Keywords: muscle strength, muscle mass, post-acute COVID-19 syndrome
Abstract
Understanding the impact of COVID-19 on muscle strength may help to elucidate the organ systems that contribute to acute and chronic COVID-19 sequelae. We questioned whether patients with postdischarge symptoms after COVID-19 had compromised muscle strength compared with a control group, and if this potential relationship was mediated by the lower appendicular lean mass index (ALMI). A total of 99 patients with long-COVID-19 and 97 control participants were screened. Maximal grip strength was assessed with a TKK 5101 digital dynamometer, and leg extension 1RM was measured using EGYM Smart Strength machines. Body composition (fat mass percentage, lean mass, visceral fat, and appendicular lean mass index) was determined using a whole body dual-energy X-ray densitometer. Results showed that grip strength and leg extension strength were significantly higher in controls than in COVID-19 survivors (mean [SD], 32.82 [10.01] vs. 26.94 [10.33] kg; difference, 5.87 kg; P < 0.001) and (mean [SD], 93.98 [33.73] vs. 71.59 [33.70] kg; difference, 22.38 kg; P < 0.001), respectively). The relationship between long-COVID syndrome and grip/leg strength levels was partly mediated by ALMI, which explained 52% of the association for grip strength and 39% for leg extension. Our findings provide novel insights into the mechanisms underlying the relationship between long-COVID syndrome and grip/leg strength levels, supporting the negative effects of long-COVID syndrome on muscle function.
NEW & NOTEWORTHY The causes of post-COVID-19 syndrome are uncertain. Limb muscle wasting common to patients with COVID-19 limits daily activities and exercise. In this cross-sectional study, we found that patients with long-COVID-19 syndrome had significantly lower absolute and relative muscle strength measurements than control participants. Interestingly, we identified that these relationships were mostly mediated by limb muscle mass. Our data thus suggest that the evident reduced upper and lower muscle mass is a putative cause of—or contributor to—the functional limitation of patients with long-COVID-19 syndrome.
INTRODUCTION
The impact of coronavirus disease 2019 (COVID-19) continues to be felt (1). The disease is characterized by respiratory illness and systemic inflammation, with serious and often long-term consequences for physiological function (2, 3). Indeed, the time course over which pathophysiological changes occur following severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) exposure remains unclear. Although the lasting complications of COVID-19 are still being studied, fatigue is a frequent and debilitating symptom and may continue for at least 6–7 months after symptoms onset (4). A recent systematic review and meta-analysis found that persistent symptoms occur in ∼80% of infected adult patients, with the most frequent being fatigue, headache, attention deficit disorder, exercise intolerance, and dyspnea (5). Other long-lasting symptoms of COVID-19 include diminished muscle strength and exercise intolerance, which is characterized by reduced physical performance with limb muscle weakness (6). Accordingly, the term long-COVID-19 or post-COVID-19 syndrome has been proposed for those patients with symptoms that persist for >3 mo after onset (7). Risk factors for long COVID included female sex, belonging to an ethnic minority, socioeconomic deprivation, smoking, obesity, and a wide range of comorbidities (8). Other contributing factors are similar to those seen in several chronic disease conditions such as critical illness myopathy and aging, which manifest with reduced physical and mental energy. In fact, this is a common definition used to describe the general fatigue associated with acute-COVID-19 and long-COVID-19 syndrome (9).
The cause of the loss of muscle strength seen in patients with COVID-19 and long-COVID syndrome is likely multifactorial. Although it is clear that multiple comorbidities exacerbate COVID-19 disease severity and symptoms, the disease per se might act as a second-hit mechanism, amplifying muscle weakness and exercise intolerance (10). Skeletal muscle-related symptoms such as pain (myalgia), muscle weakness (mild to severe), fatigue, and exercise intolerance are common in both acute-COVID-19 and long-COVID-19 syndrome (11). Limb muscle wasting common to patients with COVID-19 limits daily activities and exercise (12). It has been speculated that hypoxia, malnutrition, and medication may play a smaller role, and other factors such as low-grade systemic inflammation, physical inactivity, persisting viral load, and possibly specific genotypes (such as higher skeletal muscle ACE protein content in women) might play important roles (13).
Viruses enter susceptible cells through different mechanisms, such as cell adhesion and interaction with specific receptors, which is followed by invasion through endocytosis and fusion (14). Leung et al. (15) in their study report that SARS-CoV-1 infection impacts skeletal muscle via direct viral invasion, due to the angiotensin-converting enzyme 2 (ACE2) receptor is widely expressed in the musculoskeletal system. Thus, on the whole, skeletal muscle in SARS-CoV-2-infected patients might be more susceptible to muscle damage. For example, clinical observations have revealed that patients with SARS-CoV-2 infection are at high risk of developing sarcopenia acutely or insidiously (16), myalgias, myositis, rhabdomyolysis, and skeletal muscle atrophy (17). In addition, a few neuromuscular disorders have also been reported in COVID-19, including peripheral neuropathy and Guillain–Barre syndrome (18). Mechanisms that explain why these disorders commonly develop in the setting of COVID-19 are still lacking, but SARS-CoV-2-induced cytokine storm seems to be one of the main reasons that justify it (19). Other risk factors such as systemic inflammation, hypoxemia, extended periods of (forced) inactivity, and various medications may promote or exacerbate muscle weakness, fatigue, and exercise intolerance in patients with COVID-19 (20). However, little is known regarding the potential role of the lean mass on muscle strength performance in patients with COVID-19.
Understanding the impact of COVID-19 on muscle strength may help in elucidating the organ system(s) that initiate and contribute to acute- and long-term COVID-19 sequelae. In the present study, we investigated whether patients with postdischarge symptoms after COVID-19 had compromised muscle strength compared with a control group, and we tested if these potential relationships are mediated by appendicular lean mass index (ALMI).
METHODS
This exploratory secondary analysis used baseline data from “The EXER-COVID Crossover Study” (NCT04797871) (21). The characteristics of the study cohort have been previously described (22). All patients were from the Hospital Universitario de Navarra (Pamplona, Spain) and were screened for inclusion by a physician to confirm a diagnosis of COVID-19 and no other psychiatric or somatic conditions that could explain the persistent COVID-19 symptoms. For all identified participants, we revised patients with psychiatric disorders were revised psychiatric diagnostic codes from their electronic health records using billing/encounter diagnoses, external claim diagnoses, and inpatient hospital problems before their testing encounter such as schizophrenia spectrum disorders (12), mood disorders (23), and anxiety disorders (24). Data were collected between March 2021 and February 2022. The inclusion criteria were diagnosis of long-COVID-19, mild or moderate symptoms, no hospitalization, and no heart disease.
From a total of 105 patients with long-COVID-19 screened, 99 patients had complete muscle measurements at baseline and were included in the exploratory analysis. Control participants (n = 97) were from a published cohort that included participants tested for physical fitness in Pamplona, Spain (i.e., the same altitude as our cohort) before the first confirmed case of COVID-19 in Pamplona (on March 3, 2020). Control participants (n = 99) had no flu-like symptoms. The characteristics of the two cohorts have been previously described (22). The study was conducted according to the Declaration of Helsinki and was approved by the Ethics Committee on Human Research (CEIH, Protocol No. PI_2020/140) of the Hospital Universitario of Navarra (HUN) (Pamplona, Spain). Written informed consent was obtained from all patients and information was given about Spain’s data protection law.
All participants had their health history recorded, including physical activity and any current medication. A stadiometer and scale were used to measure height and weight (Seca model 799, Electronic Column Scale, Hamburg, Germany), with participants wearing light clothing and no shoes. Body mass index (BMI) was then calculated as the weight (in kilograms) divided by the squared height (in meters). Total and regional body composition was determined using a whole body dual-energy X-ray densitometer (Lunar DPX, General Electric, Madison, WI). Scans were imported into an updated version of the software (v.13.6) and reanalyzed using algorithms that provided automatic segmentation of body fat (%), lean mass (%), and visceral adipose tissue (25). Based on the European Working Group for Sarcopenia guidelines (EWGSOP) (26), the sum of upper and lower limbs’ lean mass, defined as appendicular lean mass (ALM), was used to quantify muscle mass, and this value was indexed to height, and ALMI = (ALM/height2) was obtained. The recent EWGSOP guidelines suggest an ALMI < 6.0 kg/m2 in women and ALMI < 7.0 kg/m2 in men as diagnostic cut-off values to define low muscle mass (18).
Maximal isometric handgrip strength was assessed with a maximal 3-s voluntary contraction using a TKK 5101 digital dynamometer (Takei Scientific Instruments Co., Ltd., Tokyo, Japan). Maximal dynamic leg extension strength (one-maximum muscle strength) was measured using a Smart Strength machine (eGym GmbH, München, Germany). Results were expressed in kilograms and both parameters were divided by body mass to give relative muscle strength, as described (27).
The short, self-administered International Physical Activity Questionnaire (IPAQ) (28) was used to assess physical activity. The IPAQ focuses on the amount of physical activity performed over the past 7-day period. The IPAQ includes questions about the time spent engaging in vigorous physical activities, moderate physical activities, and walking. Responses were converted to metabolic equivalent task minutes per week (MET-min/wk) according to the IPAQ scoring protocol. The weighted MET minutes per week were then calculated by multiplying the duration (minutes), frequency (days), and MET intensity, and then summing across the three domains, namely, vigorous, moderate, and walking, to produce a weighted estimate of total physical activity per week (MET-min/wk).
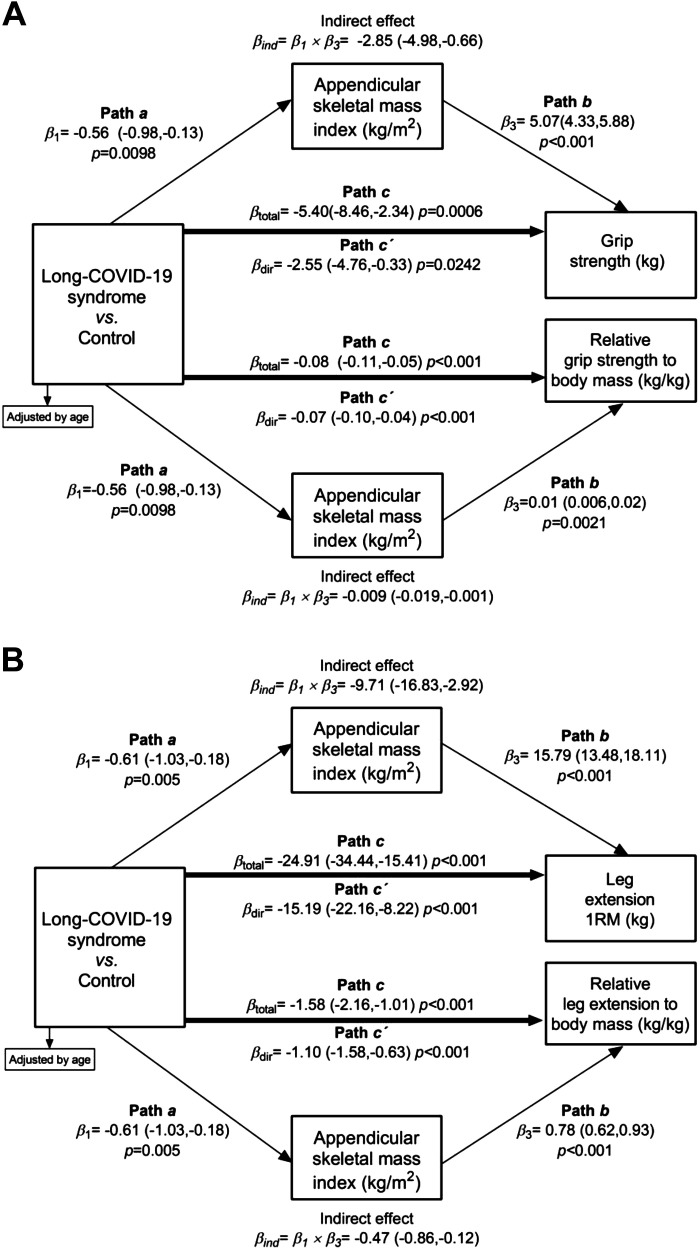
Group characteristics were compared using Student’s t test or the Vovk–Sellke Maximum p-ratio, unless otherwise stated, for continuous variables, and the χ2 test was used for categorical variables. Variables are expressed as mean (SD) or n with percentages based on a two-sided P value. Raincloud plots were produced using JASP 0.16.2 software (29) for data visualization of muscle strength parameters. The 95% confidence intervals (CIs) were further interpreted to indicate that significant within-group differences occurred if the upper or lower limits do not cross zero. In addition, we calculated Cohen’s d for the effect size, considering the effect as trivial (<0.20), small (0.20–0.50), moderate (0.50–0.80), and large (>0.80). Mediation analyses were performed using the PROCESS Statistical Package for SPSS, release 3.5.3 (IBM Corp., Portsmouth, Hampshire, UK) to test potential mediation by appendicular lean mass index for the association of groups (control vs. long-COVID-19 syndrome), on the main outcomes (muscle strength parameters). In brief, studies aimed to examine the relationship between muscle quality/metabolism, and muscle strength/power, confounding or mediator variables have been usually reported by univariate methods such as t-student (20, 30), correlation (31), or variance analysis (32), depending on the objectives of the study and the characteristics of the dependent variable. Mediation analysis is a statistical procedure that can be used to clarify the processes underlying an association between two variables and the extent to which the association can be modified, mediated, or confounded by a third variable (33). A mediation effect occurs when a third variable (the mediator) is responsible for the influence of a given independent variable on a given dependent variable. This approach uses nested models to estimate the proportion of the total adjusted association of an exposure explained by its indirect association via the mediator, with 95% CIs estimated using a nonparametric bootstrapping method. Each mediation analysis model was run using 5000 bootstrapping. We applied age covariate adjustment model for consistency across mediation analyses. Finally, we calculated the percent mediation, representing the ratio of the total effect attributable to the corresponding indirect effect (Fig. 1). A two-tailed P value of 0.05 was considered significant for all analyses.
Figure 1.
The decomposition of effects for the relationship between groups (control vs. long-COVID-19 syndrome) and muscle strength parameters, mediated through appendicular lean mass index. Nodes represent the variables being analyzed in the mediation model. Group in the equations below represents a dichotomous variable (long-COVID-19 syndrome = 1, control = 0). The total effect, denoted by path c, is the lineal regression coefficient of groups (control vs. long-COVID-19 syndrome) on the outcomes (muscle strength parameters) without controlling for the mediator. The direct effect, denoted by path c′, is the effect coefficient of exposure groups in a lineal regression with groups (control vs. long-COVID-19 syndrome), and the mediator on the outcomes. Path a denotes the effect coefficient of exposure groups on appendicular lean mass index. Path b denotes the effect coefficient of appendicular lean mass index on the outcomes, in the model with both the exposure and mediator. The indirect effect is the component of the total effect that is mediated through appendicular lean mass index (c–c′) in the relationship between exposure group and the outcomes; it is denoted by β1 × β3.
RESULTS
Clinical characteristics of the study sample are shown in Table 1. The study cohort included 196 individuals [97 control participants (n = 51, 53% female) and 99 patients with long-COVID-19 syndrome (n = 70, 71% female)]. Body mass, height, and BMI were similar between groups. Body fat percentage and visceral adiposity tissue (cm2) were significantly greater in patients with long-COVID-19 syndrome than in controls (P < 0.001), whereas lean muscle tissue (%), ALMI (kg/m2), and muscle strength parameters were significantly lower (P < 0.001). The prevalence of muscle mass loss estimated based on ALMI, muscle strength—measured by grip dynamometer—and self-reported physical activity levels were significantly higher in long-COVID-19 syndrome compared with controls participants.
Table 1.
Clinical characteristics of the study participants
| Characteristic | Long-COVID Syndrome (n = 99) | Control (n = 97) | P Value |
|---|---|---|---|
| Sex, n, women/men | 70/29 | 51/46 | 0.009 |
| Age, yr | 47.38 (9.99) | 52.22 (11.94) | 0.002 |
| Height, m | 1.66 (0.09) | 1.66 (0.10) | 0.853 |
| Body mass, kg | 74.53 (18.23) | 71.27 (16.04) | 0.259 |
| Body mass index, kg/m2 | 27.13 (5.88) | 26.04 (3.68) | 0.117 |
| Nutritional status by BMI, n (%) | |||
| Normal weight | 38 (38.4) | 39 (40.2) | 0.078 |
| Overweight | 33 (33.3) | 47 (48.5) | |
| Obesity | 28 (28.3) | 11 (11.3) | |
| Body fat, % | 38.93 (7.92) | 33.02 (9.31) | <0.001 |
| Visceral adiposity tissue, cm2 | 190.95 (102.66) | 137.70 (71.15) | <0.001 |
| Muscle tissue lean, % | 58.90 (7.34) | 64.55 (8.70) | <0.001 |
| Appendicular lean mass index, kg/m2 | 7.08 (1.44) | 7.63 (1.47) | 0.009 |
| Low muscle mass, n (%)a | |||
| <6.0 kg/m2 for women <7.0 kg/m2 for men | 12 (12.1) | 3 (3.1) | 0.0345 |
| Overall physical activity (MET-min/wk)b,c | 978.30 (1153.15) | 1763.40 (1694.69) | <0.001 |
| Physical activity levels, n (%)b,c | |||
| Light-intensity activity | 53 (55.2) | 35 (36.1) | <0.001 |
| Moderate-intensity activity | 40 (41.7) | 39 (40.2) | |
| Vigorous-intensity activity | 3 (3.1) | 23 (23.7) | |
| Grip strength, kg | 27.30 (10.50) | 32.70 (10.0) | <0.001 |
| Low muscle strength, n (%)d | |||
| <18 kg for women <28 kg for men | 17 (17.5) | 2 (2.0) | <0.001 |
| Leg extension 1RM, kg | 72.73 (34.34) | 93.45 (33.87) | <0.001 |
Data are means (SD). Two-tailed Student’s t tests for two samples of equal variance were performed between control and long-COVID-19 syndrome groups. The χ2 test was performed for sex-variable groups. aThe recent European Working Group on Sarcopenia in Older People (EWGSOP) guidelines suggest an appendicular lean mass index < 6.0 kg/m2 in women and appendicular lean mass index < 7.0 kg/m2 in men as diagnostic cut-off values to define low muscle mass. bData from 96 participants. cThe short, self-administered international physical activity questionnaire (IPAQ) was used to assess overall physical activity. The short form records four types of physical activity: vigorous activity such as aerobics; moderate-intensity activity such as leisure cycling; walking, and sitting, in the last 7 days. Responses were converted to metabolic equivalent task minutes per week (MET-min/wk) according to the IPAQ scoring protocol: total minutes over last 7 days spent on vigorous activity, moderate-intensity activity, and walking (light-intensity activity) were multiplied by 8.0, 4.0, and 3.3, respectively, to create MET scores for each activity level. MET scores across the three subcomponents were summed to indicate overall physical activity. dLow muscle strength was defined as grip strength <18 kg in women and <28 kg in men, according to EWGSOP guidelines.
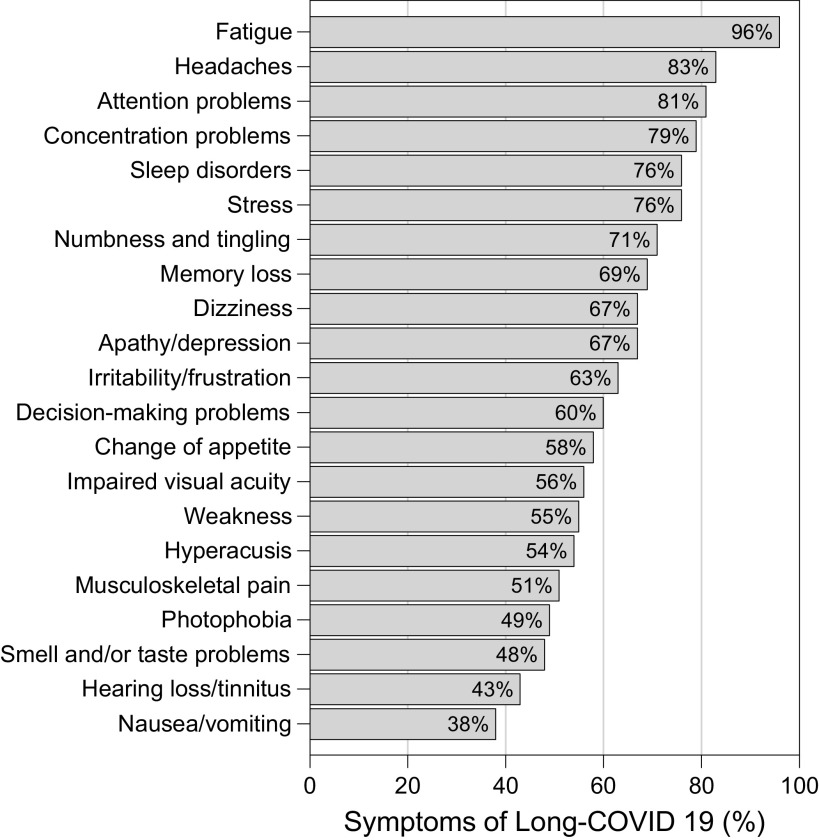
We observed a high proportion of individuals still self-reported fatigue (96%), headaches (83%), attention problems (81%), and concentration problems (79%). About 50% of all participants with long-COVID-19 syndrome have musculoskeletal disorders (weakness or musculoskeletal pain) problems linked to the disease. Other clinical characteristics of the study population were similar to other recent trials in long-COVID persistent (Fig. 2).
Figure 2.
Most common symptoms remaining after 3 mo in 99 respondents from a cohort long-COVID-19 syndrome. Patients were assessed a mean of 323 days (SD 138) days after onset of the first COVID-19 symptom, at the time of the evaluation.
Absolute grip strength was significantly greater in the control group than in the long-COVID-19 group (mean [SD], 32.82 [10.01] vs. 26.94 [10.33] kg; difference, 5.87 kg; P < 0.001) (Fig. 3A). Likewise, relative grip strength were higher in the control group than in the long-COVID-19 group (mean [SD], 0.454 [0.103] vs. 0.363 [0.106] kg; difference, 0.091 kg; P < 0.001) (Fig. 3B). In the same line, absolute leg extension group (mean [SD], 93.98 [33.73] vs. 71.59 [33.70] kg; difference, 22.38 kg; P < 0.001) and relative leg extension strength (mean [SD], 5.76 [2.26] vs. 4.22 [1.92] kg; difference, 1.54 kg; P < 0.001) were significantly higher in the control group than in the long-COVID-19 group (Fig. 3, C and D).
Figure 3.
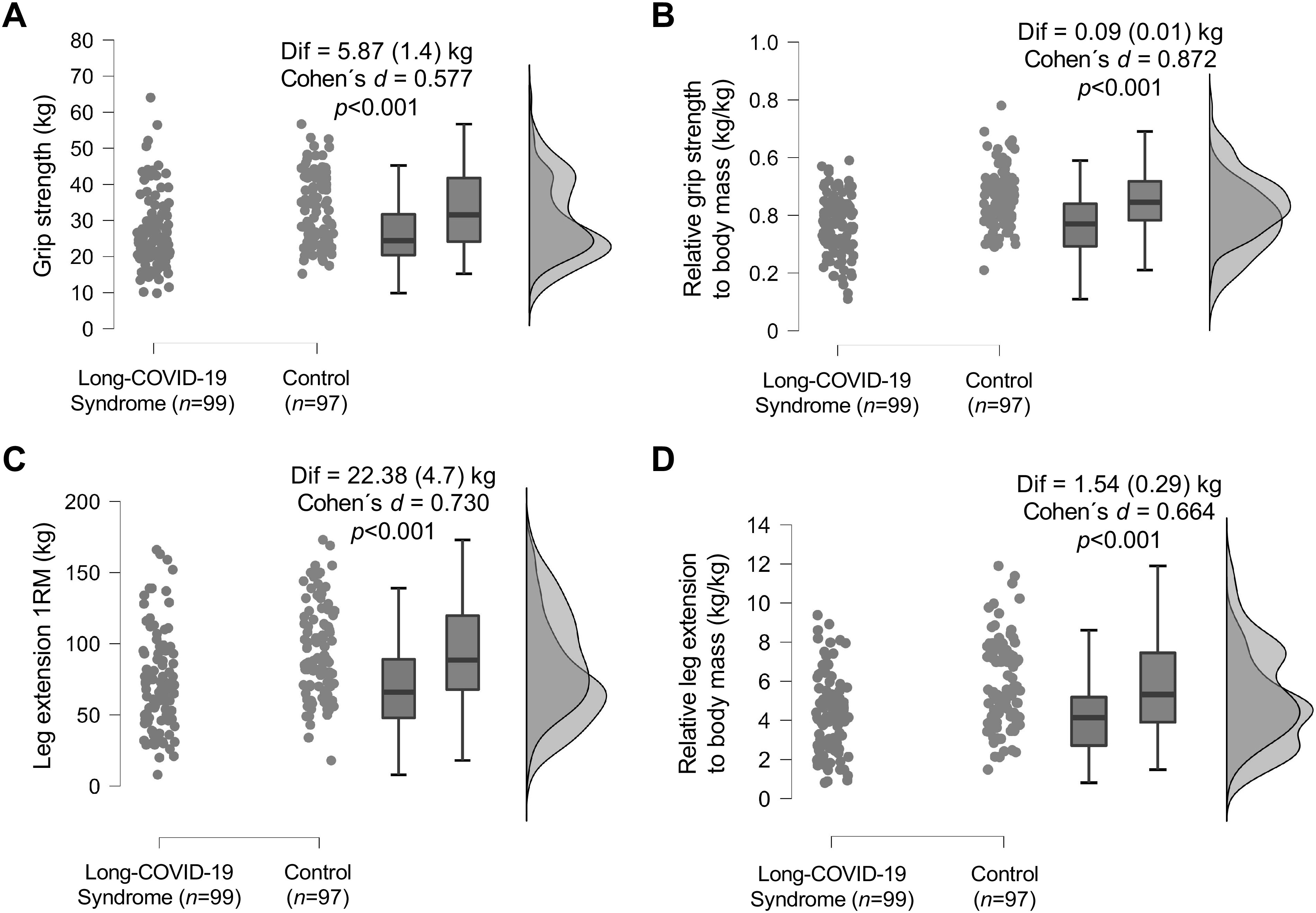
Raincloud plots for muscle strength parameters between the long-COVID-19 syndrome group and the control group. A total of 99 patients with long-COVID-19 and 97 control participants were screened. The plot displays each participant’s mean for muscle strength parameters, a boxplot, and a split-half violin plot of the density for both participant groups. A: absolute grip strength. B: relative grip strength to body mass. C: leg extension 1RM. D: relative leg extension to body mass.
We conducted a mediation analysis to estimate the effect of long-COVID-19 syndrome on muscle strength parameters. Age was included in the analysis as it was independently associated with muscle strength in the interaction analysis. Mediation analysis indicated that most of the total effect of long-COVID-19 on grip strength (–5.40 kg; 95%CI, –8.11, –2.34 kg) was attributable to an indirect effect from ALMI (–2.85 kg (95%CI, –4.85, –0.06 kg; 52% mediation). The remaining direct effect of long-COVID-19 remained statistically significant at –2.55 kg (95%CI, –4.76, –0.33 kg) after the removal of the relative ALMI association (Fig. 4A). Similarly, the analysis indicated that most of the total effect of long-COVID-19 on relative grip strength (–0.08 kg; 95%CI, –0.11, –0.05 kg) was attributable to an indirect effect from ALMI (–0.009 kg; 95%CI, –0.01, –0.001 kg; 11.2% mediation). The remaining direct effect of long-COVID-19 remained statistically significant at –0.07 kg (95%CI, –0.10, –0.04 kg) after the removal of the relative ALMI association (Fig. 4A).
Figure 4.
Effects for the relationship between control group vs. long-COVID-19 syndrome for absolute and relative grip (A) and leg (B) strength levels, mediated through appendicular lean mass index. Linear regressions models were fitted according to the procedures outlined by Baron and Kenny (34) to assess whether the association between control group vs. long-COVID-19 syndrome and absolute and relative grip/leg strength levels was mediated by appendicular lean mass index. The first equation (β1, path a) regressed the mediator (appendicular lean mass index) on the independent variable (control group vs. long-COVID-19 syndrome). The second equation (β3, path b) regressed the dependent variable (absolute or relative grip/leg strength levels) on the independent variable. The third equation regressed (βtotal, path c) the dependent variable on both the independent variable and the mediator variable. The direct effect, denoted as (βdir, path c′), is the effect coefficient of independent variable in a linear regression and the mediator on the dependent variable. The indirect effect is the component of the total effect that is mediated through appendicular lean mass index (c–c′) in the relationship between dependent variable and the independent variable; it is denoted by β1 × β3 (βind).
Regarding the lower limbs, mediation analysis indicated that the total effect of long-COVID-19 on absolute leg extension of –24.91 kg (95% CI, –34.91 to –15.41 kg) was attributable to an indirect effect from ALMI (–9.71 kg; 95% CI, –16.83 to –2.92 kg), and direct effect of absolute leg extension (–15.19 kg; 95%CI, –22.16, –8.22 kg; 39% mediation) (Fig. 4B). Similarly, mediation analysis indicated that the total effect of long-COVID-19 on relative leg extension of –1.58 kg (95% CI, –2.16 to –1.01 kg) was attributable to an indirect effect from ALMI (–0.47 kg; 95% CI, –0.86 to –0.12 kg), and direct effect of relative absolute leg extension (–1.10 kg; 95%CI, –1.58, –0.63 kg; 29.7% mediation) (Fig. 4B).
DISCUSSION
In this cross-sectional study, we found that patients with long-COVID-19 syndrome had significantly lower absolute and relative muscle strength measurements than control participants. Interestingly, we identified that these relationships were mostly mediated by ALMI. Our data thus suggest that the evident reduced upper and lower muscle mass is a putative cause of––or contributor to––the functional limitation of patients with long-COVID-19 syndrome.
Persisting skeletal muscle-related symptoms may cut across COVID-19 severity grades, but the underlying mechanisms are unclear (35). Although SARS-CoV-2 infection is known to induce systemic inflammatory responses, there is evidence of specific inflammatory responses in skeletal muscle, which may provoke altered muscle metabolic function (36). Skeletal muscle cells express ACE2, which interacts with SARS-CoV-2 in its spike domain, and likely makes skeletal muscles vulnerable to direct virus invasion (37). Indeed, the expression of both ACE2 and transmembrane protease serine 2 (TMPRSS2) in skeletal muscle cells indicates that SARS-CoV-2 might enter these cells and influence their function (38). Other potential mechanisms are the release of myotoxic cytokines, immune complex deposition in muscles, damage from homology between viral antigens and human muscle cells, and absorption of viral protein on muscle membranes, resulting in the expression of viral antigens on the myocyte surface (11). According to Beydon et al. (39), myositis secondary to COVID-19 may manifest in multiple forms, which range from muscle weakness to typical dermatomyositis identified mostly by classic rashes, or sheer back pain. Considering that SARS-CoV-2 entrance in cells occurs through ACE2 receptor, we speculated that skeletal muscle in patients with SARS-CoV-2 infection might be more susceptible to muscle damage, due to intrinsically with ACE2 expression in this tissue. In this line, viral infections may spur an interesting insight when theorizing possible skeletal muscle susceptibility to direct SARS-CoV-2 infection. One may also speculate that myoblast (14) would be the primary target of COVID-19, as they are actively replicating, as opposed to myofibers and muscle stem cells, which replicate only under specific conditions. This would further contribute to a loss of regenerative abilities of the skeletal muscle in the vicinity of infection, as myoblasts are essential in the musculoskeletal repair, once they differentiate and donate cellular and nuclear components to structure ruptured fibers (14). However, limb muscle atrophy could also affect specific fiber types, involving predominantly fast-twitch-glycolytic fibers (40, 41). Therefore, decreased usage of muscle mass could incur a greater metabolic cost characterized by lower capacity for fat oxidation and switch to glycolysis as the main fuel supply, associated with a transition from slow to fast myosin fiber types (42). Our report is consistent with that of Pleguezuelos et al. (20) who also showed that patients with post-acute COVID-19 syndrome that followed acute COVID-19 requiring admission to the intensive care unit (ICU) suffered from reduced exercise efficiency and with that of de Boer et al. (32) showing that COVID-19 does impact mitochondrial function in patients with preserved pulmonary and cardiac function; however, both studies focused on cardiorespiratory fitness values and not on muscle mass changes as we did in our study. In addition, these data of patients with long-COVID-19 syndrome complement the study published by our group (22) that reduced fitness capacity in patients with persistent COVID-19 are unknown, but it has been hypothesized that not only lower limb muscle quantity/quality decreases but also excess adiposity (as seen in this series) and low levels of physical activity could partly explain the findings of this study.
A previous study reported that severe SARS-CoV-2 infection was associated with a persistent attenuation in muscle strength (32% loss in grip strength) and endurance capacity (13% loss in 6-min walk distance), associated with deconditioning, and sustained inflammation (43). A loss of handgrip has also been reported in older patients with severe post-COVID-19 (31). A more pronounced immune response to exercise and altered muscle metabolic function is evident in severe post-COVID-19 (38), with a larger reliance on glycolysis for energy production. The use of [18]fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG-PET/CT) to investigate brain and skeletal muscles changes in patients with post-COVID-19 syndrome and persistent symptom (e.g., myalgia) revealed an increased 18F-FDG-PET uptake, which can be interpreted as greater metabolic cost in skeletal muscles during physical activity, and a probable mechanism for premature muscle fatigability (37). In this line, Topuz et al. (44) imaged the leg muscles of 68 patients with COVID-19 (mean ± SD age; 56 ± 15 yr) and found significantly higher 18F-FDG uptake in the psoas muscle during the acute stage of COVID-19 and after a 1-mo follow-up. Other studies indicate that the early onset of SARS-CoV-2 infection induces rapid changes in skeletal mitochondrial function and substrate utilization (23, 45). As exercise intolerance and muscle weakness are complex phenomena, with interactions between physical and psychological factors, it is unknown what the possible mechanisms could be to explain these symptoms in patients with long-COVID-19 syndrome. The concomitant physical inactivity and, in some cases, hypoxemia and malnutrition, are additional factors that likely contribute to modifications in skeletal muscle structure and function (46). The exact mechanisms of musculoskeletal changes related to long-COVID-19 syndrome are still not completely understood. Given the current uncertainty, building knowledge from observational studies that directly interrogates muscle tissue dynamics during both SARS-CoV-2 infection and long-COVID-19 sequalae are needed to develop public health strategies to be deployed (47).
To the best of our knowledge, this is the first study to use mediation analysis to examine the role of ALMI in the relationship between long-COVID-19 syndrome and absolute and relative grip/leg strength levels. We found that these relationships are mediated, at least partly, by ALMI, which explains 52% of the association for grip strength, 11.2% for relative grip strength, 39% for leg extension, and 29.7% for relative leg extension, overall supporting the key role of ALMI in patients with post-COVID-19 syndrome. In this line, there is agreement that ALMI may be associated with a reduction in respiratory function, which is a major characteristic of convalescent patients with COVID-19 (48). Our preliminary results provide novel insight into the mechanisms underlying this relationship. Although more in-depth research is needed to understand this phenomenon, our observations highlight an important consideration for future COVID-19 research, as skeletal muscle represents the largest metabolically active tissue in the body with the greatest mitochondrial mass (49).
The present study has several strengths. It is the first to our knowledge to characterize the association between long-COVID-19 syndrome and muscular function parameters. Also, our study aligns with the European Association of Preventive Cardiology (EAPC) recommendations for the management of patients with COVID-19 (24). Nonetheless, the present study has limitations, including the small cohort size, retrospective methodology, and lack of a contemporaneous control, relying on comparisons with historical cohorts that have distinct demographics. In addition, we recognize the limitations of a cross-sectional comparison such as this and encourage future investigations to include patients with long-COVID syndrome longitudinally to determine the recovery period for skeletal muscle parameters.
In conclusion, patients with long-COVID-19 syndrome have lower absolute and relative muscle strength than control participants, and this relationship may be mostly mediated by ALMI. These findings provide novel insights into the mechanisms underlying the relationship between long-COVID-19 syndrome and grip/leg strength levels. As the effects of long-COVID-19 syndrome on muscle function are striking future studies investigating the mechanisms of dysfunction will help accelerate the development of therapies to improve the functional status of these patients.
DATA AVAILABILITY
Data will be made available upon reasonable request.
GRANTS
The EXER-COVID study was supported by «Proyectos de I+D+i» de los Programas Estatales de Generación de Conocimiento y Fortalecimiento Científico y Tecnológico del Sistema de I+D+i Orientada a los Retos de la Sociedad, en el marco del Plan Estatal de Investigación Científica y Técnica y de Innovación 2017–2020 (PID2020-113098RB-I00). They do not have influence or authority about collection, management, analysis, and interpretation of data; writing of the report; and the decision to submit the report for publication.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.R.-V., J.O., and M.I. conceived and designed research; R.R.-V., G.L.-G., S.O.-O., Y.G.-A., N.G.-A., A.E.L., and M.C.-R. performed experiments; R.R.-V., S.O.-O,, Y.G.-A., N.G.-A., and M.C.-R. analyzed data; Y.G.-A., N.G.-A., M.C.-R., and M.I. interpreted results of experiments; R.R.-V., G.L.-G., Y.G.-A., J.O., M.C.-R., and M.I. drafted manuscript; R.R.-V., S.O.-O., M.C.-R., and M.I. edited and revised manuscript; R.R.-V., G.L.-G., S.O.-O., Y.G.-A., N.G.-A., J.O., A.E.L., M.C.-R., and M.I. approved final version of manuscript.
REFERENCES
- 1. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395: 1054–1062, 2020. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Han Q, Zheng B, Daines L, Sheikh A. Long-term sequelae of COVID-19: a systematic review and meta-analysis of one-year follow-up studies on post-COVID symptoms. Pathogens 11: 269, 2022. doi: 10.3390/pathogens11020269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lopez-Leon S, Wegman-Ostrosky T, Perelman C, Sepulveda R, Rebolledo P, Cuapio A, Villapol S. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis (Preprint). Res Sq 2021. doi: 10.21203/RS.3.RS-266574/V1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA 324: 782–793, 2020. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 5. Lopez-Leon S, Wegman-Ostrosky T, Perelman C, Sepulveda R, Rebolledo PA, Cuapio A, Villapol S. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis (Preprint). medRxiv 2021. doi: 10.1101/2021.01.27.21250617. [DOI] [PMC free article] [PubMed]
- 6. Medrinal C, Prieur G, Bonnevie T, Gravier FE, Mayard D, Desmalles E, Smondack P, Lamia B, Combret Y, Fossat G. Muscle weakness, functional capacities and recovery for COVID-19 ICU survivors. BMC Anesthesiol 21: 64, 2021. doi: 10.1186/s12871-021-01274-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yanes-Lane M, Winters N, Fregonese F, Bastos M, Perlman-Arrow S, Campbell JR, Menzies D. Proportion of asymptomatic infection among COVID-19 positive persons and their transmission potential: asystematic review and meta-analysis. PLoS One 15: e0241536, 2020. doi: 10.1371/journal.pone.0241536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Subramanian A, Nirantharakumar K, Hughes S, Myles P, Williams T, Gokhale KM, et al. Symptoms and risk factors for long COVID in non-hospitalized adults. Nat Med 28: 1706–1714, 2022. doi: 10.1038/s41591-022-01909-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hejbøl EK, Harbo T, Agergaard J, Madsen LB, Pedersen TH, Østergaard LJ, Andersen H, Schrøder HD, Tankisi H. Myopathy as a cause of fatigue in long-term post-COVID-19 symptoms: evidence of skeletal muscle histopathology. Eur J Neurol 29: 2832–2841, 2022. doi: 10.1111/ene.15435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Logue JK, Franko NM, McCulloch DJ, McDonald D, Magedson A, Wolf CR, Chu HY. Sequelae in adults at 6 months after COVID-19 infection. JAMA Netw Open 4: e210830, 2021. [Erratum in JAMA Netw Open 4: e214572, 2021]. doi: 10.1001/jamanetworkopen.2021.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rudroff T, Workman CD, Ponto LLB. 18 F-FDG-PET imaging for post-COVID-19 brain and skeletal muscle alterations. Viruses 13: 2283, 2021. doi: 10.3390/v13112283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Van Aerde N, Van den Berghe G, Wilmer A, Gosselink R, Hermans G; COVID-19 Consortium. Intensive care unit acquired muscle weakness in COVID-19 patients. Intensive Care Med 46: 2083–2085, 2020. doi: 10.1007/s00134-020-06244-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Perez-Valera M, Martinez-Canton M, Gallego-Selles A, Galván-Alvarez V, Gelabert-Rebato M, Morales-Alamo D, Santana A, Martin-Rodriguez S, Ponce-Gonzalez JG, Larsen S, Losa-Reyna J, Perez-Suarez I, Dorado C, Curtelin D, Gonzalez-Henriquez JJ, Boushel R, Hallen J, de Pablos Velasco P, Freixinet-Gilart J, Holmberg HC, Helge JW, Martin-Rincon M, Calbet JAL. Angiotensin-converting enzyme 2 (SARS-CoV-2 receptor) expression in human skeletal muscle. Scand J Med Sci Sports 31: 2249–2258, 2021. doi: 10.1111/sms.14061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Seixas MLGA, Mitre LP, Shams S, Lanzuolo GB, Bartolomeo CS, Silva EA, Prado CM, Ureshino R, Stilhano RS. Unraveling muscle impairment associated with COVID-19 and the role of 3D culture in its investigation. Front Nutr 9: 825629, 2022. doi: 10.3389/fnut.2022.825629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Leung TW, Wong KS, Hui AC, To KF, Lai ST, Ng WF, Ng HK. Myopathic changes associated with severe acute respiratory syndrome: a postmortem case series. Arch Neurol 62: 1113–1117, 2005. doi: 10.1001/archneur.62.7.1113. [DOI] [PubMed] [Google Scholar]
- 16. Piotrowicz K, Gąsowski J, Michel JP, Veronese N. Post-COVID-19 acute sarcopenia: physiopathology and management. Aging Clin Exp Res 33: 2887–2898, 2021. doi: 10.1007/s40520-021-01942-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. De Giorgio MR, Di Noia S, Morciano C, Conte D. The impact of SARS-CoV-2 on skeletal muscles. Acta Myol 39: 307–312, 2020. doi: 10.36185/2532-1900-034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Azim D, Nasim S, Kumar S, Hussain A, Patel S. Neurological consequences of 2019-nCoV infection: a comprehensive literature review. Cureus 12: e8790, 2020. doi: 10.7759/cureus.8790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Savrun A, Aydin IE, Savrun ST, Karaman U. The predictive role of biomarkers for mortality in COVID-19 patients. Trop Biomed 38: 366–370, 2021. doi: 10.47665/tb.38.3.080. [DOI] [PubMed] [Google Scholar]
- 20. Pleguezuelos E, Del Carmen A, Llorensi G, Carcole J, Casarramona P, Moreno E, Ortega P, Serra-Prat M, Palomera E, Miravitlles MM, Yebenes JC, Boixeda R, Campins L, Villelabeitia-Jaureguizar K, Garnacho-Castaño MV. Severe loss of mechanical efficiency in COVID-19 patients. J Cachexia Sarcopenia Muscle 12: 1056–1063, 2021. doi: 10.1002/jcsm.12739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ramírez-Vélez R, Oteiza J, de Tejerina JMCF, García-Alonso N, Legarra-Gorgoñon G, Oscoz-Ochandorena S, Arasanz H, García-Alonso Y, Correa-Rodríguez M, Izquierdo M. Resistance training and clinical status in patients with postdischarge symptoms after COVID-19: protocol for a randomized controlled crossover trial “The EXER-COVID Crossover Study”. Trials 23, 2022. doi: 10.1186/s13063-022-06608-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ramírez-Vélez R, García-Alonso N, Legarra-Gorgoñón G, Oscoz-Ochandorena S, Oteiza J, Izquierdo M. Ventilatory efficiency in response to maximal exercise in persistent COVID-19 syndrome patients: a cross-sectional study. Rev Esp Cardiol 2022. doi: 10.1016/J.RECESP.2022.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ajaz S, McPhail MJ, Singh KK, Mujib S, Trovato FM, Napoli S, Agarwal K. Mitochondrial metabolic manipulation by SARS-CoV-2 in peripheral blood mononuclear cells of patients with COVID-19. Am J Physiol Cell Physiol 320: C57–C65, 2021. doi: 10.1152/ajpcell.00426.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ambrosetti M, Abreu A, Cornelissen V, Hansen D, Iliou MC, Kemps H, et al. Delphi consensus recommendations on how to provide cardiovascular rehabilitation in the COVID-19 era. Eur J Prev Cardiol 28: 541–557, 2021. doi: 10.1093/eurjpc/zwaa080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kaul S, Rothney MP, Peters DM, Wacker WK, Davis CE, Shapiro MD, Ergun DL. Dual-energy X-ray absorptiometry for quantification of visceral fat. Obesity (Silver Spring) 20: 1313–1318, 2012. [Erratum in Obesity (Silver Spring) 20: 1544, 2012]. doi: 10.1038/oby.2011.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinková E, Vandewoude M, Zamboni M. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing 39: 412–423, 2010. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jaric S. Muscle strength testing: use of normalisation for body size. Sports Med 32: 615–631, 2002. doi: 10.2165/00007256-200232100-00002. [DOI] [PubMed] [Google Scholar]
- 28. Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF, Oja P. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 35: 1381–1395, 2003. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 29.JASP: A Fresh Way to Do Statistics [Online]. https://jasp-stats.org/ [10 Oct. 2022].
- 30. Tanriverdi A, Savci S, Kahraman BO, Ozpelit E. Extrapulmonary features of post-COVID-19 patients: muscle function, physical activity, mood, and sleep quality. Ir J Med Sci 191: 969–975, 2022. doi: 10.1007/s11845-021-02667-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Paneroni M, Simonelli C, Saleri M, Bertacchini L, Venturelli M, Troosters T, Ambrosino N, Vitacca M. Muscle strength and physical performance in patients without previous disabilities recovering from COVID-19 pneumonia. Am J Phys Med Rehabil 100: 105–109, 2021. doi: 10.1097/PHM.0000000000001641. [DOI] [PubMed] [Google Scholar]
- 32. de Boer E, Petrache I, Goldstein NM, Olin JT, Keith RC, Modena B, Mohning MP, Yunt ZX, San-Millán I, Swigris JJ. Decreased fatty acid oxidation and altered lactate production during exercise in patients with post-acute COVID-19 syndrome. Am J Respir Crit Care Med 205: 126–129, 2022. doi: 10.1164/rccm.202108-1903LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Díez-Fernández A, Sánchez-López M, Gulías-González R, Notario-Pacheco B, García-Prieto JC, Arias-Palencia N, Martínez-Vizcaíno V. BMI as a mediator of the relationship between muscular fitness and cardiometabolic risk in children: a mediation analysis. PLoS One 10: e0116506, 2015. doi: 10.1371/journal.pone.0116506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research. conceptual, strategic, and statistical considerations. J Pers Soc Psychol 51: 1173–1182, 1986. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 35. de Andrade-Junior MC, de Salles ICD, de Brito CMM, Pastore-Junior L, Righetti RF, Yamaguti WP. Skeletal muscle wasting and function impairment in intensive care patients with severe COVID-19. Front Physiol 12: 640973, 2021. doi: 10.3389/fphys.2021.640973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Poenaru S, Abdallah SJ, Corrales-Medina V, Cowan J. COVID-19 and post-infectious myalgic encephalomyelitis/chronic fatigue syndrome: a narrative review. Ther Adv Infect Dis 8: 20499361211009385, 2021. doi: 10.1177/20499361211009385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ferrandi PJ, Alway SE, Mohamed JS. The interaction between SARS-CoV-2 and ACE2 may have consequences for skeletal muscle viral susceptibility and myopathies. J Appl Physiol (1985) 129: 864–867, 2020. doi: 10.1152/japplphysiol.00321.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Disser NP, De Micheli AJ, Schonk MM, Konnaris MA, Piacentini AN, Edon DL, Toresdahl BG, Rodeo SA, Casey EK, Mendias CL. Musculoskeletal consequences of COVID-19. J Bone Joint Surg Am 102: 1197–1204, 2020. doi: 10.2106/JBJS.20.00847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Beydon M, Chevalier K, Al Tabaa O, Hamroun S, Delettre AS, Thomas M, Herrou J, Riviere E, Mariette X. Myositis as a manifestation of SARS-CoV-2. Ann Rheum Dis 80: e42, 2021. doi: 10.1136/annrheumdis-2020-217573. [DOI] [PubMed] [Google Scholar]
- 40. Ciciliot S, Rossi AC, Dyar KA, Blaauw B, Schiaffino S. Muscle type and fiber type specificity in muscle wasting. Int J Biochem Cell Biol 45: 2191–2199, 2013. doi: 10.1016/j.biocel.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 41. Wang Y, Pessin JE. Mechanisms for fiber-type specificity of skeletal muscle atrophy. Curr Opin Clin Nutr Metab Care 16: 243–250, 2013. doi: 10.1097/MCO.0b013e328360272d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Stein TP, Wade CE. Metabolic consequences of muscle disuse atrophy. J Nutr 135: 1824S–1828S, 2005. doi: 10.1093/jn/135.7.1824S. [DOI] [PubMed] [Google Scholar]
- 43. Lau HMC, Lee EWC, Wong CNC, Ng GYF, Jones AYM, Hui DSC. The impact of severe acute respiratory syndrome on the physical profile and quality of life. Arch Phys Med Rehabil 86: 1134–1140, 2005. doi: 10.1016/j.apmr.2004.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rudroff T, Workman CD, Ponto LLB. 18F-FDG-PET imaging for post-COVID-19 brain and skeletal muscle alterations. Viruses 13: 2283, 2021. doi: 10.3390/v13112283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Trinity JD, Craig JC, Fermoyle CC, McKenzie AI, Lewis MT, Park SH, Rondina MT, Richardson RS. Impact of presymptomatic COVID-19 on vascular and skeletal muscle function: a case study. J Appl Physiol (1985) 130: 1961–1970, 2021. doi: 10.1152/japplphysiol.00236.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gautam N, Madathil S, Tahani N, Bolton S, Parekh D, Stockley J, Goyal S, Qureshi H, Yasmin S, Cooper BG, Short J, Geberhiwot T. Medium-term outcomes in severely to critically ill patients with severe acute respiratory syndrome coronavirus 2 infection. Clin Infect Dis 74: 301–308, 2022. doi: 10.1093/cid/ciab341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Clark BC, Manini TM. What is dynapenia? Nutrition 28: 495–503, 2012. doi: 10.1016/j.nut.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jeon YK, Shin MJ, Kim MH, Mok JH, Kim SS, Kim BH, Kim SJ, Kim YK, Chang JH, Shin YB, Kim IJ. Low pulmonary function is related with a high risk of sarcopenia in community-dwelling older adults: the Korea National Health and Nutrition Examination Survey (KNHANES) 2008–2011. Osteoporos Int 26: 2423–2429, 2015. doi: 10.1007/s00198-015-3152-8. [DOI] [PubMed] [Google Scholar]
- 49. Ramírez-Vélez R, Ezzatvar Y, Izquierdo M, García-Hermoso A. Effect of exercise on myosteatosis in adults: a systematic review and meta-analysis. J Appl Physiol (1985) 130: 245–255, 2021. doi: 10.1152/japplphysiol.00738.2020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available upon reasonable request.