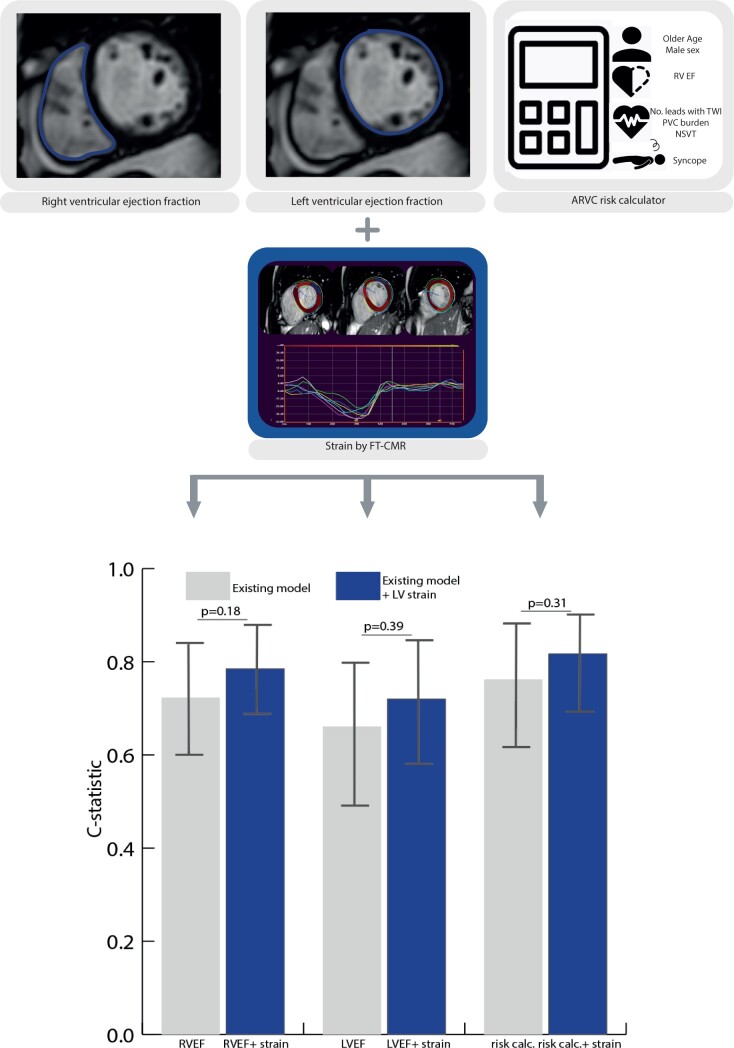
Abstract
Aims
Arrhythmogenic right ventricular cardiomyopathy (ARVC) is characterized by ventricular dysfunction and ventricular arrhythmias (VA). Adequate arrhythmic risk assessment is important to prevent sudden cardiac death. We aimed to study the incremental value of strain by feature-tracking cardiac magnetic resonance imaging (FT-CMR) in predicting sustained VA in ARVC patients.
Methods and results
CMR images of 132 ARVC patients (43% male, 40.6 ± 16.0 years) without prior VA were analysed for global and regional right and left ventricular (RV, LV) strain. Primary outcome was sustained VA during follow-up. We performed multivariable regression assessing strain, in combination with (i) RV ejection fraction (EF); (ii) LVEF; and (iii) the ARVC risk calculator. False discovery rate adjusted P-values were given to correct for multiple comparisons and c-statistics were calculated for each model. During 4.3 (2.0–7.9) years of follow-up, 19% of patients experienced sustained VA. Compared to patients without VA, those with VA had significantly reduced RV longitudinal (P ≤ 0.03) and LV circumferential (P ≤ 0.04) strain. In addition, patients with VA had significantly reduced biventricular EF (P ≤ 0.02). After correcting for RVEF, LVEF, and the ARVC risk calculator separately in multivariable analysis, both RV and LV strain lost their significance [hazard ratio 1.03–1.18, P > 0.05]. Likewise, while strain improved the c-statistic in combination with RVEF, LVEF, and the ARVC risk calculator separately, this did not reach statistical significance (P ≥ 0.18).
Conclusion
Both RV longitudinal and LV circumferential strain are reduced in ARVC patients with sustained VA during follow-up. However, strain does not have incremental value over RVEF, LVEF, and the ARVC VA risk calculator.
Keywords: arrhythmogenic right ventricular cardiomyopathy, cardiac magnetic resonance imaging, feature tracking, strain, arrhythmias
Graphical Abstract
Graphical Abstract.
Introduction
Arrhythmogenic right ventricular cardiomyopathy (ARVC) is an inherited heart disease that is characterized by ventricular dysfunction and life-threatening ventricular arrhythmias (VA).1 Right ventricular (RV) abnormalities predominate in ARVC, but left ventricular (LV) involvement is increasingly recognized.2 Although early detection of ARVC has improved over the years, risk stratification remains challenging. Adequate assessment of arrhythmic risk is important, since arrhythmias may occur early in the disease course and timely implantable cardioverter-defibrillator (ICD) implantation can be life-saving.3
Cardiac magnetic resonance (CMR) imaging is the non-invasive gold standard for the evaluation of cardiac function in ARVC given its excellent potential to accurately and reproducibly quantify global RV volumes and ejection fraction (EF). In addition, newer techniques such as myocardial strain provide a more sensitive, quantitative evaluation of myocardial function that can detect functional changes before a relevant decrease in EF occurs.4 Myocardial strain can be measured using feature-tracking CMR (FT-CMR), which quantitatively tracks myocardial features throughout the cardiac cycle on standard cine imaging. This allows quantitative regional myocardial strain assessment, which has been shown to increase diagnostic value for ARVC disease detection in a previous study.5 As lower RVEF is associated with higher arrhythmic risk in ARVC,6 and strain parameters are more sensitive than RVEF in assessing regional myocardial function,4 we hypothesized that RV strain also has incremental prognostic value over conventional arrhythmic risk markers in ARVC.6 However, the value of FT-CMR-derived strain in ARVC risk stratification remains unknown.
The purpose of this study was to (i) assess whether FT-CMR of the RV and LV is able to predict future sustained VA and (ii) evaluate the incremental value of FT-CMR over traditional arrhythmic risk factors in a multicentre cohort of ARVC patients without prior sustained VA (i.e. primary prevention patients).
Methods
Study population
We included definite ARVC patients without prior sustained VA (i.e. primary prevention patients) from the Netherlands (www.acmregistry.nl) and Johns Hopkins (www.arvd.com) ARVC registries who underwent CMR as part of their clinical work-up. ARVC diagnosis was defined according the 2010 revised task force criteria (TFC) in which ≥4 TFC points are required for ARVC diagnosis.7 A total of 158 patients met the inclusion criteria, of whom 26 patients were excluded due to CMR images unsuitable for FT-CMR analysis (e.g. artefacts/incomplete images), leading to a total cohort of 132 patients. A total of 108 patients were included in prior studies involving CMR analysis in ARVC.8–10 The study conforms to the Helsinki declaration and was approved by local ethics and/or institutional review boards.
CMR acquisition
The CMR study closest to date of diagnosis was used for analyses. CMR images were acquired on a 1.5 Tesla scanner [Avanto, Siemens Medical Imaging, Germany (Amsterdam UMC, UMC Groningen and Johns Hopkins Hospital) or Achieva Philips Medical Systems, the Netherlands (UMC Utrecht)]. Short-axis and longitudinal-axis (four-chamber, two-chamber, and three-chamber views of both ventricles) cine images were acquired using a balanced steady-state free precession sequence [field of view 350 mm, matrix size 256 × 256, slice thickness 8 mm (1.3 × 1.3 × 8 mm3), temporal resolution 40–50 ms]. Segmented phase-sensitive inversion recovery sequence was used for myocardial fibrosis evaluation using late gadolinium enhancement (LGE).
CMR analysis
Traditional measurements
Locally available software was used for semi-automatic analysis of biventricular EF, end diastolic volume (EDV), and end systolic volume (ESV, Extended MR-WorkSpace, Philips Medical Systems; QMass Medis Medical Imaging Systems or Circle CVI). Dimensions were indexed (i) to body surface area using the DuBois formula.11 The presence of RV and LV LGE was visually evaluated by an experienced cardiovascular radiologist.
Strain analysis
Global and regional biventricular longitudinal strain and LV circumferential strain were measured using Medis QStrain Software (Medis Medical Imaging Systems, version 3.1.16.8, the Netherlands) by an experienced observer blinded to the arrhythmic outcome (MB). Inter- and intra-observer variability of this observer are previously published.8 Endocardial and epicardial contours (for LV short-axis) were manually drawn during end-diastole and end-systole with subsequent automatic tracking during the cardiac cycle. This resulted in the measurement of ‘strain’ as a marker of tissue shortening during systole with more negative strain values indicating better contraction.
For the RV, peak longitudinal strain was measured in the most central slice in 4-chamber view, since strain values are most reliable in this view.5,12 For regional analysis, segments were divided into basal, mid, and apical wall.13
For the LV, peak longitudinal strain was measured in four-chamber, two-chamber, and three-chamber views to form the 16-segment American Heart Association (AHA) model.14 To measure LV circumferential strain, the basal, mid, and apical slices of the short-axis were measured to form the AHA model. Segmentation examples are included in Supplementary data online, Figure S1.
Study outcomes
The primary outcome of this study was the occurrence of sustained VA following CMR. As in previous studies,9 sustained VA was defined as sustained ventricular tachycardia lasting ≥30 s at ≥100 bpm or with haemodynamic compromise, ventricular fibrillation/flutter, sudden cardiac arrest, and/or appropriate ICD intervention.
Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics (version 25, USA) and STATA (version 11, StatCorp, USA). Continuous variables are presented as mean (±standard deviation) or median (interquartile range), and compared using independent sample t-test or Mann–Whitney U-test. Categorical variables are presented as frequencies (%), and compared using χ2 test or Fisher’s exact test. Statistical significance was determined at P 0.05. The Kaplan–Meier method was used to estimate the cumulative proportion of patients with VA as a function over time, and groups were compared using log-rank statistic. Follow-up was calculated from the date of CMR to the date of first sustained VA or censoring, which was defined as the latest follow-up visit at which the endpoint could be ascertained. The receiver operating characteristic (ROC) curve was used to determine optimal strain cut-off values for predicting the outcome.
Multivariable analysis
Multivariable Cox regression was performed to assess the association between strain and the primary outcome. To ensure that our results were not affected by over-fitting, we added each strain variable to three separate models as follows: (i) Model 1: adjusted for RVEF; (ii) Model 2: adjusted for LVEF; and (iii) Model 3: adjusted for the 5-year risk estimate of VA computed using the ‘ARVC VA risk calculator’ (www.arvcrisk.com).9 This latter risk prediction model includes male sex, age, recent cardiac syncope, prior non-sustained ventricular tachycardia, 24-h premature ventricular contraction count, number of leads with T-wave inversion anterior/inferior, and RVEF. False discovery rate (FDR) corrected P-values were calculated to correct for multiple testing. The prognostic performance of adding strain [strain parameters with the highest hazard ratio (HR) in univariable analysis] to RVEF, LVEF, or the ARVC VA risk calculator was assessed by Harrell’s concordance (c)-statistic and compared using the DeLong et al.15 method.
Results
Study population
CMR images of 132 definite ARVC patients without prior sustained VA were included. Baseline characteristics of the study population are shown in Table 1. The mean age at CMR was 40.6 ± 16.0 years and 57 (43%) subjects were male. The median TFC score was 5 (4–6) with 78 (60%) having minor or major structural TFC. A total of 107 (81%) subjects carried a pathogenic mutation, mostly in plakophilin-2 (n = 84, 64%), followed by phospholamban (n = 13, 10%), and desmoglein-2 (n = 5, 4%). None of the patients had an ICD at time of CMR, while n = 68 (52%) patients received an ICD for primary prevention after CMR.
Table 1.
Baseline characteristics of the study population
| Overall | No sustained VA in follow-up | Sustained VA in follow-up | P-value | |
|---|---|---|---|---|
| (n = 132) | (n = 107) | (n = 25) | ||
| Demographics | ||||
| Age at CMR (years) | 40.6 ± 16.0 | 40.8 ± 16.1 | 39.9 ± 15.7 | 0.80 |
| Male (%) | 57 (43) | 40 (37) | 17 (68) | <0.01 |
| Follow-up (years) | 4.3 (2.0–7.9) | 4.5 (2.1–7.8) | 3.4 (1.5–8.3) | 0.99 |
| Proband (%) | 44 (33) | 29 (27) | 15 (60) | <0.01 |
| Genetic status | ||||
| Pathogenic variant | 107 (81) | 87 (81) | 20 (80) | 0.88 |
| PKP2 (%) | 84 (64) | 67 (63) | 17 (68) | |
| DSP (%) | 2 (2) | 2 (2) | 0 | |
| DSG2 (%) | 5 (4) | 5 (5) | 0 | |
| PLN (%) | 13 (10) | 11 (10) | 2 (8) | |
| Other (%) | 3 (2) | 2 (2) | 1 (4) | |
| Clinical phenotype | ||||
| Total TFC score | 5 (4–6) | 5 (4–5) | 6 (5–7) | <0.01 |
| Repolarization criteria | ||||
| Minor | 31 (24) | 24 (22) | 7 (28) | |
| Major | 51 (39) | 37 (35) | 14 (56) | |
| Depolarization criteria | ||||
| Minor | 68 (52) | 55 (51) | 13 (52) | |
| Major | 6 (5) | 4 (4) | 2 (8) | |
| Arrhythmia criteria | ||||
| Minor | 78 (59) | 61 (57) | 17 (68) | |
| Major | 13 (10) | 8 (8) | 5 (20) | |
| Structural criteria | ||||
| Minor | 21 (16) | 18 (17) | 3 (12) | |
| Major | 58 (44) | 42 (39) | 16 (64) | |
| Family/genetic criteria | ||||
| Minor | 4 (3) | 3 (3) | 1 (4) | |
| Major | 103 (78) | 85 (79) | 18 (72) | |
| ARVC VA risk calculator, 5-year risk (%) | 21.4 ± 18.9 | 17.3 ± 14.5 | 38.9 ± 24.8 | <0.01 |
| CMR traditional parameters | ||||
| RVEF (%) | 47 ± 9 | 48 ± 9 | 40 ± 10 | <0.01 |
| RVEDVi (mL/m2) | 102 ± 30 | 100 ± 29 | 111 ± 32 | 0.15 |
| LVEF (%) | 56 ± 8 | 57 ± 7 | 51 ± 11 | 0.02 |
| LVEDVi (mL/m2) | 92 ± 20 | 92 ± 21 | 89 ± 17 | 0.55 |
| LGE total (%) | 40 (30) | 27 (25) | 13 (52) | 0.02 |
| LGE RV (%) | 24 (18) | 14 (13) | 11 (44) | <0.01 |
| LGE LV (%) | 20 (15) | 15 (14) | 5 (20) | 0.57 |
CMR, cardiac magnetic resonance; DSG2, desmoglein-2; DSP, desmoplakin; EDVi, BSA indexed end-diastolic volume; EF, ejection fraction; LGE, late gadolinium enhancement; LV, left ventricle; N, number of subjects; PKP2, plakophilin-2; PLN, phospholamban; RV, right ventricle; TFC, Task Force Criteria; VA, ventricular arrhythmia. Boldface values are statistically significant (p ≤ 0.05).
Arrhythmic outcome
During a median follow-up of 4.3 (2.0–7.9) years, 25 subjects (19%) developed sustained VA [22 (88%) appropriate ICD interventions and 3 (12%) spontaneous sustained ventricular tachycardia]. Table 1 shows their clinical characteristics. Compared to those without sustained VA, patients experiencing arrhythmic events were more often male (68% vs. 37%, P < 0.01), proband (60% vs. 29%, P < 0.01), and had a significantly higher total TFC score [6 (5–7) vs. 5 (4–5) P < 0.01]. No difference in age (39.9 ± 15.7 vs. 40.8 ± 16.1 years, P = 0.80), genetic background (80% vs. 81% with a pathogenic variant, P = 0.88), and follow-up duration [3.4 (1.5–8.3) vs. 4.5 (2.1–7.8) years, P = 0.99] was observed between the groups.
Traditional CMR parameters
As for traditional CMR parameters, ARVC patients with sustained VA had significantly reduced RVEF (40 ± 10% vs. 48 ± 9%, P < 0.01) and LVEF (51 ± 11% vs. 57 ± 7%, P = 0.02) compared to those without sustained VA. LGE was more often present in patients with sustained VA (52% vs. 25%, P = 0.02), especially in the RV (44% vs. 13%, P < 0.01). In contrast, both RVEDVi (111 ± 32 vs. 100 ± 29 mL/m2) and LVEDVi (89 ± 17 vs. 92 ± 21 mL/m2) did not significantly differ between the two groups (P ≥ 0.15). In addition, no significant difference existed in structural TFC between patients with (76% had minor or major criteria) and without (56% had minor or major criteria) sustained VA (P = 0.08).
FT-CMR as a predictive biomarker for sustained VA
RV and LV strain
Global and regional strain values stratified by the occurrence of sustained VA are shown in Table 2 (global and regional strain) and Figure 1 (regional strain).
Table 2.
RV and LV global and regional strain values stratified by patients with vs. without VA
| No sustained VA in follow-up | Sustained VA in follow-up | P-value | |
|---|---|---|---|
| (n = 107) | (n = 25) | ||
| Right ventricular strain | |||
| Global strain | |||
| GLS | −22.5 ± 8.4 | −18.5 ± 5.9 | 0.03 |
| Regional longitudinal strain | |||
| Basal | −34.4 ± 10.9 | −26.9 ± 12.8 | <0.01 |
| Mid | −25.7 ± 11.0 | −19.7 ± 12.2 | 0.02 |
| Apical | −32.5 ± 11.9 | −27.8 ± 14.5 | 0.09 |
| Left ventricular strain | |||
| Global strain | |||
| GCS | −18.8 ± 4.1 | −15.3 ± 4.4 | <0.01 |
| GLS | −21.7 ± 5.1 | −19.2 ± 5.3 | 0.06 |
| Regional circumferential strain | |||
| Anterior | −18.7 ± 6.2 | −15.3 ± 5.8 | 0.03 |
| Anterolateral | −22.2 ± 6.9 | −17.5 ± 6.2 | <0.01 |
| Posterolateral | −23.7 ± 6.2 | −18.9 ± 5.7 | <0.01 |
| Inferior | −19.6 ± 6.7 | −16.0 ± 7.5 | 0.04 |
| Septal | −20.2 ± 4.5 | −16.7 ± 4.8 | <0.01 |
| Regional longitudinal strain | |||
| Anterior | −20.0 ± 7.3 | −19.6 ± 6.6 | 0.86 |
| Anterolateral | −24.4 ± 6.1 | −23.0 ± 7.0 | 0.40 |
| Posterolateral | −23.3 ± 10.8 | −24.4 ± 9.1 | 0.77 |
| Inferior | −23.5 ± 7.2 | −20.3 ± 6.0 | 0.08 |
| Septal | −21.7 ± 5.5 | −19.4 ± 4.1 | 0.24 |
GCS, global circumferential strain; GLS, global longitudinal strain; VA, ventricular arrhythmia. Boldface values are statistically significant (p ≤ 0.05).
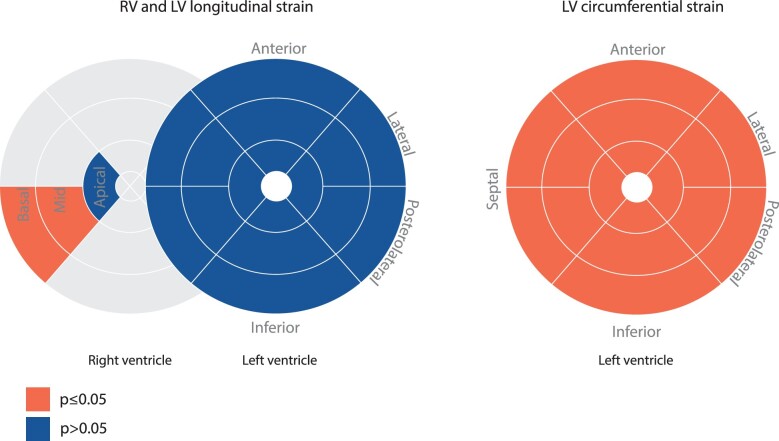
Figure 1.
RV and LV regional strain in the study population. Schematic overview of longitudinal (left) and circumferential (right) regional strain differences between patients with and without VA during follow-up. Orange; significant differences between those with and without VA. Blue; non-significant differences. LV, left ventricle; RV, right ventricle; VA, ventricular arrhythmia.
For RV strain, global longitudinal strain was significantly reduced (i.e. less negative) in patients with vs. without sustained VA (−18.5 ± 5.9% vs. −22.5 ± 8.4%, P = 0.03). For regional RV longitudinal strain, both basal (−26.9 ± 12.8% vs. −34.4 ± 10.9%, P < 0.01) and mid wall (−19.7 ± 12.2% vs. −25.7 ± 11.0%, P = 0.02) strain were reduced in patients with sustained VA. Apical strain did not significantly differ between the two groups (−27.8 ± 14.5% vs. −32.5 ± 11.9%, P = 0.09).
For LV strain, both global and regional LV longitudinal strains were comparable in patients with and without sustained VA (P ≥ 0.06). In contrast, LV global circumferential strain (GCS) was significantly reduced in patients with vs. those without sustained VA (−15.3 ± 4.4% vs. −18.8 ± 4.1%, P < 0.01), which was also observed in the LV regional circumferential strain values (P ≤ 0.04).
Predicting sustained VA using FT-CMR
Determination of cut-off values for abnormal strain using ROC analysis is displayed in Supplementary data online, Table S1. We only evaluated cut-off values for RV longitudinal and LV circumferential strain, since these parameters were significant in univariate analysis (in contrast to LV longitudinal strain). The resulting cut-offs were used for the Kaplan–Meier survival curves shown in Figure 2.
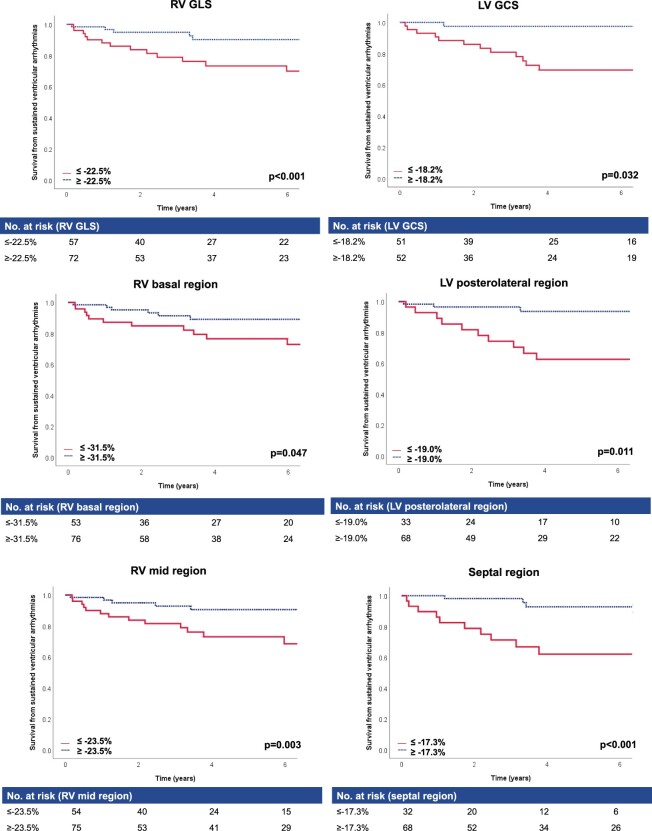
Figure 2.
Kaplan–Meier survival analysis suggests abnormal RV and LV strain in patients with VA in follow-up. Kaplan–Meier analysis of RV and LV global and regional strain. Cut-offs for abnormal strain (red) and normal strain (blue) are calculated using ROC analysis. P-values were calculated using log-rank test. GCS, global circumferential strain; GLS, global longitudinal strain; LV, left ventricle; ROC, receiver operating characteristic; RV, right ventricle; VA, ventricular arrhythmia.
For RV strain, survival without sustained VA was significantly lower in patients with reduced global longitudinal (P < 0.01) and regional basal (P = 0.05) and mid longitudinal strain (P < 0.01).
For LV strain, survival without sustained VA was significantly lower in patients with reduced global circumferential (P = 0.03) and regional posterolateral (P = 0.01) and septal (P < 0.01) circumferential strain. No statistical significance was reached for anterior and anterolateral (P ≤ 0.11) strain.
Clinical value of FT-CMR
To assess the incremental prognostic value of FT-CMR over traditional clinical parameters, we performed multivariable Cox regression analyses for each strain parameter in combination with (i) RVEF, (ii) LVEF, and (iii) the ARVC VA risk calculator.
Table 3 summarizes the univariable and multivariable regression analyses. RV and LV global and regional strain did not remain significant predictors after correcting for RVEF (HR 1.02–1.17, P > 0.05), LVEF (HR 1.06–1.18, P > 0.10), or the ARVC VA risk calculator (HR 1.05–1.18, P > 0.11). All P-values are corrected for Type I error using FDR. When only including patients with preserved RVEF and LVEF in our analysis (Supplementary data online, Table S2), none of the RV and LV strain values were independently associated with VA when included in a model with the ARVC VA risk calculator [HR 0.92–1.17 (0.80–1.52, P > 0.29)], although analyses were underpowered with a total of eight events.
Table 3.
Univariable and multivariable Cox proportional hazards model for sustained VA prediction
| Univariable model | Multivariable model 1 | Multivariable model 2 | Multivariable model 3 | |||||
|---|---|---|---|---|---|---|---|---|
| RVEF | LVEF | ARVC VA risk calculator | ||||||
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Right ventricular strain | ||||||||
| GLS | 1.05 (1.00–1.11) | 0.053 | ||||||
| Basal region | 1.06 (1.02–1.11) | 0.015 | 1.03 (0.98–1.08) | 0.238 | 1.06 (1.01–1.10) | 0.099 | 1.05 (1.00–1.10) | 0.107 |
| Mid region | 1.05 (1.01–1.10) | 0.037 | 1.02 (0.98–1.07) | 0.312 | 1.04 (1.00–1.09) | 0.099 | 1.03 (0.98–1.08) | 0.240 |
| Apical region | 1.03 (1.00–1.07) | 0.097 | ||||||
| Left ventricular strain | ||||||||
| GCS | 1.22 (1.07–1.39) | 0.015 | 1.17 (1.02–1.35) | 0.054 | 1.18 (0.99–1.42) | 0.099 | 1.18 (1.02–1.35) | 0.107 |
| Anterior region | 1.12 (1.01–1.24) | 0.047 | 1.10 (0.99–1.23) | 0.105 | 1.08 (0.97–1.21) | 0.152 | 1.10 (0.98–1.23) | 0.177 |
| Anterolateral region | 1.12 (1.03–1.23) | 0.015 | 1.10 (1.00–1.20) | 0.081 | 1.09 (0.99–1.20) | 0.116 | 1.09 (1.00–1.19) | 0.118 |
| Posterolateral region | 1.15 (1.04–1.26) | 0.020 | 1.12 (1.02–1.24) | 0.054 | 1.11 (1.00–1.23) | 0.099 | 1.10 (1.00–1.22) | 0.110 |
| Inferior region | 1.09 (1.00–1.19) | 0.053 | ||||||
| Septal region | 1.18 (1.07–1.31) | 0.015 | 1.16 (1.02–1.32) | 0.054 | 1.15 (0.99–1.32) | 0.099 | 1.17 (1.02–1.33) | 0.107 |
GCS, global circumferential strain; GLS, global longitudinal strain; LVEF, left ventricular ejection fraction; RVEF, right ventricular ejection fraction. False discovery rate corrected P-values are given in this table. Boldface values are statistically significant (p ≤ 0.05).
Figure 3 illustrates the change in c-statistic when comparing the models separately and after adding LV global and septal circumferential strain (strain parameters with highest HR on univariable analysis). The predictive value of RVEF [0.72 (0.60–0.85) vs. 0.79 (0.68–0.89)], LVEF [0.66 (0.51–0.81) vs. 0.72 (0.59–0.85)], and the ARVC VA risk calculator [0.76 (0.63–0.90) vs. 0.82 (0.72–0.92)] improved after adding LV strain (global strain and septal circumferential strain) to the model, however this did not reach statistical significance (P > 0.18).
Figure 3.
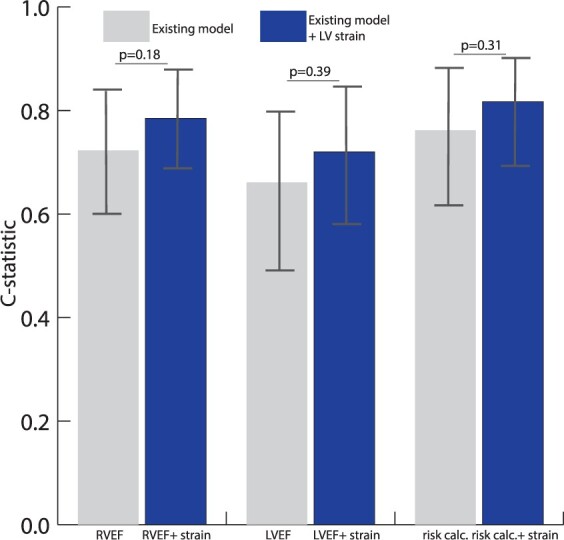
Incremental value of LV strain over conventional arrhythmic risk markers in ARVC. Bar chart with c-statstic per model. Grey bars; model 1 RVEF, model 2 LVEF, and model 3 ‘ARVC VA risk calculator’. Blue bars; addition of LV global and septal strain to these models. Addition of LV strain to the existing models is compared using the DeLong method.15 ARVC, arrhythmogenic right ventricular cardiomyopathy; LVEF, left ventricular ejection fraction; RVEF, right ventricular ejection fraction; VA, ventricular arrhythmia.
LGE was more often present in patients with VA compared to those without arrhythmic events (52% vs. 25%, P = 0.02). However, LGE did not significantly add to the predictive value of strain [0.73 (0.60–0.85) without vs. 0.77 (0.64–0.91) with LGE ] and the ARVC VA risk calculator [0.79 (0.69–0.90) without vs. 0.80 (0.70–0.91) with LGE ] (P ≥ 0.40) (Supplementary data online, Figure S2).
Discussion
Main findings
This study aimed to assess FT-CMR as a predictor of future sustained VA and to evaluate its incremental value over traditional risk markers in ARVC patients. We showed that both RV as well as LV strain are reduced in patients developing sustained VA during follow-up. Furthermore, survival without VA was significantly lower in patients with reduced RV and LV global and regional strain (basal and mid strain for the RV and posterolateral and septal strain for the LV). However, after correcting for RVEF, LVEF, and the ARVC VA risk calculator and adjusting for multiple testing, RV and LV strain did not remain a significant predictor of sustained VA.
Role of myocardial strain in ARVC
Technical groundwork
Over the years, the advent of FT-CMR in the field of deformation imaging has led to numerous studies confirming its feasibility and validating its use for biventricular regional strain assessment. Importantly, FT-CMR has shown to be a robust technique, with good inter- and intra-observer reproducibility for RV and LV strain, rendering this technique suitable for follow-up of patients.13,16 FT-CMR has been compared to several other modalities, including the gold standard for non-invasive strain assessment, myocardial tissue tagging.17 Clinical implementation of tissue tagging is limited due to prolonged imaging and post-processing times.18 In comparison, FT-CMR is less time consuming, as it uses the available cine images and has a more user-friendly post-processing method, especially for the RV. FT-CMR has also been compared to speckle tracking echocardiography which is a valuable comparison as CMR and echocardiography are both used for diagnosis and follow-up of ARVC patients and at-risk relatives. Studies have shown that trends between healthy and diseased were uniform among the modalities, however absolute strain values were not comparable.19,20 This emphasizes that FT-CMR and speckle tracking echocardiography cannot be used interchangeably during follow-up of patients. Regardless, we strongly believe that CMR and echocardiography have complimentary roles in ARVC evaluation: the high spatial resolution and multiplane tissue characterization of CMR make this technique extremely useful as a screening tool and to rule out differentials, while echocardiography is cheap and widely available, even in those with an ICD, making it a valuable tool for longitudinal follow-up.
Diagnostic value of strain parameters in ARVC
The importance of regional wall motion abnormalities in ARVC evaluation is emphasized in the diagnostic TFC, in which it is a prerequisite for fulfilment of CMR criteria.7 Visual evaluation of wall motion abnormalities is, however, subjective, and previous studies have shown the incremental diagnostic value of objective and quantitative wall motion analysis using FT-CMR or speckle tracking echocardiography.5,21 For example, Vigneault et al.5 showed a higher sensitivity and specificity for FT-CMR compared to visual assessment in 110 individuals evaluated for ARVC. Similar results were obtained for speckle tracking echocardiography, which is now recommended by the European Association of Cardiovascular Imaging for the assessment of early ARVC.21 Although LV wall motion abnormalities are not part of the diagnostic TFC for ARVC, a study by Jain et al.22 suggested a promising diagnostic role using CMR tissue tagging: the authors showed reduced LV regional circumferential strain in definite ARVC patients and patients at-risk of developing ARVC compared to controls.
Prognostic value of strain parameters in ARVC
Our study shows that reduced RV and LV strain are associated with sustained VA during follow-up in ARVC. This is not surprising, as RVEF is a known predictor of sustained VA in ARVC, and abnormal strain is thought to precede global EF changes. Similar results were previously obtained using speckle tracking echocardiography23: Lie et al.23 found significant echocardiographic RV and LV strain abnormalities (expressed as mechanical dispersion) in ARVC patients with VA in follow-up.
In contrast, we found no incremental prognostic value of RV and LV strain after correcting for RVEF and LVEF using FDR adjusted P-values for multiple testing. Of note, advanced structural disease already existed in the majority of patients developing VA: we found significantly lower RVEF and LVEF in patients developing VA during follow-up of whom 76% already had minor or major structural TFC. Indeed, this translated to a high expected 5-year VA risk of 38.9% using the ARVC VA risk calculator. While one may consider it disappointing that strain does not further risk stratify beyond conventional measures, it is not entirely unexpected since (i) strain essentially assesses the same parameter as is included in the conventional measures, namely ventricular systolic function; and (ii) arrhythmic risk in our cohort was already very high. In total, 19% of our study population experienced a VA over 4.2 years of follow-up. While one might suggest that this high event rate warrants ICD implantation in all subjects with definite ARVC, device implantation carries considerable risk in these young and active patients who need to live for decades with a device that is not complication free. As such, better risk stratification tools are required to distinguish patients who are most likely to benefit from their device. While there is proven value of adding strain to established CMR parameters for diagnostic purposes,5 no incremental value is observed in adding strain values for prognostic purposes in ARVC patients. Future studies should focus on the additional prognostic value of strain in subjects at risk of developing ARVC (i.e. family members) without disease expression.
Limitations
We only included primary prevention patients with definite ARVC, and caution should be exercised when extrapolating our results to secondary prevention patients (i.e. those with previous sustained VA) or at-risk relatives who do not fulfil the TFC. While this is the largest study to date evaluating the prognostic value of FT-CMR in ARVC, the number of VAs during follow-up was relatively small, limiting our statistical power to perform multivariable analyses. We handled this by separately adding strain to three different models (i.e. RVEF, LVEF, and the ARVC VA risk calculator), maximizing our ability to ‘correct’ for multiple risk factors. Future studies should look into the role of LGE taking into account scar quantification and localization. To date, no standardized normal values for FT-CMR derived RV and LV global and regional strain exist, which is partly due to wide inter-software variability.13 Until standardized reference values are available, centre-specific references should be used. Furthermore, the prognostic value of strain using other imaging modalities, such as speckle tracking echocardiography, should be determined.
To conclude, FT-CMR is a novel technique that quantitatively and objectively measures biventricular wall motion as strain. In the largest cohort to date of primary prevention ARVC patients evaluated by CMR, we showed that FT-CMR is able to predict sustained VA. However, after adjusting for RVEF, LVEF, and the ARVC VA risk calculator no additional value of RV and LV strain assessment in the prediction of sustained VA was observed. Although strain by FT-CMR has proven its diagnostic value in ARVC, no incremental prognostic value in predicting sustained VA is found.
Supplementary Material
Acknowledgements
We thank the ARVC patients and families who have made this work possible.
Contributor Information
M Bourfiss, Division of Cardiology, Department of Cardiology, University Medical Center Utrecht, Utrecht University, Heidelberglaan 100, 3584 CX Utrecht, The Netherlands.
N H J Prakken, Department of Radiology, University Medical Center Groningen, Hanzeplein 1, 9713 GZ Groningen, The Netherlands.
C A James, Department of Cardiology, Johns Hopkins Hospital, 1800 Orleans St, Baltimore, MD 21218, USA.
R N Planken, Department of Radiology and nuclear medicine, Amsterdam University Medical Center, Amsterdam, Location AMC, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands.
S M Boekholdt, Department of Cardiology, Amsterdam University Medical Center, Amsterdam, Location AMC, Amsterdam, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands.
D Ahmetagic, Department of Radiology, University Medical Center Utrecht, Utrecht University, Heidelberglaan 100, 3584 CX Utrecht, The Netherlands.
M P van den Berg, Department of Cardiology, University Medical Center Groningen, University of Groningen, Hanzeplein 1, 9713 GZ Groningen, The Netherlands.
C Tichnell, Department of Cardiology, Johns Hopkins Hospital, 1800 Orleans St, Baltimore, MD 21218, USA.
J F Van der Heijden, Division of Cardiology, Department of Cardiology, University Medical Center Utrecht, Utrecht University, Heidelberglaan 100, 3584 CX Utrecht, The Netherlands.
P Loh, Division of Cardiology, Department of Cardiology, University Medical Center Utrecht, Utrecht University, Heidelberglaan 100, 3584 CX Utrecht, The Netherlands.
B Murray, Department of Cardiology, Johns Hopkins Hospital, 1800 Orleans St, Baltimore, MD 21218, USA.
H Tandri, Department of Cardiology, Johns Hopkins Hospital, 1800 Orleans St, Baltimore, MD 21218, USA.
I Kamel, Department of Radiology, Johns Hopkins Hospital, 1800 Orleans St, Baltimore, MD 21218, USA.
H Calkins, Department of Cardiology, Johns Hopkins Hospital, 1800 Orleans St, Baltimore, MD 21218, USA.
F W Asselbergs, Division of Cardiology, Department of Cardiology, University Medical Center Utrecht, Utrecht University, Heidelberglaan 100, 3584 CX Utrecht, The Netherlands; Faculty of Population Health Sciences, Institute of Cardiovascular Science, University College London, Gower St, London WC1E 6BT, UK; Health Data Research UK and Institute of Health Informatics, University College London, Gower St, London WC1E 6BT, UK.
S L Zimmerman, Department of Radiology, Johns Hopkins Hospital, 1800 Orleans St, Baltimore, MD 21218, USA.
B K Velthuis, Department of Radiology, University Medical Center Utrecht, Utrecht University, Heidelberglaan 100, 3584 CX Utrecht, The Netherlands.
A S J M Te Riele, Division of Cardiology, Department of Cardiology, University Medical Center Utrecht, Utrecht University, Heidelberglaan 100, 3584 CX Utrecht, The Netherlands; Netherlands Heart Institute, Moreelsepark 1, 3511 EP Utrecht, The Netherlands.
Supplementary data
Supplementary data are available at European Heart Journal - Cardiovascular Imaging online.
Funding
Dr. M.B. is supported by the Alexandre Suerman Stipend of the UMC Utrecht (2017). Dr. A.S.J.M.T.R. is supported by the Dutch Heart Foundation (grant no. 2015T058), the UMC Utrecht Fellowship Clinical Research Talent, and the CVON PREDICT Young Talent Program. Dr. F.W.A. is supported by UCL Hospitals NIHR Biomedical Research Center. We wish to acknowledge funding from the Netherlands Cardiovascular Research Initiative, an initiative supported by the Dutch Heart Foundation (CVON2015-12 eDETECT and 2012-10 PREDICT). The Netherlands ACM Registry (www.acmregistry.nl) is supported by the Netherlands Heart Institute (project 06901). The Johns Hopkins ARVD/C Program is supported by the Leonie-Wild Foundation, the Leyla Erkan Family Fund for ARVD Research, the Dr. Francis P. Chiramonte Private Foundation, the Dr. Satish, Rupal, and Robin Shah ARVD Fund at Johns Hopkins, the Bogle Foundation, the Healing Hearts Foundation, the Campanella family, the Patrick J. Harrison Family, the Peter French Memorial Foundation, and the Wilmerding Endowments. Dr. C.A.J. was supported by Netherlands Organization for Scientific Research (NWO) 040.11.586, visitor’s travel grant.
References
- 1. Gandjbakhch E, Redheuil A, Pousset F, Charron P, Frank R.. Clinical diagnosis, imaging, and genetics of arrhythmogenic right ventricular cardiomyopathy/dysplasia: JACC state-of-the-art review. J Am Coll Cardiol 2018;72:784–804. [DOI] [PubMed] [Google Scholar]
- 2. Calkins H, Corrado D, Marcus F.. Risk stratification in arrhythmogenic right ventricular cardiomyopathy. Circulation 2017;136:2068–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Corrado D, Wichter T, Link MS, Hauer RNW, Marchlinski FE, Anastasakis Aet al. Treatment of arrhythmogenic right ventricular cardiomyopathy/dysplasia: an international task force consensus statement. Circulation 2015;132:441–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kalam K, Otahal P, Marwick TH.. Prognostic implications of global LV dysfunction: a systematic review and meta-analysis of global longitudinal strain and ejection fraction. Heart 2014;100:1673–80. [DOI] [PubMed] [Google Scholar]
- 5. Vigneault DM, Te Riele ASJM, James CA, Zimmerman SL, Selwaness M, Murray Bet al. Right ventricular strain by MR quantitatively identifies regional dysfunction in patients with arrhythmogenic right ventricular cardiomyopathy. J Magn Reson Imaging 2016;43:1132–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Canpolat U, Kabakçi G, Aytemir K, Dural M, Şahiner L, Yorgun Het al. Fragmented QRS complex predicts the arrhythmic events in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia. J Cardiovasc Electrophysiol 2013;24:1260–6. [DOI] [PubMed] [Google Scholar]
- 7. Marcus FI, McKenna WJ, Sherrill D, Basso C, Bauce B, Bluemke DAet al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Circulation 2010;121:1533–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bourfiss M, Vigneault DM, Aliyari Ghasebeh M, Murray B, James CA, Tichnell Cet al. Feature tracking CMR reveals abnormal strain in preclinical arrhythmogenic right ventricular dysplasia/cardiomyopathy: a multisoftware feasibility and clinical implementation study. J Cardiovasc Magn Reson 2017;19:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cadrin-Tourigny J, Bosman LP, Nozza A, Wang W, Tadros R, Bhonsale Aet al. A new prediction model for ventricular arrhythmias in arrhythmogenic right ventricular cardiomyopathy. Eur Heart J 2019;40:1850–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Te Riele ASJM, Bhonsale A, James CA, Rastegar N, Murray B, Burt JRet al. Incremental value of cardiac magnetic resonance imaging in arrhythmic risk stratification of arrhythmogenic right ventricular dysplasia/cardiomyopathy-associated desmosomal mutation carriers. J Am Coll Cardiol 2013;62:1761–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Du Bois D, Du Bois EF.. A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition 1989;5:303. [PubMed] [Google Scholar]
- 12. Kempny A, Fernandez-Jimenez R, Orwat S, Schuler P, Bunck AC, Maintz Det al. Quantification of biventricular myocardial function using cardiac magnetic resonance feature tracking, endocardial border delineation and echocardiographic speckle tracking in patients with repaired tetralogy of Fallot and healthy controls. J Cardiovasc Magn Reson 2012;14:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bourfiss M, Vigneault DM, Aliyari Ghasebeh M, Murray B, James CA, Tichnell Cet al. Feature tracking CMR reveals abnormal strain in preclinical arrhythmogenic right ventricular dysplasia/cardiomyopathy: a multisoftware feasibility and clinical implementation study. J Cardiovasc Magn Reson 2017;19:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WKet al. ; American Heart Association Writing Group on Myocardial Segmentation and Registration for Cardiac Imaging . Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Int J Cardiovasc Imaging 2002;105:539–42. [PubMed] [Google Scholar]
- 15. DeLong ER, DeLong DM, Clarke-Pearson DL.. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988;44:837–45. [PubMed] [Google Scholar]
- 16. Schmidt B, Dick A, Treutlein M, Schiller P, Bunck AC, Maintz Det al. Intra- and inter-observer reproducibility of global and regional magnetic resonance feature tracking derived strain parameters of the left and right ventricle. Eur J Radiol 2017;89:97–105. [DOI] [PubMed] [Google Scholar]
- 17. Hor KN, Gottliebson WM, Carson C, Wash E, Cnota J, Fleck Ret al. Comparison of magnetic resonance feature tracking for strain calculation with harmonic phase imaging analysis. JACC Cardiovasc Imaging 2010;3:144–51. [DOI] [PubMed] [Google Scholar]
- 18. Bucius P, Erley J, Tanacli R, Zieschang V, Giusca S, Korosoglou Get al. Comparison of feature tracking, fast-SENC, and myocardial tagging for global and segmental left ventricular strain. ESC Hear Fail 2020;7:523–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Taha K, Bourfiss M, Te Riele ASJM, Cramer M-JM, van der Heijden JF, Asselbergs FWet al. A head-to-head comparison of speckle tracking echocardiography and feature tracking cardiovascular magnetic resonance imaging in right ventricular deformation. Eur Heart J Cardiovasc Imaging 2020;22:950–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Obokata M, Nagata Y, Wu VC-C, Kado Y, Kurabayashi M, Otsuji Yet al. Direct comparison of cardiac magnetic resonance feature tracking and 2D/3D echocardiography speckle tracking for evaluation of global left ventricular strain. Eur Heart J Cardiovasc Imaging 2016;17:525–32. [DOI] [PubMed] [Google Scholar]
- 21. Haugaa KH, Basso C, Badano LP, Bucciarelli-Ducci C, Cardim N, Gaemperli Oet al. ; EACVI Scientific Documents Committee, EACVI Board members and external reviewers . Comprehensive multi-modality imaging approach in arrhythmogenic cardiomyopathy-an expert consensus document of the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2017;18:237–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jain A, Shehata ML, Stuber M, Berkowitz SJ, Calkins H, Lima JACet al. Prevalence of left ventricular regional dysfunction in arrhythmogenic right ventricular dysplasia: a tagged MRI study. Circ Cardiovasc Imaging 2010;3:290–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lie ØH, Rootwelt-Norberg C, Dejgaard LA, Leren IS, Stokke MK, Edvardsen Tet al. Prediction of life-threatening ventricular arrhythmia in patients with arrhythmogenic cardiomyopathy: a primary prevention cohort study. JACC Cardiovasc Imaging 2018;11:1377–86. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.