogenicity of co-administration of meningococcal type a and measles–rubella vaccines with typhoid conjugate vaccine in children aged 15–23 months in Burkina Faso. Int J Infect Dis. 2021;102:517–23. doi: 10.1016/j.ijid.2020.10.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olaru ID, Mtapuri-Zinyowera S, Feasey N, Ferrand RA, Kranzer K. Typhoid Vi-conjugate vaccine for outbreak control in Zimbabwe. Lancet Infect Dis. 2019;19(9):930. doi: 10.1016/S1473-3099(19)30425-6. [DOI] [PubMed] [Google Scholar]
- 19.Lightowler MS, Manangazira P, Nackers F, Van Herp M, Phiri I, Kuwenyi K, Panunzi I, Garone D, Marume F, Tarupiwa A, et al. Effectiveness of typhoid conjugate vaccine in Zimbabwe used in response to an outbreak among children and young adults: a matched case control study. Vaccine. 2022;40(31):4199–210. doi: 10.1016/j.vaccine.2022.04.093. [DOI] [PubMed] [Google Scholar]
- 20.Qadri F, Khanam F, Liu X, Theiss-Nyland K, Biswas PK, Bhuiyan AI, Ahmmed F, Colin-Jones R, Smith N, Tonks S, et al. Protection by vaccination of children against typhoid fever with a Vi-tetanus toxoid conjugate vaccine in urban Bangladesh: a cluster-randomised trial. Lancet. 2021;398(10301):675–84. doi: 10.1016/S0140-6736(21)01124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.WHO . Safety of typhoid conjugate vaccine. [accessed 2022 Oct 30]. https://www.who.int/groups/global-advisory-committee-on-vaccine-safety/topics/typhoid-vaccines.
- 22.WHO . Guidance on co-administration of typhoid vaccine with measles-containing vaccines.[accessed 2022 Oct 30]. https://cdn.who.int/media/docs/default-source/immunization/multiple-injections/coadministration_of_tcv_with_mcv_15aug2018.pdf?sfvrsn=ff9f92df_7.
- 23.WHO – SAGE . Background paper to SAGE on typhoid vaccine policy recommendations. [accessed 2022 Oct 30]. https://cdn.who.int/media/docs/default-source/immunization/position_paper_documents/typhoid/1-typhoid-sage-background-paper-final-v3b.pdf?sfvrsn=ddf418c3_2.
- 24.Yu JC, Khodadadi H, Malik A, Davidson B, Salles ÉDSL, Bhatia J, Hale VL, Baban B. Innate immunity of neonates and infants. Front Immunol. 2018;9:1759. doi: 10.3389/fimmu.2018.01759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vadrevu KM, Raju D, Rani S, Reddy S, Sarangi V, Ella R, Javvaji B, Mahantshetty, NS, Battu, S, Levine, MM. Persisting antibody responses to Vi polysaccharide-tetanus toxoid conjugate (Typbar TCV®) vaccine up to 7 years following primary vaccination of children < 2 years of age with, or without, a booster vaccination. Vaccine. 2021;39(45):6682–6690. doi: 10.1016/j.vaccine.2021.07.073. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


