Abstract
OBJECTIVE
We studied longitudinal differences between progressors and nonprogressors to type 1 diabetes with similar and substantial baseline risk.
RESEARCH DESIGN AND METHODS
Changes in 2-h oral glucose tolerance test indices were used to examine variability in diabetes progression in the Diabetes Prevention Trial–Type 1 (DPT-1) study (n = 246) and Type 1 Diabetes TrialNet Pathway to Prevention study (TNPTP) (n = 503) among autoantibody (Ab)+ children (aged <18.0 years) with similar baseline metabolic impairment (DPT-1 Risk Score [DPTRS] of 6.5–7.5), as well as in TNPTP Ab− children (n = 94).
RESULTS
Longitudinal analyses revealed annualized area under the curve (AUC) of C-peptide increases in nonprogressors versus decreases in progressors (P ≤ 0.026 for DPT-1 and TNPTP). Vector indices for AUC glucose and AUC C-peptide changes (on a two-dimensional grid) also differed significantly (P < 0.001). Despite marked baseline metabolic impairment of nonprogressors, changes in AUC C-peptide, AUC glucose, AUC C-peptide–to–AUC glucose ratio (AUC ratio), and Index60 did not differ from Ab− relatives during follow-up. Divergence between nonprogressors and progressors occurred by 6 months from baseline in both cohorts (AUC glucose, P ≤ 0.007; AUC ratio, P ≤ 0.034; Index60, P < 0.001; vector indices of change, P < 0.001). Differences in 6-month change were positively associated with greater diabetes risk (respectively, P < 0.001, P ≤ 0.019, P < 0.001, and P < 0.001) in DPT-1 and TNPTP, except AUC ratio, which was inversely associated with risk (P < 0.001).
CONCLUSIONS
Novel findings show that even with similarly abnormal baseline risk, progressors had appreciably more metabolic impairment than nonprogressors within 6 months and that the measures showing impairment were predictive of type 1 diabetes. Longitudinal metabolic patterns did not differ between nonprogressors and Ab− relatives, suggesting persistent β-cell responsiveness in nonprogressors.
Graphical Abstract
Introduction
Type 1 diabetes is an autoimmune disease characterized by T-cell–mediated destruction of insulin-producing β-cells within the pancreatic islets of Langerhans (1). Since insulin treatment is associated with considerable comorbidities, approaches to prevent the disorder before irremediable loss of β-cell mass are highly desirable (2). The Type 1 Diabetes TrialNet Study Group recently reported that treatment of high-risk individuals with teplizumab delays diabetes onset by 32.5 months (3,4). These encouraging results will undoubtedly lead to more prevention studies with this agent and support the rationale for additional trials applying other agents. Given this changing landscape in type 1 diabetes prevention, an improved understanding of the factors underlying disease development and progression is paramount for both optimization of risk-to-benefit ratios and assessments of therapeutic efficacy.
Seroconversion to islet autoimmunity predicts type 1 diabetes development in 14–70% of children within a decade (5). However, while an appreciable percentage of islet autoantibody–positive (Ab+) individuals are diagnosed with type 1 diabetes in prevention trials and natural history studies (i.e., progressors), the majority are not diagnosed (i.e., nonprogressors) during the periods of study (4,6,7). Further, some nonprogressors remain diabetes free for years following Ab seroconversion (8,9). Although prediction of type 1 diabetes has become increasingly accurate, the research community still lacks sufficient understanding of factors that differentiate progressors from nonprogressors (2).
The unknown length of the disease process prior to the identification of those who are Ab+ at screening (7) has been a major obstacle for studying why some progress to type 1 diabetes while others do not during follow-up. However, estimating the severity of prior metabolic decline of those who are Ab+ at the time of screening can enhance our understanding of this progression. One validated tool for determining the degree of such progression, the Diabetes Prevention Trial–Type 1 Risk Score (DPTRS), incorporates glucose and C-peptide measurements from an oral glucose tolerance test (OGTT), as well as age and BMI (10). Still, among those with similar baseline risk assessments such tools do not explain the variability in diabetes progression.
To better understand this variability, after stratification by DPTRS to address baseline metabolic risk, we compared pediatric progressors with nonprogressors for changes in C-peptide and glucose derived from serial OGTTs. We specifically sought to test the hypothesis that after adjustment for baseline risk, progressors and nonprogressors exhibit distinct longitudinal trajectories in their ability to respond to a glucose challenge.
Research Design and Methods
Subjects
We studied Ab+ children <18 years of age with available metabolic data who participated in the Diabetes Prevention Trial–Type 1 (DPT-1) (n = 523) (10,11) and the Type 1 Diabetes TrialNet Pathway to Prevention study (TNPTP) (n = 1,388) (12). None had diabetes, and all were first- and second-degree relatives of individuals with type 1 diabetes. DPT-1 participants were in treatment or placebo arms of either the parenteral insulin or oral insulin prevention trials; neither trial showed overall efficacy. For DPT-1, participants tested positive for islet cell antibodies and exhibited either abnormal first-phase insulin secretion/dysglycemia or positive insulin Ab titers with normal metabolic testing. For TNPTP, only individuals with a history of testing positive for two or more 2 biochemical Abs (insulin, GAD, islet antigen 2, or zinc transporter 8) were included. All Ab testing obtained prior to the initial OGTT was considered. Longitudinal metabolic data were available for 246 individuals participating in DPT-1 and 503 individuals in TNPTP with baseline DPTRS ranging from 6.5 to <7.5. A comparison group of 119 Ab− relatives <18 years of age in TNPTP with multiple OGTTs was also included (7). In DPT-1, progressors were followed for mean ± SD 3.19 ± 1.44 years and nonprogressors were followed for 3.89 ± 1.69 years. In TNPTP, progressors were followed for 3.05 ± 1.99 years and nonprogressors were followed for 3.68 ± 2.98 years; Ab− relative follow-up was 4.2 ± 3.2 years. Human subjects approval and informed consent were obtained as previously described (7,11).
Procedures
In both DPT-1 and TNPTP (7,12), Ab+ relatives had baseline 2-h OGTTs and then underwent biyearly OGTTs that included glucose and C-peptide measurements at 30-min intervals for monitoring of progression to diabetes. In DPT-1, intravenous glucose tolerance tests (IVGTTs) were performed to determine first-phase insulin response (FPIR) at 2, 4, and 6 years as previously described (12).
The DPTRS, which includes fasting C-peptide, sum C-peptide and sum glucose values from 30–120 min, age, and BMI, was calculated as previously described, with higher values suggesting increased risk of diabetes progression (10). We chose the DPTRS because of its prior validation for risk prediction in the DPT-1 and TrialNet cohorts and its inclusion of both metabolic and demographic risk factors (10,13). Progressors were defined as participants who received a diabetes diagnosis during study follow-up (11,12).
Area under the curve (AUC) values were calculated from OGTT data with the trapezoidal rule. AUC C-peptide–to–AUC glucose ratios (AUC ratios) were multiplied by 1,000. Index60 and FPIR were calculated as previously described (14,15). OGTT glucose and C-peptide response curves (GCRCs), plotting mean OGTT glucose and C-peptide values (30–120 min) on a two-dimensional grid, were used to visualize relationships between OGTT C-peptide and glucose values (16). For analyses of changes over the first 6 months of monitoring, the 6-month within-quadrant end point (WQE) and the 6-month ordinal directional end point (ODE) variables were calculated with use of centroid vector-based angles and quadrants of change from GCRCs as previously described (16).
Analysis
Cross-sectional analysis was performed on initial (baseline) OGTT C-peptide and glucose values and GCRC patterns in progressors to type 1 diabetes and nonprogressors. In a longitudinal analysis we examined differences in changes of metabolic indices and GCRC patterns from initial OGTT to last OGTT measurements without diabetes, annualized to address differences in length of follow-up. Values were also compared with those of Ab− controls. We also tested changes of the first repeat OGTT occurring after a mean interval of 6 ± 3 months. Associations between diabetes development and annualized or 6-month changes in metabolic variables were tested. t tests or χ2 tests were used to compare continuous characteristics or categorical characteristic frequencies. Relationships between time to diabetes development and changes in metabolic parameters/time were examined with Cox regression models with adjustments for potentially confounding variables, including baseline age, BMI, DPTRS, and baseline value. Since height was not routinely measured in Ab− relatives, comparisons including this group only included adjustment for age and baseline value. Statistical analyses were performed with SAS 9.4. Two-sided P values 0.05 were considered statistically significant.
Results
Cross-sectional Analysis of Baseline Metabolic Data
For the 523 participants in DPT-1 and 1,388 ≥2 Ab+ participants in TNPTP eligible for study, we first compared baseline metabolic features between progressors and nonprogressors after stratification by DPTRS. As shown in Supplementary Table 1, there was a marked difference between the groups in their distributions among the DPTRS categories (P < 0.001 for all DPTRS categories). In DPT-1, proportions of progressors were 84 of 115 (73.0%) in the highest DPTRS category (≥7.50) and 28 of 162 (17.3%) in the lowest DPTRS category (<6.50). In TNPTP, proportions of progressors were 152 of 256 (59.4%) in the highest DPTRS category (≥7.50) and 82 of 629 (16.7%) in the lowest DPTRS category (<6.50). However, within the same DPTRS categories, progressors and nonprogressors showed similar metabolic features (no significant differences between progressor and nonprogressor groups within DPTRS categories for DPT-1 or TNPTP) (Supplementary Table 1).
Longitudinal Analysis of Changes in Metabolic Measures During Follow-up
Progressors Versus Nonprogressors
Since the cross-sectional data indicated that progressors and nonprogressors had similar metabolic patterns within DPTRS categories at baseline, we performed a longitudinal analysis to assess the extent and nature of metabolic changes during follow-up. To study change from homogenous baseline metabolic phenotypes, we chose to focus on individuals with baseline DPTRS scores between 6.5 and 7.5; this identified individuals who not only were metabolically homogeneous but also had appreciable baseline metabolic abnormality (10). Changes in C-peptide from the baseline OGTTs to the last OGTTs (before diagnosis in progressors) were compared between progressors and nonprogressors within that baseline 6.5–7.5 DPTRS interval.
Repeat OGTT data were available for 246 children in DPT-1 (85 progressors and 161 nonprogressors) and 503 children in TNPTP (171 progressors and 332 nonprogressors) with an initial DPTRS between 6.5 and 7.5. Baseline demographic characteristics are shown in Supplementary Tables 2 and 3. No significant differences in HLA status between groups were present in either study. In DPT-1, the mean number of Abs with positive titers at any time point during monitoring was not significantly different between groups (2.73 vs. 2.60 in nonprogressors; P = 0.271); in TNPTP, the mean number of Abs with positive titers at any time point during monitoring was somewhat higher in progressors (3.29 vs. 3.03 in nonprogressors; P = 0.008).
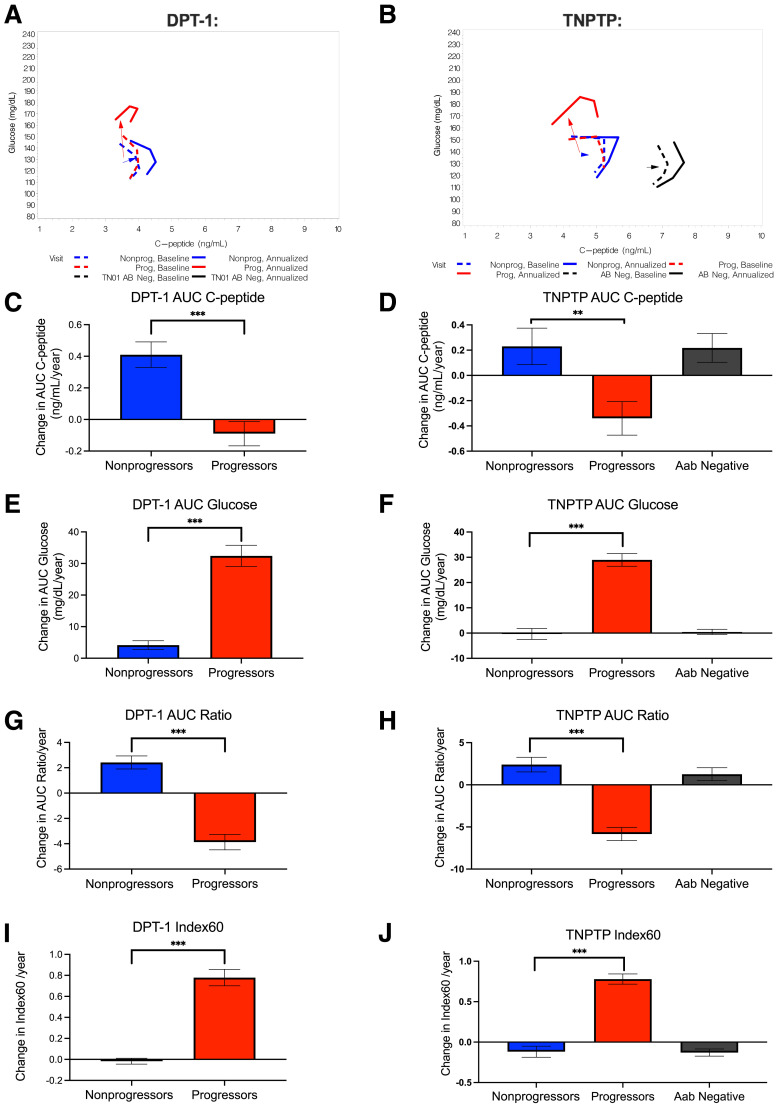
We previously showed that characteristic changes in OGTT GCRCs are associated with progression to type 1 diabetes (16). To better understand the directionality of changes in C-peptide relative to glucose during the progression to type 1 diabetes, in Fig. 1A and B we plotted mean GCRCs and vectors for annualized changes of AUC glucose and AUC C-peptide from baseline to final OGTTs (final before diagnosis in progressors). GCRCs and vectors for the group of pediatric Ab− relatives are also shown in Fig. 1B for comparison. In DPT-1, progressors exhibited an almost vertical vector, reflecting predominant increases in glucose with minimal change in C-peptide. By contrast, nonprogressors exhibited less of an increase in AUC glucose over time with an increase in AUC C-peptide, resulting in a directionality distinctly different from that of progressors. These relationships were similar for progressors and nonprogressors within the TNPTP population, although in TNPTP, loss of C-peptide appeared to be more pronounced in progressors, with less of an increase in C-peptide in nonprogressors over time compared with DPT-1 (Fig. 1B).
Figure 1.
Two-dimensional analysis shows distinct directionality of relationships between longitudinal OGTT C-peptide and glucose AUC values among progressors and nonprogressors where, over time, nonprogressors exhibit positive increases in C-peptide AUC, while progressors exhibit increases in glucose AUC. A and B: For DPT-1 (A) and TNPTP (B) participants, vectors showing directionality of changes in mean glucose and C-peptide AUCs for nonprogressors and progressors from first to final OGTT (last before diagnosis in progressors; for DPT-1, progressors were followed for mean ± SD 3.19 ± 1.44 years and nonprogressors were followed for 3.89 ± 1.69 years, and for TN-PTP, progressors were followed for an average of 3.05 ± 1.99 years and nonprogressors were followed for 3.68 ± 2.98 years). In B, black represents a group of Ab− family members (n = 119) for comparison. Average follow-up for Ab− relatives was 4.2 ± 3.2 years. C and D: For DPT-1 (C) and TNPTP (D) participants, changes in AUC C-peptide from first to final OGTT. E and F: For DPT-1 (E) and TNPTP (F) participants, changes in AUC glucose from first to final OGTT. G and H: For DPT-1 (G) and TNPTP (H) participants, changes in AUC ratio from first to final OGTT. I and J: For DPT-1 (I) and TNPTP (J) participants, changes in Index60 from first to final OGTT. Only individuals with baseline DPTRS of 6.5–7.5 were included in longitudinal analyses. In D, F, and H, plots of values from Ab− relative control subjects (Aab Negative) have also been included for comparison. For DPT-1: n = 85 progressors and n = 161 nonprogressors. For TNPTP: n = 171 progressors and n = 332 nonprogressors. Data are shown as mean SEM. ***P < 0.001. AB Neg, Ab−; Nonprog, nonprogressors; Prog, progressors; TN01, TrialNet Pathway To Prevention.
Quantification of changes in metabolic measures is shown for both DPT-1 (Fig. 1C, E, G, and I) and TNPTP (Fig. 1D, F, H, and J) participants. Absolute values for changes with statistical comparisons without and with adjustment for baseline DPTRS, age, and BMI and the baseline measure being tested are shown in Table 1.
Table 1.
Comparisons of OGTT changes in measures between progressors and nonprogressors during follow-up
| DPT-1 | TNTPT | |||||
|---|---|---|---|---|---|---|
| Progressors | Nonprogressors | P | Progressors | Nonprogressors | P | |
| Analysis from first to last OGTT (prior to diagnosis in progressors), change per year | ||||||
| n | 85 | 161 | 171 | 332 | ||
| AUC C-peptide (ng/mL) | −0.09 ± 0.71 | 0.41 ± 1.03§ | <0.001/<0.001* | −0.34 ± 1.74§ | 0.23 ± 2.64 | 0.004/0.026* |
| AUC glucose (mg/dL) | 32.43 ± 30.97 | 4.16 ± 17.49 | <0.001/<0.001* | 28.92 ± 33.09 | −0.35 ± 39.73 | <0.001/<0.001* |
| AUC ratio | −3.87 ± 5.61 | 2.42 ± 6.52§ | <0.001/<0.001* | −5.84 ± 10.21§ | 2.40 ± 15.75§ | <0.001/<0.001* |
| Index60 | 0.22 ± 0.66§ | −0.02 ± 0.35 | <0.001/<0.001* | 0.78 ± 0.82§ | −0.12 ± 1.25 | <0.001/<0.001* |
| Analysis from first to 6-month repeat OGTT | ||||||
| n | 79 | 141 | 135 | 246 | ||
| AUC C-peptide (ng/mL) | 0.17 ± 1.37 | 0.28 ± 1.38 | 0.554/0.390* | −0.08 ± 1.37 | 0.04 ± 1.16 | 0.394/0.449* |
| AUC glucose (mg/dL) | 9.80 ± 24.85 | 1.22 ± 20.87 | 0.007/<0.001* | 10.82 ± 27.63 | −2.26 ± 20.73 | <0.001/<0.001* |
| AUC ratio | −0.19 ± 9.19 | 2.56 ± 9.20 | 0.034/0.017* | −1.79 ± 8.40§ | 1.40 ± 7.75§ | <0.001/<0.001* |
| Index60 | 0.22 ± 0.67§ | −0.11 ± 0.50§ | <0.001/<0.001* | 0.27 ± 0.66§ | −0.07 ± 0.68 | <0.001/<0.001* |
| WQE | 0.68 ± 0.56 | 0.38 ± 0.50 | <0.001/<0.001† | 0.61 ± 0.54 | 0.39 ± 0.47 | <0.001/<0.001† |
| ODE | 57.3 ± 22.04 | 45.8 ± 24.43 | <0.001/<0.001† | 57.8 ± 23.99 | 44.8 ± 24.36 | <0.001/<0.001† |
Means ± SD and unadjusted/adjusted P values for comparison between progressors and nonprogressors are shown.
Adjusted for measure at baseline and baseline age, BMI, and DPTRS.
Adjusted for baseline parameters C-peptide AUC mean, glucose AUC mean, age, BMI, and DPTRS.
P value for change (N/A for WQE and ODE): <0.05.
As shown in Fig. 1C and D, for both cohorts, progressors had decreased AUC C-peptide per year. In contrast to progressors and consistent with vectors in Fig. 1A and B, over the course of the study, nonprogressors had increased AUC C-peptide per year. These annualized AUC C-peptide changes were significantly different between progressors and nonprogressors (unadjusted/adjusted P value for DPT-1 and TNPTP <0.001/<0.01 and for TNPTP 0.004/0.026). As shown in Fig. 1E and F, and consistent with vectors in Fig. 1A and B, changes in AUC glucose were significantly higher in progressors than nonprogressors (unadjusted and adjusted P values for both DPT-1 and TNPTP: <0.001).
We also examined annualized changes in AUC ratios from the baseline OGTT to the last OGTT (Fig. 1G and H). Here, nonprogressors had an increased annual change in AUC ratio over the period of follow-up, while progressors displayed a decrease (unadjusted and adjusted P values for progressor vs. nonprogressor comparison in both DPT-1 and TNPTP: <0.001). Similar to the AUC ratio (Fig. 1I and J), the Index60 composite glucose and C-peptide measure showed annualized increases in progressors but little change over time in nonprogressors (unadjusted and adjusted P values for progressor versus nonprogressor comparison for both DPT-1 and TNPTP: <0.001).
FPIR data from IVGTTs, a specific measure of β-cell function, were also examined. This was available from 245 participants in DPT-1 with DPTRS values of 6.5–7.5. From first to last IVGTTs, FPIR also differed between nonprogressors and progressors (Supplementary Fig. 1). Similar to AUC C-peptide, nonprogressors exhibited a mean FPIR increase of 7.64 ± 33.78 μU/mL/year, while progressors showed a decrease of −14.82 ± 4.55 μU/mL/year (P < 0.001 without and with adjustments for baseline FPIR, age, BMI, and baseline DPTRS), suggesting that observed increases in C-peptide are associated with improved β-cell function.
Progressors and Nonprogressors Versus Ab− Relatives
We next compared metabolic changes of the progressors and nonprogressors with the Ab− relatives in the TNPTP cohort (demographic data in Supplementary Table 4). At baseline, Ab− relatives had significantly higher C-peptide and AUC C-peptide values than both progressors and nonprogressors (P < 0.001 for both groups). AUC glucose values were significantly lower in Ab− relatives than in progressors or nonprogressors (P < 0.001 for both groups).
Annualized AUC C-peptide changes were significantly lower for progressors (P < 0.001 without and with adjustment for age and baseline value) than for Ab− relatives. In contrast, annualized AUC C-peptide changes of nonprogressors did not differ from those of Ab− relatives (P = 0.468/0.625 for unadjusted/adjusted). Annualized AUC glucose changes of progressors were significantly higher than in Ab− relatives (P < 0.001 without and with adjustment), but there was no difference between nonprogressors and Ab− relatives (P = 0.788/0.873). Annualized AUC ratio and Index60 changes were significantly different in progressors compared with Ab− relatives (P < 0.001 without and with adjustment for both variables). However, as with AUC C-peptide and AUC glucose changes, AUC ratio and Index60 changes did not differ between nonprogressors and Ab− relatives (AUC ratio, P = 0.702/0.831, and Index60, P = 0.939/0.239).
Influence of Changes in Metabolic Variables on Overall Progression to Type 1 Diabetes
To define the relationship between diabetes progression and changes in OGTT after initial risk assessments, we performed Cox regression for associations of each annualized variable with time to type 1 diabetes, without and with adjustments for age, BMI, DPTRS, and variable of interest at baseline (Table 2). Without adjustment for these potentially confounding factors, among both cohorts, type 1 diabetes was inversely related to the annual change in AUC C-peptide in both cohorts (for DPT-1 hazard ratio [HR] 0.23 [95% CI 0.15, 0.37], P < 0.001 without and with adjustment; for TNPTP 0.91 [0.87, 0.95]; P < 0.001 without and with adjustment). For annualized changes in AUC glucose, yearly increases were associated with more rapid progression to diabetes in both cohorts (P < 0.001 without and with adjustment)]. Yearly increases in AUC ratio were associated with slower progression to diabetes (P < 0.001 without and with adjustment). Conversely, yearly increases in Index60 were strongly associated with more rapid progression to diabetes in both cohorts (P < 0.001 without and with adjustment).
Table 2.
Cox regression analysis of relationship between changes in metabolic variables and time to type 1 diabetes
| Analysis from baseline to final nondiabetes OGTT result | ||||||
|---|---|---|---|---|---|---|
| DPT-1 (n = 246) | TNPTP (n = 503) | |||||
| χ2 | HR (CI) | P | χ2 | HR (CI) | P | |
| Change per year in AUC C-peptide (ng/mL) | 38.68 | 0.23 (0.15, 0.37)/0.18 (0.10, 0.30)* | <0.001/<0.001* | 18.08 | 0.91 (0.87,0.95)/0.92 (0.88, 0.96)* | <0.001/0.001* |
| Change per year in AUC glucose (mg/dL) | 149.92 | 1.05 (1.04, 1.06)/1.05 (1.04, 1.06)* | <0.001/<0.001* | 87.38 | 1.01 (1.01, 1.01)/1.01 (1.01, 1.01)* | <0.001/<0.001* |
| Change per year in AUC ratio | 118.11 | 0.73 (0.68, 0.77)/0.68 (0.63, 0.73)* | <0.001/<0.001* | 79.40 | 0.97 (0.97, 0.98)/0.98 (0.97, 0.98)* | <0.001/<0.001* |
| Change per year in Index60 | 162.73 | 10.94 (7.57, 15.80)/10.62 (7.25, 15.56)* | <0.001/<0.001* | 235.37 | 3.78 (3.19, 4.48)/3.91 (3.28, 4.65)* | <0.001/<0.001* |
| Analysis restricted from baseline to 6-month OGTT | ||||||
|---|---|---|---|---|---|---|
| DPT-1 (n = 220) | TNPTP (n = 381) | |||||
| χ2 | HR (CI) | P | χ2 | HR (CI) | P | |
| Change in AUC C-peptide (ng/mL) | 0.28 | 0.96 (0.81,1.13)/0.98 (0.81, 1.18)* | 0.598/0.809* | 4.08 | 0.86 (0.74, 1.00)/0.82 (0.69, 0.98)* | 0.043/0.025* |
| Change in AUC glucose (mg/dL) | 15.78 | 1.02 (1.01, 1.03)/1.03 (1.02, 1.04)* | <0.001/<0.001* | 47.48 | 1.03 (1.02, 1.03)/1.03 (1.02, 1.04)* | <0.001/<0.001* |
| Change in AUC ratio | 5.52 | 0.97 (0.95, 1.00)/0.96 (0.94, 0.99)* | 0.019/0.020* | 32.0 | 0.94 (0.91, 0.96)/0.92 (0.89, 0.94)* | <0.001/<0.001* |
| Change in Index60 | 27.20 | 3.26 (2.09, 5.08)/3.25 (2.05, 5.16)* | <0.001/<0.001* | 43.35 | 2.8 (2.06, 3.81)/3.06 (2.26, 4.14)* | <0.001/<0.001* |
| WQE | 19.3 | 2.33 (1.60, 3.41)/2.00 (1.35, 2.94)† | <0.001/<0.001† | 24.6 | 2.20 (1.61, 3.00)/2.44 (1.79, 3.32)† | <0.001/<0.001† |
| ODE | 11.1 | 1.02 (1.01,1.03)/1.018 (1.008, 1.028)† | 0.001/<0.001† | 28.8 | 1.02 (1.01,1.03)/1.022 (1.015, 1.028)† | <0.001/<0.001† |
Unadjusted/adjusted HRs (95% CIs) and P values for comparisons between progressors and nonprogressors are shown.
Adjustment for measure at baseline age, BMI, and DPTRS.
Adjustment for baseline C-peptide AUC, glucose AUC, age, BMI, and DPTRS.
Analysis of Changes in OGTT Measures Over the First 6 Months of OGTT Monitoring
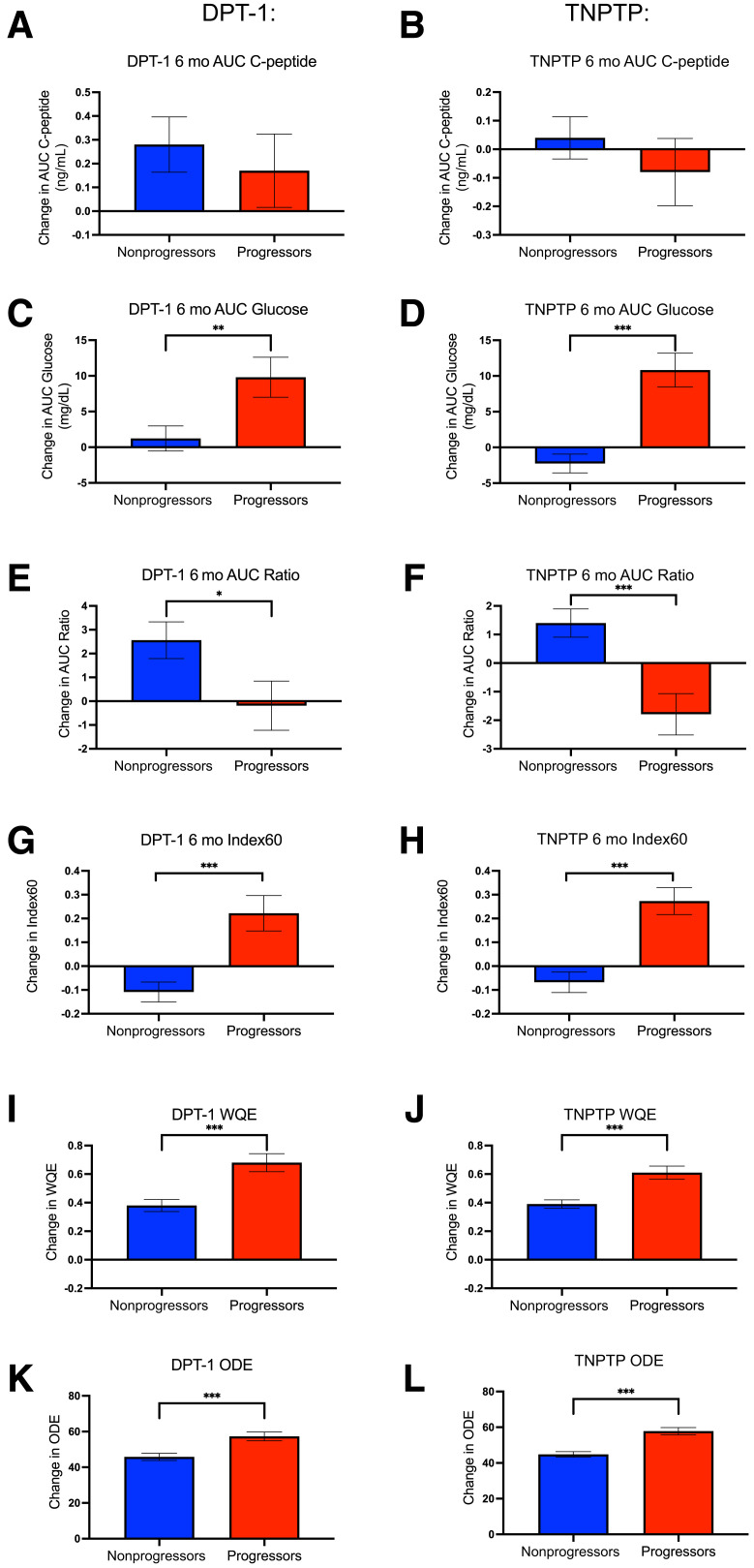
Although changes in OGTT measures over time may provide relevant insights into metabolic physiology in progressors and nonprogressors, observed changes over a short period of monitoring may have more practical use in terms of informing risk of diabetes progression. To test whether shorter-term OGTT measure changes differed between progressors and nonprogressors, we compared those changes from baseline to first follow-up OGTTs obtained ∼6 months after baseline. Graphical depiction for participants in DPT-1 and TNPTP is shown in Fig. 2, and values with statistical comparisons without and with adjustment for baseline DPTRS, age, BMI, and baseline value of the variable being tested are shown in Table 1.
Figure 2.
Changes in OGTT measures over the first 6 months of monitoring. For DPT-1 (A) and TNPTP (B) participants, changes in OGTT AUC C-peptide over the first 6 months after baseline OGTT assessment. C and D: For DPT-1 (C) and TNPTP (D) participants, 6-month changes in AUC glucose. E and F: For DPT-1 (E) and TNPTP (F) participants, 6-month changes in AUC ratio. G and H: For DPT-1 (E) and TNPTP (F) participants, 6-month changes in Index60. I and J: For DPT-1 (I) and TNPTP (J) participants, glucose C-peptide response curve vector WQE values. K and L: For DPT-1 (K) and TNPTP (L) participants, glucose C-peptide response curve vector ODE values. Only individuals with baseline DPTRS of 6.5–7.5 were included in longitudinal analyses. Data are shown as mean SEM. *P < 0.05; **P < 0.01; ***P < 0.001. mo, month.
In contrast to annualized changes, although values were directionally consistent, AUC C-peptide changes over a 6-month period were not significantly different between progressors and nonprogressors in either DPT-1 or TNPTP. Similar to annualized values, progressors had greater increases in AUC glucose compared with nonprogressors (for DPT-1, unadjusted/adjusted P = 0.007/<0.001; for TNPTP, P < 0.001 for unadjusted and adjusted). In both DPT-1 and TNPTP, over the 6-month period the AUC ratio decreased in progressors and increased in nonprogressors (DPT-1 difference between groups, P = 0.034/0.017 for unadjusted and adjusted; TNPTP difference between groups, P < 0.001 for unadjusted and adjusted). In both cohorts, Index60 increased appreciably in progressors but had little change in nonprogressors (both DPT-1 and TNPTP differences between groups, P < 0.001 for unadjusted and adjusted).
We also calculated two novel end points that we recently developed to quantify OGTT GCRC vector changes over a 6-month period, the WQE and the ordinal directional end point (ODE) (16) (Fig. 2 and Table 1). Consistent with metabolic decline, progressors exhibited significantly higher WQE and ODE values than nonprogressors in both DPT-1 and TNPTP (P < 0.001 unadjusted and adjusted in both DPT-1 and TNPTP).
Influence of 6-Month OGTT Changes in Metabolic Variables on Overall Progression to Type 1 Diabetes
To determine whether 6-month OGTT changes could predict rates of progression to type 1 diabetes, we performed Cox regression to assess relationships between type 1 diabetes and 6-month changes in metabolic measures (both unadjusted and adjusted for the baseline measure, age, BMI, and baseline DPTRS) (Table 2). Here, we observed that like annualized increases, 6-month increases in AUC C-peptide were associated with a significantly decreased occurrence of type 1 diabetes in TNPTP (HR 0.86 [95% CI 0.74, 1.00], unadjusted/adjusted P = 0.043/0.025), though this relationship was not significant in DPT-1 (0.96 [0.81, 1.13], unadjusted/adjusted P = 0.598/0.809). Increases in AUC glucose over the first 6 months of monitoring were strongly associated with more rapid progression for both cohorts (unadjusted and adjusted P < 0.001 for both cohorts). Similar to annualized changes, AUC Ratio increases over a 6-month period predicted less progression in both cohorts (unadjusted and adjusted P < 0.001). Increases in Index60 over 6 months were associated with more rapid progression in both cohorts (both unadjusted and adjusted P < 0.001), as were higher values of both WQE and ODE (each P ≤ 0.001 for unadjusted and adjusted values in both cohorts).
Conclusions
There were two phases of the above analyses that can be viewed sequentially. In the cross-sectional phase shown at baseline, OGTT GCRC patterns and other characteristics differed little between pediatric progressors and nonprogressors with nearly the same DPTRS values (range of 6.50 to <7.50). The similarity between progressors and nonprogressors was a basis for the longitudinal, second phase of the analysis, with examination of differences in changes of metabolic indices over time between progressors and nonprogressors in two separate cohorts of Ab+ children. We observed that despite the metabolic similarities between the groups at baseline, both in traditional measures and location on the GCRC two-dimensional grid, there were marked differences in longitudinal metabolic patterns. Whereas glucose increased and C-peptide decreased in the progressors, glucose changed little, and C-peptide and FPIR increased in nonprogressors. Although nonprogressors had markedly lower C-peptide at baseline than the Ab− relatives, glucose and C-peptide patterns were similar between these groups over a period of several years.
The two-dimensional grid analysis examining the directionality of change in C-peptide values relative to change in glucose uses a novel approach to demonstrate the marked divergence of metabolic change between progressors and nonprogressors. Vectors provided visual evidence of the divergence that was corroborated quantitatively by the recently developed WQE and ODE. The similar pattern between pediatric nonprogressors and Ab− relatives suggests that increases in the C-peptide response of nonprogressors are at least in part indicative of responsiveness to a normal physiologic need for more insulin secretion due to increasing insulin resistance and IGF-I from aging and pubertal development (17–21). We cannot definitively explain why nonprogressors, and not progressors, had the transition from marked impairment at baseline to a pattern of metabolic change comparable with that of Ab− relatives during follow-up. Perhaps nonprogressors had greater β-cell reserve (either functional or mass) compared with progressors and, thus, an ability to develop a compensatory response. Another possibility, supported by the improvement in FPIR in addition to AUC C-peptide, is that the increases for nonprogressors could reflect some degree of recovery from prior β-cell injury. Finally, it is plausible that differences in ongoing islet inflammation and severity of the autoimmune process may also play a role.
To our knowledge, no prior studies have included comparison of changes in C-peptide between progressors and nonprogressors with the same baseline risk and similar baseline OGTT glucose and C-peptide patterns. The increase in C-peptide of nonprogressors is, however, consistent with results of an earlier DPT-1 study showing increases in C-peptide over time during the latter part of OGTTs (22). In another DPT-1 study, OGTT AUC C-peptide did not decrease over time in a group of 11 nonprogressors (23). Among a smaller DPT-1 subgroup that had mixed-meal tolerance tests performed, slopes of C-peptide–to–BMI ratios were increased among at-risk children relative to at-risk adults and were reduced among adult progressors (24). In dysglycemic family members positive for multiple Ab, at very high risk for disease progression, improvement in early insulin secretion was associated with normalization of OGTTs (25). In aggregate these data and our findings suggest that compared with cross-sectional data in isolation, changes in metabolic measures provide valuable additional insight into type 1 diabetes progression and nonprogression.
The cross-sectional analysis demonstrated that progressors and nonprogressors had similar OGTT phenotypes within each DPTRS category, yet the relative frequency of progressors increased as DPTRS values increased. These findings could mean that nonprogressors pass through the same stages of metabolic dysfunction as progressors but at a slower pace. This could be consistent with observed WQE and ODE values shown in Fig. 2 for nonprogressors, which increased over time. It is thus likely that an appreciable number of those not diagnosed during follow-up will eventually be diagnosed. Cohorts of “slow progressors” who were positive for multiple Abs but did not progress to diabetes within 10 years were identified in other natural history studies (8). For these cohorts, abnormal insulin secretion could go unrecognized. The potential implications of our findings in nonprogressors (relative to Ab− relatives) strongly support a need for long-term follow-up after the completion of natural history studies and disease prevention trials.
Our longitudinal GCRCs in Fig. 1 showed that nonprogressors exhibited vectors of change similar to high-risk treatment responders in post hoc analyses of the DPT-1 and TrialNet oral insulin trials, with increases in both C-peptide and glucose over time (26). High-risk participants receiving teplizumab in the TrialNet teplizumab prevention study showed a more marked metabolic impact, with increases in C-peptide and decreases in glucose (16). These differences could be consistent with the relative impact of each intervention on the timing of type 1 diabetes progression.
These findings have practical implications for type 1 diabetes prediction and prevention. Type 1 diabetes prevention trials are challenging to implement, in part because of variability in rates of progression to disease (2). Although factors such as age, genetic predisposition, number and type of islet autoantibodies, C-peptide and glucose values, and risk scores help with gauging diabetes risk, variability still exists among high-risk individuals (5,14,27,28). Thus, some degree of follow-up after baseline could be helpful for selection of appropriate study participants. For example, those who exhibit increases in stimulated insulin secretion over time might not be appropriate for a trial of individuals at very high risk. Without such follow-up those individuals could be enrolled according to an inaccurate assessment of risk at baseline. As part of the longitudinal analysis, we assessed whether short-term metabolic changes over a 6-month period adds to the accuracy of type 1 diabetes prediction obtained from baseline measures. Here, we observed that changes in metabolic measures at 6 months were associated with progression to diabetes, even when accounting for baseline values. Our findings suggest that the inclusion of lower-risk study participants could be avoided by a short-term follow-up of 6 months to determine metabolic change over that period using such measures as AUC ratio, Index60, and end points (WQE and ODE) based on vectors of change in 6 months. These findings are consistent with those of a prior study with a different methodology showing the value of 6 months of follow-up of glucose data for prediction (29), but build on this prior work in providing insights on longitudinal C-peptide and relationships of C-peptide with glucose in Ab+ and Ab− relatives.
Practically, implementation of longitudinal changes in metabolic measures as part of a strategy for trial enrollment or monitoring will likely require consideration of multiple factors. For example, in prevention trials where individuals at very high risk are sought, investigators would need to consider the possibility that potential participants may progress to diabetes over the monitoring period. Thus, change in metabolic markers as enrollment criteria may be more optimal in trials targeting individuals earlier in the natural history of disease. Although we would envision continued longitudinal monitoring of high-risk children at all ranges of the DPTRS spectrum, those with improved OGTT values may require less frequent monitoring, whereas those with worsening metabolic values may need more frequent testing to catch progression.
This study has limitations. Our findings reflect diabetes progression patterns in individuals with both genetic susceptibility and established islet autoimmunity. Thus, the observations might not be applicable to the general population. Most of the participants with available data were non-Hispanic White, and so findings will need to be tested in other racial and ethnic populations. Although we considered individuals who did not progress to diabetes during follow-up to be nonprogressors, there were likely nonprogressors who subsequently developed diabetes. In addition, because Tanner staging was not collected as part of the DPT-1 or TNPTP study designs, we were unable to determine the relationship between increases in C-peptide over time and progression of puberty. However, adjustments for age and comparisons with Ab− children likely controlled at least partly for the influence of puberty. Analyses of insulin sensitivity were not performed as part of the DPT-1 or TNPTP study designs. Due to the confounding effect of autoimmune β-cell destruction on insulin secretion, fasting insulin levels cannot be assumed to reflect insulin resistance in this population (30).
Notwithstanding these limitations, among the large prospective at-risk pediatric cohorts of DPT-1 and TNPTP, the present findings strongly suggest that persistent C-peptide responsiveness, reflected by the longitudinal change in C-peptide secretion in response to glucose, is an important distinguishing feature separating individuals positive for multiple Ab who do or do not progress to type 1 diabetes over an average follow-up of nearly 4 years. This analysis should contribute to an improved understanding of the natural history of type 1 diabetes, more accurate risk prediction, and improved prevention trial design.
Article Information
Acknowledgments. The authors acknowledge the support of the Type 1 Diabetes TrialNet Study Group, which identified study participants and provided samples and follow-up data for this study.
Funding. The Type 1 Diabetes TrialNet Study Group is a clinical trials network funded by the National Institutes of Health (NIH) through the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), the National Institute of Allergy and Infectious Diseases, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development, through cooperative agreements U01 DK061010, U01 DK061034, U01 DK061042, U01 DK061058, U01 DK085465, U01 DK085453, U01 DK085461, U01 DK085466, U01 DK085499, U01 DK085504, U01 DK085509, U01 DK103180, U01 DK103153, U01 DK085476, U01 DK103266, U01 DK103282, U01 DK106984, U01 DK106994, U01 DK107013, U01 DK107014, UC4 DK097835, and UC4 DK106993, and JDRF. E.K.S. receives support from NIH grants R01 DK121929, and U01DK127382-012. E.K.S. was also supported by the Doris Duke Charitable Foundation (grant 2021258) through the COVID-19 Fund to Retain Clinical Scientists collaborative grant program and supported by the John Templeton Foundation (grant 62288). M.J.R. was supported by NIH grants R01 DK121843 and R01 DK124395.
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the NIH, JDRF, or John Templeton Foundation.
Duality of Interest. E.K.S. received compensation from Medscape in 2022 for a lecture in a symposium on general population screening. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. E.K.S. and J.M.S. designed the study and wrote and edited the manuscript. D.C. designed and performed analyses. J.L.F., H.M.I., B.M.N., L.M.J., E.P., A.P., J.P., M.A., C.E.-M., J.S.S., M.J.R., and K.C.H. edited the manuscript. All authors approved the final analysis. E.K.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 78th Scientific Sessions of the American Diabetes Association, Orlando, FL, 22–26 June 2018.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.21040171.
References
- 1. DiMeglio LA, Evans-Molina C, Oram RA. Type 1 diabetes. Lancet 2018;391:2449–2462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jacobsen LM, Haller MJ, Schatz DA. Understanding pre-type 1 diabetes: the key to prevention. Front Endocrinol (Lausanne) 2018;9:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sims EK, Bundy BN, Stier K, et al.; Type 1 Diabetes TrialNet Study Group . Teplizumab improves and stabilizes beta cell function in antibody-positive high-risk individuals. Sci Transl Med 2021;13;eabc8980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Herold KC, Bundy BN, Long SA, et al.; Type 1 Diabetes TrialNet Study Group . An anti-CD3 antibody, teplizumab, in relatives at risk for type 1 diabetes. N Engl J Med 2019;381:603–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ziegler AG, Rewers M, Simell O, et al. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA 2013;309:2473–2479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Writing Committee for the Type 1 Diabetes TrialNet Oral Insulin Study Group; Krischer JP, Schatz DA, Bundy B, Skyler JS, Greenbaum CJ. Effect of oral insulin on prevention of diabetes in relatives of patients with type 1 diabetes: a randomized clinical trial. JAMA 2017;318:1891–1902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mahon JL, Sosenko JM, Rafkin-Mervis L, et al.; TrialNet Natural History Committee; Type 1 Diabetes TrialNet Study Group . The TrialNet Natural History Study of the Development of Type 1 Diabetes: objectives, design, and initial results. Pediatr Diabetes 2009;10:97–104 [DOI] [PubMed] [Google Scholar]
- 8. Jacobsen LM, Bocchino L, Evans-Molina C, et al. The risk of progression to type 1 diabetes is highly variable in individuals with multiple autoantibodies following screening. Diabetologia 2020;63:588–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Long AE, Wilson IV, Becker DJ, et al. Characteristics of slow progression to diabetes in multiple islet autoantibody-positive individuals from five longitudinal cohorts: the SNAIL study. Diabetologia 2018;61:1484–1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sosenko JM, Skyler JS, Mahon J, et al.; Type 1 Diabetes TrialNet and Diabetes Prevention Trial-Type 1 Study Groups . Use of the Diabetes Prevention Trial-Type 1 risk score (DPTRS) for improving the accuracy of the risk classification of type 1 diabetes. Diabetes Care 2014;37:979–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Diabetes Prevention Trial-Type 1 Study Group . Effects of oral insulin in relatives of patients with type 1 diabetes: the Diabetes Prevention Trial–Type 1. Diabetes Care 2005;28:1068–1076 [DOI] [PubMed] [Google Scholar]
- 12. Diabetes Prevention Trial--Type 1 Diabetes Study Group . Effects of insulin in relatives of patients with type 1 diabetes mellitus. N Engl J Med 2002;346:1685–1691 [DOI] [PubMed] [Google Scholar]
- 13. Sosenko JM, Skyler JS, Palmer JP; Diabetes Type 1 TrialNet and Diabetes Prevention Trial-Type 1 Study Groups . The development, validation, and utility of the Diabetes Prevention Trial-Type 1 Risk Score (DPTRS). Curr Diab Rep 2015;15:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xu P, Wu Y, Zhu Y, et al.; Diabetes Prevention Trial-Type 1 (DPT-1) Study Group . Prognostic performance of metabolic indexes in predicting onset of type 1 diabetes. Diabetes Care 2010;33:2508–2513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sosenko JM, Skyler JS, DiMeglio LA, et al.; Type 1 Diabetes TrialNet Study Group; Diabetes Prevention Trial-Type 1 Study Group . A new approach for diagnosing type 1 diabetes in autoantibody-positive individuals based on prediction and natural history. Diabetes Care 2015;38:271–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sims EK, Cuthbertson D, Herold KC, Sosenko JM. The deterrence of rapid metabolic decline within 3 months after teplizumab treatment in individuals at high risk for type 1 diabetes. Diabetes 2021;70:2922–2931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Allen HF, Jeffers BW, Klingensmith GJ, Chase HP. First-phase insulin release in normal children. J Pediatr 1993;123:733–738 [DOI] [PubMed] [Google Scholar]
- 18. Bloch CA, Clemons P, Sperling MA. Puberty decreases insulin sensitivity. J Pediatr 1987;110:481–487 [DOI] [PubMed] [Google Scholar]
- 19. Lorini R; The Prediabetes Study Group of the Italian Society for Pediatric Endocrinology and Diabetology (SIEDP) . Normal values of first-phase insulin response to intravenous glucose in healthy Italian children and adolescents. J Pediatr Endocrinol Metab 1996;9:163–167 [PubMed] [Google Scholar]
- 20. Hannon TS, Janosky J, Arslanian SA. Longitudinal study of physiologic insulin resistance and metabolic changes of puberty. Pediatr Res 2006;60:759–763 [DOI] [PubMed] [Google Scholar]
- 21. Ball GD, Huang TT-K, Gower BA, et al. Longitudinal changes in insulin sensitivity, insulin secretion, and β-cell function during puberty. J Pediatr 2006;148:16–22 [DOI] [PubMed] [Google Scholar]
- 22. Sosenko JM, Palmer JP, Rafkin LE, et al.; Diabetes Prevention Trial-Type 1 Study Group . Trends of earlier and later responses of C-peptide to oral glucose challenges with progression to type 1 diabetes in diabetes prevention trial-type 1 participants. Diabetes Care 2010;33:620–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schatz D, Cuthbertson D, Atkinson M, et al. Preservation of C-peptide secretion in subjects at high risk of developing type 1 diabetes mellitus--a new surrogate measure of non-progression? Pediatr Diabetes 2004;5:72–79 [DOI] [PubMed] [Google Scholar]
- 24. Tsai EB, Sherry NA, Palmer JP, Herold KC. The rise and fall of insulin secretion in type 1 diabetes mellitus. Diabetologia 2006;49:261–270 [DOI] [PubMed] [Google Scholar]
- 25. Herold KC, Usmani-Brown S, Ghazi T, et al.; Type 1 Diabetes TrialNet Study Group . β cell death and dysfunction during type 1 diabetes development in at-risk individuals. J Clin Invest 2015;125:1163–1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sosenko JM, Skyler JS, Herold KC, et al.; Type 1 Diabetes TrialNet Study Group . Slowed metabolic decline after 1 year of oral insulin treatment among individuals at high risk for type 1 diabetes in the Diabetes Prevention Trial–Type 1 (DPT-1) and TrialNet oral insulin prevention trials. Diabetes 2020;69:1827–1832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Redondo MJ, Geyer S, Steck AK, et al.; Type 1 Diabetes TrialNet Study Group . A type 1 diabetes genetic risk score predicts progression of islet autoimmunity and development of type 1 diabetes in individuals at risk. Diabetes Care 2018;41:1887–1894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jacobsen LM, Larsson HE, Tamura RN, et al.; TEDDY Study Group . Predicting progression to type 1 diabetes from ages 3 to 6 in islet autoantibody positive TEDDY children. Pediatr Diabetes 2019;20:263–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sosenko JM, Skyler JS, Beam CA, et al.; Type 1 Diabetes TrialNet and Diabetes Prevention Trial–Type 1 Study Groups . The development and utility of a novel scale that quantifies the glycemic progression toward type 1 diabetes over 6 months. Diabetes Care 2015;38:940–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care 2004;27:1487–1495 [DOI] [PubMed] [Google Scholar]