Abstract
Introduction
The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2 or COVID-19) pandemic has had a significant impact on communities and health systems. The Federal Drug Administration (FDA) authorized Pfizer’s nirmatrelvir/ritonavir (Paxlovid™) through an EUA for the treatment of mild to moderate cases of COVID-19 at high risk for progression to severe disease. Patients with a history of transplant who test positive for COVID-19 are considered high risk because of their immunosuppression and are therefore candidates for nirmatrelvir/ritonavir.
Case Report
This is a case of a 67-year-old female with a past medical history of orthotopic heart transplant who received tacrolimus as part of her immunosuppressive regimen. She originally presented with complaints of dyspnea and cough for several days in the setting of COVID-19. The patient was started on nirmatrelvir/ritonavir due to her high risk for progression to severe disease. Four days after starting nirmatrelvir/ritonavir, she presented to the ED for slowed speech, fatigue, weakness, and loss of appetite. Upon admission she was found to have a supratherapeutic tacrolimus level of 176.4 ng/mL and an acute kidney injury. In this case, phenytoin was used as a CYP3A4 inducer to quickly decrease the tacrolimus level to within therapeutic range.
Conclusion
This case highlights the strong and important drug–drug interaction between tacrolimus and nirmatrelvir/ritonavir leading to toxic levels of tacrolimus. It also demonstrates the utility and effectiveness of phenytoin as a “rescue” medication for tacrolimus toxicity.
Keywords: Paxlovid™, nirmatrelvir/ritonavir, Tacrolimus toxicity, Drug-drug-interaction, Phenytoin
Introduction
The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2 or COVID-19) pandemic has had a significant impact on health systems. There have been extensive efforts put forth to develop a treatment for this virus. Drug manufacturers are rapidly developing new antiviral medications and releasing them through emergency use authorizations (EUA). Due to the rapid approval of these medications, there are many pharmacologic aspects of the medications that are not known upon authorization, including side effects and drug–drug interactions. The Federal Drug Administration (FDA) authorized Pfizer’s nirmatrelvir/ritonavir (Paxlovid™) through an EUA in December 2021 for the treatment of mild to moderate cases of COVID-19 at high risk for progression to severe COVID-19, including hospitalization or death. Nirmatrelvir/ritonavir consists of two antiviral medications, nirmatrelvir, a SARS-CoV-2 main protease inhibitor and ritonavir, an HIV-1 protease inhibitor; ritonavir has no antiviral activity against COVID-19 but is a well-known CYP3A4 inhibitor (a cytochrome P450 enzyme) that acts as a pharmacokinetic booster for nirmatrelvir in order to reach and maintain therapeutic concentrations [1].
Tacrolimus is a calcineurin inhibitor used for immunosuppression in solid organ transplant patients. Tacrolimus is primarily metabolized by CYP3A4 and is a substrate of P-glycoprotein and is therefore subject to numerous drug–drug interactions. Signs and symptoms of acute tacrolimus toxicity vary widely and may include gastrointestinal disturbances, nephrotoxicity, and neurotoxicity [2]. Tacrolimus requires therapeutic drug monitoring due to its narrow therapeutic index and propensity for these interactions. For most transplant patients, the goal level is 3–12 ng/mL.
Patients with a history of solid organ transplant who test positive for COVID-19 are considered to be at high risk for progression to severe disease because of their immunosuppression and are thus candidates for nirmatrelvir/ritonavir under the EUA. Ritonavir inhibits CYP3A4 and as a result is expected to increase the plasma concentrations of tacrolimus. Co-administration of nirmatrelvir/ritonavir and tacrolimus has been reported to rapidly increase tacrolimus concentrations to toxic levels [3, 4]. The clinical signs and symptoms of tacrolimus toxicity include nephrotoxicity (increase in serum creatinine or decrease in urine output) and neurotoxicity (headache, confusion, vision changes, tremors, extremity numbness, seizures, and coma) [2]. The exact mechanism of nephrotoxicity remains unknown but may result from vasoconstriction in afferent arterioles and thrombotic microangiopathy [5]. There are a few case reports that describe tacrolimus-induced neurotoxicity [6, 7]. Careful monitoring of therapeutic and adverse effects is recommended when this medication is concomitantly administered with ritonavir.
We present a case of a patient with a history of orthotopic heart transplant who received the co-administration of nirmatrelvir/ritonavir and tacrolimus in the setting of COVID-19. The patient presented with tacrolimus toxicity associated with the concomitant use of nirmatrelvir/ritonavir and tacrolimus. Phenytoin was subsequently used as a CYP3A4 inducer to quickly correct the supratherapeutic tacrolimus level.
Case Report
A 67-year-old female presented to an outside emergency department (ED) with complaints of dyspnea and cough in the setting of a recent COVID-19 diagnosis. Her past medical history is significant for an orthotopic heart transplant in 2004 secondary to familial cardiomyopathy, chronic kidney disease (baseline creatinine 1.5 mg/dL), hypertension, hyperlipidemia, hypothyroidism, and chronic obstructive pulmonary disease. She did not receive the COVID-19 vaccine. Her prior-to-admission medications included azathioprine, carvedilol, diphenoxylate-atropine, hydrochlorothiazide, levothyroxine, losartan, pravastatin, tacrolimus, and temazepam. The patient’s goal tacrolimus level was 4–6 ng/mL. Her home tacrolimus regimen was 3 mg every morning and 2 mg every evening.
The patient was initially discharged from the outside ED with a prescription for nirmatrelvir/ritonavir. According to the prescriber, the patient was not instructed to modify her tacrolimus regimen. Three days later, the patient represented to the outside ED with slowed speech, fatigue, weakness, and loss of appetite. At this point in time, the patient had completed 4 days of nirmatrelvir/ritonavir. She was then transferred to our institution for a higher level of care.
Upon admission, her tacrolimus level was 176.4 ng/mL, and she had an acute kidney injury (serum creatinine 2.5 mg/dL, BUN 111 mg/dL). Throughout hospitalization, the patient was alert but not oriented to date or time. The toxicology, transplant, and neurology services contributed to her care. Her neurologic exam was unremarkable other than the presence of encephalopathy. Neurology recommended a brain MRI and electroencephalogram (EEG). The MRI was negative, but the EEG revealed evidence of toxic/metabolic encephalopathy with diffuse slowing and generalized periodic discharges/triphasic waves; the neurology team attributed these findings to her supratherapeutic tacrolimus drug level and uremia.
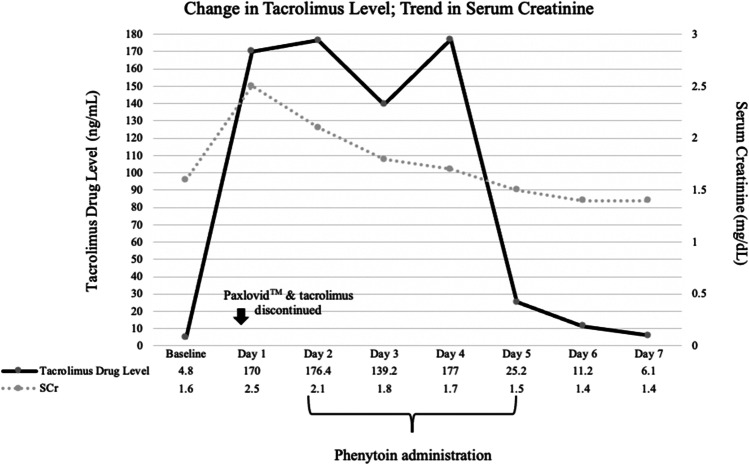
Her immunosuppressants, azathioprine and tacrolimus, were withheld throughout her admission. On the first day of her hospitalization, the team chose to initiate oral phenytoin 150 mg twice daily for its CYP3A4-inducing properties. She received a total of 7 doses of phenytoin. Five days into her admission, her tacrolimus level had returned to 6.1 ng/mL, which was slightly above her goal range (Fig. 1). She remained disoriented for several days, which resolved concomitantly with the decreasing tacrolimus level. The patient was discharged on hospital day 7 at her neurologic baseline. Tacrolimus was restarted shortly after discharge with close follow-up by her primary care provider. The dose of tacrolimus at which the patient was restarted is unclear due to follow-up at a different institution. Consent for publication of this case was obtained and provided to the journal in accordance with JMT policy.
Fig. 1.
Change in tacrolimus level in correlation to trend in serum creatinine and phenytoin administration throughout hospitalization.
Discussion
As this case illustrates, tacrolimus toxicity has high morbidity and can be precipitated by, and possibly reversed, through drug–drug interactions. The mainstay treatment of tacrolimus toxicity is supportive care as hemodialysis is ineffective due to the drug’s large volume of distribution and being 99% protein bound [2]. While limited data exists on the management of tacrolimus toxicity, several case reports describe using phenytoin for its CYP3A4-inducing property [8, 9]. Phenytoin is a dose-dependent CYP-P450 inducer meaning at higher doses, the expression of enzyme induction is enhanced. The mechanism by which phenytoin reduces tacrolimus levels through CYP3A4 is unknown, but it likely involves the activation of receptor transcription factors [10].
Enzyme induction is a gradual process. The time required for induction of an enzyme is governed by the need to either upregulate or synthesize new enzyme, assuming the elimination half-life of the inducing drug is less than the enzyme degradation half-life [10]. Evidence suggests CYP3A4 has a half-life of about 3 days [11]. However, phenytoin does not follow first-order kinetics, and its half-life increases with increasing phenytoin concentrations. The estimated half-life of phenytoin is 10–12 h. This would imply that maximal induction of CYP3A4 would likely take several weeks, although onset of induction may be evident much sooner [12].
In addition to time of exposure, the magnitude of induction of various CYP isozymes appears to be partially dependent upon the dose of the inducing drug [10]. Historically, phenytoin has been utilized at 300 to 400 mg/day for its hepatic metabolism, minimal short-term adverse effect profile, and its anti-seizure property in the setting of tacrolimus-induced neurotoxicity. Within 48 h of initiating phenytoin 300 mg/day, the patient’s tacrolimus level had decreased sevenfold, which is consistent with other reports of phenytoin induction. Phenytoin itself has the potential for several drug–drug interactions. Phenytoin induces CYP1A2, CYP2B6, CYP2C9, and CYP3A4; common drug–drug interactions with phenytoin include carbamazepine, phenobarbital, topiramate, and dexamethasone [10]. Prior to starting phenytoin for this patient, a drug–drug interaction check was completed using MicromedexⓇ.
This interaction between nirmatrelvir/ritonavir and tacrolimus has been previously described in other case reports [3, 4]; the measured tacrolimus levels, clinical manifestations, and the use of phenytoin make our case unique and reiterate the importance of avoiding nirmatrelvir/ritonavir in this high-risk patient population. Our case report also demonstrates the utility and effectiveness of phenytoin as a “rescue” medication in the setting of tacrolimus toxicity.
This case report is not without limitations. This patient was transferred from an outside institution, and the initial information prior to the patient’s arrival was limited. The patient was also lost to follow-up upon discharge from our institution making it challenging to determine the long term effects of this drug-drug interaction. Additionally, withholding tacrolimus and the CYP3A4 inhibitor, ritonavir, could have contributed to the rapid reduction in the tacrolimus level. Due to these confounding factors, we cannot directly determine a cause–effect relationship of phenytoin reducing tacrolimus levels; however, there is evidence to support phenytoin’s induction of CYP3A4. This case report supports this phenomenon.
Conclusions
This case highlights the very strong and important drug–drug interaction between tacrolimus and nirmatrelvir/ritonavir and the use of the pharmacokinetic inducer, phenytoin, to accelerate the clearance of tacrolimus. The list of possible nirmatrelvir/ritonavir drug–drug interactions through CYP inhibition or induction is extensive, and awareness of these interactions is imperative for patient safety, especially for a medication with limited efficacy. A list of drug–drug interactions can be found in the nirmatrelvir/ritonavir package insert [1]. Vigilant monitoring and awareness of tacrolimus levels is essential to prevent toxicity with its narrow therapeutic window and highly variable pharmacokinetics.
Sources of Funding
This case report did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declarations
Ethical Approval
Consent for publication of this case was obtained and provided to the journal in accordance with JMT policy.
Conflict of Interest
MS, EC, and DM declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Paxlovid™ package insert. Pfizer. New York, NY 2020.
- 2.PrografTM package insert. Astellas Pharma US, Inc. Deerfield, IL 2012.
- 3.Prikis M, Cameron A. Paxlovid™ (nirmatrelvir/ritonavir) and tacrolimus drug-drug interaction in transplant patient with SARS-2CoV infection: a case report. Transplantation Proc. 2022;54(6):1557–1560. 10.1016/j.transproceed.2022.04.015. [DOI] [PMC free article] [PubMed]
- 4.Yanay NB, Bogner I, Saker K, Tannous E. Paxlovid-tacrolimus drug-drug interaction in a 23-year-old female kidney transplant patient with COVID-19. Clin Drug Invest. 2022;42:693–695. doi: 10.1007/s40261-022-01180-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Naesens M, Kuypers DRJ, Sarwal M. Calcineurin inhibitor nephrotoxicity. CJASN. 2009;4(2):481–508. doi: 10.2215/CJN.04800908. [DOI] [PubMed] [Google Scholar]
- 6.Eidelman BH, Abu-Elmagd K, Wilson J, Fung JJ, Alessiani M, Jain A, Takaya S, Todo SN, Tzakis A, Van Thiel D, et al. Neurologic complications of KF 506. Transplant Proc. 1991;23(6):3175–3178. [PMC free article] [PubMed] [Google Scholar]
- 7.Boeve BF, Kimmel DW, Aronson AE, de Groen PC. Dysarthria and apraxia of speech associated with FK-506 (tacrolimus) Mayo Clin Proc. 1996;71(10):969–972. doi: 10.1016/S0025-6196(11)63771-3. [DOI] [PubMed] [Google Scholar]
- 8.Jantz AS, Patel SJ, Suki WN, Knight RJ, Bhimaraj A, Gaber AO. Treatment of acute tacrolimus toxicity with phenytoin in solid organ transplant recipients. Case Rep Transplant. 2013;2013:375263. doi: 10.1155/2013/375263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharma A, Wahby KA, Inany M, Lee SJ. Use of phenytoin for treatment of tacrolimus toxicity with superimposed sepsis. BMJ Case Reports CP. 2020;13:e234839. doi: 10.1136/bcr-2020-234839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brodie MJ, Mintzer S, Pack AM, Gidal BE, Vecht CJ, Schmidt D. Enzyme induction with antiepileptic drugs: cause for concern? Epilepsia. 2013;1:11–27. doi: 10.1111/j.1528-1167.2012.03671. [DOI] [PubMed] [Google Scholar]
- 11.Magnusson MO, Dahl ML, Cederberg J, Karlsson MO, Sandstrom R. Pharmacodynamics of carbamazepine-mediated induction of CYP3A4, CYP1A2, and Pgp as assessed by probe substrates midazolam, caffeine, and digoxin. Clin Pharmacol Ther. 2008;84:52–62. doi: 10.1038/sj.clpt.6100431. [DOI] [PubMed] [Google Scholar]
- 12.Kanebratt KP, Andersson TB. HepaRG cells as an in vitro model for evaluation of cytochrome P450 induction in humans. Drug Metab Dispos. 2008;36:137–145. doi: 10.1124/dmd.107.017418. [DOI] [PubMed] [Google Scholar]