Abstract
Bronchopulmonary dysplasia (BPD) is a disease with a significant sexual dimorphism where males have a disadvantage compared with their female counterparts. Although mechanisms behind this sexual dimorphism are poorly understood, sex differences in angiogenesis have been identified as one possible source of the male disadvantage in BPD. Pulmonary angiogenesis was assessed in vitro using a bead sprouting assay with pooled male or female human pulmonary microvascular endothelial cells (HPMECs, 18–19 wk gestation, canalicular stage of human lung development) in standard (sex-hormone containing) and hormone-stripped medium. We identified sex-specific phenotypes in angiogenesis where male HPMECs produce fewer but longer sprouts compared with female HPMECs. The presence of sex hormones from standard culture medium modifies the male HPMEC phenotype with shorter and fewer sprouts but does not influence the female phenotype. Using a conditioned medium model, we further characterized the influence of the sex-specific secretome. Male and female HPMECs secrete factors that increase the maximum length of sprouts in female, but not male HPMECs. The presence of sex hormones abolishes this response. The male HPMEC secretome inhibits angiogenic sprouting in male HPMECs in the absence of sex hormones. Taken together, these results demonstrate that the pulmonary endothelial cell phenotypes are influenced by sex hormones and sex-specific secreted factors in a sex-dependent manner.
NEW & NOTEWORTHY We identified a sex-specific phenotype wherein male HPMECs produce fewer but longer sprouts than females. Surprisingly, the presence of sex hormones only modifies the male phenotype, resulting in shorter and even fewer sprouts. Furthermore, we found the sex-specific secretome has a sex-dependent influence on angiogenesis that is also sex-hormone sensitive. These new and surprising findings point to the unappreciated role of sex and sex-related exogenous factors in early developmental angiogenesis.
Keywords: angiogenesis, pulmonary endothelial, sex hormone, sex specific, sexual dimorphism
INTRODUCTION
Bronchopulmonary dysplasia (BPD), a chronic lung disease characterized by impaired alveolar development and vascular rarefaction, is a disease with a prominent sexual dimorphism where male sex is considered an independent risk factor (1–3). Alveolar development cannot occur properly when pulmonary angiogenesis is inhibited (4–7). Although there exists a sexual dimorphism in angiogenesis, investigation of the underlying mechanisms that lie inherent to the lung endothelial cells, or the role sex hormones may play in these differences, have yet to be identified.
Work done on sex differences in the vascular niche during development has shown striking transcriptomic differences between female and male endothelial cells that contribute to differential cellular responses (8, 9). Sex hormones from maternal and fetal origin are crucial in development, directly impact angiogenesis, and are modified by preterm birth (9). Estradiol, integral to organ development in both sexes but commonly associated with females, has been shown to enhance endothelial cell proliferation and migration (10). Furthermore, estradiol upregulates VEGF (vascular endothelial growth factor) expression in endothelial cells, subsequently increasing angiogenesis (11). Dihydrotestosterone, a derivative of testosterone associated with males, has also been reported to converge on VEGF signaling through the androgen receptor, stimulating endothelial cell proliferation (12, 13). Few studies highlight the intersection of chromosomal and sex hormone influences on cellular sex phenotypes (14).
Accounting for the influence of sex hormones in in vitro models is critically important as standard culture practices contain physiologically relevant concentrations of sex hormones in the fetal bovine serum (FBS) (9, 15–19). Most standard culture mediums contain phenol red indicator, a weak estrogen receptor agonist (20, 21). As such, standard culture medium exposes cells to sex hormones, and an alternative medium is needed to delineate the role of sex hormones in sex-specific signaling. Using phenol red-free medium supplemented with charcoal-stripped FBS serves as a hormone-free medium (HFM) for cell culture (12, 16).
Cells also possess distinct secretomes, a profile of secreted factors, that have recently been shown to exhibit sexual dimorphism in male and female endothelial cells (22). In combination, several studies have established that the chromosomal sex of the cell determines both its secretome and how it responds to soluble external signals (8, 22–24).
Despite these previous findings, a focused study on the underlying sex differences in lung-specific endothelial cell angiogenesis and the exogenous factors that govern these differences in vitro have not been previously reported. We hypothesize that sex chromosomes mediate differences in angiogenesis in human pulmonary microvascular endothelial cells (HPMECs). Furthermore, we hypothesize that sex-specific exogenous factors, such as sex hormones or sex-specific secretome, will have a sex-specific influence on angiogenesis.
MATERIALS AND METHODS
Cell Culture
HPMECs (ScienCell, Carlsbad, CA; female lots: 17799, 17807, 15900; male lots: 11816, 11422, 16021) were cultured on fibronectin-coated plates (2 µg/cm2) in standard endothelial cell medium (SM, ScienCell) at 37°C supplemented with 5% CO2. SM was supplemented with 5% FBS, endothelial cell growth supplement (ScienCell), and 1% penicillin-streptomycin (ScienCell). Cultures maintained in HFM were grown in phenol red-free endothelial cell medium (ScienCell) supplemented the same as SM but with 5% charcoal-stripped FBS (HyClone, Logan, UT). Individual donors were equally combined to generate male- or female-pooled HPMECs and grown to near confluence before experimental use (passages 4–6).
Angiogenesis Assay
Angiogenesis was determined using a three-dimensional (3-D) fibrin gel bead assay as previously described (4, 5). Collagen-coated cytodex-3 microcarrier beads (Sigma-Aldrich, St. Louis, MO) were coated with male or female HPMECs at 20,000 cells per 750 beads, incubated for 4 h at 37°C with periodic agitation then statically overnight. Beads were resuspended in 2 mg/mL fibrin (Millipore, Burlington, MA) gels supplemented with 0.15 U/mL of aprotinin (Sigma-Aldrich) at 250 beads/mL. Gel cultures were maintained in SM or HFM for 4 days with daily medium changes. Percentage of beads to produce at least one sprout captured the cell’s ability to respond to proangiogenic factors. Number of sprouts per bead captured cell-to-cell coordination. Maximum length of sprouts captured cell proliferation. Lengths were determined in Image J by tracing the sprout from the edge of the bead to the tip of the sprout.
Conditioned Medium
Conditioned medium experiments tested the influence of sex-specific secretomes on angiogenesis. SM or HFM media was collected from male or female monolayer cultures (∼80% confluent). Conditioned medium was centrifuged (300 g for 10 min) to pellet cellular debris, collected, and stored at 4°C for a maximum of 2 days. Cells were seeded into an angiogenesis assay and were maintained in the conditioned medium with daily media changes for 4 days. These experiments were performed in parallel with male and female HPMECs angiogenesis assays in SM and HFM to serve as controls.
Immunofluorescent Imaging
HPMECs were fixed, stained, and imaged as previously described (25, 26) in 4% paraformaldehyde (Thermo Scientific, Waltham, MA) with 0.1% Triton-X-100 (Thermo Scientific) for 2 h at 4°C. Cells were counterstained with phalloidin-554 (Cell Signaling, Danvers, MA) and Hoechst (Invitrogen, Waltham, MA) overnight at 4°C. Images were taken using an epifluorescent microscope (Zeiss, Oberkochen, Germany).
Statistical Analysis of Control and Conditioned Media Experiments
Significance was determined using Student’s t test. The percentage of beads that produced a minimum of one sprout was determined by averaging across wells, whereas sprout length and number of sprouts were analyzed by averaging across beads.
RESULTS
Role of Sex Hormones in Angiogenesis
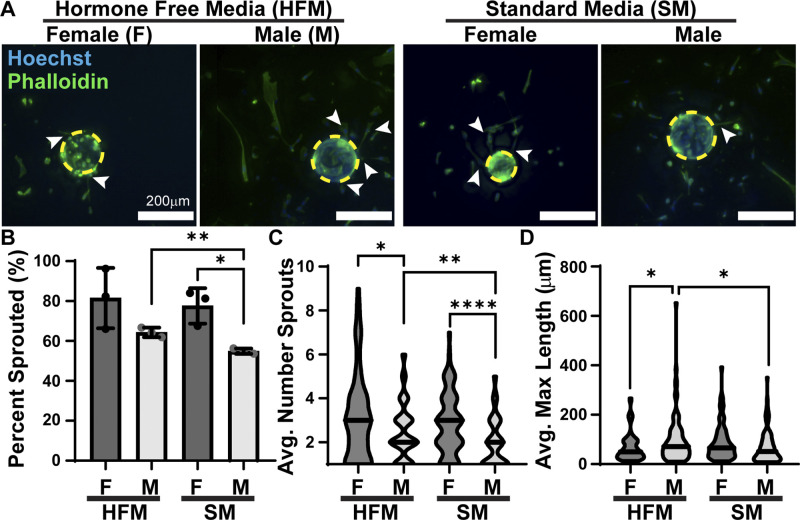
Angiogenesis was quantified in male and female HPMECs using a bead sprouting assay to establish baseline sex differences (HFM) and identify differences controlled by sex hormones (SM). Sprouts are here defined as multiple cells in a continuous line from bead to sprout tip (Fig. 1A). These data show that at baseline, there is no sex difference between male (64%) and female (82%) HPMECs in their ability to produce sprouts (Fig. 1B). However, when sex hormones are present, male HPMECs (55%) were less likely to produce sprouts. The ability to produce sprouts was unchanged in female HPMECs. Female HPMECs had more sprouts (3.1) per bead than male HPMECs (2.5) (Fig. 1C). When male HPMECs were exposed to sex hormones, the average number of sprouts decreased to 2.0 sprouts. In comparison, female HPMECs did not have a significant decrease (2.6 sprouts). Sprout elongation requires that stalk cells gain a proliferative phenotype, a process coordinated by the tip cell. Male HPMECs produced longer sprouts (99.7 μm) than female HPMECs (64.9 μm), a difference that was abolished when sex hormones were present (Fig. 1D). Male HPMEC sprout lengths decreased to 70.1 μm. In contrast, female HPMECs sprout length was not significantly different (88.5 μm) in the presence of sex hormones.
Figure 1.
Male HPMECs produce fewer, but longer, sprouts compared with female HPMECs. A: male- and female-coated beads cultured in HFM and SM. Dashed yellow circles outline beads, arrow denotes sprouts. B: percentage of beads that produced at least one sprout, means ± SD, n = 3 wells. C: average number of sprouts per bead, n = 72–86 beads. D: average maximal length of sprouts per bead, n = 72–86 beads. *P < 0.05, **P < 0.01, ****P < 0.0001. HFM, hormone-free medium; HPMEC, human pulmonary microvascular endothelial cell; SM, standard medium.
Influence of Sex-Specific Cell Secretions on Angiogenesis
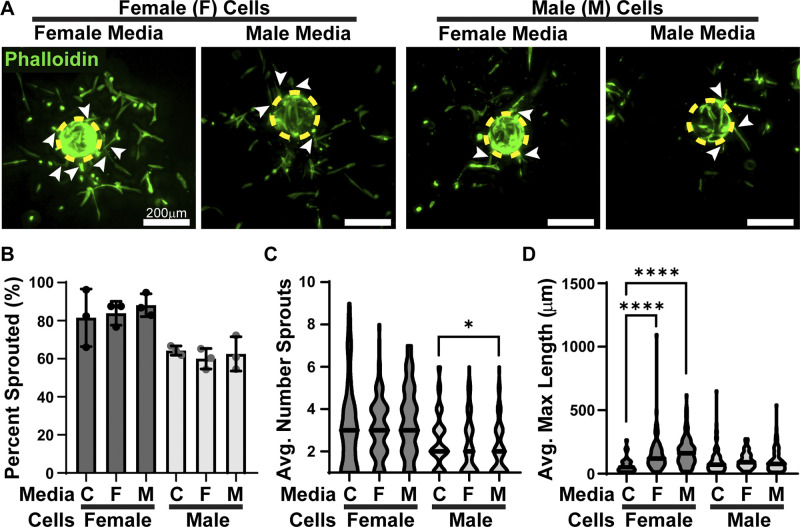
To test the influence of sex-specific secretomes, angiogenesis was assessed in the presence of conditioned media. HFM was conditioned by either male or female HPMECs grown in a monolayer and subsequently used to stimulate angiogenesis in male and female HPMECs (Fig. 2A). HPMECs cultured in a fibrin clot with unconditioned HFM served as a control. Neither male- nor female-conditioned media had a significant influence on the ability of male or female HPMECs to produce sprouts, with 60.0–88.2% beads with sprouts across conditions (Fig. 2B). Female-conditioned media had no significant effects on the average number of sprouts in male (2.2 sprouts) or female (3.1 sprouts) HPMECs. Although male-conditioned media did not significantly affect female HPMEC average number of sprouts (3.6 sprouts), it significantly decreased the average number of male sprouts (2.1) (Fig. 2C).
Figure 2.
HPMEC secretome influences angiogenesis in a sex-dependent manner. A: male- and female-coated beads cultured in HFM conditioned by male or female HPMECs. Dashed yellow circles outline beads, arrows denote sprouts. B: percentage of beads that produced at least one sprout, means ± SD, n = 3 wells. C: average number of sprouts per bead, n = 72–104 beads. D: average maximal length of sprouts, n = 72–104 beads. C denotes control, *P < 0.05 and ****P < 0.0001 compared with the sex-matched control. HFM, hormone-free medium; HPMEC, human pulmonary microvascular endothelial cell.
The average maximal length of sprouts in male HPMECs remained constant in all three conditions (99.7–105.4 μm). In contrast, female HPMECs had dramatically longer sprouts than the control (64.9 μm) compared with female- (151.1 μm) or male (178.3 μm)-conditioned media (Fig. 2D). This increase in length in the female HPMECs is ∼2.5 times longer than control.
Influence of Sex Hormones on Cell Secretions on Angiogenesis
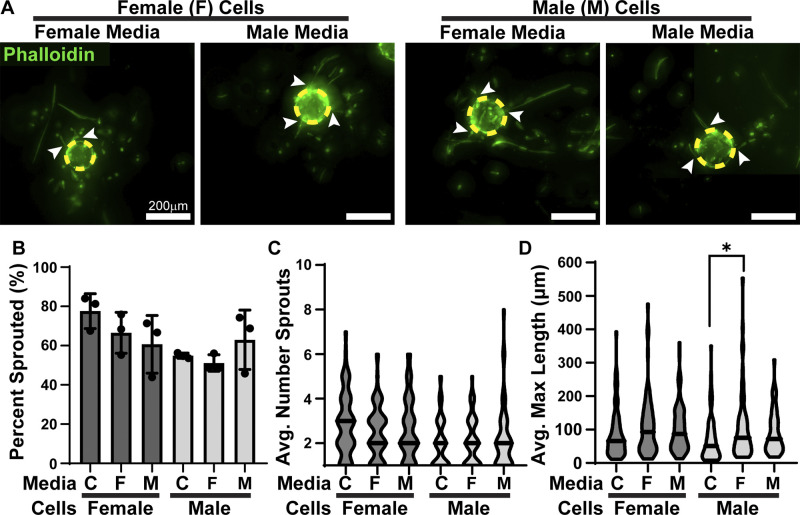
The presence of sex hormones can influence the secretome of cells. To test the influence of sex hormones on sex-specific secretions, we conditioned sex hormone-containing SM with male or female HPMECs grown in a monolayer and subsequently used it to stimulate angiogenesis in male and female HPMECs (Fig. 3A). HPMECs cultured in unconditioned SM served as a control. Neither male- nor female-conditioned media in the presence of sex hormones had a significant influence on the ability of female (60–84%) or male (54–81%) HPMECs to produce a sprout (Fig. 3B). These conditioned media also did not influence female (2.2–3.3) or male (1.8–2.5) average number of sprouts (Fig. 3C).
Figure 3.
HPMEC response to the secretome is sex-hormone dependent. A: male- and female-coated beads cultured in SM (hormone containing) conditioned by male or female HPMECs. Dashed yellow circles outline beads, arrows denote sprouts. B: percentage of beads that produced at least one sprout, means ± SD, n = 3 wells. C: average number of sprouts per bead, n = 59–86 beads. D: average maximal length of sprouts, n = 59–86 beads. C denotes control, *P < 0.05 compared with the sex-matched control. HPMEC, human pulmonary microvascular endothelial cell; SM, standard medium.
The presence of sex hormones with male- and female-conditioned media abolished the HFM secretome response of female HPMECs having longer sprouts. Female HPMECs in conditioned SM (89.6–110.8 µm) had lengths comparable to controls (Fig. 3D). Female HPMECs secrete factors that male HPMECs responded to in the presence of sex hormones, producing longer sprouts (107 µm) than controls (69.8 µm). In the presence of sex hormones, male HPMEC secretions had no significant influence over maximal sprout length in male HPMECs (92.8 µm).
DISCUSSION
Sex differences are prominent in pulmonary angiogenesis, a critical process for proper lung development (1). In this study, we characterized sex differences of human pulmonary microvascular endothelial cells. Our objective was to identify sex differences in 3-D angiogenesis and determine the role of exogenous factors in this process.
In the absence of sex hormones, male and female HPMECs were equally likely to produce at least one sprout in response to proangiogenic factors. This demonstrates that both male and female lung endothelial cells respond to proangiogenic factors regardless of chromosomal identity. Both estrogen and testosterone play a role in sex-specific signaling and are expected to have some role in the observed phenotype of the HPMEC response to sprouting. When sex hormones were present in the medium, angiogenic sprouting was inhibited only in male HPMECs, with a lower percentage of beads containing sprouts. Therefore, we hypothesized that the inherent ability to respond to a proangiogenic factor by male and female HPMECs is the same; however, when exogenous sex hormones are present, tip cell formation is inhibited in male cells but not in female cells. Identifying which sex hormone is responsible for this inhibition in male pulmonary endothelial cells, as well as the underlying mechanism of that inhibition, should be pursued.
Once an endothelial cell responds to a proangiogenic factor, a complex signaling cascade inhibits neighboring cells from responding (27–30). This is canonically achieved through Delta-like ligand 4 and Notch signaling (27, 28). In the absence of sex hormones, female HPMECs produced more sprouts per bead than male HPMECs (Fig. 1C). This suggests that the area of inhibition over neighboring cells becoming tip cells is increased in male HPMECs. The presence of sex hormones did not influence the number of sprouts per bead in female HPMECs but did have a further inhibitory effect on male HPMECs, with fewer angiogenic sprouts per bead observed.
Angiogenesis requires not only the formation of a tip cell but also the coordination of a proliferative stalk (30, 31). Contrary to the tip cell behaviors, in the absence of sex hormones, male HPMECs had longer sprouts than female HPMECs (Fig 1C). Combined, this represents a male and female phenotype where males produce fewer but longer sprouts than female HPMECs (Fig. 4A). The functional advantage of one phenotype over another is unclear. However, it is likely that the ability to change between different phenotypes in response to stressors such as hyperoxia and inflammation, which are superimposed onto the requirements of the developing and growing lung, may underpin functional outcomes. Consistent with the other parameters of angiogenesis, the sexual dimorphism of maximum sprout length was abolished when male and female HPMECs were exposed to sex hormone-containing medium. Female HPMECs were unaffected by the presence of sex hormones, whereas male HPMECs exhibited decreased sprout length, further demonstrating that the presence of sex hormones had an overall inhibitory role in male angiogenesis (Fig. 4B).
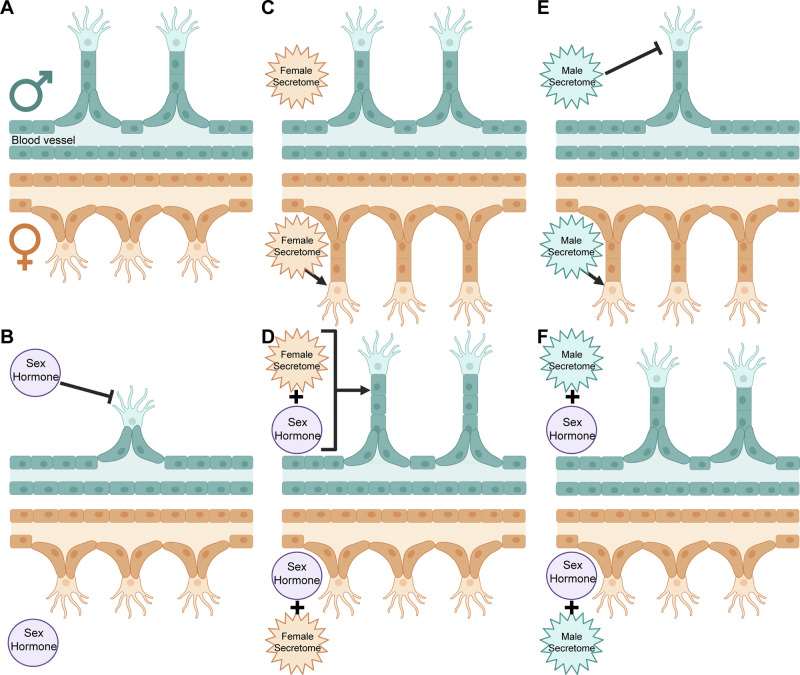
Figure 4.
Sex-specific angiogenesis phenotype and the influence of exogenous factors. A: baseline phenotype: male HPMECs produce longer but fewer sprouts than females. B: male HPMECs produce shorter and fewer sprouts in the presence of sex hormones. C: female HPMECs produce longer sprouts in the presence of a female secretome. D: sex hormones and a female secretome increase sprout length in male HPMECs but eliminate changes from the female secretome alone in female HPMECs. E: male HPMECs produce fewer sprouts, whereas female HPMECs produce longer sprouts in the presence of a male secretome. F: sex hormones in combination with the male secretome nullify the effects of either source independently, with male and female angiogenesis resembling the baseline phenotype. HPMEC, human pulmonary microvascular endothelial cell. Created with Biorender.com and published with permission.
To address the secretome, we performed a bead-sprouting angiogenesis assay with media conditioned by male or female HPMECs in the absence of exogenous sex hormones. The ability of male and female HPMECs to respond to proangiogenic factors and produce at least one sprout remained unchanged when male and female HPMECs were exposed to male- or female-conditioned media (Fig 2B). This was consistent even when sex hormones were present in the conditioned medium (Fig. 3B). Interestingly, conditioned media from male HPMECs contain factors that decreased the number of sprouts from male but not female HPMECs (Fig 2C). The presence of sex hormones abolished this further decrease in sprout number of male HPMECs in response to male-conditioned media (Fig. 3C). In contrast, both male and female HPMECs secreted factors that stimulated the elongation of sprouts in female HPMECs but had no influence on male HPMECs (Fig 2C). Again, the presence of sex hormones in the conditioned media abolished this effect on female HPMECs with the average maximal sprout length similar to control cells (Fig. 3C). In contrast, male HPMECs had increased maximal sprout lengths when exposed to the secretome of female HPMECs in the presence of sex hormones. Together, this demonstrates that male and female HPMECs not only have distinct secretomes but also these secretomes produce a response that is dependent upon both the presence of sex hormones and the sex of the receiving cells (Fig. 4, C–F).
Conclusions
These findings represent the first steps to identifying how male and female lung endothelial cells undergo angiogenesis differently and what exogenous factors influence this process. Sex hormones, as well as sex-specific secretomes, play a pivotal role in angiogenesis and need to be considered in future studies. Identification of molecular targets of these sex differences in lung angiogenesis has implications for therapeutic targets for the treatment of BPD.
GRANTS
This work was partly supported by National Institutes of Health Grants T32GM133395 and F31HL152611 (to B.H-P.); R01HL144775, R01HL146395, and R21HD100862 (to K.L.); and R01HL133163 and R01HL145147 (to J.P.G.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
B.H-P. and J.P.G. conceived and designed research; B.H-P. and S.C.P. performed experiments; B.H-P., S.C.P., and J.P.G. analyzed data; B.H-P., C.R.G., S.C.P., K.L., and J.P.G. interpreted results of experiments; B.H-P. and J.P.G. prepared figures; B.H-P. and J.P.G. drafted manuscript; B.H-P., C.R.G., S.C.P., K.L., and J.P.G. edited and revised manuscript; B.H-P., C.R.G., S.C.P., K.L., and J.P.G. approved final version of manuscript.
REFERENCES
- 1. Lingappan K, Jiang W, Wang L, Moorthy B. Sex-specific differences in neonatal hyperoxic lung injury. Am J Physiol Lung Cell Mol Physiol 311: L481–L493, 2016. doi: 10.1152/ajplung.00047.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Coarfa C, Zhang Y, Maity S, Perera DN, Jiang W, Wang L, Couroucli X, Moorthy B, Lingappan K. Sexual dimorphism of the pulmonary transcriptome in neonatal hyperoxic lung injury: identification of angiogenesis as a key pathway. Am J Physiol Lung Cell Mol Physiol 313: L991–L1005, 2017. doi: 10.1152/ajplung.00230.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Collaco JM, Aherrera AD, McGrath-Morrow SA. The influence of gender on respiratory outcomes in children with bronchopulmonary dysplasia during the first 3 years of life. Pediatr Pulmonol 52: 217–224, 2017. doi: 10.1002/ppul.23520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhang Y, Coarfa C, Dong X, Jiang W, Hayward-Piatkovskyi B, Gleghorn JP, Lingappan K. MicroRNA-30a as a candidate underlying sex-specific differences in neonatal hyperoxic lung injury: implications for BPD. Am J Physiol Lung Cell Mol Physiol 316: L144–L156, 2019. doi: 10.1152/ajplung.00372.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang Y, Dong X, Shirazi J, Gleghorn JP, Lingappan K. Pulmonary endothelial cells exhibit sexual dimorphism in their response to hyperoxia. Am J Physiol Heart Circ Physiol 315: H1287–H1292, 2018. doi: 10.1152/ajpheart.00416.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Morgan JT, Stewart WG, McKee RA, Gleghorn JP. The mechanosensitive ion channel TRPV4 is a regulator of lung development and pulmonary vasculature stabilization. Cell Mol Bioeng 11: 309–320, 2018. doi: 10.1007/s12195-018-0538-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gilbert RM, Morgan JT, Marcin ES, Gleghorn JP. Fluid mechanics as a driver of tissue-scale mechanical signaling in organogenesis. Curr Pathobiol Rep 4: 199–208, 2016. doi: 10.1007/s40139-016-0117-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lorenz M, Koschate J, Kaufmann K, Kreye C, Mertens M, Kuebler WM, Baumann G, Gossing G, Marki A, Zakrzewicz A, Miéville C, Benn A, Horbelt D, Wratil PR, Stangl K, Stangl V. Does cellular sex matter? Dimorphic transcriptional differences between female and male endothelial cells. Atherosclerosis 240: 61–72, 2015. doi: 10.1016/j.atherosclerosis.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 9. Lingappan K, Hayward-Piatkovskyi B, Gleghorn JP. Neonatal lung disease: mechanisms driving sex differences. In: Sex-Based Differences in Lung Physiology, edited by Silveyra P, Tigno XT.. Cham: Springer International Publishing, 2021, p. 115–144. [Google Scholar]
- 10. Losordo DW, Isner JM. Estrogen and angiogenesis. Arterioscler Thromb Vasc Biol 21: 6–12, 2001. doi: 10.1161/01.ATV.21.1.6. [DOI] [PubMed] [Google Scholar]
- 11. Barnabas O, Wang H, Gao X-M. Role of estrogen in angiogenesis in cardiovascular diseases. J Geriatr Cardiol 10: 377–382, 2013. doi: 10.3969/j.issn.1671-5411.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cai J, Hong Y, Weng C, Tan C, Imperato-McGinley J, Zhu Y-S. Androgen stimulates endothelial cell proliferation via an androgen receptor/VEGF/cyclin A-mediated mechanism. Am J Physiol Heart Circ Physiol 300: H1210–H1221, 2011. doi: 10.1152/ajpheart.01210.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sieveking DP, Lim P, Chow RWY, Dunn LL, Bao S, McGrath KCY, Heather AK, Handelsman DJ, Celermajer DS, Ng MKC. A sex-specific role for androgens in angiogenesis. J Exp Med 207: 345–352, 2010. doi: 10.1084/jem.20091924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Arnold AP. A general theory of sexual differentiation. J Neurosci Res 95: 291–300, 2017. doi: 10.1002/jnr.23884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hollier LP, Keelan JA, Hickey M, Maybery MT, Whitehouse AJO. Measurement of androgen and estrogen concentrations in cord blood: accuracy, biological interpretation, and applications to understanding human behavioral development. Front Endocrinol (Lausanne) 5: 64, 2014. doi: 10.3389/fendo.2014.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Song W, Khera M. Physiological normal levels of androgen inhibit proliferation of prostate cancer cells in vitro. Asian J Androl 16: 864–868, 2014. doi: 10.4103/1008-682X.129132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kim J, Kim M, Nahm S-S, Lee D-M, Pokharel S, Choi I. Characterization of gendr-specific bovine serum. Anim Cells Syst 15: 147–154, 2011. doi: 10.1080/19768354.2011.577584. [DOI] [Google Scholar]
- 18. Hill M, Pašková A, Kančeva R, Velíková M, Kubátová J, Kancheva L, Adamcová K, Mikešová M, Žižka Z, Koucký M, Šarapatková H, Kačer V, Matucha P, Meloun M, Pařízek A. Steroid profiling in pregnancy: a focus on the human fetus. J Steroid Biochem Mol Biol 139: 201–222, 2014. doi: 10.1016/j.jsbmb.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 19. Lagiou P, Samoli E, Okulicz W, Xu B, Lagiou A, Lipworth L, Georgila C, Vatten L, Adami HO, Trichopoulos D, Hsieh CC. Maternal and cord blood hormone levels in the United States and China and the intrauterine origin of breast cancer. Ann Oncol 22: 1102–1108, 2011. doi: 10.1093/annonc/mdq565. [DOI] [PubMed] [Google Scholar]
- 20. Berthois Y, Katzenellenbogen JA, Katzenellenbogen BS. Phenol red in tissue culture media is a weak estrogen: implications concerning the study of estrogen-responsive cells in culture. Proc Natl Acad Sci USA 83: 2496–2500, 1986. doi: 10.1073/pnas.83.8.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Welshons WV, Wolf MF, Murphy CS, Jordan VC. Estrogenic activity of phenol red. Mol Cell Endocrinol 57: 169–178, 1988. doi: 10.1016/0303-7207(88)90072-x. [DOI] [PubMed] [Google Scholar]
- 22. Cattaneo MG, Banfi C, Brioschi M, Lattuada D, Vicentini LM. Sex-dependent differences in the secretome of human endothelial cells. Biol Sex Differ 12: 7, 2021. doi: 10.1186/s13293-020-00350-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Boscaro C, Trenti A, Baggio C, Scapin C, Trevisi L, Cignarella A, Bolego C. Sex pdifferences in the pro-angiogenic response of human endothelial cells: focus on PFKFB3 and FAK activation. Front Pharmacol 11: 587221, 2020. doi: 10.3389/fphar.2020.587221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stanhewicz AE, Wenner MM, Stachenfeld NS. Sex differences in endothelial function important to vascular health and overall cardiovascular disease risk across the lifespan. Am J Physiol Heart Circ Physiol 315: H1569–H1588, 2018. doi: 10.1152/ajpheart.00396.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Morgan JT, Shirazi J, Comber EM, Eschenburg C, Gleghorn JP. Fabrication of centimeter-scale and geometrically arbitrary vascular networks using in vitro self-assembly. Biomaterials 189: 37–47, 2019. doi: 10.1016/j.biomaterials.2018.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gilbert RM, Schappell LE, Gleghorn JP. Defective mesothelium and limited physical space are drivers of dysregulated lung development in a genetic model of congenital diaphragmatic hernia. Development 148: dev199460, 2021. doi: 10.1242/dev.199460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kangsamaksin T, Tattersall IW, Kitajewski J. Notch functions in developmental and tumour angiogenesis by diverse mechanisms1. Biochem Soc Trans 42: 1563–1568, 2014. doi: 10.1042/BST20140233. [DOI] [PubMed] [Google Scholar]
- 28. Gore AV, Swift MR, Cha YR, Lo B, McKinney MC, Li W, Castranova D, Davis A, Mukouyama Y-S, Weinstein BM. Rspo1/Wnt signaling promotes angiogenesis via Vegfc/Vegfr3. Development 138: 4875–4886, 2011. doi: 10.1242/dev.068460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hellström M, Phng L-K, Hofmann JJ, Wallgard E, Coultas L, Lindblom P, Alva J, Nilsson A-K, Karlsson L, Gaiano N, Yoon K, Rossant J, Iruela-Arispe ML, Kalén M, Gerhardt H, Betsholtz C. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature 445: 776–780, 2007. doi: 10.1038/nature05571. [DOI] [PubMed] [Google Scholar]
- 30. Lobov IB, Renard RA, Papadopoulos N, Gale NW, Thurston G, Yancopoulos GD, Wiegand SJ. Delta-like ligand 4 (Dll4) is induced by VEGF as a negative regulator of angiogenic sprouting. Proc Natl Acad Sci USA 104: 3219–3224, 2007. doi: 10.1073/pnas.0611206104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Phng L-K, Gerhardt H. Angiogenesis: a team effort coordinated by notch. Dev Cell 16: 196–208, 2009. doi: 10.1016/j.devcel.2009.01.015. [DOI] [PubMed] [Google Scholar]