Keywords: aorta, arterial stiffness, in utero, vaping, vascular reactivity
Abstract
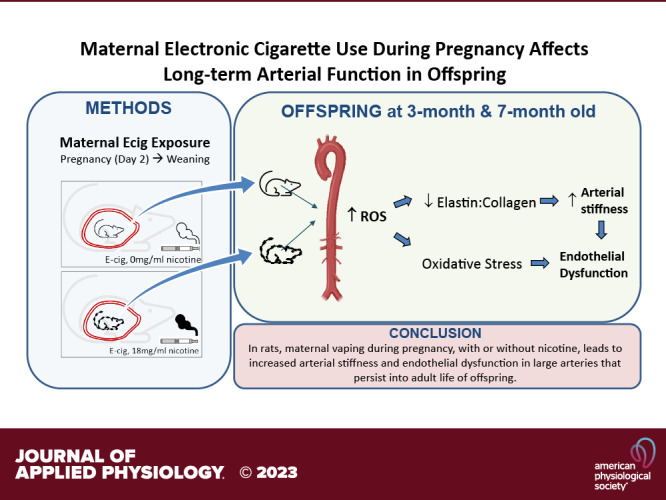
Vaping, or electronic cigarette (ecig) use, is prevalent among pregnant women, although little is known about the effects of perinatal ecig use on cardiovascular health of the progeny (even when using nicotine-free e-liquid). Maternal toxicant inhalation may adversely affect vital conduit vessel development. We tested the hypothesis that perinatal exposure to maternal vaping would lead to a dose-dependent dysfunction that would persist into later life of offspring. Pregnant Sprague–Dawley rats were exposed to either nicotine-free (ecig0) or nicotine-containing ecig aerosol (18 mg/mL, ecig18) starting on gestational day 2 and continued until pups were weaned (postnatal day 21). Pups were never directly exposed. Conduit artery function (stiffness and reactivity) and structure were assessed in 3- and 7-mo-old offspring. At 3 mo, pulse wave velocity (PWV) in the ecig0 and ecig18 offspring was significantly higher than controls in both the 20 puffs/day (6.6 ± 2.1 and 4.8 ± 1.3 vs. 3.2 ± 0.7 m/s, respectively, P < 0.05, means ± SD) and in 60 puffs/day exposure cohort (7.5 ± 2.8 and 7.5 ± 2.5 vs. 3.2 ± 0.5 m/s, respectively, P < 0.01). Wire myography revealed (range of 23%–31%) impaired aortic relaxation in all ecig exposure groups (with or without nicotine). Incubation of vessels with TEMPOL or Febuxostat reversed the aortic dysfunction, implicating the involvement of reactive oxygen species. Nearly identical changes and pattern was seen in vascular outcomes of 7-mo-old offspring. The take-home message from this preclinical study is that maternal vaping during pregnancy, with or without nicotine, leads to maladaptations in vascular (aortic) development that persist into adult life of offspring.
NEW & NOTEWORTHY We observe a significant alteration in arterial structure and function in adolescent and adult offspring due to developmental exposure to toxicants resulting from perinatal maternal vaping. Taken together with previous work that described lasting dysfunction in cerebral microvasculature in offspring, these data underscore the adverse consequences of maternal exposure to electronic cigarette aerosol in conduit and resistance vessels alike, irrespective of nicotine content.
INTRODUCTION
Electronic cigarette (ecig) use, or vaping, is increasingly prevalent among pregnant women, ranging between 5% and 15% of the surveyed pregnant population within the last 5 yr (1–5). Pregnant women who have a history of smoking conventional tobacco cigarettes are attracted to ecigs for fear of harming their unborn baby from the widely accepted dangers of smoking, which include preterm birth, intrauterine growth restriction, and sudden infant death syndrome. Indeed, the perception that “vaping is safer than smoking” pervades forums for pregnant women (1, 6–9). However, emerging reports on adverse health effects of vaping on the maternal-fetal dyad indicate there are many reasons for concern, which include adverse developmental outcomes due to disruptions in placental function (10–14), impaired brain glucose utilization (15), altered lung organogenesis, remodeling and function (16, 17), liver dysfunction (18), epigenetic changes (19, 20), and cardiovascular deficits (11, 12, 14, 21).
Nicotine addiction is the primary driver of smoking or vaping during pregnancy, and regardless of intrinsic factors (e.g., age, smoking history) or delivery vehicle (e.g., cigarettes or ecigs), the consequences of perinatal nicotine exposure on the growth and development of the fetus are numerous and well-described (for review, see Ref. 22, 23). Unlike cigarettes, the ability to use ecigs without nicotine provides a unique opportunity to evaluate pregnancy exposure on health outcomes of offspring to e-liquid solution in the absence of nicotine. Although humans are unlikely to vape without nicotine, we (24) and others (25–27) have exploited this experimental capability and found that vaping without nicotine induces similar microvascular dysfunction as vaping with nicotine. Thus, the reproductive toxicological impact of ecigs is only just beginning to be studied and realized.
In humans (28, 29) and animals (30, 31), smoking and vaping have been reported to increase arterial stiffness and endothelial dysfunction, both of which are prodromic for cardiovascular disease. Endothelial cells play a critical role in cardiovascular homeostasis by regulating vascular tone, angiogenesis, and adhesion/aggregation of cells in circulation. Endothelial dysfunction and arterial stiffness are positively associated with hypertension, coronary artery disease, stroke, heart failure, and arrhythmias. Moreover, maternal factors such as nutrition status, obesity, hypertensive pregnancy or essential hypertension, diabetes, alcohol intake, and uteroplacental insufficiency have been associated with increased arterial stiffness in offspring, but the effects of maternal vaping during pregnancy on gestational disruptions to large vessel development are yet to be fully understood. Given that even low levels of smoking and exposure to ambient air pollution are known to significantly increase cardiovascular risk (for review, see Ref 32) and adversely influence pregnancy outcomes (for review, see Ref 33), understanding the risks from electronic cigarettes is of great public health importance.
The objective of this study was to evaluate maternal vaping exposure during pregnancy (with and without nicotine) on large conduit artery structure and function in adolescent and adult offspring with perinatal exposure. We tested the hypothesis that perinatal exposure of progeny to maternal vaping would alter vascular development and lead to a dose-dependent dysfunction that would persist into adolescent and adult life of offspring.
MATERIALS AND METHODS
Animal Breeding and Maternal Exposure Conditions
All procedures were approved by the West Virginia University Animal Care and Use Committee. Timed pregnancies were achieved by breeding male (250–275 g) and female (200–250 g) Sprague–Dawley rats (Charles River, Wilmington, MA) in a pathogen-free vivarium facility at West Virginia University. Standard rat chow and tap water were provided to dams and offspring, and animals were kept on 12:12 day:night cycle during the study. Determination of pregnancy and gestational day (GD) 0 was made with the observation of sperm and/or vaginal plug on the female. Rats were then randomly assigned to: 1) ambient air (control, n = 5), 2) ecig liquid with no nicotine (ecig0, n = 5), or 3) ecig liquid with 18 mg/mL e-liquid nicotine (ecig18, n = 5; Table 1). E-liquid used for both vape exposure consisted of 75/25 vegetable glyercin:propylene glycol (VG:PG) composition with French Vanilla flavor and was obtained from a local vape shop. Up to two pregnant dams experiencing the same exposure were housed together until giving birth (i.e., GD20).
Table 1.
Exposure and animal characteristics
| Ecig0 |
Ecig18 |
|||||
|---|---|---|---|---|---|---|
| Air | 20 Puffs | 60 Puffs | 20 Puffs | 60 Puffs | P, ANOVA | |
| Chamber conditions | ||||||
| Total particle count, no. of particles/cm3 | 2.8 ± 0.4 × 104 | 1.4 ± 0.5 × 1010 | 2.5 ± 2.4 × 1010 | 1.5 ± 0.3 × 1010 | 1.8 ± 1.1 × 1010 | P = 0.613 |
| Average particle count, no. of particles/cm3 | 1.56 ± 0.2 | 1.4 ± 0.4 × 106 | 1.6 ± 2.0 × 106 | 1.5 ± 0.3 × 106 | 0.99 ± 0.6 × 106 | P = 0.565 |
| Temperature, °C | 20.2 ± 0.2 | 22.7 ± 0.7 | 22.7 ± 1.1 | 21.2 ± 0.8 | 22.6 ± 1.4 | P < 0.002 |
| Relative humidity, % | 44 ± 14 | 62 ± 6 | 48 ± 16 | 57 ± 7 | 56 ± 18 | P = 0.592 |
| Dams | ||||||
| Dam, n | 5 | 5 | 3 | 5 | 3 | |
| Dam age @ birth, mo | 5.8 ± 1.1 | 5.1 ± 2.1 | 7.7 ± 0.4 | 5.4 ± 1.9 | 7.8 ± 0.3 | P = 0.143 |
| Litter size, no. of pups | 14 ± 1 | 13 ± 1 | 11 ± 1 | 12 ± 2 | 11 ± 4 | P = 0.561 |
| Litter size, range | 12–16 | 10–16 | 11–12 | 9–16 | 5–17 | n/a |
| Male pups, % of litter | 44 | 43 | 42 | 40 | 39 | P = 0.879 |
| Offspring | ||||||
| Body mass @ birth, g | 7.0 ± 0.1 | 6.4 ± 0.2 | 6.1 ± 0.2 | 6.6 ± 0.2 | 6.4 ± 0.2 | P = 0.210 |
| 21–23 days old (weaning) | ||||||
| Body mass, g | 40.9 ± 0.7 | 39.2 ± 1.1 | 40.2 ± 0.7 | 39.2 ± 1.1 | 41.3 ± 0.4 | P = 0.411 |
| Lean body mass,a % | 89.7 ± 0.01 | 88.3 ± 0.01* | 87.5 ± 0.01*# | 89.2 ± 0.02*^ | 88.8 ± 0.02*^ | P < 0.001 |
| Body fat mass,a % | 9.8 ± 0.01 | 11.4 ± 0.01* | 12.0 ± 0.01* | 10.0 ± 0.01^ | 10.5 ± 0.01*^ | P < 0.001 |
| 3-mo old | ||||||
| Total n, no. of males | 15 (7) | 14 (7) | 12 (6) | 10 (5) | 12 (6) | |
| Age, days | 94 ± 1 | 96 ± 2 | 94 ± 2 | 99 ± 3 | 94 ± 2 | P = 0.957 |
| Body mass, g | 398 ± 36 | 388 ± 38 | 425 ± 45 | 293 ± 38 | 407 ± 36 | P = 0.224 |
| 7-mo group | ||||||
| Total n, no. of males | 10 (5) | 8 (3) | 6 (3) | 8 (3) | 6 (3) | |
| Age, days | 228 ± 3 | 226 ± 4 | 227 ± 7 | 226 ± 3 | 227 ± 4 | P = 0.111 |
| Body mass, g | 536 ± 45 | 377 ± 50 | 508 ± 38 | 482 ± 29 | 424 ± 36 | P = 0.761 |
| Lean body mass,b % | 68.0 ± 0.1 | 73.9 ± 0.1* | 64.3 ± 0.1*# | 72.1 ± 0.1*^ | 69.1 ± 0.1 | P < 0.001 |
| Body fat mass,b % | 32.0 ± 1.3 | 26.1 ± 1.4* | 35.6 ± 1.2*# | 27.9 ± 0.9* | 30.9 ± 1.0 | P < 0.001 |
| Blood pressure | ||||||
| Systolic BP, mmHg | 102 ± 4 | 109 ± 6 | 110 ± 4 | 103 ± 5 | 107 ± 5 | P = 0.645 |
| Diastolic BP, mmHg | 76 ± 4 | 83 ± 6 | 72 ± 3 | 77 ± 4 | 78 ± 2 | P = 0.411 |
| Mean arterial pressure, mmHg | 85 ± 4 | 92 ± 6 | 85 ± 3 | 86 ± 5 | 87 ± 2 | P = 0.713 |
| Heart rate, beats/min | 342 ± 9 | 348 ± 13 | 350 ± 8 | 306 ± 7 | 344 ± 8 | P = 0.299 |
Data presented as means ± SE. BP, blood pressure; DEXA, dual X-ray absorptiometry; ecig, electronic cigarette; ecig0, nicotine-free ecig aerosol; ecig18, nicotine-containing ecig aerosol (18 mg/mL); nd, not detected; TPM, total particulate matter measured gravimetrically.
Measured by echoMRI.
Measured by DEXA. P < 0.05 compared with *air, #20 vs. 60 puff for same ecig condition, ^ecig0 vs. ecig18 with same puff number. P values reported for ANOVA are Exposure main-effect.
Maternal exposure began on GD2 using a whole body exposure (InExpose, Scireq Inc., Montreal, Canada). After birth, exposure to dams (not pups) continued during the weaning period until postnatal day 21 (PD21). Pups were never directly exposed to ecig aerosol. Ecig0 and ecig18 exposures were performed concurrently using two separate but identical ecig devices and exposure chambers that were independently operated and monitored. Control (air exposed) dams were handled and transported daily in a similar fashion to exposed dams. Our maternal exposure was limited to the window of gestation and lactation to provide a direct assessment of vaping during the perinatal period only, and to eliminate any potential feed-forward effects that might occur with vaping experiences before becoming pregnant. Although this design is not strictly mimicking the human condition (i.e., who likely would be smoking or vaping before becoming pregnant), it does provide direct experimental evidence toward the consequences of vaping solely while pregnant. After birth, pups were never directly exposed, but cohabited with dams (when dams were not being exposed to vape aerosol) until weaning.
Maternal exposure was conducted for 1 h/day, 5 days/wk. For dose-effect determination, the frequency of ecig activation was increased threefold between the ecig cohorts. The first cohort of dams was administered 1 puff every 3 min for 60 min (i.e., 20 puffs in 1 h) of either ecig0 or ecig18, whereas the second cohort received 1 puff every minute for 60 min (i.e., 60 puffs in 1 h). We used identical 3rd-generation, tank-style, ecig devices purchased online (Joyetech eGrip OLED). The ecig device was controlled by an external computer-activated solenoid in a custom cradle (to hold the Ecig device), which allowed precise and reliable triggering of the ecig device without any modification to the device itself. Ecig puff duration was set to 5 s and with watts set at 17.5 W. An inhalation draw of ∼1 lpm was generated by the computer-controlled exposure system and a continuous bias flow of 5 lpm of air was flushed in to the chambers throughout the exposure. This resulted in an intermittent (i.e., saw-tooth) exposure pattern to the ecig aerosol that more closely mimics human vaping patterns compared with traditional whole body chamber environments. Atomizers were changed once a week.
Aerosol Analysis
We have previously reported the concentration and size of the aerosol particles produced for maternal exposures in this study (24). In brief, the concentration and size of the aerosol particles were analyzed separately using condensation particle counters (CPC Model; Cat. No. 3775, TSI Inc.) and an electrical low-pressure impactor (ELPI+, Dekati Ltd), respectively. Assessment of the vape cloud showed a complex, but similar distribution pattern between the respective devices/chambers, resulting in a median particle diameter of 0.395 and 0.336 μm for ecig0 and ecig18, respectively. Control conditions showed very wide and negligible detection of particles in ambient air.
Vascular Assessments
At the appropriate study group age (3 and 7 mo), vascular stiffness was first assessed using in vivo ultrasonography for pulse wave velocity (PWV), after which ex vivo aorta structure and function were assessed (details provided below).
In vivo ultrasonography.
PWV provides an indirect measure of arterial stiffness (with stiffer vessels having higher PWV) and is useful for characterizing cardiovascular disease and progression. Under sedation (isoflurane, 5% induction, 2%–3% maintenance), left common carotid arteries (LCCA) were noninvasively imaged using Vevo2100 high-frequency microultrasound (VisualSonics Inc, Toronto, ON, Canada). Doppler ultrasound images and ECG signal detection were saved for offline analysis using VisualSonics analysis software. Distance (d) between proximal (downstream to aortic arch) and distal (upstream of bifurcation) points on the LCCA and the arrival time of pulse wave upstroke relative to R-wave peak (t) were measured. Repeated (≥3) measurements for each variable were averaged. Pulse wave velocity was calculated using the regional transit-time (TT) method (PWV = Δd/Δt). We choose to evaluate PWV in the carotid artery since the distance between proximal and distal points can reliability be measured in a single image, and that aging effects on PWV in the carotid artery closely mirror that in the aorta (34).
Ex vivo aorta structure and function.
Offspring were anesthetized, and blood pressure (via tail cuff plethysmography, Kent Scientific), body temperature, and heart rate were also assessed and recorded. Thereafter, rats were euthanized by exsanguination using phosphate-buffered solution (PBS) to flush the vascular system of blood via intracardiac (left ventricle) puncture. Thoracic aorta was excised and placed in ice-cold buffer solution to be used for wire myography experiments (described below), whereas abdominal aorta was preserved in 10% neutral buffered formalin and paraffin-embedded for histological analysis.
Freshly excised thoracic aortas were carefully cleaned of surrounding adipose tissue and cut into 2-mm rings. The aortic rings were immediately mounted on a 4-chamber wire myograph system (DMT, ADInstruments) containing warm aerated (37°C; 95% O2 and 5% CO2) Krebs–Henseleit buffer solution [1.18 mM KH2PO4, 1.2 mM MgSO4·7H2O, 4.7 mM KCl, 25 mM NaHCO3, 118 mM NaCl, 5.5 mM glucose, 0.026 mM ethylenediaminetetraacetic acid (EDTA)]. Following a 1-h equilibration and gradual 2 g preload tension, the vessels were challenged with KCl to confirm viability and determine maximum constrictor response. After washout and preconstriction with thromboxane A2 mimetic U-46619 (U46; 10−8M), the vessels were subjected to methacholine (Mch;10−9M to 10−4M) to assess endothelial-dependent relaxation. Vessel rings were washed 3× and methacholine concentration-response curves were repeated following a 30-min incubation with either: 1) nitro-l-argininemethylester, l-NAME (a nitric oxide synthase inhibitor, 10−5 M), 2) TEMPOL (superoxide dismutase mimetic,10−5 M), or 3) febuxostat (xanthine oxidase inhibitor, 10−10 M). For endothelial-independent relaxation, preconstricted vessels were challenged with sodium nitroprusside (SNP, NO donor; 10−9 M to 10−5 M). Finally, cumulative concentration-response with constrictor U46619 (U46; 10−12 M to 10−8 M) was reported as a percentage of KCl standard response.
For histologic assessment, 5-µm sections of the aorta were deparaffinized and stained with Verhoeff-Van Gieson for elastin fibers (Sigma-Aldrich) or Masson’s trichrome (Sigma-Aldrich) for collagen fibers. ImageJ (Fiji) software was used to calculate the total area of aorta wall and quantify elastin and collagen content. Relative densities of elastin and collagen fibers were calculated and reported as percent of the aorta wall area.
Body Composition Analyses
Lean and fat body mass were measured at weaning (PND21-23) and at 7 mo of age. At weaning, we used EchoMRI system (Model 500, EchoMRI LLC, Houston, TX) in alive unanesthesized rats to assess body composition. At 7 mo of age, we used Dual X-ray absorptiometry (DEXA, Model 8056 Discovery-SL) calibrated with rat phantom to assess body composition in isoflurane-anesthetized rats. All measurements were made according to the manufacturer’s guidelines and instructions for animal body composition testing for each apparatus.
Data and Statistical Analyses
All data are expressed as means ± SE. Two-way analysis of variance was used to identify main-effects for dose (20 vs. 60 puffs) and exposure condition (air vs. ecig0 vs. ecig18). One-way analysis of variance (ANOVA) tests were conducted for dependent variables to address comparisons with exposure groups, and where appropriate, repeated-measures ANOVA was used for comparison of multiple measurements within the same animal. Post hoc tests were done in instances of significant main effect in ANOVA. In all cases, P ≤ 0.05 was taken to reflect statistical significance.
RESULTS
Maternal Exposures
Table 1 summarizes chamber exposure conditions, litter characteristics, and anthropometric/clinical measures of offspring. Litter size and body mass at birth were not different between groups. At weaning, ecig0 pups have significantly higher body fat (BF) mass and lower lean body mass (LBM) than controls (Table 1). At 7 mo of age, body mass was not different between any of the groups, but 20-puff-ecig-exposed offspring show lower BF and higher LBM (P < 0.05). Sixty-puff exposure shows high BF and lower LBM with ecig0 (P < 0.05), but not ecig18.
Arterial Stiffness and Histology
Pulse wave velocity (PWV) was significantly greater in 3-mo-old ecig0 and ecig18 offspring compared with air (P < 0.05 ANOVA) (Fig. 1). The increase in carotid PWV (i.e., stiffness) was not dose-dependent to the number of maternal puffs (i.e., 20 vs. 60 puffs) exposure and not different based on presence or absence of nicotine (i.e., ecig0 vs. ecig18; Fig. 1). PWV at 7 mo trends toward the same pattern (P = 0.057 ANOVA).
Figure 1.
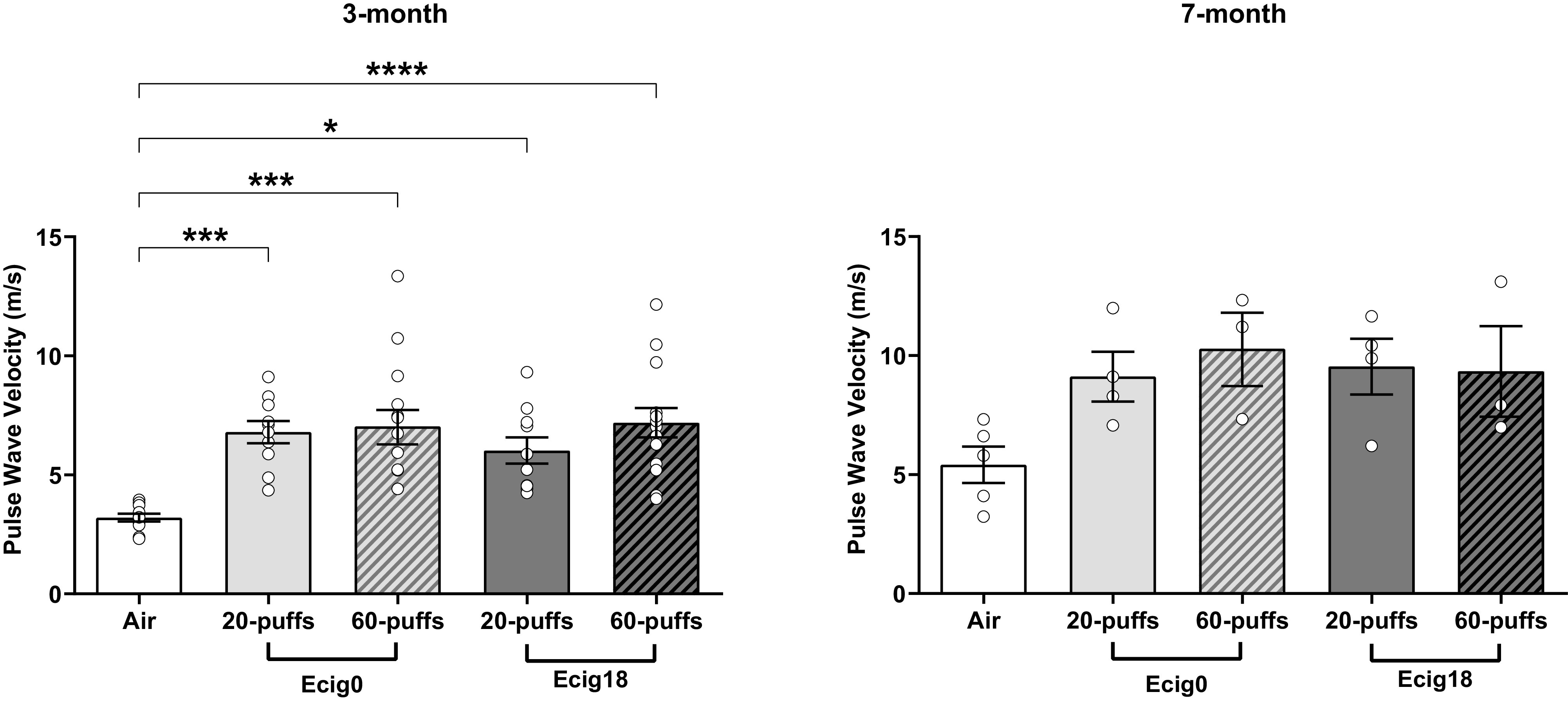
In vivo arterial stiffness data [measured as pulse wave velocity (PWV)] using Doppler microultrasound of left common carotid artery in (isoflurane) anesthetized offspring at 3 and 7 mo of age. Offspring only received in utero exposure from maternal vaping with (18 mg/mL) or without (0 mg) nicotine (ecig18 and ecig0, respectively). n = 10–14/offspring for 3 mo; N = 3–5/group for 7 mo. All data shown are means ± SE. *P < 0.05, ***P < 0.001, ****P < 0.0001 vs. controls. ecig, electronic cigarette.
We also assessed potential structural changes or adaptations in the aorta of offspring by selectively staining for elastin and collagen (Fig. 2). We observed a significant reduction in elastin (ANOVA P < 0.001) coupled with increase in collagen (ANOVA P < 0.001) under all vape conditions compared with controls (Fig. 2, A and B). Examination of the elastin:collagen (E:C) ratio reveals a range of 41%–54% decline in E:C ratio compared with controls (ANOVA P < 0.001; Fig. 2C), which is consistent with a stiffer vessel wall. All ecig-exposed groups, regardless of age, ecig dose, or nicotine, exhibit significant E:C decline compared with air-exposed controls (Fig. 2C).
Figure 2.
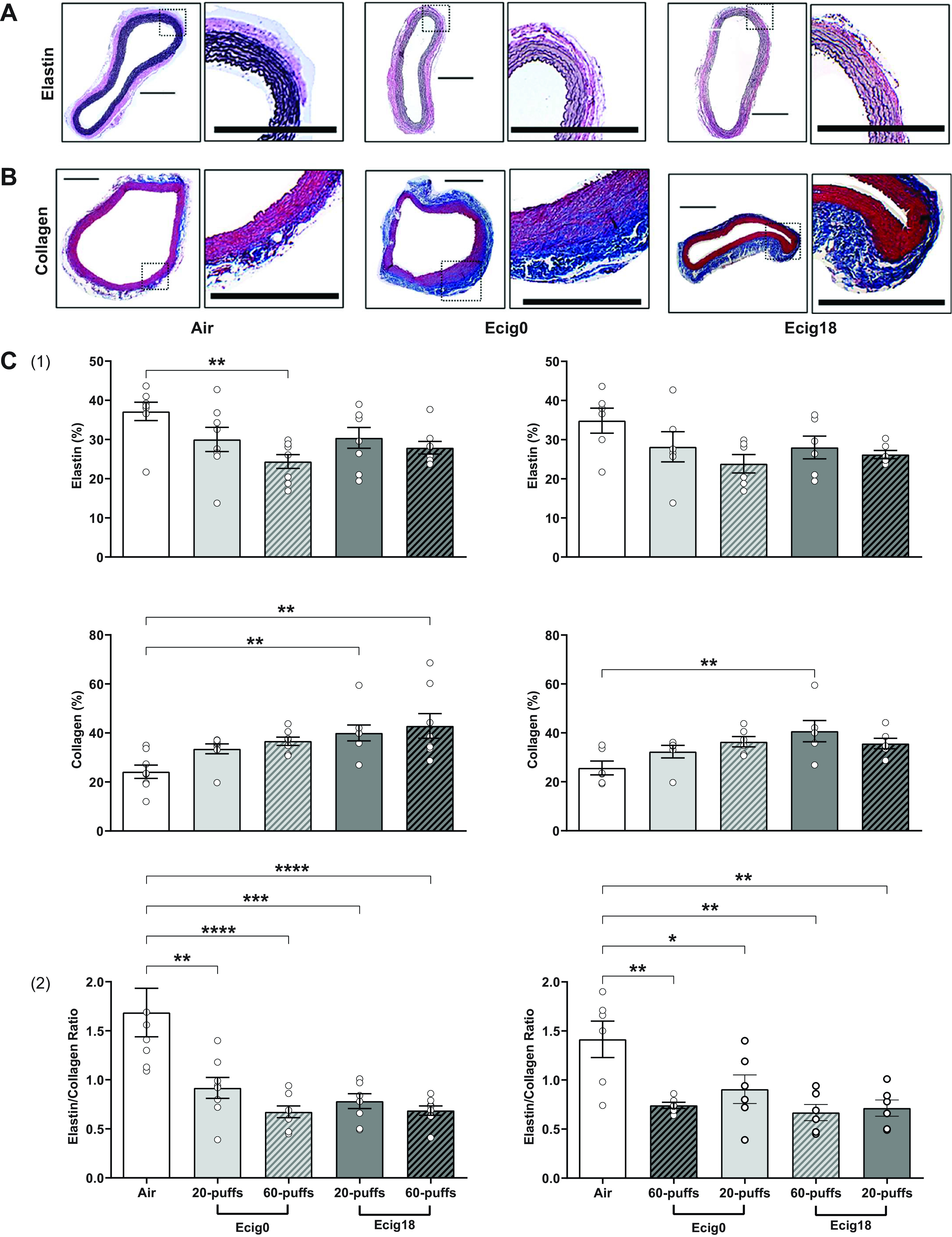
Representative photomicrographs (scale bar = 1 mm) of abdominal aorta stained with (A) Verhoeff–Van Gieson for elastin fibers (purple-black) and (B) Masson’s Trichrome for collagen fibers (blue) showing decreased elastin and increased collagen content in aorta offspring with history of maternal exposed to e-cigarette containing 18 mg/mL or 0 mg nicotine (ecig18 and ecig0, respectively) during pregnancy. Controls are offspring with maternal exposure to ambient air. C: density quantification from respective elastin and collagen images (panel 1) from 3- (left) and 7-mo-old (right) offspring (expressed relative % to whole aorta area) and the ratio of elastin/collagen (panel 2). Data are means ± SE. N = 8 rats per group for 3 mo, N = 6 rats per group for 7 mo old. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 vs. controls. ecig, electronic cigarette.
Aorta Reactivity and Function
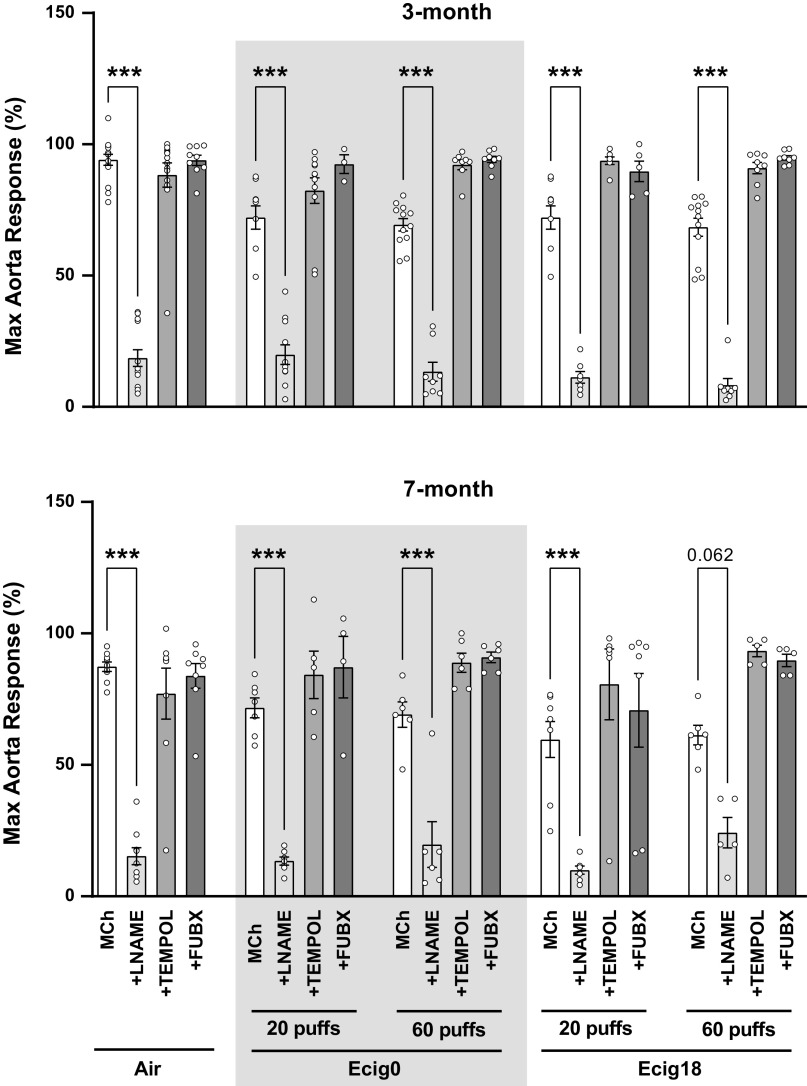
Aortic reactivity to increasing doses of methacholine (MCh) was assessed (Fig. 3). At 3 mo of age, nicotine versus no nicotine (ecig0 and ecig18, respectively) groups showed similar impaired endothelial-dependent responses to MCh-induced relaxation compared with control offspring (with a 24 ± 2% average reduction in aortic reactivity compared with controls, P < 0.01, Fig. 3, A and B). Impaired MCh-induced relaxation was not different in offspring with maternal exposure to 20 versus 60 puffs, where reductions in maximal MCh reactivity was 24 ± 5% and 22 ± 4% for ecig0 and ecig18, respectively (for 20-puff cohort) and 25 ± 2% and 26 ± 3% in ecig0 and ecig18, respectively (for 60-puff cohort). Endothelial-independent reactivity to SNP (Fig. 4A) and increased constriction tension to U44619 (Fig. 4B) were not different between groups.
Figure 3.
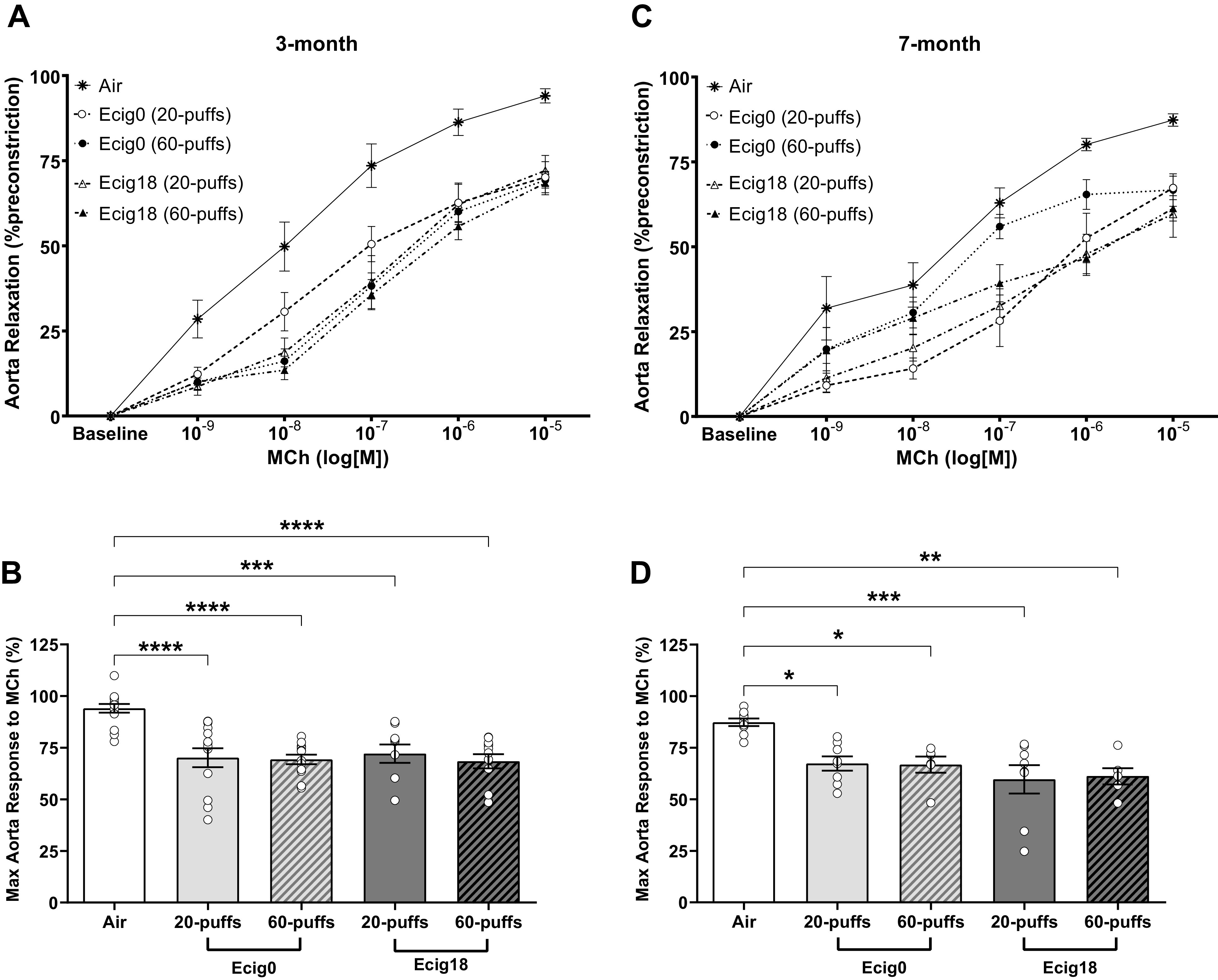
Ex vivo wire myography data showing relaxation responses of preconstricted thoracic aortic segments to increasing concentrations of methacholine (MCh) in offspring at 3 (A and B) and 7 mo (C and D) of age. Maternal exposure occurred with e-cigarettes with nicotine (ecig18 = 18 mg/mL) or without nicotine (ecig0 = 0 mg/mL). Controls are offspring with maternal exposure to ambient air. B and D: the maximal MCh dose (i.e., 10−5 M) from 3- and 7-mo-old offspring, respectively. All data are means ± SE. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 vs. controls. ecig, electronic cigarette.
Figure 4.
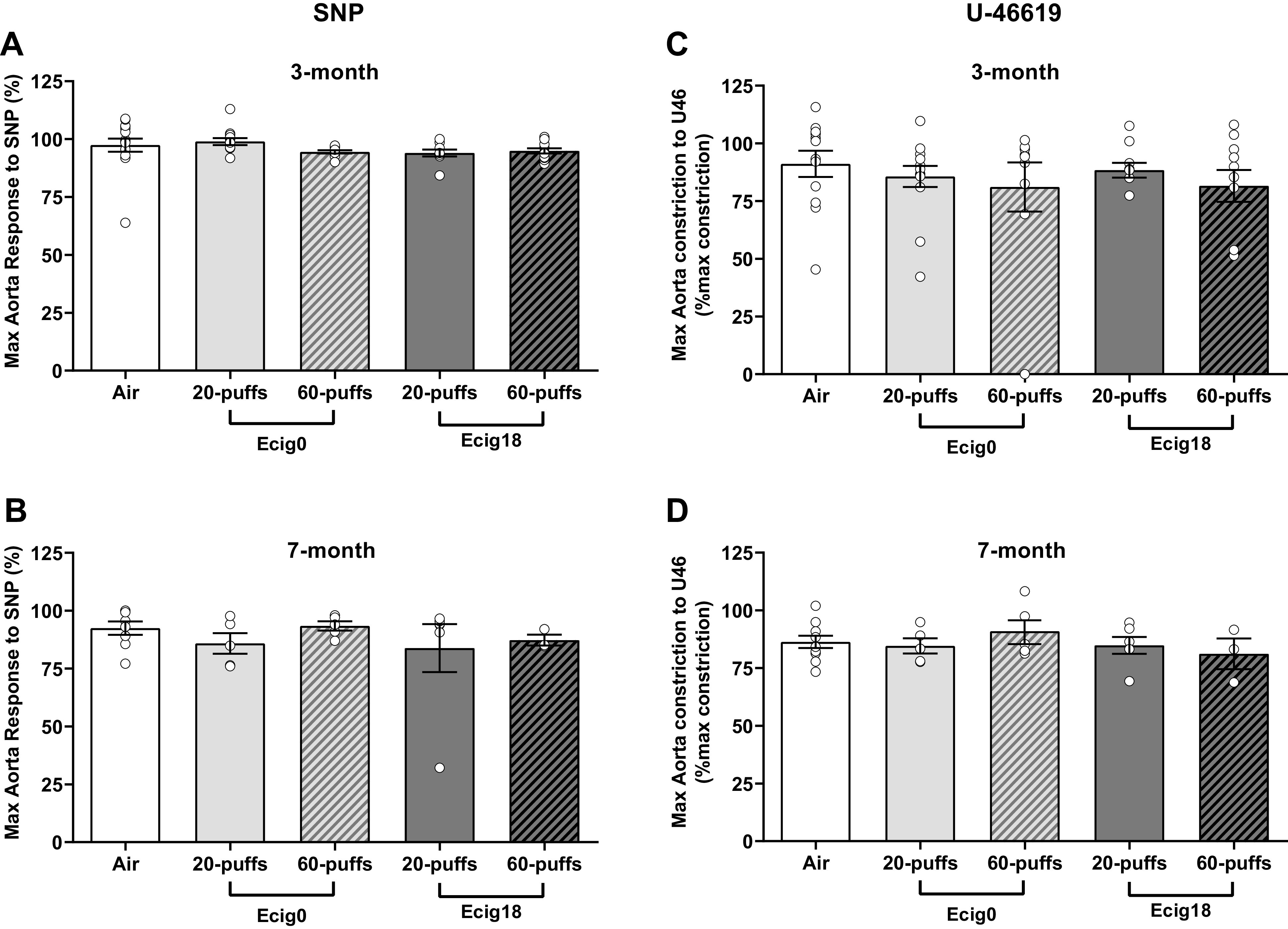
Ex vivo wire myography data showing thoracic aorta maximal responses in 3 (A and C) or 7 mo old (B and D) to either sodium nitroprusside (SNP, an endothelium-independent nitric oxide donor; A and B) or U46619 (U46, a vasoconstrictor; C and D). All responses are shown as a percentage of maximal KCl constriction. ecig, electronic cigarette.
At 7 mo of age, we observed similar levels and patterns of impairment in aortic reactivity in the offspring noted at 3 mo of age, with a 24 ± 3% average reduction in aortic relaxation compared with controls (P < 0.01, Fig. 3, C and D). The impaired relaxation was not different in offspring between maternal exposure to 20 versus 60 puffs, where reductions in maximal relaxation were 20 ± 2% and 28 ± 7% for ecig0 and ecig18, respectively (for 20-puff cohort) and 21 ± 3% and 26 ± 4% in ecig0 and ecig18, respectively (for 60-puff cohort). As before, there were no differences with respect to nicotine, and the endothelial-independent reactivity to SNP (Fig. 4B) and the vasoconstriction responses to U46619 (Fig. 4D) were not different between groups.
NO Bioavailability and Oxidative Stress
Using l-nG-nitro arginine methyl ester (l-NAME) we observed that aortic relaxation was nearly abolished in all groups (Fig. 5), suggesting NO bioavailability as a key mechanism for aortic dysfunction. The fact we observed that all vaping conditions (ecig0 vs. ecig18, 20 vs. 60 puffs) equally impaired aortic reactivity (by ∼14%) demonstrates the importance of NO in this response and suggests that reduced NO bioavailability is not due to nicotine (or least, not at this nicotine dose).
Figure 5.
Data showing effect on the maximal methacholine dose (Mch 10−5M) responses (previously shown in Fig. 3) when treated with 1) nitric oxide inhibitor (l-NAME), 2) superoxide dismutase mimetic (TEMPOL), and 3) xanthine oxidase (XO)-specific inhibitor Febuxostat. Thoracic aorta are from 3- and 7-mo-old offspring who were only exposed in utero from maternal exposure to e-cigarette aerosol with (18 mg/mL) or without (0 mg) nicotine (ecig18 and ecig0, respectively). Controls are offspring with maternal exposure to ambient air. All data shown are means ± SE. ***P < 0.0001. ecig, electronic cigarette.
Next, we explored the role of oxidative stress on aortic EDD with acute co-incubation of aorta with Tempol (a superoxide dismutase mimetic) and Febuxostat (a selective inhibitor of xanthine oxidase). Tempol has no effect on EDD in air group (as expected), but Tempol was effective in restoring the impaired maximal EDD for all vape conditions (Fig. 5). Likewise, we found that Febuxostat was equally effective in restoring the impaired maximal EDD for all the vaping conditions (Fig. 5). Taken together, these data suggest the ROS-induced reductions of endothelial cell-mediated NO exert a critical influence on the vascular dysfunction observed.
DISCUSSION
Epidemiological, clinical, and experimental data show that pregnancy and early life are critical sensitive windows of susceptibility and that periconceptional, perinatal and postnatal environments can have a significant influence on offspring’s risk for later-life chronic disease (35). In this study, we describe the consequences of maternal ecig exposure on central arterial stiffness and aortic reactivity in the F1 adolescent- and adult-age progeny. The results of this study extend our previous work (showing vascular dysfunction in the middle cerebral artery, a resistance vessel) (24) to health risks toward central conducting vessels (i.e., aorta and carotid arteries). In offspring that have only received perinatal exposure to ecig aerosol, we observe two- to threefold increase in carotid artery stiffness and ∼20%–30% deficit in aortic reactivity compared with controls. Increasing maternal exposure threefold (from 20 to 60 puffs/hour) in our paradigm did not create a dose-dependent effect on the progeny’s vascular outcomes. Likewise, the presence or absence of nicotine in the e-liquid did not significantly alter the deficits observed within any of the ecig-exposed offspring. Notably the changes in arterial stiffness and endothelial dysfunction were observed in adolescent (3 mo old) offspring persisted into adult life (7 mo of age).
Arterial Stiffness and Aortic Dysfunction
Increased arterial stiffness and vascular dysfunction (e.g., flow-mediated dilation) are reported with direct ecig exposure in humans (36) and animals (30, 37). Our finding of elevated PWV in 7-mo-old animals without direct exposure to ecig aerosol (and only perinatal exposure from maternal vaping) suggest a developmental and/or epigenetic mechanism(s) producing the dysfunction we observed in response to maternal exposure during pregnancy. The observation of increased arterial stiffness is significant because vessel stiffness is an independent predictor of cardiovascular disease, and is associated with worsening ventricular-vascular coupling (38). Prior research has also shown that infant aortic PWV may be a useful index that is sensitive to the gestational environment (39), which is consistent with our finding that offspring up to 7 mo old have increased arterial stiffness from only perinatal exposure. It is important to note greater arterial stiffness is seen in offspring of parents with hypertension and is an early prognostic indicator in the pathogenesis of hypertension (40). Indeed, elevated blood pressure alone can also increase PWV; thus it is critical to know the underlying hemodynamic status (e.g., blood pressure) when evaluating our data. We measured blood pressure (using tail-cuff method; Table 1) and did not find significant differences between our groups, but admittedly this method is a peripheral measure and does not exclude the possibility the central blood pressure could have been elevated. Given that histological assessment shows that there were intrinsic or structural changes in the vessels in offspring with perinatal exposure (i.e., reductions in the E:C ratio in aorta; Fig. 2), which is consistent with elevated arterial stiffness, it is tempting to speculate the effects we see may be a precursor to hypertension in the offspring.
It is interesting to note that the dose effect for maternal exposure we used (i.e., 20 vs. 60 puffs) did not result in significant differences in the vascular outcomes we reported. This could be because threefold greater number of puffs (with all other settings staying the same) did not significantly change the total (or average) aerosol density in the exposure chamber (Table 1). So even though the 60-puff regime did tend to produce greater number of particles, our exposure paradigm was designed to quickly flush the chamber with clean air (after each puff). This allowed us to expose the dams to an intermittent exposure and allowing some clear air breaths between each puff, which is more similar to human use (rather than a constant and continuous exposure often achieve with whole body exposures). We would also emphasize that similar vascular impairment between 20- and 60-puff exposures suggests the threshold for vascular dysfunction ensuing from vaping is likely to be quite low. One important interpretation from this is that a reduction in exposure by reducing the number puffs by pregnant users (who might resolve to cut back on their vaping habit during pregnancy) may not be successful in preventing harm or risks to their offspring. Given that 20 puffs/day is relatively low (particularly in terms of human behavior/use), there may be no safe level of exposure during pregnancy.
Endothelial Dysfunction and Oxidative Stress
Endothelial dysfunction is widely recognized as a preclinical risk factor for cardiovascular and cerebrovascular disease and is linked to an imbalance between endothelium-derived relaxing and/or contracting factors (e.g., reduction in the bioavailability of nitric oxide, NO). Both smoking (41) and vaping (42) are found to reduce NO bioavailability, which is consistent with our observation that MCh stimulation with l-NAME reduced aortic endothelial-dependent dilation in control animals, and in the ecig0 and ecig18 mice, indicating that reduced NO has a major influence in the response we report. Among the many etiologies that can underpin endothelial dysfunction (e.g., cellular senesce, inflammation, hypertension, obesity, etc.), oxidative stress is a likely contributor to vaping-induced vascular dysfunction. For example, treating human vascular endothelial cells with ecig aerosol extract has been found to induce reactive oxygen species, cause DNA damage, reduce cell viability, and trigger apoptotic pathways, whereas antioxidant treatment partially rescues the induced cell death (43). Moreover, chronic ecig use in humans and animals is associated with greater oxidative stress (44, 45), and as little as 10 puffs of ecig aerosol inhalation can trigger increases in circulating endothelial progenitor cells (EPC) (46, 47). EPCs are thought to be biologic marker for vascular function and correlate with cumulative vascular risk (48). These mechanisms, including the potential for epigenetic, transcriptional, and endoplasmic reticulum stress, have all been implicated in developmental origins of disease (35, 49), highlighting the potential for vaping to compromise the maternal-fetal dyad circulatory system (46, 47). In our study, when we sought to evaluate the role of oxidative stress with tempol (i.e., a SOD mimic that catalyzes the disproportionation of superoxide) or febuxostat (i.e., an inhibitor of xanthine oxidase, which under hypoxic or inflammatory conditions produces hydrogen peroxide and superoxide), both restored aortic endothelial-dependent dilation suggesting that presence of a prooxidative environment. These findings highlight that oxidative stress is an important and critical factor in the etiology of the vascular dysfunction we see young- and adult-age offspring whose risk stem only from perinatal ecig exposure due to maternal vaping, but they do not exclude the potential for other factors to be involved too. For example, in our previous report (24), we have also seen changes in the number of circulating extracellular vesicles (EVs). EVs are released from many different cell types and are thought to be an early sign of cellular injury leading to chronic disease, including endothelial dysfunction. Though we have not yet identified the source(s) of EVs in our animals, given that aorta responses to SNP and U46619 were not different that controls (Fig. 4) suggests the smooth muscle function was not impaired and might suggest the changes we see may stem from EVs originating by endothelial cells. Indeed, EVs are implicated in epigenetic alterations following ecig exposures (19, 20), which could also contribute to the vascular impairments we observe here.
Electronic Cigarette Aerosol
We know the ecig cloud is a complex aerosol mixture comprising of particles and gaseous components (50, 51), and although it is still unclear which subset(s) of potential toxicants triggered the response(s) that led to the developmental cascade that manifested the phenotype in our offspring, it is clear from our physical analyses of the ecig cloud (24) the particles produced are optimal for lung parenchymal distribution that are implicated in cardiovascular pathogenesis (10). Although we cannot exclude the possibility that the flavoring (French Vanilla) we used contributed in some way to the phenotype observed, reports from recent studies suggest similar level of vascular dysfunction performed without any additives (i.e., only VG and PG) (52, 53). We can exclude nicotine in the etiology of vascular dysfunction we observed (at least at the concentration we used 18 mg/mL), since ecig0 and ecig18 exhibited similar levels of dysfunction. Thus, we believe the main contributor to vascular phenotype we observe stems directly from the base solution. Indeed, there are many known toxicants (e.g., carbonyl compounds, volatile organic compounds, and metals) that are released when the base component of e-liquid [i.e., propylene glycol (PG) and vegetable glycerin (VG)] is heated. Many of these compounds are proven to be developmentally toxic (54–56). For example, cardiotoxic metals (such as lead, nickel, chromium, and manganese, and sometimes even arsenic) have been measured in ecig users. At least one study has confirmed ecigs as a source of cardiotoxic metals that uniquely damage the vascular system (57). A possible mechanistic pathway may involve sequestration of intrinsic metals in the body due to Humic-like substances (HULIS), which has been proven to disrupt iron homeostasis resulting in adverse cardiovascular, pregnancy, and perinatal outcomes (58). Thermal degradation of the base solution into carbonyl compounds continues to be an area of great interest and concern. A recent study has confirmed that inhalation exposure to formaldehyde alone produces similar impairment to aortic function as with ecig aerosol (59), supporting the notion the aerosolization of the base solution may be the primary source of harm. That is not say that nicotine and/or flavoring may not also have the potential to cause harm, but rather their effects are likely to be added on top of that stemming from the base components (VG/PG). The extent to which any or all of these players impact the gestational and postnatal dynamic is an area that requires further research.
Most pregnant women who are dual users (i.e., smoke and vape) use their ecigs daily with a nicotine concentration between 1 and 6 mg (60) and a national sample of pregnant women with vaping during late pregnancy, either exclusively or dual-use, shows an increased risk of small-for-gestational age compared with nonusers (adjusted OR 2.4, 95% CI 1.0–5.7 for sole vapers, and OR 2.3 95% CI 1.3–4.1 for dual-users) (61). The developmental toxicity of nicotine (22) is well described, with nicotine exposure leading to altered size, weight, tracheal development, metabolic changes, and liver dysfunction (18, 62). Some cardiovascular effects of nicotine can plausibly arise via conversion into cotinine, a metabolite of nicotine, which has subtle yet enduring developmental consequences. However, in this study, no significant differences in vascular deficits were observed in pups born to mothers who were administered e-liquid with nicotine absent or present, indicating the nicotine is not the principal factor driving vascular dysfunction. We did observe some subtle changes in body composition (i.e., lean vs. fat mass; Table 1) in ecig compared with air offspring, but we did not see significant differences in birth weight or body mass (up to 7 mo postnatally) as might be expected. Although a full explanation for this is not yet clear, we note that others, which have shown reductions in body mass with ecig exposure, have used much greater exposure to nicotine. For example, Orzabal et al. (63) showed nicotine-containing ecig-aerosol-exposed Sprague–Dawley dams exhibited decreased fetal weight by 47% but used up to 100 mg/mL nicotine in the e-liquid and a more intense vaping paradigm (1-s puffs every 20 s for 3 h a day). At present, it is not clear what the nicotine threshold is to trigger the changes others have observed, but based on our data, it would seem above the 18 mg/mL.
Strengths and Limitations
This is a preclinical study involving pregnancy exposure in animals and thus caution must be extended if/when attempting to infer human outcomes. Nevertheless, in terms of inhalation exposures, it is notable that animal studies have consistently and reliably provided valuable insights into the mechanisms and pathways involved in programming peripheral disease risk (64). Thus, based on the rapidly growing body of evidence from animal studies, it is more logical to expect the same risks to apply to humans, rather than assume ecigs confer minimal risk. An added strength of this study is the inclusion of male and female animals in equal or near-equal ratios. Although the numbers available are not powered to discern sex differences statistically, they do suggest the vascular outcomes we assessed for these conditions are not likely to be sex-based. However, an important caveat and potential limitation in this regard is that estrous status of reproductively mature females can impact vascular reactivity. Since we did not account for the estrous cycle in our females, we cannot fully exclude the possibility that sex differences might be present.
We narrowed the timing of exposure to during gestation and lactation to understand the consequences of a compromised perinatal environment. Preclinical and clinical studies have shown reduced fecundability/fertility due to nicotine-containing ecig aerosol (62, 65). Prepregnancy toxicant exposure may change morphology of maternal vasculature and subsequently placental and uterine vascular structure/function. A study design in which the female rodents be exposed before pregnancy, although potentially more reflective of human patterns of ecig use, would call into question the effects of vaping on implantation and fertility. Although this question is indeed of great interest, it was not the focus of this study. In this work, we cannot separate whether our effect occurred primarily from the gestational or weaning periods in development, but early reports from ongoing studies with gestational-only exposure (limited to GD2-GD21) suggest that it is the perinatal exposure that is inducing nearly, if not, all of the effect (52, 53). It should be noted that reproductive studies are typically based on the number of dams studied, so the relatively small number dams we studied is a limitation when considering the potential for biological variability. However, we would point out that the histological and physiological effects in the offspring were robust and consistent (strong in magnitude and low variability) across litters of the same maternal exposure; thus it also seems unlikely that adding more dams would ultimately change the outcomes we see and report.
It is important to recognize that there are considerable number of permutations for ecig parameters (e.g., wattage, nicotine content, puff topography, etc.) and uncertainty surrounding the interactions of these properties and their potential individual effects. The two levels of nicotine exposure (0 and 18 mg/mL) in this study serve as a starting point to understand the vulnerability of a fetus to maternal vaping. But it is important to remember the contribution of each parameter is a complexity that could also influence the outcome. For example, keeping the same nicotine concentration and simply increasing the watts (i.e., the temperature used to create the vape cloud) could easily be expected to produce a very different toxicity profile. The wattage we used (i.e., 17.5 watts) is well within the normal operating range many humans would/could use with variable-power tank-style ecig devices (so called “mods”) (66) but is higher compared with most pod-type ecig devices that operate between 5 and 10 watts (e.g., JUUL, Puff Bar, etc.). With the many permutations that can exist with ecigs and/or other rapidly evolving tobacco technologies, it will be important for future studies to better understand and explore the potential of harm.
Clinical Relevance
Inhalational pollutants affect pregnancy adversely leading to preterm birth, fetal growth restriction, increased uterine vascular resistance, impaired placental vascularization, and/or increased gestational diabetes (67). Although direct ecig exposure is known to trigger adverse cardiovascular outcomes, this study expands our understanding to include adverse developmental outcomes in offspring due solely to perinatal ecig exposure (i.e., indirect exposure from maternal vaping). Importantly these preclinical data suggest maternal vaping creates a hostile fetal environment that has long-lasting impact to the vascular health in offspring. Given the growing interest by some health care providers/agency to promote vaping (vs. smoking) during pregnancy, it becomes increasingly important to assess the vascular health of individuals born with a history of indirect ecig exposure due to maternal vaping. It is notable that the relative magnitude of vascular changes we observe in this study is comparable to other risk factors (such as high-fat diet, diabetes, obesity and aging) (24, 30) and there is great concern for the future morbidity and mortality in progeny already conditioned with vascular risk factor, particularly when these individuals when faced with cardiovascular (e.g., myocardial infarct, hypertension, etc.) or cerebrovascular (e.g., stroke) challenge in adolescent or adult life.
Conclusions
Our study objective was to probe the vascular deficits in offspring that result from perinatal exposure of maternal electronic cigarette aerosol. Despite the marketing as healthier alternatives to cigarette smoking and subsequent widespread public perception of ecig harmlessness (68, 69), we observed significant long-lasting vascular dysfunction in offspring with a history of perinatal exposure to ecig aerosols (with or without nicotine). Here, we report [similar to previous evidence in a resistance vessel (24)] that fetal exposure from maternal vaping during pregnancy triggers subtle, but significant, disruptions in conduit artery structure and function in offspring that persist into adult life. A stiffer vessel poses a disease risk factor by itself, and when considering the added potential of combining added risk factors that can develop during life (e.g., obesity, diet, sedentary lifestyle, etc.), there is a great concern for vascular health and disease in offspring experiencing perinatal exposures. These data add to the growing base of evidence that counters the notion that ecigs are safe, even compared with cigarettes, and highlight the need for greater awareness that the effects ecigs should be evaluated from a broader holistic view in health and disease. These data should be used to inform users and clinicians to track and monitor the vascular health of ecig users and, equally important, all those born with any history of perinatal exposure.
DATA AVAILABILITY
Data will be made available upon reasonable request.
GRANTS
Funding support was provided by Philip R Dino Innovative Research Grant from the WVU Cancer Institute (to I.M.O.), Transition Grant Support from the Office of Research and Graduate Education, WVU Health Sciences Center (to I.M.O.), and NIH U54-GM104942-03 (to P.D.C.) and American Heart Association 20CSA35320107 (to I.M.O. and P.D.C.). NanoSight NS300 Grant: Stroke CoBRE GM109098 and WV-CTSI Grant #GM103434, ZetaSizer NanoZ Grant: Stroke CoBRE GM109098 and WV-CTSI Grant #GM103434.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
E.A., P.D.C., and I.M.O. conceived and designed research; E.A., K.F., and L.H. performed experiments; E.A., P.D.C., and I.M.O. analyzed data; E.A., K.F., P.D.C., and I.M.O. interpreted results of experiments; E.A., P.D.C., and I.M.O. prepared figures; E.A. drafted manuscript; E.A., K.F., L.H., P.D.C., and I.M.O. edited and revised manuscript; E.A., K.F., L.H., P.D.C., and I.M.O. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge the many hours and time devoted by Sarah Reppert, Sydney Dangnott, and Jaclyn Montag (undergraduate students in the WVU Exercise Physiology program) who helped in carrying out the daily exposure.
REFERENCES
- 1. Wagner NJ, Camerota M, Propper C. Prevalence and Perceptions of electronic cigarette use during pregnancy. Matern Child Health J 21: 1655–1661, 2017. doi: 10.1007/s10995-016-2257-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kurti AN, Redner R, Lopez AA, Keith DR, Villanti AC, Stanton CA, Gaalema DE, Bunn JY, Doogan NJ, Cepeda-Benito A, Roberts ME, Phillips J, Higgins ST. Tobacco and nicotine delivery product use in a national sample of pregnant women. Prev Med 104: 50–56, 2017. doi: 10.1016/j.ypmed.2017.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Oncken C, Ricci KA, Kuo CL, Dornelas E, Kranzler HR, Sankey HZ. Correlates of electronic cigarettes use before and during pregnancy. Nicotine Tob Res 19: 585–590, 2017. doi: 10.1093/ntr/ntw225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kapaya M, D'Angelo DV, Tong VT, England L, Ruffo N, Cox S, Warner L, Bombard J, Guthrie T, Lampkins A, King BA. Use of electronic vapor products before, during, and after pregnancy among women with a recent live birth - Oklahoma and Texas, 2015. MMWR Morb Mortal Wkly Rep 68: 189–194, 2019. doi: 10.15585/mmwr.mm6808a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bhandari NR, Day KD, Payakachat N, Franks AM, McCain KR, Ragland D. Use and risk perception of electronic nicotine delivery systems and tobacco in pregnancy. Womens Health Issues 28: 251–257, 2018. doi: 10.1016/j.whi.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 6. Whittington JR, Simmons PM, Phillips AM, Gammill SK, Cen R, Magann EF, Cardenas VM. The use of electronic cigarettes in pregnancy: a review of the literature. Obstet Gynecol Surv 73: 544–549, 2018. doi: 10.1097/OGX.0000000000000595. [DOI] [PubMed] [Google Scholar]
- 7. England LJ, Tong VT, Koblitz A, Kish-Doto J, Lynch MM, Southwell BG. Perceptions of emerging tobacco products and nicotine replacement therapy among pregnant women and women planning a pregnancy. Prev Med Rep 4: 481–485, 2016. doi: 10.1016/j.pmedr.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Breland A, McCubbin A, Ashford K. Electronic nicotine delivery systems and pregnancy: recent research on perceptions, cessation, and toxicant delivery. Birth Defects Res 111: 1284–1293, 2019. doi: 10.1002/bdr2.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ashford K, Wiggins A, Butler K, Ickes M, Rayens MK, Hahn E. e-Cigarette use and perceived harm among women of childbearing age who reported tobacco use during the past year. Nurs Res 65: 408–414, 2016. doi: 10.1097/NNR.0000000000000176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stapleton PA, Wingard CJ, Nurkiewicz TR, Holloway AC, Zelikoff JT, Knudsen TB, Rogers LK. Cardiopulmonary consequences of gestational toxicant exposure: symposium overview at the 56th annual SOT meeting, Baltimore, MD. Reprod Toxicol 79: 16–20, 2018. doi: 10.1016/j.reprotox.2018.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shao XM, López-Valdés HE, Liang J, Feldman JL. Inhaled nicotine equivalent to cigarette smoking disrupts systemic and uterine hemodynamics and induces cardiac arrhythmia in pregnant rats. Sci Rep 7: 16974, 2017. doi: 10.1038/s41598-017-17301-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lim R, Sobey CG. Maternal nicotine exposure and fetal programming of vascular oxidative stress in adult offspring. Br J Pharmacol 164: 1397–1399, 2011. doi: 10.1111/j.1476-5381.2011.01488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Holloway AC, Salomon A, Soares MJ, Garnier V, Raha S, Sergent F, Nicholson CJ, Feige JJ, Benharouga M, Alfaidy N. Characterization of the adverse effects of nicotine on placental development: in vivo and in vitro studies. Am J Physiol Endocrinol Physiol 306: E443–E456, 2014. doi: 10.1152/ajpendo.00478.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gopalakrishnan K, More AS, Hankins GD, Nanovskaya TN, Kumar S. Postnatal cardiovascular consequences in the offspring of pregnant rats exposed to smoking and smoking cessation pharmacotherapies. Reprod Sci 24: 919–933, 2017. doi: 10.1177/1933719116673199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sifat AE, Nozohouri S, Villalba H, Al Shoyaib A, Vaidya B, Karamyan VT, Abbruscato T. Prenatal electronic cigarette exposure decreases brain glucose utilization and worsens outcome in offspring hypoxic-ischemic brain injury. J Neurochem 153: 63–79, 2020. doi: 10.1111/jnc.14947. [DOI] [PubMed] [Google Scholar]
- 16. Noël A, Hansen S, Zaman A, Perveen Z, Pinkston R, Hossain E, Xiao R, Penn A. In utero exposures to electronic-cigarette aerosols impair the Wnt signaling during mouse lung development. Am J Physiol Lung Cell Mol Physiol 318: L705–L722, 2020. doi: 10.1152/ajplung.00408.2019. [DOI] [PubMed] [Google Scholar]
- 17. Wang Q, Sundar IK, Blum JL, Ratner JR, Lucas JH, Chuang TD, Wang Y, Liu J, Rehan VK, Zelikoff JT, Rahman I. Prenatal exposure to E-cigarette aerosols leads to sex-dependent pulmonary extracellular matrix remodeling and myogenesis in offspring mice. Am J Respir Cell Mol Biol 63: 794–805, 2020. doi: 10.1165/rcmb.2020-0036OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li G, Chan YL, Wang B, Saad S, George J, Oliver BG, Chen H. E-cigarettes damage the liver and alter nutrient metabolism in pregnant mice and their offspring. Ann N Y Acad Sci 1475: 64–77, 2020. doi: 10.1111/nyas.14411. [DOI] [PubMed] [Google Scholar]
- 19. Chen H, Li G, Chan YL, Chapman DG, Sukjamnong S, Nguyen T, Annissa T, McGrath KC, Sharma P, Oliver BG. Maternal E-cigarette exposure in mice alters DNA methylation and lung cytokine expression in offspring. Am J Respir Cell Mol Biol 58: 366–377, 2018. doi: 10.1165/rcmb.2017-0206RC. [DOI] [PubMed] [Google Scholar]
- 20. Nguyen T, Li GE, Chen H, Cranfield CG, McGrath KC, Gorrie CA. Maternal E-cigarette exposure results in cognitive and epigenetic alterations in offspring in a mouse model. Chem Res Toxicol 31: 601–611, 2018. doi: 10.1021/acs.chemrestox.8b00084. [DOI] [PubMed] [Google Scholar]
- 21. Ahmed N, Kalininskiy A, Gandhi H, Shin JJ. Spontaneous coronary artery dissection in a postpartum e-cigarette smoker. BMJ Case Rep 2018: bcr2018225194, 2018. doi: 10.1136/bcr-2018-225194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. England LJ, Aagaard K, Bloch M, Conway K, Cosgrove K, Grana R, Gould TJ, Hatsukami D, Jensen F, Kandel D, Lanphear B, Leslie F, Pauly JR, Neiderhiser J, Rubinstein M, Slotkin TA, Spindel E, Stroud L, Wakschlag L. Developmental toxicity of nicotine: a transdisciplinary synthesis and implications for emerging tobacco products. Neurosci Biobehav Rev 72: 176–189, 2017. doi: 10.1016/j.neubiorev.2016.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Glover M, Phillips CV. Potential effects of using non-combustible tobacco and nicotine products during pregnancy: a systematic review. Harm Reduct J 17: 16, 2020. doi: 10.1186/s12954-020-00359-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Burrage EN, Aboaziza E, Hare L, Reppert S, Moore J, Goldsmith WT, Kelley EE, Mills A, Dakhlallah D, Chantler PD, Olfert IM. Long-term cerebrovascular dysfunction in the offspring from maternal electronic cigarette use during pregnancy. Am J Physiol Heart Circ Physiol 321: H339–H352, 2021. doi: 10.1152/ajpheart.00206.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. El-Mahdy MA, Ewees MG, Eid MS, Mahgoup EM, Khaleel SA, Zweier JL. Electronic cigarette exposure causes vascular endothelial dysfunction due to NADPH oxidase activation and eNOS uncoupling. Am J Physiol Heart Circ Physiol 322: H549–H567, 2022. doi: 10.1152/ajpheart.00460.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. El-Mahdy MA, Mahgoup EM, Ewees MG, Eid MS, Abdelghany TM, Zweier JL. Long-term electronic cigarette exposure induces cardiovascular dysfunction similar to tobacco cigarettes: role of nicotine and exposure duration. Am J Physiol Heart Circ Physiol 320: H2112–H2129, 2021. doi: 10.1152/ajpheart.00997.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nabavizadeh P, Liu J, Rao P, Ibrahim S, Han DD, Derakhshandeh R, Qiu H, Wang X, Glantz SA, Schick SF, Springer ML. Impairment of endothelial function by cigarette smoke is not caused by a specific smoke constituent, but by vagal input from the airway. Arterioscler Thromb Vasc Biol 42: 1324–1332, 2022. doi: 10.1161/ATVBAHA.122.318051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vlachopoulos C, Ioakeimidis N, Abdelrasoul M, Terentes-Printzios D, Georgakopoulos C, Pietri P, Stefanadis C, Tousoulis D. Electronic cigarette smoking increases aortic stiffness and blood pressure in young smokers. J Am Coll Cardiol 67: 2802–2803, 2016. doi: 10.1016/j.jacc.2016.03.569. [DOI] [PubMed] [Google Scholar]
- 29. Mohammadi L, Han DD, Xu F, Huang A, Derakhshandeh R, Rao P, Whitlatch A, Cheng J, Keith RJ, Hamburg NM, Ganz P, Hellman J, Schick SF, Springer ML. Chronic E-cigarette use impairs endothelial function on the physiological and cellular levels. Arterioscler Thromb Vasc Biol 42: 1333–1350, 2022. doi: 10.1161/ATVBAHA.121.317749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Olfert IM, DeVallance E, Hoskinson H, Branyan KW, Clayton S, Pitzer CR, Sullivan DP, Breit MJ, Wu Z, Klinkhachorn P, Mandler WK, Erdreich BH, Ducatman BS, Bryner RW, Dasgupta P, Chantler PD. Chronic exposure to electronic cigarettes results in impaired cardiovascular function in mice. J Appl Physiol (1985) 124: 573–582, 2018. doi: 10.1152/japplphysiol.00713.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rao P, Han DD, Tan K, Mohammadi L, Derakhshandeh R, Navabzadeh M, Goyal N, Springer ML. Comparable impairment of vascular endothelial function by a wide range of electronic nicotine delivery devices. Nicotine Tob Res 24: 1055–1062, 2022. doi: 10.1093/ntr/ntac019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang M, Li H, Huang S, Qian Y, Steenland K, Xie Y, Papatheodorou S, Shi L. Short-term exposure to nitrogen dioxide and mortality: a systematic review and meta-analysis. Environ Res 202: 111766, 2021. doi: 10.1016/j.envres.2021.111766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Johnson NM, Hoffmann AR, Behlen JC, Lau C, Pendleton D, Harvey N, Shore R, Li Y, Chen J, Tian Y, Zhang R. Air pollution and children's health-a review of adverse effects associated with prenatal exposure from fine to ultrafine particulate matter. Environ Health Prev Med 26: 72, 2021. doi: 10.1186/s12199-021-00995-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fabricio MF, Jordão MT, Miotto DS, Ruiz TFR, Vicentini CA, Lacchini S, Santos CF, Michelini LC, Amaral SL. Standardization of a new non-invasive device for assessment of arterial stiffness in rats: correlation with age-related arteries' structure. MethodsX 7: 100901, 2020. doi: 10.1016/j.mex.2020.100901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. von Ehr J, von Versen-Höynck F. Implications of maternal conditions and pregnancy course on offspring's medical problems in adult life. Arch Gynecol Obstet 294: 673–679, 2016. doi: 10.1007/s00404-016-4178-7. [DOI] [PubMed] [Google Scholar]
- 36. Wold LE, Tarran R, Alexander LEC, Hamburg NM, Kheradmand F, Helen GS, Wu JC; American Heart Association Council on Basic Cardiovascular Sciences; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Hypertension; and Stroke Council. Cardiopulmonary consequences of vaping in adolescents: a scientific statement from the American Heart Association. Circ Res 131: e70–e82, 2022. doi: 10.1161/RES.0000000000000544. [DOI] [PubMed] [Google Scholar]
- 37. Szostak J, Wong ET, Titz B, Lee T, Wong SK, Low T, Lee KM, Zhang J, Kumar A, Schlage WK, Guedj E, Phillips B, Leroy P, Buettner A, Xiang Y, Martin F, Sewer A, Kuczaj A, Ivanov NV, Luettich K, Vanscheeuwijck P, Peitsch MC, Hoeng J. A 6-month systems toxicology inhalation study in ApoE(-/-) mice demonstrates reduced cardiovascular effects of E-vapor aerosols compared with cigarette smoke. Am J Physiol Heart Circ Physiol 318: H604–H631, 2020. doi: 10.1152/ajpheart.00613.2019. [DOI] [PubMed] [Google Scholar]
- 38. Bell V, McCabe EL, Larson MG, Rong J, Merz AA, Osypiuk E, Lehman BT, Stantchev P, Aragam J, Benjamin EJ, Hamburg NM, Vasan RS, Mitchell GF, Cheng S. Relations between aortic stiffness and left ventricular mechanical function in the community. J Am Heart Assoc 6: e004903, 2017. doi: 10.1161/JAHA.116.004903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Koudsi A, Oldroyd J, McElduff P, Banerjee M, Vyas A, Cruickshank JK. Maternal and neonatal influences on, and reproducibility of, neonatal aortic pulse wave velocity. Hypertension 49: 225–231, 2007. doi: 10.1161/01.HYP.0000250434.73119.7a. [DOI] [PubMed] [Google Scholar]
- 40. Andersson C, Quiroz R, Enserro D, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Mitchell GF, Vasan RS. Association of parental hypertension with arterial stiffness in nonhypertensive offspring: the Framingham Heart Study. Hypertension 68: 584–589, 2016. doi: 10.1161/HYPERTENSIONAHA.116.07426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Golbidi S, Edvinsson L, Laher I. Smoking and endothelial dysfunction. Curr Vasc Pharmacol 18: 1–11, 2020. doi: 10.2174/1573403X14666180913120015. [DOI] [PubMed] [Google Scholar]
- 42. Carnevale R, Sciarretta S, Violi F, Nocella C, Loffredo L, Perri L, Peruzzi M, Marullo AG, De Falco E, Chimenti I, Valenti V, Biondi-Zoccai G, Frati G. Acute impact of tobacco vs electronic cigarette smoking on oxidative stress and vascular function. Chest 150: 606–612, 2016. doi: 10.1016/j.chest.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 43. Anderson C, Majeste A, Hanus J, Wang S. E-Cigarette aerosol exposure induces reactive oxygen species, DNA damage, and cell death in vascular endothelial cells. Toxicol Sci 154: 332–340, 2016. doi: 10.1093/toxsci/kfw166. [DOI] [PubMed] [Google Scholar]
- 44. Moheimani RS, Bhetraratana M, Yin F, Peters KM, Gornbein J, Araujo JA, Middlekauff HR. Increased cardiac sympathetic activity and oxidative stress in habitual electronic cigarette users: implications for cardiovascular risk. JAMA Cardiol 2: 278–284, 2017. doi: 10.1001/jamacardio.2016.5303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cai H, Wang C. Graphical review: The redox dark side of e-cigarettes; exposure to oxidants and public health concerns. Redox Biol 13: 402–406, 2017. doi: 10.1016/j.redox.2017.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Antoniewicz L, Bosson JA, Kuhl J, Abdel-Halim SM, Kiessling A, Mobarrez F, Lundbäck M. Electronic cigarettes increase endothelial progenitor cells in the blood of healthy volunteers. Atherosclerosis 255: 179–185, 2016. doi: 10.1016/j.atherosclerosis.2016.09.064. [DOI] [PubMed] [Google Scholar]
- 47. Heiss C. Electronic cigarettes increase EPCs. Atherosclerosis 255: 119–121, 2016. doi: 10.1016/j.atherosclerosis.2016.10.033. [DOI] [PubMed] [Google Scholar]
- 48. Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med 348: 593–600, 2003. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 49. Allison BJ, Kaandorp JJ, Kane AD, Camm EJ, Lusby C, Cross CM, Nevin-Dolan R, Thakor AS, Derks JB, Tarry-Adkins JL, Ozanne SE, Giussani DA. Divergence of mechanistic pathways mediating cardiovascular aging and developmental programming of cardiovascular disease. FASEB J 30: 1968–1975, 2016. doi: 10.1096/fj.201500057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Conklin DJ, Ogunwale MA, Chen Y, Theis WS, Nantz MH, Fu XA, Chen LC, Riggs DW, Lorkiewicz P, Bhatnagar A, Srivastava S. Electronic cigarette-generated aldehydes: the contribution of e-liquid components to their formation and the use of urinary aldehyde metabolites as biomarkers of exposure. Aerosol Sci Technol 52: 1219–1232, 2018. doi: 10.1080/02786826.2018.1500013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Grana R, Benowitz N, Glantz SA. E-cigarettes: a scientific review. Circulation 129: 1972–1986, 2014. doi: 10.1161/CIRCULATIONAHA.114.007667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Frazier JI, Mills A, Nassabeh S, Plants R, Robinson M, Kincaid S, Kottapalli D, Prabhu S, Chantler PD, Olfert IM. Low vs. high wattage vaping during pregnancy influences vascular function in rat offspring. FASEB J 36: S1, May 2022. doi: 10.1096/fasebj.2022.36.S1.R5928. [DOI] [Google Scholar]
- 53. Frazier J, Coblentz T, Bruce J, Nassabeh S, Plants R, Burrage E, Mills A, Chantler P, Olfert I. Effect of E-liquid base solution (vegetable glycerin or propylene glycol) on aortic function in mice. FASEB J 35: S1, May 2021. doi: 10.1096/fasebj.2021.35.S1.01638. [DOI] [Google Scholar]
- 54. Greene RM, Pisano MM. Developmental toxicity of e-cigarette aerosols. Birth Defects Res 111: 1294–1301, 2019. doi: 10.1002/bdr2.1571. [DOI] [PubMed] [Google Scholar]
- 55. Lippi G, Favaloro EJ, Meschi T, Mattiuzzi C, Borghi L, Cervellin G. E-cigarettes and cardiovascular risk: beyond science and mysticism. Semin Thromb Hemost 40: 60–65, 2014. doi: 10.1055/s-0033-1363468. [DOI] [PubMed] [Google Scholar]
- 56. Wylie BJ, Hauptman M, Hacker MR, Hawkins SS. Understanding rising electronic cigarette use. Obstet Gynecol 137: 521–527, 2021. doi: 10.1097/AOG.0000000000004282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Navas-Acien A, Martinez-Morata I, Hilpert M, Rule A, Shimbo D, LoIacono NJ. Early cardiovascular risk in E-cigarette users: the potential role of metals. Curr Environ Health Rep 7: 353–361, 2020. [Erratum in Curr Environ Health Rep 7: 362, 2020]. doi: 10.1007/s40572-020-00297-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ghio AJ, Soukup JM, Madden MC. The toxicology of air pollution predicts its epidemiology. Inhal Toxicol 30: 327–334, 2018. doi: 10.1080/08958378.2018.1530316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Jin L, Lynch J, Richardson A, Lorkiewicz P, Srivastava S, Theis W, Shirk G, Hand A, Bhatnagar A, Srivastava S, Conklin DJ. Electronic cigarette solvents, pulmonary irritation, and endothelial dysfunction: role of acetaldehyde and formaldehyde. Am J Physiol Heart Circ Physiol 320: H1510–H1525, 2021. doi: 10.1152/ajpheart.00878.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. McCubbin A, Wiggins A, Barnett J, Ashford K. Perceptions, characteristics, and behaviors of cigarette and electronic cigarette use among pregnant smokers. Womens Health Issues 30: 221–229, 2020. doi: 10.1016/j.whi.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wang X, Lee NL, Burstyn I. Smoking and use of electronic cigarettes (vaping) in relation to preterm birth and small-for-gestational-age in a 2016 U.S. national sample. Prev Med 134: 106041, 2020. doi: 10.1016/j.ypmed.2020.106041. [DOI] [PubMed] [Google Scholar]
- 62. El-Merhie N, Krüger A, Uliczka K, Papenmeier S, Roeder T, Rabe KF, Wagner C, Angstmann H, Krauss-Etschmann S. Sex dependent effect of maternal e-nicotine on F1 Drosophila development and airways. Sci Rep 11: 4441, 2021. doi: 10.1038/s41598-021-81607-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Orzabal MR, Lunde-Young ER, Ramirez JI, Howe SYF, Naik VD, Lee J, Heaps CL, Threadgill DW, Ramadoss J. Chronic exposure to e-cig aerosols during early development causes vascular dysfunction and offspring growth deficits. Transl Res 207: 70–82, 2019. doi: 10.1016/j.trsl.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Shrestha N, Ezechukwu HC, Holland OJ, Hryciw DH. Developmental programming of peripheral diseases in offspring exposed to maternal obesity during pregnancy. Am J Physiol Regul Integr Comp Physiol 319: R507–R516, 2020. doi: 10.1152/ajpregu.00214.2020. [DOI] [PubMed] [Google Scholar]
- 65. Harlow AF, Hatch EE, Wesselink AK, Rothman KJ, Wise LA. Electronic cigarettes and fecundability: results from a prospective preconception cohort study. Am J Epidemiol 190: 353–361, 2021. doi: 10.1093/aje/kwaa067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Cohen JE, Hardesty JJ, Nian Q, Crespi E, Sinamo JK, Kennedy RD, Welding K, Kaplan B, Soule E, Eissenberg T, Breland AB. Combinations of electronic nicotine delivery system device and liquid characteristics among U.S. adults. Addict Behav 135: 107441, 2022. doi: 10.1016/j.addbeh.2022.107441. [DOI] [PubMed] [Google Scholar]
- 67. Gómez-Roig MD, Pascal R, Cahuana MJ, García-Algar O, Sebastiani G, Andreu-Fernández V, Martínez L, Rodríguez G, Iglesia I, Ortiz-Arrabal O, Mesa MD, Cabero MJ, Guerra L, Llurba E, Domínguez C, Zanini MJ, Foraster M, Larqué E, Cabañas F, Lopez-Azorín M, Pérez A, Loureiro B, Pallás-Alonso CR, Escuder-Vieco D, Vento M. Environmental exposure during pregnancy: influence on prenatal development and early life: a comprehensive review. Fetal Diagn Ther 48: 245–257, 2021. doi: 10.1159/000514884. [DOI] [PubMed] [Google Scholar]
- 68. Tsai M, Byun MK, Shin J, Crotty Alexander LE. Effects of e-cigarettes and vaping devices on cardiac and pulmonary physiology. J Physiol 598: 5039–5062, 2020. doi: 10.1113/JP279754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kim S, Oancea SC. Electronic cigarettes may not be a “safer alternative” of conventional cigarettes during pregnancy: evidence from the nationally representative PRAMS data. BMC Pregnancy Childbirth 20: 557, 2020. doi: 10.1186/s12884-020-03247-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available upon reasonable request.