ABSTRACT
Cancer patients with autoimmune disease (AID) are usually excluded from clinical trials involving immune checkpoint inhibitors (ICIs). The available electronic databases were systematically searched from inception until July 3, 2022. We recorded the incidence of immune-related adverse events (irAEs), progression-free survival (PFS), and overall survival (OS) data of included studies. This meta-analysis included 14 studies comprising 11511 participants; however, only 8716 participants were treated with ICI. Therefore, the analysis was conducted on 8716 patients (769 patients with AID compared to 7947 patients without AID). The pooled risk ratio (RR) for any grade and grade ≥3 irAEs was 1.74 (95% confidence interval [CI]: 1.27-2.37) and 1.43 (95% CI: 1.10-1.88), respectively. The irAEs in the same system as that of the AID were referred to as AID-homogeneous irAEs; in the other cases, there were referred to as AID-heterogeneous irAEs. Subgroup analysis found that the higher risk of AID-homogeneous irAEs contributed to the higher risk of overall irAEs among patients with AID. The pooled hazard ratio (HR) for PFS and OS was 1.09 (95% CI: 0.96–1.24) and 1.07 (95% CI: 0.94-1.22), respectively. The results of PFS and OS subgroup analyses matched the overall results. Patients with AID had a significantly higher risk of developing any grade and ≥3 grade irAEs under ICI therapy, specifically AID-homogeneous irAEs; however, the frequency of AID-heterogeneous irAEs in patients with AID was similar to irAEs in patients without AID. No statistically significant differences in PFS and OS were observed between the two groups.
KEYWORDS: Cancer, autoimmune disease, immune checkpoint inhibitors, immune-related adverse events, progression-free survival, overall survival
1. Introduction
The therapeutic outlook for many malignancies has fundamentally changed owing to the advent of immune checkpoint inhibitors (ICIs),1–3 a radical transformation of the antitumor concept that directly kills tumor cells to regulate the immune microenvironment. ICIs aim to block negative co-stimulation of T lymphocytes, which can activate anergic T cells, thus restoring their antitumor effects.4 This differs from chemotherapy and targeted therapies. However, immune-related side events caused by ICIs have gradually attracted the attention of clinicians.
The immune system is finely tuned to distinguish between self and non-self, in order to maintain host integrity.5 Autoimmune disease (AID) occurs when the immune system over-activates autoantigens.6 Various autoimmune diseases can occur in any body system. The commonly accepted pathogenesis is the loss of tolerance to autoantigens, leading to an attack on the self-organs.7 Autoinflammatory diseases include a diverse group of diseases caused by the over-activation of the innate immune system, typically in the absence of autoantibodies and antigen specific T cells.8 Autoimmune diseases are mainly caused by the over-activation of adaptive immunity; however, many diseases have characteristics of both autoimmune diseases and autoinflammatory diseases.9 The pathogenesis of systemic lupus erythematous was initially associated with the dysfunction of adaptive immunity, but current evidence indicates the involvement of innate immune dysfunction, especially in impaired apoptotic cell clearance.10 Thus, autoimmune inflammatory disease can be regarded as a broad range of autoimmune diseases. It is unclear whether the immunological and molecular mechanisms of each autoimmune disease are related to the breakdown of tolerance to autoantigens.11 The generation of ectopic germinal cells and defect of Treg cells are possible mechanisms of autoimmune diseases.12 B-lymphocytes produce antibodies by somatic hypermutation and category transformation of immunoglobulin genes in germinal centers.12 The common clinical manifestations of autoimmune diseases are similar to the immunological side effects associated with ICIs.13 The possible mechanisms leading to immune-related adverse events (irAEs) include excitation of B cells and cytotoxic T cells, activation of intracellular signaling, and production of cytokines causing inflammation.14 The pathophysiological process of AIDs and irAEs are very complex, and AIDs are highly- heterogeneous entities. Fearing deterioration of preexisting AID, cancer patients with AID are often excluded from clinical trials involving ICIs.15,16
Individuals with AID are often at higher risk of tumorigenesis,17 which in turn increases the risk of AID.18 Owing to the abnormalities of the immune system, it is unclear whether patients with AIDs will benefit from ICIs. A previous study reported that ICIs were effective in patients with AIDs, with often manageable irAEs.19 However, this study did not compare progression-free survival (PFS) and overall survival (OS) between patients with and without AID. Based on this background, published data from several studies that treated patients with AID with ICIs were systematically collected. We aim to assess the rate of irAEs, PFS, and OS among patients on ICI therapy.
2. Methods
2.1. Literature search
Electronic databases, including PubMed, Web of Science, EMBASE, and Google Scholar from inception until 3 July 2022, were searched to identify eligible studies, without language restrictions. We used the following Medical Subject Headings (MeSH) for our search: cancer, melanoma, leukemia, lymphoma, multiple myeloma, sarcoma, malignant mesothelioma, immune-related adverse event, irAEs, autoimmune disease, autoimmune disorder, lupus, interstitial lung disease, autoimmune thyroiditis, inflammatory bowel disease, rheumatoid arthritis, CTLA-4, ipilimumab, PD-1, nivolumab, pembrolizumab, cemiplimab, PD-L1, atezolizumab, durvalumab, avelumab, PFS, and OS. In addition, the related bibliographies of the retrieved articles were also searched in case any articles were missing. The full search strategy is presented in Appendix S1.
2.2. Eligibility criteria
Studies were eligible if the following conditions were met: (1) patients had advanced cancer or other malignant diseases; (2) patients had preexisting AID; (3) patients were treated with ICIs, including anti-PD-1, anti-PD-L1, and CTLA-4 agents; and (4) studies reporting the incidence of irAEs, exact hazard ratio (HR) values, or survival curve for PFS and/or OS. Studies were excluded if (1) they only reported irAEs without PFS or OS; (2) they reported PFS and OS as survival rates in percentage terms; (3) they included patients diagnosed with AIDs after the treatment with ICIs; (4) they discussed autoimmune antibodies instead of AIDs; and (5) they were case reports.
2.3. Data extraction and quality assessment
Two authors (QC and GW H) reviewed and screened the content of all eligible studies using a predefined data extraction form. All the patients in each study were divided into AID (cancer patients with AID) and non-AID (cancer patients without AID) groups. We calculated the total number of patients in the AID and non-AID groups, number of patients developing any grade or grade ≥3 irAEs, and incidence of irAEs, which was reported as risk ratio (RR). The outcome measures, PFS, and OS, were assessed and consistently reported as HR. The following information was collected independently: the first author’s name, publication time, country, and patient characteristics, such as number of patients, proportion of sex, type of autoimmune disease, class of malignancy, and category of immunotherapy. The New Castle – Ottawa Scale was used to evaluate the quality of each study. Any disagreements were resolved by an experienced reviewer (FY Z) or through discussions.
2.4. Statistical analysis
R software (version 4.1.3) was used for data analysis. RR was used to reflect the incidence of irAEs of any grade and grades ≥3.20–27 If the studies recorded HR for PFS or OS, we adopted the results. For some studies20–22–24–26–28,29 that lacked HR for PFS or OS values, we downloaded the Kaplan – Meier curves, used the specialized software (Engauge Digitizer) to extract the curves’ data, and estimated HR using Jayne F Tierney’s spreadsheet.30 Calculations were independently repeated twice to obtain precise results.31 We reported the pooled RR with 95% CI for irAEs and the pooled HR with 95% CI for PFS and OS. Forest plots were used to represent the pooled results. Statistical heterogeneity was estimated using the I2 test. I2 <50% and >50% were considered as low and high heterogeneity, respectively. Statistical significance was set at p < .05.
3. Result
3.1. Literature search results
In total, 5,754 relevant articles were initially retrieved from database searches; 5,502 studies were excluded either because they were repetitive or did not meet the study’s requirements after the screening of titles and abstracts. In total, 252 studies were considered eligible for further assessment. Fourteen studies were included in the analysis. Details of the inclusion and exclusion process are presented as a flowchart (Figure 1).
Figure 1.
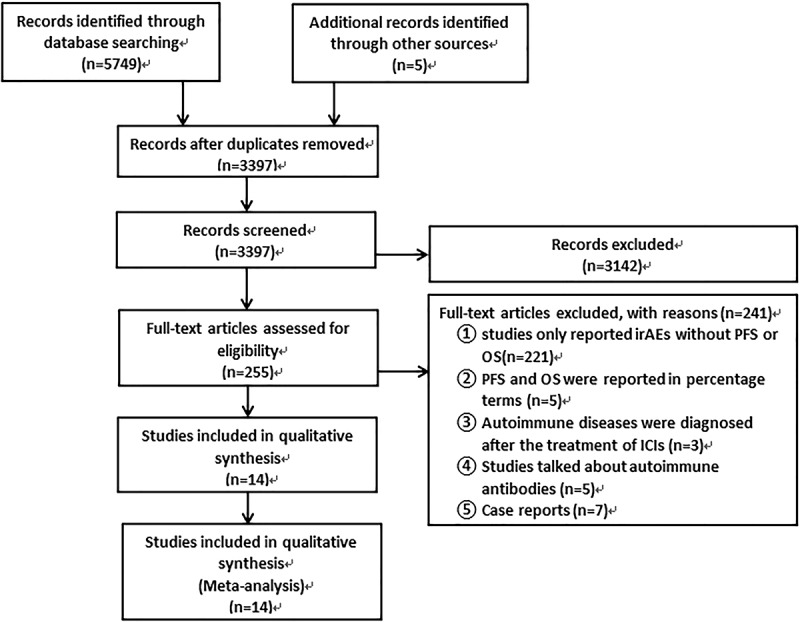
Selection process for the studies included in the meta-analysis.
3.2. Characteristics of the selected studies
Fourteen eligible citations were included. Eleven of the fourteen studies were retrospective, two were prospective, and one was a clinical trial. All articles were published between 2014 and 2022. A total of 11,511 participants were included; however, only 8716 participants were treated with ICIs. Therefore, the analysis was conducted on 8716 patients; a detailed description of the patients’ characteristics is contained in the meta-analysis presented in Table 1. Of these, 769 were AID patients and 7947 were non-AID patients. Detailed information about each category of AID is presented in Table S1. These studies mainly took place in Asia and Europe, with only two in the US. Among these, three studies were from Japan,20,21,24 one from Korea,28 two from Italy,22,32 one from France,25 two from Germany,26,33 one from the UK,29 one from Switzerland,23 one from Holland,27 and two from the US.34,35 Nine studies reported on non-small cell lung cancer (NSCLC),20–22–24,25-28–29-33–35 seven on melanoma,22–25–27–32–34,35 and three on metastatic renal cell cancer and urothelial carcinomas.22,23,35 Among AIDs, ILD and endocrine AID were most frequently mentioned. Specific information on irAEs, survival, and quality characteristics is presented in Tables 2–5, respectively.
Table 1.
Detailed description of the characteristics of included studies.
| Author | Year | Gender Male (%) Female (%) |
Age | Type of Autoimmune Disease | Type of Cancer | Immunotherapy Regimen |
|---|---|---|---|---|---|---|
| Bottoni et al.32 | 2014 | 48 (24.1%) 151 (75.9%) |
<60 56.3% ≥60 43.7% |
Autoimmune thyroiditis 55.1% Rheumatoid arthritis12.2% Others32.7% |
Melanoma | CTLA-4 |
| Danlos et al.25 | 2017 | 215 (54.2%) 182 (45.8%) |
62.3 (23–88) | Psoriasis Autoimmune thyroiditis Sjogren’s syndrome Rheumatoid arthritis Other |
Melanoma NSCLC Others |
anti-PD-1 (Pembrolizumab) (Nivolumab) (Avelumab) |
| Schadendorf et al.26 | 2019 | 557(55.3%) 451(44.7%) |
62(18–89) | Endocrine 56.0% Skin 28.0% Gastrointestinal 8.0% Hepatic 4% |
Melanoma | anti-PD-1 (Nivolumab) CTLA-4+anti-PD-1 (Ipilimumab+ Nivolumab) |
| aGulati et al.34 | 2021 | 295 (61.1%) 188 (38.9%) |
Mean 63.29 | Asthma Inflammatory bowel disease Psoriasis Rheumatoid arthritis Eczema Polymyalgia rheumatic Other |
Melanoma | CTLA-4 CTLA-4+anti-PD-1 PD-1 |
| Van der Kooij et al.27 | 2021 | 2538 (58.1%) 1829 (41.9%) |
<65 67.8% ≥65 32.2% |
Rheumatologic AID Endocrine AID Inflammatory bowel disease Other |
Melanoma | CTLA-4 (Ipilimumab) anti-PD-1 (Pembrolizumab) (Nivolumab) CTLA-4+anti-PD-1 |
| Kanai et al.24 | 2018 | 154 (71.3%) 62 (28.7%) |
69 (30–89) | Interstitial lung disease | NSCLC | anti-PD-1 (Nivolumab) |
| Cortellini et al.22 | 2019 | 499 (66.4%) 252 (33.6%) |
69 (24–92) | Thyroid disorders60% Dermatologic16.4% Rheumatologic11.8% Others 11.8% |
NSCLC65.5% Melanoma21.2% Kidney cancer 12.5% Others0.8% |
anti-PD-1 (Pembrolizumab) (Nivolumab) |
| Shibaki et al.20 | 2019 | 223 (62.3%) 135 (37.7%) |
62 (27–84) | Interstitial lung disease | NSCLC | anti-PD-1 (Nivolumab) (Pembrolizumab) |
| Byeon et al.28 | 2020 | 167 (70.5%) 70 (29.5%) |
60 (35–80) | Rheumatoid arthritis7.1% Behcet’s disease7.1% Interstitial lung disease85.3% |
NSCLC | anti-PD-1 (Pembrolizumab) (Nivolumab) |
| Tasaka et al.21 | 2020 | 337 (73.1%) 124 (26.9%) |
69 (34–88) | Interstitial lung disease | NSCLC | anti-PD-L1 (Atezolizumab) |
| Faehling et al.33 | 2020 | 82 (65.1%) 44 (34.9%) |
62.4 (33.5–81.6) | NA | NSCLC | anti-PD-L1 (Durvalumab) |
| Ansel et al.29 | 2020 | 41 (50%) 41 (50%) |
65 (60.6–73.2) | Rheumatoid Arthritis Psoriasis Idiopathic pulmonary fibrosis Raynaud’s Disease Fibromyalgia Post-polio Syndrome Multiple sclerosis |
NSCLC | ati-PD-1 (Pembrolizumab) |
| Han et al.35 | 2022 | 991 (54.4%) 838 (45.6%) |
66.43 (57.5–74) 63.54 (54.4–71) |
Psoriasis Rheumatoid arthritis |
NSCLC Melanoma Kidney Other |
anti-PD-L anti-PD-L1 |
| aLoriot et al.23 | 2020 | 772 (77.4%) 225 (22.6%) |
69 (41–82) 68 (34–93) |
Psoriasis | Urinary tract carcinoma | anti-PD-L1 (Atezolizumab) |
aWe chose baseline characteristics of cohort studies; NSCLC: non-small cell lung cancer; PD-1: programmed cell death protein 1; PD-L1: programmed cell death protein ligand 1; CTLA-4: cytotoxic T-lymphocyte – associated protein 4; NA: not available.
Table 2.
Detailed information of irAEs of included studies.
| Author | Sum AID | Any irAEs (%) | ≥G3 irAEs (%) | Sum non-AID | Any irAEs (%) | ≥G3 irAEs (%) |
|---|---|---|---|---|---|---|
| Danlos et al.25 | 45 | 20 (44.4%) | NA | 352 | 84 (23.9%) | NA |
| Kanai et al.24 | 26 | 8 (31%) | 5 (19%) | 190 | 22 (12%) | 10 (5%) |
| Schadendorf et al.26 | 25 | 25 (100%) | 14 (56%) | 983 | 948 (96.4%) | 446 (45.4%) |
| Cortellini et al.22 | 85 | 56 (65.9%) | 8 (9.4%) | 666 | 266 (39.9%) | 59 (8.8%) |
| Shibaki et al.20 | 14 | 4 (29%) | 1 (7.1%) | 196 | 22 (11%) | 8 (4.1%) |
| Tasaka et al.21 | 49 | 15 (30.6%) | NA | 412 | 39 (9.5%) | NA |
| Loriot et al.23 | 35 | 24 (69%) | 9(26%) | 962 | 506 (53%) | 112 (12%) |
| bVan der Kooij et al.27 | 187 | NA | 31 (16.6%) | 1540 | NA | 206 (13.4%) |
aWe chose the patients treated with anti-PD-1 because they are the most in this study; AID: autoimmune disease; irAEs: immune-related adverse events; G3: grade 3; NA: not available.
Table 3.
Detailed information for PFS and OS of included studies.
| Author | Country | Patients AID (%) Non-AID (%) |
PFS (month) (AID: Non AID) |
OS (month) (AID: Non AID) |
HR for PFS (95%CI) | HR for OS (95%CI) | Quality | |
|---|---|---|---|---|---|---|---|---|
| Bottoni et al.32 | Italy | 49 24.6% 150 75.4% |
NA | 109:187 | NA | 3.01 (1.26–7.19) | 7 | |
| Danlos et al.25 | France | 45 11.3% 352 88.7% |
NA | NA | NA | 1.39 (0.74–2.61) | 7 | |
| Kanai et al.24 | Japan | 26 12% 190 88% |
2.7:2.9 | NA | 1.86 (1.11–3.83) | NA | 7 | |
| Schadendorf et al.26 | Germany | 25 2.5% 983 97.5% |
NA | 18.6:21.4 | NA | 1.32(0.76–2.30) | 8 | |
| Cortellini et al.22 | Italy | 85 11.3 666 88.7% |
6.8:8.0 | 9.8:16.5 | 1.3 (0.95–1.77) | 1.04 (0.73–1.48) | 6 | |
| Shibaki et al.20 | Japan | 14 6.6% 196 93.4% |
4.3:5.3 | NA | 0.97 (0.67–1.44) | NA | 6 | |
| Byeon et al.28 | Korea | 14 5.9% 223 94.1% |
3.6:2.3 | NA | 1.28 (0.61–2.69) | NA | 6 | |
| Tasaka et al.21 | Japan | 49 10.6% 412 89.4% |
5.9:3.5 | 27.8:25.2 | 0.94 (0.63–1.42) | 1.70 (0.75–3.82) | 6 | |
| Loriot et al.23 | Switzerland | 35 3.5% 962 96.5% |
NA | 8.2:8.8 | NA | 1.80 (0.85–3.81) | 7 | |
| Faehling et al.33 | Germany | 9 7.1% 116 92.9% 1 UN |
NA | NA | 1.01 (0.41–2.54) | 1.34 (0.42–4.28) | 7 | |
| Ansel et al.29 | UK | 10 12% 72 88% |
33.32:6.4 | 42:10.7 | 1.16 (0.36–3.77) | 0.5 (0.19–1.3) | 6 | |
| Gulati et al.34 | US | 74 15.3% 409 84.7% |
NA | NA | 0.49 (0.26–0.91) | 0.21 (0.07–0.65) | 8 | |
| bVan der Kooij et al.27 | Holland | 187 10.8% 1540 89.2% |
NA | NA | 1.11 (0.92–1.34) | 1.08 (0.87–1.34) | 8 | |
| Han et al.35 | US | 147 8.1% 1675 91.9% |
NA | NA | NA | 0.95 (0.76–1.17) | 6 | |
aWe chose the patients treated with anti-PD-1 they are the most in this study; UN: whether he/she had AID or not was uncertain; UK: the United Kingdom; US: the United States; NA: not available.
Table 4.
Quality assessment of the included retrospective studies with New Castle – Ottawa quality assessment scale.
| Study | Case definition adequate | Representativeness of the case | Selection of Controls | Definition of Controls | Comparability of cases and controls on the basis of the design or analysis | Assessment of exposure |
Same method of ascertainment for cases and controls | Non-Response rate |
|---|---|---|---|---|---|---|---|---|
| Bottoni et al.32 | √ | √ | √ | √ | √ | √ | ||
| Danlos et al.25 | √ | √ | √ | √ | √ | √ | √ | |
| Kanai et al.24 | √ | √ | √ | √ | √ | √ | ||
| Cortellini et al.22 | √ | √ | √ | √ | √ | |||
| Shibaki et al.20 | √ | √ | √ | √ | √ | |||
| Byeon et al.28 | √ | √ | √ | √ | √ | |||
| Tasaka et al.21 | √ | √ | √ | √ | √ | |||
| Faehling et al.33 | √ | √ | √ | √ | √ | √ | ||
| Ansel et al.29 | √ | √ | √ | √ | √ | |||
| Van der Kooij et al.27 | √ | √ | √ | √ | √ | √ | √ | |
| Han et al.35 | √ | √ | √ | √ | √ | √ |
√ Adequacy of criteria and its absence represents inadequacy.
Table 5.
Quality assessment of the included cohort studies with New Castle – Ottawa quality assessment scale.
| Study | Representativeness of exposed cohort |
Selection of Non exposed cohort |
Ascertainment of exposure |
Demonstration that outcome of interest was not present at start of study |
Comparability of cohorts on the basis of the design or analysis | Assessment of outcome |
Was follow-up long enough for outcomes to occur |
Adequacy of follow-up completion of cohorts |
|---|---|---|---|---|---|---|---|---|
| Schadendorf et al.26 | √ | √ | √ | √ | √ | √ | √ | √ |
| Loriot et al.23 | √ | √ | √ | √ | √ | √ | ||
| Gulati et al.34 | √ | √ | √ | √ | √ | √ | √ |
√ Adequacy of criteria and its absence represents inadequacy.
3.3. Safety
Eight studies reported irAEs, including 466 and 5301 patients in the AID and non-AID groups, respectively. Seven studies reported irAEs of any grade, six of which were grade ≥3 irAEs. These studies used CTCAE 4.0 to assess the irAEs. Sonam Ansel et al. study also reported irAEs but used CTCAE 5.0, so we did not include the study.29 The incidence of irAEs of any grade ranged from 29% to 100% in the AID group and 9.5% to 96.4% in the non-AID group, whereas the incidence of grade ≥3 irAEs ranged from 7.1% to 56% in the AID group and 4.1% to 45.4% in the non-AID group. The AID group (RR: 1.74, 95% CI 1.27–2.37; Figure 2) was associated with a significantly higher risk of developing irAEs; nevertheless, significant heterogeneity was detected (I2 = 91.9%, p < .01). The incidence of grade ≥3 irAEs (RR, 1.43; 95% CI 1.10–1.88; Figure 3) was also higher in the AID group, and low heterogeneity was found (I2 = 33.0%; p = .19).
Figure 2.
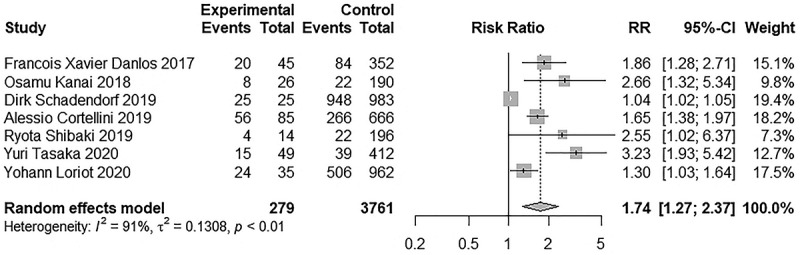
Forest plot of any grade irAEs in patients with and without AID receiving ICIs.
Figure 3.
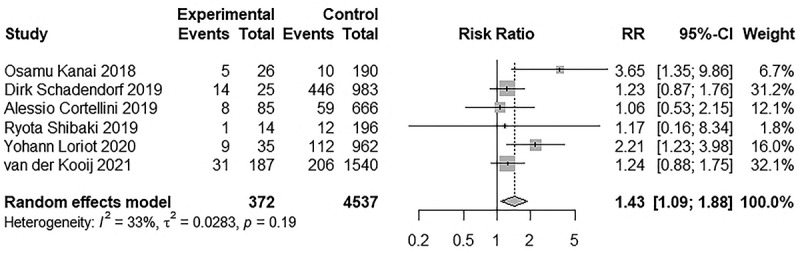
Forest plot of grade ≥3 irAEs in patients with and without AID receiving ICIs.
Subsequently, a subgroup analysis for irAEs of any grade was carried out to determine the reason for the high heterogeneity. When systemic autoimmune diseases were excluded, such as rheumatoid arthritis and systemic lupus erythematosus, we found that AIDs tended to aggravate irAEs in the same systems involved by it, but generally did not exacerbate irAEs in other systems. When irAEs occurred in the same system as that of the AID, we referred to them as AID-homogeneous irAEs; in the other cases, they were referred to as AID-heterogeneous irAEs. Three studies mainly discussed interstitial lung disease, and pulmonary toxicity was higher in the interstitial lung disease group than in the non-interstitial lung disease group (RR = 2.93, 95% CI 2.01–4.28). The incidence of irAEs in the following AIDs were: psoriasis, cutaneous side effects were AID-homogeneous irAEs (RR = 1.74, 95% CI 1.16–2.60); autoimmune thyroiditis, endocrine toxicity (RR = 1.57, 95% CI 1.11–2.21); and inflammatory bowel disease, gastrointestinal toxicity (RR = 3.43, 95% CI 1.39–8.49). For AID-heterogeneous irAEs, no difference was detected in the incidence of pulmonary toxicity (RR = 1.78, 95% CI 0.69–4.58), endocrine toxicity (RR = 0.91, 95% CI 0.38–2.19), gastrointestinal toxicity (RR = 1.42, 95%CI 0.93–2.17), and hepatotoxicity (RR = 1.24, 95% CI 0.70–2.19) between the two groups. Detailed information is presented in Figure 4. Next, we compared the incidence of irAEs in different cancer types when using the same ICI. When anti-PD-1s were used, there were no difference in any grade (RR = 0.66, 95% CI 0.38–1.13) and ≥3 grade irAEs (RR = 0.61, 95% CI 0.32–1.16) between NSCLC and melanoma. Limited by the data, we did not analyze the incidence of irAEs among other cancer types when using other ICIs. In addition, we analyzed the incidence of irAEs in the same cancer type with different ICIs. No difference was found in any grade irAEs (RR = 1.38, 95% CI 0.77–2.48) between anti-PD-1 and anti-PD-L1 in patients with NSCLC. Similarly, we did not analyze the incidence of irAEs in other cancer types due to limited data. Detailed information is presented in Figures 5a,b and 6. In addition, we analyzed the incidence of irAEs associated with the use of anti-PD-1 monotherapy versus anti-PD-1 and CTLA-4 combination therapy in melanoma patients with autoimmune disease. We did not analyze the incidence of irAEs in other cancer types or other ICIs limited by the data. The incidence of irAEs in combination therapy was significantly higher than that in monotherapy at any grade (RR = 0.58, 95% CI 0.38–0.88) and ≥3 grades (RR = 0.29, 95% CI 0.16–0.51), which are consistent with the findings in Audrey Simonaggio et al.36 and Alice Tison et al.37 studies. Detailed information is presented in Figure 7. Two studies reported discontinuation rates due to immunotoxicity. There was no significant difference (RR = 0.71, 95% CI 0.34–1.48) in patients with and without AID. Detailed information is presented in Figure 8. Three studies reported on immune-related mortality. There was no significant difference (RR = 2.03 95% CI 0.76–5.46) in Grade 5 irAEs between patients with AID and without AID. Detailed information is presented in Figure 9. Immune-related deaths have also been reported in the following studies on patients with preexisting AID who received treatment with ICIs. Alice Tison et al. study reported immune-related deaths in different pre-existing autoimmune diseases treated with different ICIs.37 Elena Fountzilas et al. study reported that 2 of 123 patients with AID died of immunotoxicity, but did not report immune-related mortality in patients without AID.38
Figure 4.

Forest plot of AID- homogeneous and AID-heterogeneous irAEs in patients with and without AID receiving ICIs.
Figure 5.

Forest plots of any grade irAEs and grade ≥3 irAEs in NSCLC compared to melanoma using PD-1. (a) Forest plot of any grade irAEs in NSCLC compared to melanoma using PD-1. (b) Forest plot grade ≥3 irAEs in NSCLC compared to melanoma using PD-1.
Figure 6.

Forest plot of any grade irAEs in NSCLC using PD-1 compared to PD-L1.
Figure 7.

Forest plots of any grade irAEs and grade ≥3 irAEs in melanoma patients with AID using anti-PD-1 monotherapy compared to anti-PD-1 and CTLA-4 combined therapy.
Figure 8.
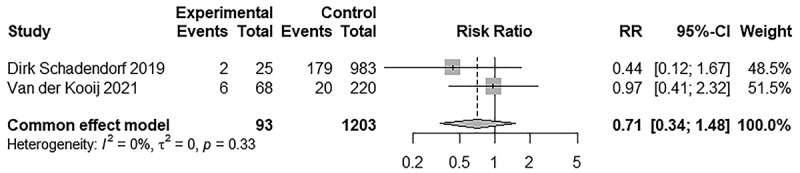
Forest plot of discontinuation rate due to immunotoxicity in patients with and without AID receiving ICIs.
Figure 9.
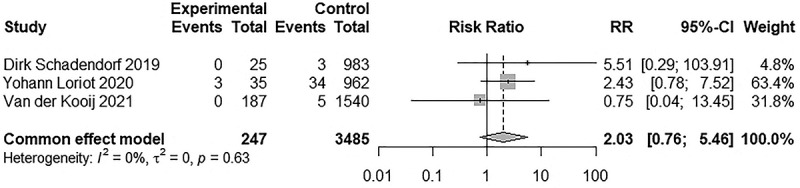
Forest plot of immune-related mortality due to immunotoxicity in patients with and without AID receiving ICIs.
3.4. Efficacy
Nine and ten studies reported PFS and OS, respectively. In total, 8716 participants were treated with ICIs, of these, PFS was assessed in 4,293 participants and OS in 8053 participants. There was no significant difference in PFS (HR: 1.09, 95% CI 0.96–1.24) and OS (HR: 1.07, 95% CI 0.94–1.22) between AID and non-AID groups when they were treated with ICIs. There was low heterogeneity (I2 = 30.1%; p = .18) in PFS; however, high heterogeneity was found (I2 = 53.9%; p = .02) in OS. Forest plots of these outcomes are shown in Figures 10 and 11 respectively.
Figure 10.
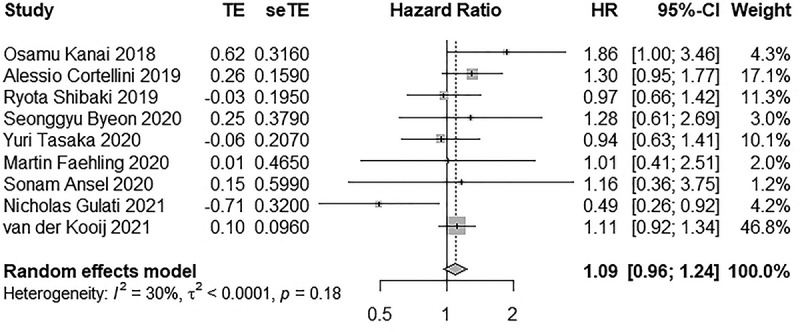
Forest plot of PFS in patients with and without AID receiving ICIs.
Figure 11.

Forest plot of OS in patients with and without AID receiving ICIs.
Furthermore, subgroup analyses were performed analyze the PFS and OS in different cancer types and in different ICIs. The PFS and OS assessed were as follows: PFS: HR = 1.16 (0.96–1.39) in NSCLC group; HR = 0.78 (0.35–1.73) in melanoma group; HR = 1.23 (0.99–1.54) using anti-PD-1; HR = 0.95 (0.66–1.38) using PD-L1; HR = 0.73 (0.39–1.38) using CTLA-4; OS: HR = 1.02 (0.81–1.28) in NSCLC group; HR = 1.11 (0.56–2.23) in melanoma group; HR = 1.04 (0.87–1.25) using anti-PD-1; HR = 1.57 (0.81–3.06) using anti-PD-L1; HR = 0.86 (0.21–3.85) using CTLA-4. Forest plots of these outcomes are shown in Figures 12a,b and 13a,b, respectively. Besides, we conducted subgroup analysis by region and literature quality. High heterogeneity was discovered in subgroups of region and literature quality, but it did not change the results of the analysis. Detailed information is presented in Figure 14. Each subgroup demonstrated that using ICI did not shorten PFS and OS in patients with AID. The results of each subgroup matched the overall results.
Figure 12.

Forest plots of PFS and OS in NSCLC or melanoma using anti-PD-1. (a) Forest plot of PFS in NSCLC or melanoma using anti-PD-1. (b) Forest plot of OS in NSCLC or melanoma using anti-PD-1.
Figure 13.
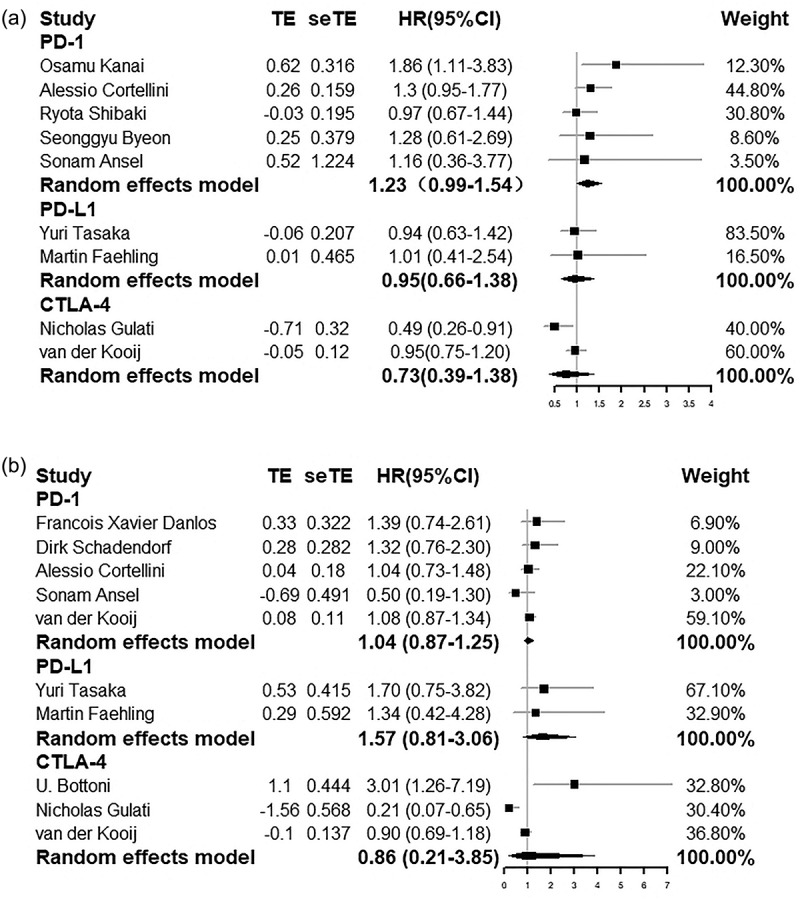
Forest plots of PFS and OS in NSCLC using anti-PD-1 or in NSCLC using anti-PD-L1 or in melanoma using CTLA-4. (a) Forest plot of PFS in NSCLC using anti-PD-1 or in NSCLC using anti-PD-L1 or in melanoma using CTLA-4. (b) Forest plot of OS in NSCLC using anti-PD-1 or in NSCLC using anti-PD-L1 or in melanoma using CTLA-4.
Figure 14.
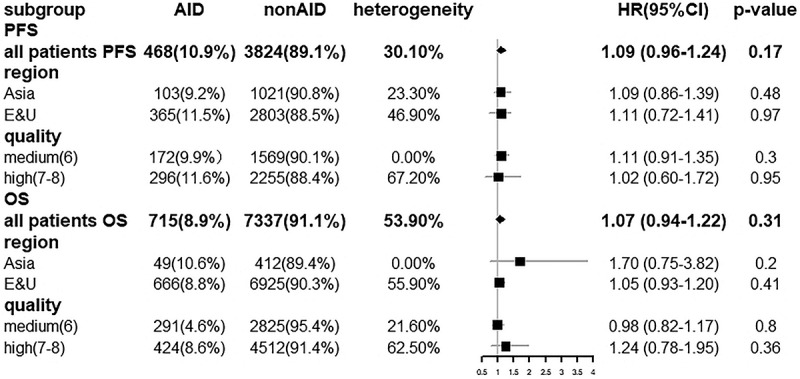
Forest plot of PFS and OS in regional factors and quality characteristics subgroup analysis.
3.5. Publication bias and sensitivity analysis
Publication bias was verified using a funnel plot (Supplemental Material: Figure S1) and Egger’s linear regression test. There was no obvious publication bias for any grade irAEs (t = 1.95, p = .11), grade ≥3 irAEs (t = 1.02, p = .37), PFS (t = −0.32, p = .76), or OS (t = 0.46, p = .65). However, funnel plots for irAEs of any grade and OS were asymmetrical on visual inspection, indicating that the underlying publication bias should be considered. From the sensitivity analysis, our study results were reliable because the pooled results of any grade irAEs, grade ≥3 irAEs, PFS, and OS remained significant regardless of the studies omitted. Forest plots of sensitivity analysis are shown in Figure S2.
4. Discussion
From our findings, the incidence of irAEs was higher in the AID group than in the non-AID group treated with ICIs. Specifically, the AID group is more likely to develop irAEs in the same system that their autoimmune disease has involved, whereas the frequency of irAEs involving systems other than that affected by their autoimmune disease is similar to the frequency of irAEs in the non-AID group. Survival outcomes in the AID group were almost unaffected compared to those in the non-AID group. To the best of our knowledge, this is the first study to assess the types of irAEs that patients with AIDs are prone to and compare PFS and OS in cancer patients with and without AID on ICI therapy, thus filling a gap in previous studies of this kind.
The major pathological and clinical manifestations of AIDs mainly manifest in which system, indicating that the immune cells of this system are in an abnormal activation state. The immune cells of other systems are generally in a normal state; hence, the frequency of irAEs is similar to that in normal people. Clinicians should pay more attention to irAEs involving the same system as that of the preexisting AID.
Although patients with AID are at a higher risk of developing irAEs, no statistically significant reductions in PFS and OS were observed in the AID group compared to the non-AID group. Meanwhile, the curative effect of ICIs on patients with AID is worthy of affirmation. This may suggest that the abnormal immune system in AID does not affect the killing function of tumor cells by T lymphocytes, or aggravation of irAEs does not shorten the survival of patients with AID. Considering the fatality of some irAEs, the means of controlling the occurrence of irAEs during treatment are yet to be determined. Clinically, immunosuppressants such as steroids are commonly used to control irAEs. Since only two studies have discussed the use of immunosuppressants to control irAEs, we cannot rely on the limited data to accurately analyze the results.20,29 Tison et al. found that the OS of patients with AID was significantly shortened with the administration of corticosteroids.37 However, Fausto et al. reported that the use of steroids to control irAEs in patients with cancer did not shorten OS.38,39 There are no definitive answers on whether, when, and how steroids should be used in treatment with ICIs. Therefore, it is necessary to perform patient stratification strategies based on the severity of irAEs to determine subsequent treatments.40
Although irAEs may be mild and manageable in most cases, immune checkpoint inhibitors can still lead to severe, even life-threatening side effects.41 It is widely acknowledged that the use of different types of ICI resulted in different incidences of irAEs. Immune-related adverse events have been reported in more than 50% of patients treated with ipilimumab, and in 30% of patients treated with PD-1 inhibitors; instead, the combination of ipilimumab and nivolumab had the highest incidence of irAEs.42 However, there are no effective biomarkers to detect the emergence of irAEs when a patient using ICIs. It is essential to manage irAEs effectively by optimizing identification and response strategies. For example, popularize relevant knowledge to patients, standardize the measurement of adverse reactions, optimize the choice of agents, and individualize the treatment of irAEs.42 Cancer patients with AID have a greater chance of developing irAEs when using ICIs. Collaboration between oncologists and rheumatologists should be further encouraged, as well as the implementation of prospective clinical trials to investigate the prevention and management of irAEs. A clinical trial (NCT03816345) is currently underway to use nivolumab in treating advanced cancer patients with autoimmune disorders. The primary aims of the clinical trial are to assess the specific toxicity and efficacy associated with the use of nivolumab in patients with varying severity of the autoimmune disease.
Previous studies reported that irAEs significantly correlated with better curative effect in patients with cancer.43,44–48 Therefore, some clinicians doubt that patients with AID may benefit more from ICIs because of their immune-activated tendency.49 Although our findings demonstrated that patients with AID were at a higher risk of developing irAEs, we did not conclude that the survival would be prolonged in the AID group. All the above studies were conducted in non-AID cancer patients, unlike the population we studied. Moreover, several factors can affect the final PFS and OS. First, regarding the Eastern Cooperative Oncology Group (ECOG) status of patients with cancer, it is necessary to evaluate the survival time separately, according to the performance of the ECOG status. Second, various AIDs may have mild or severe effects on the body. For instance, systemic lupus erythematous (SLE) can affect nearly all organs and produce multiple autoantibodies,50 whereas psoriasis causes relatively minor impairments in other systems.
This study has some limitations. First, because most studies did not use ICIs in patients with AID, the number of included studies was insufficient. Further, we did not access the information about the severity of patients with AID treated with ICI in our analysis. Patients with a severe AID were less likely to be treated with ICI, so selection bias was unavoidable. Second, most data collected were from retrospective studies rather than prospective clinical trials; therefore, the veracity of the information may not be sufficiently objective. Third, most of the malignancies included in the study were NSCLC and melanoma, with only few other cancers involved. Furthermore, seven studies only reported survival curves; hence, HR was estimated from the survival curve using specialized tools. Thus, there may be a certain degree of deviation from the actual situation.
5. Conclusion
In summary, patients with AID were at a higher risk of developing irAEs, and AID-homogeneous irAEs were higher in the AID group than in the non-AID group, whereas no significant difference was detected for AID-heterogeneous irAEs. No statistically significant reductions in PFS and OS were observed in cancer patients with AID. Therefore, the indications of ICI treatment can be broadened to include AID populations in routine clinic practice. Further large-scale prospective studies are required to validate our findings.
Supplementary Material
Acknowledgment
The authors would like to thank Editage (www.editage.cn) for English language editing.
Funding Statement
The author(s) reported there is no funding associated with the work featured in this article.
Abbreviations
- ICIs
immune checkpoint inhibitors
- AID
autoimmune disease
- irAEs
immune-related adverse events
- EMBASE
Excerpta Medica Database
- PFS
progression-free survival
- OS
overall survival
- CI
confidence interval
- RR
HR:risk ratiohazard ratio
- PD-1
programmed cell death protein 1
- PD-L1
programmed cell death protein ligand 1
- CTLA-4
cytotoxic T-lymphocyte – associated protein 4
- MeSH
medical subject headings
- NSCLC
Non-small cell lung cancer
- ECOG
Eastern Cooperative Oncology Group
- SLE
systemic lupus erythematous
Authors’ contributions
QC and GW H conducted literature search, data extraction, risk of bias assessment. QC performed statistical analysis and wrote the original manuscript. GW H revised the manuscript and rectified some information of tables and Figures. FY Z resolved differences in data collection and revised the manuscript critically. P Y and DQ Y advised on the writing and revised the manuscript critically. All authors read and approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2022.2145102.
References
- 1.Sharma P, Allison JP.. The future of immune checkpoint therapy. Science. 2015;348(6230):56–13. doi: 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- 2.Homet Moreno B, Ribas A. Anti-programmed cell death protein-1/ligand-1 therapy in different cancers. Br J Cancer. 2015;112(9):1421–27. doi: 10.1038/bjc.2015.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dine J, Gordon R, Shames Y, Kasler MK, Barton-Burke M. Immune checkpoint inhibitors: an innovation in immunotherapy for the treatment and management of patients with cancer. Asia Pac J Oncol Nurs. 2017;4(2):127–35. doi: 10.4103/apjon.apjon_4_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sanmamed MF, Chen L. A paradigm shift in cancer immunotherapy: from enhancement to normalization. Cell. 2018;175(2):313–26. doi: 10.1016/j.cell.2018.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Münz C, Lünemann JD, Getts MT, Miller SD. Antiviral immune responses: triggers of or triggered by autoimmunity. Nat Rev Immunol. 2009;9(4):246–58. doi: 10.1038/nri2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vojdani A. A potential link between environmental triggers and autoimmunity. Autoimmune Dis. 2014;2014:437231. doi: 10.1155/2014/437231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rose NR. Prediction and prevention of autoimmune disease in the 21st century: a review and preview. Am J Epidemiol. 2016;183(5):403–06. doi: 10.1093/aje/kwv292. [DOI] [PubMed] [Google Scholar]
- 8.Milchert M, Makowska J, Brzezińska O, Brzosko M, Więsik-Szewczyk E. Monogenic autoinflammatory diseases in adults - a challenge to rheumatologic practice at the onset of the Polish national programme of interleukin 1 inhibitor treatment. Reumatologia. 2019;57(6):326–35. doi: 10.5114/reum.2019.91298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simon D, Tascilar K, Fagni F, Krönke G, Kleyer A, Meder C, Atreya R, Leppkes M, Kremer AE, Ramming A, et al. Efficacy and safety of SARS-CoV-2 revaccination in non-responders with immune-mediated inflammatory disease. Ann Rheum Dis. 2021;80(10):1312–16. doi: 10.1136/annrheumdis-2021-220461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dörner T, Jacobi AM, Lee J, Lipsky PE. Abnormalities of B cell subsets in patients with systemic lupus erythematosus. J Immunol Methods. 2011;363(2):187–97. doi: 10.1016/j.jim.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 11.Yang Y, Santamaria P. Evolution of nanomedicines for the treatment of autoimmune disease: from vehicles for drug delivery to inducers of bystander immunoregulation. Adv Drug Deliv Rev. 2021;176:113898. doi: 10.1016/j.addr.2021.113898. [DOI] [PubMed] [Google Scholar]
- 12.Sudres M, Verdier J, Truffault F, Le Panse R, Berrih-Aknin S. Pathophysiological mechanisms of autoimmunity. Ann N Y Acad Sci. 2018;1413(1):59–68. doi: 10.1111/nyas.13560. [DOI] [PubMed] [Google Scholar]
- 13.Kennedy LC, Bhatia S, Thompson JA, Grivas P. Preexisting autoimmune disease: implications for immune checkpoint inhibitor therapy in solid tumors. J Natl Compr Cancer Netw. 2019;17(6):750–57. doi: 10.6004/jnccn.2019.7310. [DOI] [PubMed] [Google Scholar]
- 14.Lee DJ, Lee HJ, Jr, Farmer JR, Reynolds KL. Mechanisms driving immune-related adverse events in cancer patients treated with immune checkpoint inhibitors. Curr Cardiol Rep. 2021;23(8):98. doi: 10.1007/s11886-021-01530-2. [DOI] [PubMed] [Google Scholar]
- 15.Horn L, Mansfield AS, Szczęsna A, Havel L, Krzakowski M, Hochmair MJ, Huemer F, Losonczy G, Johnson ML, Nishio M, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018;379(23):2220–29. doi: 10.1056/NEJMoa1809064. [DOI] [PubMed] [Google Scholar]
- 16.Paz-Ares L, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, Statsenko G, Hochmair MJ, Özgüroğlu M, Ji JH, et al. Durvalumab plus platinum–etoposide versus platinum–etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet. 2019;394(10212):1929–39. doi: 10.1016/S0140-6736(19)32222-6. [DOI] [PubMed] [Google Scholar]
- 17.Franks AL, Slansky JE. Multiple associations between a broad spectrum of autoimmune diseases, chronic inflammatory diseases and cancer. Anticancer Res. 2012;32:1119–36. [PMC free article] [PubMed] [Google Scholar]
- 18.Kazarian M, Laird-Offringa IA. Small-cell lung cancer-associated autoantibodies: potential applications to cancer diagnosis, early detection, and therapy. Mol Cancer. 2011;10:33. doi: 10.1186/1476-4598-10-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xie W, Huang H, Xiao S, Fan Y, Deng X, Zhang Z. Immune checkpoint inhibitors therapies in patients with cancer and preexisting autoimmune diseases: a meta-analysis of observational studies. Autoimmun Rev. 2020;19(12):102687. doi: 10.1016/j.autrev.2020.102687. [DOI] [PubMed] [Google Scholar]
- 20.Shibaki R, Murakami S, Matsumoto Y, Goto Y, Kanda S, Horinouchi H, Fujiwara Y, Yamamoto N, Motoi N, Kusumoto M, et al. Tumor expression and usefulness as a biomarker of programmed death ligand 1 in advanced non-small cell lung cancer patients with preexisting interstitial lung disease. Med Oncol. 2019;36(6):49. doi: 10.1007/s12032-019-1274-0. [DOI] [PubMed] [Google Scholar]
- 21.Tasaka Y, Honda T, Nishiyama N, Tsutsui T, Saito H, Watabe H, Shimaya K, Mochizuki A, Tsuyuki S, Kawahara T, et al. Non-inferior clinical outcomes of immune checkpoint inhibitors in non-small cell lung cancer patients with interstitial lung disease. Lung Cancer. 2021;155:120–26. doi: 10.1016/j.lungcan.2021.03.014. [DOI] [PubMed] [Google Scholar]
- 22.Cortellini A, Buti S, Santini D, Perrone F, Giusti R, Tiseo M, Bersanelli M, Michiara M, Grassadonia A, Brocco D, et al. Clinical outcomes of patients with advanced cancer and pre-existing autoimmune diseases treated with anti-programmed death-1 immunotherapy: a real-world transverse study. Oncologist. 2019;24(6):e327–37. doi: 10.1634/theoncologist.2018-0618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loriot Y, Sternberg CN, Castellano D, Oosting SF, Dumez H, Huddart R, Vianna K, Alonso Gordoa T, Skoneczna I, Fay AP, et al. Safety and efficacy of atezolizumab in patients with autoimmune disease: subgroup analysis of the SAUL study in locally advanced/metastatic urinary tract carcinoma. Eur J Cancer. 2020;138:202–11. doi: 10.1016/j.ejca.2020.07.023. [DOI] [PubMed] [Google Scholar]
- 24.Kanai O, Kim YH, Demura Y, Kanai M, Ito T, Fujita K, Yoshida H, Akai M, Mio T, Hirai T. Efficacy and safety of nivolumab in non-small cell lung cancer with preexisting interstitial lung disease. Thorac Cancer. 2018;9(7):847–55. doi: 10.1111/1759-7714.12759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Danlos FX, Voisin AL, Dyevre V, Michot J-M, Routier E, Taillade L, Champiat S, Aspeslagh S, Haroche J, Albiges L, et al. Safety and efficacy of anti-programmed death 1 antibodies in patients with cancer and pre-existing autoimmune or inflammatory disease. Eur J Cancer. 2018;91:21–29. doi: 10.1016/j.ejca.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 26.Schadendorf D, Ascierto PA, Haanen J, Espinosa E, Demidov L, Garbe C, Guida M, Lorigan P, Chiarion-Sileni V, Gogas H, et al. Safety and efficacy of nivolumab in challenging subgroups with advanced melanoma who progressed on or after ipilimumab treatment: a single-arm, open-label, phase II study (CheckMate 172). Eur J Cancer. 2019;121:144–53. doi: 10.1016/j.ejca.2019.08.014. [DOI] [PubMed] [Google Scholar]
- 27.van der Kooij MK, Suijkerbuijk K, Aarts M, van den Berkmortel FW, Blank CU, Boers-Sonderen MJ, van Breeschoten J, van den Eertwegh AJ, de Groot JW, Haanen JB, et al. Safety and efficacy of checkpoint inhibition in patients with melanoma and preexisting autoimmune disease : a cohort study. Ann Intern Med. 2021;174(5):641–48. doi: 10.7326/M20-3419. [DOI] [PubMed] [Google Scholar]
- 28.Byeon S, Cho JH, Jung HA, Sun J-M, Lee S-H, Ahn JS, Park K, Ahn M-J. PD-1 inhibitors for non-small cell lung cancer patients with special issues: real-world evidence. Cancer Med. 2020;9(7):2352–62. doi: 10.1002/cam4.2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ansel S, Rulach R, Trotter N, Steele N. Pembrolizumab for advanced non-small cell lung cancer (NSCLC): impact of autoimmune comorbidity and outcomes following treatment completion. J Oncol Pharm Pract. 2022:10781552221079356. doi: 10.1177/10781552221079356. [DOI] [PubMed] [Google Scholar]
- 30.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang X, Karapetyan L, Kirkwood JM. Safety and efficacy of checkpoint inhibition in patients with melanoma and preexisting autoimmune disease. Ann Intern Med. 2021;174(9):1345. doi: 10.7326/L21-0441. [DOI] [PubMed] [Google Scholar]
- 32.Bottoni U, Paolino G, Ambrifi M, Didona D, Albanesi M, Clerico R, Lido P, Brachini A, Corsetti P, Richetta AG, et al. Association between autoimmune disease and cutaneous melanoma with regard to melanoma prognosis. Clin Exp Dermatol. 2015;40(3):254–59. doi: 10.1111/ced.12531. [DOI] [PubMed] [Google Scholar]
- 33.Faehling M, Schumann C, Christopoulos P, Didona D, Albanesi M, Clerico R, Lido P, Brachini A, Corsetti P, Richetta AG, et al. Durvalumab after definitive chemoradiotherapy in locally advanced unresectable non-small cell lung cancer (NSCLC): real-world data on survival and safety from the German expanded-access program (EAP). Lung Cancer. 2020;150:114–22. doi: 10.1016/j.lungcan.2020.10.006. [DOI] [PubMed] [Google Scholar]
- 34.Gulati N, Celen A, Johannet P, Mehnert JM, Weber J, Krogsgaard M, Osman I, Zhong J. Preexisting immune-mediated inflammatory disease is associated with improved survival and increased toxicity in melanoma patients who receive immune checkpoint inhibitors. Cancer Med. 2021;10(21):7457–65. doi: 10.1002/cam4.4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Han CY, Fitzgerald C, Lee M, Valero C, Gönen M, Shoushtari A, Morris LGT. Association between toxic effects and survival in patients with cancer and autoimmune disease treated with checkpoint inhibitor immunotherapy. JAMA Oncol. 2022;8(9):1352. doi: 10.1001/jamaoncol.2022.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simonaggio A, Michot JM, Voisin AL, Le Pavec J, Collins M, Lallart A, Cengizalp G, Vozy A, Laparra A, Varga A, et al. Evaluation of readministration of immune checkpoint inhibitors after immune-related adverse events in patients with cancer. JAMA Oncol. 2019;5(9):1310–17. doi: 10.1001/jamaoncol.2019.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tison A, Quéré G, Misery L, Funck‐brentano E, Danlos F-X, Routier E, Robert C, Loriot Y, Lambotte O, Bonniaud B, et al. Safety and efficacy of immune checkpoint inhibitors in patients with cancer and preexisting autoimmune disease: a nationwide, multicenter cohort study. Arthritis Rheumatol. 2019;71(12):2100–11. doi: 10.1002/art.41068. [DOI] [PubMed] [Google Scholar]
- 38.Fountzilas E, Lampaki S, Koliou GA, Koumarianou A, Levva S, Vagionas A, Christopoulou A, Laloysis A, Psyrri A, Binas I, et al. Real-world safety and efficacy data of immunotherapy in patients with cancer and autoimmune disease: the experience of the Hellenic Cooperative Oncology Group. Cancer Immunol Immunother. 2022;71(2):327–37. doi: 10.1007/s00262-021-02985-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petrelli F, Signorelli D, Ghidini M, Ghidini A, Pizzutilo EG, Ruggieri L, Cabiddu M, Borgonovo K, Dognini G, Brighenti M, et al. Association of steroids use with survival in patients treated with immune checkpoint inhibitors: a systematic review and meta-analysis. Cancers (Basel). 2020;12(3). doi: 10.3390/cancers12030546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schneider BJ, Naidoo J, Santomasso BD, Lacchetti C, Adkins S, Anadkat M, Atkins MB, Brassil KJ, Caterino JM, Chau I, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: aSCO guideline update. J Clin Oncol. 2021;39(36):4073–126. doi: 10.1200/JCO.21.01440. [DOI] [PubMed] [Google Scholar]
- 41.Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, Chau I, Ernstoff MS, Gardner JM, Ginex P, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American society of clinical oncology clinical practice guideline. J Clin Oncol. 2018;36:1714–68. doi: 10.1200/JCO.2017.77.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Naing A, Hajjar J, Gulley JL, Atkins MB, Ciliberto G, Meric-Bernstam F, Hwu P. Strategies for improving the management of immune-related adverse events. J Immunother Cancer. 2020;8(2):e001754. doi: 10.1136/jitc-2020-001754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wagner G, Stollenwerk HK, Klerings I, Pecherstorfer M, Gartlehner G, Singer J. Efficacy and safety of immune checkpoint inhibitors in patients with advanced non-small cell lung cancer (NSCLC): a systematic literature review. Oncoimmunology. 2020;9(1):1774314. doi: 10.1080/2162402X.2020.1774314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Freeman-Keller M, Kim Y, Cronin H, Richards A, Gibney G, Weber JS. Nivolumab in resected and unresectable metastatic melanoma: characteristics of immune-related adverse events and association with outcomes. Clin Cancer Res. 2016;22(4):886–94. doi: 10.1158/1078-0432.CCR-15-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haratani K, Hayashi H, Chiba Y, Kudo K, Yonesaka K, Kato R, Kaneda H, Hasegawa Y, Tanaka K, Takeda M, et al. Association of immune-related adverse events with nivolumab efficacy in non–small-cell lung cancer. JAMA Oncol. 2018;4(3):374–78. doi: 10.1001/jamaoncol.2017.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ahn BC, Pyo KH, Xin CF, Jung D, Shim HS, Lee CY, Park SY, Yoon HI, Hong MH, Cho BC, et al. Comprehensive analysis of the characteristics and treatment outcomes of patients with non-small cell lung cancer treated with anti-PD-1 therapy in real-world practice. J Cancer Res Clin Oncol. 2019;145(6):1613–23. doi: 10.1007/s00432-019-02899-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grangeon M, Tomasini P, Chaleat S, Jeanson A, Souquet-Bressand M, Khobta N, Bermudez J, Trigui Y, Greillier L, Blanchon M, et al. Association between immune-related adverse events and efficacy of immune checkpoint inhibitors in non–small-cell lung cancer. Clin Lung Cancer. 2019;20(3):201–07. doi: 10.1016/j.cllc.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 48.Menzies AM, Johnson DB, Ramanujam S, Atkinson VG, Wong ANM, Park JJ, McQuade JL, Shoushtari AN, Tsai KK, Eroglu Z, et al. Anti-PD-1 therapy in patients with advanced melanoma and preexisting autoimmune disorders or major toxicity with ipilimumab. Ann Oncol. 2017;28(2):368–76. doi: 10.1093/annonc/mdw443. [DOI] [PubMed] [Google Scholar]
- 49.Zhou X, Yao Z, Yang H, Liang N, Zhang X, Zhang F. Are immune-related adverse events associated with the efficacy of immune checkpoint inhibitors in patients with cancer? A systematic review and meta-analysis. BMC Med. 2020;18(1):87. doi: 10.1186/s12916-020-01549-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guo J, Gao Y, Wang Y, Zou Y, Du Y, Luo C, Shi Y, Yang Y, Wu X, Su Y, et al. Investigation of C1-complex regions reveals new C1Q variants associated with protection from systemic lupus erythematosus, and affect its transcript abundance. Sci Rep. 2018;8(1):8048. doi: 10.1038/s41598-018-26380-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.


