Abstract
Subclinical depressive symptoms are associated with increased risk of Alzheimer’s disease (AD), but the brain mechanisms underlying this relationship are still unclear. We aimed to provide a comprehensive overview of the brain substrates of subclinical depressive symptoms in cognitively unimpaired older adults using complementary multimodal neuroimaging data. We included cognitively unimpaired older adults from the baseline data of the primary cohort Age-Well (n = 135), and from the replication cohort ADNI (n = 252). In both cohorts, subclinical depressive symptoms were assessed using the 15-item version of the Geriatric Depression Scale; based on this scale, participants were classified as having depressive symptoms (>0) or not (0). Voxel-wise between-group comparisons were performed to highlight differences in gray matter volume, glucose metabolism and amyloid deposition; as well as white matter integrity (only available in Age-Well). Age-Well participants with subclinical depressive symptoms had lower gray matter volume in the hippocampus and lower white matter integrity in the fornix and the posterior parts of the cingulum and corpus callosum, compared to participants without symptoms. Hippocampal atrophy was recovered in ADNI, where participants with subclinical depressive symptoms also showed glucose hypometabolism in the hippocampus, amygdala, precuneus/posterior cingulate cortex, medial and dorsolateral prefrontal cortex, insula, and temporoparietal cortex. Subclinical depressive symptoms were not associated with brain amyloid deposition in either cohort. Subclinical depressive symptoms in ageing are linked with neurodegeneration biomarkers in the frontolimbic network including brain areas particularly sensitive to AD. The relationship between depressive symptoms and AD may be partly underpinned by neurodegeneration in common brain regions.
Subject terms: Neuroscience, Biomarkers, Depression
Introduction
Late-life depression appears as one of the main potentially modifiable late-life risk factors for dementia [1], and it has been projected that a significant number of Alzheimer’s disease (AD) cases could be prevented if depression is reduced throughout life [2]. Even subclinical depressive symptoms—that do not meet diagnostic criteria for clinical depressive disorder—worsen quality of life and health in older adults, increasing disability, morbidity and mortality [3, 4]. They are also associated with increased risk for both clinical depressive disorder and AD. Thus, subclinical depressive symptoms are associated with higher risk of cognitive decline in cognitively unimpaired (CU) older adults, with each additional symptom increasing the risk of AD by about 20% [5, 6]. Moreover, depressive symptoms are frequent in patients with mild cognitive impairment (MCI) or dementia, and they increase the risk of progression to AD dementia in MCI patients [7, 8]. However, the mechanisms linking depressive symptoms and AD risk are still unclear. Neuroimaging studies investigating the brain changes related to depressive symptoms in ageing may help to better understand these mechanisms underlying this relationship. Studying the preclinical stages of these states, namely, subclinical depressive symptoms and AD biomarkers in CU older adults, could help disentangle their possible interactions as they develop.
Previous magnetic resonance imaging (MRI) studies in CU older adults have reported that subclinical depressive symptoms were associated with lower gray matter (GM) volume or cortical thickness mainly in the medial prefrontal cortex and temporal regions including the hippocampus [9–17]. Studies with positron emission tomography (PET) are sparse; they suggest that depressive symptoms are associated with lower glucose metabolism or perfusion in AD-related brain regions such as the precuneus and posterior cingulate cortex, and in fronto-temporal regions including the hippocampus [12, 18–20]. Regarding amyloid-PET studies, findings are mixed, with some studies reporting an association between depressive symptoms and higher amyloid deposition both cross-sectionally and longitudinally [21–24], while others did not find such a relationship [25, 26].
Most of the previous studies used regions-of-interest (ROI)-based analyses, which did not allow for a global picture of the brain changes associated with subclinical depressive symptoms. Moreover, a majority of studies included only one imaging modality, most often focusing on changes in GM volume. Our main goal with this study was to provide a more comprehensive overview of the brain substrates of subclinical depressive symptoms in CU older adults using whole brain voxel-wise analyses with structural, functional and molecular neuroimaging data in two independent cohorts. With this approach, we aim to offer a better understanding of the early brain mechanisms underlying the links between subclinical depressive symptoms in ageing and the preclinical stage of AD, which are both associated with increased risk of developing clinical stages of these states. We hypothesized that the presence of subclinical depressive symptoms would be associated with brain structural and functional alterations related to late-life depression and AD—especially in frontolimbic regions—as well as with higher neocortical amyloid deposition.
Materials and methods
Study design
Data from CU older adults from two independent protocols were selected. The analyses were first conducted in the primary cohort Age-Well (monocentric study) and replicated in a larger cohort with the Alzheimer’s disease Neuroimaging Initiative data (ADNI; multicentric study).
Participants
Age-Well cohort
One hundred thirty-five CU older adults were included from the baseline visit of the Age-Well randomized controlled trial of the Medit-Ageing European project [27], sponsored by the French National Institute of Health and Medical Research (INSERM). Participants were recruited from the general population with the main following eligibility criteria: native French speaker, aged at least 65 years, retired for at least 1 year, educated for at least 7 years and showing performance within the normal range for age and educational level on standardized cognitive tests (see Tables 1 and 2 in [27] for details). Participants had no evidence of a major neurological or psychiatric disorder, chronic disease or acute unstable illness, no history of cerebrovascular disease, and no current or recent medication that may interfere with cognitive functioning (including antidepressants and anxiolytics). Notably, the absence of major depression was assessed using a clinician-administered questionnaire, the Montgomery-Åsberg Depression Rating Scale (MADRS) [28], with a cut-off value of 6 (participants with MADRS > 6 were excluded). All participants gave their written informed consent prior to the examinations, and the Age-Well randomized clinical trial was approved by the ethics committee (Comité de Protection des Personnes Nord-Ouest III, Caen, France; trial registration number: EudraCT: 2016-002441-36; IDRCB: 2016-A01767-44; ClinicalTrials.gov Identifier: NCT02977819).
ADNI cohort
Two hundred fifty-two CU older adults from ADNI were included in our study as a replication cohort. The main exclusion criteria included the presence of psychiatric illness (major depression or bipolar disorder) or neurological disease (see [29] for details). The absence of major depression was assessed using the self-reported Geriatric Depression Scale (GDS) [30], and the investigators indicated that “although differing sensitivities and specificities have been obtained across studies, a score >5 was considered suggestive of depression and warranted a follow-up interview” (in our study, only one ADNI participant was concerned with a GDS score = 6) and “scores >10 are almost always depression”. Details regarding the cohort recruitment and all data collection methods is available online (http://adni.loni.usc.edu/). Only participants with an available score of depressive symptoms and whose multimodal neuroimaging scans were acquired no more than 3 months after the assessment of depressive symptoms were selected. The ADNI study was approved by the institutional review boards of all of the participating institutions. Informed written consent was obtained from all participants at each site.
Assessment of subclinical depressive symptoms and classification of participants
In this study we were specifically interested in the presence of subclinical depressive symptoms, which was assessed using the 15-item version of the GDS. This self-reported questionnaire ranges from 0 to 15, and a higher score reflects the presence of more depressive symptoms [30, 31]. Given that all participants were screened for the lack of depression, GDS scores were rather low showing a floor-effect and a non-linear distribution. Therefore, instead of the severity of the symptoms (which lacked variability) we were primarily interested in comparing participants with or without subclinical depressive symptoms. Thus, based on the GDS, participants were classified as having subclinical depressive symptoms (DepS; GDS > 0) or not (NoDepS; GDS = 0). This threshold was selected as, while there is no consensus to date in the current literature to define “subclinical” depressive symptoms [32], previous studies have shown that each additional depressive symptom (including 1-point increase in 15-item GDS) significantly increases the risk of AD in CU older adults [5, 33]. In Age-Well, the DepS and NoDepS groups included 77 and 58 participants respectively. In ADNI, the DepS and NoDepS groups included 134 and 118 participants respectively. Additional analyses were also performed with the severity (number) of subclinical depressive symptoms, for the sake of completeness.
Assessment of cognitive performance
Global cognition was measured using the Mini-Mental State Examination (MMSE [34], scores from 0 to 30) within each cohort, as well as using the Mattis Dementia Rating Scale (DRS [35], scores from 0 to 144) in Age-Well, and the Montreal Cognitive Assessment (MoCA [36], scores from 0 to 30) in ADNI. Verbal episodic memory was assessed using the immediate free recall subscore of the California Verbal Learning Test (sum of scores from the five trials of the 16-word list) (CVLT [37], scores from 0 to 80) in Age-Well and the Immediate subscore of the Rey Auditory Verbal Learning Test (sum of scores from the five trials of the 15-word list) (RAVLT [38], scores from 0 to 75) in ADNI. The MoCA and RAVLT scores were not available for 5 and 3 participants, respectively.
Neuroimaging procedure
Age-Well cohort
All participants were scanned on the same MRI scanner (Philips Achieva; 3.0 T) and PET camera (Discovery RX VCT 64 PET-CT; General Electric Healthcare) at the Cyceron Center (Caen, France). High-resolution T1-weighted structural imaging were acquired to measure GM volume and an echo-planar imaging/spin echo diffusion weighted sequence (DKI) was performed to obtain white matter (WM) microstructural integrity measurements. Mean kurtosis parameter maps reflected WM microstructural integrity based on the number, density, orientation, and degree of organization of WM microstructures [39]. Myelin and axonal integrity was also estimated from the radial and axial parameters of DKI, respectively [40]. Fluorine 18-labeled (18F) florbetapir-PET scans were obtained with a 10-min acquisition beginning 50 min after the intravenous injection reflecting amyloid burden. 18F-fluorodeoxyglucose (FDG)-PET scans were acquired on a subset of participants (n = 92) to measure brain glucose metabolism. The detailed acquisition and preprocessing procedures [27] are available in Supplementary Material 1. The sample size for each imaging modality is reported in Supplementary Table 1.
ADNI cohort
Acquisition processes of structural MRI, FDG- and florbetapir-PET imaging are described at http://adni.loni.usc.edu/data-samples/data-types/. DKI images were not available. The same preprocessing procedures as in Age-Well were applied on ADNI data, except that the segmentation of the MRI scans was only based on the T1-weighted images in ADNI (while both the T1 and FLAIR were used in Age-Well).
Statistical analyses
Between-group comparisons
Between-group differences for demographic, cognitive, and psychoaffective variables were assessed both within and across cohorts, using Student’s t tests for continuous variables and χ2 tests for categorical variables with statistical significance set to p < 0.05.
Voxel-wise group differences in GM volume, WM integrity, glucose metabolism and amyloid burden were explored using analyses of covariance (ANCOVA) in SPM12. In both cohorts, all voxel-wise analyses were adjusted for age, sex and education, as well as self-reported anxiety symptoms when available (only for Age-Well). Results were evaluated for significance at puncorrected < 0.005 combined with a minimum cluster size determined by Monte–Carlo simulations using the AFNI’s 3dClustSim program to achieve a corrected statistical significance of p < 0.05.
Results
Participants’ characteristics
Demographic data, cognitive performance and psychoaffective symptoms for each group in both cohorts, as well as between-group differences, are reported in Table 1.
Table 1.
Participants’ characteristics and between-group comparisons within and between cohorts.
| Age-Well—Primary cohort (N = 135) | NoDepS group | DepS group | Between-group comparisons | ||
|---|---|---|---|---|---|
| p value | t or χ² value | Mean difference [95% CI] | |||
| N (%) | 58 (43) | 77 (57) | |||
| Demographic data | |||||
| Gender: Female N (%) | 28 (48.27) | 55 (71.43) | 0.01 | 6.54 | |
| Age, years (range) | 69.41 ± 3.91 (64–83) | 68.45 ± 3.63 (65–79) | 0.14 | −1.47 | −0.96 [−2.25–0.33] |
| Education, years (range) | 13.12 ± 3.17 (7–22) | 13.18 ± 3.04 (7–20) | 0.91 | 0.11 | 0.06 [−1.00–1.13] |
| Florbetapir SUVR (range) | 0.96 ± 0.21 (0.73–1.76) | 0.98 ± 0.20 (0.72–1.73) | 0.47 | 0.72 | 0.03 [−0.05–0.10] |
| Amyloid positive N (%) | 10 (17.24) | 20 (26.31) | 0.30 | 1.08 | |
| APOEε4 carriers N (%) | 15 (25.86) | 21 (27.27) | 1.00 | <0.001 | |
| Cognition measures | |||||
| MMSE (range) | 28.93 ± 0.95 (26–30) | 29.11 ± 1.09 (26–30) | 0.34 | 0.97 | 0.17 [−0.18–0.53] |
| DRS (range) | 140.90 ± 2.79 (130–144) | 141.03 ± 2.56 (133–144) | 0.78 | 0.28 | 0.13 [−0.78–1.04] |
| CVLT Immediate (range) | 57.36 ± 8.61 (30–71) | 57.69 ± 7.70 (37–73) | 0.82 | 0.23 | 0.33 [−2.46–3.11] |
| Psychoaffective variables | |||||
| GDS (range) | 0.00 ± 0.00 (0–0) | 2.25 ± 1.77 (1–11) | <0.001 | 9.65 | 2.25 [1.79–2.71] |
| STAI-B (range) | 31.60 ± 6.18 (20–51) | 36.82 ± 6.77 (24–54) | <0.001 | 4.59 | 5.21 [2.97–7.46] |
| ADNI—Replication cohort (N = 252) | NoDepS group | DepS group | Between-group comparisons | ||
|---|---|---|---|---|---|
| p value | t or χ² value | Mean difference [95% CI] | |||
| N (%) | 118 (47) | 134 (53) | |||
| Demographic data | |||||
| Gender: Female N (%) | 65 (55.08) | 78 (58.20) | 0.71 | 0.14 | |
| Age, years (range) | 73.35 ± 5.69a (63–85) | 73.66 ± 6.32a (59–95) | 0.68 | 0.41 | 0.31 [−1.19–1.81] |
| Education, years (range) | 16.50 ± 2.59a (8–20) | 16.71 ± 2.35a (12–20) | 0.50 | 0.67 | 0.21 [−0.40–0.82] |
| Florbetapir SUVR (range) | 1.21 ± 0.37a (0.76–2.55) | 1.13 ± 0.36a (0.74–3.24) | 0.11 | −1.60 | −0.07 [−0.17–0.02] |
| Amyloid positive N (%) | 69 (58.47)a | 63 (47.01)a | 0.09 | 2.86 | |
| APOEε4 carriers N (%) | 39 (33.05) | 33 (24.63) | 0.11 | 2.58 | |
| Cognition measures | |||||
| MMSE (range) | 28.99 ± 1.16 (25–30) | 28.96 ± 1.26 (24–30) | 0.81 | −0.24 | −0.04 [−0.34–0.27] |
| MoCA (range) | 25.67 ± 2.25 (20–30) | 25.63 ± 2.47 (19–30) | 0.89 | −0.14 | −0.04 [−0.64–0.56] |
| RAVLT Immediate (range) | 46.08 ± 10.16 (20–70) | 45.56 ± 10.21 (23–69) | 0.71 | 0.37 | 0.48 [−2.07–3.03] |
| Psychoaffective variables | |||||
| GDS (range) | 0.00 ± 0.00 (0–0) | 1.78 ± 1.11a (1–6) | <0.001 | 17.41 | 1.78 [1.58–1.98] |
| STAI-B (range) | NC | NC | |||
Data are presented as mean ± standard deviation of participants unless otherwise indicated.
Between-group differences in each cohort were assessed using Student’s t tests for continuous variables and χ2 tests for categorical variables. Statistical significance was set to p < 0.05 for all analyses.
N sample size, NoDepS group without depressive symptoms, DepS group with subclinical depressive symptoms, SUVR standard uptake value ratio, MMSE Mini-Mental State Examination, DRS Mattis Dementia Rating Scale, CVLT California Verbal Learning Test, GDS Geriatric Depression Scale, STAI-B State-Trait Anxiety Inventory form Y-B, MoCA Montreal Cognitive Assessment, RAVLT Rey Auditory Verbal Learning Test, NC not collected.
aSignificant between-group differences between cohorts (see details in Supplementary Table 2).
Bold values indicate statistical significance p < 0.05.
In Age-Well, participants with depressive symptoms had a higher proportion of women and a higher score of anxiety symptoms than those without symptoms. The two groups did not differ in any other demographic or cognitive variables. There was no difference between participants with versus without depressive symptoms in ADNI.
When comparing groups between cohorts, the proportion of participants classified as having subclinical depressive symptoms was similar between cohorts (χ2 = 0.39 p = 0.54). However, Age-Well participants were younger, less educated, and had lower florbetapir SUVR and proportion of amyloid-positive individuals than ADNI participants. Moreover, those with depressive symptoms in Age-Well had a higher GDS score.
Brain changes associated with the presence of subclinical depressive symptoms
Gray matter volume
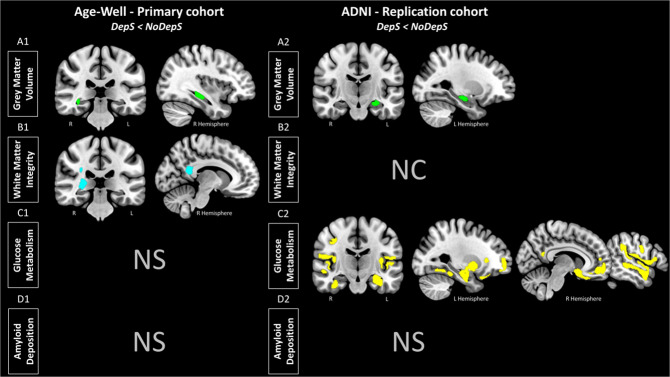
Participants with subclinical depressive symptoms showed lower GM volume compared with participants without symptoms in the right hippocampus in Age-Well (Fig. 1A1), and in the left hippocampus in ADNI (Fig. 1A2). Peak statistics and coordinates of significant clusters are detailed in Supplementary Table 3. In both cohorts, the volume of the contralateral hippocampus was also found when using a more permissive threshold (i.e., at puncorrected < 0.005 in Age-Well and at puncorrected < 0.01 in ADNI).
Fig. 1. Brain substrates of subclinical depressive symptoms in cognitively unimpaired older adults.
Results of the voxel-wise between-group differences in gray matter volume (A, green), white matter integrity (mean kurtosis) (B, blue), glucose metabolism (C, yellow) and amyloid deposition (D) in Age-Well (left panel) and ADNI (right panel). Analyses were adjusted for age, sex and education. Anxiety symptoms were added as a covariate in Age-Well. All results are presented at a puncorrected < 0.005 threshold combined with a cluster-level multiple comparisons correction. DepS group with subclinical depressive symptoms, NoDepS group without depressive symptoms, NS not significant, NC not collected, R right, L left.
White matter integrity
In Age-Well, participants with subclinical depressive symptoms showed lower WM microstructural integrity (mean kurtosis) than participants without depressive symptoms mainly in the posterior cingulum and corpus callosum (splenium part), fornix and inferior longitudinal fasciculus (Fig. 1B1). They also showed lower myelin integrity (radial kurtosis) in the posterior cingulum and corpus callosum (splenium part) (Supplementary Fig. 1). No significant differences were observed regarding the axonal integrity of the WM (axonal kurtosis).
Glucose metabolism
While in Age-Well, no significant difference in glucose metabolism was observed between the two groups, in ADNI, participants with subclinical depressive symptoms showed lower glucose metabolism mainly in the medial temporal lobe, temporal cortex, and medial and dorsolateral prefrontal, anterior cingulate, temporoparietal, precuneus/posterior cingulate cortices, and insula (Fig. 1C2).
Amyloid deposition
No difference in brain amyloid deposition was observed between participants with depressive symptoms versus those without symptoms in either Age-Well or ADNI.
Additional analyses
Neuroimaging measures
Neuroimaging analyses were replicated without partial volume effects (PVE) correction for PET images. Results were similar with differences in cluster sizes for glucose metabolism analyses in ADNI, as illustrated in Supplementary Fig 2. Neuroimaging results were also similar when adding the MMSE as a covariate (data not shown).
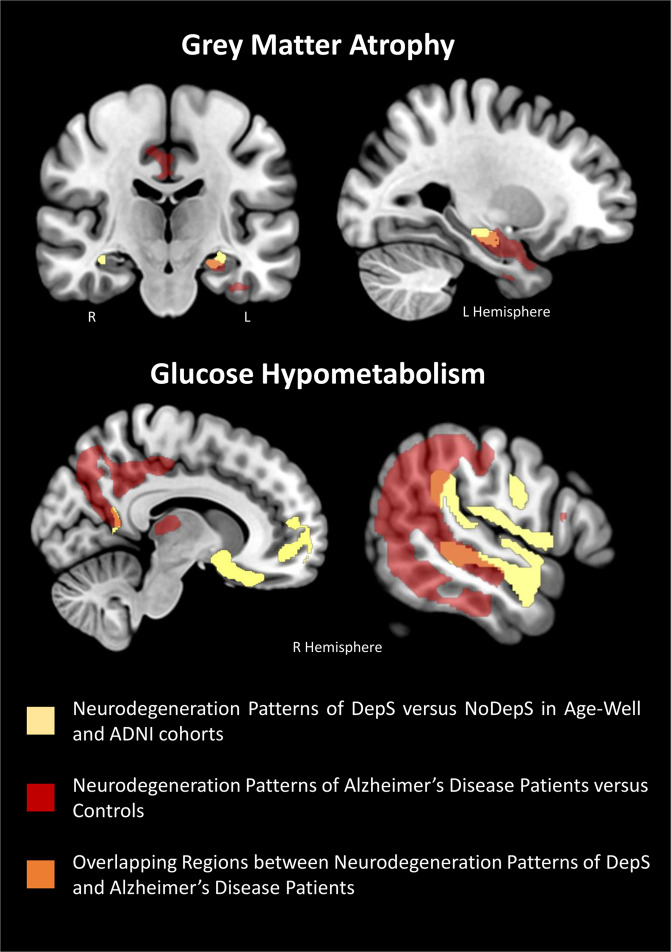
To highlight the overlap between the brain substrates of subclinical depressive symptoms from both cohorts and the pattern of neurodegeneration typically found in patients with AD, we superimposed our findings over patterns of GM atrophy and hypometabolism from a group of 56 cognitively impaired amyloid-positive patients on the Alzheimer’s continuum compared to 28 controls from the independent Imagerie Multimodale de la maladie d’Alzheimer à un stade Précoce (IMAP+) cohort ([41]; see the Supplementary Material 2 for details). The superimposition of both patterns overlapped notably in the hippocampus, precuneus, posterior cingulate—retrosplenial area and temporoparietal region, as illustrated in Fig. 2.
Fig. 2. Illustration of the overlap between the brain substrates of subclinical depressive symptoms and the pattern of neurodegeneration in Alzheimer’s disease (AD).
The patterns of gray matter atrophy (at the top) and hypometabolism (at the bottom) in participants with subclinical depressive symptoms compared to those without are represented in yellow. They are overlapped on the respective patterns of gray matter atrophy (at the top) and hypometabolism (at the bottom) typically found in patients with AD and obtained here by comparing a group of 56 cognitively impaired amyloid-positive patients on the Alzheimer’s continuum to 28 controls from the IMAP + cohort ([41]; see Supplementary Material 2 for details). The superimposition allows areas of overlap to be highlighted (in orange), notably in the hippocampus, precuneus, posterior cingulate—retrosplenial area and temporoparietal region. DepS group with subclinical depressive symptoms, NoDepS group without depressive symptoms, R right, L left.
We then aimed to further assess whether the severity of subclinical depressive symptoms was correlated with the brain changes found in the main neuroimaging analyses. We extracted the mean value within the clusters previously highlighted in the voxel-wise between-group comparison analyses (i.e., GM volume and WM integrity in Age-Well, as well as GM volume and glucose metabolism in ADNI) on the corresponding non-smoothed MRI and PET images for each participant. Given that all participants were screened for the lack of depression, GDS scores were rather low showing a floor-effect and a non-linear distribution. Therefore, we performed non-parametric analyses using Spearman’s partial correlations to assess the associations between the severity of subclinical depressive symptoms and the extracted neuroimaging measures within the group of participants with subclinical depressive symptoms, as well as within the entire sample, for both cohorts. Analyses were corrected for age, sex and education, as well as anxiety symptoms (only for Age-Well).
We found that higher subclinical depressive symptoms were associated with lower GM volume (rho = −0.211 p = 0.016 in Age-Well and rho = −0.251 p < 0.001 in ADNI), WM integrity (rho = −0.232 p = 0.008 for mean kurtosis and rho = −0.236 p = 0.007 for radial kurtosis) and glucose metabolism (rho = −0.277 p < 0.001) in the clusters of interest within the entire samples, for both cohorts (Supplementary Table 4). These associations were not significant within the group of participants with subclinical depressive symptoms in either Age-Well or ADNI.
Psychoaffective measures
We sought to better characterize the psychoaffective difficulties experienced by participants with subclinical depressive symptoms compared to those without depressive symptoms in Age-Well (assessments not available in ADNI). For this purpose, we investigated between-group differences in self-report of positive and negative affect (i.e., positive and negative emotions or feelings) based on the Positive and Negative Affect Schedule (PANAS [42]), ruminative brooding (i.e., repetitive passive and judgmental thoughts about one’s mood) based on the Rumination Response Scale (RRS [43], defusion (i.e., ability to achieve psychological distance from one’s thoughts and feelings) based on the Drexel Defusion Scale (DDS [44]) and emotion regulation abilities (i.e., ability to regulate one’s emotions through cognitive reappraisal and/or expressive suppression strategies) based on the Emotion Regulation Questionnaire (ERQ [45]) (see Supplementary Material 3 for details). We used ANCOVAs adjusted for age, sex and education. Participants with subclinical depressive symptoms showed higher negative affect (F = 5.82 p = 0.017) and ruminative brooding (F = 4.32 p = 0.040), as well as lower psychological defusion (F = 9.33 p = 0.003) than those without symptoms (Supplementary Table 5). No significant differences were observed regarding positive affect and emotion regulation abilities.
Discussion
The aim of this study was to provide a comprehensive overview of the brain substrates of subclinical depressive symptoms in CU older adults using complementary multimodal neuroimaging data in two independent cohorts. We showed that participants with subclinical depressive symptoms from both cohorts presented lower GM volume in the hippocampus. In ADNI, glucose hypometabolism was also found in the hippocampus and extended to the amygdala, precuneus/posterior cingulate cortex, medial and dorsolateral prefrontal cortex, insula, and temporoparietal cortex. In Age-Well, we also found lower WM integrity mainly in the fornix, posterior cingulum and corpus callosum and inferior longitudinal fasciculus. Furthermore, the presence of subclinical depressive symptoms was not associated with brain amyloid deposition in either cohort.
Subclinical depressive symptoms are consistently associated with neurodegeneration biomarkers in the hippocampus
The association between subclinical depressive symptoms and lower GM volume in the hippocampus in CU older adults is in line with previous studies using both group comparisons or correlation analyses—cross-sectionally and longitudinally [10, 12–14, 16, 17, 46–49]. Interestingly, lower glucose metabolism colocalized with lower GM volume in the hippocampus in ADNI, which corroborates a previous FDG-PET study using a ROI-based approach [12]. This finding was not recovered in Age-Well but this might reflect a lack of power related to the smaller sample size (n = 92, against n = 252 in ADNI). Our findings thus suggest that the hippocampus is particularly sensitive to subclinical depressive symptoms, as it is to AD, being the main target of structural and functional neurodegeneration in both conditions [50, 51]. Thus, we assume that these common alterations in the hippocampus could partly underlie the link between subclinical depressive symptoms, increased risk of clinical depressive disorder, and AD. Potential mechanisms mediating the association between subclinical depressive symptoms and hippocampal neurodegeneration could involve cortisol neurotoxicity, neuroinflammation and/or preclinical AD tau aggregates. In late-life depression, cortisol-mediated hippocampal neurotoxicity has been proposed as a main etiological mechanism [52], with the hippocampus being particularly vulnerable to high levels of cortisol, resulting in neuronal death and/or suppressed neurogenesis [52, 53]. Neuroinflammatory processes may also be involved, as increased inflammatory cytokine levels was reported in older adults with subclinical or clinical depressive disorders, and elevated cytokines levels may impair hippocampal neurogenesis—leading to GM volume loss [54]. Another mechanism could involve tau aggregates in the hippocampus, as increased tau accumulation has been associated with depressive disorders, and the hippocampus is one of the first regions to show tau pathology in preclinical AD [51, 55, 56].
Brain substrates of subclinical depressive symptoms extend beyond the hippocampus to a frontolimbic network
In ADNI, the pattern of glucose metabolism associated with subclinical depressive symptoms extended beyond the hippocampus to the precuneus/posterior cingulate, temporoparietal and medial prefrontal cortex—known to be vulnerable to metabolic changes in AD [51]. Our results are consistent with the few PET studies in the field reporting lower glucose metabolism or perfusion associated with depressive symptoms in the precuneus, posterior cingulate and fronto-temporal regions [18–20]. Here, the presence of subclinical depressive symptoms was also associated with lower WM integrity mainly in the fornix, posterior cingulum and corpus callosum, and inferior longitudinal fasciculus. These results are in line with previous studies in CU older adults showing an association between subclinical depressive symptoms and reduced WM integrity in the corpus callosum and inferior longitudinal fasciculus [57–59] as well as in frontal regions and in the global WM [9, 11, 60]. Similar links were reported in late-life depression, especially in the cingulum, corpus callosum, but also in the uncinate fasciculus and frontal lobe [61]. Furthermore, the results we obtained with the radial and axonal parameters of DKI suggest that these WM alterations rather reflect demyelination processes than axonal degeneration. This demyelination may result from neuroinflammation; as mentioned above, depressive disorders have been related to high level of inflammatory markers which may alter myelin sheaths [54, 62]. Overall, our findings highlight that the presence of subclinical depressive symptoms is associated with lower integrity of WM microstructure in regions similar to those found to be associated with clinical depressive disorder in ageing.
Interestingly, the fornix and the posterior cingulum and corpus callosum are also among the WM tracts the most altered in early AD stages [63, 64]. Moreover these tracts are structurally connected to the brain regions found to be associated with subclinical depressive symptoms in our study—including specific AD biomarkers such as hippocampal atrophy and posterior cingulate hypometabolism [65, 66]. Previous work highlighted that cingulum alteration was related to hippocampal atrophy and posterior cingulate hypometabolism in MCI patients [67]. Thus, it is possible that the functional alterations notably observed with FDG-PET in older adults with depressive symptoms are related to disconnection from the atrophied hippocampus associated with the disruption of the connecting WM tracts, as proposed in AD [67].
Subclinical depressive symptoms are associated with neurodegeneration in large brain networks involved in emotion regulation, self-referential processes and memory
Most of the structures associated with subclinical depressive symptoms, namely the hippocampus, amygdala, cingulum, fornix, insula, medial and dorsolateral prefrontal cortex, are key components of the limbic/paralimbic and frontal brain networks—referred to as the frontolimbic network here [68, 69]. This network is mainly involved in emotional and mood processes, including identification of emotional stimuli, generation and/or regulation of the affective state and emotional behavior, as well as memory processes [69]. In line with these findings, participants with subclinical depressive symptoms showed higher negative affect (i.e., negative emotions and feelings) than those without depressive symptoms in Age-Well. Moreover, in late-life depression the frontolimbic network is also particularly impaired and its dysfunction may contribute to the severity of the symptoms [70, 71]. Interestingly, when considering depressive symptoms as a continuous variable, we also found that subclinical depressive symptom severity was associated with levels of brain alterations reported above in the entire samples, for both cohorts. The brain substrates of subclinical depressive symptoms also involve regions of the default mode network (DMN), including the precuneus/cingulate posterior, medial prefrontal, temporoparietal and temporal cortex and the hippocampus. This network is involved in self-referential processes and memory. Changes in the activity and functional connectivity of this network have been observed in late-life depression, and are thought to be related to a dysregulation of mental content in favor of negative thoughts and rumination [72–75]. Our findings complement this literature by showing that participants with subclinical depressive symptoms also exhibited mental regulation difficulties, with higher ruminative brooding (i.e., repetitive passive and judgmental thoughts about one’s mood) and lower defusion (i.e., lower ability to achieve psychological distance from one’s thoughts and feelings) in Age-Well. Furthermore, the DMN includes the regions the most sensitive to AD, as neurodegenerative changes and amyloid deposition are mainly located in this network [76–78]. The insula, dorsolateral prefrontal cortex and precuneus are also part of the salience and cognitive control networks [79, 80]. The salience network is involved in assessing the relevance of stimuli and events, and its dysfunction in the case of depressive disorders—associated with aberrant switching between the DMN and the cognitive control network—may contribute to patients’ difficulties in disengaging self-focus processes involving negatively biased thoughts [79, 81]. The cognitive control network is more involved in high-level cognitive processes, including executive functions such as attention, planning or working memory to achieve a specific goal; functional disruption in this network is thought to reflect decreased cognitive control of attention and emotion regulation [80]. Thus, the brain substrates related to subclinical depressive symptoms overlap in several interconnected brain networks; these alterations may contribute to subsequent network brain dysfunctions as described in clinical depressive disorder and AD, which partly underly the core symptoms of these diseases.
Subclinical depressive symptoms and AD neuroimaging biomarkers
Although the brain substrates of subclinical depressive symptoms partly overlap with AD neurodegeneration biomarkers, they were not associated with brain amyloid deposition in either cohort. This is consistent with most [22–25], but not all [21] previous cross-sectional studies. Longitudinal studies led to more consistent findings, showing that higher baseline level of depressive symptoms was related to increased amyloid deposition over time. The opposite direction was also observed with higher baseline amyloid deposition associated with higher level of depressive symptoms over time [22–24]. Thus, the links between subclinical depressive symptoms and AD may not initially involve amyloid deposition but rather neurodegeneration in partially common brain regions, suggesting that subclinical depressive symptoms may be a risk factor for AD rather than a prodromal manifestation of the disease.
Strengths and limitations
The main strength of our study was to provide an overview of the brain substrates of subclinical depressive symptoms in CU older adults using complementary multimodal neuroimaging data (i.e., GM volume, WM integrity, glucose metabolism, and amyloid deposition). Furthermore, the results were partly replicated in two independent cohorts with large sample sizes (i.e., Age-Well and ADNI)—using the same scale to assess depressive symptoms (i.e., GDS).
However, the cross-sectional design of our study is a limitation as it prevents us from assessing the links between baseline levels and changes over time in depressive symptoms and neuroimaging biomarkers. Moreover, the threshold we used to define “subclinical” depressive symptoms was not validated; further studies might allow researchers to select the specific threshold for “clinically relevant” subclinical symptoms. In addition, these symptoms were assessed using a self-report questionnaire; as it is subjective, the measure could be biased by the subject’s honesty, awareness and introspective ability. Further investigations are also needed to clarify the involvement of tau pathology, and of physiological mechanisms related to stress and/or inflammation (e.g., cortisol, cytokines), in the relationship between subclinical depressive symptoms and their brain substrates (especially the hippocampus). In addition, assessing the brain substrates of the subdimensions of depressive symptoms would help specify which of these symptoms are most relevant for future targeted intervention; repetitive negative thinking for instance has been proposed as a risk factor for AD and has been associated with structural alterations in some brain regions identified in our study [82, 83]. In this context, mental training through meditation practice—targeting emotional and attentional regulation and stress reduction—could be a promising way to alleviate depressive symptoms and their adverse factors in ageing. Post-intervention data in Age-Well will be used to address this question [27].
Conclusion
In conclusion, the presence of subclinical depressive symptoms in CU older adults was associated with brain structural and functional changes in regions mainly belonging to the frontolimbic network, some of which are particularly sensitive to AD. Notably, neurodegeneration markers overlapped in the hippocampus, which alteration may underlie the links between subclinical depressive symptoms in ageing and the risk of clinical depressive disorder and AD dementia. The lack of positive association with amyloid deposition indicates that this link may not involve amyloid deposition, but rather neurodegeneration in partially common brain regions, suggesting that, at this subclinical stage, depressive symptoms is a risk factor for AD rather than a prodromal manifestation of the disease.
Supplementary information
Acknowledgements
A. Cognet, C. Gaubert, T. Jorand, M. Botton, MSc, A. Joret Philippe, MSc, S. Egret, MSc, P. Lacheray, MSc, J. Lebahar, PhD, C. Tomadesso, PhD, H. Espérou, MD, PhD, E. Frison, MD, PhD, and the Cyceron MRI-PET staff members for their help with recruitment and data acquisition or administrative support. We also acknowledge the Euclid team, the sponsor (INSERM) and all the participants of the study for their contribution.
Author contributions
GC had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: ET, OK, GP, NM, and GC. Acquisition, analysis, or interpretation of data: ET, IM, EK, SS, VO, BL, FM, DV, OK, GP, NLM, and GC. Drafting of the manuscript: ET and GC. Critical revision of the manuscript for important intellectual content: ET, IM, EK, SS, VO, BL, FM, DV, OK, GP, NM, and GC. Statistical analysis: ET. Obtained funding: ET, OK, GP, NM, and GC. Administrative, technical, or material support: BL, FM, SS, OK, GP, and DV. Supervision: GC. Other—principal investigators: GC, Vincent De La Sayette (MD, principal investigator).
Funding
The Age-Well randomized clinical trial is part of the Medit-Ageing project and is funded through the European Union’s Horizon 2020 Research and Innovation Program (grant 667696), Institut National de la Santé et de la Recherche Médicale, Région Normandie, and Fondation MMA des Entrepreneurs du Futur. Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California. The funders and sponsor had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Competing interests
OK, GP, NM and GC reported grants from European Union’s Horizon 2020 Research and Innovation Program under grant agreement No. 667696 during the conduct of the study. NM reported grants from a Senior Fellowship from the Alzheimer’s Society (AS-SF-15b-002). GC reported grants, personal fees and nonfinancial support from Institut National de la Santé et de la Recherche Médicale (INSERM); personal fees from Fondation Entrepreneurs MMA, grants and personal fees from Fondation Alzheimer, grants from Région Normandie, grants from Fondation Recherche Alzheimer, grants from Association France Alzheimer, outside the submitted work. No other disclosures were reported.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Part of the data used in preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found at: http://adni.loni.usc.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf.
A list of authors and their affiliations appears at the end of the paper.
Contributor Information
Gaël Chételat, Email: chetelat@cyceron.fr.
the Medit-Ageing Research Group:
Eider M. Arenaza-Urquijo, Florence Allais, Claire André, Julien Asselineau, Sebastian Baez Lugo, Martine Batchelor, Axel Beaugonin, Alexandre Bejanin, Pierre Champetier, Anne Chocat, Fabienne Collette, Sophie Dautricourt, Eglantine Ferrand-Devouge, Robin De Flores, Vincent De La Sayette, Pascal Delamillieure, Marion Delarue, Yacila I. Deza-Araujo, Hélène Esperou, Francesca Felisatti, Eric Frison, Francis Gheysen, Julie Gonneaud, Marc Heidmann, Thien Huong Tran, Frank Jessen, Pierre Krolak-Salmon, Gwendoline Le Du, Valérie Lefranc, Antoine Lutz, Jose-Luis Molinuevo, Cassandre Palix, Léo Paly, Géraldine Rauchs, Stéphane Réhel, Florence Requier, Eric Salmon, Raquel Sanchez, Corinne Schimmer, Matthieu Vanhoutte, Patrik Vuilleumier, Caitlin Ware, and Miranka Wirth
Supplementary information
The online version contains supplementary material available at 10.1038/s41380-022-01772-8.
References
- 1.Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396:413–46. doi: 10.1016/S0140-6736(20)30367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011;10:819–28. doi: 10.1016/S1474-4422(11)70072-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lyness JM, Kim J, Tang W, Tu X, Conwell Y, King DA, et al. The clinical significance of subsyndromal depression in older primary care patients. Am J Geriatr Psychiatry. 2007;15:214–23. doi: 10.1097/01.JGP.0000235763.50230.83. [DOI] [PubMed] [Google Scholar]
- 4.Meeks TW, Vahia IV, Lavretsky H, Kulkarni G, Jeste DV. A tune in “a minor” can “b major”: a review of epidemiology, illness course, and public health implications of subthreshold depression in older adults. J Affect Disord. 2011;129:126–42. doi: 10.1016/j.jad.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilson RS, Barnes LL, Leon CFM, de, Aggarwal NT, Schneider JS, Bach J, et al. Depressive symptoms, cognitive decline, and risk of AD in older persons. Neurology. 2002;59:364–70. doi: 10.1212/wnl.59.3.364. [DOI] [PubMed] [Google Scholar]
- 6.Barnes DE, Alexopoulos GS, Lopez OL, Williamson JD, Yaffe K. Depressive symptoms, vascular disease, and mild cognitive impairment: findings from the Cardiovascular Health Study. Arch Gen Psychiatry. 2006;63:273–279. doi: 10.1001/archpsyc.63.3.273. [DOI] [PubMed] [Google Scholar]
- 7.Rosenberg PB, Mielke MM, Appleby BS, Oh ES, Geda YE, Lyketsos CG. The association of neuropsychiatric symptoms in MCI with incident dementia and Alzheimer disease. Am J Geriatr Psychiatry. 2013;21:685–95. doi: 10.1016/j.jagp.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lanctôt KL, Amatniek J, Ancoli-Israel S, Arnold SE, Ballard C, Cohen-Mansfield J, et al. Neuropsychiatric signs and symptoms of Alzheimer’s disease: new treatment paradigms. Alzheimers Dement. 2017;3:440–9. doi: 10.1016/j.trci.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dotson VM, Davatzikos C, Kraut MA, Resnick SM. Depressive symptoms and brain volumes in older adults: a longitudinal magnetic resonance imaging study. J Psychiatry Neurosci. 2009;34:367–75. [PMC free article] [PubMed] [Google Scholar]
- 10.Brown ES, Hughes CW, McColl R, Peshock R, King KS, Rush AJ. Association of depressive symptoms with hippocampal volume in 1936 adults. Neuropsychopharmacology. 2014;39:770–9. doi: 10.1038/npp.2013.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tudorascu DL, Rosano C, Venkatraman VK, MacCloud RL, Harris T, Yaffe K, et al. Multimodal MRI markers support a model of small vessel ischemia for depressive symptoms in very old adults. Psychiatry Res. 2014;224:73–80. doi: 10.1016/j.pscychresns.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donovan NJ, Hsu DC, Dagley AS, Schultz AP, Amariglio RE, Mormino EC, et al. Depressive symptoms and biomarkers of Alzheimer’s disease in cognitively normal older adults. J Alzheimers Dis. 2015;46:63–73. doi: 10.3233/JAD-142940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elbejjani M, Fuhrer R, Abrahamowicz M, Mazoyer B, Crivello F, Tzourio C, et al. Depression, depressive symptoms, and rate of hippocampal atrophy in a longitudinal cohort of older men and women. Psychol Med. 2015;45:1931–44. doi: 10.1017/S0033291714003055. [DOI] [PubMed] [Google Scholar]
- 14.Zhou H, Li R, Ma Z, Rossi S, Zhu X, Li J. Smaller gray matter volume of hippocampus/parahippocampus in elderly people with subthreshold depression: a cross-sectional study. BMC Psychiatry. 2016;16:219. doi: 10.1186/s12888-016-0928-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pink A, Przybelski SA, Krell-Roesch J, Stokin GB, Roberts RO, Mielke MM, et al. Cortical thickness and depressive symptoms in cognitively normal individuals: the Mayo Clinic Study of Aging. J Alzheimers Dis. 2017;58:1273–81. doi: 10.3233/JAD-170041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Shea DM, Dotson VM, Woods AJ, Porges EC, Williamson JB, O’Shea A, et al. Depressive symptom dimensions and their association with hippocampal and entorhinal cortex volumes in community dwelling older adults. Front Aging Neurosci. 2018;10:40. [DOI] [PMC free article] [PubMed]
- 17.Szymkowicz SM, Woods AJ, Dotson VM, Porges EC, Nissim NR, O’Shea A, et al. Associations between subclinical depressive symptoms and reduced brain volume in middle-aged to older adults. Aging Ment Health. 2019;23:819–30. doi: 10.1080/13607863.2018.1432030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dotson VM, Beason-Held L, Kraut MA, Resnick SM. Longitudinal study of chronic depressive symptoms and regional cerebral blood flow in older men and women. Int J Geriatr Psychiatry. 2009;24:809–19. doi: 10.1002/gps.2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brendel M, Reinisch V, Kalinowski E, Levin J, Delker A, Därr S, et al. Hypometabolism in brain of cognitively normal patients with depressive symptoms is accompanied by atrophy-related partial volume effects. Curr Alzheimer Res. 2016;13:475–86. doi: 10.2174/1567205013666160314143922. [DOI] [PubMed] [Google Scholar]
- 20.Krell-Roesch J, Ruider H, Lowe VJ, Stokin GB, Pink A, Roberts RO, et al. FDG-PET and neuropsychiatric symptoms among cognitively normal elderly persons: the Mayo Clinic Study of Aging. J Alzheimer’s Dis. 2016;53:1609–16. doi: 10.3233/JAD-160326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yasuno F, Kazui H, Morita N, Kajimoto K, Ihara M, Taguchi A, et al. High amyloid-β deposition related to depressive symptoms in older individuals with normal cognition: a pilot study. Int J Geriatr Psychiatry. 2016;31:920–8. doi: 10.1002/gps.4409. [DOI] [PubMed] [Google Scholar]
- 22.Donovan NJ, Locascio JJ, Marshall GA, Gatchel J, Hanseeuw BJ, Rentz DM, et al. Longitudinal association of amyloid beta and anxious-depressive symptoms in cognitively normal older adults. Am J Psychiatry. 2018;175:530–7. doi: 10.1176/appi.ajp.2017.17040442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harrington KD, Gould E, Lim YY, Ames D, Pietrzak RH, Rembach A, et al. Amyloid burden and incident depressive symptoms in cognitively normal older adults. Int J Geriatr Psychiatry. 2017;32:455–63. doi: 10.1002/gps.4489. [DOI] [PubMed] [Google Scholar]
- 24.Babulal GM, Ghoshal N, Head D, Vernon EK, Holtzman DM, Benzinger TLS, et al. Mood changes in cognitively normal older adults are linked to Alzheimer disease biomarker levels. Am J Geriatr Psychiatry. 2016;24:1095–104. doi: 10.1016/j.jagp.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krell-Roesch J, Lowe VJ, Neureiter J, Pink A, Roberts RO, Mielke MM, et al. Depressive and anxiety symptoms and cortical amyloid deposition among cognitively normal elderly persons: the Mayo Clinic Study of Aging. Int Psychogeriatr. 2018;30:245–51. doi: 10.1017/S1041610217002368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perin S, Harrington KD, Lim YY, Ellis K, Ames D, Pietrzak RH, et al. Amyloid burden and incident depressive symptoms in preclinical Alzheimer’s disease. J Affect Disord. 2018;229:269–74. doi: 10.1016/j.jad.2017.12.101. [DOI] [PubMed] [Google Scholar]
- 27.Poisnel G, Arenaza-Urquijo E, Collette F, Klimecki OM, Marchant NL, Wirth M, et al. The Age-Well randomized controlled trial of the Medit-Ageing European project: effect of meditation or foreign language training on brain and mental health in older adults. Alzheimers Dement. 2018;4:714–23. doi: 10.1016/j.trci.2018.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–9. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 29.Petersen RC, Aisen PS, Beckett LA, Donohue MC, Gamst AC, Harvey DJ, et al. Alzheimer’s Disease Neuroimaging Initiative (ADNI): clinical characterization. Neurology. 2010;74:201–9. doi: 10.1212/WNL.0b013e3181cb3e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. Clin Gerontologist J Aging Ment Health. 1986;5:165–73. [Google Scholar]
- 31.Wancata J, Alexandrowicz R, Marquart B, Weiss M, Friedrich F. The criterion validity of the Geriatric Depression Scale: a systematic review. Acta Psychiatr Scand. 2006;114:398–410. doi: 10.1111/j.1600-0447.2006.00888.x. [DOI] [PubMed] [Google Scholar]
- 32.Rodríguez MR, Nuevo R, Chatterji S, Ayuso-Mateos JL. Definitions and factors associated with subthreshold depressive conditions: a systematic review. BMC Psychiatry. 2012;12:181. doi: 10.1186/1471-244X-12-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ezzati A, Katz MJ, Derby CA, Zimmerman ME, Lipton RB. Depressive symptoms predict incident dementia in a community sample of older adults: results from the Einstein Aging Study. J Geriatr Psychiatry Neurol. 2019;32:97–103. doi: 10.1177/0891988718824036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Folstein MF, Folstein SE, McHugh PR. Mini-mental state’. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 35.Mattis S. Mental status examination for organic mental syndrome in the elderly patient. In: Bellack L, Karusu TB, editors. Geriatric Psychiatry. New York: Grune & Stratton; 1976. p. 77–121.
- 36.Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatrics Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 37.Delis D. California Verbal Learning Test®—Second Edition. 2018. https://www.pearsonclinical.com/psychology/products/100000166/california-verballearning-test-second-edition-cvlt-ii.html.
- 38.Moradi E, Hallikainen I, Hänninen T, Tohka J. Alzheimer’s Disease Neuroimaging Initiative. Rey’s Auditory Verbal Learning Test scores can be predicted from whole brain MRI in Alzheimer’s disease. Neuroimage Clin. 2017;13:415–27. doi: 10.1016/j.nicl.2016.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Falangola MF, Jensen JH, Babb JS, Hu C, Castellanos FX, Martino AD, et al. Age-related non-Gaussian diffusion patterns in the prefrontal brain. J Magn Reson Imaging. 2008;28:1345–50. doi: 10.1002/jmri.21604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Falangola MF, Jensen JH, Tabesh A, Hu C, Deardorff RL, Babb JS, et al. Non-Gaussian diffusion MRI assessment of brain microstructure in mild cognitive impairment and Alzheimer’s disease. Magn Reson Imaging. 2013;31:840–6. doi: 10.1016/j.mri.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.La Joie R, Perrotin A, de La Sayette V, Egret S, Doeuvre L, Belliard S, et al. Hippocampal subfield volumetry in mild cognitive impairment, Alzheimer’s disease and semantic dementia. Neuroimage Clin. 2013;3:155–62. doi: 10.1016/j.nicl.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54:1063–70. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 43.Treynor W, Gonzalez R, Nolen-Hoeksema S. Rumination reconsidered: a psychometric analysis. Cogn Ther Res. 2003;27:247–59. [Google Scholar]
- 44.Forman EM, Herbert JD, Juarascio AS, Yeomans PD, Zebell JA, Goetter EM, et al. The Drexel defusion scale: a new measure of experiential distancing. J Contextual Behav Sci. 2012;1:55–65. [Google Scholar]
- 45.Gross JJ, John OP. Individual differences in two emotion regulation processes: implications for affect, relationships, and well-being. J Pers Soc Psychol. 2003;85:348–62. doi: 10.1037/0022-3514.85.2.348. [DOI] [PubMed] [Google Scholar]
- 46.den Heijer T, Tiemeier H, Luijendijk HJ, van der Lijn F, Koudstaal PJ, Hofman A, et al. A study of the bidirectional association between hippocampal volume on magnetic resonance imaging and depression in the elderly. Biol Psychiatry. 2011;70:191–7. doi: 10.1016/j.biopsych.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 47.Elbejjani M, Fuhrer R, Abrahamowicz M, Mazoyer B, Crivello F, Tzourio C, et al. Hippocampal atrophy and subsequent depressive symptoms in older men and women: results from a 10-year prospective cohort. Am J Epidemiol. 2014;180:385–93. doi: 10.1093/aje/kwu132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Buddeke J, Kooistra M, Zuithoff NPA, Gerritsen L, Biessels GJ, Graaf Y, et al. Hippocampal volume and the course of depressive symptoms over eight years of follow-up. Acta Psychiatr Scand. 2017;135:78–86. doi: 10.1111/acps.12662. [DOI] [PubMed] [Google Scholar]
- 49.Zhang Z, Wei F, Shen X-N, Ma Y-H, Chen K-L, Dong Q, et al. Associations of subsyndromal symptomatic depression with cognitive decline and brain atrophy in elderly individuals without dementia: a longitudinal study. J Affect Disord. 2020;274:262–8. doi: 10.1016/j.jad.2020.05.097. [DOI] [PubMed] [Google Scholar]
- 50.Sexton CE, Mackay CE, Ebmeier KP. A systematic review and meta-analysis of magnetic resonance imaging studies in late-life depression. Am J Geriatr Psychiatry. 2013;21:184–95. doi: 10.1016/j.jagp.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 51.Teipel S, Drzezga A, Grothe MJ, Barthel H, Chételat G, Schuff N, et al. Multimodal imaging in Alzheimer’s disease: validity and usefulness for early detection. Lancet Neurol. 2015;14:1037–53. doi: 10.1016/S1474-4422(15)00093-9. [DOI] [PubMed] [Google Scholar]
- 52.Geerlings MI, Gerritsen L. Late-life depression, hippocampal volumes, and hypothalamic-pituitary-adrenal axis regulation: a systematic review and meta-analysis. Biol Psychiatry. 2017;82:339–50. doi: 10.1016/j.biopsych.2016.12.032. [DOI] [PubMed] [Google Scholar]
- 53.Wang Q, Van Heerikhuize J, Aronica E, Kawata M, Seress L, Joels M, et al. Glucocorticoid receptor protein expression in human hippocampus; stability with age. Neurobiol Aging. 2013;34:1662–73. doi: 10.1016/j.neurobiolaging.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 54.Alexopoulos GS, Morimoto SS. The inflammation hypothesis in geriatric depression. Int J Geriatr Psychiatry. 2011;26:1109–18. doi: 10.1002/gps.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gatchel JR, Donovan NJ, Locascio JJ, Schultz AP, Becker JA, Chhatwal J, et al. Depressive symptoms and tau accumulation in the inferior temporal lobe and entorhinal cortex in cognitively normal older adults: a pilot study. J Alzheimers Dis. 2017;59:975–85. doi: 10.3233/JAD-170001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Babulal GM, Roe CM, Stout SH, Rajasekar G, Wisch JK, Benzinger TLS, et al. Depression is associated with tau and not amyloid positron emission tomography in cognitively normal adults. J Alzheimers Dis. 2020;74:1045–55. doi: 10.3233/JAD-191078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hayakawa YK, Sasaki H, Takao H, Mori H, Hayashi N, Kunimatsu A, et al. Structural brain abnormalities in women with subclinical depression, as revealed by voxel-based morphometry and diffusion tensor imaging. J Affect Disord. 2013;144:263–8. doi: 10.1016/j.jad.2012.10.023. [DOI] [PubMed] [Google Scholar]
- 58.Hayakawa YK, Sasaki H, Takao H, Hayashi N, Kunimatsu A, Ohtomo K, et al. Depressive symptoms and neuroanatomical structures in community-dwelling women: a combined voxel-based morphometry and diffusion tensor imaging study with tract-based spatial statistics. Neuroimage Clin. 2014;4:481–7. doi: 10.1016/j.nicl.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Allan CL, Sexton CE, Filippini N, Topiwala A, Mahmood A, Zsoldos E, et al. Sub-threshold depressive symptoms and brain structure: a magnetic resonance imaging study within the Whitehall II cohort. J Affect Disord. 2016;204:219–25. doi: 10.1016/j.jad.2016.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lamar M, Charlton RA, Morris RG, Markus HS. The impact of subcortical white matter disease on mood in euthymic older adults: a diffusion tensor imaging study. Am J Geriatr Psychiatry. 2010;18:634–42. doi: 10.1097/JGP.0b013e3181cabad1. [DOI] [PubMed] [Google Scholar]
- 61.Wen M-C, Steffens DC, Chen M-K, Zainal NH. Diffusion tensor imaging studies in late-life depression: systematic review and meta-analysis. Int J Geriatr Psychiatry. 2014;29:1173–84. doi: 10.1002/gps.4129. [DOI] [PubMed] [Google Scholar]
- 62.Benedetti F, Poletti S, Hoogenboezem TA, Mazza E, Ambrée O, de Wit H, et al. Inflammatory cytokines influence measures of white matter integrity in Bipolar Disorder. J Affect Disord. 2016;202:1–9. doi: 10.1016/j.jad.2016.05.047. [DOI] [PubMed] [Google Scholar]
- 63.Sexton CE, Kalu UG, Filippini N, Mackay CE, Ebmeier KP. A meta-analysis of diffusion tensor imaging in mild cognitive impairment and Alzheimer’s disease. Neurobiol Aging. 2011;32:2322.e5–18.. doi: 10.1016/j.neurobiolaging.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 64.Oishi K, Lyketsos CG. Alzheimer’s disease and the fornix. Front Aging Neurosci. 2014;6:241. doi: 10.3389/fnagi.2014.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Knyazeva MG. Splenium of Corpus Callosum: patterns of interhemispheric interaction in children and adults. Neural Plasticity. 2013;2013:e639430. doi: 10.1155/2013/639430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bubb EJ, Metzler-Baddeley C, Aggleton JP. The cingulum bundle: anatomy, function, and dysfunction. Neurosci Biobehav Rev. 2018;92:104–127. doi: 10.1016/j.neubiorev.2018.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Villain N, Fouquet M, Baron J-C, Mézenge F, Landeau B, de La Sayette V, et al. Sequential relationships between grey matter and white matter atrophy and brain metabolic abnormalities in early Alzheimer’s disease. Brain. 2010;133:3301–14. doi: 10.1093/brain/awq203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:1125–65. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lindquist KA, Wager TD, Kober H, Bliss-Moreau E, Barrett LF. The brain basis of emotion: a meta-analytic review. Behav Brain Sci. 2012;35:121–43. doi: 10.1017/S0140525X11000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tadayonnejad R, Ajilore O. Brain network dysfunction in late-life depression: a literature review. J Geriatr Psychiatry Neurol. 2014;27:5–12. doi: 10.1177/0891988713516539. [DOI] [PubMed] [Google Scholar]
- 71.Alexopoulos GS. Mechanisms and treatment of late-life depression. Transl Psychiatry. 2019;9:1–16.. doi: 10.1038/s41398-019-0514-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Alexopoulos GS, Hoptman MJ, Kanellopoulos D, Murphy CF, Lim KO, Gunning FM. Functional connectivity in the cognitive control network and the default mode network in late-life depression. J Affect Disord. 2012;139:56–65. doi: 10.1016/j.jad.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Andrews-Hanna JR, Smallwood J, Spreng RN. The default network and self-generated thought: component processes, dynamic control, and clinical relevance. Ann N Y Acad Sci. 2014;1316:29–52. doi: 10.1111/nyas.12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Makovac E, Fagioli S, Rae CL, Critchley HD, Ottaviani C. Can’t get it off my brain: meta-analysis of neuroimaging studies on perseverative cognition. Psychiatry Res Neuroimaging. 2020;295:0925–4927. [DOI] [PubMed]
- 75.Lugo SB, Deza-Araujo Y, Collette F, Vuilleumier P, Klimecki O. The Medit-Ageing Research. Exposure to negative socio-emotional events induces sustained alteration of resting-state brain networks in the elderly. 2020 (Preprint at Research Square). [DOI] [PubMed]
- 76.Buckner RL. Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25:7709–17. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sheline YI, Raichle ME. Resting state functional connectivity in preclinical Alzheimer’s disease. Biol Psychiatry. 2013;74:340–7. doi: 10.1016/j.biopsych.2012.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Badhwar A, Tam A, Dansereau C, Orban P, Hoffstaedter F, Bellec P. Resting-state network dysfunction in Alzheimer’s disease: a systematic review and meta-analysis. Alzheimers Dement. 2017;8:73–85. doi: 10.1016/j.dadm.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Menon V. Salience network. In: Toga AW, editor. Brain mapping. Waltham: Academic Press; 2015. p. 597–611.
- 80.Breukelaar IA, Antees C, Grieve SM, Foster SL, Gomes L, Williams LM, et al. Cognitive control network anatomy correlates with neurocognitive behavior: a longitudinal study. Hum Brain Mapp. 2017;38:631–43. doi: 10.1002/hbm.23401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Manoliu A, Meng C, Brandl F, Doll A, Tahmasian M, Scherr M, et al. Insular dysfunction within the salience network is associated with severity of symptoms and aberrant inter-network connectivity in major depressive disorder. Front Hum Neurosci. 2014;7:930. [DOI] [PMC free article] [PubMed]
- 82.Demnitz-King H, Göehre I, Marchant NL. The neuroanatomical correlates of repetitive negative thinking: a systematic review. Psychiatry Res Neuroimaging. 2021;316:111353. doi: 10.1016/j.pscychresns.2021.111353. [DOI] [PubMed] [Google Scholar]
- 83.Marchant NL, Lovland LR, Jones R, Pichet Binette A, Gonneaud J, Arenaza-Urquijo EM, et al. Repetitive negative thinking is associated with amyloid, tau, and cognitive decline. Alzheimers Dement. 2020;16:1054–64. doi: 10.1002/alz.12116. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.