Abstract
The binding characteristics of iota toxin, a binary enterotoxin produced by Clostridium perfringens type E, were studied by fluorescence-activated cytometry. The proteolytically activated binding component of iota toxin, iota b (Ib), bound to various cell types when incubated at 4, 25, or 37°C for 10 min. The binding of Ib was inhibited by antisera against C. perfringens type E or Clostridium spiroforme culture supernatants, but not C. perfringens types C or D. Pretreatment of Vero cells with glycosidases or lectins did not affect Ib interactions, while pronase effectively prevented Ib binding to the cell surface. The Ib protomer (Ibp) bound to the cell surface, but trypsinization of Ibp was necessary for docking of the ADP-ribosylating component, iota a (Ia). Ia attached to cell-bound Ib within 10 min at 37°C, but surface levels of Ia decreased 90% after 30 min and were undetectable by 60 min. Detectable surface levels of Ib also diminished over time, and Western blot analysis suggested internalization or embedment of Ib into the membrane.
Clostridium perfringens is an anaerobic, gram-positive bacillus responsible for various diseases of animals and humans (14, 26, 27). This ubiquitous microorganism consists of five toxin types (A, B, C, D, and E), based on the production of one or more lethal, dermonecrotic proteins designated alpha, beta, epsilon, and iota (11). In addition to these “major” toxins, C. perfringens produces at least 10 “minor” toxins that exhibit diverse biological activities and may play a role in pathogenesis.
Among C. perfringens, only type E strains produce iota toxin, which has been implicated in sporadic outbreaks of diarrhea in calves, lambs, and guinea pigs (4, 8, 10, 22). Another clostridium, Clostridium spiroforme, produces a cross-reacting, iota-like toxin involved in rapidly fatal enterotoxemias among rabbits (3, 17). The iota and iota-like toxins each consist of two nonlinked proteins that work synergistically (20, 29). Complementary components of each toxin can form a hybrid, biologically active toxin (28). One subunit of C. perfringens iota toxin, iota a (Ia), has a molecular mass of ∼47 kDa and ADP ribosylates monomeric actin (18, 20, 30–32), thus preventing the formation of actin filaments within a cell and subsequently disrupting the cytoskeletal structure (1). The other subunit, iota b (Ib), is immunologically distinct from Ia, lacks detectable enzymatic activity, and has a molecular mass of ∼71 kDa following proteolysis of an Ib protomer (Ibp) by serine type proteases (22, 28). Ib binds to an uncharacterized cell surface receptor and facilitates the entry of Ia into a target cell.
Previous studies have shown that iota and iota-like toxins are cytotoxic to various cell types (5, 20, 21), thus suggesting a ubiquitous surface receptor. However, no one has characterized the binding of Ib and ensuing interactions with Ia. We investigated, by fluorescence-activated cytometry, the properties of C. perfringens Ib to various cell binding types with a particular emphasis on Vero cells. The interactions of Ia with cell-bound Ib were also studied using this technology. Finally, the receptor for Ib was initially characterized by pretreating Vero cells with various glycosidases, lectins, or proteases.
MATERIALS AND METHODS
Toxin and antiserum.
C. perfringens Ia, Ib, and Ibp were purified from C. perfringens using established procedures (30). The homogeneity of Ia, Ib, and Ibp was confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis via a single band following Coomassie staining. Monospecific anti-Ia and anti-Ib sera were generated in rabbits as described previously (21).
Binding studies.
Cell lines were purchased from the American Type Culture Collection (Manassas, Va.) and included A549 (human lung carcinoma), BHK-21 (Syrian hamster kidney), FRHL-103 (fetal rhesus monkey lung), GPK (guinea pig kidney), MDCK (canine kidney), MRC-5 (human lung), SW-13 (human adenocarcinoma), Vero (African green monkey kidney), and WISH (human amnion). Cells were detached from culture flasks by 50 mM EDTA in Hanks balanced salt solution (HBSS) lacking Ca2+ and Mg2+. Cells (4 × 105 to 8 × 105 per tube) were washed with ice-cold HBSS containing 0.2% bovine serum albumin (BSA), gently vortexed, and pelleted by centrifugation (600 × g). Various concentrations of Ib and/or Ia (diluted in HBSS-BSA) were added to cells incubated at 4, 25, or 37°C for various times. Cells were immediately washed with ice-cold HBSS-BSA and then incubated for 1 h on ice with a 1:400 dilution of rabbit anti-Ia or anti-Ib serum. After being washed, cells were incubated (1 h on ice) with a fluorescein isothiocyanate conjugate of goat anti-rabbit immunoglobulin G (IgG) (Organon Teknika, West Chester, Pa.). Following a final wash, cells were fixed with 0.5% paraformaldehyde and analyzed by FACSort flow cytometry (Becton Dickinson, Mountain View, Calif.).
Binding experiments were also done with Ib (5 μg/ml) heated at 60°C for 15 min and subsequently incubated with paraformaldehyde-fixed Vero cells for 10 min at 37°C. In some experiments, Ia was added to the heated Ib preparation after it was cooled to room temperature (2.5 μg/ml, final concentration of each) and the mixture was subsequently incubated with Vero cells for 10 min at 37°C.
Ibp, with and without trypsin treatment, was tested for its ability to bind Vero cells and subsequently dock with Ia. Vero cells were preincubated for 10 min at 37°C with a 2.5-μg/ml concentration of Ia and Ibp, previously treated with or without trypsin (1 mg/ml) for 30 min at 37°C. Cells were processed as described before.
Time course experiments were done with an Ia-Ib mixture (2.5-μg/ml final concentration of each) incubated with Vero cells at 37°C. The Ia and Ib molecules were detected on the cell surface with monospecific antiserum. Further analysis of Ib associated with Vero cells was done by mixing 5 × 106 Vero cells with Ib (60 μg/ml) for various times (10, 30, 60, and 120 min) at 37°C. Cells were extensively washed with ice-cold HBSS, solubilized with sodium dodecyl sulfate disruption buffer, and electrophoresed on a Bis-Tris NuPage (4 to 12%) gradient gel (Novex, San Diego, Calif.). Proteins were blotted onto nitrocellulose and probed with rabbit anti-Ib sera plus goat anti-rabbit IgG conjugated to horseradish peroxidase, and immunoreactive proteins were detected on film by the ECL system (Amersham Pharmacia Biotech, Piscataway, N.J.).
Binding inhibition studies.
Lectins were purchased from Sigma (St. Louis, Mo.) and included those from the plants and animals shown in Table 1. All lectins were diluted in HBSS to a 5-mg/ml concentration, and 50 μl of a single lectin was added to Vero cells for 30 min at room temperature. Cells were washed and processed as described above.
TABLE 1.
Sources and binding specificities of lectins
| Lectin source | Binding specificity |
|---|---|
| Agaricus bisporus (mushroom) | β-Gal(1-3)GalNAc |
| Anguilla anguilla (freshwater eel) | α-l-Fucose |
| Artocarpus integrifolia (jacalin) | α-Gal-OMe |
| Bauhinia purpurea (camel's foot tree) | β-Gal(1-3)GalNAc |
| Canavalia ensiformis (concanavalin A) | α-Mannose, α-glucose |
| Caragana arborescens (Siberian pea tree) | GalNAc |
| Cicer arietinum (chick-pea) | Fetuin |
| Cytisus scoparius (scotch broom) | GalNAc, galactose |
| Datura stramonium (jimson weed) | (GlcNAc)2 |
| Dolichos biflorus (horse gram) | α-GalNAc |
| Erythrina cristagalli (coral tree) | β-Gal(1-4)GlcNAc |
| Euonymus europaeus (spindle tree) | α-Gal(1-3)Gal |
| Glycine max (soybean) | GalNAc |
| Limulus polyphemus (horseshoe crab) | NeuNAc, GalNAc, GlcNAc |
| Lycopersican esculentum (tomato) | (GlcNAc)3 |
| Maackia amurensis (legume) | Sialic acid |
| Narcissus pseudonarcissus (daffodil) | α-d-Mannose |
| Phaseolus vulgaris (red kidney bean) | Oligosaccharide |
| Ptilota plumosa (red marine algae) | α-Galactose |
| Triticum vulgaris (wheat germ) | (GlcNAc)2, NeuNAc |
Additional studies, based on cleaving potential carbohydrate receptor sites, were done by pretreating Vero cells for 30 min at room temperature with one of the following glycosidases (2.5 mg/ml): baker's yeast α-glucosidase, bovine kidney α-fucosidase, C. perfringens neuraminidase, Escherichia coli α-galactosidase or β-glucuronidase, jack bean α-mannosidase or β-N-acetylglucosaminidase, or snail β-mannosidase (Sigma). Vero cells were then washed with ice-cold HBSS-BSA before the addition of Ib (10 μg/ml) and processed as stated above.
Inhibition of Ib binding by various proteases was initially tested by preincubating Vero cells with a protease (2.5 mg/ml) for 30 min at room temperature. Additional studies were done with cells fixed in paraformaldehyde prior to protease treatment. The proteases included carboxypeptidase Y, chymotrypsin, clostripain, collagenase, elastase, papain, pepsin, pronase, proteinase K, thermolysin, and trypsin (Sigma). To minimize the effects of any residual protease on Ib, cells were extensively washed five times with HBSS (Ca2+ and Mg2+ free) containing 1% BSA and protease inhibitors (5 mM EDTA, 100 μg of phenylmethylsulfonyl fluoride/ml, and leupeptin). Following the washes, Ib (10 μg/ml) was incubated for 10 min at 37°C with cells in fresh medium containing BSA and protease inhibitors. Cells were subsequently washed with ice-cold medium and processed as described previously.
The binding specificity of Ib was partly determined with TechLab (Blacksburg, Va.) goat anti-C. spiroforme and -C. perfringens type C, D, or E sera (1:5 dilution in HBSS-BSA) that were preincubated with an equal volume of Ib (20 μg/ml) for 30 min at room temperature. This mixture was then added to cells for 10 min at 37°C, followed by processing as stated above.
The binding of rabbit anti-Ib sera to Ib preincubated with C. spiroforme antisera was tested by an enzyme-linked immunosorbent assay. A 1-μg/ml concentration of Ib in carbonate buffer was adsorbed overnight (4°C) onto Immulon II plates (Dynatech, McLean, Va.). Wells were blocked with 3% milk in phosphate-buffered saline (PBS), and 1:5 dilutions of the goat anti-C. spiroforme sera (or diluent only) were added to wells for 1 h at 37°C. Wells were washed with PBS containing 0.1% Tween 20 (PBST), and 1:400 dilutions of rabbit anti-Ib sera were incubated for 1 h at 4 or 37°C. After the wells were washed with PBST, goat anti-rabbit Ig conjugated to alkaline phosphatase was incubated in each well for 1 h at 4 or 37°C. Wells were washed with PBST, substrate was added, absorbance at 405 nm (A405) was recorded, and the mean +/− standard deviation was calculated for triplicate samples.
Cytotoxicity assays.
Cells were grown in 96-well culture plates and treated with various concentrations of Ia and/or Ib diluted in media consisting of Eagle's minimal essential medium containing nonessential amino acids and 5% fetal bovine serum. Cytotoxicity, as evidenced by cell rounding, was usually detected within 2 h, but final results were recorded 24 h after the addition of iota toxin.
RESULTS
Binding of C. perfringens Ib to different cell types.
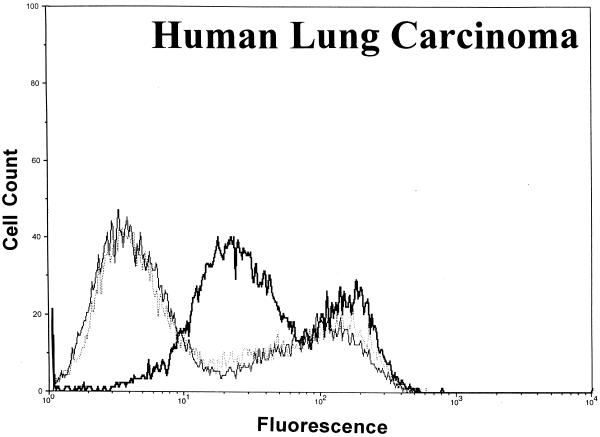
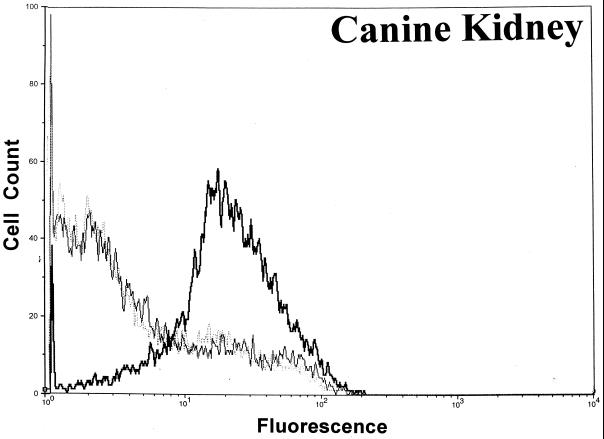
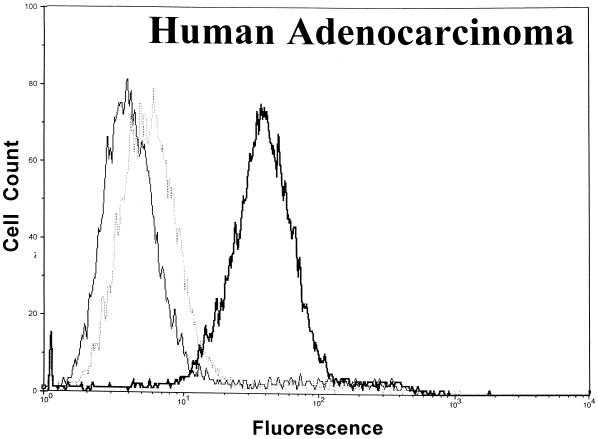
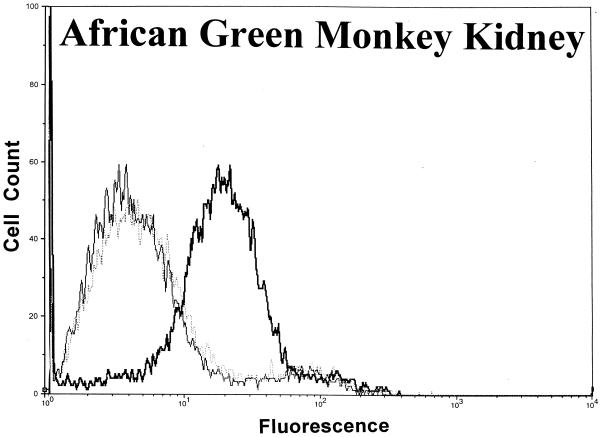
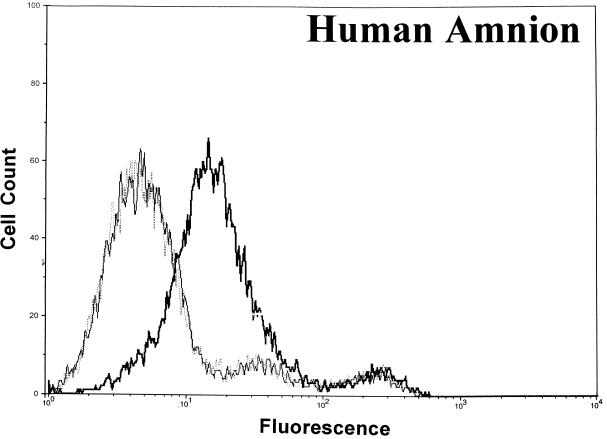
Significant surface binding of the Ib molecule was observed via flow cytometry with various cell lines including human lung carcinoma, canine kidney, human adenocarcinoma, African green monkey kidney (Vero), and human amnion (Fig. 1). However, the binding of Ib to hamster and guinea pig kidney cells was very weak, and binding to fetal rhesus or human lung cells was nonexistent (data not shown). Cell lines that bound Ib were susceptible to the cytotoxic effects of iota toxin. Cells that did not bind Ib, as determined by flow cytometry, were resistant to iota toxin. In all cases, the binding of Ib to a cell surface was inhibited by C. spiroforme antiserum, which contains antibodies that recognize C. perfringens Ib and neutralize the in vivo and in vitro effects of iota toxin (28). The negative-control antiserum, C. perfringens type D, lacks antibodies against iota toxin and did not affect Ib binding to any cell line. Additional studies with Vero cells revealed that C. perfringens type E antiserum, but not type C, effectively inhibited the binding of Ib (data not shown).
FIG. 1.
Binding of C. perfringens Ib to various cell lines as determined by flow cytometry using 10,000 cells to provide a fluorescence profile. Ib (20 μg/ml) was preincubated with either C. spiroforme (⋯) or C. perfringens type D (▄▄) antisera for 30 min at room temperature before addition to cells. Controls included cells incubated with media plus type D antisera (——).
A lack of an Ib signal following preincubation of Ib with C. spiroforme antiserum was not due to the binding of all available epitopes recognized by the rabbit anti-Ib sera. An enzyme-linked immunosorbent assay confirmed that plate-adsorbed Ib, preincubated with the C. spiroforme antiserum and then incubated with the rabbit monospecific anti-Ib, was readily recognized by anti-Ib to the same degree (A405 = 1.74 +/− 0.18) as Ib not pretreated with the C. spiroforme antiserum (A405 = 1.74 +/− 0.05).
Because many cytotoxicity studies for iota toxin previously used Vero cells (5, 19, 20, 28) and our flow cytometry data clearly showed significant binding of Ib, all subsequent experiments were done with this cell line.
Temperature effects on C. perfringens Ib binding to Vero cells.
The Ib molecule (10 μg/ml) readily bound to Vero cells within 10 min at 4, 25, or 37°C, although the mean fluorescence signals at 4 and 25°C were, respectively, 69 and 80% of that at 37°C. As evidenced by earlier experiments with the various cell lines, binding of Ib to Vero cells at any of these temperatures was inhibited by C. spiroforme, but not C. perfringens type D, antisera.
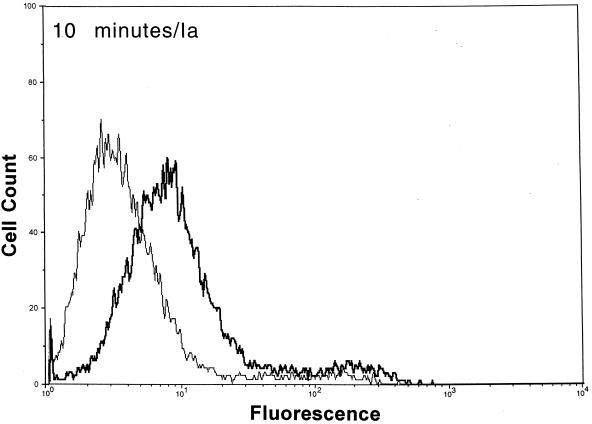
Docking of C. perfringens Ia to cell-bound Ib.
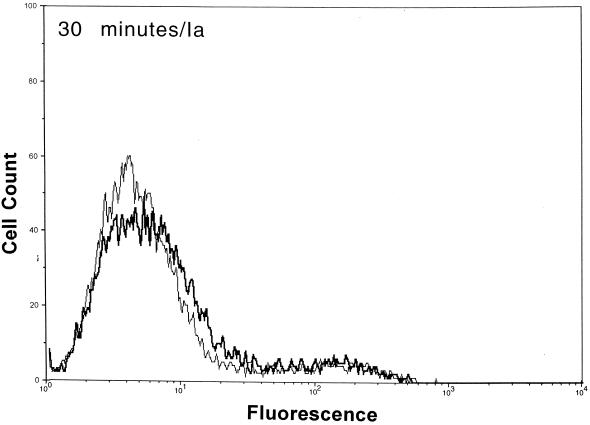
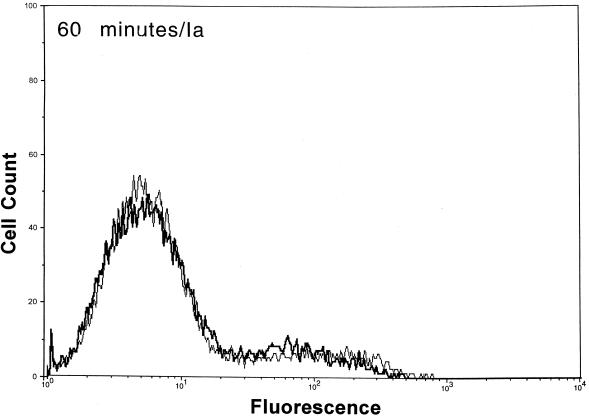
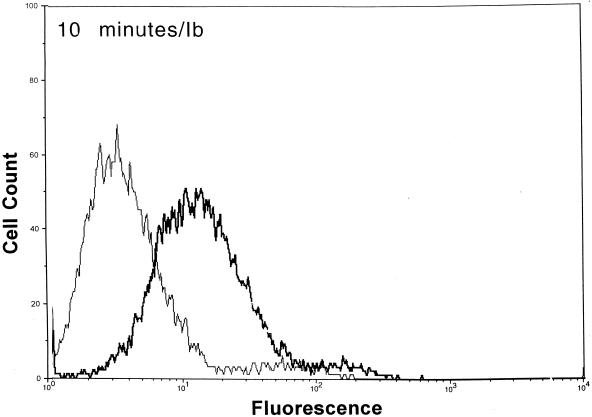
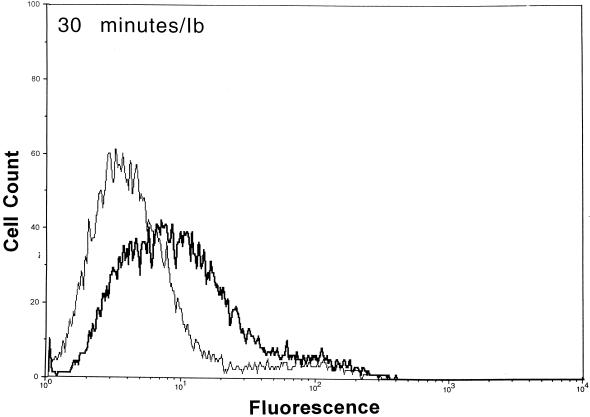
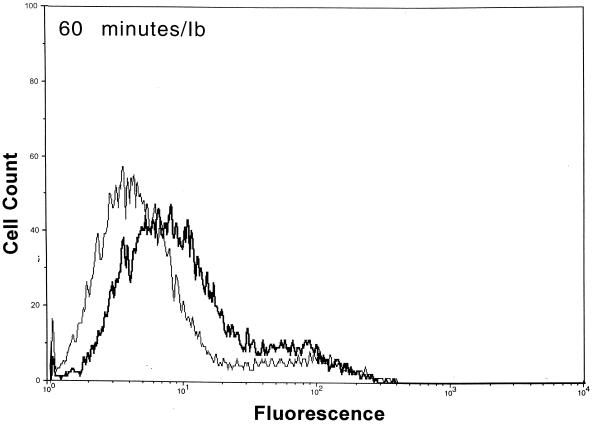
After functional binding of Ib to Vero cells was established, the next series of experiments explored the interaction of Ia with cell-bound Ib. Results of a time course study revealed that Ia bound to cell-attached Ib within 10 min at 37°C (Fig. 2). Detectable levels of Ia on the cell surface were diminished by ∼90% within 30 min, and levels were nonexistent by 60 min. The Ia molecule did not bind to cells without Ib. Detectable levels of Ib on the cell surface also decreased over time (Fig. 2). Relative to the 10-min incubation, the fluorescence of Ib-treated cells dropped 33 and 50% after 30 and 60 min, respectively. The Ib-specific fluorescence decreased by 80% after 90 and 120 min (data not shown). Western blot analysis of Vero cells incubated with Ib did not show a decrease in cell-associated levels over time (10 to 120 min), thus suggesting internalization or embedment into the cell membrane (data not shown).
FIG. 2.
Detection of Ia and Ib on the surfaces of Vero cells following incubation of each component (2.5 μg/ml, final concentration) at 37°C for various times. The Ia or Ib components were detected with monospecific antiserum (▄▄), and control cells (——) were not incubated with Ia or Ib but with all other reagents.
Additional binding studies determined that Ibp, treated with or without trypsin, effectively bound to the cell surface (Table 2). However, trypsinization was essential for the docking of Ia to cell-bound Ib, as evidenced by a 43-fold increase in Ia-specific fluorescence following proteolysis of Ibp.
TABLE 2.
Binding of Ia to Vero cell-bound Ib or Ibp
| Seruma | Presence of subunit or protomerb:
|
Trypsinc | Signald | ||
|---|---|---|---|---|---|
| Ib | Ibp | Ia | |||
| Anti-Ib | − | − | + | − | 0.49 |
| + | − | − | − | 26.01 | |
| + | − | − | + | 18.86 | |
| − | + | − | − | 15.40 | |
| − | + | − | + | 24.53 | |
| Anti-Ia | + | − | + | − | 7.32 |
| + | − | + | + | 5.28 | |
| − | + | + | − | 0.21 | |
| − | + | + | + | 8.93 | |
Vero cells were incubated with Ia, Ib, or Ibp (2.5 μg/ml each) for 10 min at 37°C and then processed as described in Materials and Methods, using specific antisera to Ia or Ib.
+, present; −, absent.
+, trypsin (1 mg/ml) treatment of Ib or Ibp for 30 min at 37°C before incubation of either iota protein to the cells; −, no treatment.
Mean intensity of the fluorescence signal due to Ia or Ib on the cell surface minus the mean signal from control cells incubated with Ib plus anti-Ia.
Heat-inactivated Ib binds to Vero cells.
Previous in vivo studies revealed that heated Ib (55°C for 15 min), when combined with unheated Ia, lacks biological activity (28). Our next experiments addressed the question of whether the heating of Ib affected binding to the cell surface receptor or docking with Ia. Flow cytometry experiments with heated Ib (60°C for 15 min) clearly revealed binding to Vero cells within 10 min that represented 68% of the binding by unheated Ib. However, heated Ib bound to the cell surface did not effectively interact with Ia in a 10-min period, as determined by flow cytometry experiments with Ia-specific antisera. Cytotoxic effects of heated Ib combined with Ia on Vero cells were also greatly diminished, but not to background levels. High concentrations (86 to 690 ng/ml) of each component resulted in 20 to 90% cell rounding. Unheated Ib plus Ia produced 100% cell rounding down to 43 ng of each component/ml and 20% cell rounding down to 2.8 ng/ml. Although their morphology was different from that of untreated controls, cells affected by heated Ib plus Ia were noticeably less rounded than cells exposed to equivalent concentrations of unheated Ib plus Ia. Finally, preincubation of Vero cells for 15 min with heated Ib (7.5 μg/ml) and then unheated Ib plus Ia (750 ng/ml each) resulted in transient protection within a 2-h period. However, 24 h after the addition of iota toxin there were no signs of protection due to heated Ib.
Characterization of the receptor for C. perfringens Ib.
Studies were done to determine the physicochemical properties of the cell surface receptor for Ib. Pretreatment of Vero cells with individual lectins (n = 20) or glycosidases (n = 8) did not prevent the binding of Ib to the surface receptor (data not shown), suggesting that carbohydrates do not play a role in Ib-receptor interactions.
Vero cells were also pretreated with individual proteases (n = 11). Pronase was the only enzyme that effectively inhibited binding of Ib to the cell surface in a dose-response fashion (Table 3), suggesting that the receptor is proteinaceous but relatively resistant to proteolysis.
TABLE 3.
Pronase pretreatment of Vero cells diminishes binding of Iba
| Pronase (mg/ml) | Signalb |
|---|---|
| 0.02 | 27.8 |
| 0.1 | 12.2 |
| 0.5 | 8.5 |
| 2.5 | 0.6 |
Binding studies were done with Vero cells incubated with or without Ib (10 μg/ml) for 10 min, and all other reagents were used as outlined in Materials and Methods.
Mean fluorescence due to Ib on the cell surface minus the mean fluorescence from control cells not incubated with Ib.
DISCUSSION
A group of protein toxins produced by C. perfringens type E, C. spiroforme, Clostridium botulinum types C and D, and Bacillus anthracis use a common method for intoxicating cells. Basically, a receptor binding protein facilitates the entry of an enzyme into a target cell. However, unlike the noncovalently bound A-B model originally described for cholera toxin (7), the iota, iota-like, C2, and anthrax toxin subunits are physically independent in solution. Interaction of the binding component from any of these toxins with a cell surface receptor subsequently converts the binding protein into a “receptor” that effectively docks with an enzyme and facilitates its internalization into a target cell.
The iota family and C2 toxins are cytotoxic for various cell lines, and these results suggest a ubiquitous receptor (16, 33). Previous mutagenesis studies with CHO cells show that the C2 toxin and C. perfringens iota toxin bind to different receptors (6). The receptor for C2 toxin may regulate cellular growth, similar to a precursor for a heparin-binding epidermal growth factor recognized by diphtheria toxin (13). Competition studies and flow cytometry analysis of Vero cells incubated with Ib and an excess of staphylococcal enterotoxin A, cholera toxin, C. perfringens enterotoxin, or E. coli heat-stable enterotoxin A did not yield an identity for the Ib receptor (B. G. Stiles, unpublished data). However, based on pretreatment of Vero cells with pronase, the receptor for Ib is proteinaceous.
Flow cytometry studies confirmed that Ib binds to various cell types, which were also susceptible to iota toxin in cytotoxicity assays. However, fetal rhesus and human lung cells did not bind Ib and were subsequently not susceptible to iota toxin. Various experiments were done to generate saturable dose-response curves. A clear dose response was evident; however, saturation levels varied from experiment to experiment. Although reagents and methodology were kept constant between experiments, including cell manipulations, it is likely that variation (perhaps cell cycling?) among Vero cells led to our findings. The labeling of Ib, via a fluor or radioiodine, would have further established specific binding using classical competition techniques. Unfortunately, labeling of Ib results in a biologically attenuated, weakly binding molecule (M. R. Popoff, unpublished data). We tried fluor-tagged (Alexa-488) Ib for competition binding studies; however, the detectable signal by flow cytometry was weak and nonspecific. Indirect detection of surface-bound Ib via monospecific antisera correlated well with biological activity, thus suggesting that the binding of Ib detected by flow cytometry resulted in functional iota toxicity. Nonspecific binding of Ib would not likely result in docking with, or internalization of, Ia and subsequent cytotoxicity.
Like early log-phase cultures of C. botulinum types C and D, which contain a protoxin form of C2, C. perfringens type E produces an iota protoxin activated by trypsin (18, 22). Proteolysis of Ibp results in a 19.8-kDa fragment released from the amino terminus (18). As with C2 toxin, proteolysis of Ibp clearly affects docking with Ia. The recorded signal for cell-associated Ibp was 68% of that for an equal protein (not molar) concentration of proteolytically activated Ib. Differences in fluorescence readings between the binding of Ib and Ibp were likely due to a 20% disparity in molecular weights and decreased ability of antibody to bind masked epitopes buried within the Ibp molecule (28).
A previous study showed that proteolysis of C2 component II leads to oligomerization in solution (15). However, similar experiments with iota toxin revealed only monomeric forms of Ib following proteolysis and no complexing with Ia in solution (28). Another study also reported that neither Ib nor the binding component of C. spiroforme iota-like toxin forms oligomers in acidic or neutral pH conditions (19). Additionally, trypsin-treated component II of C2 toxin forms cation-selective channels in artificial membranes (24), but neither unprocessed component II nor the enzymatic subunit causes channel formation. We observed that >1 μg of highly purified Ib/ml, without Ia, caused some cytotonic effects on Vero cells, which may be due to gross alterations (channel formation?) of the cell membrane. Similar biological effects have also been noted with high concentrations of purified Ib in a guinea pig dermonecrosis assay (28).
Previous experiments with mice suggest that C. perfringens Ib functionally remains on the cell surface up to 120 min (23). Our flow cytometry experiments revealed that Ib was still detectable on the surface of Vero cells 120 min after the addition of Ib plus Ia at 37°C. However, the fluorescence signals for Ib (and Ia) dramatically decreased over time. During the 120-min time period, Western blot analysis showed that Ib is internalized and/or embedded within the cell membrane. The binding of Ia to cell-bound Ib was rapid (<10 min) at 4, 25, or 37°C. Within 30 min at 37°C, surface levels of Ia were reduced by ∼90%, and by 60 min the Ia molecule was not found on the cell surface. These data suggest that docking with Ib and subsequent internalization of Ia into a target cell are relatively efficient processes.
Based on experiments with pronase-treated Vero cells, the receptor for Ib appears to be proteinaceous. However, 10 other proteases did not inhibit the binding of Ib, thus suggesting that the receptor is relatively resistant to proteolysis. Studies with various lectins and glycosidases also suggest that carbohydrates do not likely play an important role in the Ib-receptor interaction. Additional work with Vero cells pretreated with tunicamycin or swainsonine, both of which inhibit the formation of asparagine-linked glycoproteins, did not alter the binding of Ib as determined by flow cytometry. Obviously, our studies to determine the role of carbohydrates in Ib binding to the cell surface are not unequivocal. It is possible that we did not select the correct lectin or glycosidase that recognizes a specific carbohydrate moiety important for binding. Furthermore, there is a binding avidity issue, as Ib may readily displace a poorly bound lectin from the cell surface and thus lead us to our current conclusion that carbohydrates do not likely play a role in the binding of Ib to the cell surface.
In addition to structural similarities with component II of C. botulinum C2 toxin, the Ib subunit of C. perfringens iota toxin also shares 34% sequence homology with the protective antigen (PA) of anthrax toxin (18) and cross-reacting epitopes (19). The anthrax toxin produced by B. anthracis consists of three nonlinked proteins. A 20-kDa amino-terminal fragment is released from PA via proteolysis by a cell-associated protein called furin (9), thus resulting in the formation of a PA heptamer on the cell surface (12) and the docking of either the lethal or edema factor. The Ib molecule is activated by another serine-type protease such as trypsin, but does not contain a furin cleavage site. There is no evidence suggesting that Ib oligomerizes on the cell surface. Furthermore, components of anthrax toxin do not functionally complement the iota toxin subunits (19). Similar to the binding component of C. botulinum C2 toxin (24), only protease-treated PA induces a multimeric structure that forms cation-selective channels in artificial membranes (2). These findings suggest conformational changes following proteolysis that lead to the formation of transmembrane channels. However, proteolysis is not necessary for the initial interaction of either PA, component II of C2 toxin, or Ib with a cell surface receptor. Monomeric or oligomeric forms of PA bind lethal factor in a 1:1 ratio (25), and 50% of the receptor-bound PA is internalized within 30 min. The Ib-specific fluorescence levels of Vero cells decreased 50% over 60 min at 37°C, suggesting internalization or membrane embedment of Ib into a target cell. It is possible that a single Ib molecule facilitates the entry of multiple Ia molecules into a cell, although this is not known.
These studies are the first to characterize the binding interactions of C. perfringens Ib to a cell surface receptor. Further work with deletion or site-directed mutants of Ib will help discern the sites on this fascinating molecule that are critical for targeting a cell and subsequent docking with Ia.
ACKNOWLEDGMENTS
The technical help afforded by Yvette Campbell, Beverly Dyas, Marilyn Buckley, and the cell culture group of USAMRIID is always greatly appreciated.
REFERENCES
- 1.Aktories K, Wegner A. Mechanisms of the cytopathic action of actin-ADP-ribosylating toxins. Mol Microbiol. 1992;6:2905–2908. doi: 10.1111/j.1365-2958.1992.tb01749.x. [DOI] [PubMed] [Google Scholar]
- 2.Blaustein R O, Koehler T M, Collier R J, Finkelstein A. Anthrax toxin: channel-forming activity of protective antigen in planar phospholipid bilayers. Proc Natl Acad Sci USA. 1989;86:2209–2213. doi: 10.1073/pnas.86.7.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borriello S P, Carman R J. Association of iota-like toxin and Clostridium spiroforme with both spontaneous and antibiotic-associated diarrhoea and colitis in rabbits. J Clin Microbiol. 1983;17:414–418. doi: 10.1128/jcm.17.3.414-418.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bosworth T J. On a new type of toxin produced by Clostridium welchii. J Comp Pathol. 1943;53:245–255. [Google Scholar]
- 5.Butt M T, Papendick R E, Carbone L G, Quimby F W. A cytotoxicity assay for Clostridium spiroforme enterotoxin in cecal fluid of rabbits. Lab Anim Sci. 1994;44:52–54. [PubMed] [Google Scholar]
- 6.Fritz G, Schroeder P, Aktories K. Isolation and characterization of a Clostridium botulinum C2 toxin-resistant cell line: evidence for possible involvement of the cellular C2II receptor in growth regulation. Infect Immun. 1995;63:2334–2340. doi: 10.1128/iai.63.6.2334-2340.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gill D. The arrangement of subunits in cholera toxin. Biochemistry. 1976;15:1242–1248. doi: 10.1021/bi00651a011. [DOI] [PubMed] [Google Scholar]
- 8.Hart B, Hooper P T. Enterotoxaemia of calves due to Clostridium welchii type E. Aust Vet J. 1967;43:360–363. doi: 10.1111/j.1751-0813.1967.tb04882.x. [DOI] [PubMed] [Google Scholar]
- 9.Klimpel K R, Molloy S S, Thomas G, Leppla S H. Anthrax toxin protective antigen is activated by a cell-surface protease with the sequence specificity and catalytic properties of furin. Proc Natl Acad Sci USA. 1992;89:10277–10281. doi: 10.1073/pnas.89.21.10277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Madden D L, Horton R E, McCullough N B. Spontaneous infection in ex-germfree guinea pigs due to Clostridium perfringens. Lab Anim Sci. 1970;20:454–455. [PubMed] [Google Scholar]
- 11.McDonel J. Toxins of Clostridium perfringens types A, B, C, D, E. In: Dorner F, Drews J, editors. Pharmacology of bacterial toxins. Oxford, United Kingdom: Pergamon Press; 1986. pp. 477–517. [Google Scholar]
- 12.Milne J C, Furlong D, Hanna P C, Wall J S, Collier R J. Anthrax protective antigen forms oligomers during intoxication of mammalian cells. J Biol Chem. 1994;269:20607–20612. [PubMed] [Google Scholar]
- 13.Naglich J G, Metherall J E, Russell D W, Eidels L. Expression cloning of a diphtheria toxin receptor: identity with a heparin-binding EGF-like growth factor precursor. Cell. 1992;69:1051–1061. doi: 10.1016/0092-8674(92)90623-k. [DOI] [PubMed] [Google Scholar]
- 14.Niilo L. Clostridium perfringens in animal diseases: a review of current knowledge. Can Vet J. 1980;21:141–148. [PMC free article] [PubMed] [Google Scholar]
- 15.Ohishi I. Activation of botulinum C2 toxin by trypsin. Infect Immun. 1987;55:1461–1465. doi: 10.1128/iai.55.6.1461-1465.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ohishi I, Miyake M, Ogura H, Nakamura S. Cytopathic effect of botulinum C2 toxin on tissue-culture cells. FEMS Microbiol Lett. 1984;23:281–284. [Google Scholar]
- 17.Peeters J E, Geeroms R, Carman R J, Wilkins T D. Significance of Clostridium spiroforme in the enteritis-complex of commercial rabbits. Vet Microbiol. 1986;12:25–31. doi: 10.1016/0378-1135(86)90038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perelle S, Gibert M, Boquet P, Popoff M R. Characterization of Clostridium perfringens iota-toxin genes and expression in Escherichia coli. Infect Immun. 1993;61:5147–5156. doi: 10.1128/iai.61.12.5147-5156.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perelle S, Scalzo S, Kochi S, Mock M, Popoff M R. Immunological and functional comparison between Clostridium perfringens iota toxin, C. spiroforme toxin, and anthrax toxins. FEMS Microbiol Lett. 1997;146:117–121. doi: 10.1111/j.1574-6968.1997.tb10180.x. [DOI] [PubMed] [Google Scholar]
- 20.Popoff M R, Boquet P. Clostridium spiroforme toxin is a binary toxin which ADP-ribosylates cellular actin. Biochem Biophys Res Commun. 1988;152:1361–1368. doi: 10.1016/s0006-291x(88)80435-2. [DOI] [PubMed] [Google Scholar]
- 21.Popoff M R, Milward F W, Bancillon B, Boquet P. Purification of the Clostridium spiroforme binary toxin and activity of the toxin on HEp-2 cells. Infect Immun. 1989;57:2462–2469. doi: 10.1128/iai.57.8.2462-2469.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ross H, Warren M, Barnes J. Clostridium welchii iota toxin: its activation by trypsin. J Gen Microbiol. 1949;3:148–152. doi: 10.1099/00221287-3-1-148. [DOI] [PubMed] [Google Scholar]
- 23.Sakurai J, Kobayashi K. Lethal and dermonecrotic activities of Clostridium perfringens iota toxin: biological activities induced by cooperation of two nonlinked components. Microbiol Immunol. 1995;39:249–253. doi: 10.1111/j.1348-0421.1995.tb02197.x. [DOI] [PubMed] [Google Scholar]
- 24.Schmid A, Benz R, Just I, Aktories K. Interaction of Clostridium botulinum C2 toxin with lipid bilayer membranes. Formation of cation-selective channels and inhibition of channel function by chloroquine. J Biol Chem. 1994;269:16706–16711. [PubMed] [Google Scholar]
- 25.Singh Y, Klimpel K R, Goel S, Swain P K, Leppla S H. Oligomerization of anthrax toxin protective antigen and binding of lethal factor during endocytic uptake into mammalian cells. Infect Immun. 1999;67:1853–1859. doi: 10.1128/iai.67.4.1853-1859.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith L D, Williams B L. The pathogenic anaerobic bacteria. 3rd ed. Springfield, Ill: Charles C. Thomas; 1984. [Google Scholar]
- 27.Songer J G. Clostridial enteric diseases of domestic animals. Clin Microbiol Rev. 1996;9:216–234. doi: 10.1128/cmr.9.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stiles B G. Purification and characterization of Clostridium perfringens iota toxin. Ph.D thesis. Blacksburg: Virginia Polytechnic Institute and State University; 1987. [Google Scholar]
- 29.Stiles B G, Wilkins T D. Clostridium perfringens iota toxin: synergism between two proteins. Toxicon. 1986;24:767–773. doi: 10.1016/0041-0101(86)90101-7. [DOI] [PubMed] [Google Scholar]
- 30.Stiles B G, Wilkins T D. Purification and characterization of Clostridium perfringens iota toxin: dependence on two nonlinked proteins for biological activity. Infect Immun. 1986;54:683–688. doi: 10.1128/iai.54.3.683-688.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Damme J, Jung M, Hofmann F, Just I, Vandekerckhove J, Aktories K. Analysis of the catalytic site of the actin ADP-ribosylating Clostridium perfringens iota toxin. FEBS Lett. 1996;380:291–295. doi: 10.1016/0014-5793(96)00052-x. [DOI] [PubMed] [Google Scholar]
- 32.Vandekerckhove J, Schering B, Barmann M, Aktories K. Clostridium perfringens iota toxin ADP-ribosylates skeletal muscle actin in Arg-177. FEBS Lett. 1987;225:48–52. doi: 10.1016/0014-5793(87)81129-8. [DOI] [PubMed] [Google Scholar]
- 33.Zepeda H, Considine R V, Smith H L, Sherwin J R, Ohishi I, Simpson L L. Actions of the Clostridium botulinum binary toxin on the structure and function of Y-1 adrenal cells. J Pharmacol Exp Ther. 1988;246:1183–1189. [PubMed] [Google Scholar]