Abstract
The anterior optic pathway is one of the preferential sites of involvement in CNS inflammatory demyelinating diseases, such as multiple sclerosis and neuromyelitis optica, with optic neuritis being a common presenting symptom. What is more, optic nerve involvement in these diseases is often subclinical, with optical coherence tomography demonstrating progressive neuroretinal thinning in the absence of optic neuritis. The pathological substrate for these findings is poorly understood and requires investigation. We had access to post-mortem tissue samples of optic nerves, chiasms and tracts from 29 multiple sclerosis (mean age 59.5, range 25–84 years; 73 samples), six neuromyelitis optica spectrum disorders (mean age 56, range 18–84 years; 22 samples), six acute disseminated encephalomyelitis (mean age 25, range 10–39 years; 12 samples) cases and five non-neurological controls (mean age 55.2, range 44–64 years; 16 samples). Formalin-fixed paraffin-embedded samples were immunolabelled for myelin, inflammation (microglial/macrophage, T- and B-cells, complement), acute axonal injury and astrocytes. We assessed the extent and distribution of these markers along the anterior optic pathway for each case in all compartments (i.e. parenchymal, perivascular and meningeal), where relevant. Demyelinated plaques were classified as active based on established criteria. In multiple sclerosis, demyelination was present in 82.8% of cases, of which 75% showed activity. Microglia/macrophage and lymphocyte inflammation were frequently found both in the parenchymal and meningeal compartments in non-demyelinated regions. Acute axonal injury affected 41.4% of cases and correlated with extent of inflammatory activity in each compartment, even in cases that died at advanced age with over 20 years of disease duration. An antero-posterior gradient of anterior optic pathway involvement was observed with optic nerves being most severely affected by inflammation and acute axonal injury compared with the optic tract, where a higher proportion of remyelinated plaques were seen. In neuromyelitis optica spectrum disorder, cases with a history of optic neuritis had extensive demyelination and lost aquaporin-4 reactivity. In contrast, those without prior optic neuritis did not have demyelination but rather diffuse microglial/macrophage, T- and B-lymphocyte inflammation in both parenchymal and meningeal compartments, and acute axonal injury was present in 75% of cases. Acute demyelinating encephalomyelitis featured intense inflammation, and perivenular demyelination in 33% of cases. Our findings suggest that chronic inflammation is frequent and leads to neurodegeneration in multiple sclerosis and neuromyelitis optica, regardless of disease stage. The chronic inflammation and subsequent neurodegeneration occurring along the optic pathway broadens the plaque-centred view of these diseases and partly explains the progressive neuroretinal changes observed in optic coherence tomography studies.
Keywords: multiple sclerosis, neuromyelitis optica spectrum disorder, optic nerve, optic pathway, pathology
Pisa et al. show that CNS demyelinating diseases display extensive inflammation along the anterior optic pathway. Inflammation beyond the lesional milieu is associated with acute axonal injury even after long-standing, end-stage disease in MS and in the absence of a history of optic neuritis or demyelination in NMOSD.
Introduction
Anterior optic pathway involvement is a common feature in inflammatory demyelinating diseases of the CNS, such as multiple sclerosis and neuromyelitis spectrum disorders. In multiple sclerosis, optic neuritis is the presenting symptom in 25% and affects up to 70% throughout their disease course.1 Moreover, subclinical optic nerve demyelination is increasingly frequent with longer disease duration,2,3 being invariably present at end-stage disease.4 In neuromyelitis optica spectrum disorders, optic neuritis is the presenting symptom in 50% of patients and is typically more severe and longitudinally extensive compared with multiple sclerosis and is rarely subclinical.5,6 Extensive anterior optic pathway involvement in neuromyelitis optica has been observed with involvement of the intracranial portion of the optic nerve, the chiasm and the optic tract being seen in the majority.7
In the last decade, the introduction of optical coherence tomography into clinical practice has cast light onto the nature of optic pathway involvement in multiple sclerosis and neuromyelitis optica spectrum disorders. In multiple sclerosis, progressive neuro-retinal atrophy suggestive of neurodegeneration is a characteristic feature,8 occurs in the absence of a history or subclinical evidence of optic neuritis and associates with measures of disease activity,9,10 physical and cognitive disability11 and atrophy in the brain and spinal cord.12–14 Progressive neuro-retinal atrophy and reduced integrity of the optic pathway have also been observed in neuromyelitis optica,15–17 even in patients who never experienced optic neuritis. These findings challenge the plaque-centred view of anterior optic pathway degeneration in these inflammatory demyelinating diseases.
Post-mortem studies evaluating the anterior optic pathway in inflammatory demyelinating diseases have focused on the extent, distribution, and associated features of demyelination in multiple sclerosis4,18–20 and neuromyelitis optica.6 However, the pathological substrate for progressive neurodegeneration of the anterior optic pathway in multiple sclerosis and neuromyelitis optica, illuminated by recent optic coherence tomography studies, is not known and is the focus of the current study.
We hypothesized that chronic inflammation in the anterior optic pathway leads to neurodegeneration, even in the absence of demyelination in CNS diseases where demyelination is a main feature. To address this, we characterized the distribution and extent of demyelination, inflammation and neurodegeneration in anterior optic pathways in an internationally exceptional cohort of multiple sclerosis, neuromyelitis spectrum disorders and acute disseminated encephalomyelitis post-mortem cases. We show that anterior optic pathway demyelination is frequent, can occur early and be highly inflammatory across the spectrum of inflammatory demyelinating diseases and associates with acute axonal injury. Parenchymal inflammation in non-lesional white matter is also common and associates with axonal injury in multiple sclerosis even at long disease duration and in neuromyelitis spectrum disorders in the absence of demyelination or a clinical history of optic neuritis. We describe an anteroposterior pathology severity gradient in multiple sclerosis with the optic nerve being more commonly affected than the optic chiasm and tract. These findings broaden the plaque-centred view of anterior optic pathway neurodegeneration in these diseases and provides a plausible mechanism for the progressive neuroretinal changes observed in optic coherence tomography studies obtained during life.
Materials and methods
Study population
A human autopsy cohort encompassing the spectrum of inflammatory demyelinating CNS diseases was derived from the Oxford Brain Bank, with relevant ethics committee approval (REC 15/SC/0639). Pathologically confirmed cases of multiple sclerosis, neuromyelitis optica spectrum disorders, acute disseminated encephalomyelitis and non-neurological controls were selected based on availability of anterior optic pathway samples (optic nerves, chiasms and optic tracts). The anterior optic pathway was routinely collected during autopsy of CNS demyelinating diseases and, occasionally, in other conditions, regardless of a prior history of visual symptoms. Clinical information, including age, sex, disease duration and history of optic neuritis were included in sensitivity analyses, where relevant.
Neuropathological evaluation
Formalin-fixed, paraffin-embedded tissue blocks were cut into 6 µm-thick adjacent sections for immunohistochemistry using optimized methods.21 Briefly, adjacent sections were baked at 60°C for 20 min, deparaffinized in xylene and rehydrated with successive ethanol baths before removal of endogenous peroxidase and appropriate antigen retrieval procedures. Adjacent sections were incubated with primary antibodies for: myelin (proteolipid protein, PLP), microglial/macrophage inflammation (CD68/PG-M1), T-lymphocytes (CD3), B-lymphocytes (CD20), complement deposition (C9neo), acute axonal injury (beta-amyloid precursor protein, β-APP), glial fibrillary acid protein (GFAP) and aquaporin-4 channel (AQP4), as specified in Supplementary Table 1, with subsequent labelling with secondary antibody and 3,3′-diaminobenzidine (DAB) visualization.21 Sections were counter-stained with haematoxylin. Omission of the primary antibody was performed to confirm specificity of the immunoreaction. Observers were blind to disease category. Immunohistochemistry sections were imaged and visualized using AxioVision software (v4.9.1, Zeiss) and digitally scanned using Aperio ScanScope AT Turbo system (Leica Biosystems) at ×400 magnification for downstream analyses.
Demyelination
Areas of demyelination were defined as complete loss of myelin in PLP-stained sections. The total area of demyelination in the sample was related to total sampled area. Optic tract samples sometimes included areas of grey matter derived from the lateral geniculate nucleus, which were not considered.
Stage of lesion (active, mixed active/inactive and inactive) was determined using established criteria based on the intensity and distribution of microglial infiltrate in demyelinated regions, as described elsewhere.22,23 ‘Active’ lesions were those with evidence of activity (i.e. active and active/inactive) compared with ‘inactive’ lesions. Areas of remyelination were defined as sharply demarcated areas of thinned myelin on PLP staining.
Inflammation
Microglial/macrophage (CD68+) and T-lymphocyte (CD3+) infiltrates were quantified in each sample distinguishing the demyelinated lesion, peri-lesional and non-lesional white matter areas. In order to do so, PLP stained slides were used as reference for the positioning of the regions of interest in the CD68 and CD3 stained slides. The regions of interest were 1 mm × 0.5 mm in size (0.5 mm2) and were placed using two methods: (i) For areas of demyelination with surrounding non-lesional areas, a region of interest was placed in the lesion centre, border and non-lesional white matter area (defined as a distance greater than 0.5 mm from the lesion border), whenever the area was sufficiently large to fit a region of interest; (ii) Systematic analysis of the whole sampled area was undertaken by randomly superimposing a 3 mm × 3 mm grid onto the sample, wherein an additional area of interest was placed in each square not already sampled in (i). Areas with tissue artifacts (damaged tissue, small wrinkles, etc.) were avoided.
For each region of interest, a semi-quantitative scoring method was used to estimate the extent of microglial/macrophage (CD68), T-cell (CD3) inflammation for each compartment, as follows: Perivascular inflammation was quantified as: 0 = no cells; 1 = 1–3 cells; 2 = ≥4 cells; 3 = presence of perivascular cuffs; Parenchymal inflammation was quantified as: 0 = no inflammatory cells; 1 = average of one positively-labelled cell per 200 × field (≈7.1 cells/mm2); 2 = average of 2–4 positively-labelled cells per 200 × field (≈14.3–28.5 cells/mm2); 3 = average >4 positively-labelled cells per 200 × field (>28.5 cells/mm2), as previous described.24 Microglial/macrophage inflammation (CD68) was quantified using a semi-automated colour-based extraction software and expressed as chromogen-positive pixels/mm2.25 In each sample, the average CD68 and CD3 semi-quantitative score was calculated in each compartment (i.e. parenchyma and perivascular) for each area (i.e. lesion, border, non-lesional white matter). In the meningeal compartment, CD68 and CD3 immunoreactivity was analysed in each sample as follows: 0 = similar to controls; 1 = mildly increased compared to controls; 2 = severely increased compared to controls. Given the rare occurrence of CD20 immunoreactive cells in all compartments, the qualitative characteristics of these cells were described with the samples classified based on the presence/absence of these cell types.
Astrocytes—GFAP and AQP4
GFAP and AQP4 staining was assessed both qualitatively and quantitatively, the latter using an optimized automated positive pixel-count analysis, in the same regions of interest used for the scoring of the inflammatory infiltrates.
Acute axonal injury
Samples were classified according to the presence of acute axonal injury, which was defined as presence of β-APP positive axons. Where present, an optimized semi-automated colour-based extraction method was used to quantify the extent of β-APP immunoreactivity.25 The entire sampled area was considered due to the non-homogenous expression of β-APP immunoreactivity taking into account demyelination status. Therefore, the sum total of β-APP immunoreactive positive pixels was related to the total sampled area (pixels/mm2).
Statistical analyses
Data are presented as number/proportion of samples, given that each case contributed with a different number of samples. When considering the relation between clinical or demographic variables, proportion of donors has also been reported together with the proportion of samples to facilitate interpretation.
Analyses were performed with SPSS statistics 26.0 (IBM SPSS, NY, USA). The association between presence/absence of pathological features and disease type or dichotomous variable was tested using Pearson chi-square or Fisher’s exact test (if expected count <5), while a Linear-by-Linear association analysis was used when considering the association with ordinal variables (e.g. meningeal inflammation: no, mild, severe; plaque activity: active, mixed active-inactive, chronic inactive). In order to test the differences between continuous variables, such as inflammatory scores (both semi-quantitative scores and pixel count), β-APP pixel count, percentage demyelination and nominal or ordinal variables (such as disease type, plaque activity, sampled type), we used a generalized estimating equation model (GEE). This model allows to take into account intra-subject variability to permit modelling of characteristics of a populations/cohort from multiple observations taken from each case (i.e. values/observations that are not independent but rather clustered by case). This is of particular importance in the current study, given that each subject contributed a different number of samples with all sampled areas being adjacent within the same pathway.26 Generalized estimating equation is a semi-parametric test that uses a quasi-likelihood function to estimate a population-average of the parameters and can handle normal and non-normal distributions. A corrected robust covariance matrix estimator was used with the assumption of independent within-subject dependency.
To test the difference of a continuous variable (semi-quantitative and quantitative inflammatory markers, β-APP, GFAP and AQP4 positive pixel counts) between different classes of a factor variable (e.g. lesion stage, sample type, presence of meningeal inflammation), we used a linear generalized estimating equation model with the continuous variable as dependent, and the factor as independent variable. The mean value and confidence interval (CI, 95%) of the dependent variable in each class are reported, except for the inflammatory markers, which are reported in the Supplementary material. The Wald chi-square of the association with the independent factor and the P-value of the pairwise comparison is reported. P-values were corrected for multiple comparisons using the last significant difference method. To determine the predictors of meningeal inflammation, we used a binary logistic generalized estimating equation model with presence of meningeal inflammation as the dependent variable and parenchymal inflammatory scores as independent variables in univariate models. The Wald chi-square of the association with each predictor and its P-value are reported. Only P-values <0.05 were considered significant.
Data availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Results
Demographics
A total of 46 cases with 123 anterior optic pathway samples were included for study as follows: multiple sclerosis, n = 29 cases with 73 samples (34 nerves, 19 chiasms, 20 tracts); neuromyelitis optica spectrum disorders, n = 6 cases with 22 samples (eight nerves, five chiasms, nine tracts); acute disseminated encephalomyelitis, n = 6 cases with 12 samples (two nerves, three chiasms, seven tracts); and non-neurological controls, n = 5 cases with 16 samples (eight nerves, two chiasms, six tracts). Meninges were available in 78.9% of samples included in the study (in particular: multiple sclerosis 83.6%; neuromyelitis optica 72.7%; acute disseminated encephalomyelitis 50%; controls 87.5%. Demographic characteristics of the study cohort are presented in Table 1.
Table 1.
Demographic and clinical characteristics
| Controls | MS | NMOSD | ADEM | |
|---|---|---|---|---|
| Sex, n | F3; M2 | F21; M8 | F6; M0 | F2; M4 |
| Age, years (mean) | 55.2 (44–64) | 59.48 (25–84) | 56 (18–84) | 25 (10–39) |
| Disease duration (mean) | — | 12.22 years (0.33–32) | 9 years (1–15) | 6.98 days (2–14) |
| PMI, h (mean) | 84 (72–96) | 54.36 (24–264) | N/A | 128 (96–168) |
ADEM = acute disseminated encephalomyelitis; F = female; M = male; MS = multiple sclerosis; N/A = not available; MNOSD = neuromyelitis optica spectrum disorder; PMI = post-mortem interval
Acute disseminated encephalomyelitis cases were younger with shorter disease duration than multiple sclerosis and neuromyelitis optica spectrum disorder cases. All acute disseminated encephalomyelitis cases presented with a fulminant disease course with death occurring on average within one week from disease onset. All neuromyelitis optica spectrum disorder cases tested positive for AQP4 antibodies except for one donor who died before the anti-AQP4 assay was available but who had typical clinical, radiographic and pathological features of AQP4-positive neuromyelitis optica spectrum disorder. Of the confirmed AQP4-positive cases (n = 5), all experienced recurrent longitudinally extensive myelitis with four having no history of visual symptoms or evidence of optic neuritis. An AQP4-positive neuromyelitis optica spectrum disorder case (n = 1) and the pathologically confirmed case without AQP4 status available had recurrent longitudinally extensive transverse myelitis and optic neuritis. Information on anti-MOG antibody status was only available in the three most recent AQP4-positive cases, and these were all negative. Serum for the three historical samples (of which two tested AQP4-positive during life) were not available.
Demyelination
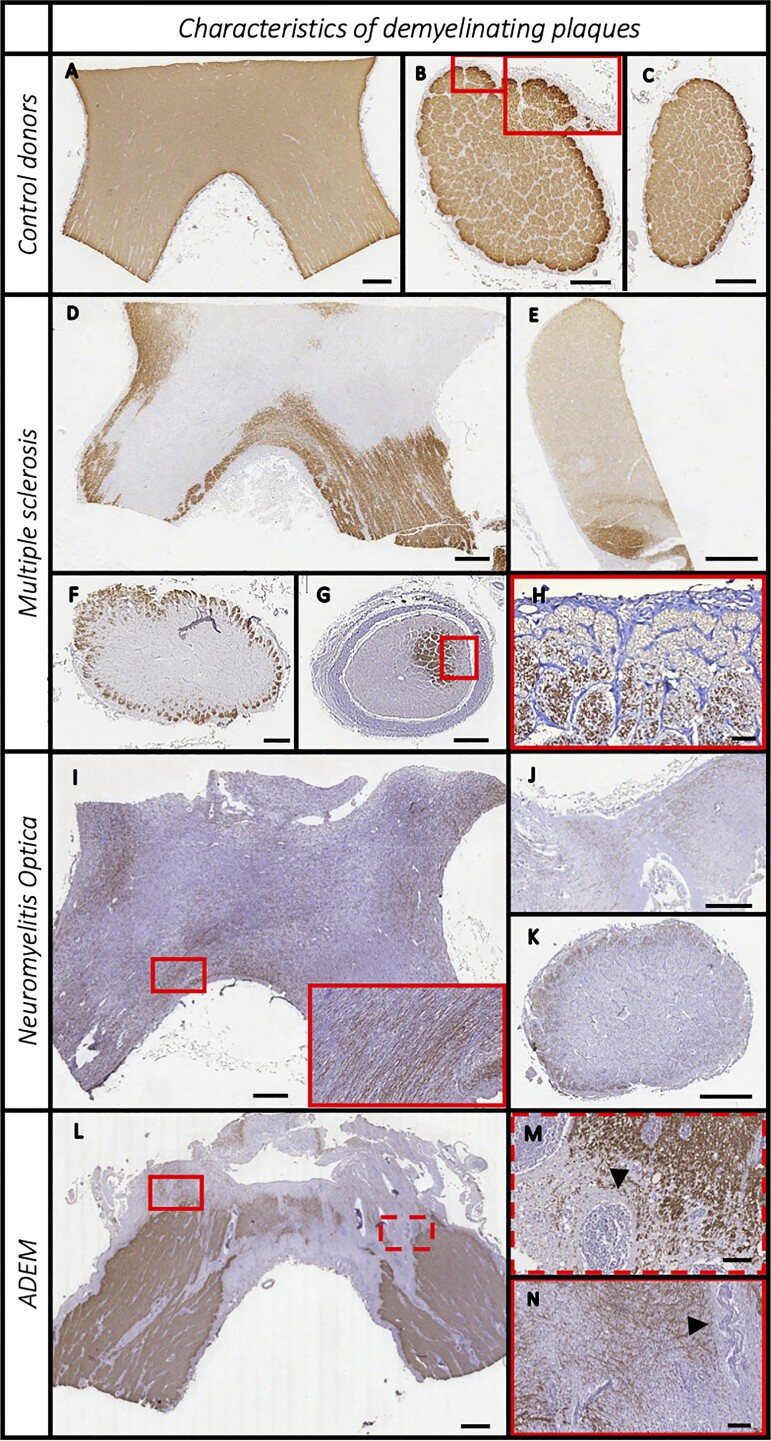
In non-neurological controls, the parenchyma showed a densely packed and linearly organised pattern of PLP expression throughout the length of the anterior optic pathway. No morphological differences in myelin were observed between the optic nerves, chiasms and tracts. A rim of absent PLP staining was invariably present in the glia limitans of the subpial zone and in the connective tissue sheaths surrounding axonal bundles in the optic nerve and tract. No control cases showed evidence of demyelination (Fig. 1).
Figure 1.
Features of demyelinating plaques (PLP staining) in the pre-geniculate optic pathway. Non-neurological donors (A–C), multiple sclerosis (D–G), neuromyelitis optica spectrum disorder (H–J) and acute disseminated encephalomyelitis (K–M) cases. Controls display densely packed PLP-positive fibres, and a rim of absent PLP staining in the glia limitans of the subpial zone and in the connective tissue sheaths surrounding axonal bundles (A–C). Multiple sclerosis samples often presented multiple and confluent plaques (D) and a very heterogenous patter of involvement with either central (F) or sub-pial demyelination (G, with rotated magnification image in H). In 13% of samples, remyelinated shadow plaques were also observed (E, faint PLP stained plaque at the top). The neuromyelitis optica spectrum disorder cases showed extensive demyelination, with some sparing of PLP-positive fibres invariably located in the sub-pial area (I–K). Acute disseminated encephalomyelitis (ADEM) cases displayed a typical perivenular demyelination around hyper-cellular perivascular spaces (L, indicated by arrowheads in the two magnifications M and N). Scale bar = 1 mm, except H, M and N where scale bar = 100 µm.
Multiple sclerosis
In multiple sclerosis, anterior optic pathway demyelination occurred in 46 (63%) samples from 24 (82.8%) cases. Demyelination showed heterogeneous patterns in terms of extent of the area involved, number and location of plaques (Fig. 1).
Mean lesion area was 52.6% (range 1% to 100%), and lesions were often multiple when present. Lesions commonly involved the central part of the anterior optic pathway with relative sparing of the subpial zone (41.3% of samples); a subpial lesion pattern of demyelination was observed in 15.2% of samples (Fig. 1). The remaining samples either showed a combined central and subpial pattern of demyelination (30.4%) or extensive demyelination (13%) not making it possible to specify a pattern.
Lesions in the majority of multiple sclerosis cases showed evidence of inflammatory activity (i.e. active and mixed active/inactive) as it was found in 34 (73.9%) samples from 24 cases (75%) (see Supplementary material for further details). In particular, four had acute active plaques (16.7%—six samples), 14 cases (58.3% of cases—28 samples) had mixed active-inactive lesions and six had chronic inactive plaques only (25% of donors—12 samples).
While active lesions were only present in donors with less than 20 years of disease duration, the proportion of mixed active-inactive plaques was common even in donors with more than 20 years of disease duration (2/3 donors, 2/8 samples versus 8/10 donors, 12/19 samples in those with shorter disease duration) and more than 60 years of age (5/9 donors, 5/18 samples versus 9/11 donors, 15/25 samples in younger cases).
Of note, CD20+ B-cells were detected in the perivascular space of four active multiple sclerosis lesions (two active lesions in one case and two mixed active-inactive lesions from another case).
Remyelination was detected in 13% of samples (six samples from four cases).
Neuromyelitis optic spectrum disorder
In neuromyelitis optica spectrum disorder cases, lesions were observed in two out of six cases (33.3%; four out of 22 samples) with the proportional demyelinated area affecting 64.9% of the total area sampled (range 50.3% to 75.5%). In both cases with lesions, demyelination was present in all the available samples (which included: the chiasm with the posterior part of the optic nerves from one donor; one optic nerve, the chiasm and one tract from the other donor). Areas of normal myelination were not found anywhere in the samples of cases where demyelination was observed. In fact, myelin in non-lesional areas appeared less dense and organized compared with controls. Unlike in multiple sclerosis, myelin loss typically spared the subpial zone (Fig. 1) and all lesions showed an ill-defined edge.
All lesions surveyed had evidence of inflammatory activity as shown by microglial/macrophage and T-lymphocytes infiltrates (Supplementary material), but B-cells were not detected. None of the neuromyelitis optica cases showed evidence of remyelination.
Acute disseminated encephalomyelitis
In acute disseminated encephalomyelitis, demyelination was found in two of the six cases (33.3% of cases, two out of 12 samples) in a typical perivenular distribution affecting approximately 9% of the total sampled area (range 6.6–11%) (Fig. 1).
All lesions showed inflammatory activity with evidence of a pronounced perivascular CD3+ T-cell infiltrate being a characteristic feature (see Fig. 1 and Supplementary material). No B-cells were found in lesions.
Non-lesional white matter—inflammation
Multiple sclerosis
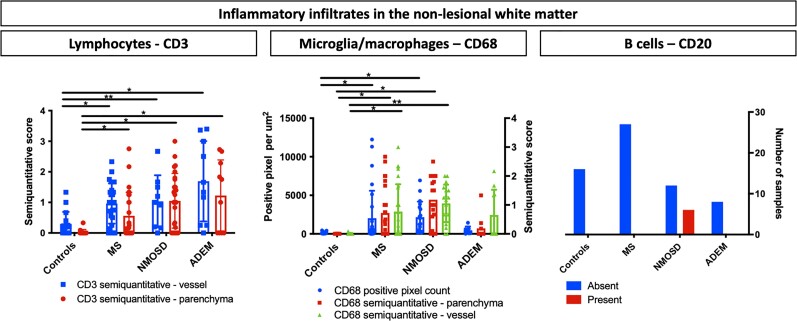
On evaluation of samples without evidence of demyelination (27 samples from 14 cases), diffuse CD68+ microglial/macrophage and CD3+ lymphocyte infiltration was observed in the parenchyma and in the perivascular space (Fig. 2 and Supplementary material). Sensitivity analysis considering only the multiple sclerosis cases without demyelinating lesions in the whole sampled area (five donors, seven samples) yielded similar findings. CD20+ B-cells were not found.
Figure 2.
Inflammation in the non-lesional white matter. Bar graphs show the characteristics of the inflammatory infiltrate in the non-lesional white matter of five controls, five acute disseminated encephalomyelitis (ADEM), 14 multiple sclerosis (MS) and four neuromyelitis optica spectrum disorder (NMOSD) donors. Only samples without demyelination were considered. Left, CD3+ lymphocytic infiltration was increased compared with controls in all the demyelinating CNS diseases with highest values observed in ADEM cases. Middle, There was a significant increase in CD68+ microglial infiltration in MS and NMOSD cases compared with controls. Left, B-cells were typically present in NMOSD cases but were not found in MS and ADEM cases (chi-square 14, P = 0.001). CD3+ lymphocytic and CD68+ microglial infiltration were quantified using a semiquantitative scores for parenchymal and one for perivascular inflammation; automated pixel counting software was also used for CD68. B-cell inflammation was classified as with or without CD20-positive cells in the non-lesional white matter. Bars display the mean values, the whiskers represent CI95%. The asterisks indicate significant post hoc pairwise comparisons (*P < 0.05, **P < 0.001).
Cases older than 60 years or with over 20 years of disease duration tended to have higher inflammation in the non-lesional white matter compared with cases younger cases (Supplementary material).
Neuromyelitis optica spectrum disorder
On considering the neuromyelitis optica spectrum disorder cases without evidence of demyelination (four cases, 18 samples), microglial/macrophage and T-cell inflammatory scores were higher than controls. CD20+ B-cell lymphocytes were detected in 33.3% of the samples (6/18 samples, 2/4 cases) (Fig. 2 and Supplementary material). Of note, none of these four neuromyelitis spectrum disorder cases without demyelination had a history of optic neuritis.
Acute disseminated encephalomyelitis
Non-lesional white matter (five subjects, 10 samples) showed a predominant T-cell lymphocyte infiltration (Fig. 2) above that of controls. However, the extent CD68+ microglial/macrophage infiltration in the parenchyma or perivascular space did not differ from controls. (Fig. 2 and Supplementary material). CD20+ B-cells were not found.
Meningeal inflammation
Control cases (14 samples) displayed sparse CD3+ and CD68+ positive cells, which, when present, were mostly located near pial vessels. CD20+ B-cells were not detected.
Meningeal inflammation (CD3/CD68) was present in 100% of neuromyelitis optica spectrum disorder cases (16 samples), 83.3% of acute disseminated encephalomyelitis cases (six samples) and 59% of multiple sclerosis cases (61 samples). Severe meningeal involvement was frequent in neuromyelitis optica spectrum disorder (31.3%) and acute disseminated encephalomyelitis (50%), while it was rarer in multiple sclerosis (9.8%).
Multiple sclerosis
In multiple sclerosis cases, meningeal inflammation (CD3/CD68) was associated with presence of demyelination and with lesional activity.
Meningeal inflammation was invariably present in samples with active lesions, and was intense in 50% of cases. Mixed active-inactive and chronic inactive lesions often had evidence of meningeal inflammation (70 and 71%, respectively), but was rarely intense (10 and 11.7%, respectively). Finally, meningeal inflammation was in 30% of cases without demyeliation where it was never severe in intensity (Linear-by-Linear association for presence of meningeal inflammation 9.41, P = 0.002; for presence of severe inflammation: 5.49, P = 0.022).
Moreover, the presence of meningeal inflammation (CD3/CD68) was predicted by microglial/macrophage and T-cell lymphocyte infiltration in non-lesional white matter (CD68 perivascular score, Wald 12.37, P < 0.001; CD68 parenchymal score, Wald 31.66, P < 0.001; pixel count, Wald 8.24, P = 0.004; CD3 perivascular score, Wald 4.79, P = 0.029; CD3 parenchymal score, Wald 9.27, P = 0.002).
Twenty-five out of 61 meningeal samples (41%) showed CD20 B-cell infiltration and nine (14.8%) had several CD20+ cells (more than 10 cells per sample), usually grouped into clusters. The presence and the extent of meningeal B-cell infiltration was associated with the severity of CD3/CD68 meningeal inflammation (Linear-by-linear association: 19.71, P < 0.001). The presence of meningeal B-cells was also predicted by the microglial macrophage inflammatory infiltrate in non-lesional white matter (perivascular score, Wald 4.29, P = 0.038; parenchymal score, Wald 5.79, P = 0.016; pixel count, Wald 5.24, P = 0.022). We found no association between B-cell infiltration, presence of demyelination or lesional activity.
The presence of subpial demyelination did not relate to the nature or severity of meningeal inflammation.
Neuromyelitis optica spectrum disorder
As meningeal inflammation (CD3/CD68) was present in all cases, the relationship between meningeal inflammation, presence of demyelination, and lesional activity could not be explored. CD20+ B-cells were detected in 14 of the 16 available samples (87.5%) from five donors. Among those, 10 samples had evidence of B-cell clusters and four had sparse lymphocytes related to the pial layer. Similar to the multiple sclerosis cohort, B-cell meningeal inflammation was not associated with the presence of demyelination.
Acute disseminated encephalomyelitis
Despite severe meningeal involvement (CD3/CD68), CD20+ B-cell meningeal infiltration was sparse (1–2 cells in two out of seven samples). No association was found between plaque location and pattern (e.g. subpial versus central) and the presence of CD3/CD68 or CD20 meningeal inflammation in any of the disease groups.
Axonal injury
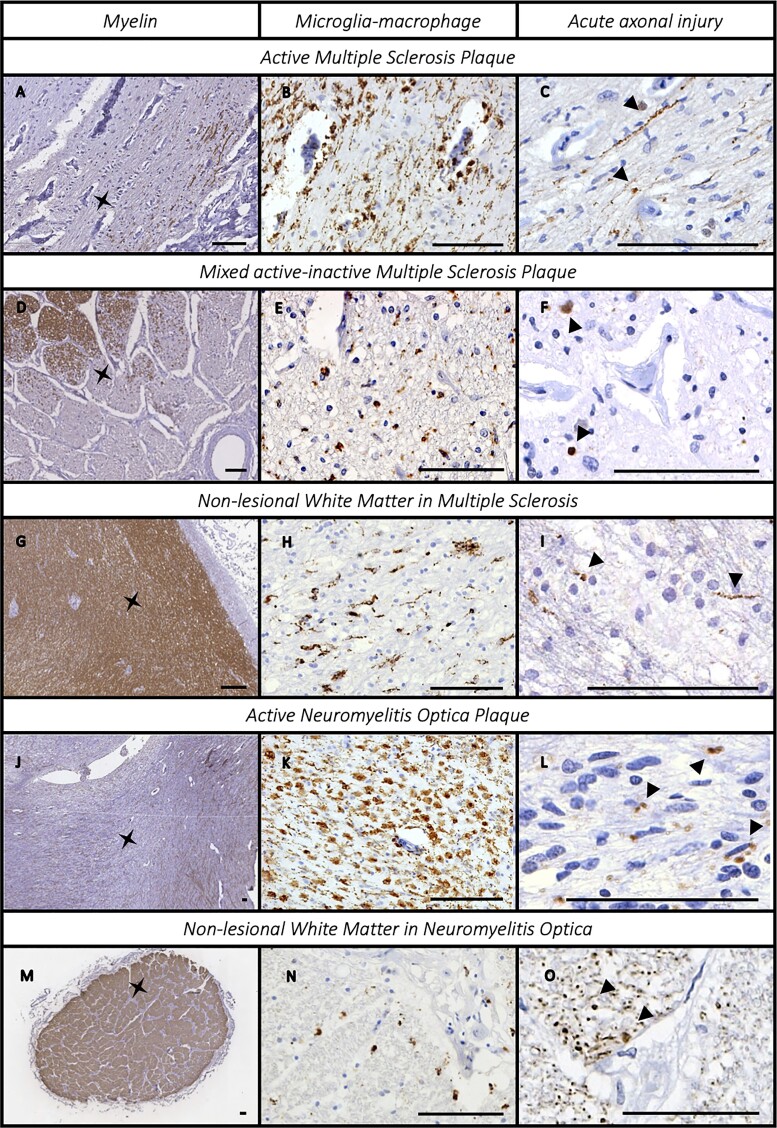
Acute axonal injury (β-APP positive axons) was frequently found in all CNS demyelinating disease groups studied. β-APP positive axons were often grouped in small clusters and were found in lesional and non-lesional areas alike and related to the presence and extent of inflammatory activity (Fig. 3).
Figure 3.
Inflammation and acute axonal injury in the anterior optic pathway. The association between microglial–macrophage inflammatory infiltrate and acute axonal injury β-APP in multiple sclerosis [active plaques (A–C); mixed active/inactive plaques (D–F); and non-lesional white matter (G–I)] and neuromyelitis spectrum disorder [active plaques (J–L); non-lesional white matter (M–O)]. We compared PLP (left column) with CD68+ elements (middle column) and β-APP positive axons (right column, some indicated by arrowheads). The asterisk on the PLP section indicates the site where the CD68 and β-APP images were acquired. Scale bar = 100 µm.
Multiple sclerosis
In the multiple sclerosis cohort, 41.4% of donors (12/29) and 27.4% of samples (20/73) displayed acute axonal injury, which associated with lesion activity, inflammation in non-lesional white matter and meninges. The presence of acute axonal injury was not associated with age or disease duration (5/9 donors, 11/39 samples aged ≥60 years versus 5/14 donors, 9/34 samples aged <60 years; 3/7 donors, 5/12 samples with disease duration ≥20 years versus 6/13 donors, 12/40 samples with shorter disease duration).
Axonal damage was present in 100% of active lesions (6/6 samples), 35% of mixed active-inactive lesions (7/20 samples), 13% of inactive plaques (3/23 samples) and in 16.7% of samples without lesions (4/24 samples). Lesion activity was associated with presence of axonal injury (Linear-by-Linear association 12.12, P < 0.001). The extent of acute axonal injury was greater in samples with active lesions compared with mixed active-inactive and inactive lesions (active: 3225.99 pixels/nm2; CI95% −420.6–6872.6; mixed active-inactive: 532.7 pixels/nm2; CI95% 364–701; inactive: 214 pixels/nm2; CI95% 102–326; Wald 17.38, P < 0.001). In samples without demyelination, β-APP staining was associated with the extent of microglial/macrophage and T-cell lymphocyte infiltration in the parenchyma (CD68+: Wald 10.9, P = 0.001; CD3+: Wald 5.04, P = 0.025), while perivascular CD68+ and CD3+ inflammation was not associated.
Acute axonal injury was associated with meningeal inflammation. Β-APP+ axonal expression was more common and the extent more severe in samples with meningeal inflammation compared to samples without (meningeal inflammation: positive—38.9% (14/36 samples) versus negative—12% (3/25 samples), chi-square 5.3, P = 0.04; positive—1462.76 pixels/nm2; CI95% 92.9–2833 versus negative—249.2 pixels/nm2; CI95% 206–292; Wald 3.01, P = 0.083).
Neuromyelitis optica spectrum disorder
Acute axonal injury was detected in 66.7% (4/6) neuromyelitis optica spectrum disorder cases and 54.5% (12/22) of samples. Interestingly, β-APP+ axons were present in three of the four donors (9/18 samples) without demyelination or a prior history of optic neuritis and was associated with inflammation in non-lesional white matter. The severity of axonal injury correlated with microglial/macrophage inflammation (CD68 parenchymal score: Wald 71.91, P < 0.001; CD68 perivascular score: Wald 14.04, P < 0.001; CD68 pixel count: Wald 9.19, P = 0.002) and T-cell lymphocyte infiltration in non-lesional white matter (CD3 parenchymal score: Wald 7.31, P = 0.007; CD3 perivascular score: Wald 9.225, P = 0.002).
Acute disseminated encephalomyelitis
Evidence of acute axonal injury was detected in 50% (3/6) acute disseminated encephalomyelitis cases and 33.3% (4/12) of samples. The presence or extent of acute axonal injury did not relate to measures of inflammation.
GFAP and AQP4 expression
In controls, the pattern of GFAP and AQP4 immunoreactivity in the anterior optic pathway conformed to classical expression patterns described in other CNS areas. In brief, GFAP expression was seen in astrocyte cell bodies and processes with most intense expression in the glia limitans and perivascular area of larger vessels. AQP4 immunolabelling was mainly concentrated on astrocyte foot processes with increased expression in the glia limitans and at the abluminal area of all vessels and capillaries. In multiple sclerosis, GFAP and AQP4 showed increased perivascular staining intensity in lesional and non-lesional areas compared to controls. In contrast, neuromyelitis optica spectrum disorder cases showed almost complete loss of the perivascular GFAP and AQP4 expression patterns in lesional areas with relative preservation in non-lesional white matter compared to controls. Of note, complement deposition (C9neo) was detected only in lesional areas of neuromyelitis optica spectrum disorder cases. Interestingly, the plane of the tissue sample (i.e. cross-sectional versus longitudinal) significantly impacted quantitative pixel counts of GFAP and AQP4 perivascular expression in controls precluding meaningful comparisons between groups (see Supplementary material).
Antero-posterior gradient of pathological burden in multiple sclerosis
Pathological burden followed a clear antero-posterior gradient in multiple sclerosis cases. Optic nerves showed more severe demyelination, inflammatory activity, and acute axonal injury, and less remyelination, compared to the optic chiasm and tracts (Table 2). Neuromyelitis optica spectrum disorder and acute disseminated encephalomyelitis cases did not display a preferential antero-posterior pattern of pathological involvement.
Table 2.
Antero-posterior gradient of disease involvement in the anterior optic pathway of multiple sclerosis donors
| Optic nerves | Chiasms | Optic tracts |
|---|---|---|
| Extension of demyelinated area (%) | ||
| 65.2% | 45.2% | 36.2% |
| CI95%, 46.9–83.4% | CI95%, 18.9–71.4% | CI95%, 25.5–46.9% |
| Wald chi-square 8.22, P = 0.016 | ||
| Remyelinated shadow plaques (presence) | ||
| 0% | 15.4% | 27.3% |
| 0 out of 22 samples | 2 out of 13 samples | 3 out of 11 samples |
| Linear-by-Linear Association 5.85, P = 0.025 | ||
| Inflammatory activity (presence of active plaques) | ||
| 69.6% | 50% | 25% |
| 16 out of 23 samples | 7 out of 14 samples | 3 out of 12 samples |
| Linear-by-Linear Association 6.2, P = 0.014 | ||
| Acute axonal injury (presence of β-APP-positive axons) | ||
| 38.2% | 31.6% | 5% |
| 13 out of 34 samples | 6 out of 19 samples | 1 out of 20 samples |
| Linear-by-Linear Association 6.44, P = 0.012 | ||
Pathological burden followed a clear antero-posterior gradient in multiple sclerosis samples with optic nerves showing a more severe involvement. In particular, the percentage of demyelinated area was more extensive in the optic nerves and gradually reduced in the chiasms and tracts. Moreover, the percentage of active plaques was over two thirds in the optic nerves, half in the chiasms, and less than a third in the tracts. Also, when dividing active and mixed active-inactive lesions, we found that acute active lesions were more likely to be located in the anterior part of the optic pathway (Linear-by-Linear association: chi-square 6.16, P = 0.015). Conversely, shadow plaques were never found in the optic nerves but in 15.4% of chiasm lesions and in 27.3% of the optic tract plaques. Finally, in multiple sclerosis cases the anterior part of the pre-geniculate optic pathway more frequently showed acute axonal injury regardless of demyelination as it was present in 38.2% of optic nerve samples, in 31.6% of the chiasms and in 5% of the optic tracts sampled.
Discussion
The pathological features of inflammatory demyelinating CNS disease have been widely explored to date but few studies have specifically assessed the characteristics of anterior optic pathway involvement. Moreover, the relationship between neuroinflammation and neurodegeneration has been inadequately assessed in this CNS area. We show that anterior optic pathway demyelination is frequent, can occur early and be highly inflammatory across the spectrum of inflammatory demyelinating diseases. Our findings implicate inflammation rather than demyelination as an important substrate of axonal injury in multiple sclerosis and neuromyelitis spectrum disorders. This work broadens the plaque-centred view of anterior optic pathway neurodegeneration and provides an explanation for the progressive neuroretinal changes observed in optic coherence tomography studies in multiple sclerosis and neuromyelitis optica spectrum disorders.
Anterior optic pathway demyelination is a common feature of CNS inflammatory demyelinating diseases. Demyelination was present in 82.8% of multiple sclerosis cases, which is in line with previous work.4,18–20 Proportionally fewer cases of neuromyelitis optica spectrum disorder and acute disseminated encephalomyelitis had evidence of demyelination (33.3% in each group) in our cohort, which, in the case of neuromyelitis optica spectrum disorders, is less than anticipated based on clinical series where over 70% of patients experience optic neuritis during life.27 Ascertainment bias and relatively small sample size intrinsic to pathological studies likely explain the discrepancy.
Regarding the characteristics of the pattern of demyelination, a subpial distribution was found only in a minority of multiple sclerosis cases regardless of the nature and extent of meningeal inflammation. In fact, many lesions had a tendency to be more centrally located with subpial sparing. This pattern differs from that seen in the cerebral cortex and periventricular area, where demyelination is more common at CSF interfaces, which highlights the complexity of lesion distribution throughout the neuraxis and warrants reflection on its substrate.28,29 It may be that CSF soluble factors play a role but other mechanisms require consideration. In contrast to multiple sclerosis, neuromyelitis optica spectrum disorder cases showed a more extensive pattern of demyelination but similarly showed relative sparing of the subpial area, supporting a more vessel-centric antibody-mediated mechanism of lesion formation.30 Demyelination was preferentially perivascular in acute disseminated encephalomyelitis as has been well established in this disease.31
In all disease groups, significant inflammatory activity was observed regardless of demyelination status. In neuromyelitis optica spectrum disorder and acute disseminated encephalomyelitis cases, all demyelinated lesions showed evidence of active inflammation with non-lesional white matter areas also showing heightened inflammation compared to controls. While this is to be expected in the acute disseminated encephalomyelitis group where disease duration was short and the severity was fulminant, these findings are surprising in these neuromyelitis optica spectrum disorder cases where clinically evident optic neuritis was not a feature at death. The presence of ongoing inflammatory activity in plaques and non-lesional white matter areas in neuromyelitis optica spectrum disorders, even at end-stage disease, highlights the importance of effective immunosuppressive treatment in preventing downstream sequelae, such as neurodegeneration. Similar findings are seen in multiple sclerosis, where the majority of plaques showed inflammatory activity, even with long disease duration (>20 years) and advanced age (>60 years). While the proportion of acute lesions decreased with disease duration in multiple sclerosis, the persistence of mixed active-inactive lesions throughout the disease course is a striking feature similar to other CNS areas.32 Prior studies have shown a dissociation between the prevalence of gadolinium-enhancing lesions—which are active lesions—and disability progression in the later stages of the disease.33,34 Mixed active-inactive plaques may partly explain this gap, as they largely elude detection in vivo but are associated with progressive neurodegeneration, which is the main determinant of disability accumulation.35,36 Further, the severe extent of inflammation outside of the lesional milieu, as observed in our study, is under-appreciated in vivo and likely contributes to the degenerative profile commonly encountered at all disease stages.
A key finding of this work is the frequency of acute axonal injury and its link to inflammation beyond the lesional milieu in end-stage multiple sclerosis and neuromyelitis optica spectrum disorders. In this study, we used β-APP to define acute axonal injury. This protein accumulates in the proximal ends of damaged axons and can be detected up to 30 days from the time of injury thus providing an opportunity to explore the determinants of axonal damage post-mortem.37 In our series, acute axonal injury was detected in 41% of multiple sclerosis cases and 67% (four of six) of neuromyelitis optica spectrum disorder cases. In multiple sclerosis, acute axonal injury related to plaque activity, as expected, but also to inflammation in the meninges and non-lesional white matter supporting a more diffuse inflammatory-driven neurodegenerative process.29,38,39 This concept extends to neuromyelitis optica spectrum disorder cases wherein acute axonal injury was present in the optic nerve of three of four cases without a history of optic neuritis during life or evidence of demyelination or AQP4 loss in the sampled tissue. However, these cases exhibited striking lymphocytic and microglial inflammation in non-lesional white matter and invariable meningeal inflammation, often with B-cell infiltrates. These findings are in line with those by Saji et al.,40 who reported cerebral cortical neuronal/axonal pathology alongside parenchymal microglial and meningeal inflammation in the absence of AQP4 loss and myelin loss. A prior description of intense peri-optic meningeal inflammation and presence of acute axonal injury in optic nerve areas with normal myelination further substantiate our findings.6 We acknowledge that the frequencies of inflammation and acute axonal injury we report may not be generalisable to the whole neuromyelitis optica spectrum disorder population due to the small sample size. However, our observations challenge the widely accepted notion that neurodegeneration in neuromyelitis optica spectrum disorders is only driven by clinically apparent (i.e. attack associated) inflammatory disease activity.41 The high proportion of both multiple sclerosis and neuromyelitis optica spectrum disorder cases with ongoing inflammation and axonal injury at death challenges fundamental clinical paradigms, namely that only a negligible proportion of end-stage progressive multiple sclerosis is considered active and that neuromyelitis optica spectrum disorder is deemed stable outside attacks.42 Our findings may extend beyond the visual system and should encourage reflection about their implication on the approach to treating these conditions, especially at later disease stages.
The present findings also put into perspective the results of optical coherence tomography studies in both multiple sclerosis and neuromyelitis optica spectrum disorder. These studies showed that progressive axonal and neuronal loss occurs at retinal level in absence of optic neuritis or changes in the latency of visual evoked potentials—which is a proxy marker of demyelination.9,15,43 In multiple sclerosis, the rate of neuroretinal thinning is increasingly recognized as a marker of neurodegeneration due to its correlation with other measures of disability and disease burden.12–14 Our findings provide useful insight on the pathogenesis of this phenomenon and support its biological validity as a biomarker. Moreover, we provide a plausible explanation for progressive retinal thinning in neuromyelitis optica spectrum disorder,15,16 where retinal changes are usually considered to be only due to a primary damage of retinal AQP4-rich structures.16
While demyelination, inflammation and acute axonal injury are prevalent features of the diseases studied, we observed a striking antero-posterior gradient of pathologic burden in multiple sclerosis cases. In our series, optic nerves showed more severe demyelination, inflammatory activity, and acute axonal injury, and less remyelination, compared to the optic chiasm and tracts. A similar pathological gradient was not observed in neuromyelitis optica disease spectrum and acute disseminated encephalomyelitis cases. It is known that optic nerve is preferentially affected by multiple sclerosis at the disease onset7 and we provide evidence that this preference persists throughout the disease course. An interesting finding is the inverse relationship between inflammation and remyelination along the anterior visual pathway, which aligns with observations in other brain areas.44 The mechanisms underpinning preferential optic nerve involvement in multiple sclerosis are poorly understood. Topographical differences in vascular anatomy and blood-brain barrier integrity, extracellular matrix proteins and glial cell abundance and phenotypes, relative abundance of specific antigens, preferential affectation by pathogens, and even mechanical forces may play a role. With regards to the latter, the orbit and optic nerve are highly mobile on extraocular movement. Historical observations regarding another highly mobile structure preferentially affected by multiple sclerosis, the cervical cord, led to the hypothesis that stretching forces may act on vascular structures and facilitate an inflammatory response.45 Could a similar mechanical mechanism explain the anteroposterior gradient of pathological burden in the anterior visual system in multiple sclerosis?
We acknowledge that our study has limitations intrinsic to studies using archival human tissue. Case collection is biased towards more severe and end-stage disease, which the heterogeneity of age at death and disease duration of our cohorts partly addresses. That being said, we recognise that the small sample sizes for neuromyelitis optica spectrum disorder, acute disseminated encephalomyelitis and control cases, which reflects the rarity of these conditions and lack of access to high-quality post-mortem material, may limit the generalisability of our findings to clinical practice. However, pathological studies of the anterior visual pathway provide a level of resolution well beyond any other in vivo technique used in the clinic. In addition, one must consider an ascertainment bias with sample collection at the time of the autopsy, where the most anterior aspects of the optic nerve (near the globe) are not routinely collected due to technical factors. Despite this, we were still able to demonstrate a clear anteroposterior gradient of pathological burden in multiple sclerosis but appreciate that more comprehensive assessment of the anterior visual pathway may cast additional insight into the nature and extent of pathology than described herein.
We also aknowledge that MOG antibody disease is a recently-recognized entity which needs to be differentiated from other CNS demyelinating diseases. Given that the anti-MOG antibody test was not available at the time of death in the majority of our cases, it is not possible to rule out completely whether a subgroup was positive. Multiple sclerosis cases and the neuromyelitis optica spectrum disorder case without available serology showed pathological features highly typical of their disease group which have been extensively recorded throughout the neuraxis at autopsy as well as in subsequent histological studies.21,22,24,46 Moreover, prototypical clinical features, such as the presence of a progressive phenotype in the multiple sclerosis cohort, were commonly recorded. For the acute disseminated encephalomyelitis cases, pathological and clinical boundaries with MOG antibody disease are less defined, although the fulminant disease course of non-paediatric cases would be an atypical feature of MOG antibody disease.47,48 Overall, it is unlikely that main results of the study are influenced by the lack of anti-MOG antibody testing, particularly in the multiple sclerosis cases and the one neuromyelitis optica spectrum disorder case for which serology was not available.
Finally, given the heterogeneity of the planes (i.e. cross-sectional versus longitudinal) of tissue sections available for study, it was not possible to reliably quantify axonal loss within and between cases. While axonal loss represents an irreversible phenomenon that accumulates over decades, assessment of β-APP immunohistochemistry allows for detection of axonal injury within 30 days of death, provided a unique opportunity to cast light on the relationships between inflammation and neurodegeneration at autopsy.
In summary, we show that anterior optic pathway demyelination is frequent, can occur early and be highly inflammatory across the spectrum of inflammatory demyelinating diseases. We provide evidence that inflammation, beyond the lesional milieu, is associated with acute axonal injury even after long-standing, end-stage disease in multiple sclerosis and in the absence of a history of optic neuritis or demyelination in neuromyelitis optica spectrum disorder. The striking anteroposterior gradient of pathological burden in multiple sclerosis might provide important clues into disease pathogenesis worthy of further study. Our findings provide important context to the results of optical coherence tomography studies in multiple sclerosis and neuromyelitis optica spectrum disorder, offering a plausible aetiological cause of progressive inner retinal thinning, even in the absence of overt clinical attacks or neurophysiological evidence of demyelination often encountered in these conditions. What is more, our findings should make the scientific community pause on the way inflammation and treatment response are assessed in clinical practice and how we approach therapeutic intervention, especially in longstanding and end-stage disease.
Supplementary Material
Contributor Information
Marco Pisa, Nuffield Department of Clinical Neurosciences, University of Oxford, Level 6 West Wing, John Radcliffe Hospital, Oxford, UK.
Jonathan Pansieri, Nuffield Department of Clinical Neurosciences, University of Oxford, Level 6 West Wing, John Radcliffe Hospital, Oxford, UK.
Sydney Yee, Nuffield Department of Clinical Neurosciences, University of Oxford, Level 6 West Wing, John Radcliffe Hospital, Oxford, UK.
Jennifer Ruiz, Joyce D. and Andrew J. Mandell Center for Comprehensive Multiple Sclerosis Care and Neuroscience Research, Mount Sinai Rehabilitation Hospital, Trinity Health of New England, Hartford, CT, USA.
M Isabel Leite, Nuffield Department of Clinical Neurosciences, University of Oxford, Level 6 West Wing, John Radcliffe Hospital, Oxford, UK.
Jacqueline Palace, Nuffield Department of Clinical Neurosciences, University of Oxford, Level 6 West Wing, John Radcliffe Hospital, Oxford, UK.
Giancarlo Comi, Department of Neurology, Vita-Salute San Raffaele University, Milan, Italy; Casa di Cura del Policlinico, Milan, Italy.
Margaret M Esiri, Nuffield Department of Clinical Neurosciences, University of Oxford, Level 6 West Wing, John Radcliffe Hospital, Oxford, UK.
Letizia Leocani, Department of Neurology, Vita-Salute San Raffaele University, Milan, Italy; Experimental Neurophysiology Unit, Institute of Experimental Neurology-INSPE, IRCCS Scientific Institute San Raffaele, Milan, Italy.
Gabriele C DeLuca, Nuffield Department of Clinical Neurosciences, University of Oxford, Level 6 West Wing, John Radcliffe Hospital, Oxford, UK.
Funding
This research work was supported by the National Institute for Health Research Oxford Biomedical Research Centre (BRC) Oxford and funded by the Oxford-Quinnipiac partnership.
Competing interests
The authors report no competing interests.
Supplementary material
Supplementary material is available at Brain online.
References
- 1. Toosy AT, Mason DF, Miller DH. Optic neuritis. Lancet Neurol. 2014;13:83–99. [DOI] [PubMed] [Google Scholar]
- 2. Leocani L, Rovaris M, Boneschi FM, et al. Multimodal evoked potentials to assess the evolution of multiple sclerosis: A longitudinal study. J Neurol Neurosurg Psychiatry. 2006;77:1030–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Filippi M, Preziosa P, Meani A, et al. Prediction of a multiple sclerosis diagnosis in patients with clinically isolated syndrome using the 2016 MAGNIMS and 2010 McDonald criteria: a retrospective study. Lancet Neurol. 2018;17:133–142. [DOI] [PubMed] [Google Scholar]
- 4. Toussaint D, Périer O, Verstappen A, Bervoets S. Clinicopathological study of the visual pathways, eyes, and cerebral hemispheres in 32 cases of disseminated sclerosis. J Clin Neuroophthalmol. 1983;3:211–220. [PubMed] [Google Scholar]
- 5. Vabanesi M, Pisa M, Guerrieri S, et al. In vivo structural and functional assessment of optic nerve damage in neuromyelitis optica spectrum disorders and multiple sclerosis. Sci Rep. 2019;9:10371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hokari M, Yokoseki A, Arakawa M, et al. Clinicopathological features in anterior visual pathway in neuromyelitis optica. Ann Neurol. 2016;79:605–624. [DOI] [PubMed] [Google Scholar]
- 7. Ramanathan S, Prelog K, Barnes EH, et al. Radiological differentiation of optic neuritis with myelin oligodendrocyte glycoprotein antibodies, aquaporin-4 antibodies, and multiple sclerosis. Mult Scler. 2016;22:470–482. [DOI] [PubMed] [Google Scholar]
- 8. Petzold A, de Boer JF, Schippling S, et al. Optical coherence tomography in multiple sclerosis: a systematic review and meta-analysis. Lancet Neurol. 2010;9:921–932. [DOI] [PubMed] [Google Scholar]
- 9. Pisa M, Guerrieri S, Di Maggio G, et al. No evidence of disease activity is associated with reduced rate of axonal retinal atrophy in MS. Neurology. 2017;89:2469–2475. [DOI] [PubMed] [Google Scholar]
- 10. Pisa M, Croese T, Dalla Costa G, et al. Subclinical anterior optic pathway involvement in early multiple sclerosis and clinically isolated syndromes. Brain. 2021;144:848–862. [DOI] [PubMed] [Google Scholar]
- 11. Coric D, Balk LJ, Verrijp M, et al. Cognitive impairment in patients with multiple sclerosis is associated with atrophy of the inner retinal layers. Mult Scler. 2018;24:158–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Saidha S, Al-Louzi O, Ratchford JN, et al. Optical coherence tomography reflects brain atrophy in multiple sclerosis: A four-year study. Ann Neurol. 2015;78:801–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Oh J, Sotirchos ES, Saidha S, et al. Relationships between quantitative spinal cord MRI and retinal layers in multiple sclerosis. Neurology. 2015;84:720–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Petracca M, Cordano C, Cellerino M, et al. Retinal degeneration in primary-progressive multiple sclerosis: A role for cortical lesions? Mult Scler. 2017;23:43–50. [DOI] [PubMed] [Google Scholar]
- 15. Pisa M, Ratti F, Vabanesi M, et al. Subclinical neurodegeneration in multiple sclerosis and neuromyelitis optica spectrum disorder revealed by optical coherence tomography. Mult Scler. 2020;26:1197–1206. [DOI] [PubMed] [Google Scholar]
- 16. Oertel FC, Havla J, Roca-Fernández A, et al. Retinal ganglion cell loss in neuromyelitis optica: a longitudinal study. J Neurol Neurosurg Psychiatry. 2018;89:1259–1265. [DOI] [PubMed] [Google Scholar]
- 17. Tian D-C, Su L, Fan M, et al. Bidirectional degeneration in the visual pathway in neuromyelitis optica spectrum disorder (NMOSD). Mult Scler. 2018;24:1585–1593. [DOI] [PubMed] [Google Scholar]
- 18. Gartner S. Optic neuropathy in multiple sclerosis; optic neuritis. AMA Arch Ophthalmol. 1953;50:718–726. [DOI] [PubMed] [Google Scholar]
- 19. Ulrich J, Waltraut GL. The optic nerve in multiple sclerosis: A morphological study with retrospective clinico-pathological correlations. Neuro-Ophthalmology 1983;3:149–159. [Google Scholar]
- 20. Mogensen PH. Histopathology of anterior parts of the optic pathway in patients with multiple sclerose. Acta Ophthalmol. 1990;68:218–220. [DOI] [PubMed] [Google Scholar]
- 21. Yates RL, Esiri MM, Palace J, et al. The influence of HLA-DRB1*15 on motor cortical pathology in multiple sclerosis. Neuropathol Appl Neurobiol. 2015;41:371–384. [DOI] [PubMed] [Google Scholar]
- 22. Diaz-Sanchez M, Williams K, DeLuca GC, Esiri MM. Protein co-expression with axonal injury in multiple sclerosis plaques. Acta Neuropathol. 2006;111:289–299. [DOI] [PubMed] [Google Scholar]
- 23. Kuhlmann T, Ludwin S, Prat A, Antel J, Brück W, Lassmann H. An updated histological classification system for multiple sclerosis lesions. Acta Neuropathol. 2017;133:13–24. [DOI] [PubMed] [Google Scholar]
- 24. DeLuca GC, Joseph A, George J, et al. Olfactory pathology in central nervous system demyelinating diseases. Brain Pathol. 2015;25:543–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bankhead P, Loughrey MB, Fernández JA, et al. QuPath: Open source software for digital pathology image analysis. Sci Rep. 2017;7:16878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hanley JA, Negassa A, Edwardes MD, Forrester JE. Statistical analysis of correlated data using generalized estimating equations: an orientation. Am J Epidemiol. 2003;157:364–375. [DOI] [PubMed] [Google Scholar]
- 27. Mealy MA, Wingerchuk DM, Greenberg BM, Levy M. Epidemiology of neuromyelitis optica in the United States: a multicenter analysis. Arch Neurol. 2012;69:1176–1180. [DOI] [PubMed] [Google Scholar]
- 28. Haider L, Zrzavy T, Hametner S, et al. The topograpy of demyelination and neurodegeneration in the multiple sclerosis brain. Brain. 2016;139(Pt 3):807–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lassmann H. Multiple sclerosis pathology. Cold Spring Harb Perspect Med. 2018;8:a028936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lucchinetti CF, Mandler RN, McGavern D, et al. A role for humoral mechanisms in the pathogenesis of Devic’s neuromyelitis optica. Brain. 2002;125(Pt 7):1450–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lassmann H. Acute disseminated encephalomyelitis and multiple sclerosis. Brain. 2010;133(Pt 2):317–319. [DOI] [PubMed] [Google Scholar]
- 32. Frischer JM, Weigand SD, Guo Y, et al. Clinical and pathological insights into the dynamic nature of the white matter multiple sclerosis plaque. Ann Neurol. 2015;78:710–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bielekova B, Kadom N, Fisher E, et al. MRI as a marker for disease heterogeneity in multiple sclerosis. Neurology. 2005;65:1071–1076. [DOI] [PubMed] [Google Scholar]
- 34. Filippi M, Rocca MA. MRI evidence for multiple sclerosis as a diffuse disease of the central nervous system. J Neurol. 2005;252(Suppl 5):v16–v24. [DOI] [PubMed] [Google Scholar]
- 35. Filippi M, Rocca MA, Barkhof F, et al. Association between pathological and MRI findings in multiple sclerosis. Lancet Neurol. 2012;11:349–360. [DOI] [PubMed] [Google Scholar]
- 36. Kornek B, Storch MK, Weissert R, et al. Multiple sclerosis and chronic autoimmune encephalomyelitis: a comparative quantitative study of axonal injury in active, inactive, and remyelinated lesions. Am J Pathol. 2000;157:267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bramlett HM, Kraydieh S, Green EJ, Dietrich WD. Temporal and regional patterns of axonal damage following traumatic brain injury: a beta-amyloid precursor protein immunocytochemical study in rats. J Neuropathol Exp Neurol. 1997;56:1132–1141. [DOI] [PubMed] [Google Scholar]
- 38. Rissanen E, Tuisku J, Vahlberg T, et al. Microglial activation, white matter tract damage, and disability in MS. Neurol Neuroimmunol Neuroinflamm. 2018;5:e443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sucksdorff M, Matilainen M, Tuisku J, et al. Brain TSPO-PET predicts later disease progression independent of relapses in multiple sclerosis. Brain. 2020;143:3318–3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Saji E, Arakawa M, Yanagawa K, et al. Cognitive impairment and cortical degeneration in neuromyelitis optica. Ann Neurol. 2013;73:65–76. [DOI] [PubMed] [Google Scholar]
- 41. Wingerchuk DM, Pittock SJ, Lucchinetti CF, Lennon VA, Weinshenker BG. A secondary progressive clinical course is uncommon in neuromyelitis optica. Neurology. 2007;68:603–605. [DOI] [PubMed] [Google Scholar]
- 42. University of California, San Francisco MS-EPIC Team, Cree BAC, Hollenbach JA, et al. Silent progression in disease activity-free relapsing multiple sclerosis. Ann Neurol. 2019;85:653–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Klistorner A, Graham EC, Yiannikas C, et al. Progression of retinal ganglion cell loss in multiple sclerosis is associated with new lesions in the optic radiations. Eur J Neurol. 2017;24:1392–1398. [DOI] [PubMed] [Google Scholar]
- 44. Patrikios P, Stadelmann C, Kutzelnigg A, et al. Remyelination is extensive in a subset of multiple sclerosis patients. Brain. 2006;129(Pt 12):3165–3172. [DOI] [PubMed] [Google Scholar]
- 45. Oppenheimer DR. The cervical cord in multiple sclerosis. Neuropathol Appl Neurobiol. 1978;4:151–162. [DOI] [PubMed] [Google Scholar]
- 46. Geraldes R, Esiri MM, Perera R, et al. Vascular disease and multiple sclerosis: a post-mortem study exploring their relationships. Brain. 2020;143:2998–3012. [DOI] [PubMed] [Google Scholar]
- 47. Juryńczyk, Jacob A, Fujihara K, Palace J. Myelin oligodendrocyte glycoprotein (MOG) antibody-associated disease: practical considerations. Pract Neurol. 2019;19:187–195. [DOI] [PubMed] [Google Scholar]
- 48. Baumann M, Sahin K, Lechner C, et al. Clinical and neuroradiological differences of paediatric acute disseminating encephalomyelitis with and without antibodies to the myelin oligodendrocyte glycoprotein. J Neurol Neurosurg Psychiatry. 2015;86:265–272. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.