Abstract
Background
The outbreak of severe acute respiratory syndrome coronavirus 2 has had an enormous impact on global health. Vaccination remains one of the most effective interventions for disease prevention. Clinically significant vaccine side effects are uncommon, though autoimmune-mediated disease occurs in a small percentage of vaccine recipients. Systemic lupus erythematosus (SLE) is a multisystem autoimmune disease that is associated with significant morbidity and mortality. Childhood-onset SLE tends to have more severe disease manifestations than adult-onset SLE. In adults, there are a few reported cases of SLE developing soon after coronavirus disease 2019 (COVID-19) mRNA vaccination.
Case presentation
A 14-year-old previously healthy male developed laboratory and clinical evidence of SLE, including maculopapular malar rash, arthritis, pleuritic chest pain, and class V (membranous) lupus nephritis, 2 days after his third dose of the Pfizer-BioNTech COVID-19 vaccine. The patient’s symptoms improved after initiation of prednisone and mycophenolate mofetil. We also summarize eleven prior case reports describing SLE after COVID-19 vaccine in adults.
Conclusion
To our knowledge, this is the first reported pediatric patient with new onset SLE following COVID-19 mRNA vaccination. While potential mechanistic links exist between COVID-19 vaccination and SLE development, additional studies are necessary to elucidate the exact nature of this relationship.
Keywords: Systemic lupus erythematosus, COVID-19, Vaccination, SARS-CoV2, Lupus nephritis, Case report
Background
Systemic lupus erythematosus (SLE) is an autoimmune disease with multiple manifestations that most often presents in the second and third decades, and is much more common in females [1, 2]. Childhood onset SLE (cSLE) is more likely to cause kidney and neuropsychiatric disease, and has overall increased disease activity. Vaccinations have been proposed as potential triggers for the onset of SLE given their role in antigen stimulation, although these associations have not been confirmed in epidemiologic studies [1–5].
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has caused 6.2 million deaths worldwide as of May 2022 [6]. Literature suggests SLE patients may be at an increased risk of poor outcomes with coronavirus disease 2019 (COVID-19) and vaccination is encouraged in SLE patients, especially those receiving potent immunosuppressive therapy [2, 7]. However, recent literature has shown that SLE patients exhibit more vaccine reactogenicity, with more frequent reports of fever, vomiting, and injection site redness following the SARS-CoV-2 mRNA Pfizer- BioNTech vaccine [8]. Furthermore, there are a number of case reports in adults describing SLE presenting after COVID-19 vaccination [7–12]. There are also reports of SLE exacerbations, including relapse of class V (membranous) lupus nephritis, after SARS-CoV2-vaccination in the adult population [12–15]. However, we could not identify any cases describing the development of cSLE or exacerbation of lupus nephritis during childhood. We report a pediatric patient who developed clinical symptoms of cSLE two days after administration of the 3rd dose of the SARS-CoV2 vaccination; he also had nephrotic-range proteinuria and a kidney biopsy demonstrated class V lupus nephritis.
Case presentation
A fourteen-year-old Asian male (51 kg in weight) with no significant past medical history developed a non-photosensitive facial rash two days following his third dose of the SARS-CoV-2 mRNA Pfizer-BioNTech vaccine, and approximately eight months after his second vaccine. The patient was not receiving any medications and had not received any medications during the two months prior to the most recent vaccine. The rash quickly spread to his knees and arms; it was unresponsive to topical steroids and he was referred to dermatology. The dermatologist prescribed oral cephalexin given concern for secondary infection, but his rash did not improve. The patient subsequently developed bilateral arthralgias of his shoulders, hands, and knees. He also developed worsening hair loss, pleuritic chest pain, and photophobia. The patient had no relevant past medical history or family history of SLE or other autoimmune disorders. Additionally, he did not report any reactions with his previous COVID vaccines.
At his pediatrician visit three weeks after the vaccine, labs were notable for a positive antinuclear antibody (ANA 1:80 titer, nuclear speckled pattern) and positive autoantibodies against double-stranded deoxyribonucleic acid (dsDNA), Ro, Smith and ribonucleoprotein (RNP). He also had hypocomplementemia and an elevated erythrocyte sedimentation rate (ESR).
The patient was subsequently evaluated in pediatric rheumatology clinic five weeks after the COVID-19 vaccination. His blood pressure was 115/63, which is mildly elevated for age, sex and height (168.3 cm). His positive physical exam findings included arthritis of bilateral elbows, palatal erythema, maculopapular malar rash on face with flat, violaceous lesions on extremities. Patient also had capillary loop dilatation on nailfold capillaroscopy.
Laboratory evaluation at his initial rheumatology clinic visit included leucopenia (white blood cell count 3500 per µl), hemoglobin of 14.1 g/dL, platelet count of 140,000 per µl, hypoalbuminemia (albumin 2.6 g/dL), elevated ESR (126 mm/hour) and normal C-reactive protein. Repeat serology testing confirmed a positive ANA with a titer of 1:1280 (nuclear speckled pattern) and positive anti-dsDNA, anti-Smith, anti-RNP, and anti-Ro antibodies. The patient’s anti-histone antibody was also found to be high-titer positive (2.7 Units, reference < 1.0 negative). Lupus anticoagulant, anti-cardiolipin antibodies, beta-2-glycoprotein antibodies, and direct Coombs were negative. His immunoglobin G level was elevated at 1806 mg/dL (reference, 500–1590 mg/dL). His creatinine was 0.46 mg/dL and his urinalysis had 3 + protein and no red blood cells; the urine protein/urine creatinine ratio was 13.5 mg/mg (reference, < 0.2 mg/mg). His calculated glomerular filtration rate was 155 ml/min/1.73 m2 [16]. Chest X-ray as well as echocardiogram were normal. Table 1 summarizes the clinical findings, laboratory results, and management modalities in chronological order.
Table 1.
Chronology of clinical features, laboratory results, and treatment modalities
| Day 0 (administration of 3rd SARS-CoV-2 Pfizer-BioNTech mRNA vaccine | Day + 4, saw pediatrician and was referred to dermatologist | Day + 20, seen by pediatric dermatology | Day + 35–38, seen by pediatric rheumatology and nephrology | |
|---|---|---|---|---|
| Clinical features | Rash (face, with spread to knees and arms) | Persistent rash, joint pains, hair loss, and light sensitivity | Arthritis bilateral elbows, also found to have palatal erythema and persistent rash | |
| Laboratory results | +ANA (1:80), +dsDNA, +Smith, +RNP, +Ro antibodies, low complements (C3 43, C4 7) | ANA 1:1280 with confirmed Smith/RNP, dsDNA, Ro+ antibodies; urine protein: creatinine elevated to 13.5 mg/mg | ||
| Treatment | Topical steroid cream | Oral cephalexin |
Mycophenolate mofetil 1000 mg twice daily; hydroxychloroquine 300 mg daily; Prednisone 60 mg’ daily; famotidine 20 mg daily |
ANA antinuclear antibody, dsDNA double-stranded deoxyribonucleic acid, RNP ribonucleoprotein
The patient was started on hydroxychloroquine 300 mg daily, prednisone 60 mg daily, and famotidine 20 mg daily. He was referred to pediatric nephrology, which led to a kidney biopsy. The kidney biopsy revealed class V lupus nephritis (Fig. 1). On light microscopy, glomeruli had a mild increase in mesangial matrix and cells; the basement membranes appeared intact (Fig. 1a). The immunofluorescent evaluation demonstrated “full house” staining; he was positive for IgA, IgG, IgM, C3, and C1q (Fig. 1b). Electron microscopic examination revealed numerous subepithelial immune complex deposits with minimal basement membrane remodeling and extensive foot process effacement (Fig. 1c). Numerous deposits were noted in the mesangium along with tubuloreticular inclusions. The patient was started on mycophenolate mofetil 1000 mg twice daily and losartan 12.5 mg daily. Three weeks after initiation of prednisone, the patient had resolution of his malar rash and arthritis with improvement of his capillary changes. Additionally, his urine protein/creatinine ratio dramatically improved to 0.8 mg/mg four weeks after starting treatment.
Fig. 1.
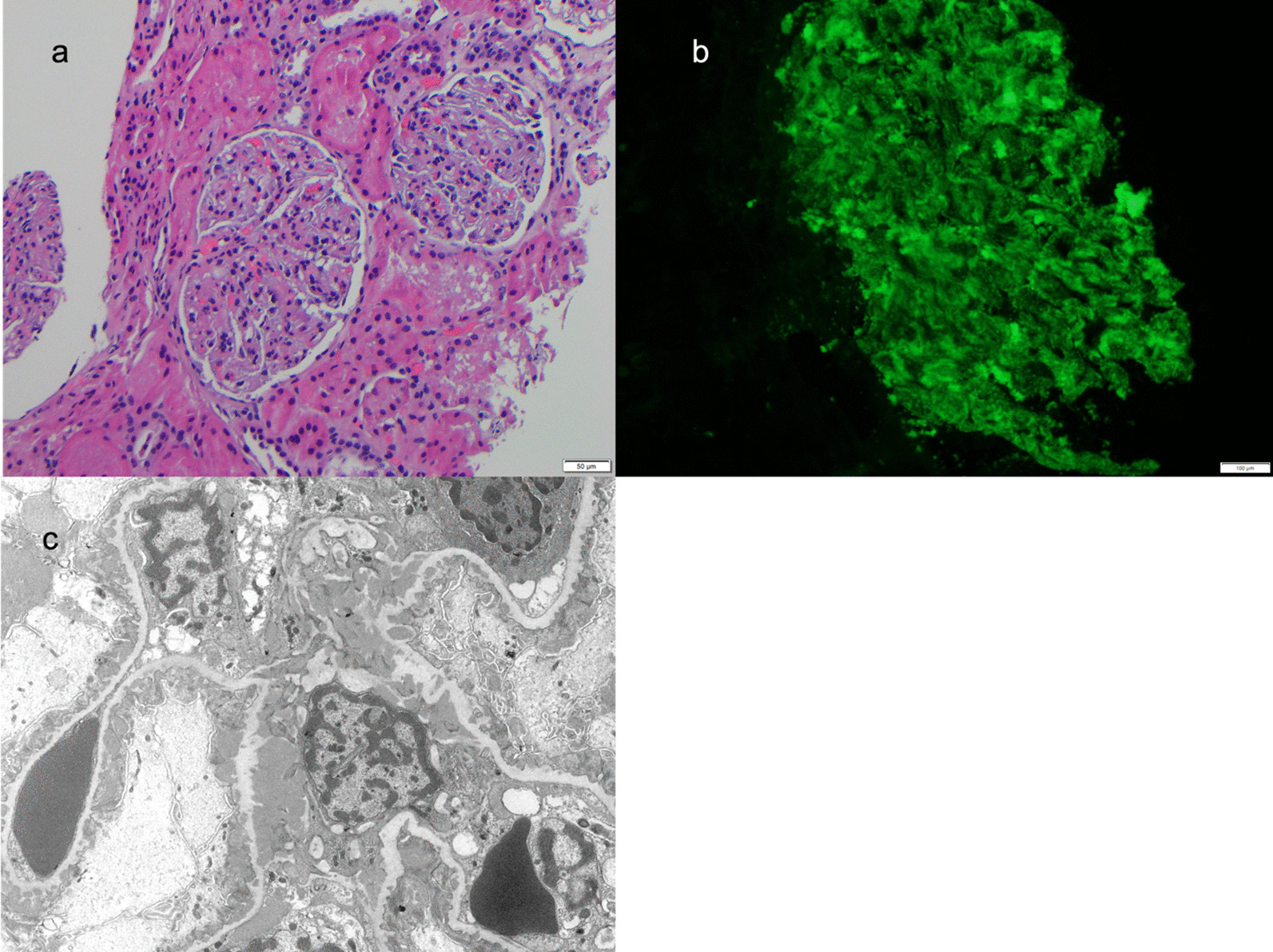
Kidney Biopsy showing a light microscopy, b immunofluorescence and c electron microscopy. a By light microscopy, glomeruli had a mild increase in mesangial matrix and cells with intact appearing basement membranes. The interstitium, tubules, and small vessels were normal except for abundant protein droplets the tubular epithelium. b Granular mesangial and peripheral staining was present on direct antibody immunofluorescence with antibodies against IgA, IgG, IgM, C3, and C1q. c Ultrastructural examination confirmed abundant mesangial and paramesangial deposits. Numerous early subepithelial membranous deposits were associated with small basement membrane spikes. Subendothelial tubuloreticular bodies were present (not shown)
Discussion and conclusion
We describe a 14-year-old male patient who developed cSLE with class V lupus nephritis two days after SARS-CoV-2 mRNA Pfizer-BioNTech vaccination. This association does not prove causality. Indeed, there have been billions of COVID-19 vaccine doses given worldwide so some medical events will inevitably occur after vaccination. At the time of this case, the Centers for Disease Control was recommending a two-dose primary series for those 5–17 years of age (Pfizer and Moderna) and booster vaccine for those who had received the Pfizer vaccine [17]. However, COVID-19 vaccination has been linked with rare autoimmune-mediated adverse events, and thus it s important to be aware of potential associations given the limited data on rare adverse events with these vaccinations [18, 19].
There are a number of prior cases in adults describing an association between COVID-19 vaccination and development of SLE. Cases reporting an association of COVID-19 vaccination and the development of SLE are summarized in Table 2 [9–11, 20–27]. Overall, these cases do not demonstrate any clear pattern. Six of the 11 cases occurred in patients in their 20’s, a peak time for onset of SLE. Three different vaccines were associated with the development of SLE, and six and five cases occurred after the 2nd dose and 1st dose, respectively. The clinical manifestations were typical of SLE, but variable, with two patients having lupus nephritis. Most patients developed symptoms one to two weeks following vaccination (Table 2), in contrast with our case which occurred approximately two days after vaccination. Additionally, there have been reports of other vaccines triggering development of SLE [1–5, 13, 28, 29].
Table 2.
Review of adult case reports associating SLE development and COVID-19 vaccination
| Case report | Vaccine administered | Clinical symptoms | Family history of autoimmunity | Laboratory values/imaging/ biopsy results | Medications utilized |
|---|---|---|---|---|---|
|
Baez et al. Case Rep Rheumatology Feb 2022 |
Moderna COVID-19 vaccine (2nd dose) | 27-year-old Female with Type 1 Diabetes developed arthritis two weeks after administration | Mother with SLE | Positive antinuclear antibody, anti-dsDNA, anti-Ro, and anti-La/SSB antibodies; low C4 levels | Low dose prednisone and hydroxychloroquine |
|
Kaur et al Cureus 2022 Feb |
Pfizer-BioNTech COVID-19 vaccine (2nd dose) | 54-year-old male with history of Sjögren’s syndrome; developed fever, lymphadenopathy, and purpuric lesions two weeks after vaccine | None reported | Hypocomplementemia, Positive antinuclear antibody, anti-dsDNA antibodies, anti-Smith antibodies, anti-ribonucleoprotein antibodies, anti-histone antibodies | High dose prednisone |
|
Hidaka et al. Int J Hematol 2022 Feb |
Pfizer-BioNTech COVID-19 vaccine (2nd dose) | 53-year -old female with history of bronchial asthma, Vogt–Koyanagi–Harada disease, and Hashimoto disease; developed wheezing and conjunctival pallor two weeks after vaccine | None reported | Positive antinuclear antibody, hemolytic anemia, positive Coombs, thrombocytopenia, hypocomplementemia, positive lupus anticoagulant | High dose prednisone |
|
Nune et al. Int Journal Medicine 2021 |
Pfizer-BioNTech SARS-CoV-2 vaccine (2nd dose) | 24-year-old male developed polyarthralgia, fever and fatigue two weeks after vaccine | None reported | Positive antinuclear antibody, anti-dsDNA, lymphopenia, hypocomplementemia | High dose prednisone, methotrexate |
|
Mousa et al. Clin Rheumatol. 2022 May |
Pfizer-BioNTech COVID-19 vaccine (1st dose) | 22-year-old female developed abdominal pain, vomiting, and rash one week after vaccine | No family history of autoimmunity | Positive antinuclear antibody, anti-dsDNA, lymphopenia, anemia, thrombocytopenia, transaminitis, elevated lipase and amylase, hypocomplementemia | High dose prednisone, hydroxychloroquine, azathioprine |
|
Zavala-Miranda et al. Kidney Int. 2021 Dec |
AstraZeneca CoV-19 vaccine (1st dose) | 23-year-old woman who presented with nephrotic syndrome 1 week after vaccine | No family history of autoimmunity | Positive antinuclear antibody, anti-dsDNA, lymphopenia, elevated protein-to-creatinine ratio, class V Lupus Nephritis | High dose prednisone, mycophenolate mofetil, hydroxychloroquine, and diuretics |
|
Kim et al. Kidney Int. 2022 April |
AstraZeneca CoV-19 vaccine (2nd dose) | 60-year-old woman with history of positive ANA developed fevers and pitting edema eight weeks after administration | None reported | Positive antinuclear antibody, anti-dsDNA, anti-Smith, lymphopenia, anemia, thrombocytopenia, elevated creatinine, elevated protein-to-creatinine ratio, class III Lupus Nephritis | Intravenous methylprednisolone, cyclophosphamide, High dose prednisone, hydroxychloroquine |
|
Rios et al. Mod Rheumatol Case Rep. 2022 March |
Pfizer/BioNTech COVID-19 vaccine (1st dose) | 42-year-old woman who developed inflammatory arthritis with sudden onset dyspnea and hypoxemia 2 weeks after administration | None reported | Positive antinuclear antibody, anti-dsDNA, lymphopenia, hypocomplementemia, positive lupus anticoagulant, elevated D-dimer, CT pulmonary angiogram consistent with filling defect in the right pulmonary artery | Intravenous methylprednisolone, high dose prednisone, hydroxychloroquine, anticoagulation, azathioprine |
|
Lemoine et al. Clin Rheumatol. 2022 |
Pfizer-BioNTech COVID-19 vaccine (1st dose) | 68-year-old woman who presented with upper and lower extremity muscle weakness, stiffness, and pain along with subsequent rash one week after administration | None reported | Positive antinuclear antibody, anti-dsDNA | High dose prednisone, azathioprine, methotrexate |
|
Raviv et al. Case Rep Rheumatol. 2022 Feb |
Pfizer-BioNTech COVID-19 vaccine (1st dose) | 24-year-old male developed facial rash 2 days after vaccine administration, followed by development of inflammatory arthritis with hair loss eight weeks after administration | No family history of autoimmunity | Positive antinuclear antibody, antichromatin antibody, ribosome P antibody, hypocomplementemia | Topical steroid cream, hydroxychloroquine etoricoxib |
|
Patil et al. J Cosmet Dermatol. 2021 Oct |
AstraZeneca CoV-19 vaccine (2nd dose) | 22‐year‐old female with history of jaundice developed fever, polyarthralgia, rash, lower extremity edema and petechia one week after administration | Sister with autoimmune thyroiditis | Positive antinuclear antibody, anti-dsDNA antibody, anti-histone antibody, hemolytic anemia, positive Coombs, thrombocytopenia, elevated urine albumin 1+ | High dose prednisone, hydroxychloroquine, mycophenolate mofetil, furosemide, telmisartan |
ANA antinuclear antibody, dsDNA double-stranded deoxyribonucleic acid, RNP ribonucleoprotein, CT computed tomography scan
A number of studies in adults have systematically assessed SLE patient for flares following COVID-19 immunization. In one study, patients had SLE Disease Activity Index (SLEDAI) measured before and after COVID-19 vaccination (BNT162b2 [Pfizer/ BioNTech], mRNA-1273 [Moderna] or Ad26.COV2.S [Johnson & Johnson]). The SLEDAI score did not change significantly (3.2 pre and 2.9 post). There were post-vaccination flares in 11.4% of patients, but all except for one of the 11 flares were considered mild, and most did not require intervention [14].
In a prospective study of patients with rheumatic and musculoskeletal disease, including 273 patients with SLE, disease flares requiring treatment occurred in 11% of SLE patients, though none were severe [30]. Interestingly, in this study prior COVID-19 was a risk factor for a disease flare. Another study assessed disease flares in 100 patients who received the BNT162b2 (Pfizer /BioNTech) vaccine (10 only received one dose) [15]. There were 27 disease flares, more commonly after the 2nd dose. Most flares were arthritis or dermal. Importantly, there were 28 flares in the six months prior to immunization [15]. In an international cross-sectional survey of SLE patients, the flare rate was low (3%), but the severity of the flares required a change in treatment in over 70% of patients [31]. In addition, there are a number of reported cases describing flares of SLE or even transition from cutaneous SLE to systemic SLE following COVID-19 vaccination [12, 32–36].
The case reports of new onset SLE are anecdotal and are limited by their prospective, observational nature. Epidemiologic studies of immune thrombocytopenia following COVID-19 vaccine emphasize considering the baseline rate of disease before attributing a disease entity to a vaccine [19, 37]. Unfortunately, there are no current epidemiologic studies of SLE with and without preceding COVID-19 vaccination.
The pathophysiology of SLE is complex, with some individuals being at increased risk due to genetic predisposition [38]. SLE is more common in females and in people with African, Asian or Hispanic ancestry. In addition, a variety of environmental factors may have a role in initiating SLE. Ultimately, SLE is an autoimmune disease caused by disturbances in the regulation of the immune system [38].
There are potential mechanistic links between COVID-19 vaccination and SLE. The COVID-19 mRNA vaccines increase type I interferon, which is also increased and believed to be important in the pathogenesis of SLE [39]. In addition, molecular mimicry of the SARS-CoV-2 spike protein could lead to autoantibodies to self-antigens. Vaccination may also directly activate B cells. However, SLE is clearly a rare complication and may only occur in a genetically susceptible patient [40].
In summary, we have reported a pediatric patient who developed symptoms of SLE two days after his third COVID-19 mRNA vaccination. Large epidemiologic studies are needed to assess whether this is more than an association, but it would clearly be a rare complication. It is possible that the vaccination led to SLE in a genetically susceptible individual. To the best of our knowledge, this is the first reported pediatric patient with new onset SLE following COVID-19 vaccination.
Acknowledgements
Not applicable.
Abbreviations
- SLE
Systemic lupus erythematosus
- cSLE
Childhood onset SLE
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- ANA
Antinuclear antibody
- dsDNA
Double-stranded deoxyribonucleic acid
- RNP
Ribonucleoprotein
- ESR
Erythrocyte sedimentation rate
- LN
Lupus nephritis
- Ig
Immunoglobulins
Author contributions
MN assisted in data collection, prepared the figure, and wrote the original draft of the manuscript. BG and LG treated the patient and reviewed and edited the manuscript. HR provided the figures, wrote the pathology section of the manuscript and reviewed and edited the manuscript. All authors read and approved the final manuscript.
Funding
No funding was obtained for this study.
Availability of data and materials
Data were ethically extracted from the patient’s file. Data used in this study is available from the corresponding author upon request.
Declarations
Ethical approval and consent to participate
This study was not considered Human Research by the Children’s Healthcare of Atlanta Institutional Review Board.
Consent for publication
Written Consent for publishing of clinical data and identifying images was obtained from parent of the patient prior to case report submission.
Competing interests
The authors declare no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Barbhaiya M, Costenbader KH. Environmental exposures and the development of systemic lupus erythematosus. Curr Opin Rheumatol. 2016;28(5):497–505. doi: 10.1097/BOR.0000000000000318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abu-Shakra M, Press J, Sukenik S, Buskila D. Influenza virus vaccination of patients with SLE: effects on generation of autoantibodies. Clin Rheumatol. 2002;21(5):369–372. doi: 10.1007/s100670200099. [DOI] [PubMed] [Google Scholar]
- 3.Agmon-Levin N, Zafrir Y, Paz Z, Shilton T, Zandman-Goddard G, Shoenfeld Y. Ten cases of systemic lupus erythematosus related to hepatitis B vaccine. Lupus. 2009;18(13):1192–1197. doi: 10.1177/0961203309345732. [DOI] [PubMed] [Google Scholar]
- 4.Mok CC, Ho LY, Fong LS, To CH. Immunogenicity and safety of a quadrivalent human papillomavirus vaccine in patients with systemic lupus erythematosus: a case-control study. Ann Rheum Dis. 2013;72(5):659–664. doi: 10.1136/annrheumdis-2012-201393. [DOI] [PubMed] [Google Scholar]
- 5.Grimaldi-Bensouda L, Le Guern V, Kone-Paut I, Aubrun E, Fain O, Ruel M, et al. The risk of systemic lupus erythematosus associated with vaccines: an international case-control study. Arthritis Rheumatol. 2014;66(6):1559–1567. doi: 10.1002/art.38429. [DOI] [PubMed] [Google Scholar]
- 6.El-Morshedy M, Altun E, Eliwa MS. A new statistical approach to model the counts of novel coronavirus cases. Math Sci (Karaj) 2022;16(1):37–50. doi: 10.1007/s40096-021-00390-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mason A, Anver H, Lwin M, Holroyd C, Faust SN, Edwards CJ. Lupus, vaccinations and COVID-19: what we know now. Lupus. 2021;30(10):1541–1552. doi: 10.1177/09612033211024355. [DOI] [PubMed] [Google Scholar]
- 8.Bartels LE, Ammitzbøll C, Andersen JB, Vils SR, Mistegaard CE, Johannsen AD, et al. Local and systemic reactogenicity of COVID-19 vaccine BNT162b2 in patients with systemic lupus erythematosus and rheumatoid arthritis. Rheumatol Int. 2021;41(11):1925–1931. doi: 10.1007/s00296-021-04972-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patil S, Patil A. Systemic lupus erythematosus after COVID-19 vaccination: a case report. J Cosmet Dermatol. 2021;20(10):3103–3104. doi: 10.1111/jocd.14386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raviv Y, Betesh-Abay B, Valdman-Grinshpoun Y, Boehm-Cohen L, Kassirer M, Sagy I. First presentation of systemic lupus erythematosus in a 24-year-old male following mRNA COVID-19 vaccine. Case Rep Rheumatol. 2022;2022:9698138. doi: 10.1155/2022/9698138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lemoine C, Padilla C, Krampe N, Doerfler S, Morgenlander A, Thiel B, et al. Systemic lupus erythematous after Pfizer COVID-19 vaccine: a case report. Clin Rheumatol. 2022;41(5):1597–1601. doi: 10.1007/s10067-022-06126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tuschen K, Bräsen JH, Schmitz J, Vischedyk M, Weidemann A. Relapse of class V lupus nephritis after vaccination with COVID-19 mRNA vaccine. Kidney Int. 2021;100(4):941–944. doi: 10.1016/j.kint.2021.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang B, Shao X, Wang D, Xu D, Zhang JA. Vaccinations and risk of systemic lupus erythematosus and rheumatoid arthritis: a systematic review and meta-analysis. Autoimmun Rev. 2017;16(7):756–765. doi: 10.1016/j.autrev.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 14.Izmirly PM, Kim MY, Samanovic M, Fernandez-Ruiz R, Ohana S, Deonaraine KK, et al. Evaluation of immune response and disease status in systemic lupus erythematosus patients following SARS-CoV-2 vaccination. Arthritis Rheumatol. 2022;74(2):284–294. doi: 10.1002/art.41937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zavala-Flores E, Salcedo-Matienzo J, Quiroz-Alva A, Berrocal-Kasay A. Side effects and flares risk after SARS-CoV-2 vaccination in patients with systemic lupus erythematosus. Clin Rheumatol. 2022;41(5):1349–1357. doi: 10.1007/s10067-021-05980-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pierce CB, Muñoz A, Ng DK, Warady BA, Furth SL, Schwartz GJ. Age- and sex-dependent clinical equations to estimate glomerular filtration rates in children and young adults with chronic kidney disease. Kidney Int. 2021;99(4):948–956. doi: 10.1016/j.kint.2020.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. COVI-19 Vaccines for Children and Teens. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/children-teens.html. Accessed August 1 2022.
- 18.Witberg G, Barda N, Hoss S, Richter I, Wiessman M, Aviv Y, et al. Myocarditis after Covid-19 vaccination in a large health care organization. N Engl J Med. 2021;385(23):2132–2139. doi: 10.1056/NEJMoa2110737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wise J. Covid-19: AstraZeneca vaccine linked with small risk of ITP, real world data show. BMJ. 2021;373:n1489. doi: 10.1136/bmj.n1489. [DOI] [PubMed] [Google Scholar]
- 20.Báez-Negrón L, Vilá LM. New-Onset systemic lupus erythematosus after mRNA SARS-CoV-2 vaccination. Case Rep Rheumatol. 2022;2022:6436839. doi: 10.1155/2022/6436839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaur I, Zafar S, Capitle E, Khianey R. COVID-19 vaccination as a potential trigger for new-onset systemic lupus erythematosus. Cureus. 2022;14(2):e21917. doi: 10.7759/cureus.21917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hidaka D, Ogasawara R, Sugimura S, Fujii F, Kojima K, Nagai J, et al. New-onset Evans syndrome associated with systemic lupus erythematosus after BNT162b2 mRNA COVID-19 vaccination. Int J Hematol. 2022;115(3):424–427. doi: 10.1007/s12185-021-03243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nune A, Iyengar KP, Ish P, Varupula B, Musat CA, Sapkota HR. The Emergence of new-onset SLE following SARS-CoV-2 vaccination. QJM. 2021;114(10):739–740. doi: 10.1093/qjmed/hcab229. [DOI] [PubMed] [Google Scholar]
- 24.Alrashdi Mousa N, Saleh AM, Khalid A, Alshaya AK, Alanazi SMM. Systemic lupus erythematosus with acute pancreatitis and vasculitic rash following COVID-19 vaccine: a case report and literature review. Clin Rheumatol. 2022;41(5):1577–1582. doi: 10.1007/s10067-022-06097-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zavala-Miranda MF, González-Ibarra SG, Pérez-Arias AA, Uribe-Uribe NO, Mejia-Vilet JM. New-onset systemic lupus erythematosus beginning as class V lupus nephritis after COVID-19 vaccination. Kidney Int. 2021;100(6):1340–1341. doi: 10.1016/j.kint.2021.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim HJ, Jung M, Lim BJ, Han SH. New-onset class III lupus nephritis with multi-organ involvement after COVID-19 vaccination. Kidney Int. 2022;101(4):826–828. doi: 10.1016/j.kint.2022.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Molina Rios S, Rojas Martinez R, Estévez Ramirez GM, Medina YF. Systemic lupus erythematosus and antiphospholipid syndrome after COVID-19 vaccination. A case report. Mod Rheumatol Case Rep. 2022 doi: 10.1093/mrcr/rxac018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soldevilla HF, Briones SF, Navarra SV. Systemic lupus erythematosus following HPV immunization or infection? Lupus. 2012;21(2):158–161. doi: 10.1177/0961203311429556. [DOI] [PubMed] [Google Scholar]
- 29.de Mattos ABN, Garbo Baroni L, Zanotto LL, Furian MEA. Subacute cutaneous lupus erythematosus triggered after measles vaccination. Lupus. 2021;30(5):833–835. doi: 10.1177/0961203321990087. [DOI] [PubMed] [Google Scholar]
- 30.Connolly CM, Ruddy JA, Boyarsky BJ, Barbur I, Werbel WA, Geetha D, et al. Disease flare and reactogenicity in patients with rheumatic and musculoskeletal diseases following two-dose SARS-CoV-2 messenger RNA vaccination. Arthritis Rheumatol. 2022;74(1):28–32. doi: 10.1002/art.41924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Felten R, Kawka L, Dubois M, Ugarte-Gil MF, Fuentes-Silva Y, Piga M, et al. Tolerance of COVID-19 vaccination in patients with systemic lupus erythematosus: the international VACOLUP study. Lancet Rheumatol. 2021;3(9):e613–e615. doi: 10.1016/S2665-9913(21)00221-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Niebel D, Ralser-Isselstein V, Jaschke K, Braegelmann C, Bieber T, Wenzel J. Exacerbation of subacute cutaneous lupus erythematosus following vaccination with BNT162b2 mRNA vaccine. Dermatol Ther. 2021;34(4):e15017. doi: 10.1111/dth.15017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sugimoto T, Yorishima A, Oka N, Masuda S, Yoshida Y, Hirata S. Exacerbation of systemic lupus erythematosus after receiving mRNA-1273-based coronavirus disease 2019 vaccine. J Dermatol. 2022;49(6):e199–e200. doi: 10.1111/1346-8138.16327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sekar A. Lupus nephritis flare post Moderna mRNA-1273 coronavirus vaccine. QJM. 2022;114(12):882–883. doi: 10.1093/qjmed/hcab284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Joseph AK, Chong BF. Subacute cutaneous lupus erythematosus flare triggered by COVID-19 vaccine. Dermatol Ther. 2021;34(6):e15114. doi: 10.1111/dth.15114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kreuter A, Burmann SN, Burkert B, Oellig F, Michalowitz AL. Transition of cutaneous into systemic lupus erythematosus following adenoviral vector-based SARS-CoV-2 vaccination. J Eur Acad Dermatol Venereol. 2021;35(11):e733–e735. doi: 10.1111/jdv.17514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee EJ, Cines DB, Gernsheimer T, Kessler C, Michel M, Tarantino MD, et al. Thrombocytopenia following Pfizer and Moderna SARS-CoV-2 vaccination. Am J Hematol. 2021;96(5):534–537. doi: 10.1002/ajh.26132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsokos GC. Autoimmunity and organ damage in systemic lupus erythematosus. Nat Immunol. 2020;21(6):605–614. doi: 10.1038/s41590-020-0677-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tang W, Askanase AD, Khalili L, Merrill JT. SARS-CoV-2 vaccines in patients with SLE. Lupus Sci Med. 2021;8(1):1–2. doi: 10.1136/lupus-2021-000479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Caso F, Costa L, Ruscitti P, Navarini L, Del Puente A, Giacomelli R, et al. Could Sars-coronavirus-2 trigger autoimmune and/or autoinflammatory mechanisms in genetically predisposed subjects? Autoimmun Rev. 2020;19(5):102524. doi: 10.1016/j.autrev.2020.102524. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data were ethically extracted from the patient’s file. Data used in this study is available from the corresponding author upon request.


