Abstract
Aims
The 2016 European Society of Cardiology Heart Failure Guidelines defined a new category: heart failure with mid-range ejection fraction (HFmrEF) of 40–49%. This new category was highlighted as having limited evidence and research was advocated into underlying characteristics, pathophysiology, and diagnosis. We used multi-parametric cardiovascular magnetic resonance (CMR) to define the cardiac phenotype of presumed non-ischaemic HFmrEF.
Methods and results
Patients (N = 300, 62.7 ± 13 years, 63% males) with a clinical diagnosis of heart failure with no angina symptoms, history of myocardial infarction, or coronary intervention were prospectively recruited. Patients underwent clinical assessment and CMR including T1 mapping, extracellular volume (ECV) mapping, late gadolinium enhancement, and measurement of myocardial blood flow at rest and maximal hyperaemia. Of 273 patients in the final analysis, 93 (34%) patients were categorized as HFmrEF, 46 (17%) as heart failure with preserved ejection fraction (HFpEF), and 134 (49%) as heart failure with reduced ejection fraction (HFrEF). Nineteen (20%) patients with HFmrEF had evidence of occult ischaemic heart disease. Diffuse fibrosis and hyperaemic myocardial blood flow were similar in HFmrEF and HFpEF, but HFmrEF showed significantly lower native T1 (1311 ± 32 vs. 1340 ± 45 ms, P < 0.001), ECV (24.6 ± 3.2 vs. 26.3 ± 3.1%, P < 0.001), and higher myocardial perfusion reserve (2.75 ± 0.84 vs. 2.28 ± 0.84, P < 0.001) compared with HFrEF.
Conclusion
Patients with HFmrEF share most phenotypic characteristics with HFpEF, including the degree of microvascular impairment and fibrosis, but have a high prevalence of occult ischaemic heart disease similar to HFrEF. Further work is needed to confirm how the phenotype of HFmrEF responds to medical therapy.
Keywords: heart failure, mildly reduced, ejection fraction, cardiovascular magnetic resonance, HFmrEF
Graphical Abstract
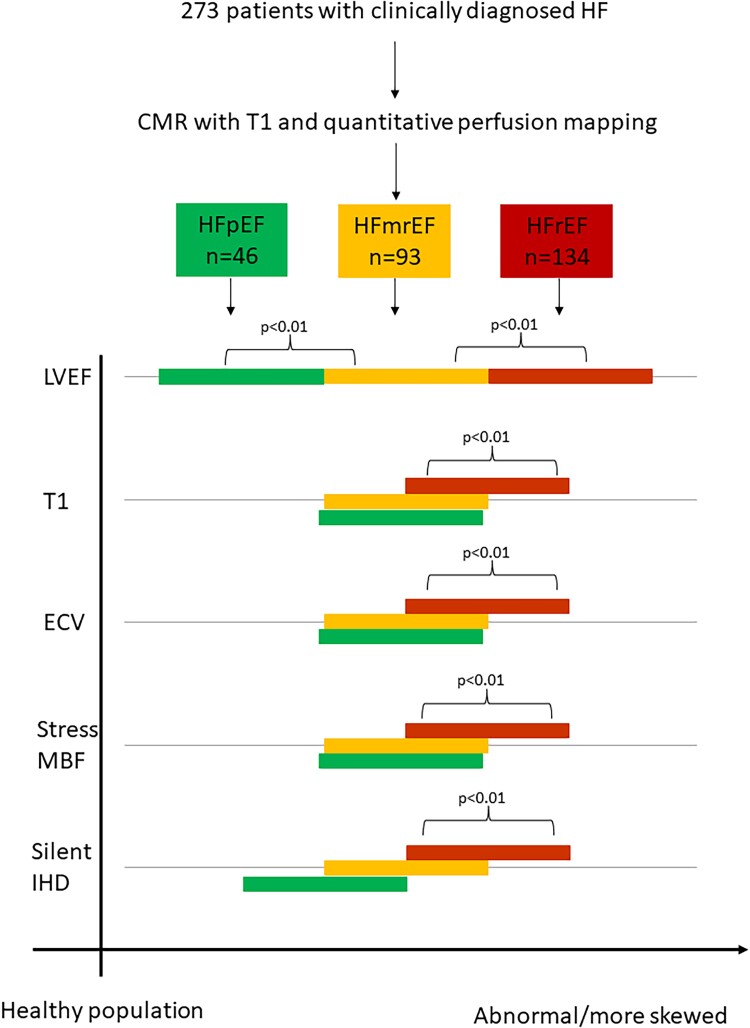
Graphical Abstract.
There is limited research into the mechanisms of heart failure with mildly reduced ejection fraction (HFmrEF, 40–49%). Using cardiovascular magnetic resonance, we have shown that patients with HFmrEF share most phenotypic characteristics with heart failure with preserved ejection fraction (HFpEF, ≥50%), including the degree of microvascular impairment and fibrosis. However, they also have a high prevalence of occult ischaemic heart disease similar to heart failure with reduced ejection fraction (HFrEF, <40%).
Introduction
Heart failure (HF) has historically been classified based on left-ventricular (LV) ejection fraction (EF) and divided into two groups: HF with reduced ejection fraction (HFrEF) and HF with preserved ejection fraction (HFpEF). The 2016 European Society of Cardiology (ESC) Heart Failure Guidelines defined a new, middle, category: HF with mid-range ejection fraction (HFmrEF) of 40–49% between HFrEF (EF ≤ 40%) and HFpEF (EF ≥ 50%),1 recently renamed as HF with mildly reduced ejection fraction.2
Heart failure with rEF and HFpEF have been shown to have different patient characteristics, outcomes, and therapeutic response.3,4 Within clinical trials, the HFmrEF group has often been excluded, split, or grouped with HFpEF.4,5 The 2016 ESC guidelines highlighted this new category as an area with a gap in evidence and advocate research into underlying characteristics, pathophysiology, and diagnosis.
The categorical classification of HF based solely on LVEF has been challenged reflecting overlap in epidemiology, pathophysiology, and clinical manifestation across the defined groups. Large epidemiological registries have shown HFmrEF to most closely resemble HFrEF in terms of age and sex distribution and presence of ischaemic heart disease (IHD), but with less ventricular and atrial dilatation.6–8
Cardiovascular magnetic resonance (CMR) can identify high risk features in HF such as inducible ischaemia, ischaemic scar, focal, and diffuse fibrosis. These characteristics have not previously been described for HFmrEF. However, across the HF spectrum, they have been shown to be potential markers of prognosis and may be helpful in guiding therapy.
We used CMR to define the cardiac phenotype of presumed non-ischaemic HFmrEF specifically comparing the prevalence of occult IHD and tissue characteristics to HFpEF and HFrEF.
Methods
Study population
Patients seen in cardiology clinics and referred for a CMR scan following a clinical diagnosis of HF were prospectively recruited (N = 300). Patients were excluded if they had a known history of coronary artery disease (stenosis >70% on angiography, myocardial infarction, previous percutaneous coronary intervention, or coronary artery bypass grafting) or symptoms of angina. Other exclusion criteria included hypertrophic cardiomyopathy, amyloidosis, congenital heart disease, advanced renal failure, or contraindication to CMR or gadolinium-based contrast agents
Patient characteristics
Patients underwent clinical assessment on the day of their CMR appointment, including medical history, New York Heart Association (NYHA) function class, risk factors, and medical management. In patients with an LVEF ≥50%, an H2FPEF score9 was calculated using previous echocardiogram results, where available, and medical history. Where the H2FPEF score indicated that a diagnosis of HFpEF was unlikely (score < 2), these patients were excluded from further analysis. Haematocrit (Hct) was measured from a blood sample taken at the time of the CMR scan.
Study protocol
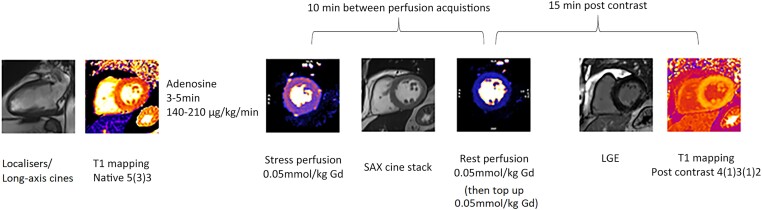
All CMR studies were undertaken on a 3T system (Siemens Magnetom Prisma, Erlangen, Germany). Participants were advised to avoid caffeine for 24 h before the study. The protocol (Figure 1) consisted of cine imaging, native, and post-contrast T1 mapping using a MOdified Look Locker Inversion recovery (MOLLI) sequence, stress and rest perfusion using free breathing, motion corrected (MOCO) automated in-line perfusion mapping,10 and MOCO bright blood late gadolinium enhancement (LGE). T1 and perfusion maps were acquired as three short axis, 8 mm slices, at basal, mid, and apical levels, with slice spacing varied on a per patient basis to cover the left ventricle. LGE images were acquired as a short-axis stack and in four-, three-, and two-chamber views. When it was unclear if enhancement seen on bright blood LGE was ischaemic, a dark blood LGE stack was also acquired.11 A full protocol is available in Supplementary data online, Supplement 1.
Figure 1.
CMR protocol. At least 10 min was left between stress and rest perfusion acquisitions. Post-contrast T1 mapping was carried out at least 15 min after contrast injection.
For perfusion imaging, adenosine was infused for a minimum of 3 min, at a rate of 140 µg/kg/min and increased up to a maximum of 210 µg/kg/min if there was insufficient haemodynamic response (heart rate increase <10 b.p.m.) or there was no symptomatic response. Images were acquired over 90 dynamics to allow for poor ventricular function. A 10 min interval was kept between perfusion acquisitions. An intravenous bolus of 0.05 mmol/kg gadobutrol (Gadovist, Leverkusen, Germany) was administered at 5 mL/s followed by a 20 mL saline flush using an automated injection pump (Medrad MRXperion Injection System, Bayer). Perfusion mapping was performed using the Gadgetron streaming software image reconstruction framework.10
Qualitative analysis
LGE was reported if enhancement was identified on two orthogonal planes or, where available, on both bright and dark blood LGE images. Ischaemic LGE was defined as involving the subendocardium in a typical coronary distribution. Inducible ischaemia was defined as a visual perfusion defect affecting >1 segment present at stress but not at rest, or matching infarct on LGE imaging, in a coronary distribution.
Quantitative analysis
T1 and perfusion maps were analysed using cvi42 software (Circle Cardiovascular Imaging, Calgary, Canada). Endocardial and epicardial borders were drawn excluding papillary muscles, right-ventricular insertion points marked, and a 16-segment American Heart Association (AHA) model was used.12 In order to minimize partial volume effect, a 10% offset was applied to endocardial and epicardial borders. T1 times and myocardial blood flow (MBF) were measured for each of the 16 segments. Where the left-ventricular outflow tract was included, or partial volume effect meant segments were too thin to contour, these segments were excluded from further analysis (184 segments in total; 4%). In order to report global microvascular function (rather than the effects of occult coronary artery disease or replacement fibrosis) segments with ischaemia or late gadolinium enhancement were also excluded from analysis. T1 times and MBF values for all remaining segments were averaged to provide a global value.
Myocardial perfusion reserve (MPR) was calculated as stress:rest MBF. Extracellular volume (ECV) was calculated using the formula ‘myocardial ECV = (1−Hct) × (ΔR1myocardium/ΔR1blood), where R1 = 1/T1’. These were calculated for each segment and averaged to provide a global value.
Statistical analysis
Analysis was performed using SPSS 23 (IBM SPSS, Armonk, NY, USA). Normality of distribution was assessed using the Shapiro–Wilk test. Data are presented as mean (±standard deviation) for continuous data and number (percentage) for categorical data.
Continuous variables were analysed using analysis of variance with post-hoc Bonferroni correction to compare groups. Categorical data were analysed using χ2 test or Fisher’s exact test. Correlation was assessed using Pearson r correlation. Statistical tests were two-tailed and P < 0.05 was considered significant. Multiple regression analysis was performed to examine the relationship between tissue characteristics and ejection fraction, age, and sex.
This study complies with the Declaration of Helsinki; the locally appointed ethics committee has approved the research protocol (Ref. 17/YH/0300) and informed consent was obtained from all subjects.
Results
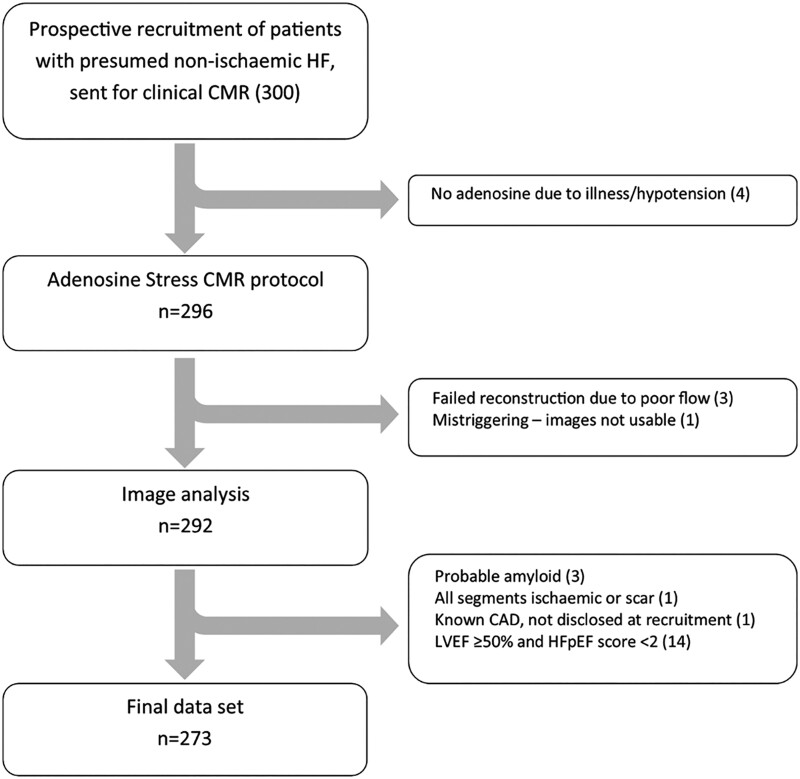
Of the 300 patients who were prospectively recruited, a total of 27 were excluded from analysis due to adenosine contraindications (n = 4), because data could not be analysed (n = 4) or because of exclusion criteria identified after the CMR scan (n = 19; Figure 2).
Figure 2.
Recruitment into study. Three hundred patients were prospectively recruited prior to clinical CMR. About 273 were included in final analysis.
Of the 273 patients included in the final analysis, 134 (49%) met criteria for HFrEF, 93 (34%) had HFmrEF, and 46 (17%) had HFpEF (HFpEF score median 4, interquartile range 3–6).
Clinical characteristics
Age and sex distribution of HFmrEF patients fell between HFpEF and HFrEF but no significant differences were seen in cardiovascular risk factors between HFmrEF patients and other groups (Table 1). Groups were comparable for co-morbidities. There were no differences in functional class between the groups. HFmrEF patients reported symptoms of breathlessness in similar proportions to HFpEF patients, and less than HFrEF patients. Diuretic and mineralocorticoid antagonist therapy was lowest in HFpEF and highest in HFrEF.
Table 1.
Clinical features
| All patients 273 |
HFpEF 46 |
HFmrEF 93 |
HFrEF 134 |
P-value | HFpEF:HFmrEF | HFmrEF:HFrEF | |
|---|---|---|---|---|---|---|---|
| Age | 62.88 ± 12.4 | 60.39 ± 11.0 | 61.2 ± 14.3 | 64.9 ± 12.3 | 0.022 | 1.0 | 0.058 |
| Female, n (%) | 104 (36.2) | 24 (52.2) | 36 (38.7) | 34 (25.3) | 0.002 | 0.132 | 0.032 |
| Hct | 43.7 (4.4) | 42.6 (4.2) | 42.9 (4.1) | 44.6 (4.4) | 0.003 | 1.0 | 0.014 |
| Symptoms | |||||||
| NYHA 1 | 183 (63.8) | 33 (71.7) | 65 (69.9) | 75 (56.0) | |||
| 2 | 90 (31.4) | 9 (19.6) | 24 (25.8) | 53 (39.6) | 0.051 | 0.460 | 0.091 |
| 3 | 14 (4.9) | 4 (8.7) | 4 (4.3) | 6 (4.5) | |||
| SOBOE | 106 (36.9) | 15 (32.6) | 28 (30.0) | 60 (44.8) | 0.059 | 0.764 | 0.026 |
| Orthopnoea | 48 (16.7) | 7 (15.2) | 11 (11.8) | 28 (20.9) | 0.189 | 0.575 | 0.075 |
| Peripheral oedema | 45 (15.7) | 8 (17.4) | 14 (15.0) | 22 (16.4) | 0.931 | 0.722 | 0.782 |
| Risk factors | |||||||
| Diabetes | 52 (18.1) | 4 (8.7) | 16 (17.2) | 29 (21.6) | 0.139 | 0.179 | 0.410 |
| Hypertension | 122 (42.5) | 18 (39.1) | 40 (43.0) | 60 (44.8) | 0.800 | 0.662 | 0.792 |
| Hypercholesterolaemia | 72 (25.1) | 9 (19.6) | 29 (31.2) | 31 (23.1) | 0.242 | 0.148 | 0.176 |
| Stroke/TIA | 38 (13.2) | 4 (8.7) | 8 (8.6) | 23 (17.2) | 0.108 | 0.985 | 0.065 |
| AF | 112 (39) | 15 (32.6) | 40 (43.0) | 57 (42.5) | 0.444 | 0.238 | 0.943 |
| Smoking history | 159 (55.4) | 23 (50.0) | 52 (55.9) | 77 (57.4) | 0.678 | 0.510 | 0.817 |
| BMI (kg/m2) | 27.96 ± 4.94 | 28.7 ± 5.30 | 28.1 ± 4.9 | 27.6 ± 4.9 | 0.420 | 1.000 | 1.000 |
| Medications | |||||||
| Antiplatelet | 55 (19.2) | 10 (21.7) | 16 (17.2) | 26 (19.4) | 0.806 | 0.519 | 0.675 |
| Beta-blocker | 225 (78.4) | 38 (82.6) | 76 (81.7) | 106 (79.6) | 0.825 | 0.898 | 0.627 |
| Statin | 119 (41.5) | 15 (32.6) | 35 (37.6) | 67 (50.3) | 0.055 | 0.561 | 0.065 |
| ACEi/ARB | 241 (84.0) | 36 (78.3) | 81 (87.1) | 117 (87.3) | 0.285 | 0.179 | 0.962 |
| MRA | 64 (22.3) | 4 (8.7) | 14 (15.1) | 45 (33.6) | <0.001 | 0.293 | 0.002 |
| Diuretic | 116 (40.4) | 10 (21.7) | 27 (29.0) | 78 (58.2) | <0.001 | 0.360 | <0.001 |
AF, atrial fibrillation; BMI, body mass index; ACEi, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; MRA, mineralocorticoid antagonist; NYHA, New York Heart Association class; SOBOE, shortness of breath on exertion; TIA, transient ischaemic attack.
HFmrEF fell between HFpEF and HFrEF for left- and right-ventricular volumes and mass (Table 2).
Table 2.
Volumetrics
| All patients 273 |
HFpEF 46 |
HFmrEF 93 |
HFrEF 134 |
P-value (all HF) | HFpEF:HFmrEF | HFmrEF:HFrEF | |
|---|---|---|---|---|---|---|---|
| LVEF (%) | 39.8 ± 12 | 55.3 ± 4.4 | 45.2 ± 2.8 | 28.9 ± 8.0 | <0.001 | <0.001 | <0.001 |
| LVEDVi (mL/m2) | 111 ± 36 | 85.0 ± 15 | 101 ± 25 | 130 ± 39 | <0.001 | <0.001 | <0.001 |
| LV mass indexed (g/m2) | 67.6 ± 19 | 58.8 ± 15 | 63.2 ± 18 | 75.5 ± 18 | <0.001 | 0.236 | <0.001 |
| RVEDVi (mL/m2) | 77.8 ± 22 | 71.3 ± 18 | 75.7 ± 20 | 81.9 ± 25 | 0.011 | 0.812 | 0.124 |
| RVEF (%) | 49.6 ± 13 | 56.8 ± 10 | 52.5 ± 10 | 44.1 ± 13 | <0.001 | 0.124 | <0.001 |
LVEDVi, indexed left-ventricular end-diastolic volume; RVEDVi, indexed right-ventricular end-diastolic volume; RVEF, right-ventricular ejection fraction.
Prevalence of occult ischaemic heart disease
Ischaemic LGE was seen in 58 patients (21%) and was more frequent in both HFmrEF and HFrEF (17 and 31%) than in HFpEF (2%), P < 0.001. Inducible ischaemia was seen in 20 (7%) overall, most commonly HFmrEF (9.7%) and HFrEF (7.5%) and less often in HFpEF (2.2%), P = 0.278. Non-ischaemic LGE was detected in 91 patients (33%) and the prevalence was not significantly different between groups. The presence of occult IHD (defined as either inducible ischaemia or ischaemic LGE) was 20% in HFmrEF and 33% in HFrEF but only 4% in the HFpEF group (P < 0.001 for trend; Table 3).
Table 3.
Presence of ischaemic heart disease or late gadolinium enhancement
| All patients 273 |
HFpEF 46 | HFmrEF 93 |
HFrEF 134 | P-value | HFpEF:HFmrEF | HFmrEF:HFrEF | |
|---|---|---|---|---|---|---|---|
| Regional ischaemia | 20 (7.3) | 1 (2.2) | 9 (9.7) | 10 (7.5) | 0.278 | 0.107 | 0.553 |
| Ischaemic LGE | 58 (21.2) | 1 (2.2) | 16 (17.2) | 41 (30.6) | <0.001 | 0.011 | 0.022 |
| IHD | 65 (23.8) | 2 (4.3) | 19 (20.4) | 44 (32.8) | <0.001 | 0.013 | 0.040 |
| Non-ischaemic LGE | 91 (33.3) | 16 (34.8) | 25 (26.9) | 50 (37.3) | 0.254 | 0.336 | 0.100 |
Ischaemic heart disease was seen in a significantly higher proportion of patients with HFmrEF compared with HFpEF, and lower compared with HFrEF.
Tissue characteristics
Native T1 was highest in HFrEF with no significant difference between HFmrEF and HFpEF; HFpEF 1310 ± 34 ms, HFmrEF 1311 ± 32 ms, and HFrEF 1340 ± 45 ms, HFpEF vs. HFmrEF P = 1.0, HFmrEF vs. HFrEF P < 0.001 (Table 4).
Table 4.
Parametric data
| All patients 273 |
HFpEF 46 |
HFmrEF 93 |
HFrEF 134 |
P-value (all HF) | HFpEF:HFmrEF | HFmrEF:HFrEF | |
|---|---|---|---|---|---|---|---|
| Native T1 (ms) | 1325 ± 41 | 1310 ± 34 | 1311 ± 32 | 1340 ± 45 | <0.001 | 1.0 | <0.001 |
| ECV (%) | 25.5 ± 3.1 | 25.0 ± 2.7 | 24.6 ± 3.2 | 26.3 ± 3.1 | <0.001 | 1.0 | <0.001 |
| Stress MBF (mL/g/min) | 1.72 ± 0.59 | 1.96 ± 0.61 | 1.89 ± 0.62 | 1.51 ± 0.50 | <0.001 | 1.0 | <0.001 |
| Rest MBF(mL/g/min) | 0.72 ± 0.22 | 0.78 ± 0.27 | 0.71 ± 0.19 | 0.70 ± 0.22 | 0.070 | 0.170 | 1.0 |
| MPR | 2.50 ± 0.84 | 2.62 ± 0.73 | 2.75 ± 0.84 | 2.28 ± 0.84 | <0.001 | 1.0 | <0.001 |
Values of native T1, ECV, and stress MBF were not significantly different between HFpEF and HFmrEF. T1 and ECV were significantly higher in HFrEF, and stress MBF significantly lower, when compared with HFmrEF.
ECV showed the same pattern with highest ECV in HFrEF (implying more interstitial expansion); HFpEF 25.0 ± 2.7%, HFmrEF 24.6 ± 3.2% and HFrEF 26.3 ± 3.1%, HFpEF vs. HFmrEF P = 1.0, HFmrEF vs. HFrEF P < 0.001.
Stress MBF showed no difference between HFmrEF and HFpEF, but significantly lower values in HFrEF then the other groups. HFpEF 1.96 ± 0.27 mL/g/min, HFmrEF 1.89 ± 0.62 mL/g/min, and HFrEF 1.51 ± 0.50 mL/g/min, HFpEF vs. HFmrEF P = 1.0, HFmrEF vs. HFrEF P < 0.001.
Within HF patients, rest MBF was not significantly different between the three groups; HFpEF 0.78 ± 0.27 mL/g/min, HFmrEF 0.71 ± 0.19 mL/g/min, and HFrEF 0.70 ± 0.22 mL/g/min, HFpEF vs. HFmrEF P = 0.170, HFmrEF vs. HFrEF P = 1.0.
MPR was not different between HFmrEF and HFpEF (P = 1.0) but was lower in HFrEF compared with HFmrEF (2.28 ± 0.84 vs. 2.75 ± 0.84 ml/g/min, P < 0.001; Table 4).
These findings remained when those with imaging evidence of occult IHD were removed from analysis (see Supplementary data online, Supplement 2).
Correlation between LVEF and tissue characteristics
When EF was considered as a continuous variable in all patients, there were weak but significant negative correlations between EF and T1 (r = −0.39, P < 0.001), and EF and ECV (r = −0.26, P < 0.001).
There was a significant correlation between stress MBF and EF (r = 0.34, P < 0.001) and MPR and EF (r = 0.30, P < 0.001). In addition, regression analysis was performed confirming the correlation of EF with T1 (or ECV) and stress MBF (or MPR) independent of age and sex (see Supplementary data online, Supplement 3).
Discussion
This study has shown that among 273 patients being investigated for the aetiology of HF, those with HFmrEF share most phenotypical characteristics including extent of diffuse fibrosis and microvascular function with HFpEF but have a similar incidence of occult IHD as patients with HFrEF.
Incidence of occult IHD
The incidence of IHD in HFmrEF in unselected HF populations has been reported to range from 41 to 61%,6,13 similar to HFrEF and higher than in HFpEF.6,7,13–16 Our cohort was more selective and excluded patients with symptoms or history of IHD and the incidence of IHD in all subgroups was therefore lower than in previous studies. However, the overall trend of IHD being more prevalent in HFmrEF than in HFpEF and similar to HFrEF remained. At 20%, the incidence of occult IHD in HFmrEF patients was comparable with that in high risk groups such as older adults17 and those with Type 2 diabetes18 with important potential prognostic implications. Silent ischaemia and infarction are associated with an increased risk of adverse cardiac events.17,19–21 The subset of patients with ischaemic HFmrEF may therefore have a worse prognosis than those with no IHD. Within our study, 26% of the HFmrEF population with occult IHD were not receiving either antiplatelet agents or anticoagulation and 60% of patients were not receiving statin therapy, suggesting opportunities for better secondary prevention therapy in this patient group22 although it remains to be shown if such treatment can modulate risk.23
Diffuse fibrosis
Within our patient group, T1 and ECV in HFmrEF were similar to HFpEF but significantly lower than in HFrEF. Previous studies have examined the significance of ECV in both preserved and reduced LV ejection fraction. In HFpEF, ECV has been shown to be increased when compared with controls, it was a predictor of disease severity such as LV stiffness24 and baseline brain natriuretic peptide,25 and was associated with adverse outcomes including hospitalization for HF and death.25,26 CMR registry data have shown that increased ECV is associated with increased risk of HF hospitalization and mortality across the spectrum of ejection fraction, including patients with HFmrEF.27 The evidence for ECV as a prognostic marker in both HFpEF and HFrEF makes it likely that it also has prognostic relevance in HFmrEF and assessment of ECV may help identify subgroups of patients for targeted anti-fibrotic treatment.
Myocardial perfusion
Patients with HFmrEF had reduced stress MBF compared with controls similar to patients with HFpEF, but significantly higher values compared with HFrEF. These findings remained consistent when those with evidence of occult IHD were removed from the analysis.
Microvascular dysfunction leading to chronic hypoperfusion and progressive deterioration in LV function has been suggested as a pathophysiological mechanism for HF.28 The correlation between EF and stress MBF that we have demonstrated supports this hypothesis, although cannot provide evidence of a causal relationship. Previous studies have shown decreased stress MBF in DCM29,30 and severe systolic HF, which has been presumed to be secondary to microvascular disease. In addition, impaired stress MBF is associated with poor prognosis in patients with LV dysfunction, independent of the level of impairment.30 This theory also presents a potential target for medical therapy to slow progression, including in patients with HFmrEF.
We found no correlation between EF and resting MBF. Some previous reports had shown reduced MBF in DCM,30,31 suggesting that it may contribute to the development of LV dysfunction through a state of chronic, low-grade hypoperfusion. However, these findings are less consistent between studies, with one PET (positron emission tomography) study showing no difference between controls and DCM groups,28 and another CMR study showing higher resting MBF in DCM compared with controls.29 Resting MBF is subject to multiple factors which can be difficult to control in clinical studies, which may explain the heterogeneity of reported correlations between resting MBF and EF.
Nomenclature
The classification of patients with HF and ejection fraction between 40 and 50% remains inconsistent. ESC guidelines classify this group as a separate entity, suggesting potential unique characteristics, while the AHA guidelines have broadly included this group with HFpEF.32 Within the AHA group, there are two categories, either HFpEF borderline (EF 40–49%) or HFpEF recovered (40–49%) with evidence of previous EF <40%. These two groups would differ significantly in treatment options, with one behaving similarly to HFpEF and unlikely to benefit prognostically from medical therapies, and the other requiring traditional HFrEF treatments to maintain this recovered EF. Distinguishing between these groups without prior imaging evidence is difficult, and tissue characteristics may differ between the two and help inform diagnosis. This distinction points to the potential importance of aetiology rather than EF as a marker for treatment, an area where CMR plays a key role, as demonstrated by the proportion of silent IHD identified in our cohort.
An alternative to the classification of HF according to LVEF is to consider it as a continuous spectrum of disease, defined by phenotype and aetiology.33,34 A key argument in support of this approach is that LVEF is not fixed but can fluctuate significantly over time. In a prospective study of patients with a diagnosis of HF, 57% of those initially categorized as HFmrEF changed their EF class within one year, with 24% reducing to HFrEF and 33% increasing in EF to the HFpEF category. Despite the change in LVEF, the underlying disease aetiology in these patients will not have changed. In this context, HFmrEF would be a ‘milder’ form of HFrEF, demonstrating impaired systolic function, and may warrant treatment with conventional HF therapies,34 indeed, retrospective analysis from previous trials have suggested that patients with HFmrEF show some benefit from treatment similar to those with HFrEF,35–37 leading to the recent change in nomenclature from ‘mid-range’ to ‘mildly reduced’.2 Our results demonstrate correlations in T1, ECV, and stress MBF with EF across all patients with HF and thus support the view of HF as a continuum with the most important distinction being its aetiology rather than the degree of LV impairment. CMR may play an important role in the identification of different phenotypes of HFmrEF to optimize their risk stratification.
Limitations
Patients in this study were prospectively recruited from clinical care, where a CMR scan had been requested by the treating clinician. This may have introduced a degree of referral bias where patients not deemed suitable for CMR may have been excluded reducing the proportion of patients with AF, more frail or elderly patients, or those with decompensated HF symptoms. A large proportion of our patients had NYHA Class I symptoms at the time of scanning, potentially because they had been commenced on appropriate medical therapy prior to their scan. This may mean that categorization of HF by EF has changed from what it would have been at first clinical diagnosis, but represents real-world assessment of this patient group. While patients were advised to avoid caffeine prior to their scan, this was not tested for, and previous studies have shown that some patients will still have detectable levels of caffeine at the time of their scan, which may influence the effects of adenosine.
Conclusion
This study provides a detailed description of the HFmrEF phenotype. Patients with HFmrEF share most phenotypic characteristics with HFpEF, including the degree of microvascular impairment and fibrosis, but have a high prevalence of occult IHD similar to HFrEF. Detailed imaging assessment of patients with HFmrEF may be appropriate to optimize secondary prevention. Further work is needed to confirm how the unique phenotype of HFmrEF responds to medical therapy.
Supplementary Material
Contributor Information
Louise A E Brown, Multidisciplinary Cardiovascular Research Centre (MCRC) and Biomedical Imaging Science Department, Leeds Institute of Cardiovascular and Metabolic Medicine, University of Leeds, Clarendon Way, Leeds LS2 9JT, UK.
Ali Wahab, Multidisciplinary Cardiovascular Research Centre (MCRC) and Biomedical Imaging Science Department, Leeds Institute of Cardiovascular and Metabolic Medicine, University of Leeds, Clarendon Way, Leeds LS2 9JT, UK.
Eunice Ikongo, Multidisciplinary Cardiovascular Research Centre (MCRC) and Biomedical Imaging Science Department, Leeds Institute of Cardiovascular and Metabolic Medicine, University of Leeds, Clarendon Way, Leeds LS2 9JT, UK.
Chirstopher E D Saunderson, Multidisciplinary Cardiovascular Research Centre (MCRC) and Biomedical Imaging Science Department, Leeds Institute of Cardiovascular and Metabolic Medicine, University of Leeds, Clarendon Way, Leeds LS2 9JT, UK.
Nicholas Jex, Multidisciplinary Cardiovascular Research Centre (MCRC) and Biomedical Imaging Science Department, Leeds Institute of Cardiovascular and Metabolic Medicine, University of Leeds, Clarendon Way, Leeds LS2 9JT, UK.
Sharmaine Thirunavukarasu, Multidisciplinary Cardiovascular Research Centre (MCRC) and Biomedical Imaging Science Department, Leeds Institute of Cardiovascular and Metabolic Medicine, University of Leeds, Clarendon Way, Leeds LS2 9JT, UK.
Amrit Chowdhary, Multidisciplinary Cardiovascular Research Centre (MCRC) and Biomedical Imaging Science Department, Leeds Institute of Cardiovascular and Metabolic Medicine, University of Leeds, Clarendon Way, Leeds LS2 9JT, UK.
Arka Das, Multidisciplinary Cardiovascular Research Centre (MCRC) and Biomedical Imaging Science Department, Leeds Institute of Cardiovascular and Metabolic Medicine, University of Leeds, Clarendon Way, Leeds LS2 9JT, UK.
Thomas P Craven, Multidisciplinary Cardiovascular Research Centre (MCRC) and Biomedical Imaging Science Department, Leeds Institute of Cardiovascular and Metabolic Medicine, University of Leeds, Clarendon Way, Leeds LS2 9JT, UK.
Eylem Levelt, Multidisciplinary Cardiovascular Research Centre (MCRC) and Biomedical Imaging Science Department, Leeds Institute of Cardiovascular and Metabolic Medicine, University of Leeds, Clarendon Way, Leeds LS2 9JT, UK.
Erica Dall’Armellina, Multidisciplinary Cardiovascular Research Centre (MCRC) and Biomedical Imaging Science Department, Leeds Institute of Cardiovascular and Metabolic Medicine, University of Leeds, Clarendon Way, Leeds LS2 9JT, UK.
Kristopher D Knott, The Cardiovascular Magnetic Resonance Imaging Unit and The Inherited Cardiovascular Diseases Unit, Barts Heart Centre, St Bartholomew’s Hospital, West Smithfield, London, UK.
John P Greenwood, Multidisciplinary Cardiovascular Research Centre (MCRC) and Biomedical Imaging Science Department, Leeds Institute of Cardiovascular and Metabolic Medicine, University of Leeds, Clarendon Way, Leeds LS2 9JT, UK.
James C Moon, The Cardiovascular Magnetic Resonance Imaging Unit and The Inherited Cardiovascular Diseases Unit, Barts Heart Centre, St Bartholomew’s Hospital, West Smithfield, London, UK.
Hui Xue, National Heart, Lung, and Blood Institute, National Institutes of Health, DHHS, Bethesda, MD, USA.
Peter Kellman, National Heart, Lung, and Blood Institute, National Institutes of Health, DHHS, Bethesda, MD, USA.
Sven Plein, Multidisciplinary Cardiovascular Research Centre (MCRC) and Biomedical Imaging Science Department, Leeds Institute of Cardiovascular and Metabolic Medicine, University of Leeds, Clarendon Way, Leeds LS2 9JT, UK.
Peter P Swoboda, Multidisciplinary Cardiovascular Research Centre (MCRC) and Biomedical Imaging Science Department, Leeds Institute of Cardiovascular and Metabolic Medicine, University of Leeds, Clarendon Way, Leeds LS2 9JT, UK.
Supplementary data
Supplementary data are available at European Heart Journal – Cardiovascular Imaging online.
Funding
This research was funded by the British Heart Foundation (RG/16/1/32092) and was supported by the National Institute for Health Research (NIHR), through the Local Clinical Research Networks and the Leeds Clinical Research Facility.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJSet al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2016;37:2129–200. [DOI] [PubMed] [Google Scholar]
- 2. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm Met al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;42:3599–726. [DOI] [PubMed] [Google Scholar]
- 3. Lam CSP, Gamble GD, Ling LH, Sim D, Leong KTG, Yeo PSDet al. Mortality associated with heart failure with preserved vs. Reduced ejection fraction in a prospective international multi-ethnic cohort study. Eur Heart J 2018;39:1770–80. [DOI] [PubMed] [Google Scholar]
- 4. Bhatia RS, Tu J V, Lee DS, Austin PC, Fang J, Haouzi Aet al. Outcome of heart failure with preserved ejection fraction in a population-based study. NEJM2006;355:260–9. [DOI] [PubMed] [Google Scholar]
- 5. Fonarow GC, Stough WG, Abraham WT, Albert NM, Gheorghiade M, Greenberg BHet al. Heart failure characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure A report from the OPTIMIZE-HF registry. JACC2007;50:768–77. [DOI] [PubMed] [Google Scholar]
- 6. Chioncel O, Lainscak M, Seferovic PM, Anker SD, Crespo-Leiro MG, Harjola VPet al. Epidemiology and one-year outcomes in patients with chronic heart failure and preserved, mid-range and reduced ejection fraction: an analysis of the ESC Heart Failure Long-Term Registry. Eur J Heart Fail 2017;19:1574–85. [DOI] [PubMed] [Google Scholar]
- 7. Fröhlich H, Rosenfeld N, Täger T, Goode K, Kazmi S, Hole Tet al. Epidemiology and long-term outcome in outpatients with chronic heart failure in Northwestern Europe. Heart 2019;105:1252–9. [DOI] [PubMed] [Google Scholar]
- 8. Koh AS, Tay WT, Teng THK, Vedin O, Benson L, Dahlstrom Uet al. A comprehensive population-based characterization of heart failure with mid-range ejection fraction. Eur J Heart Fail 2017;19:1624–34. [DOI] [PubMed] [Google Scholar]
- 9. Reddy YNV V, Carter RE, Obokata M, Redfield MM, Borlaug BA. A simple, evidence-based approach to help guide diagnosis of heart failure with preserved ejection fraction. Circulation 2018;138:861–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kellman P, Hansen MS, Nielles-Vallespin S, Nickander J, Themudo R, Ugander Met al. Myocardial perfusion cardiovascular magnetic resonance: optimized dual sequence and reconstruction for quantification. J Cardiovasc Magn Reson 2017;19:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Francis R, Kellman P, Kotecha T, Baggiano A, Norrington K, Martinez-Naharro Aet al. Prospective comparison of novel dark blood late gadolinium enhancement with conventional bright blood imaging for the detection of scar. J Cardiovasc Magn Reson 2017;19:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WKet al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the cardiac imaging committee of the council on clinical cardiology of the American Heart Association. Circulation 2002;105:539–42. [DOI] [PubMed] [Google Scholar]
- 13. Vedin O, Lam CSP, Koh AS, Benson L, Teng THK, Tay WTet al. Significance of ischemic heart disease in patients with heart failure and preserved, midrange, and reduced ejection fraction: a nationwide cohort study. Circ Hear Fail. 2017;10:e003875. [DOI] [PubMed] [Google Scholar]
- 14. Farré N, Lupon J, Roig E, Gonzalez-Costello J, Vila J, Perez Set al. Clinical characteristics, one-year change in ejection fraction and long-term outcomes in patients with heart failure with mid-range ejection fraction: a multicentre prospective observational study in Catalonia (Spain). BMJ Open 2017;7:e018719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pascual-Figal DA, Ferrero-Gregori A, Gomez-Otero I, Vazquez R, Delgado-Jimenez J, Alvarez-Garcia Jet al. Mid-range left ventricular ejection fraction: clinical profile and cause of death in ambulatory patients with chronic heart failure. Int J Cardiol 2017;240:265–70. [DOI] [PubMed] [Google Scholar]
- 16. Webb J, Draper J, Fovargue L, Sieniewicz B, Gould J, Claridge Set al. Is heart failure with mid range ejection fraction (HFmrEF) a distinct clinical entity or an overlap group? IJC Hear Vasc 2018;21:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Acharya T, Aspelund T, Jonasson TF, Schelbert EB, Cao JJ, Sathya Bet al. Association of unrecognized myocardial infarction with long-term outcomes in community-dwelling older adults: the ICELAND MI study. JAMA Cardiol 2018;3:1101–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Swoboda PP, McDiarmid AK, Erhayiem B, Ripley DP, Dobson LE, Garg Pet al. Diabetes mellitus, microalbuminuria, and subclinical cardiac disease: identification and monitoring of individuals at risk of heart failure. J Am Heart Assoc 2017;6:e005539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kwong RY, Sattar H, Wu H, Vorobiof G, Gandla V, Steel Ket al. Incidence and prognostic implication of unrecognized myocardial scar characterized by cardiac magnetic resonance in diabetic patients without clinical evidence of myocardial infarction. Circulation 2008;118:1011–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Giusca S, Kelle S, Nagel E, Buss SJ, Voss A, Puntmann Vet al. Differences in the prognostic relevance of myocardial ischaemia and scar by cardiac magnetic resonance in patients with and without diabetes mellitus. Eur Heart J Cardiovasc Imaging 2016;17:812–20. [DOI] [PubMed] [Google Scholar]
- 21. Zellweger MJ, Hachamovitch R, Kang X, Hayes SW, Friedman JD, Germano Get al. Prognostic relevance of symptoms versus objective evidence of coronary artery disease in diabetic patients. Eur Heart J 2004;25:543–50. [DOI] [PubMed] [Google Scholar]
- 22. Knuuti J, Wijns W, Achenbach S, Agewall S, Barbato E, Bax JJet al. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J 2020;41:407–77. [DOI] [PubMed] [Google Scholar]
- 23. Selvanayagam JB, Hartshorne T, Billot L, Grover S, Hillis GS, Jung Wet al. Cardiovascular magnetic resonance-GUIDEd management of mild to moderate left ventricular systolic dysfunction (CMR GUIDE): study protocol for a randomized controlled trial. Ann Noninvasive Electrocardiol 2017;22:e12420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rommel KP, Von Roeder M, Latuscynski K, Oberueck C, Blazek S, Fengler Ket al. Extracellular volume fraction for characterization of patients with heart failure and preserved ejection fraction. J Am Coll Cardiol 2016;67:1815–25. [DOI] [PubMed] [Google Scholar]
- 25. Schelbert EB, Fridman Y, Wong TC, Abu Daya H, Piehler KM, Kadakkal Aet al. Temporal relation between myocardial fibrosis and heart failure with preserved ejection fraction: association with baseline disease severity and subsequent outcome. JAMA Cardiol 2017;2:995–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kanagala P, Cheng ASH, Singh A, Khan JN, Gulsin GS, Patel Pet al. Relationship between focal and diffuse fibrosis assessed by CMR and clinical outcomes in heart failure with preserved ejection fraction. JACC Cardiovasc Imaging 2019;12:2291–301. [DOI] [PubMed] [Google Scholar]
- 27. Schelbert EB, Piehler KM, Zareba KM, Moon JC, Ugander M, Messroghli DRet al. Myocardial fibrosis quantified by extracellular volume is associated with subsequent hospitalization for heart failure, death, or both across the spectrum of ejection fraction and heart failure stage. J Am Heart Assoc 2015;4:e002613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Van Den Heuvel AFM, Van Veldhuisen DJ, Van Der Wall EE, Blanksma PK, Siebelink HMJ, Vaalburg WMet al. Regional myocardial blood flow reserve impairment and metabolic changes suggesting myocardial ischemia in patients with idiopathic dilated cardiomyopathy. J Am Coll Cardiol 2000;35:19–28. [DOI] [PubMed] [Google Scholar]
- 29. Gulati A, Ismail TF, Ali A, Hsu L-Y, Gonçalves C, Ismail NAet al. Microvascular dysfunction in dilated cardiomyopathy. JACC Cardiovasc Imaging 2019;12:1699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Neglia D, Michelassi C, Giovanna Trivieri M, Sambuceti G, Giorgetti A, Pratali Let al. Prognostic role of myocardial blood flow impairment in idiopathic left ventricular dysfunction. Circ 2002;105:186–93. [DOI] [PubMed]
- 31. Jerosch-Herold M, Sheridan DC, Kushner JD, Nauman D, Burgess D, Dutton Det al. Cardiac magnetic resonance imaging of myocardial contrast uptake and blood flow in patients affected with idiopathic or familial dilated cardiomyopathy. Am J Physiol Hear Circ Physiol 2008;295:1234–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MHet al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol 2013;62:e147–239. [DOI] [PubMed] [Google Scholar]
- 33. Triposkiadis F, Butler J, Abboud FM, Armstrong PW, Adamopoulos S, Atherton JJet al. The continuous heart failure spectrum: moving beyond an ejection fraction classification. Eur Heart J 2019;40:2155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lam CSP, Voors AA, Piotr P, McMurray JJV, Solomon SD. Time to rename the middle child of heart failure: heart failure with mildly reduced ejection fraction. Eur Heart J 2020;41:2353–5. [DOI] [PubMed] [Google Scholar]
- 35. Lund LH, Claggett B, Liu J, Lam CS, Jhund PS, Rosano GMet al. Heart failure with mid-range ejection fraction in CHARM: characteristics, outcomes and effect of candesartan across the entire ejection fraction spectrum. Eur J Heart Fail 2018;20:1230–9. [DOI] [PubMed] [Google Scholar]
- 36. Abdul-Rahim AH, Shen L, Rush CJ, Jhund PS, Lees KR, McMurray JJV. Effect of digoxin in patients with heart failure and mid-range (borderline) left ventricular ejection fraction. Eur J Heart Fail 2018;20:1139–45. [DOI] [PubMed] [Google Scholar]
- 37. Cleland JGF, Bunting K V., Flather MD, Altman DG, Holmes J, Coats AJSet al. Beta-blockers for heart failure with reduced, mid-range, and preserved ejection fraction: an individual patient-level analysis of double-blind randomized trials. Eur Heart J 2018;39:26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.